The Effect of Electrochemical Surface Properties on Molybdenite Flotation in Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Electrochemical Studies
2.3. Grinding and Flotation
3. Results and Discussion
3.1. Electrochemical Studies
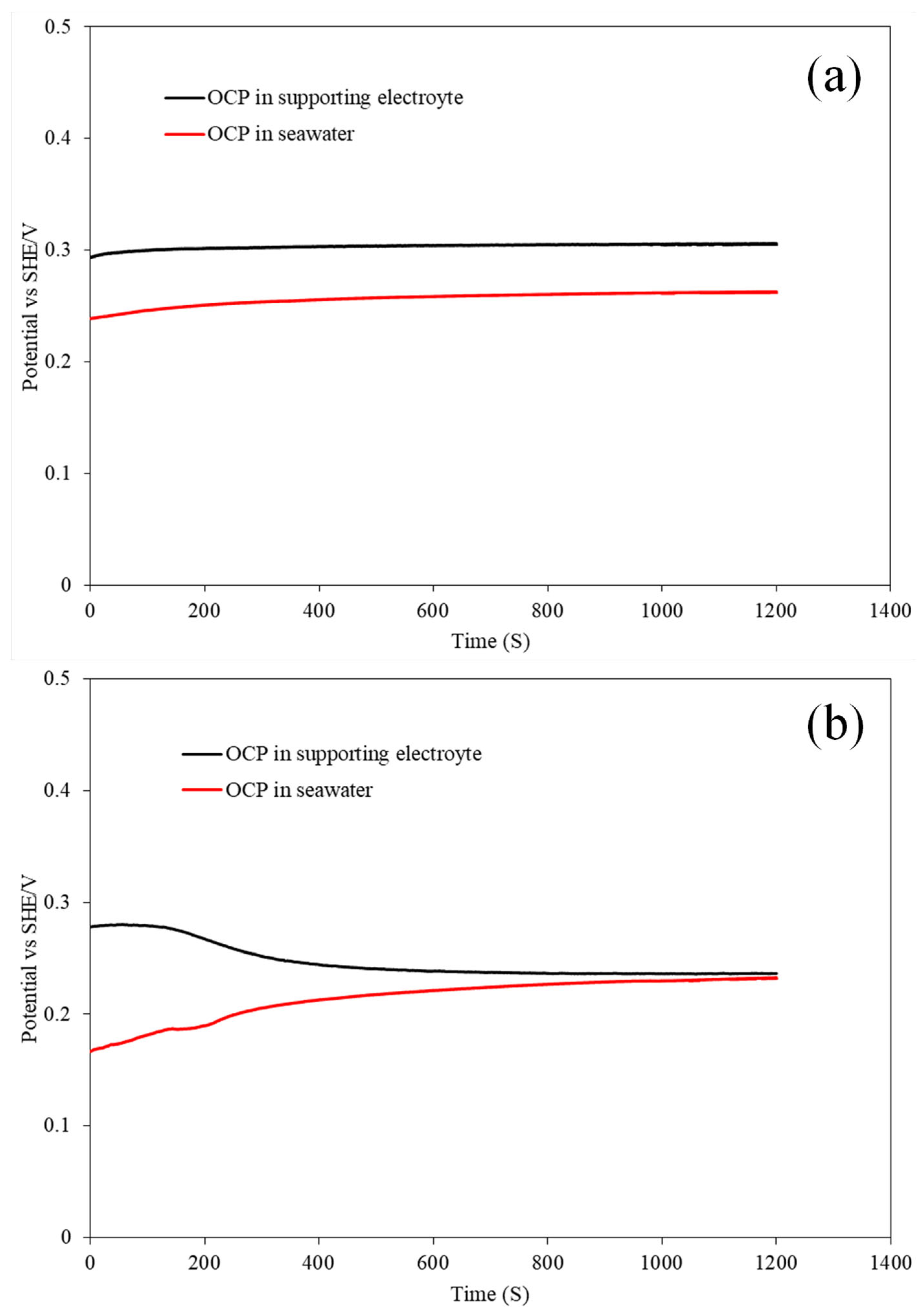
3.1.1. Open Circuit Potential Measurements
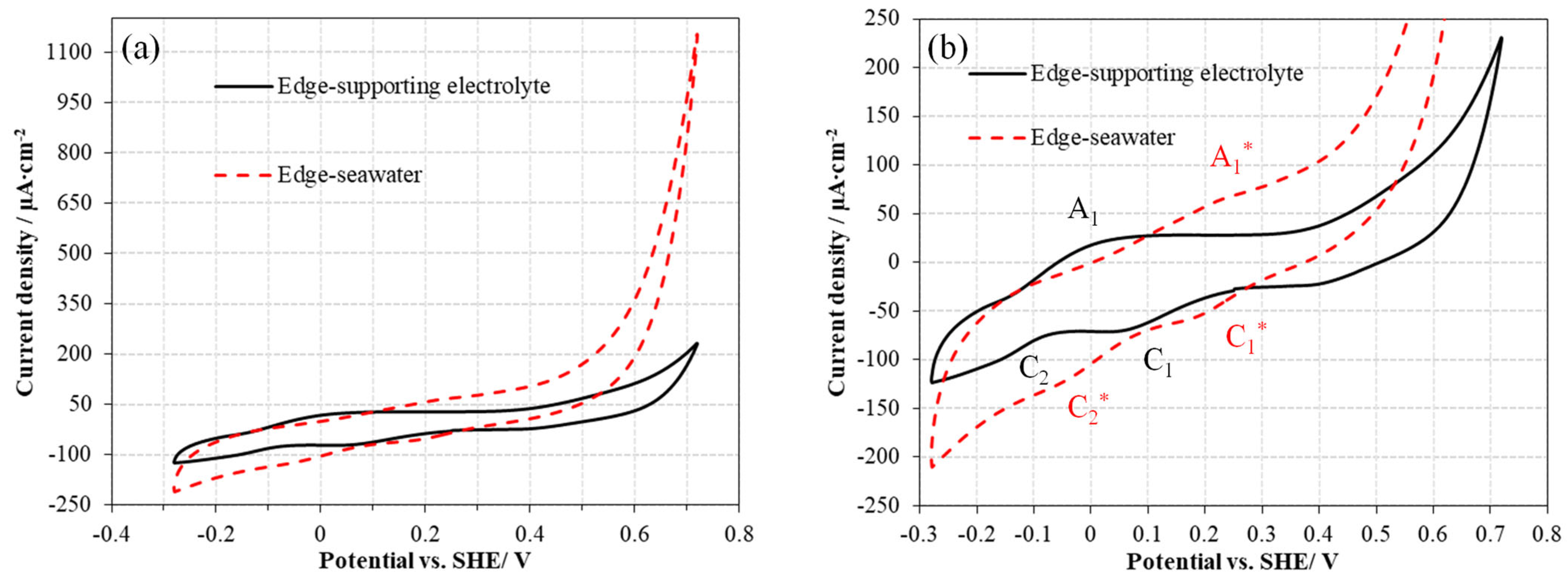
3.1.2. Cyclic Voltammetry Measurements
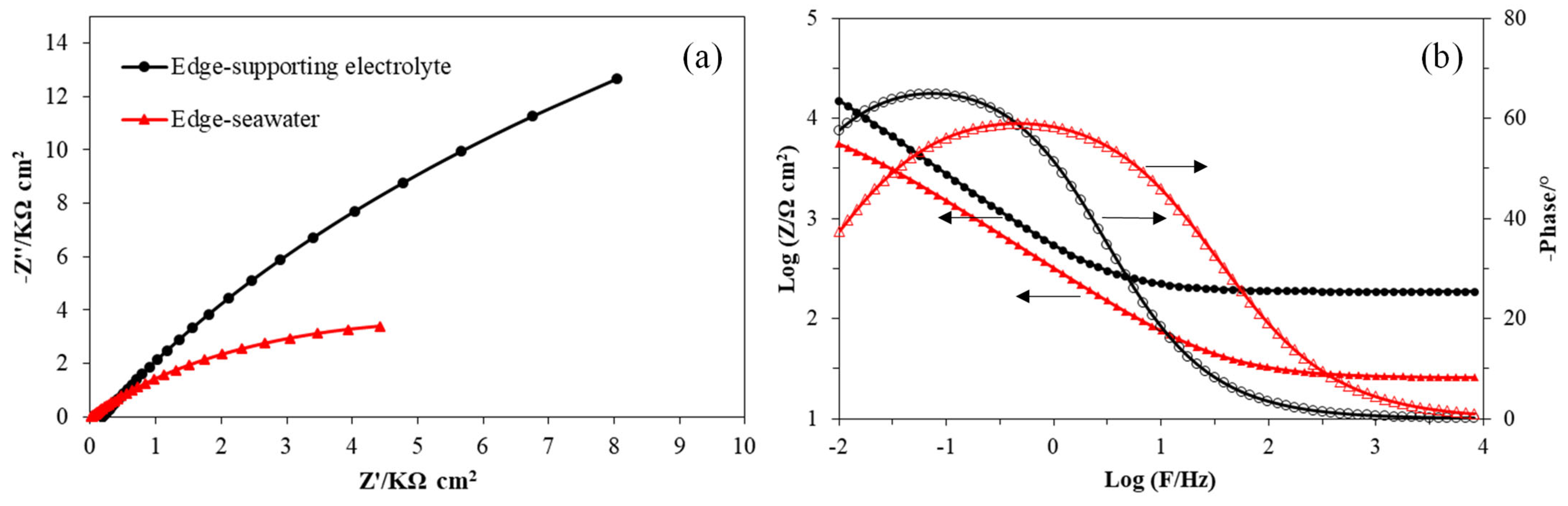
3.1.3. Electrochemical Impedance Spectroscopy Measurements
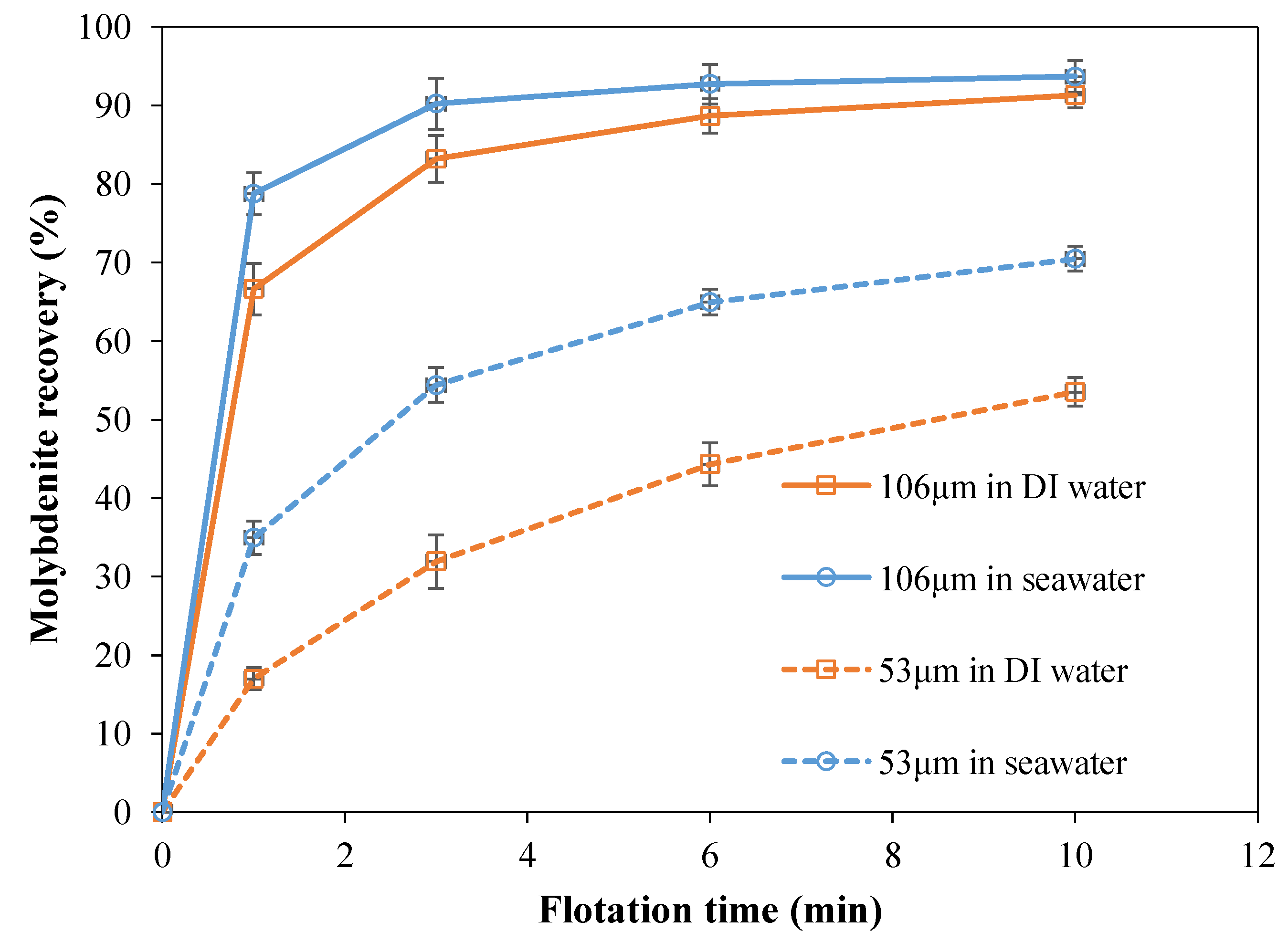
3.2. Flotation Tests
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Castro, S.; Lopez-Valdivieso, A.; Laskowski, J.S. Review of the flotation of molybdenite. Part I: Surface properties and floatability. Int. J. Miner. Process. 2016, 148, 48–58. [Google Scholar] [CrossRef]
- Moreno, P.A.; Aral, H.; Cuevas, J.; Monardes, A.; Adaro, M.; Norgate, T.; Bruckard, W. The use of seawater as process water at Las Luces copper–molybdenum beneficiation plant in Taltal (Chile). Miner. Eng. 2011, 24, 852–858. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, S.; Bradshaw, D. Role of saline water in the selective flotation of fine particles. Water Miner. Process. 2012, 15, 61–71. [Google Scholar]
- Pan, L.; Yoon, R.-H. Effects of elctrolytes on the stability of wetting films: Implications on seawater flotation. Miner. Eng. 2018, 122, 1–9. [Google Scholar] [CrossRef]
- Castro, S.; Miranda, C.; Toledo, P.; Laskowski, J.S. Effect of frothers on bubble coalescence and foaming in electrolyte solutions and seawater. Int. J. Miner. Process. 2013, 124, 8–14. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Arancibia-Bravo, M.P.; Reyes, A.; Aguirre, C.E.; Cortes, L.; Cisternas, L.A. The impact of seawater with calcium and magnesium removal for the flotation of copper-molybdenum sulphide ores. Miner. Eng. 2017, 109, 10–13. [Google Scholar] [CrossRef]
- Hirajima, T.; Suyantara, G.P.W.; Ichikawa, O.; Elmahdy, A.M.; Miki, H.; Sasaki, K. Effect of Mg2+ and Ca2+ as divalent seawater cations on the floatability of molybdenite and chalcopyrite. Miner. Eng. 2016, 96–97, 83–93. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, X.; Peng, Y. The effect of sodium hydrosulfide on molybdenite flotation in seawater and diluted seawater. Miner. Eng. 2020, 158, 106589. [Google Scholar] [CrossRef]
- Becker, U.; Rosso, K.M.; Weaver, R.; Warren, M.; Hochella, M.F. Metal island growth and dynamics on molybdenite surfaces. Geochim. Cosmochim. Acta 2003, 67, 923–934. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, Q.; Xu, Z.; Zeng, H. Probing Anisotropic Surface Properties of Molybdenite by Direct Force Measurements. Langmuir 2015, 31, 11409–11418. [Google Scholar] [CrossRef]
- Tan, S.M.; Ambrosi, A.; Sofer, Z.; Huber, Š.; Sedmidubský, D.; Pumera, M. Pristine Basal- and Edge-Plane-Oriented Molybdenite MoS2 Exhibiting Highly Anisotropic Properties. Chem. A Eur. J. 2015, 21, 7170–7178. [Google Scholar] [CrossRef]
- Heising, J.; Kanatzidis, M.G. Structure of restacked MoS2 and WS2 elucidated by electron crystallography. J. Am. Chem. Soc. 1999, 121, 638–643. [Google Scholar] [CrossRef]
- Xie, L. Probing Surface Heterogeneity, Electrochemical Properties and Bubble-Solid Interaction Mechanisms of Sulfide Minerals in Flotation. Ph.D. Thesis, University of Alberta, Edmonton, Canada, 2017. [Google Scholar]
- Zanin, M.; Ametov, I.; Grano, S.; Zhou, L.; Skinner, W. A study of mechanisms affecting molybdenite recovery in a bulk copper/molybdenum flotation circuit. Int. J. Miner. Process. 2009, 93, 256–266. [Google Scholar] [CrossRef]
- Wang, H.; Gu, G.-H.; Fu, J.-G.; Chen, L.; Hao, Y. Study of the interfacial interactions in the molybdenite floatation system. J. China Univ. Min. Technol. 2008, 18, 82–87. [Google Scholar] [CrossRef]
- Yang, B.; Song, S.; Lopez-Valdivieso, A. Morphology of Hydrophobic Agglomerates of Molybdenite Fines in Aqueous Suspensions. Sep. Sci. Technol. 2015, 50, 1560–1564. [Google Scholar] [CrossRef]
- Chanturiya, V.A.; Krasavtseva, E.A.; Makarov, D.V. Electrochemistry of Sulfides: Process and Environmental Aspects. Sustainability 2022, 14, 11285. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, H. Recent advances in electrochemical techniques for characterizing surface properties of minerals. Adv. Colloid Interface Sci. 2020, 288, 102346. [Google Scholar] [CrossRef]
- Chander, S. Electrochemistry of sulfide flotation: Growth characteristics of surface coatings and their properties, with special reference to chalcopyrite and pyrite. Int. J. Miner. Process. 1991, 33, 121–134. [Google Scholar] [CrossRef]
- Yang, B.; Song, S.; Lopez-Valdivieso, A. Effect of Particle Size on the Contact Angle of Molybdenite Powders. Miner. Process. Extr. Metall. Rev. 2014, 35, 208–215. [Google Scholar] [CrossRef]
- Wang, E.; Wan, H.; Qu, J.; Yi, P.; Bu, X. Inhibiting Mechanism of High pH on Molybdenite Flotation. Exp. DFT Study. Miner. 2024, 14, 663. [Google Scholar]
- Zhang, Q.D.; Li, X.L.; Hu, Z.F.; Gao, B.W.; Liu, C. Study on Floatation Separation of Molybdenite and Talc Based on Crystal Surface Anisotropy. Separations 2025, 12, 123. [Google Scholar] [CrossRef]
- Chao, Y.D.; Li, S.L.; Gao, L.H.; Sun, L.J.; Li, L.N.; Chai, N.; Cao, Y.J. Enhanced Flotation Recovery of Fine Molybdenite Particles Using a Coal Tar-Based Collector. Minerals 2021, 11, 1439. [Google Scholar] [CrossRef]
- Miki, H.; Matsuoka, H.; Hirajima, T.; Suyantara, G.P.W.; Sasaki, K. Electrolysis Oxidation of Chalcopyrite and Molybdenite for Selective Flotation. Mater. Trans. 2017, 58, 761–767. [Google Scholar] [CrossRef]
- Kester, D.R.; Duedall, I.W.; Connors, D.N.; Pytkowicz, R.M. Preparation of artificial seawater 1. Limnology and oceanography 1967, 12, 176–179. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. Electrochemistry aspects of pyrite in the presence of potassium amyl xanthate and a lignosulfonate-based biopolymer depressant. Electrochim. Acta 2015, 174, 133–142. [Google Scholar] [CrossRef]
- Wang, J.; Xie, L.; Lu, Q.; Wang, X.; Wang, J.; Zeng, H. Electrochemical investigation of the interactions of organic and inorganic depressants on basal and edge planes of molybdenite. J. Colloid Interface Sci. 2020, 570, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Yang, W.; Cao, W.; He, T.; Liu, Y.; Yang, J.; Guo, L.; Peng, Y. The Interaction between Ca2+ and Molybdenite Edges and Its Effect on Molybdenum Flotation. Minerals 2017, 7, 141. [Google Scholar] [CrossRef]
- Hirajima, T.; Miki, H.; Suyantara, G.P.W.; Matsuoka, H.; Elmahdy, A.M.; Sasaki, K.; Imaizumi, Y.; Kuroiwa, S. Selective flotation of chalcopyrite and molybdenite with H2O2 oxidation. Miner. Eng. 2017, 100, 83–92. [Google Scholar] [CrossRef]
- Spevack, P.A.; McIntyre, N. A Raman and XPS investigation of supported molybdenum oxide thin films. 1. Calcination and reduction studies. J. Phys. Chem. 1993, 97, 11020–11030. [Google Scholar] [CrossRef]
- Mu, Y.; Peng, Y.; Lauten, R.A. The depression of copper-activated pyrite in flotation by biopolymers with different compositions. Miner. Eng. 2016, 96–97, 113–122. [Google Scholar] [CrossRef]
- Ertekin, Z.; Pekmez, K.; Ekmekçi, Z. Evaluation of collector adsorption by electrochemical impedance spectroscopy. Int. J. Miner. Process. 2016, 154, 16–23. [Google Scholar] [CrossRef]
- Lucay, F.; Cisternas, L.; Gálvez, E.; López-Valdivieso, A. Study of the natural floatability of molybdenite fines in saline solutions. Effect of gypsum precipitation. Miner. Metall. Process. J. 2015, 32, 203–208. [Google Scholar] [CrossRef]
- Yepsen, R.; Roa, J.; Toledo, P.G.; Gutiérrez, L. Chalcopyrite and Molybdenite Flotation in Seawater: The Use of Inorganic Dispersants to Reduce the Depressing Effects of Micas. Minerals 2021, 11, 539. [Google Scholar] [CrossRef]




| Species Present (wt.%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al2O3 | Bi | CaO | Co | Cu | Fe | Mo | Pb | S | SiO2 | Ti | Zn | |
| Molybdenite | 0.13 | 0.23 | 0.07 | 0.01 | 0.06 | 0.14 | 58.3 | 0.06 | 39.5 | 1.52 | - | 0.01 |
| Type | Ca2+ | K+ | Mg2+ | Na+ | SO42− | Cl− | CO32−/HCO3− |
|---|---|---|---|---|---|---|---|
| Synthetic seawater | 3494 | 588 | 2897 | 14,236 | 8447 | 30,300 | 341 |
| Average seawater | 400 | 280 | 1272 | 10,556 | 2649 | 18,980 | 140 |
| Electrode | Supporting Electrolyte | Seawater |
|---|---|---|
| MoS2-Basal | 293 | 239 |
| MoS2-Edge | 270 | 167 |
| Rs Ω cm2 | Rct kΩ cm2 | Cdl µF cm−2 | n | |
|---|---|---|---|---|
| Basal-Supporting electrolyte | 745.4 | 354.4 | 128.3 | 0.68 |
| Basal-Seawater | 696.7 | 350.5 | 132.9 | 0.68 |
| Edge-Supporting electrolyte | 184.9 | 65.5 | 529.3 | 0.78 |
| Edge-Seawater | 25.7 | 11.3 | 870.9 | 0.71 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Zhang, N.; Cui, H. The Effect of Electrochemical Surface Properties on Molybdenite Flotation in Seawater. Minerals 2025, 15, 1049. https://doi.org/10.3390/min15101049
Chen Y, Zhang N, Cui H. The Effect of Electrochemical Surface Properties on Molybdenite Flotation in Seawater. Minerals. 2025; 15(10):1049. https://doi.org/10.3390/min15101049
Chicago/Turabian StyleChen, Yang, Na Zhang, and Haoran Cui. 2025. "The Effect of Electrochemical Surface Properties on Molybdenite Flotation in Seawater" Minerals 15, no. 10: 1049. https://doi.org/10.3390/min15101049
APA StyleChen, Y., Zhang, N., & Cui, H. (2025). The Effect of Electrochemical Surface Properties on Molybdenite Flotation in Seawater. Minerals, 15(10), 1049. https://doi.org/10.3390/min15101049









