Scheelite as a Strategic Tungsten Resource: A Bibliometric Study of Global and Chinese Technology Trends (1999–2024)
Abstract
1. Introduction
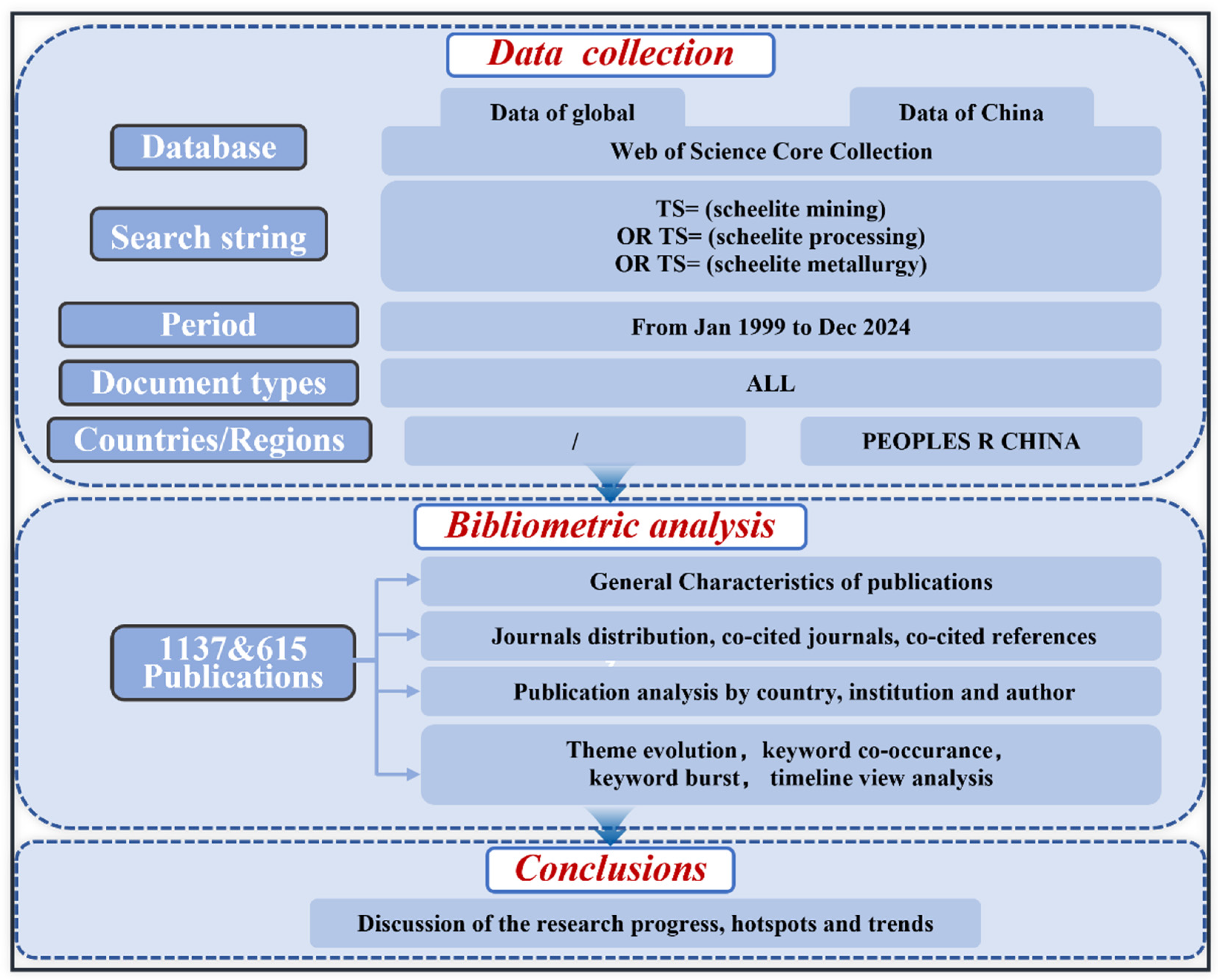
2. Data Sources and Methods
2.1. Data Source and Retrieval
2.2. Data Processing and Graphing
3. Results and Discussions
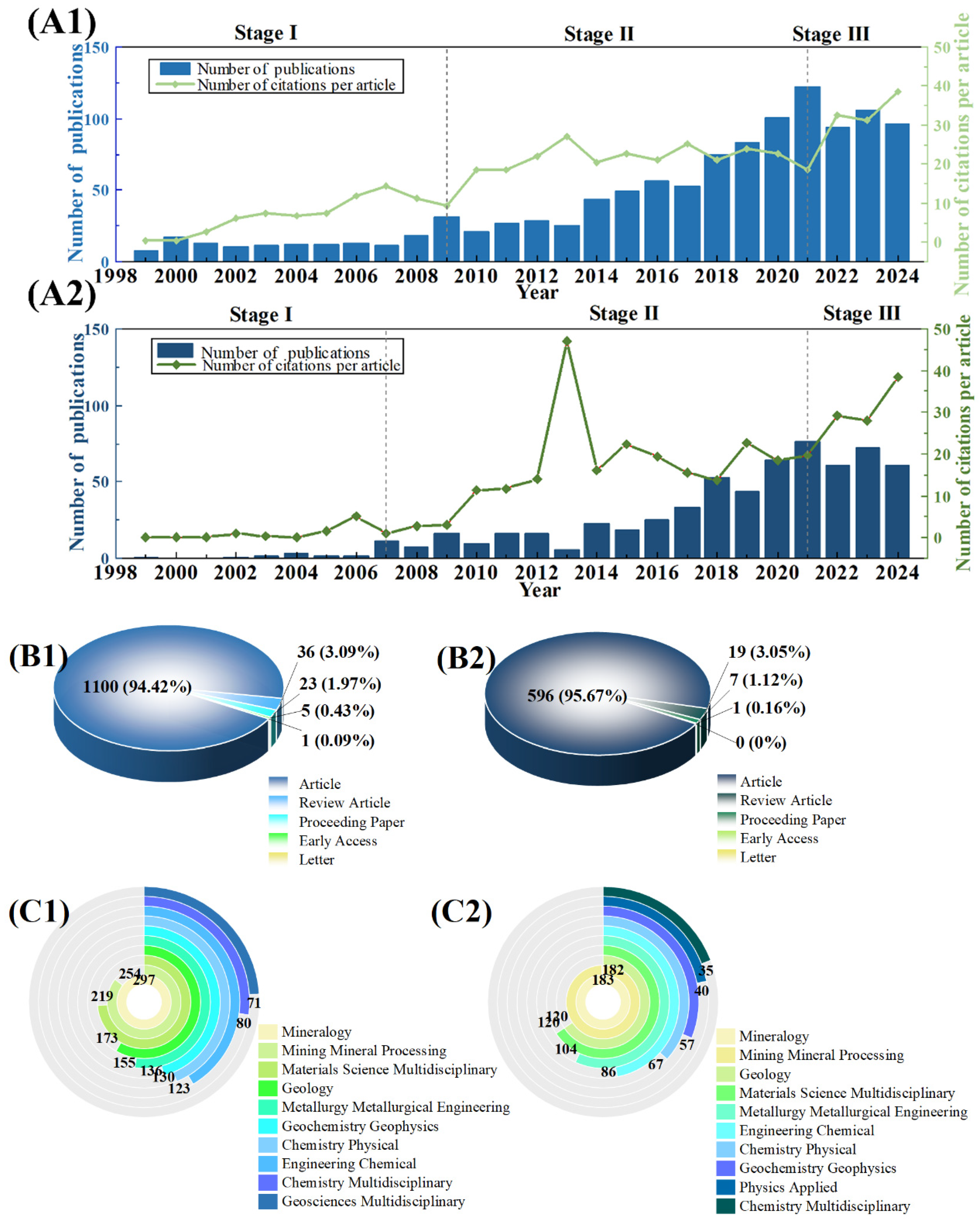
3.1. General Characteristics of Publications
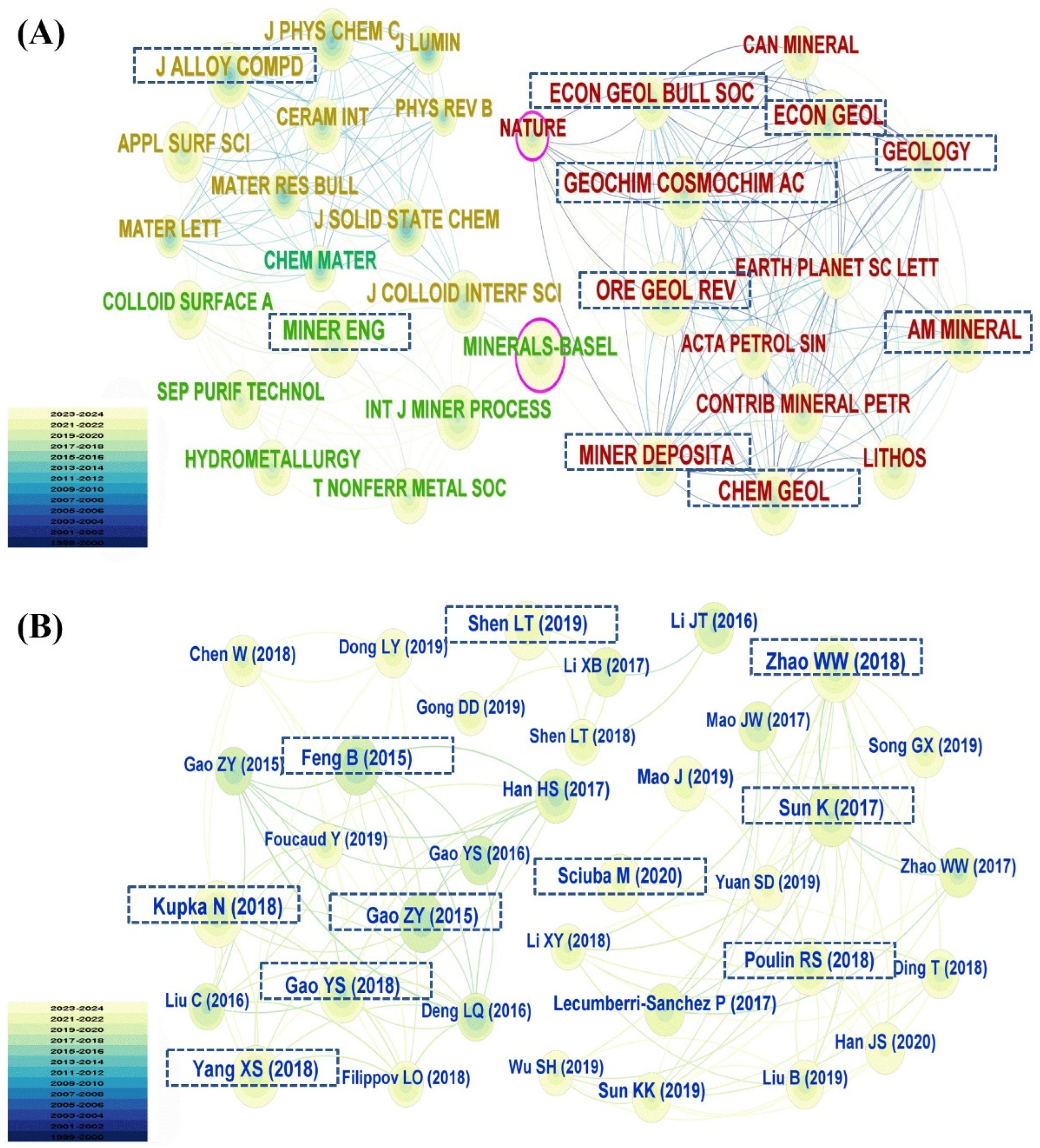
3.2. Publication Analysis by Journal and Reference
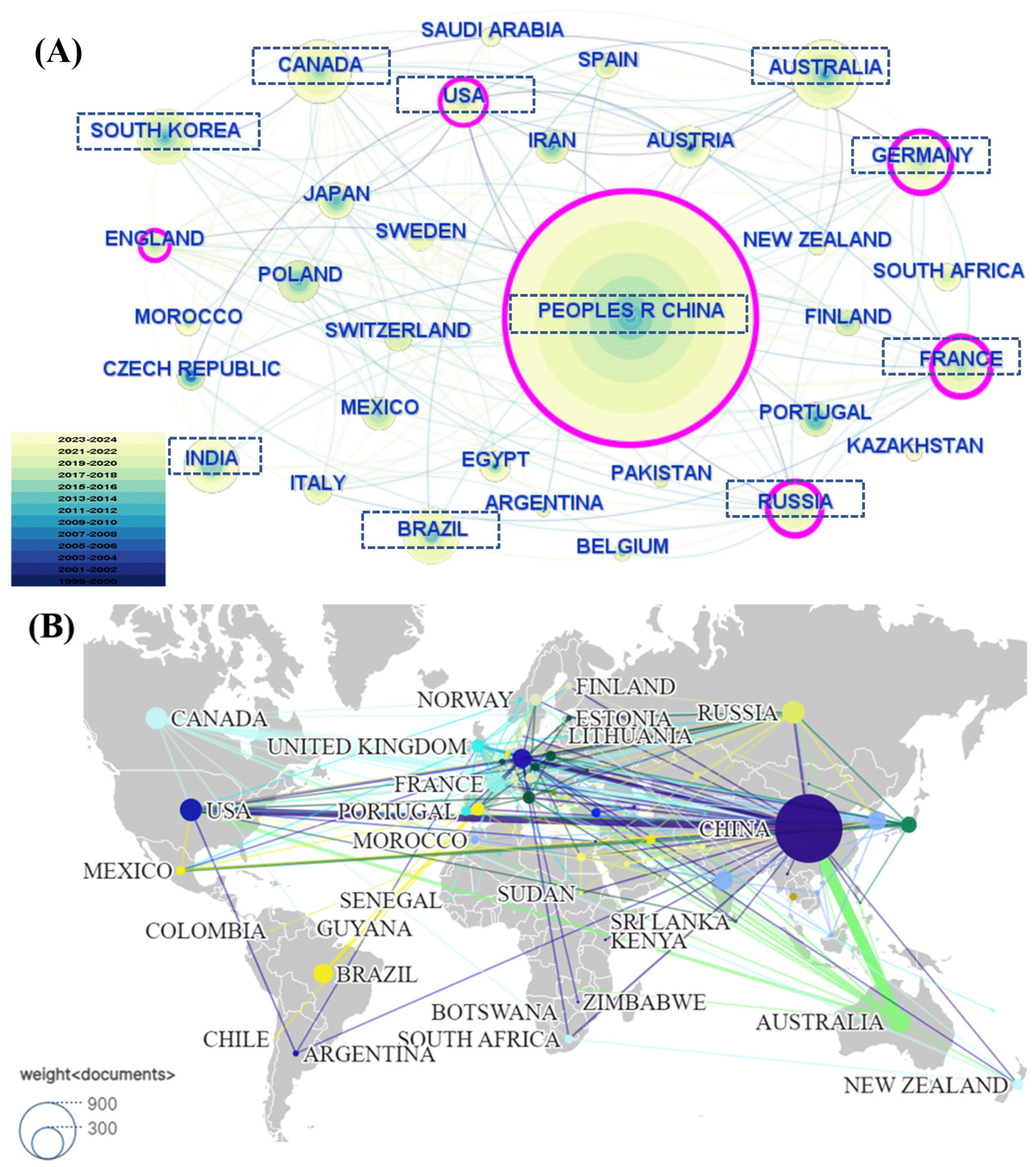
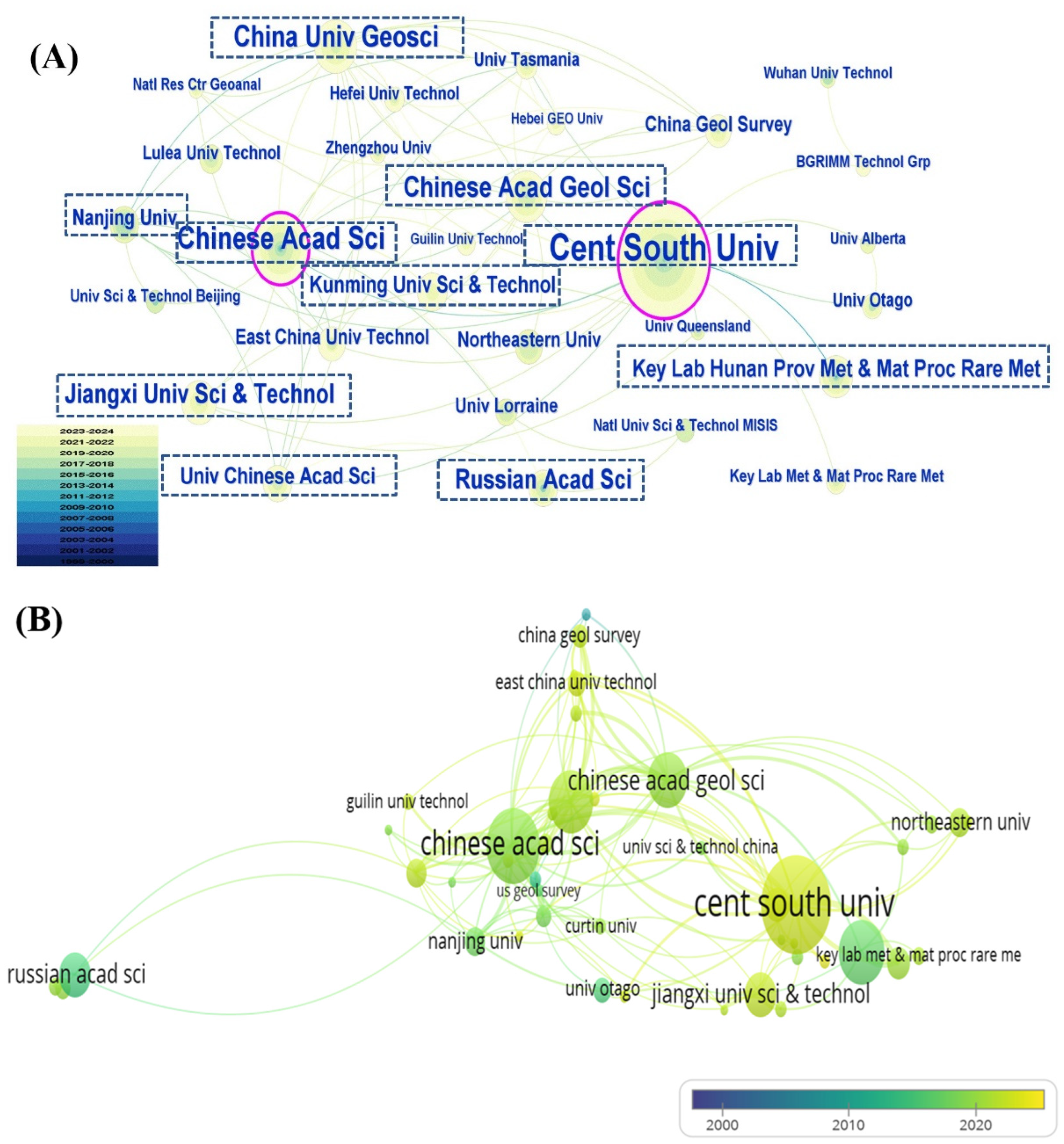
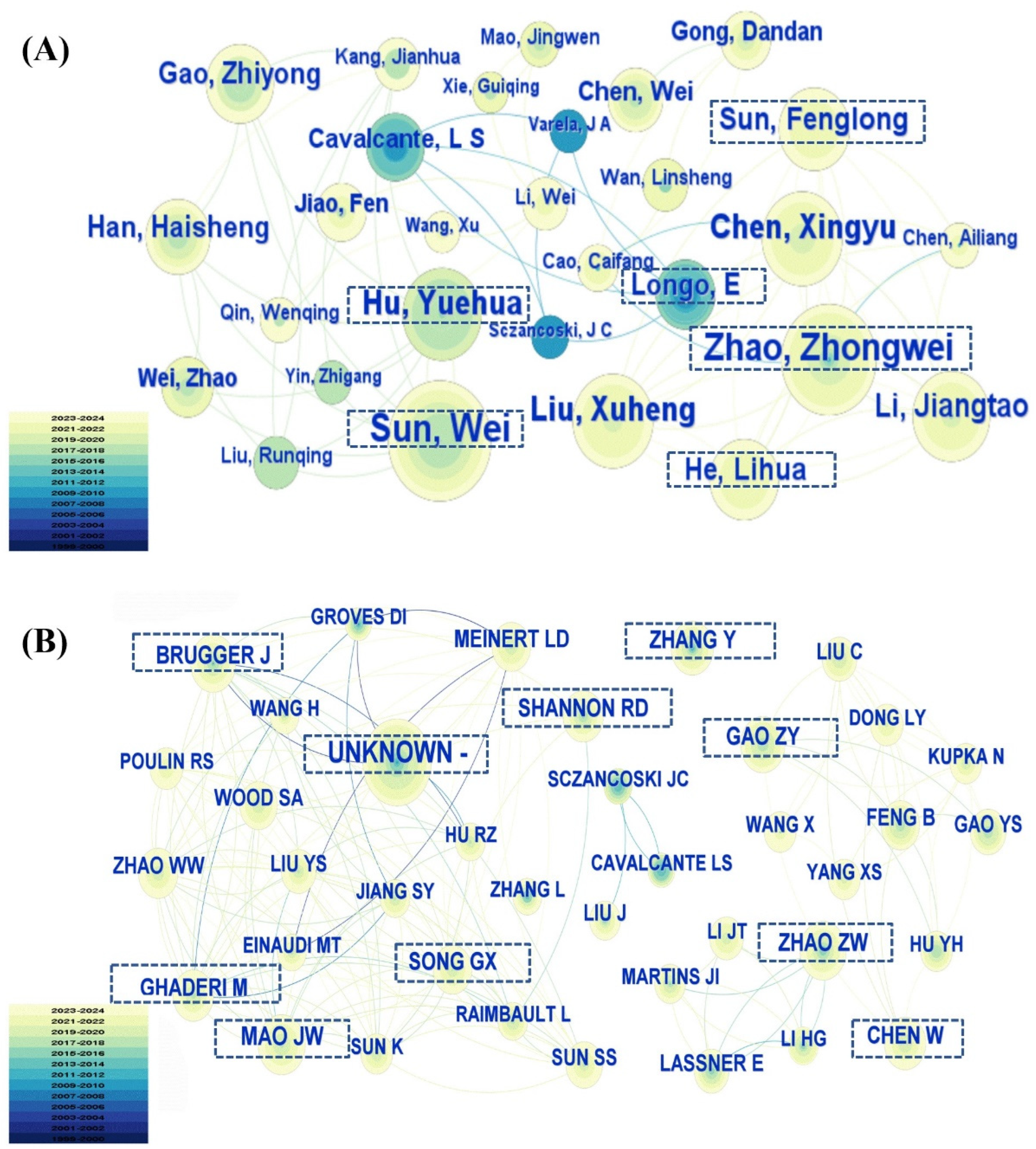
3.3. Publication Analysis by Country, Institution, and Author
3.3.1. Publication Analysis by Country
3.3.2. Publication Analysis by Institution
3.3.3. Publication Analysis by Author
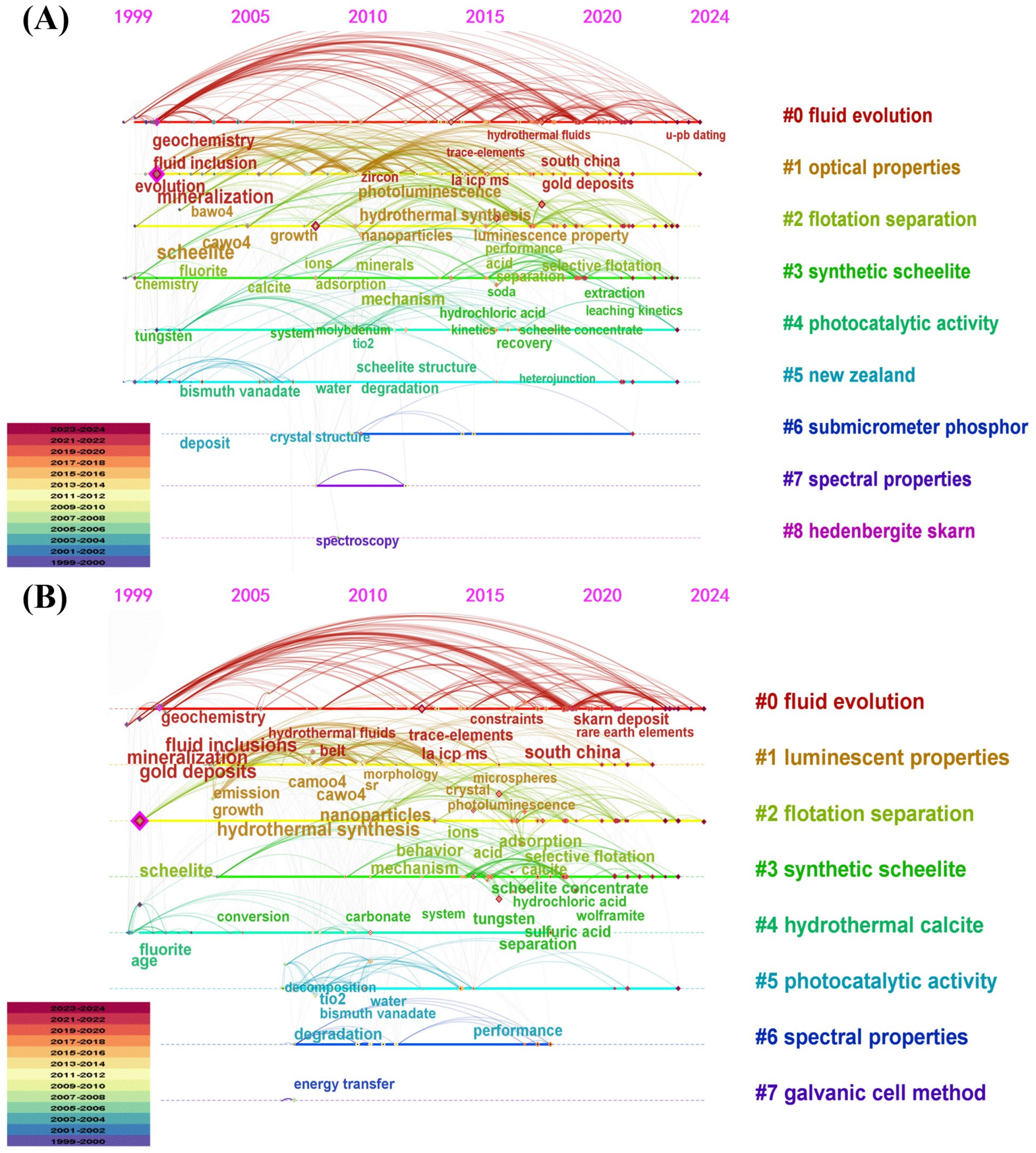
3.4. Keyword Analysis and Emerging Trends
3.4.1. Keyword Co-Occurrence Analysis
3.4.2. Timeline View Analysis
3.4.3. Keyword Burst Analysis
4. Conclusions and Perspectives
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Qin, W.Q.; Jiao, F.; Dong, L.Y.; Guo, J.G.; Zhang, J.; Yang, C.R. Review of tungsten resource reserves, tungsten concentrate production and tungsten beneficiation technology in China. Trans. Nonferrous Met. Soc. China 2022, 32, 2318–2338. [Google Scholar] [CrossRef]
- Zhao, Z.W.; Li, J.T.; Wang, S.B.; Li, H.G.; Liu, M.S.; Sun, P.M.; Li, Y.J. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Srinivas, K.; Sreenivas, T.; Natarajan, R.; Padmanabhan, N.P.H. Studies on the recovery of tungsten from a composite wolframite-scheelite concentrate. Hydrometallurgy 2000, 58, 43–50. [Google Scholar] [CrossRef]
- Chen, Y.; Huo, G.; Guo, X.; Chen, J. A review of flowsheets for tungsten recovery from scheelite, wolframite and secondary resources and challenges for sustainable production. Hydrometallurgy 2025, 234, 106455. [Google Scholar] [CrossRef]
- Brazdil, J.F. Scheelite: A versatile structural template for selective alkene oxidation catalysts. Catal. Sci. Technol. 2015, 5, 3452–3458. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, Y.; Qiao, L.; Shen, P.; Mao, Y.; Dong, L.; Liu, D. Selective adsorption of soluble starch on the cassiterite surface for effective flotation separation of scheelite from cassiterite. Surf. Interfaces 2024, 48, 104238. [Google Scholar] [CrossRef]
- Li, T.T.; Shen, Y.B.; Zhao, S.K.; Zhou, P.F.; Zhong, X.X.; Gao, S.L.; Wei, D.Z.; Meng, F.L. Synthesis and in-situ noble metal modification of WO3•0.33H2O nanorods from a tungsten-containing mineral for enhancing NH3 sensing performance. Chin. Chem. Lett. 2020, 31, 2037–2040. [Google Scholar] [CrossRef]
- Errandonea, D.; Manjón, F.J.; Somayazulu, M.; Häusermann, D. Effects of pressure on the local atomic structure of CaWO4 and YLiF4: Mechanism of the scheelite-to-wolframite and scheelite-to-fergusonite transitions. J. Solid State Chem. 2004, 177, 1087–1097. [Google Scholar] [CrossRef]
- Kempe, U.; Belyatsky, B.V.; Krymsky, R.S.; Kremenetsky, A.A.; Ivanov, P.A. Sm-Nd and Sr isotope systematics of scheelite from the giant Au(-W) deposit Muruntau (Uzbekistan): Implications for the age and sources of Au mineralization. Miner. Depos. 2001, 36, 379–392. [Google Scholar] [CrossRef]
- Deng, R.D.; Yang, X.F.; Hu, Y.; Ku, J.G.; Zuo, W.R.; Ma, Y.Q. Effect of Fe(II) as assistant depressant on flotation separation of scheelite from calcite. Miner. Eng. 2018, 118, 133–140. [Google Scholar] [CrossRef]
- Qiu, Z.W.; Deng, X.H.; Qi, N.; Huang, P.C.; Yao, J.M.; Li, Z.S.; Chen, Y.J. Geochemistry of garnet and scheelite as indicators for skarn-type Mo-W mineralization: A case study from the Shibaogou deposit, Qinling Orogen, China. Ore Geol. Rev. 2024, 175, 106370. [Google Scholar] [CrossRef]
- Lu, H.Z.; Liu, Y.M.; Wang, C.L.; Xu, Y.Z.; Li, H.Q. Mineralization and fluid inclusion study of the Shizhuyuan W-Sn-Bi-Mo-F skarn deposit, Hunan province, Cehina. Econ. Geol. Bull. Soc. Econ. Geol. 2003, 98, 955–974. [Google Scholar] [CrossRef]
- Ai, G.; Yang, X.; Li, X. Flotation characteristics and flotation kinetics of fine wolframite. Powder Technol. 2017, 305, 377–381. [Google Scholar] [CrossRef]
- Bohlouli, A.; Afshar, M.R.; Aboutalebi, M.R.; Seyedein, S.H. Optimization of tungsten leaching from low manganese wolframite concentrate using Response Surface Methodology (RSM). Int. J. Refract. Met. Hard Mater. 2016, 61, 107–114. [Google Scholar] [CrossRef]
- Calvo, G.; Valero, A. Strategic mineral resources: Availability and future estimations for the renewable energy sector. Environ. Dev. 2022, 41, 100640. [Google Scholar] [CrossRef]
- Vikentiev, I.V. Critical and Strategic Minerals in the Russian Federation. Geol. Ore Depos. 2023, 65, 481–493. [Google Scholar] [CrossRef]
- Zuo, Z.; Cheng, J.; Guo, H.; Li, Y. Knowledge mapping of research on strategic mineral resource security: A visual analysis using CiteSpace. Resour. Policy 2021, 74, 102372. [Google Scholar] [CrossRef]
- Kalantzakos, S. The Race for Critical Minerals in an Era of Geopolitical Realignments. Int. Spect. 2020, 55, 1–16. [Google Scholar] [CrossRef]
- Brugger, J.; Lahaye, Y.; Costa, S.; Lambert, D.; Bateman, R. Inhomogeneous distribution of REE in scheelite and dynamics of Archaean hydrothermal systems (Mt. Charlotte and Drysdale gold deposits, Western Australia). Contrib. Mineral. Petrol. 2000, 139, 251–264. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Marana, N.L.; da Silva, R.O.; Tranquilin, R.L.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Sambrano, J.R.; Li, M.S.; et al. Electronic structure and optical properties of BaMoO4 powders. Curr. Appl. Phys. 2010, 10, 614–624. [Google Scholar] [CrossRef]
- Zhu, L.J.; Zhu, Y.M.; Xiong, W.L.; Zhou, Z.; Gao, P.; Han, Y.X. Aluminium-Modified sodium silicate as a selective depressant in Fluorite-Dolomite Flotation: Experimental and DFT analysis. Miner. Eng. 2025, 228, 109318. [Google Scholar] [CrossRef]
- Zheng, F.; Qin, K.; Cook, N.J.; Li, G.; Ciobanu, C.L.; Xu, Y.; Song, G. Geology, geochronology, and geochemistry of the Gaojiabang tungsten-molybdenum deposit, Anhui Province, Southeast China. Ore Geol. Rev. 2023, 157, 105432. [Google Scholar] [CrossRef]
- Yue, T.; Han, H.S.; Hu, Y.H.; Wei, Z.; Wang, J.J.; Wang, L.; Sun, W.; Yang, Y.; Sun, L.; Liu, R.H.; et al. Beneficiation and Purification of Tungsten and Cassiterite Minerals Using Pb-BHA Complexes Flotation and Centrifugal Separation. Minerals 2018, 8, 566. [Google Scholar] [CrossRef]
- Corpas-Martinez, J.R.; Perez, A.; Navarro-Dominguez, R.; Amor-Castillo, C.; Martin-Lara, M.A.; Calero, M. Comparison Between Performance of Fluorite Flotation Under Different Depressants Reagents in Two Pieces of Laboratory Equipment. Appl. Sci. 2020, 10, 5667. [Google Scholar] [CrossRef]
- Chen, X.D.; Liu, W.G.; Zhang, J.; Bao, L.Y.; Liu, W.B.; Shen, Y.B. Application and mechanistic insights of high-performance sodium phytate-based combined depressant in flotation of dolomite-rich magnesite ore. J. Clean. Prod. 2025, 503, 145415. [Google Scholar] [CrossRef]
- Lei, Y.; Sun, F.; Liu, X.; Zhao, Z. Understanding the wet decomposition processes of tungsten ore: Phase, thermodynamics and kinetics. Hydrometallurgy 2022, 213, 105928. [Google Scholar] [CrossRef]
- Rutledge, J.; Anderson, C.G. Tannins in Mineral Processing and Extractive Metallurgy. Metals 2015, 5, 1520–1542. [Google Scholar] [CrossRef]
- Li, H.G. Production of high purity APT from scheelite and complex tungsten raw material with high Mo content. Trans. Nonferrous Met. Soc. China 2004, 14, 366–369. [Google Scholar]
- Alguacil, F.J.; Alonso, M. Recovery of Tungsten from Raw and Secondary Materials Using Hydrometallurgical Processing. Metals 2025, 15, 799. [Google Scholar] [CrossRef]
- El-bahi, A.; Taha, Y.; Ait-Khouia, Y.; Hakkou, R.; Benzaazoua, M. Advancing phosphate ore minerals separation with sustainable flotation reagents: An investigation into highly selective biobased depressants. Adv. Colloid Interface Sci. 2023, 317, 102921. [Google Scholar] [CrossRef]
- Wei, Z.; Sun, W.; Han, H.S.; Gui, X.H.; Xing, Y.W. Flotation chemistry of scheelite and its practice: A comprehensive review. Miner. Eng. 2023, 204, 108404. [Google Scholar] [CrossRef]
- Wang, X.; Qin, W.Q.; Jiao, F.; Yang, C.R.; Li, W.; Zhang, Z.Q.; Zhou, J.M.; Guo, J.G.; Zhang, J. Review on development of low-grade scheelite recovery from molybdenum tailings in Luanchuan, China: A case study of Luoyang Yulu Mining Company. Trans. Nonferrous Met. Soc. China 2022, 32, 980–998. [Google Scholar] [CrossRef]
- Mukherjee, D.; Lim, W.M.; Kumar, S.; Donthu, N. Guidelines for advancing theory and practice through bibliometric research. J. Bus. Res. 2022, 148, 101–115. [Google Scholar] [CrossRef]
- Donthu, N.; Kumar, S.; Mukherjee, D.; Pandey, N.; Lim, W.M. How to conduct a bibliometric analysis: An overview and guidelines. J. Bus. Res. 2021, 133, 285–296. [Google Scholar] [CrossRef]
- Wei, P.Y.; Abid, M.; Adun, H.; Awoh, D.K.; Cai, D.S.; Zaini, J.H.; Bamisile, O. Progress in Energy Storage Technologies and Methods for Renewable Energy Systems Application. Appl. Sci. 2023, 13, 5626. [Google Scholar] [CrossRef]
- Mishra, D.; Gunasekaran, A.; Papadopoulos, T.; Hazen, B. Green supply chain performance measures: A review and bibliometric analysis. Sustain. Prod. Consum. 2017, 10, 85–99. [Google Scholar] [CrossRef]
- Cobo, M.J.; López-Herrera, A.G.; Herrera-Viedma, E.; Herrera, F. Science Mapping Software Tools: Review, Analysis, and Cooperative Study Among Tools. J. Am. Soc. Inf. Sci. Technol. 2011, 62, 1382–1402. [Google Scholar] [CrossRef]
- Chen, C.M.; Song, M. Visualizing a field of research: A methodology of systematic scientometric reviews. PLoS ONE 2019, 14, e0223994. [Google Scholar] [CrossRef]
- Bornmann, L.; Mutz, R. Growth rates of modern science: A bibliometric analysis based on the number of publications and cited references. J. Assoc. Inf. Sci. Technol. 2015, 66, 2215–2222. [Google Scholar] [CrossRef]
- Singh, V.K.; Singh, P.; Karmakar, M.; Leta, J.; Mayr, P. The journal coverage of Web of Science, Scopus and Dimensions: A comparative analysis. Scientometrics 2021, 126, 5113–5142. [Google Scholar] [CrossRef]
- Wu, L.; Miao, H.; Liu, T. Development in Agricultural Ecosystems’ Carbon Emissions Research: A Visual Analysis Using CiteSpace. Agronomy 2024, 14, 1288. [Google Scholar] [CrossRef]
- Li, Z.; Du, C. Current status and research hotspots of pesticide-containing wastewater treatment: Systematic review and bibliometric analysis. J. Water Process Eng. 2025, 69, 106738. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Xing, Z.; Fu, W.; Li, L.; Wu, S. Bibliometric analysis of microplastics research: Advances and future directions (2020–2024). Cont. Shelf Res. 2025, 285, 105371. [Google Scholar] [CrossRef]
- Gürmen, S.; Timur, S.; Arslan, C.; Duman, I. Acidic leaching of scheelite concentrate and production of hetero-poly-tungstate salt. Hydrometallurgy 1999, 51, 227–238. [Google Scholar] [CrossRef]
- Welham, N.J. Non-thermal production of tungsten from scheelite. Mater. Sci. Technol. 1999, 15, 456–458. [Google Scholar] [CrossRef]
- Craw, D.; Windle, S.J.; Angus, P.V. Gold mineralization without quartz veins in a ductile-brittle shear zone, Macraes Mine, Otago Schist, New Zealand. Miner. Depos. 1999, 34, 382–394. [Google Scholar] [CrossRef]
- Olawumi, T.O.; Chan, D.W.M. A scientometric review of global research on sustainability and sustainable development. J. Clean. Prod. 2018, 183, 231–250. [Google Scholar] [CrossRef]
- Yeung, A.W.K. A revisit to the specification of sub-datasets and corresponding coverage timespans when using Web of Science Core Collection. Heliyon 2023, 9, e21527. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2017, 11, 959–975. [Google Scholar] [CrossRef]
- Zhou, X.; Li, T.; Ma, X. A bibliometric analysis of comparative research on the evolution of international and Chinese green supply chain research hotspots and frontiers. Environ. Sci. Pollut. Res. 2021, 28, 6302–6323. [Google Scholar] [CrossRef]
- Kemec, A.; Altinay, A.T. Sustainable Energy Research Trend: A Bibliometric Analysis Using VOSviewer, RStudio Bibliometrix, and CiteSpace Software Tools. Sustainability 2023, 15, 3618. [Google Scholar] [CrossRef]
- van Eck, N.J.; Waltman, L. Citation-based clustering of publications using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef]
- Chen, C.; Hu, Z.; Liu, S.; Tseng, H. Emerging trends in regenerative medicine: A scientometric analysis in CiteSpace. Expert Opin. Biol. Ther. 2012, 12, 593–608. [Google Scholar] [CrossRef]
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubik, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Chen, C. A Glimpse of the First Eight Months of the COVID-19 Literature on Microsoft Academic Graph: Themes, Citation Contexts, and Uncertainties. Front. Res. Metr. Anal. 2020, 5, 607286. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhao, S.; Tan, L.; Tan, Y.; Wang, Y.; Ye, Z.; Hou, C.; Xu, Y.; Liu, S.; Wang, G. Frontier and hot topics in electrochemiluminescence sensing technology based on CiteSpace bibliometric analysis. Biosens. Bioelectron. 2022, 201, 113932. [Google Scholar] [CrossRef]
- Hassan-Montero, Y.; De-Moya-Anegon, F.; Guerrero-Bote, V.P. SCImago Graphica: A new too for exploring and visually communicating data. Prof. De La Inf. 2022, 31, e310502. [Google Scholar] [CrossRef]
- Chen, H.; Chen, F.; Luo, J.; Chen, S. Analysis of research trends and hotspots in emergency department overcrowding: A bibliometric study based on VOSview and Scimago Graphica. Technol. Health Care 2025, 33, 1159–1168. [Google Scholar] [CrossRef]
- Gao, Z.; Zhang, C.; McFadzean, B. A Bibliometric Analysis of Fluorite Resource Utilization Technology: Global and Chinese Development in the Past 25 Years. Minerals 2025, 15, 679. [Google Scholar] [CrossRef]
- Li, J.; Jovanovic, A.; Klimek, P.; Guo, X. Bibliometric analysis of fracking scientific literature. Scientometrics 2015, 105, 1273–1284. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef]
- Silva, L.A.; Garrot, T.G.; Pereira, A.M.; Correia, J.C.G. Historical perspective and bibliometric analysis of molecular modeling applied in mineral flotation systems. Miner. Eng. 2021, 170, 107062. [Google Scholar] [CrossRef]
- Gazni, A.; Sugimoto, C.R.; Didegah, F. Mapping World Scientific Collaboration: Authors, Institutions, and Countries. J. Am. Soc. Inf. Sci. Technol. 2012, 63, 323–335. [Google Scholar] [CrossRef]
- Shen, L.; Li, X.; Zhou, Q.; Peng, Z.; Liu, G.; Qi, T.; Taskinen, P. Sustainable and efficient leaching of tungsten in ammoniacal ammonium carbonate solution from the sulfuric acid converted product of scheelite. J. Clean. Prod. 2018, 197, 690–698. [Google Scholar] [CrossRef]
- Graupner, T.; Niedermann, S.; Kempe, U.; Klemd, R.; Bechtel, A. Origin of ore fluids in the Muruntau gold system: Constraints from noble gas, carbon isotope and halogen data. Geochim. Et Cosmochim. Acta 2006, 70, 5356–5370. [Google Scholar] [CrossRef]
- Choi, S. Core-periphery, new clusters, or rising stars?: International scientific collaboration among ‘advanced’ countries in the era of globalization. Scientometrics 2012, 90, 25–41. [Google Scholar] [CrossRef]
- Shi, W.; Yang, W.; Du, D. The scientific cooperation network of chinese scientists and its proximity mechanism. Sustainability 2020, 12, 660. [Google Scholar] [CrossRef]
- Zhou, P.; Glanzel, W. In-depth analysis on China’s international cooperation in science. Scientometrics 2010, 82, 597–612. [Google Scholar] [CrossRef]
- Qin, W.Q.; Hu, J.J.; Zhu, H.L.; Jiao, F.; Jia, W.H.; Han, J.W.; Chen, C. Effect of depressants on flotation separation of magnesite from dolomite and calcite. Int. J. Min. Sci. Technol. 2023, 33, 83–91. [Google Scholar] [CrossRef]
- Jiang, Z.S.; He, G.C.; Shi, Y.; Duan, Y.L.; Lin, Y.; Jiang, Y.M. Contrasting effects of waste glass and scheelite tailings additions upon the properties of tailings-based foam ceramics and its mechanisms. J. Clean. Prod. 2024, 450, 142025. [Google Scholar] [CrossRef]
- Dong, L.Y.; Cui, Y.R.; Qiao, L.D.; Lan, S.Z.; Zheng, Q.F.; Shen, P.L.; Liu, D.W. A critical review on the flotation of calcium-containing minerals. Sep. Purif. Technol. 2025, 360, 131082. [Google Scholar] [CrossRef]
- Liu, X.H.; Zhai, J.P.; Chen, X.Y.; Li, J.T.; He, L.H.; Sun, F.L.; Zhao, Z.W. Recovery of Tungsten in the Process of Preparation of Calcium Sulfate Whiskers from Scheelite Decomposed Residue. Acs Sustain. Chem. Eng. 2022, 10, 13194–13204. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Fan, R.Y.; Ralston, J.; Sun, W.; Hu, Y.H. Surface broken bonds: An efficient way to assess the surface behaviour of fluorite. Miner. Eng. 2019, 130, 15–23. [Google Scholar] [CrossRef]
- Marlton, F.P.; Mullens, B.G.; Chater, P.A.; Kennedy, B.J. Tetrahedral Displacive Disorder in the Scheelite-Type Oxide RbReO4. Inorg. Chem. 2022, 61, 15130–15137. [Google Scholar] [CrossRef]
- Song, G.X.; Qin, K.Z.; Li, G.M.; Evans, N.J.; Chen, L. Scheelite elemental and isotopic signatures: Implications for the genesis of skarn-type W-Mo deposits in the Chizhou Area, Anhui Province, Eastern China. Am. Mineral. 2014, 99, 303–317. [Google Scholar] [CrossRef]
- Sun, X.M.; Zhang, Y.; Xiong, D.X.; Sun, W.D.; Shi, G.Y.; Zhai, W.; Wang, S.W. Crust and mantle contributions to gold-forming process at the Daping deposit, Ailaoshan gold belt, Yunnan, China. Ore Geol. Rev. 2009, 36, 235–249. [Google Scholar] [CrossRef]
- Guo, Y.N.; Yang, X.; Ma, F.Y.; Li, K.X.; Xu, L.; Yuan, X.; Guo, Y.H. Additive-free controllable fabrication of bismuth vanadates and their photocatalytic activity toward dye degradation. Appl. Surf. Sci. 2010, 256, 2215–2222. [Google Scholar] [CrossRef]
- Ren, L.; Jin, L.; Wang, J.B.; Yang, F.; Qiu, M.Q.; Yu, Y. Template-free synthesis of BiVO4 nanostructures: I. Nanotubes with hexagonal cross sections by oriented attachment and their photocatalytic property for water splitting under visible light. Nanotechnology 2009, 20, 115603. [Google Scholar] [CrossRef]
- Zhou, D.; Li, J.; Pang, L.X.; Wang, D.W.; Reaney, I.M. Novel water insoluble (NaxAg2-x) MoO4 (0 ≤ x ≤ 2) microwave dielectric ceramics with spinel structure sintered at 410 degrees. J. Mater. Chem. C 2017, 5, 6086–6091. [Google Scholar] [CrossRef]
- Bittermann, A.; McNamara, D.; Simonsmeier, B.A.A.; Schneider, M. The Landscape of Research on Prior Knowledge and Learning: A Bibliometric Analysis. Educ. Psychol. Rev. 2023, 35, 58. [Google Scholar] [CrossRef]
- Liu, H.; Hong, R.; Xiang, C.; Lv, C.; Li, H. Visualization and analysis of mapping knowledge domains for spontaneous combustion studies. Fuel 2020, 262, 116598. [Google Scholar] [CrossRef]
- Yang, B.; Huang, K.; Sun, D.; Zhang, Y. Mapping the scientific research on non-point source pollution: A bibliometric analysis. Environ. Sci. Pollut. Res. 2017, 24, 4352–4366. [Google Scholar] [CrossRef]
- Liu, Y.F.; Jiang, S.H.; Bagas, L. The genesis of metal zonation in the Weilasituo and Bairendaba Ag-Zn-Pb-Cu-(Sn-W) deposits in the shallow part of a porphyry Sn-W-Rb system, Inner Mongolia, China. Ore Geol. Rev. 2016, 75, 150–173. [Google Scholar] [CrossRef]
- Schmidt, C.; Romer, R.L.; Wohlgemuth-Ueberwasser, C.C.; Appelt, O. Partitioning of Sn and W between granitic melt and aqueous fluid. Ore Geol. Rev. 2020, 117, 103263. [Google Scholar] [CrossRef]
- Zhu, H.L.; Qin, W.Q.; Chen, C.; Chai, L.Y.; Jiao, F.; Jia, W.H. Flotation separation of fluorite from calcite using polyaspartate as depressant. Miner. Eng. 2018, 120, 80–86. [Google Scholar] [CrossRef]
- Gao, Z.Y.; Gao, Y.S.; Zhu, Y.Y.; Hu, Y.H.; Sun, W. Selective Flotation of Calcite from Fluorite: A Novel Reagent Schedule. Minerals 2016, 6, 114. [Google Scholar] [CrossRef]
- Jiang, S.Y.; Peng, N.J.; Huang, L.C.; Xu, Y.M.; Zhan, G.L.; Dan, X.H. Geological characteristic and ore genesis of the giant tungsten deposits from the Dahutang ore-concentrated district in northern Jiangxi Province. Acta Petrol. Sin. 2015, 31, 639–655. [Google Scholar]
- Sun, X.M.; Xiong, D.X.; Wang, S.W.; Shi, G.Y.; Zhai, W. Noble gases isotopic composition of fluid inclusions in scheelites collected from Daping gold mine, Yunnan province, China, and its application for ore genesis. Acta Petrol. Sin. 2006, 22, 725–732. [Google Scholar]
- Upham, S.P.; Small, H. Emerging research fronts in science and technology: Patterns of new knowledge development. Scientometrics 2010, 83, 15–38. [Google Scholar] [CrossRef]
- Thongtem, T.; Kungwankunakorn, S.; Kuntalue, B.; Phuruangrat, A.; Thongtem, S. Luminescence and absorbance of highly crystalline CaMoO4, SrMoO4, CaWO4 and SrWO4 nanoparticles synthesized by co-precipitation method at room temperature. J. Alloys Compd. 2010, 506, 475–481. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Z. Kinetics of scheelite concentrate digestion with sulfuric acid in the presence of phosphoric acid. Hydrometallurgy 2016, 163, 55–60. [Google Scholar] [CrossRef]
- Hoskin, P.W.O. Trace-element composition of hydrothermal zircon and the alteration of Hadean zircon from the Jack Hills, Australia. Geochim. Et Cosmochim. Acta 2005, 69, 637–648. [Google Scholar] [CrossRef]
- Brugger, J.; Etschmann, B.; Pownceby, M.; Liu, W.; Grundler, P.; Brewe, D. Oxidation state of europium in scheelite: Tracking fluid-rock interaction in gold deposits. Chem. Geol. 2008, 257, 26–33. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhou, M.-F.; Williams-Jones, A.E.; Zhao, Z. Constraints on the uptake of REE by scheelite in the Baoshan tungsten skarn deposit, South China. Chem. Geol. 2018, 477, 123–136. [Google Scholar] [CrossRef]
- Yang, X.S. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Sun, K.; Chen, B. Trace elements and Sr-Nd isotopes of scheelite: Implications for the W-Cu-Mo polymetallic mineralization of the Shimensi deposit, South China. Am. Mineral. 2017, 102, 1114–1128. [Google Scholar] [CrossRef]
- Gao, Y.; Gao, Z.; Sun, W.; Yin, Z.; Wang, J.; Hu, Y. Adsorption of a novel reagent scheme on scheelite and calcite causing an effective flotation separation. J. Colloid Interface Sci. 2018, 512, 39–46. [Google Scholar] [CrossRef]
- Gao, Z.; Bai, D.; Sun, W.; Cao, X.; Hu, Y. Selective flotation of scheelite from calcite and fluorite using a collector mixture. Miner. Eng. 2015, 72, 23–26. [Google Scholar] [CrossRef]
- Shen, L.T.; Li, X.B.; Lindberg, D.; Taskinen, P. Tungsten extractive metallurgy: A review of processes and their challenges for sustainability. Miner. Eng. 2019, 142, 105934. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.; Wang, J.; Wang, P. The flotation separation of scheelite from calcite using acidified sodium silicate as depressant. Miner. Eng. 2015, 80, 45–49. [Google Scholar] [CrossRef]
- Poulin, R.S.; Kontak, D.J.; McDonald, A.; McClenaghan, M.B. Assessing scheelite as an ore-deposit discriminator using its trace-element and Ree chemistry. Can. Mineral. 2018, 56, 265–302. [Google Scholar] [CrossRef]
- Sciuba, M.; Beaudoin, G.; Grzela, D.; Makvandi, S. Trace element composition of scheelite in orogenic gold deposits. Miner. Depos. 2020, 55, 1149–1172. [Google Scholar] [CrossRef]
- Glanzel, W.; Moed, H.F. Journal impact measures in bibliometric research. Scientometrics 2002, 53, 171–193. [Google Scholar] [CrossRef]
- Lariviere, V.; Gingras, Y. On the Relationship Between Interdisciplinarity and Scientific Impact. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 126–131. [Google Scholar] [CrossRef]
- Kaminskii, A.A.; McCray, C.L.; Lee, H.R.; Lee, S.W.; Temple, D.A.; Chyba, T.H.; Marsh, W.D.; Barnes, J.C.; Annanenkov, A.N.; Legun, V.D.; et al. High efficiency nanosecond Raman lasers based on tetragonal PbWO4 crystals. Opt. Commun. 2000, 183, 277–287. [Google Scholar] [CrossRef]
- Seiler, R.L.; Stollenwerk, K.G.; Garbarino, J.R. Factors controlling tungsten concentrations in ground water, Carson Desert, Nevada. Appl. Geochem. 2005, 20, 423–441. [Google Scholar] [CrossRef]
- Bateman, R.; Hagemann, S. Gold mineralisation throughout about 45 Ma of Archaean orogenesis: Protracted flux of gold in the Golden Mile, Yilgarn craton, Western Australia. Miner. Depos. 2004, 39, 536–559. [Google Scholar] [CrossRef]
- Brugger, J.; Maas, R.; Lahaye, Y.; McRae, C.; Ghaderi, M.; Costa, S.; Lambert, D.; Bateman, R.; Prince, K. Origins of Nd-Sr-Pb isotopic variations in single scheelite grains from Archaean gold deposits, Western Australia. Chem. Geol. 2002, 182, 203–225. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Cavalcante, L.S.; Joya, M.R.; Espinosa, J.W.M.; Pizani, P.S.; Varela, J.A.; Longo, E. Synthesis, growth process and photoluminescence properties of SrWO4 powders. J. Colloid Interface Sci. 2009, 330, 227–236. [Google Scholar] [CrossRef]
- Cavalcante, L.S.; Sczancoski, J.C.; Lima, L.F.; Espinosa, J.W.M.; Pizani, P.S.; Varela, J.A.; Longo, E. Synthesis, Characterization, Anisotropic Growth and Photoluminescence of BaWO4. Cryst. Growth Des. 2009, 9, 1002–1012. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting with the aim at photon energy conversion. J. Ceram. Soc. Jpn. 2001, 109, S81–S88. [Google Scholar] [CrossRef]
- Han, Z.D.; Golev, A.; Edraki, M. A Review of Tungsten Resources and Potential Extraction from Mine Waste. Minerals 2021, 11, 701. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Li, Y.; Hong, J.; Hua, F. A bibliometric mapping study of the literature on oral health-related quality of life. J. Evid.-Based Dent. Pract. 2023, 23, 101780. [Google Scholar] [CrossRef] [PubMed]
- Thangadurai, V.; Knittlmayer, C.; Weppner, W. Metathetic room temperature preparation and characterization of scheelite-type ABO4 (A = Ca, Sr, Ba, Pb; B = Mo, W) powders. Mater. Sci. Eng. B-Solid State Mater. Adv. Technol. 2004, 106, 228–233. [Google Scholar] [CrossRef]
- Abdalla, M.A.M.; Peng, H.Q.; Wu, D.; Abusin, L.; Mbah, T.J. Prediction of Hydrophobic Reagent for Flotation Process Using Molecular Modeling. Acs Omega 2018, 3, 6483–6496. [Google Scholar] [CrossRef]
- Babicki, S.; Arndt, D.; Marcu, A.; Liang, Y.; Grant, J.R.; Maciejewski, A.; Wishart, D.S. Heatmapper: Web-enabled heat mapping for all. Nucleic Acids Res. 2016, 44, W147–W153. [Google Scholar] [CrossRef]
- Boyack, K.W. Thesaurus-based methods for mapping contents of publication sets. Scientometrics 2017, 111, 1141–1155. [Google Scholar] [CrossRef]
- Zhang, C.H.; Wei, S.; Hu, Y.H.; Tang, H.H.; Gao, J.D.; Yin, Z.G.; Guan, Q.J. Selective adsorption of tannic acid on calcite and implications for separation of fluorite minerals. J. Colloid Interface Sci. 2018, 512, 55–63. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Peng, J.T. Infrared microthermometric and noble gas isotope study of fluid inclusions in ore minerals at the Woxi orogenic Au-Sb-W deposit, western Hunan, South China. Ore Geol. Rev. 2015, 65, 55–69. [Google Scholar] [CrossRef]
- Zhou, W.G.; Chen, H.; Ou, L.M.; Shi, Q. Aggregation of ultra-fine scheelite particles induced by hydrodynamic cavitation. Int. J. Miner. Process. 2016, 157, 236–240. [Google Scholar] [CrossRef]
- Milojevic, S. Modes of Collaboration in Modern Science: Beyond Power Laws and Preferential Attachment. J. Am. Soc. Inf. Sci. Technol. 2010, 61, 1410–1423. [Google Scholar] [CrossRef]
- Elidrissi, B.; Addou, M.; Regragui, M.; Monty, C.; Bougrine, A.; Kachouane, A. Structural and optical properties of CeO2 thin films prepared by spray pyrolysis. Thin Solid Film. 2000, 379, 23–27. [Google Scholar] [CrossRef]
- Bukar, U.A.; Sayeed, M.S.; Razak, S.F.A.; Yogarayan, S.; Amodu, O.A.; Mahmood, R.A.R. A method for analyzing text using VOSviewer. Methodsx 2023, 11, 102339. [Google Scholar] [CrossRef]
- Ding, X.; Yang, Z. Knowledge mapping of platform research: A visual analysis using VOSviewer and CiteSpace. Electron. Commer. Res. 2022, 22, 787–809. [Google Scholar] [CrossRef]
- Zyryanov, V.V. Mechanochemical synthesis of M′MO4 oxides with the scheelite structure. Inorg. Mater. 2000, 36, 54–59. [Google Scholar] [CrossRef]
- Orlovskii, Y.V.; Basiev, T.T.; Vorob’ev, I.N.; Orlovskaya, E.O.; Barnes, N.P.; Mirov, S.B. Temperature dependencies of excited states lifetimes and relaxation rates of 3–5 phonon (4–6 μm) transitions in the YAG, LuAG and YLF crystals doped with trivalent holmium, thulium, and erbium. Opt. Mater. 2002, 18, 355–365. [Google Scholar] [CrossRef]
- Peng, J.T.; Hu, R.Z.; Burnard, P.G. Samarium-neodymium isotope systematics of hydrothermal calcites from the Xikuangshan antimony deposit (Hunan, China): The potential of calcite as a geochronometer. Chem. Geol. 2003, 200, 129–136. [Google Scholar] [CrossRef]
- Cheng, Y.B.; Mao, J.W.; Rusk, B.; Yang, Z.X. Geology and genesis of Kafang Cu-Sn deposit, Gejiu district, SW China. Ore Geol. Rev. 2012, 48, 180–196. [Google Scholar] [CrossRef]
- Li, C.; Zhou, L.M.; Zhao, Z.; Zhang, Z.Y.; Zhao, H.; Li, X.W.; Qu, W.J. In-situ Sr isotopic measurement of scheelite using fs-LA-MC-ICPMS. J. Asian Earth Sci. 2018, 160, 38–47. [Google Scholar] [CrossRef]
- Cavosie, A.J.; Erickson, T.M.; Timms, N.E. Nanoscale records of ancient shock deformation: Reidite (ZrSiO4) in sandstone at the Ordovician Rock Elm impact crater. Geology 2015, 43, 315–318. [Google Scholar] [CrossRef]
- Wang, T.; Feng, B.; Guo, Y.T.; Zhang, W.P.; Rao, Y.B.; Zhong, C.H.; Zhang, L.Z.; Cheng, C.; Wang, H.H.; Luo, X.P. The flotation separation behavior of apatite from calcite using carboxymethyl chitosan as depressant. Miner. Eng. 2020, 159, 106635. [Google Scholar] [CrossRef]
- Yao, X.; Yu, X.Y.; Wang, L.P.; Zeng, Y.H.; Mao, L.H.; Liu, S.M.; Xie, H.H.; He, G.C.; Huang, Z.Q.; Liu, Z.L. Preparation of cinnamic hydroxamic acid collector and study on flotation characteristics and mechanism of scheelite. Int. J. Min. Sci. Technol. 2023, 33, 773–781. [Google Scholar] [CrossRef]
- Pereira, L.; Kupka, N.; Hoang, D.H.; Michaux, B.; Saquran, S.; Ebert, D.; Rudolph, M. On the impact of grinding conditions in the flotation of semi-soluble salt-type mineral-containing ores driven by surface or particle geometry effects? Int. J. Min. Sci. Technol. 2023, 33, 855–872. [Google Scholar] [CrossRef]
- Reinhardt, N.; Frenzel, M.; Meinert, L.D.; Gutzmer, J.; Kürschner, T.; Burisch, M. Mineralogy and fluid characteristics of the Waschleithe Zn skarn-a distal part of the Schwarzenberg mineral system, Erzgebirge, Germany. Ore Geol. Rev. 2021, 131, 104007. [Google Scholar] [CrossRef]
- Peng, B.; Frei, R. Nd-Sr-Pb isotopic constraints on metal and fluid sources in W-Sb-Au mineralization at Woxi and Liaojiaping (Western Hunan, China). Miner. Depos. 2004, 39, 313–327. [Google Scholar] [CrossRef]
- Dong, S.Y.; Feng, J.L.; Li, Y.K.; Hu, L.M.; Liu, M.L.; Wang, Y.F.; Pi, Y.Q.; Sun, J.Y.; Sun, J.H. Shape-controlled synthesis of BiVO4 hierarchical structures with unique natural-sunlight-driven photocatalytic activity. Appl. Catal. B Environ. 2014, 152, 413–424. [Google Scholar] [CrossRef]
- Guan, Z.H.; Zhang, Y.; Wen, S.M.; Wu, Y.; Li, X.K.; Li, X.W. Mn-SS as a novel depressant of the flotation process of scheelite and calcite: Role and mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2024, 686, 133443. [Google Scholar] [CrossRef]
- Pontes, F.M.; Maurera, M.; Souza, A.G.; Longo, E.; Leite, E.R.; Magnani, R.; Machado, M.A.C.; Pizani, P.S.; Varela, J.A. Preparation, structural and optical characterization of BaWO4 and PbWO4 thin films prepared by a chemical route. J. Eur. Ceram. Soc. 2003, 23, 3001–3007. [Google Scholar] [CrossRef]
- Rodrigues, M.H.D.; Borges, K.C.M.; Tello, A.C.M.; Roca, R.A.; Gonçalves, R.D.; da Silva, A.B.F.; Longo, E.; Godinho, M., Jr. Effect of pH on the synthesis of BiVO4 to improve photocatalysis and antimicrobial properties. Mater. Chem. Phys. 2023, 296, 127198. [Google Scholar] [CrossRef]
- Edwards, P.N.; Mayernik, M.S.; Batcheller, A.L.; Bowker, G.C.; Borgman, C.L. Science friction: Data, metadata, and collaboration. Soc. Stud. Sci. 2011, 41, 667–690. [Google Scholar] [CrossRef]
- Ding, Y.; Yan, E.; Frazho, A.; Caverlee, J. PageRank for Ranking Authors in Co-citation Networks. J. Am. Soc. Inf. Sci. Technol. 2009, 60, 2229–2243. [Google Scholar] [CrossRef]
- Jeong, Y.K.; Song, M.; Ding, Y. Content-based author co-citation analysis. J. Informetr. 2014, 8, 197–211. [Google Scholar] [CrossRef]
- Hu, K.; Govindjee, G.; Tan, J.; Xia, Q.; Dai, Z.; Guo, Y. Co-author and co-cited reference network analysis for chlorophyll fluorescence research from 1991 to 2018. Photosynthetica 2020, 58, 110–124. [Google Scholar] [CrossRef]
- Yu, J.Q.; Kudo, A. Effects of structural variation on the photocatalytic performance of hydrothermally synthesized BiVO4. Adv. Funct. Mater. 2006, 16, 2163–2169. [Google Scholar] [CrossRef]
- Li, G.S.; Zhang, D.Q.; Yu, J.C. Ordered mesoporous BiVO4 through nanocasting:: A superior visible light-driven photocatalyst. Chem. Mater. 2008, 20, 3983–3992. [Google Scholar] [CrossRef]
- Yoon, S.H.; Kim, D.W.; Cho, S.Y.; Hong, K.S. Investigation of the relations between structure and microwave dielectric properties of divalent metal tungstate compounds. J. Eur. Ceram. Soc. 2006, 26, 2051–2054. [Google Scholar] [CrossRef]
- Yu, J.Q.; Zhang, Y.; Kudo, A. Synthesis and photocatalytic performances of BiVO4 by ammonia co-precipitation process. J. Solid State Chem. 2009, 182, 223–228. [Google Scholar] [CrossRef]
- Wang, F.Y.; Ge, G.; Ning, S.Y.; Nie, L.Q.; Zhong, G.X.; White, N.C. A new approach to LA-ICP-MS mapping and application in geology. Acta Petrol. Sin. 2017, 33, 3422–3436. [Google Scholar]
- Cavalcante, L.S.; Longo, V.M.; Sczancoski, J.C.; Almeida, M.A.P.; Batista, A.A.; Varela, J.A.; Orlandi, M.O.; Longo, E.; Li, M.S. Electronic structure, growth mechanism and photoluminescence of CaWO4 crystals. Crystengcomm 2012, 14, 853–868. [Google Scholar] [CrossRef]
- Marques, V.S.; Cavalcante, L.S.; Sczancoski, J.C.; Alcântara, A.F.P.; Orlandi, M.O.; Moraes, E.; Longo, E.; Varela, J.A.; Li, M.S.; Santos, M. Effect of Different Solvent Ratios (Water/Ethylene Glycol) on the Growth Process of CaMoO4 Crystals and Their Optical Properties. Cryst. Growth Des. 2010, 10, 4752–4768. [Google Scholar] [CrossRef]
- Sczancoski, J.C.; Bomio, M.D.R.; Cavalcante, L.S.; Joya, M.R.; Pizani, P.S.; Varela, J.A.; Longo, E.; Li, M.S.; Andrés, J. Morphology and Blue Photoluminescence Emission of PbMoO4 Processed in Conventional Hydrothermal. J. Phys. Chem. C 2009, 113, 5812–5822. [Google Scholar] [CrossRef]
- Dai, C.; Wu, X.; Wang, Q.; Bai, Y.; Zhao, D.; Fu, J.; Fu, B.; Ding, H. Layered double hydroxides for efficient treatment of heavy metals and organic pollutants: Recent progress and future perspectives. Sep. Purif. Technol. 2025, 352, 128277. [Google Scholar] [CrossRef]
- de Sousa, J.T.F.; dos Anjos, M.A.S.; Neto, J.A.D.; de Farias, E.C.; Branco, F.G.; Pederneiras, C.M. Self-Compacting Concrete with Artificial Lightweight Aggregates from Sugarcane Ash and Calcined Scheelite Mining Waste. Appl. Sci. 2025, 15, 452. [Google Scholar] [CrossRef]
- Essenni, S.; Khan, M.A.; Billah, R.E.; Jeon, B.H.; Sundaramurthy, S.; Agunaou, M. Template assisted hydrothermal synthesis of bismuth vanadate for Rhodamine B photodegradation. J. Mol. Liq. 2024, 398, 124270. [Google Scholar] [CrossRef]
- Pizzi, S.; Caputo, A.; Corvino, A.; Venturelli, A. Management research and the UN sustainable development goals (SDGs): A bibliometric investigation and systematic review. J. Clean. Prod. 2020, 276, 124033. [Google Scholar] [CrossRef]
- Gaviria-Marin, M.; Merigo, J.M.; Baier-Fuentes, H. Knowledge management: A global examination based on bibliometric analysis. Technol. Forecast. Soc. Change 2019, 140, 194–220. [Google Scholar] [CrossRef]
- Duque-Acevedo, M.; Belmonte-Urena, L.J.; Joaquin Cortes-Garcia, F.; Camacho-Ferre, F. Agricultural waste: Review of the evolution, approaches and perspectives on alternative uses. Glob. Ecol. Conserv. 2020, 22, e00902. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wen, P.C.; Xia, L.; Chen, J.; Che, J.Y.; Wang, C.Y.; Ma, B.Z. Understanding the role of hydrogen peroxide in sulfuric acid system for leaching low-grade scheelite from the perspective of phase transformation and kinetics. Sep. Purif. Technol. 2021, 277, 119407. [Google Scholar] [CrossRef]
- Niu, N.; Yang, P.A.P.; Wang, W.X.; He, F.; Gai, S.L.; Wang, D.; Lin, J. Solvothermal synthesis of SrMoO4:Ln (Ln = Eu3+, Tb3+, Dy3+) nanoparticles and its photoluminescence properties at room temperature. Mater. Res. Bull. 2011, 46, 333–339. [Google Scholar] [CrossRef]
- Ryu, J.H.; Yoon, J.W.; Shim, K.B. Microwave-assisted synthesis of BaMoO4 nanocrystallites by a citrate complex method and their anisotropic aggregation. J. Alloys Compd. 2006, 413, 144–149. [Google Scholar] [CrossRef]
- Han, H.S.; Liu, W.L.; Hu, Y.H.; Sun, W.; Li, X.D. A novel flotation scheme: Selective flotation of tungsten minerals from calcium minerals using Pb-BHA complexes in Shizhuyuan. Rare Met. 2017, 36, 533–540. [Google Scholar] [CrossRef]
- Wei, Z.; Hu, Y.H.; Han, H.S.; Sun, W.; Wang, R.L.; Wang, J.J. Selective flotation of scheelite from calcite using Al-Na2SiO3 polymer as depressant and Pb-BHA complexes as collector. Miner. Eng. 2018, 120, 29–34. [Google Scholar] [CrossRef]
- Cera, M.; Trudu, S.; Amadou, A.O.; Asunis, F.; Farru, G.; De Gaudenzi, G.P.; De Gioannis, G.; Serpe, A. Trends and perspectives in the use of organic acids for critical metal recycling from hard-metal scraps. Int. J. Refract. Met. Hard Mater. 2023, 114, 106249. [Google Scholar] [CrossRef]
- He, D.; Hu, H.; Jiao, F.; Zuo, W.; Liu, C.; Xie, H.; Dong, L.; Wang, X. Thermal separation of heavy metals from municipal solid waste incineration fly ash: A review. Chem. Eng. J. 2023, 467, 143344. [Google Scholar] [CrossRef]
- Tejado-Ramos, J.-J.; Chocarro-Leon, M.; Barrero-Bejar, I.; Giraldo-Pavon, F.; Tarragona-Perez, C.; Morales-Sotaminga, E.S.; Fernandez-Cedron, L. Drones and ultraviolet radiation for the detection of scheelite mineral. Remote Sens. Appl. Soc. Environ. 2023, 30, 100949. [Google Scholar] [CrossRef]
- Zhang, W.; Ralston, J.; Zheng, R.; Sun, W.; Xu, S.; Cao, J.; Jin, X.; Feng, Z.; Gao, Z. Quantitative evaluation of collector flotation performance II: The creation of a collector property index based on molecular structure. Sep. Purif. Technol. 2024, 332, 125855. [Google Scholar] [CrossRef]
- Espeche, M.J.; Wan, B.; Lira, R.; Seltmann, R. Mineral Chemistry and U-Pb Garnet Geochronology of Strongly Reduced Tungsten Skarns at the Pampa de Olaen Mining district, Cordoba, Argentina. Ore Geol. Rev. 2021, 138, 104379. [Google Scholar] [CrossRef]
- Kundu, T.; Dash, N.; Angadi, S.I. Separation behavior of Falcon concentrator for the recovery of ultrafine scheelite particles from the gold mine tailings. Sep. Purif. Technol. 2023, 309, 123065. [Google Scholar] [CrossRef]
- Das, S.K.; Nagesh, C.; Sreenivas, T.; Kundu, T.; Angadi, S.I. A treatise on occurrence, beneficiation and plant practices of tungsten-bearing ores. Powder Technol. 2023, 429, 118938. [Google Scholar] [CrossRef]
- Jiao, F.; Li, W.; Wang, X.; Yang, C.; Zhang, Z.; Fu, L.; Qin, W. Application of EDTMPS as a novel calcite depressant in scheelite flotation. Int. J. Min. Sci. Technol. 2023, 33, 639–647. [Google Scholar] [CrossRef]
- Huang, Z.; Shuai, S.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Application of a new amidoxime surfactant in flotation separation of scheelite and calcite: Adsorption mechanism and DFT calculation. J. Mol. Liq. 2022, 364, 120036. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, D.; Du, C.; Xu, D.-M.; Li, R.-T.; Shi, Z.-Q.; Darwish, M.A.; Zhou, T.; Jantunen, H. Design and Fabrication of a Satellite Communication Dielectric Resonator Antenna with Novel Low Loss and Temperature-Stabilized (Sm1-XCaX) (Nb1-XMoX)O4 (X = 0.15 − 0.7) Microwave Ceramics. Chem. Mater. 2023, 35, 104–115. [Google Scholar] [CrossRef]
- Shi, W.; Chen, J.; Kong, J.; Ma, Z.; Gao, J.; Guo, J.; Hu, Z.; Lv, Q.; Deng, B.; Chen, W.; et al. A novel highly thermal-stable red-emitting CaGdSbWO8:Eu3+ phosphor with scheelite structure for high CRI w-LEDs,security ink, and latent fingerprint. J. Alloys Compd. 2022, 914, 165134. [Google Scholar] [CrossRef]
- Xiao, L.; Ji, L.; Yin, C.; Chen, A.; Chen, X.; Liu, X.; Li, J.; He, L.; Sun, F.; Zhao, Z. Tungsten extraction from scheelite hydrochloric acid decomposition residue by hydrogen peroxide. Miner. Eng. 2022, 179, 107461. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, C.; Kamaruzzaman, S.N.; Aziz, N.M. A Systematic Review of Green Building Development in China: Advantages, Challenges and Future Directions. Sustainability 2022, 14, 12293. [Google Scholar] [CrossRef]
- Li, Q.; Long, R.; Chen, H.; Chen, F.; Wang, J. Visualized analysis of global green buildings: Development, barriers and future directions. J. Clean. Prod. 2020, 245, 118775. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Wu, Z.S.; Chen, W.Q.; Du, Q.A.; Tang, L.W.; Shi, H.Z.; Ma, G.T.; Zhang, Z.; Liang, W.; Wu, B.; et al. Genesis of the Nuri Cu-W-Mo Deposit, Tibet, China: Constraints from in situ Trace Elements and Sr Isotopic Analysis of Scheelite. Acta Geol. Sin.-Engl. Ed. 2024, 98, 117–131. [Google Scholar] [CrossRef]
- Duan, X.X.; Ju, Y.F.; Wang, S.D.; Wang, Z.Q. Scheelite geochemistry implications for ore-forming fluid evolution of Zhuxiling and Xiaoyao tungsten deposits, southern Anhui Province. Acta Petrol. Sin. 2023, 39, 2741–2760. [Google Scholar] [CrossRef]
- Chen, Z.Y.; Zhang, L.; Sun, X.W.; Sun, S.C.; Shen, G.W.; Yu, T.W.; Chen, X.G.; Ning, Z.W.; Xu, Y.H.; Wang, J.Y. Tungsten mineralization in the Huangjindong gold deposit, southern China: Insights from scheelite texture, in-situ trace elements and Sr isotope compositions. Ore Geol. Rev. 2024, 166, 105960. [Google Scholar] [CrossRef]
- Jena, P.; Nallamuthu, N.; Prasad, K.H.; Venkateswarlu, M.; Satyanarayana, N. Structural characterization and electrical conductivity studies of BaMoO4 nanofibers prepared by sol-gel and electrospinning techniques. J. Sol-Gel Sci. Technol. 2014, 72, 480–489. [Google Scholar] [CrossRef]
- Sadegh, M.; Badiei, A. Synthesis of CaWO4:Er3+@SiO2 and CaWO4:Tm3+@SiO2 nano-particles via a combustion pathway and study of their optical properties. Res. Chem. Intermed. 2014, 40, 2007–2014. [Google Scholar] [CrossRef]
- Ghosh, S.; Hajra, P.; Kundu, S.; Baduri, S.; Ray, D.; Bhattacharya, C. Associative Role of g-C3N4 to BiVO4 via Favorable Crystallinity and Rapid Charge-Carrier Transport for an Improved Photoelectrochemical Water Oxidation Process: An In Situ Composite Explored through Different Carbon Nitride Precursors. Acs Appl. Eng. Mater. 2023, 1, 2892–2902. [Google Scholar] [CrossRef]
- Lu, Y.J.; Shang, H.S.; Guan, H.J.; Zhao, Y.F.; Zhang, H.S.; Zhang, B. Enhanced visible-light photocatalytic activity of BiVO4 microstructures via annealing process. Superlattices Microstruct. 2015, 88, 591–599. [Google Scholar] [CrossRef]
- Foucaud, Y.; Filippova, I.; Dehaine, Q.; Hubert, P.; Filippov, L. Integrated approach for the processing of a complex tungsten Skarn ore (Tabuaco, Portugal). Miner. Eng. 2019, 143, 105896. [Google Scholar] [CrossRef]
- Gong, G.C.; Liu, J.; Han, Y.X.; Zhu, Y.M. An atomic scale investigation of the adsorption of sodium oleate on Ca2+ activated quartz surface. Physicochem. Probl. Miner. Process. 2019, 55, 426–436. [Google Scholar] [CrossRef]
- Zhang, W.J.; Chen, Y.Q.; Che, J.Y.; Wang, C.Y.; Ma, B.Z. Green leaching of tungsten from synthetic scheelite with sulfuric acid-hydrogen peroxide solution to prepare tungstic acid. Sep. Purif. Technol. 2020, 241, 116752. [Google Scholar] [CrossRef]
- Zhang, W.J.; Wang, C.Y.; Ma, B.Z. Leaching kinetics of calcium molybdate with hydrochloric acid in presence of phosphoric acid. Trans. Nonferrous Met. Soc. China 2019, 29, 859–867. [Google Scholar] [CrossRef]
- Chen, Y.L.; Huo, G.S.; Guo, X.Y.; Wang, Q.M. Sustainable extraction of tungsten from the acid digestion product of tungsten concentrate by leaching-solvent extraction together with raffinate recycling. J. Clean. Prod. 2022, 375, 133924. [Google Scholar] [CrossRef]
- Du, J.W.; Li, J.; He, D.M.; Xu, M.Y.; Zhang, G.Q.; Cao, Z.Y.; Wu, S.X. Green separation and recovery of molybdenum from tungstate solution achieved by using a recyclable vulcanizing agent. J. Clean. Prod. 2021, 278, 123930. [Google Scholar] [CrossRef]
- Li, Y.J.; Ying, Y.C.; Li, W.C.; Jiang, X.J.; Liu, Y.D.; Chen, W.; Jiang, S.Y. Genesis of W mineralization in the Yangla Cu-polymetallic deposit (NW Yunnan, China): Constraints from scheelite microstructure, trace element, U-Pb dating and Sr isotope geochemistry. Ore Geol. Rev. 2024, 169, 106098. [Google Scholar] [CrossRef]
- Di, H.F.; Shao, Y.J.; Xiong, Y.Q.; Zheng, H.; Fang, X.; Fang, W.J. Scheelite as a microtextural and geochemical tracer of multistage ore-forming processes in skarn mineralization: A case study from the giant Xintianling W deposit, South China. Gondwana Res. 2024, 136, 104–125. [Google Scholar] [CrossRef]
- Wu, K.Y.; Liu, B.; Wu, Q.H.; Chen, S.F.; Kong, H.; Li, H.; Elatikpo, S.M. Trace element geochemistry, oxygen isotope and U-Pb geochronology of multistage scheelite: Implications for W-mineralization and fluid evolution of Shizhuyuan W-Sn deposit, South China. J. Geochem. Explor. 2023, 248, 107192. [Google Scholar] [CrossRef]
- Yang, P.P.; Li, C.X.; Wang, W.X.; Quan, Z.W.; Gai, S.L.; Lin, J. Uniform AMoO4:Ln (A = Sr2+, Ba2+; Ln = Eu3+, Tb3+) submicron particles: Solvothermal synthesis and luminescent properties. J. Solid State Chem. 2009, 182, 2510–2520. [Google Scholar] [CrossRef]
- Lai, X.; Wei, Y.Y.; Qin, D.; Zhao, Y.; Wu, Y.; Gao, D.J.; Bi, J.; Lin, D.M.; Xu, G.L. Controlled Synthesis of CaWO4 Microcrystalline via Surfactant-Assisted Precipitation Method. Integr. Ferroelectr. 2013, 142, 7–15. [Google Scholar] [CrossRef]
- Liu, C.; Zhu, Y.H.; Huang, K.H.; Yang, S.Y.; Liang, Z. Studies of benzyl hydroxamic acid/calcium lignosulphonate addition order in the flotation separation of smithsonite from calcite. Int. J. Min. Sci. Technol. 2021, 31, 1153–1158. [Google Scholar] [CrossRef]
- Liao, R.P.; Wen, S.M.; Liu, J.; Feng, Q.C. Flotation separation of fine smithsonite from calcite using sodium hexametaphosphate as the depressant in the Na2S-Pb(II)-KIAX system. Sep. Purif. Technol. 2022, 295, 121245. [Google Scholar] [CrossRef]
- Gong, D.D.; Zhou, K.G.; Li, J.J.; Peng, C.H.; Chen, W. Kinetics of Roasting Reaction Between Synthetic Scheelite and Magnesium Chloride. Jom 2019, 71, 2827–2833. [Google Scholar] [CrossRef]
- Zhang, G.Q.; Guan, W.J.; Xiao, L.S.; Zhang, Q.X. A novel process for tungsten hydrometallurgy based on direct solvent extraction in alkaline medium. Hydrometallurgy 2016, 165, 233–237. [Google Scholar] [CrossRef]
- Zhang, L.M.; Shen, L.T.; Zhou, Q.S.; Qi, T.G.; Peng, Z.H.; Liu, G.H.; Li, X.B. Leaching of WO3 from Sulfuric Acid Converted Product of Scheelite in NH3•H2O-(NH4)2C2O4 Solution. J. Sustain. Metall. 2023, 9, 1589–1600. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; Yang, B.; Yin, W.; Guo, J.; Song, N.; Wang, Y.; Sun, H. Activation-inhibition mechanism of diammonium hydrogen phosphate in flotation separation of brucite and calcite. J. Environ. Chem. Eng. 2023, 11, 110184. [Google Scholar] [CrossRef]
- Mao, J.-W.; Han, D.-D.; Zhou, H.; Sun, H.-B.; Zhang, Y.-L. Bioinspired Superhydrophobic Swimming Robots with Embedded Microfluidic Networks and Photothermal Switch for Controllable Marangoni Propulsion. Adv. Funct. Mater. 2023, 33, 2208677. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Zhu, Z.; Sun, H.; Sheng, Q.; Fu, Y.; Yao, J.; Zhao, K. Differential adsorption of hydrolytic polymaleic anhydride as an eco-friendly depressant for the selective flotation of apatite from dolomite. Sep. Purif. Technol. 2021, 256, 117803. [Google Scholar] [CrossRef]
- Kim, W.; Khan, G.F.; Wood, J.; Mahmood, M.T. Employee Engagement for Sustainable Organizations: Keyword Analysis Using Social Network Analysis and Burst Detection Approach. Sustainability 2016, 8, 631. [Google Scholar] [CrossRef]
- Kim, E.S.; Chun, B.S.; Freer, R.; Cernik, R.J. Effects of packing fraction and bond valence on microwave dielectric properties of A2+B6+O4 (A2+: Ca, Pb, Ba; B6+: Mo, W) ceramics. J. Eur. Ceram. Soc. 2010, 30, 1731–1736. [Google Scholar] [CrossRef]
- Dutta, S.; Som, S.; Sharma, S.K. Luminescence and photometric characterization of K+ compensated CaMoO4:Dy3+ nanophosphors. Dalton Trans. 2009, 42, 9654–9661. [Google Scholar] [CrossRef]
- Choi, G.-K.; Kim, J.-R.; Yoon, S.H.; Hong, K.S. Microwave dielectric properties of scheelite (A = Ca, Sr, Ba) and wolframite (A = Mg, Zn, Mn) AMoO4 compounds. J. Eur. Ceram. Soc. 2007, 27, 3063–3067. [Google Scholar] [CrossRef]
- Tokunaga, S.; Kato, H.; Kudo, A. Selective preparation of monoclinic and tetragonal BiVO4 with scheelite structure and their photocatalytic properties. Chem. Mater. 2001, 13, 4624–4628. [Google Scholar] [CrossRef]
- Zhou, D.; Pang, L.-X.; Wang, D.-W.; Reaney, I.M. BiVO4 based high k microwave dielectric materials: A review. J. Mater. Chem. C 2018, 6, 9290–9313. [Google Scholar] [CrossRef]
- Chen, W.; Feng, Q.; Zhang, G.; Yang, Q.; Zhang, C. The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner. Eng. 2017, 113, 1–7. [Google Scholar] [CrossRef]
- Guo, H.-H.; Zhou, D.; Pang, L.-X.; Qi, Z.-M. Microwave dielectric properties of low firing temperature stable scheelite structured (Ca,Bi)(Mo,V)O4 solid solution ceramics for LTCC applications. J. Eur. Ceram. Soc. 2019, 39, 2365–2373. [Google Scholar] [CrossRef]
- Yu, Y.; Ma, L.; Cao, M.; Liu, Q. Slime coatings in froth flotation: A review. Miner. Eng. 2017, 114, 26–36. [Google Scholar] [CrossRef]
- Su, Y.; Li, L.; Li, G. Synthesis and Optimum Luminescence of CaWO4-Based Red Phosphors with Codoping of EU3+ and Na+. Chem. Mater. 2008, 20, 6060–6067. [Google Scholar] [CrossRef]
- Gong, X.-f.; Yao, J.; Yang, B.; Yin, W.-z.; Wang, Y.-l.; Fu, Y.-f. Flotation separation of brucite and calcite in dodecylamine system enhanced by regulator potassium dihydrogen phosphate. Trans. Nonferrous Met. Soc. China 2024, 34, 2658–2670. [Google Scholar] [CrossRef]
- Yang, B.; He, J. New insights into selective depression mechanism of Tamarindus indica kernel gum in flotation separation of fluorapatite and calcite. Sep. Purif. Technol. 2025, 354, 128787. [Google Scholar] [CrossRef]
- Derhy, M.; Taha, Y.; El-Bahi, A.; Ait-Khouia, Y.; Benzaazoua, M.; Hakkou, R. Selective flotation of calcite and dolomite from apatite using bio-based alternatives to conventional collectors: Castor and mustard oils. Miner. Eng. 2024, 208, 108597. [Google Scholar] [CrossRef]
- Bai, R.; Zhao, G.; Liu, G. Selective flotation separation of bastnaesite from calcite using p-methyl/ methoxy benzohydroxamic acid collectors. J. Ind. Eng. Chem. 2025, 143, 283–292. [Google Scholar] [CrossRef]
- Shen, C.; Yang, X.; Li, Z.; Wu, D.; Cao, Y.; Zhang, Y.; Chai, W. Efficient flotation separation mechanism of scheelite from calcite and fluorite using carboxymethyl sulfonated lignin as environmentally friendly depressant. Colloids Surf. A Physicochem. Eng. Asp. 2025, 711, 136311. [Google Scholar] [CrossRef]
- Bucci, L.A.; Hagemann, S.G.; Groves, D.T.; Standing, J.G. The Archean Chalice gold deposit: A record of complex, multistage, high-temperature hydrothermal activity and gold mineralisation associated with granitic rocks in the Yilgarn Craton, Western Australia. Ore Geol. Rev. 2002, 19, 23–67. [Google Scholar] [CrossRef]
- Martins, J.I. Leaching Systems of Wolframite and Scheelite: A Thermodynamic Approach. Miner. Process. Extr. Metall. Rev. 2014, 35, 23–43. [Google Scholar] [CrossRef]
- Voicu, G.; Bardoux, M.; Stevenson, R. Lithostratigraphy, geochronology and gold metallogeny in the northern Guiana Shield, South America: A review. Ore Geol. Rev. 2001, 18, 211–236. [Google Scholar] [CrossRef]
- Mueller, A.G.; Nemchin, A.A.; Frei, R. The Nevoria gold skarn deposit, Southern Cross greenstone belt, western Australia: II. Pressure-temperature-time path and relationship to postorogenic granites. Econ. Geol. Bull. Soc. Econ. Geol. 2004, 99, 453–478. [Google Scholar] [CrossRef]
- Sun, J.H.; Yang, H. A polyacrylamide gel route to photocatalytically active BiVO4 particles with monoclinic scheelite structure. Ceram. Int. 2014, 40, 6399–6404. [Google Scholar] [CrossRef]
- Gu, X.; Schulz, O.; Vavtar, F.; Liu, J.; Zheng, M.; Fu, S. Rare earth element geochemistry of the Woxi W-Sb-Au deposit, Hunan Province, South China. Ore Geol. Rev. 2007, 31, 319–336. [Google Scholar] [CrossRef]
- Brazdil, J.F. Designing Multifunctionality into Single Phase and Multiphase Metal-Oxide-Selective Propylene Ammoxidation Catalysts. Catalysts 2018, 8, 103. [Google Scholar] [CrossRef]
- Dill, H.G.; Melcher, F.; Botz, R. Meso- to epithermal W-bearing Sb vein-type deposits in calcareous rocks in western Thailand; with special reference to their metallogenetic position in SE Asia. Ore Geol. Rev. 2008, 34, 242–262. [Google Scholar] [CrossRef]
- Bolan, S.; Wijesekara, H.; Ireshika, A.; Zhang, T.; Pu, M.; Petruzzelli, G.; Pedron, F.; Hou, D.; Wang, L.; Zhou, S.; et al. Tungsten contamination, behavior and remediation in complex environmental settings. Environ. Int. 2023, 181, 108276. [Google Scholar] [CrossRef]
- Shuai, S.; Huang, Z.; Burov, V.E.; Poilov, V.Z.; Li, F.; Wang, H.; Liu, R.; Zhang, S.; Cheng, C.; Li, W.; et al. Flotation separation of wolframite from calcite using a new trisiloxane surfactant as collector. Int. J. Min. Sci. Technol. 2023, 33, 379–387. [Google Scholar] [CrossRef]


| Keywords | Year | Strength | Begin | End | 1999–2024 |
|---|---|---|---|---|---|
| deposits | 1999 | 5.46 | 1999 | 2006 | ▃▃▃▂▂▂▂▂▂▂ |
| geochemistry | 2000 | 4.95 | 2000 | 2008 | ▂▃▃▃▂▂▂▂▂▂ |
| cawo4 | 2002 | 10.84 | 2002 | 2016 | ▂▃▃▃▃▃▃▂▂▂ |
| thin films | 2003 | 5.91 | 2003 | 2014 | ▂▃▃▃▃▃▂▂▂▂ |
| origin | 2003 | 5 | 2003 | 2012 | ▂▃▃▃▃▂▂▂▂▂ |
| growth | 2005 | 5.91 | 2005 | 2016 | ▂▂▃▃▃▃▃▂▂▂ |
| crystals | 2007 | 10.43 | 2007 | 2014 | ▂▂▂▃▃▃▂▂▂▂ |
| camoo4 | 2007 | 8.57 | 2007 | 2016 | ▂▂▂▃▃▃▃▂▂▂ |
| luminescence | 2007 | 8.22 | 2007 | 2012 | ▂▂▂▃▃▂▂▂▂▂ |
| ca | 2003 | 6.06 | 2007 | 2014 | ▂▂▂▃▃▃▂▂▂▂ |
| powders | 2007 | 5.72 | 2007 | 2014 | ▂▂▂▃▃▃▂▂▂▂ |
| molybdate | 2007 | 5.39 | 2007 | 2012 | ▂▂▂▃▃▂▂▂▂▂ |
| hydrothermal synthesis | 2009 | 7.92 | 2009 | 2018 | ▂▂▂▂▃▃▃▃▂▂ |
| sr | 2009 | 7.31 | 2009 | 2018 | ▂▂▂▂▃▃▃▃▂▂ |
| nanoparticles | 2009 | 6.67 | 2009 | 2018 | ▂▂▂▂▃▃▃▃▂▂ |
| nanocrystals | 2009 | 6.35 | 2009 | 2018 | ▂▂▂▂▃▃▃▃▂▂ |
| luminescent property | 2009 | 5.53 | 2009 | 2016 | ▂▂▂▂▃▃▃▂▂▂ |
| energy transfer | 2009 | 4.75 | 2009 | 2020 | ▂▂▂▂▃▃▃▃▃▂ |
| route | 2009 | 4.73 | 2009 | 2016 | ▂▂▂▂▃▃▃▂▂▂ |
| morphology | 2011 | 8.77 | 2011 | 2016 | ▂▂▂▂▂▃▃▂▂▂ |
| photoluminescence | 2009 | 6.72 | 2013 | 2016 | ▂▂▂▂▂▂▃▂▂▂ |
| collector | 2017 | 6.66 | 2017 | 2022 | ▂▂▂▂▂▂▂▃▃▂ |
| calcite | 2004 | 4.7 | 2017 | 2020 | ▂▂▂▂▂▂▂▃▃▂ |
| separation | 2015 | 6.44 | 2019 | 2024 | ▂▂▂▂▂▂▂▂▃▃ |
| la icp ms | 2013 | 5.64 | 2019 | 2024 | ▂▂▂▂▂▂▂▂▃▃ |
| Keywords | Year | Strength | Begin | End | 1999–2024 |
|---|---|---|---|---|---|
| crystals | 2005 | 5.45 | 2005 | 2014 | ▂▂▃▃▃▃▂▂▂▂ |
| hydrothermal synthesis | 2005 | 5.43 | 2005 | 2018 | ▂▂▃▃▃▃▃▃▂▂ |
| growth | 2005 | 5.31 | 2005 | 2016 | ▂▂▃▃▃▃▃▂▂▂ |
| degradation | 2008 | 5.18 | 2008 | 2018 | ▂▂▂▃▃▃▃▃▂▂ |
| luminescence | 2008 | 5.13 | 2008 | 2012 | ▂▂▂▃▃▂▂▂▂▂ |
| camoo4 | 2008 | 3.9 | 2008 | 2014 | ▂▂▂▃▃▃▂▂▂▂ |
| nanoparticles | 2009 | 5.95 | 2009 | 2018 | ▂▂▂▃▃▃▃▃▂▂ |
| cawo4 | 2009 | 5.36 | 2009 | 2014 | ▂▂▂▃▃▃▂▂▂▂ |
| nanocrystals | 2009 | 4.54 | 2009 | 2018 | ▂▂▂▃▃▃▃▃▂▂ |
| irradiation | 2009 | 3.65 | 2009 | 2012 | ▂▂▂▃▃▂▂▂▂▂ |
| optical property | 2009 | 3.65 | 2009 | 2012 | ▂▂▂▃▃▂▂▂▂▂ |
| morphology | 2011 | 4.84 | 2011 | 2014 | ▂▂▂▂▃▃▂▂▂▂ |
| sr | 2011 | 3.44 | 2011 | 2016 | ▂▂▂▂▃▃▃▂▂▂ |
| water | 2011 | 3.33 | 2011 | 2018 | ▂▂▂▂▃▃▃▃▂▂ |
| eu3+ | 2014 | 4.62 | 2014 | 2016 | ▂▂▂▂▂▂▃▂▂▂ |
| photoluminescence | 2014 | 3.85 | 2014 | 2020 | ▂▂▂▂▂▂▃▃▃▂ |
| luminescence property | 2014 | 3.31 | 2014 | 2018 | ▂▂▂▂▂▂▃▃▂▂ |
| soda | 2015 | 4.34 | 2015 | 2020 | ▂▂▂▂▂▂▃▃▃▂ |
| zircon u pb | 2018 | 3.72 | 2018 | 2020 | ▂▂▂▂▂▂▂▂▃▂ |
| calcite | 2017 | 3.58 | 2017 | 2020 | ▂▂▂▂▂▂▂▃▂▂ |
| collector | 2017 | 3.42 | 2017 | 2018 | ▂▂▂▂▂▂▂▂▃▂ |
| leaching kinetics | 2019 | 3.41 | 2021 | 2022 | ▂▂▂▂▂▂▂▂▂▃ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.; Gao, L.; Cao, J. Scheelite as a Strategic Tungsten Resource: A Bibliometric Study of Global and Chinese Technology Trends (1999–2024). Minerals 2025, 15, 1181. https://doi.org/10.3390/min15111181
Gao Z, Gao L, Cao J. Scheelite as a Strategic Tungsten Resource: A Bibliometric Study of Global and Chinese Technology Trends (1999–2024). Minerals. 2025; 15(11):1181. https://doi.org/10.3390/min15111181
Chicago/Turabian StyleGao, Zhengbo, Lingxiao Gao, and Jian Cao. 2025. "Scheelite as a Strategic Tungsten Resource: A Bibliometric Study of Global and Chinese Technology Trends (1999–2024)" Minerals 15, no. 11: 1181. https://doi.org/10.3390/min15111181
APA StyleGao, Z., Gao, L., & Cao, J. (2025). Scheelite as a Strategic Tungsten Resource: A Bibliometric Study of Global and Chinese Technology Trends (1999–2024). Minerals, 15(11), 1181. https://doi.org/10.3390/min15111181







