Mineral Sources and Vertical Distribution of Nutrients in Extremely Acidic Pit Lakes: Impact on Microbial Ecology
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Water and Sediment Sampling
2.3. Chemical Analyses
3. Results and Discussion
3.1. Sources and Chemical Species of Essential Nutrients
3.1.1. Dissolved Inorganic Carbon (DIC)
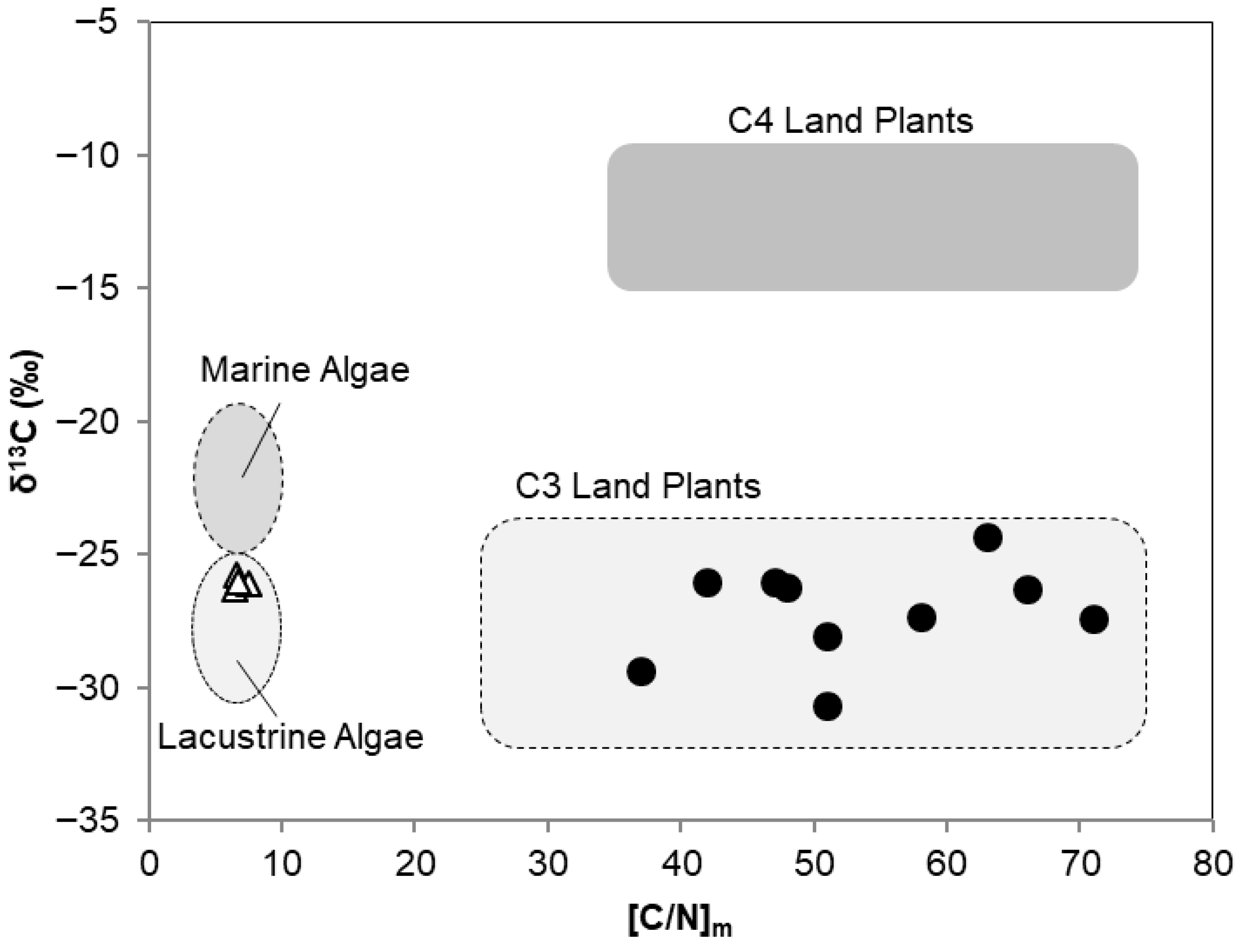
3.1.2. Total Organic Carbon (TOC)
3.1.3. Phosphorus
3.1.4. Nitrogen
3.1.5. Silicon
3.2. Nutrient Concentrations in the Water Column: Vertical and Seasonal Variations
3.2.1. Dissolved Inorganic Carbon
3.2.2. Total Organic Carbon
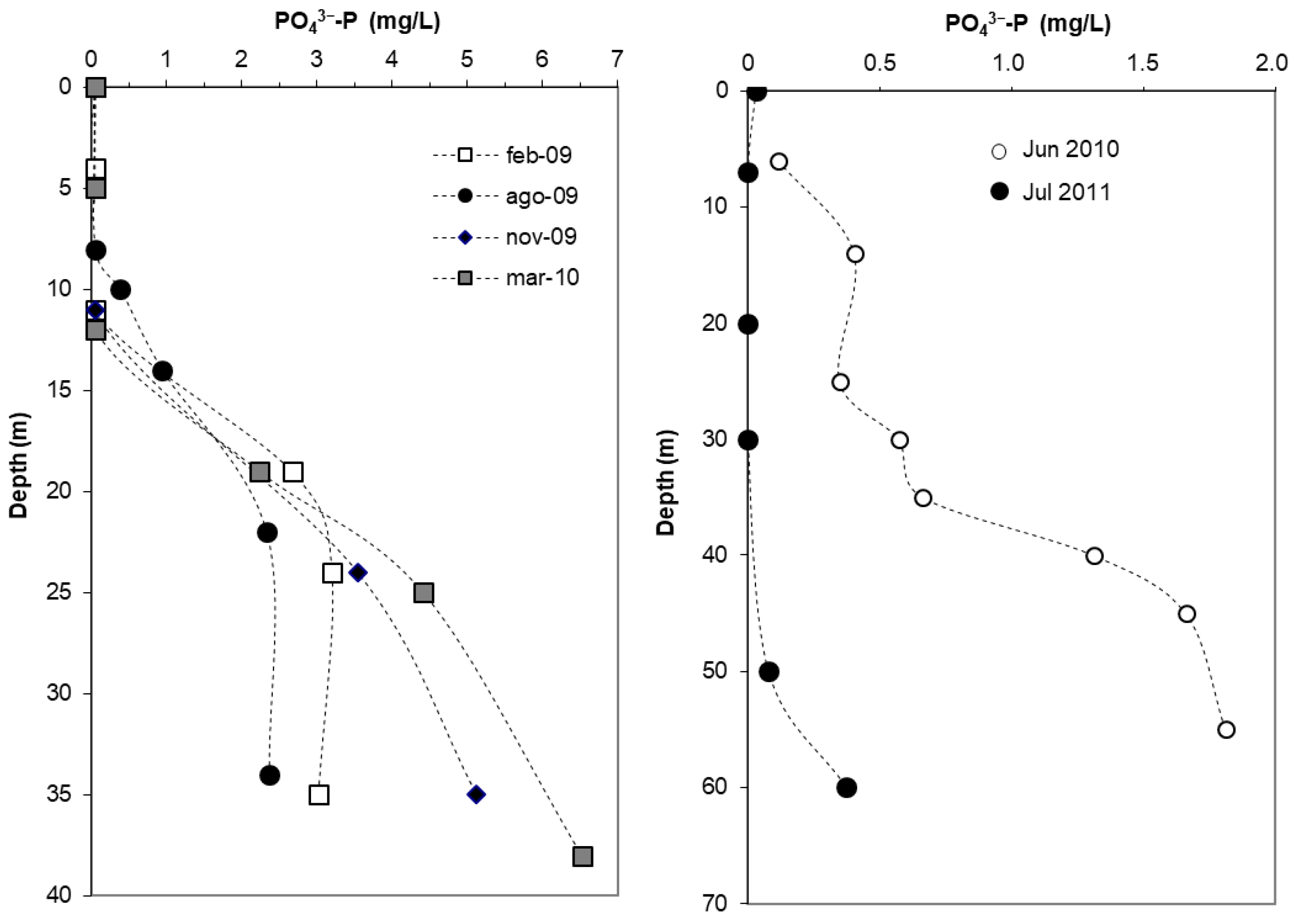
3.2.3. Phosphorus
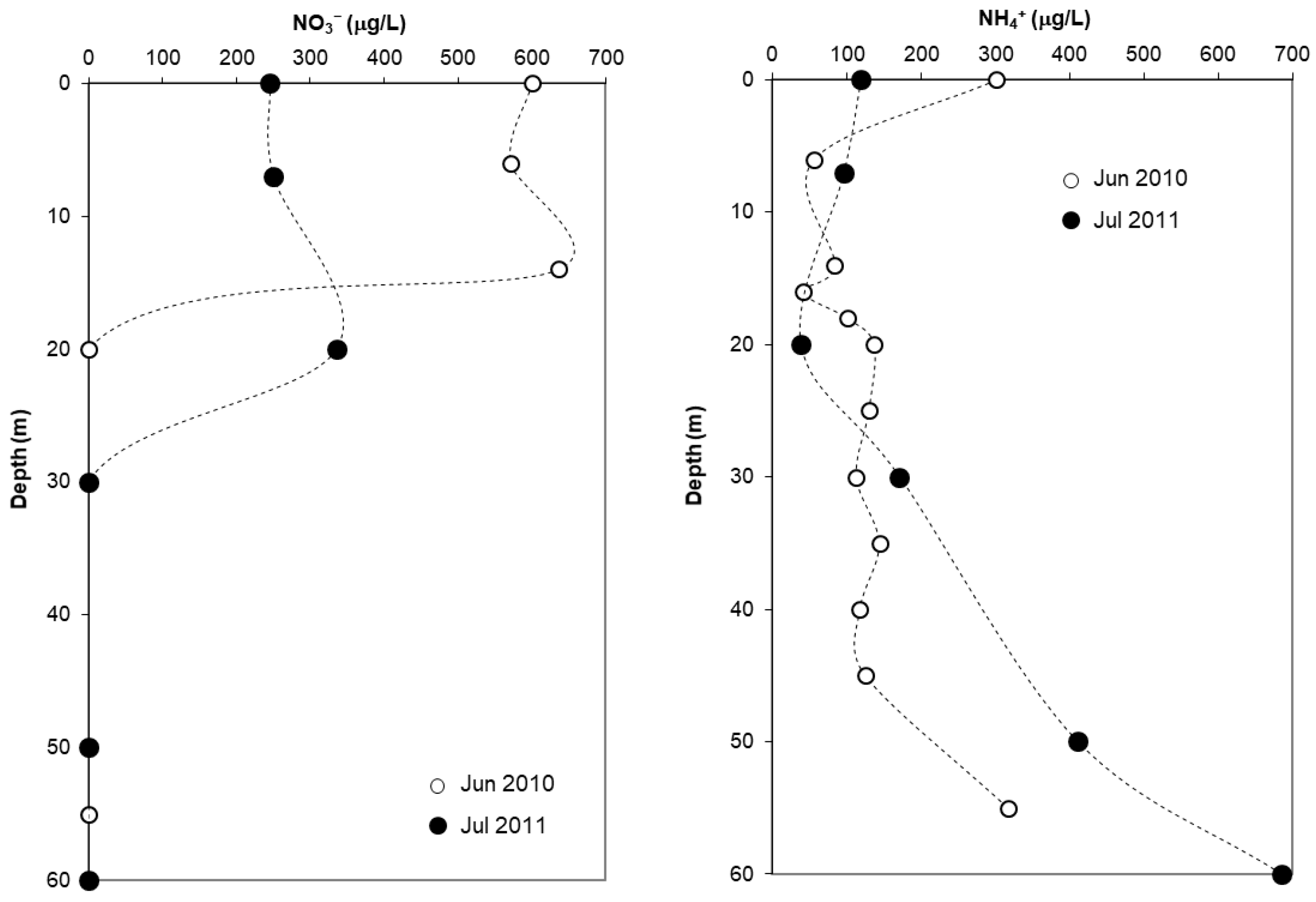
3.2.4. Dissolved Inorganic Nitrogen
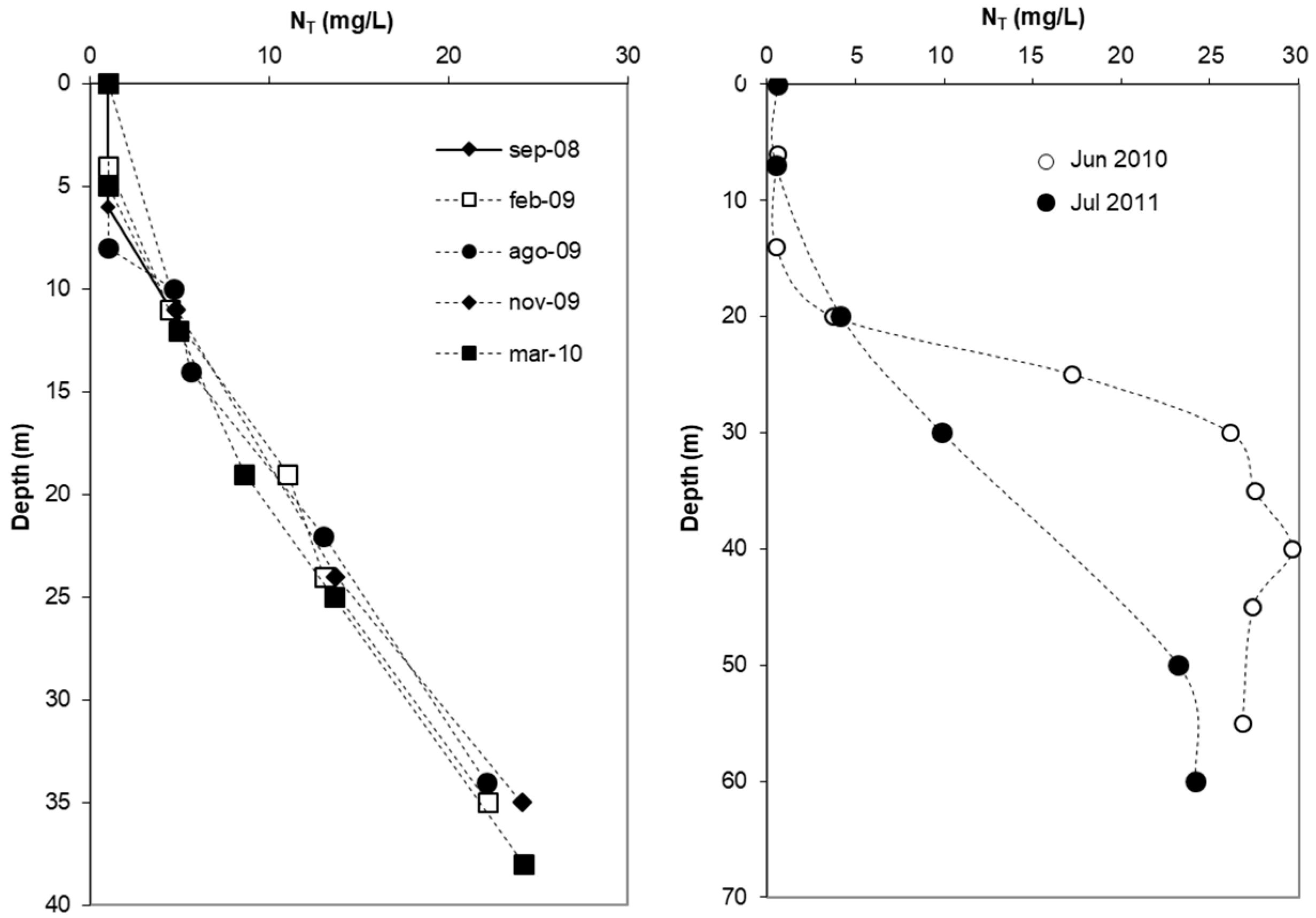
3.2.5. Total Nitrogen
3.2.6. Soluble Silica
3.3. Nutrient Concentrations in Shallow and Deep Sediments
3.4. Vertical Fluxes of Nutrients Across the Water Column
3.5. Influence of Nutrient Distribution on Phytoplankton Dynamics
3.6. Influence of Nutrient Distribution on Bacterial Ecology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetzel, D.B. Limnology: Lake and River Ecosystems, 3rd ed.; Academic Press: San Diego, CA, USA, 2001; 1006p. [Google Scholar]
- Konhauser, K. Introduction to Geomicrobiology; Blackwell Publishing: Oxford, UK, 2007; 425p. [Google Scholar]
- Lessmann, D.; Nixdorf, B. Phytoplankton (of acidic pit lakes). In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, B., Wolkersdorfer, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–116. [Google Scholar]
- Geller, W.; Klapper, H.; Salomons, W. (Eds.) Acidic Mining Lakes: Acid Mine Drainage, Limnology and Reclamation; Springer: Berlin, Germany, 1998; 435p. [Google Scholar]
- Geller, W.; Schultze, M.; Kleinmann, R.; Wolkersdorfer, C. Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Springer: Heidelberg, Germany, 2013; 525p. [Google Scholar]
- McCullough, C.D. Approaches to remediation of acid mine drainage water in pit lakes. Int. J. Min. Reclam. Environ. 2008, 22, 105–119. [Google Scholar] [CrossRef]
- Castendyk, D.E.; Eary, L.E. Mine Pit Lakes; Society for Mining, Metallurgy, and Exploration: Littleton, CO, USA, 2009. [Google Scholar]
- Wendt-Potthoff, K. The biology and ecosystems of acidic pit lakes. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, B., Wolkersdorfern, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 107–186. [Google Scholar]
- Wendt-Potthoff, K.; Koschorreck, M.; Diez, M.; Sánchez-España, J. High microbial activity in a nutrient-rich, acidic mine pit lake. Limnologica 2012, 42, 175–188. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Diez, M.; Santofimia, E. Mine pit lakes of the Iberian Pyrite Belt: Some basic limnological, hydrogeochemical and microbiological considerations. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kelinmann, R., Wolkerdorfer, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 315–342. [Google Scholar] [CrossRef]
- Diez-Ercilla, M.; Sánchez-España, J.; Yusta, I.; Wendt-Potthoff, K.; Koschorreck, M. Formation of biogenic sulphides in the water column of an acidic pit lake: Biogeochemical controls and effects on trace metal dynamics. Biogeochemistry 2014, 121, 519–536. [Google Scholar] [CrossRef]
- Falagán, C.; Sánchez-España, J.; Johnson, D.B. New insights into the biogeochemistry of extremely acidic environments revealed by a combined cultivation-based and culture-independent study of two stratified pit lakes. FEMS Microbiol. Ecol. 2014, 87, 231–243. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Ilin, A.; van der Graaf, C.; Sánchez-Andrea, I. Microbial Geochemistry of the Acidic Saline Pit Lake of Brunita Mine (La Unión, SE Spain). Mine Water Environ. 2020, 39, 535–555. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Falagán, C.; Ayala-Muñoz, D.; Wendt-Potthoff, K. Adaptation of Coccomyxa sp. to extremely low light conditions causes deep chlorophyll and oxygen maxima in acidic pit lakes. Microorganisms 2020, 8, 1218. [Google Scholar] [CrossRef]
- van der Graaf, C.M.; Sánchez-España, J.; Yusta, I.; Ilin, A.; Shetty, S.A.; Bale, N.J.; Villanueva, L.; Stams, A.J.M.; Sánchez-Andrea, I. Biosulfidogenesis Mediates Natural Attenuation in Acidic Mine Pit Lakes. Microorganisms 2020, 8, 1275. [Google Scholar] [CrossRef]
- Ayala-Muñoz, D.; Macalady, J.L.; Sánchez-España, J.; Falagán, C.; Couradeau, E.; Burgos, W.D. Microbial carbon, sulfur, iron, and nitrogen cycling linked to the potential remediation of a meromictic acidic pit lake. ISME J. 2022, 16, 2666–2679. [Google Scholar] [CrossRef]
- Sánchez-España, J. From Volcanic Craters to Mine Pits: The Microbial Ecology of Extremophiles Inhabiting the Most Acidic Lakes in the World. Geomicrobiol. J. 2025, 42, 633–650. [Google Scholar] [CrossRef]
- Liu, Y.; Macalady, J.L.; Sánchez-España, J.; Burgos, W.D. Enrichment of acid-tolerant sulfide-producing microbes from an acidic pit lake. Front. Microbiol. 2024, 15, 1475137. [Google Scholar] [CrossRef]
- Sánchez-España, J.; López-Pamo, E.; Santofimia, E.; Diez-Ercilla, M. The acidic mine pit lakes of the Iberian Pyrite Belt: An approach to their physical limnology and hydrogeochemistry. Appl. Geochem. 2008, 23, 1260–1287. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; López, G.A. Schwertmannite to jarosite conversion in the water column of an acidic mine pit lake. Mineral. Mag. 2012, 76, 2659–2682. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Santofimia, E.; González-Toril, E.; San Martín-Úriz, P.; López-Pamo, E.; Amils, R. Physicochemical and Microbiological Stratification of a Meromictic Pit Lake (San Telmo, IPB). In Proceedings of the IMWA Symposium 2007: Water in Mining Environment, Cagliari, Italy, 27–31 May 2007; Cidu, R., Frau, F., Eds.; pp. 447–451. [Google Scholar]
- Sánchez-España, J.; López-Pamo, E.; Diez, M.; Santofimia, E. Physico-chemical gradients and meromictic stratification in Cueva de la Mora and other acidic pit lakes of the Iberian Pyrite Belt. Mine Water Environ. 2009, 28, 15–29. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Yusta, I.; Diez-Ercilla, M. Schwertmannite and hydrobasaluminite: A re-evaluation of their solubility and control on the iron and aluminum concentration in acidic pit lakes. Appl. Geochem. 2011, 26, 1752–1774. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Diez, M.; Pérez-Cerdán, F.; Yusta, I.; Boyce, A.J. Hydrological investigation of a multi-stratified pit lake using radioactive and stable isotopes combined with hydrometric monitoring. J. Hydrol. 2014, 511, 494–508. [Google Scholar] [CrossRef]
- Sánchez-España, J.; Boehrer, B.; Yusta, I. Extreme Carbon dioxide concentrations in acidic pit lakes provoked by water/rock interaction. Environ. Sci. Technol. 2014, 48, 4273–4281. [Google Scholar] [CrossRef]
- Fernández-Caliani, J.C.; Barba-Brioso, C.; González, I.; Galán, E. Heavy metal pollution in soils around the abandoned mine sites of the Iberian Pyrite Belt (Southwest Spain). Water Air Soil Pollut. 2009, 200, 211–226. [Google Scholar] [CrossRef]
- Leistel, J.M.; Marcoux, E.; Thiéblemont, D.; Quesada, C.; Sánchez, A.; Almodóvar, G.R.; Pacual, E.; Sáez, R. The volcanic-hosted massive sulphide deposits of the Iberian Pyrite Belt. Miner. Depos. 1998, 33, 2–30. [Google Scholar] [CrossRef]
- Sánchez España, J. Mineralogy and Geochemistry of Massive Sulphide Deposits in the Northern Iberian Pyrite Belt (San Telmo-San Miguel-Peña del Hierro). Ph.D. Thesis, Universidad del País Vasco (UPV/EH), Huelva, Spain, 2000; 307p. [Google Scholar]
- López-García, J.A.; Manteca, J.I.; Prieto, A.C.; Calvo, B. Primera aparición en España de cronstedtita. Caracterización estructural. Bol. Soc. Esp. Miner. 1992, 15, 21–25. [Google Scholar]
- Cánovas, M.; Alhama, I.; López, G. La paragénesis mineralógica de la Cantera “Brunita”. In Introducción a la Investigación de la UPCT, Investigación EICM, 6th ed.; Universidad Politécnia de Cartagena: Cartagena, Spain, 2013; pp. 40–42. (In Spanish) [Google Scholar]
- Sánchez-España, J.; López-Pamo, E.; Santofimia, E.; Aduvire, O.; Reyes, J.; Barettino, D. Acid mine drainage in the Iberian Pyrite Belt (Odiel river watershed, Huelva, SW Spain): Geochemistry, mineralogy and environmental implications. Appl. Geochem. 2005, 20, 1320–1356. [Google Scholar] [CrossRef]
- Salminen, J.; Kobylin, P.; Ojala, A. Solubility of carbon dioxide in natural systems. In Thermodynamics, Solubility and Environmental Issues; Letcher, T.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2007; pp. 189–203. [Google Scholar]
- Stumm, W.; Morgan, J.J. Aquatic Chemistry: Chemical Equilibria and Rates in Natural Waters, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1996; p. 1022. [Google Scholar]
- Yusta, I.; Martínez, A.; Velasco, F. Litogeoquímica de las pizarras negras de la Faja Pirítica Ibérica: Implicaciones en la génesis de los sulfuros masivos. Bol. Soc. Esp. Miner. 2002, 25, 107–108. [Google Scholar]
- Poerschmann, J.; Spijkerman, E.; Langer, U. Fatty acid patterns in Chlamydomonas sp. as a marker for nutritional regimes and temperature under extremely acidic conditions. Microb. Ecol. 2004, 48, 78–89. [Google Scholar] [CrossRef]
- Friese, K.; Herzsprung, P.; Witter, B. Photochemical degradation of organic carbon in acidic mining lakes. Acta Hydrochim. Hydrobiol. 2002, 30, 141–148. [Google Scholar] [CrossRef]
- Diez-Ercilla, M.; López-Pamo, E.; Sánchez-España, J. Photoreduction of Fe(III) in the Acidic Mine Pit Lake of San Telmo (Iberian Pyrite Belt): Field and Experimental Work. Aquat. Geochem. 2009, 15, 391–419. [Google Scholar] [CrossRef]
- Kamjunke, N.; Tittel, J.; Krumbeck, H.; Beulker, C.; Poerschmann, J. High heterotrophic bacterial production in acidic, iron-rich mining lakes. Microb. Ecol. 2005, 49, 425–433. [Google Scholar] [CrossRef]
- Nixdorf, B.; Wollmann, K.; Deneke, R. Ecological potentials for planktonic development and food web interactions in extremely acidic mining lakes in Lusatia. In Acid Mining Lakes: Acid Mine Drainage, Limnology and Reclamation; Geller, W., Klapper, H., Salomons, W., Eds.; Springer: Berlin, Germany, 1998; pp. 147–167. [Google Scholar]
- Beulker, C.; Lessmann, D.; Nixdorf, B. Aspects of phytoplankton succession and spatial distribution in an acidic mining lake (Plessa 117, Germany). Acta Oecol. 2003, 24, 25–31. [Google Scholar] [CrossRef]
- Spijkerman, E. Phosphorus limitation of algae living in iron-rich, acidic lakes. Aquat. Microbiol. Ecol. 2008, 53, 201–210. [Google Scholar] [CrossRef]
- Kemp, A.L.W.; Mudrochova, A. Distribution and forms of nitrogen in a Lake Ontario sediment core. Limnol. Oceanogr. 1972, 17, 855–867. [Google Scholar] [CrossRef]
- McCarthy, M.; Pratum, T.; Hedges, J.; Benner, R. Chemical composition of dissolved organic nitrogen in the ocean. Nature 1997, 390, 150–154. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Kraus, T.E.C.; Dahlgren, R.A.; Anastasio, C.; Zasoski, R.J. Contribution of amino compounds to dissolved organic nitrogen in forest soils. Biogeochemistry 2002, 61, 173–198. [Google Scholar] [CrossRef]
- Pehlivanoglu, E.; Sedlak, D.L. Measurement of dissolved organic nitrogen forms in wastewater effluents: Concentrations, size distribution and NDMA formation potential. Water Res. 2008, 42, 3890–3898. [Google Scholar] [CrossRef]
- Stevenson, F.J. Chemical state of the nitrogen in rock. Geochim. Chosmochim. Acta 1962, 26, 797–809. [Google Scholar] [CrossRef]
- Müller, P.J. C/N ratios in Pacific deep-sea sediments: Effect of inorganic ammonium and organic nitrogen compounds sorbed by clays. Geochim. Cosmochim. Acta 1997, 41, 765–776. [Google Scholar] [CrossRef]
- Nieto, F. Characterization of coexisting NH4- and K-micas in very low-grade metapelites. Am. Mineral. 2002, 87, 205–216. [Google Scholar] [CrossRef]
- Schubert, C.J.; Calvert, S.E. Nitrogen and carbon isotopic composition of marine and terrestrial organic matter in Arctic Ocean sediments: Implications for nutrient utilization and organic matter composition. Deep-Sea Res. I 2002, 48, 789–810. [Google Scholar] [CrossRef]
- Ager, F.J.; Mata, M.P.; Ynsa, M.D.; Respaldiza, M.A.; Goffé, B.; Nieto, F. Nitrogen determination in micas of metamorphic rocks. Nucl. Instrum. Methods Phys. Res. B 2006, 249, 642–645. [Google Scholar] [CrossRef]
- Meybeck, M. Carbon, nitrogen and phosphorus transport by world rivers. Am. J. Sci. 1982, 282, 401–450. [Google Scholar] [CrossRef]
- Seitzinger, S.P.; Sanders, S.P. Contribution of dissolved organic nitrogen from rivers to estuarine eutrophication. Mar. Ecol. Prog. Ser. 1997, 159, 1–12. [Google Scholar] [CrossRef]
- Castro, A.M.; Calvo, M.; García, G.; Alonso, A. La mina de Reocín. Bocamina 2001, 8, 14–67. [Google Scholar]
- Yinon, J. Toxicity and Metabolism of Explosives; CRC Press Inc.: Boca Raton, FL, USA, 1990. [Google Scholar]
- Yoon, J.M.; Van Aken, B.; Schnoor, J.L. Leaching of contaminated leaves following uptake and phytoremediation of RDX, HMX, and TNT by poplar. Int. J. Phytoremediat. 2006, 8, 81–94. [Google Scholar] [CrossRef]
- Ju, K.S.; Perales, R.E. Nitroaromatic compounds, from synthesis to biodegradation. Microbiol. Mol. Biol. Rev. 2010, 74, 250–272. [Google Scholar] [CrossRef] [PubMed]
- Farling, S. Generation of Biodegradation-Sorption Barriers for Munitions Constituents. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2013; 93p. [Google Scholar]
- Wani, A.H.; Davis, J.L. RDX biodegradation column study: Influence of ubiquitous electron acceptors on anaerobic biotransformation of RDX. J. Chem. Technol. Biotechnol. 2003, 78, 1082–1092. [Google Scholar] [CrossRef]
- Smets, B.F.; Yin, H.; Esteve-Nuñez, A. TNT biotransformation: When chemistry confronts mineralization. Appl. Microbiol. 2007, 76, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Sungsoo, H. In Situ Bioremediation and Natural Attenuation of Dinitrotoluenes and Trinitrotoluene. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2008; 238p. [Google Scholar]
- Bernstein, A.; Ronen, Z. Biodegradation of the Explosives TNT, RDX and HMX. In Microbial Degradation of Xenobiotics, Environmental Science and Engineering; Singh, S.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 135–176. [Google Scholar]
- Davis, C.C. Aqueous Silica in the Environment: Effects on Iron Hydroxide Surface Chemistry and Implications for Natural and Engineered Systems. Master’s Thesis, Virginia Polytechnic Institute and State University, Blacksburg, Virginia, 2000; 49p. [Google Scholar]
- Davis, C.; Chen, H.W.; Edwards, M. Modeling Silica Sorption to Iron Hydroxide. Environ. Sci. Technol. 2002, 36, 582–587. [Google Scholar] [CrossRef]
- Alcolea, A.; Fernández-López, C.; Vázquez, M.; Caparrós, A.; Ibarra, I.; García, C.; Zarroca, M.; Rodríguez, R. An assessment of the influence of sulfidic mine wastes on rainwater quality in a semiarid climate (SE Spain). Atmos. Environ. 2015, 107, 85–94. [Google Scholar] [CrossRef]
- Noges, T. Relationships between morphology, geographic location and water quality parameters of European lakes. Hydrobiologia 2009, 633, 33–43. [Google Scholar] [CrossRef]
- Jeschke, C.; Falagán, C.; Knöller, K.; Schultze, M.; Koschorreck, M. No nitrification in lakes below pH 3. Environ. Sci. Technol. 2014, 47, 14018–14023. [Google Scholar] [CrossRef]
- Jackson, G.A.; Williams, P.M. Importance of dissolved organic nitrogen and phosphorus to biological nutrient cycling. Deep Sea Res. 1985, 32, 223–235. [Google Scholar]
- Quirós, R. The nitrogen to phosphorus ratio for lakes: A cause or a consequence of aquatic biology? In El Agua en Iberoamérica: De la Limnología a la Gestión en Sudamerica; Fernández, A., Chalar, G., Eds.; CYTED: Buenos Aires, Argentina, 2002; pp. 11–26. [Google Scholar]
- Sánchez-España, J.; Yusta, I.; Burgos, W.D. Geochemistry of dissolved aluminum at low pH: Hydrobasaluminite formation and interaction with trace metals, silica and microbial cells under anoxic conditions. Chem. Geol. 2016, 441, 124–137. [Google Scholar] [CrossRef]
- Meyers, P. Organic geochemical proxies of paleoceanographic, paleolimnologic, and paleoclimatic processes. Org. Geochem. 1997, 27, 213–250. [Google Scholar] [CrossRef]
- Lessmann, D.; Fyson, A.; Nixdorf, B. Phytoplankton of the extremely acidic mining lakes of Lusatia (Germany) with pH≤3. Hydrobiologia 2000, 506, 753–758. [Google Scholar] [CrossRef]
- Kleeberg, A. Zygnematalean green algae (Streptophyta, Zygnematales) in lake impacted by acidic precipitation, experimental acidification, and acid mine drainage. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, R., Wolkersdorfer, C., Eds.; Springer: Heidelberg, Germany, 2013; pp. 159–172. [Google Scholar]
- Spijkerman, E.; Barua, D.; Gerloff-Elias, A.; Kern, J.; Gaedke, U.; Heckathorn, S.A. Stress responses and metal tolerance of Chlamydomonas acidophila in metal-enriched lake water and artificial medium. Extremophiles 2007, 11, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Weithoff, G.; Spijkerman, E.; Kamjunke, N.; Tittle, J. Trophic interactions and energy flow. In Acidic Pit Lakes: The Legacy of Coal and Metal Surface Mines; Geller, W., Schultze, M., Kleinmann, B., Wolkersdorfern, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 135–149. [Google Scholar]
- Borowitzka, L.J.; Brown, A.D. The salt regulations of marine and halophilic species of the unicelular green alga Dunaliella: The role of glycerol as a compatible solute. Arch. Microbiol. 1974, 96, 37–52. [Google Scholar] [CrossRef]
- Herzsprung, P.; Friese, K.; Packroff, G.; Schimmele, M.; Wendt-Potthoff, K.; Winkler, M. Vertical and annual distribution of ferric and ferrous iron in acidic mining lakes. Acta Hydrochim. Hydrobiol. 1998, 26, 253–262. [Google Scholar] [CrossRef]
- Brinkmann, T.; Hörsch, P.; Sartorius, D.; Frimmel, F.H. Photoformation of low-molecular weight organic acids from brown water dissolved organic matter. Environ. Sci. Technol. 2003, 37, 4190–4198. [Google Scholar] [CrossRef]




| Depth | Total Organic Carbon | Total Nitrogen | Phosphate | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (m) | Unfiltered | Filtered | Filtered | Unfiltered | Filtered | Filtered | Unfiltered | Filtered | |||
| (0.45 µm) | (0.1 µm) | (0.45 µm) | (0.1 µm) | (0.1 µm) | |||||||
| 10 | 3.36 | 2.84 | 2.12 | 4.80 | 4.80 | 2.12 | - | - | |||
| 19 | 2.27 | - | 2.11 | 13.70 | 12.40 | 12.40 | 2.68 | 1.28 | |||
| 35 | 10.70 | 8.44 | 7.98 | 24.10 | 23.10 | 22.00 | 3.03 | 0.68 | |||
| Site | Effluent Type | Total N | N-NH4+ | P-PO43− | TOC |
|---|---|---|---|---|---|
| Olivargas | Unpolluted river | 21.20 | b.d. | 0.04 | 3.06 |
| Olivargas | Unpolluted river | 6.27 | b.d. | b.d. | 5.09 |
| Río Tinto | Waste-rock pile | 6.24 | 1.58 | 0.48 | 4.30 |
| La Zarza | Mine portal | 6.39 | 0.13 | 0.12 | 4.48 |
| Pit Lake | Water Layer Type | Depth | DIC | TOC | PO43−-P | NT | NO3−-N | NH4+-N | SiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Units | m | mg/L | mg/L | µg/L | µg/L | µg/L | µg/L | mg/L | |
| San Telmo | Mixolimnion, oxygenic | 0 | 40 | 1.61 | b.d. | 704 | n.a. | 27 | n.a. |
| Mixolimnion, oxygenic | 10 | 48 | 1.20 | b.d. | b.d. | 16 | 30 | 70.4 | |
| Mixolimnion, oxygenic | 20 | 45 | 2.22 | n.a. | 1070 | n.a. | 31 | n.a. | |
| Monimolimnion, anoxic | 40 | 88 | 0.91 | b.d. | 1330 | n.a. | 47 | n.a. | |
| Monimolimnion, anoxic | 95 | 104 | 0.61 | b.d. | 1620 | 16 | 40 | 69.2 | |
| Cueva de la Mora | Mixolimnion, oxygenic | 0 | 57 | 0.54 | b.d. | b.d. | 410 | 24 | 116 |
| Mixolimnion, oxygenic | 4 | 56 | 0.57 | b.d. | b.d. | 420 | 25 | 116 | |
| Monimolimnion, anoxic | 11 | 310 | 2.70 | b.d. | 4420 | n.a. | 455 | 128 | |
| Monimolimnion, anoxic | 19 | 640 | 2.11 | 2676 | 11,000 | n.a. | 399 | 121 | |
| Monimolimnion, anoxic | 24 | 768 | 1.70 | 3210 | 13,050 | 25 | 352 | 112 | |
| Monimolimnion, anoxic | 35 | 1270 | 10.70 | 3030 | 22,200 | 25 | 580 | 80 | |
| Herrerías-Guadiana | Mixolimnion, oxygenic | 0 | 71 | 0.90 | b.d. | b.d. | 601 | 53 | 20.7 |
| Mixolimnion, oxygenic | 6 | 83 | 1.00 | 115 | 536 | 572 | 56 | 78.6 | |
| Mixolimnion, oxygenic | 14 | 67 | 1.10 | 403 | 512 | 637 | 84 | 140 | |
| Monimolimnion, anoxic | 20 | 1100 | 3.04 | b.d. | 3720 | b.d. | 137 | 70.8 | |
| Monimolimnion, anoxic | 40 | 2600 | 19.10 | 1310 | 29,600 | b.d. | 118 | 101 | |
| Monimolimnion, anoxic | 55 | 4962 | 14.20 | 1810 | 26,800 | b.d. | 317 | 20.2 | |
| Brunita | Mixolimnion, oxygenic | 0 | n.a. | 1.74 | 40 | n.a. | 1200 | 30 | 75 |
| Mixolimnion, oxygenic | 12 | n.a. | 2.87 | 40 | n.a. | 2040 | 66 | 71 | |
| Monimolimnion, anoxic | 16 | n.a. | 1.58 | 97 | n.a. | 1890 | 77 | 36 | |
| Monimolimnion, anoxic | 20 | n.a. | 1.87 | 484 | n.a. | 1190 | 494 | 20.1 | |
| Monimolimnion, anoxic | 24 | n.a. | 3.05 | 946 | n.a. | 1040 | 682 | 22.5 |
| Pit Lake | Depth | Layer | P2O5 | Ctotal | Cinorg | Corg | Ntotal |
|---|---|---|---|---|---|---|---|
| (m) | (cm) | (%) | (%) | (%) | (%) | (%) | |
| Guadiana | 20 | 0–2 | 0.26 | 1.53 | 0.03 | 1.50 | 0.02 |
| Guadiana | 60 | 0–2 | 0.31 | n.a. | n.a. | n.a. | n.a. |
| Guadiana | 60 | 2–4 | 0.27 | 1.04 | 0.03 | 1.01 | <0.01 |
| San Telmo | 0 | 0–2.5 | 0.06 | 0.87 | 0.02 | 0.85 | n.a. |
| San Telmo | 30 | 0–2.5 | 0.05 | 0.60 | 0.01 | 0.59 | n.a. |
| San Telmo | 40 | 0–1 | 0.10 | 2.04 | 0.02 | 2.02 | n.a. |
| Cueva de la Mora | 5 | 0–2.5 | 0.23 | 9.31 | 0.20 | 9.11 | n.a. |
| Cueva de la Mora | 5 | 3.5–4.5 | 0.21 | 10.27 | 0.16 | 10.12 | n.a. |
| Cueva de la Mora | 5 | 5–6 | 0.23 | 16.63 | 0.78 | 15.85 | n.a. |
| Cueva de la Mora | 35 | 0–2 | 0.21 | 1.23 | 0.02 | 1.21 | n.a. |
| Cueva de la Mora | 37 | 0–3 | 0.08 | 1.40 | 0.01 | 1.39 | n.a. |
| Cueva de la Mora | 38 | 8–10 | 0.12 | 1.30 | 0.12 | 1.18 | n.a. |
| Cueva de la Mora | 38 | 13–15 | 0.12 | 1.81 | <0.10 | 1.86 | n.a. |
| Sample | Depth | Sampling | CT | NT | PT | [C/N]m | δ13CPDB | |
|---|---|---|---|---|---|---|---|---|
| m | wt.% | wt.% | wt.% | ‰ | ||||
| ST-10 | 10 | October 2014 | 2.37 | 0.42 | 0.04 | 6.64 | −25.79 | |
| ST-40 | 40 | October 2014 | 2.04 | 0.36 | 0.03 | 6.51 | −26.28 | |
| ST-40 | 40 | April 2011 | 1.28 | 0.20 | 0.02 | 7.47 | n.d. | |
| ST-40 | 40 | May 2012 | 15.24 | n.d. | 0.09 | - | n.d. | |
| ST-100 | 100 | October 2014 | 1.92 | 0.33 | 0.03 | 6.80 | −26.04 |
| San Telmo Pit Lake | 10 m | 40 m | 100 m | |
| Annual sedimentation rate (1) | cm/year | 1.0 | 1.1 | 1.1 |
| Total sediment thickness (2) | cm | 23.2 | 23.5 | 23.8 |
| C flux (3) | g m−2 year−1 | 8.0 | 8.5 | 10 |
| N flux (3) | g m−2 year−1 | 1.3 | 1.4 | 1.6 |
| P flux (3) | g m−2 year−1 | 0.03 | 0.03 | 0.04 |
| Cueva de la Mora pit lake | 11 m | 21 m | 38 m | |
| Annual sedimentation rate (1) | cm/year | 0.2 | 0.4 | 0.6 |
| Total sediment thickness (2) | cm | 8.3 | 14.5 | 24.9 |
| C flux (3) | g m−2 year−1 | 33 | 36 | 24 |
| N flux (3) | g m−2 year−1 | 0.8 | 1.2 | 1.2 |
| P flux (3) | g m−2 year−1 | 0.2 | 0.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-España, J.; Falagán, C.; Ilin, A.M.; Yusta, I. Mineral Sources and Vertical Distribution of Nutrients in Extremely Acidic Pit Lakes: Impact on Microbial Ecology. Minerals 2025, 15, 1223. https://doi.org/10.3390/min15111223
Sánchez-España J, Falagán C, Ilin AM, Yusta I. Mineral Sources and Vertical Distribution of Nutrients in Extremely Acidic Pit Lakes: Impact on Microbial Ecology. Minerals. 2025; 15(11):1223. https://doi.org/10.3390/min15111223
Chicago/Turabian StyleSánchez-España, Javier, Carmen Falagán, Andrey M. Ilin, and Iñaki Yusta. 2025. "Mineral Sources and Vertical Distribution of Nutrients in Extremely Acidic Pit Lakes: Impact on Microbial Ecology" Minerals 15, no. 11: 1223. https://doi.org/10.3390/min15111223
APA StyleSánchez-España, J., Falagán, C., Ilin, A. M., & Yusta, I. (2025). Mineral Sources and Vertical Distribution of Nutrients in Extremely Acidic Pit Lakes: Impact on Microbial Ecology. Minerals, 15(11), 1223. https://doi.org/10.3390/min15111223






