Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review
Abstract
1. Introduction
2. Brief Geology
2.1. The Khibiny Massif and Apatite-Nepheline Deposits
2.2. The Lovozero Massif and Co-Named Loparite Deposit
3. Methods
4. Morphological Types of Gas Phase
4.1. Occluded Gases
4.2. Diffusely Dispersed Gases
4.3. Free Gases
4.4. On the Spatial Relationship of Gas Occurrence Forms
5. On the Origin of HHCGs in Nepheline-Syenite Massifs
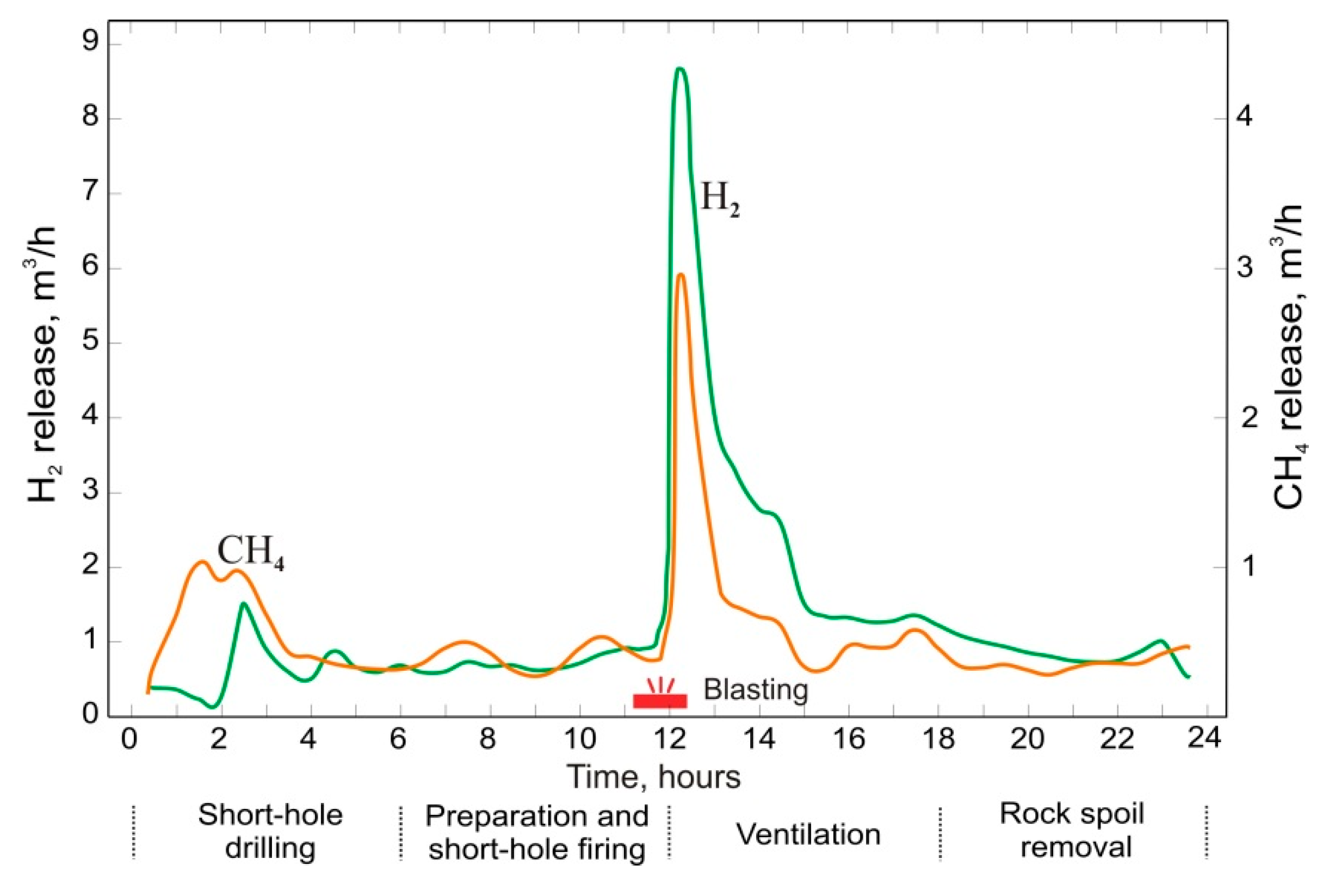
6. Gas Content of Underground Mine Openings, Basic Principles, and Measures for Gas-safe Mining
7. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Eliseev, N.A.; Fedorov, E.E. The Lovozero Pluton and its Ore Deposits; Transactions of the Precambrian Laboratory: Leningrad, Russia, 1953. (In Russian) [Google Scholar]
- Vlasov, K.A.; Kuz’menko, M.Z.; Es’kova, E.M. The Lovozero Alkali Massif; Fry, D.G.; Syers, K., Translators; Oliver & Boyd: Edinburgh and London, UK, 1966. [Google Scholar]
- Ivanova, T.N. Apatite Deposits of the Khibiny Tundres; Gosgeoltekhizlat: Moscow, Russia, 1963. (In Russian) [Google Scholar]
- Onokhin, F.M. Peculiarities of the Khibiny Massif Structure and Apatite-Nepheline Deposits; Nauka: Leningrad, Russia, 1975. (In Russian) [Google Scholar]
- Kamenev, Y.A.; Mineev, D.A. (Eds.) New Khibiny Apatite Deposits; Nedra: Moscow, Russia, 1982. (In Russian) [Google Scholar]
- Kamenev, Y.A. Prospecting, Exploring and Geological Industrial Assessment of the Khibiny Type Apatite Deposits; Nedra: Moscow, Russia, 1987. (In Russian) [Google Scholar]
- Arzamastsev, A.A. Unique Paleozoic Intrusions of the Kola Peninsula; KSC RAS: Apatity, Russia, 1994. [Google Scholar]
- Kogarko, L.N.; Lahaye, Y.; Brey, G.P. Plume-related mantle source of super-large rare metal deposits from the Lovozero and Khibina massifs on the Kola Peninsula, Eastern part of Baltic shield: Sr, Nd and Hf isotope systematics. Miner. Petrol. 2010, 98, 197–208. [Google Scholar] [CrossRef]
- Ivanyuk, G.; Yakovenchuk, V.; Pakhomovsky, Y.; Kalashnikov, A.; Mikhailova, J.; Goryainov, P. Self-Organization of the Khibiny Alkaline Massif (Kola Peninsula, Russia). In Earth Sciences; Dar, I.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 131–156. ISBN 978-953-307-861-8. [Google Scholar]
- Pakhomovsky, Y.A.; Ivanyuk, G.Y.; Yakovenchuk, V.N. Loparite-(Ce) in rocks of the Lovozero layered complex at Mt. Karnasurt and Mt. Kedykvyrpakhk. Geol. Ore Deposit. 2014, 56, 685–698. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Konopleva, N.G.; Pakhomovsky, Y.A.; Ivanyuk, G.Y. Rare earth deposits of the Murmansk Region, Russia—A review. Econ. Geol. 2016, 111, 1529–1559. [Google Scholar] [CrossRef]
- Kogarko, L. Chemical composition and petrogenetic implications of apatite in the Khibiny apatite-nepheline deposits (Kola Peninsula). Minerals 2018, 8, 532. [Google Scholar] [CrossRef]
- Krivovichev, V.G.; Charykova, M.V. Mineral systems, their types, and distribution in nature. I. Khibiny, Lovozero, and the Mont Saint-Hilaire. Geol. Ore Dep. 2016, 58, 551–558. [Google Scholar] [CrossRef]
- Petersilie, I.A. Hydrocarbonic gases and bitumens if igneous massifs in the central part of the Kola Peninsula. Dokl. AN USSR 1958, 122, 1086–1089. (In Russian) [Google Scholar]
- Petersilie, I.A. Geology and Geochemistry of Natural Gases and Disperse Bitumens of Some Geological Formations of the Kola Peninsula; Nauka: Moscow, Russia, 1964. (In Russian) [Google Scholar]
- Ikorsky, S.V.; Nivin, V.A.; Pripachkin, V.A. Gas Geochemistry of Endogenic Formations; Nauka: St.-Petersburg, Russia, 1992. (In Russian) [Google Scholar]
- Nivin, V.A. Diffusely disseminated hydrogen–hydrocarbon gases in rocks of nepheline syenite complexes. Geochem. Int. 2009, 47, 672–691. [Google Scholar] [CrossRef]
- Nivin, V.A. Gas Components in Magmatic Rocks: Geochemical, Mineragenic and Environmental Aspects and Results. Doctor of Sciences (Geol.-Miner.) Dissertation, Vernadsky Institute of Geochemistry and Analytical Chemistry of Russian Academу of Sciences, Moscow, Russia, 2013; p. 354. (In Russian). [Google Scholar]
- Nivin, V.A. Free hydrogen-hydrocarbon gases from the Lovozero loparite deposit (Kola Peninsula, NW Russia). Appl. Geochem. 2016, 74, 44–55. [Google Scholar] [CrossRef]
- Wallace, P.J. Volatiles in subduction zone magmas: concentrations and fluxes based on melt inclusion and volcanic gas data. J. Volcanol. Geotherm. Res. 2005, 140, 217–240. [Google Scholar] [CrossRef]
- Fischer, T.P.; Chiodini, G. Volcanic, magmatic and hydrothermal gas discharges. In Encyclopaedia of Volcanoes, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 779–797. [Google Scholar]
- Petersilie, I.A.; Sørensen, H. Hydrocarbon gases and bituminous substances in rocks from the Ilímaussaq alkaline intrusion, South Greenland. Lithos 1970, 3, 59–76. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J.; Larsen, E.; Rose-Hansen, J. Hydrocarbon-rich fluid inclusions in minerals from the alkaline Ilímaussaq intrusion. South Greenland. Bull. Minéral. 1979, 102, 642–653. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J.; Rose-Hansen, J. Volatiles associated with alkaline igneous rift activity: fluid inclusions in the Ilímaussaq intrusion and the Gardar granitic complexes (South Greenland). Chem. Geol. 1982, 37, 79–93. [Google Scholar] [CrossRef]
- Konnerup-Madsen, J. A review of the composition and evolution of hydrocarbon gases during solidification of the Ilímaussaq alkaline complex, South Greenland. Geol. Greenl. Surv. Bull. 2001, 190, 159–166. [Google Scholar]
- Markl, G.; Baumgartner, L. pH changes in peralkaline late-magmatic fluids. Contrib. Mineral. Petrol. 2002, 144, 331–346. [Google Scholar] [CrossRef]
- Krumrei, T.V.; Pernicka, E.; Kaliwoda, M.; Markl, G. Volatiles in a peralkaline system: abiogenic hydrocarbons and F–Cl–Br systematics in the naujaite of the Ilímaussaq intrusion, South Greenland. Lithos 2007, 95, 298–314. [Google Scholar] [CrossRef]
- Graser, G.; Potter, J.; Kohler, J.; Markl, G. Isotope, major, minor and trace element geochemistry of late-magmatic fluids in the peralkaline Ilimaussaq intrusion, South Greenland. Lithos 2008, 106, 207–221. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Reduced orthomagmatic C-O-H-N-NaCl fluids in the Strange Lake rare-metal granitic complex, Quebec Labrador, Canada. Eur. J. Mineral. 1992, 4, 1155–1174. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Fischer–Tropsch synthesis of hydrocarbons during sub-solidus alteration of the Strange Lake peralkaline granite, Quebec/Labrador, Canada. Geochim. Cosmochim. Acta 1997, 61, 83–99. [Google Scholar] [CrossRef]
- Salvi, S.; Williams-Jones, A.E. Alteration, HFSE mineralisation and hydrocarbon formation in peralkaline igneous systems: insights from the Strange Lake Pluton, Canada. Lithos 2006, 91, 19–34. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Williams-Jones, A.E.; Blamey, N.J.F. Fluid evolution in the Strange Lake granitic pluton, Canada: Implications for HFSE mobilisation. Chem. Geol. 2016, 444, 83–100. [Google Scholar] [CrossRef]
- Ikorsky, S.V.; Nivin, V.A. Free phase gases in rocks of the Lovozero alkaline massif (Kola Peninsula). Dokl. Acad. Nauk S.S.S.R. 1983, 269, 934–936. (In Russian) [Google Scholar]
- Potter, J.; Konnerup-Madsen, J. A review of the occurrence and origin of abiogenic hydrocarbons in igneous rocks. In Hydrocarbons in Crystalline Rocks; Petford, N., McCaffrey, K.J.W., Eds.; The Geological Society of London: London, UK, 2003; Geological Society Special Publication; Volume 214, pp. 151–173. [Google Scholar] [CrossRef]
- Nivin, V.A.; Treloar, P.J.; Konopleva, N.G.; Ikorsky, S.V. A review of the occurrence, form and origin of C-bearing species in the Khibiny alkaline igneous complex, Kola Peninsula, NW Russia. Lithos 2005, 85, 93–112. [Google Scholar] [CrossRef]
- Beeskow, B.; Treloar, P.J.; Rankin, A.H.; Vennemann, T.W.; Spangenberg, J. A reassessment of models for hydrocarbon generation in the Khibiny nepheline syenite complex, Kola Peninsula, Russia. Lithos 2006, 91, 1–18. [Google Scholar] [CrossRef]
- Gottikh, R.P.; Pisotskii, B.I.; Kulakova, I.I. Geochemistry of reduced fluids from alkaline igneous rocks of the Khibiny Pluton. Dokl. Earth Sci. 2006, 407, 298–303. [Google Scholar] [CrossRef]
- Potter, J.; Longstaffe, F.J. A gas-chromatograph, continuous flow-isotope ratio mass-spectrometry method for δ13 C and δD measurement of complex fluid inclusion volatiles: Examples from the Khibina alkaline igneous complex, northwest Russia and the south Wales coalfields. Chem. Geol. 2007, 244, 186–201. [Google Scholar] [CrossRef]
- Schonenberger, J.; Markl, G. The magmatic and fluid evolution of the Motzfeldt intrusion in South Greenland: Insights into the formation of agpaitic and miaskitic rocks. J. Petrol. 2008, 49, 1549–1577. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Redox potential of the Khibiny magmatic system and genesis of abiogenic hydrocarbons in alkaline plutons. Geol. Ore Deposit. 2009, 51, 425–440. [Google Scholar] [CrossRef]
- Markl, G.; Marks, M.A.W.; Frost, B.R. On the controls of oxygen fugacity in the generation and crystallization of peralkaline melts. J. Petrol. 2010, 51, 1831–1847. [Google Scholar] [CrossRef]
- Kendrick, M.A.; Honda, M.; Walshe, J.; Petersen, K. Fluid sources and the role of abiogenic-CH4 in Archean gold mineralization: Constraints from noble gases and halogens. Precambrian Res. 2011, 189, 313–327. [Google Scholar] [CrossRef]
- Nivin, V.A. Variations in the composition and origin of hydrocarbon gases from inclusions in minerals of the Khibiny and Lovozero plutons, Kola Peninsula, Russia. Geol. Ore Deposit. 2011, 53, 699–707. [Google Scholar] [CrossRef]
- Laier, T.; Nytoft, H.P. Bitumen biomarkers in the Mid-Proterozoic Ilimaussaq intrusion, Southwest Greenland–A challenge to the mantle gas theory. Mar. Petrol. Geol. 2012, 30, 50–65. [Google Scholar] [CrossRef]
- Potter, J.; Salvi, S.; Longstaffe, F. Abiogenic hydrocarbon isotopic signatures in granitic rocks: Identifying pathways of formation. Lithos 2013, 182–183, 114–124. [Google Scholar] [CrossRef]
- Kerr, M.; Hanley, J.; Morrison, G.; Everest, J.; Bray, C. Preliminary evaluation of trace hydrocarbon speciation and abundance as an exploration tool for footwall-style sulfide ores associated with the Sudbury Igneous Complex, Ontario, Canada. Econ. Geol. 2015, 110, 531–556. [Google Scholar] [CrossRef]
- Burisch, M.; Gerdes, A.; Walter, B.F.; Neumann, U.; Fettel, M.; Markl, G. Methane and the origin of five-element veins: Mineralogy, age, fluid inclusion chemistry and ore forming processes in the Odenwald, SW Germany. Ore Geol. Rev. 2017, 81, 42–61. [Google Scholar] [CrossRef]
- Chinnasamy, S.S.; Mishra, B. Genetic implications of fluid-deposited disordered graphite and methane-rich inclusions in the Jonnagiri granodiorite-hosted gold deposit, Eastern Dharwar Craton, India. Ore Geol. Rev. 2017, 89, 587–593. [Google Scholar] [CrossRef]
- Migdisov, A.A.; Guo, X.; Xu, H.; Williams-Jones, A.E.; Sun, C.J.; Vasyukova, O.; Sugiyama, I.; Fuchs, S.; Pearce, K.; Roback, R. Hydrocarbons as ore fluids. Geochem. Persp. Let. 2017, 5, 47–52. [Google Scholar] [CrossRef]
- Lecumberri-Sancheza, P.; Bouabdellah, M.; Zemri, O. Transport of rare earth elements by hydrocarbon-bearing brines: Implications for ore deposition and the use of REEs as fluid source tracers. Chem. Geol. 2018, 479, 204–215. [Google Scholar] [CrossRef]
- Vasyukova, O.V.; Williams-Jones, A.E. Direct measurement of metal concentrations in fluid inclusions, a tale of hydrothermal alteration and REE ore formation from Strange Lake, Canada. Chem. Geol. 2018, 483, 385–396. [Google Scholar] [CrossRef]
- Nivin, V.A. The main principles and measures of gas-safe work at the underground mines of the “Apatit” corporation. Gornyi Zhurnal 1991, 8, 34–36. (In Russian) [Google Scholar]
- Gerasimovsky, V.I.; Volkov, V.P.; Kogarko, L.N.; Polyakov, A.I.; Saprykina, T.V.; Balashov, Y.A. The Geochemistry of the Lovozero Alkaline Massif; Part 1 and Part 2; Australian National University Press: Canberra, Australia, 1966. [Google Scholar]
- Bussen, I.V.; Sakharov, A.S. Petrology of the Lovozero Alkaline Massif; Nauka: Leningrad, Russia, 1972. (In Russian) [Google Scholar]
- Galakhov, A.V. Petrology of the Khibiny Alkaline Massif; Nauka: Leningrad, Russia, 1975. (In Russian) [Google Scholar]
- Kogarko, L.N. Problems of Genesis of Agpaitic Magmas; Nauka: Moscow, Russia, 1977. (In Russian) [Google Scholar]
- Kostyleva-Labuntsova, E.E.; Borutsky, B.E.; Sokolova, M.N.; Shlyukova, Z.V.; Dorfman, M.D.; Dudkin, O.B.; Kozyreva, L.V.; Ikorsky, S.V. Mineralogy of the Khibiny Massif; Nauka: Moscow, Russia, 1978. [Google Scholar]
- Arzamastsev, A.A.; Ivanova, T.N.; Korobeinikov, A.N. Petrology of the Khibiny Ijolite-Urtite and Laws of Localization of Apatite Bodies; Nauka: Leningrad, Russia, 1987. (In Russian) [Google Scholar]
- Pekov, I.V. Lovozero Massif: History, Pegmatites, Minerals; Ocean Pictures Ltd.: Moscow, Russia, 2000. [Google Scholar]
- Yakovenchuk, V.N.; Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Men’shikov, Y.P. Khibiny; Wall, F., Ed.; Laplandia Minerals: Apatity, Russia, 2005. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. New data on pyrochlore- and perovskite-group minerals from the Lovozero alkaline complex, Russia. Eur. J. Mineral. 2002, 14, 821–836. [Google Scholar] [CrossRef]
- Downes, H.; Balaganskaya, E.; Beard, A.; Liferovich, R.; Demaiffe, D. Petrogenetic processes in the ultramafic, alkaline and carbonatitic magmatism in the Kola Alkaline Province: A review. Lithos 2005, 85, 48–75. [Google Scholar] [CrossRef]
- Goryainov, P.M.; Konopleva, N.G.; Ivanyuk, G.Y.; Yakovenchuk, V.N. Structural organization of the ore zone of the Koashva apatite–nepheline deposit. Otech. Geol. 2007, 2, 55–60. (In Russian) [Google Scholar]
- Arzamastsev, A.; Yakovenchuk, V.; Pakhomovsky, Y.; Ivanyuk, G. The Khibina and Lovozero Alkaline Massifs: Geology and Unique Mineralization. In Proceedings of the Guidebook for 33rd International Gtological Congress Excursion, Apatity, Russia, 22 July–2 August 2008; p. 58. [Google Scholar]
- Korchak, Y.A.; Men’shikov, Y.P.; Pakhomovsky, Y.A.; Yakovenchuk, V.N.; Ivanyuk, G.Y. Trap formation of the Kola Peninsula. Petrology 2011, 19, 87–101. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare earth mineralization in igneous rocks: sources and processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Arzamastsev, A.A.; Arzamastseva, L.V.; Zhirova, A.M.; Glaznev, V.N. Model of formation of the Khibiny–Lovozero ore-bearing volcanic–plutonic complex. Geol. Ore Deposit. 2013, 55, 341–356. [Google Scholar] [CrossRef]
- Ivanyuk, G.Y.; Pakhomovsky, Y.A.; Yakovenchuk, V.N. Eudialyte-group minerals in rocks of Lovozero layered complex at Mt. Karnasurt and Mt. Kedykvyrpakhk. Geol. Ore Deposit. 2015, 57, 600–613. [Google Scholar] [CrossRef]
- Veselovskiy, R.V.; Thomson, S.N.; Arzamastsev, A.A.; Zakharov, V.S. Apatite fission track thermochronology of Khibina massif (Kola Peninsula, Russia): Implications for post-Devonian tectonics of the NE Fennoscandia. Tectonophysics 2015, 665, 157–163. [Google Scholar] [CrossRef]
- Kogarko, L.N. Geochemistry of fractionation of coherent elements (Zr and Hf) during the profound differentiation of peralkaline magmatic systems: A case study of the Lovozero complex. Geoch. Int. 2016, 54, 1–6. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review on agpaitic rocks. Earth Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Zartman, R.E.; Kogarko, L.N. Lead isotopic evidence for interaction between plume and lower crust during emplacement of peralkaline Lovozero rocks and related rare-metal deposits, East Fennoscandia, Kola Peninsula, Russia. Contrib. Mineral. Petrol. 2017, 172, 1–14. [Google Scholar] [CrossRef]
- Rodionov, N.; Lepekhina, E.N.; Antonov, A.V.; Kapitonov, I.N.; Balashova, Y.S.; Belyatsky, B.V.; Arzamastsev, A.A.; Sergeev, S.A. U-Pb SHRIMP-II ages of titanite and timing constraints on apatite-nepheline mineralization in the Khibiny and Lovozero alkaline massifs (Kola Peninsula). Russ. Geol. Geophys. 2018, 59, 962–974. [Google Scholar] [CrossRef]
- Timashev, S.F.; Nivin, V.A.; Syvorotkin, V.L.; Polyakov, Y.S. Flicker-noise spectroscopy in dynamic analysis of hydrogen evolution in the Lovozero and Khibiny massifs (Kola Peninsula). In Dynamic Phenomena in Complicated Systems; MESRT: Kazan, Russia, 2011; pp. 263–278. (In Russian) [Google Scholar]
- Nivin, V.A.; Pukha, V.V.; Lovchikov, A.V.; Rakhimov, R.G. Changes in the molecular hydrogen concentration in an underground mine (Lovozero rare-metal deposit, Kola Peninsula). Dokl. Earth Sci. 2016, 471, 1261–1264. [Google Scholar] [CrossRef]
- Nivin, V.A.; Pukha, V.V.; Lovchikov, A.V.; Rakhimov, R.G. Features and factors of time variations in hydrogen release at Lovozersky rare-metal deposit (Kola Peninsula). Geochem. Int. 2018, 56, 688–701. [Google Scholar] [CrossRef]
- Kryvdik, S.G.; Nivin, V.A.; Kul’chitskaya, A.A.; Voznyak, D.K.; Kalinichenko, A.M.; Zagnitko, V.N.; Dubina, A.V. Hydrocarbons and other volatile components in alkaline rocks from the Ukrainian Shield and Kola Peninsula. Geochem. Int. 2007, 45, 270–294. [Google Scholar] [CrossRef]
- Kul’chitskaya, A.A.; Nivin, V.A.; Avedisyan, A.A.; Voznyak, D.K.; Vasyuta, Y.V. Comparison of the results of extracting methane from minerals by mechanical and thermal methods. Mineral. J. 2009, 31, 84–94. (In Russian) [Google Scholar]
- Ermolaeva, V.N.; Chukanov, N.V.; Pekov, I.V.; Kogarko, L.N. The geochemical and genetic role of organic substances in postmagmatic derivatives of alkaline plutons. Geol. Ore Deposit. 2009, 51, 513–524. [Google Scholar] [CrossRef]
- Potter, J.; Rankin, A.H.; Treloar, P.J.; Nivin, V.A.; Ting, W.; Ni, P. A preliminary study of methane inclusions in alkaline igneous rocks of the Kola igneous province, Russia: Implications for the origin of methane in igneous rocks. Eur. J. Miner. 1998, 10, 1167–1180. [Google Scholar] [CrossRef]
- Potter, J. The Characterisation and Origin of Hydrocarbons in Alkaline Rocks of the Kola Alkaline Province. Ph. D. Thesis, Kingston University, Kingston, UK, 2000. [Google Scholar]
- Potter, J.; Rankin, A.H.; Treloar, P.J. Abiogenic Fischer-Tropsch synthesis of hydrocarbons in alkaline igneous rocks; fluid inclusion, textural and isotopic evidence from the Lovozero complex, NW Russia. Lithos 2004, 75, 311–330. [Google Scholar] [CrossRef]
- Beeskow, B. The Occurrence, Distribution and Origin of Hydrocarbons in the Khibiny Nepheline Syenite Complex, Kola Peninsula, Russia. Ph. D. Thesis, Kingston University, Kingston, UK, 2007. [Google Scholar]
- Pokrovskiy, B.G. Crustal Contamination of Mantle Magmas According to Isotope Geochemistry; Nauka: Moscow, Russia, 2000. (In Russian) [Google Scholar]
- Nivin, V.A. Helium and argon isotopes in rocks and minerals of the Lovozero alkaline massif. Geochem. Int. 2008, 46, 482–502. [Google Scholar] [CrossRef]
- Mokrushina, O.D.; Sharygin, V.V. Fluid inclusions in nepheline of the loparite deposit in the Lovozero alkaline massif. In All-Russian Conference on Thermobarogeochemistry, Dedicated to the 100th Anniversary of the Birth of Prof. Yu.A. Dolgov (1918–1993); Mineralogical Museum Named after Fersman, RAS: Moscow, Russia, 2018; pp. 82–84. (In Russian) [Google Scholar]
- Nivin, V.A.; Belov, N.I. Correlations between gas-geochemical and geomechanical parameters of alkali rocks at ore deposits. Geochem. Int. 1993, 30, 34–40. [Google Scholar]
- Nivin, V.A.; Belov, N.I.; Treloar, P.J.; Timofeyev, V.V. Relationships between gas geochemistry and release rates and the geomechanical state of igneous rock massifs. Tectonophysics 2001, 336, 233–244. [Google Scholar] [CrossRef]
- Goryainov, P.M.; Ivanyuk, G.Y.; Yakovenchuk, V.N. Tectonic percolation zones in the Khibiny massif: Morphology, geochemistry, and genesis. Izv. Phys. Solid Earth 1998, 34, 822–827. [Google Scholar]
- Lovchikov, A.V. Review of the strongest rock bursts and mining-induced earthquakes in Russia. J. Mining Sci. 2013, 49, 572–575. [Google Scholar] [CrossRef]
- Gokhberg, M.B.; Adushkin, V.V.; Voytov, G.I.; Pushkin, M.G.; Krivomazova, N.G.; Zel’dina, B.B. On the reaction of free gases in the Khibiny massif to a powerful industrial explosion. Dokl. AN USSR 1989, 308, 1082–1086. (In Russian) [Google Scholar]
- Proskurowski, G.; Lilley, M.D.; Seewald, J.S.; Fruh-Green, G.L.; Olson, E.J.; Lupton, J.E.; Sylva, S.P.; Kelley, D.S. Abiogenic hydrocarbon production at Lost City hydrothermal field. Science 2008, 319, 604–607. [Google Scholar] [CrossRef] [PubMed]
- Etiope, G.; Sherwood Lollar, B. Abiotic methane on Earth. Rev. Geophys. 2013, 51, 276–299. [Google Scholar] [CrossRef]
- Deville, E.; Prinzhofer, A. The origin of N2-H2-CH4-rich natural gas seepages in ophiolitic context: A major and noble gases study of fluid seepages in New Caledonia. Chem. Geol. 2016, 440, 139–147. [Google Scholar] [CrossRef]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofer, A. Reduced gas seepages in ophiolitic complexes: Evidences for multiple origins of the H2-CH4-N2 gas mixtures. Geochim. Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Etiope, G.; Whiticar, M.J. Abiotic methane in continental ultramafic rock systems: Towards a genetic model. Appl. Geochem. 2019, 102, 139–152. [Google Scholar] [CrossRef]
- Zgonnik, V.; Beaumont, V.; Larin, N.; Pillot, D.; Deville, E. Diffused flow of molecular hydrogen through the Western Hajar mountains, Northern Oman. Arab. J. Geosci. 2019, 12. [Google Scholar] [CrossRef]
- Brovarone, A.V.; Martinez, I.; Elmaleh, A.; Compagnoni, R.; Chaduteau, C.; Ferraris, C.; Esteve, I. Massive production of abiotic methane during subduction evidenced in metamorphosed ophicarbonates from the Italian Alps. Nat. Commun. 2017, 8, 14134. [Google Scholar] [CrossRef] [PubMed]
- Nivin, V.A. Gas concentrations in minerals with reference to the problem of the genesis of hydrocarbon gases in rocks of the Khibiny and Lovozero massifs. Geochem. Int. 2002, 40, 883–898. [Google Scholar]
- Nivin, V.A. Molecular–mass distribution of saturated hydrocarbons in gas of the Lovozerskii nepheline–syenite massif. Dokl. Earth Sci. 2009, 429, 1580–1582. [Google Scholar] [CrossRef]
- Nivin, V.A. Estimation of the Ore and Country Rock Gas Content at Deep (down to +10 m) Horizons of the Kukisvumchorr and Yuksporr Apatite-Nepheline Deposits Developed by the Kirovsk Mine of JSC Apatit; Unpublished report; Archives of KSC RAS: Apatity, Russia, 2016. (In Russian) [Google Scholar]
- Nivin, V.A. Estimation of Ore and Country Rock Gas Content of the Plateau Rasvumchorr Deposit; Unpublished report; Archives of KSC RAS: Apatity, Russia, 2016. (In Russian) [Google Scholar]
- Nivin, V.A.; Pukha, V.V. Additional Study of the Gas Content at Kurnasurt and Kedykvyrpakhk of the Lovozero Rare-Metal Deposit; Unpublished report; Archives of KSC RAS: Apatity, Russia, 2016. (In Russian) [Google Scholar]
- Lyutkevich, Y.M. Statement in the debate at Vsesoyuznyi meeting on the problem of oil origin. In The Problem of Oil and Gas Origin and Conditions of Their Deposit’s Formation; Gostekhizdat: Moscow, Russia, 1960; pp. 286–289. (In Russian) [Google Scholar]
- Guseva, A.N.; Krasil’nikova, M.P. About composition of organic matter in rocks of the Khibiny alkaline massif. Vestnik MGU 1960, 2, 68–69. (In Russian) [Google Scholar]
- Galimov, E.M.; Petersilie, I.A. On the carbon isotope composition in hydrocarbon gases and CO2 contained in alkaline igneous rocks of the Khibiny, Lovozero and Issimaussaq massifs. Dokl. AN USSR 1967, 176, 914–917. (In Russian) [Google Scholar]
- Galimov, E.M. Carbon Isotopes in Oil-Gas Geology; Aeronautics and Space Administration: Washington, DC, USA, 1975. [Google Scholar]
- Voytov, G.I. Isotope characteristics of the Khibiny spontaneous gases. Dokl. AN USSR 1977, 236, 975–978. (In Russian) [Google Scholar]
- Voytov, G.I.; Adushkin, V.V.; Gokhberg, M.B.; Nosik, L.P.; Kucher, M.I.; Nikulina, I.V.; Pushkin, M.G.; Zhogina, L.M. About chemical and isotope instabilities of gas jets in Khibiny. Dokl. AN USSR 1990, 312, 567–571. (In Russian) [Google Scholar]
- Nivin, V.A.; Devirts, A.L.; Lagutina, Y.P. The origin of the gas phase in the Lovozero massif based on hydrogen-isotope data. Geochem. Int. 1995, 32, 65–71. [Google Scholar]
- Kogarko, L.N.; Kosztolanyi, C.; Ryabchikov, I.D. Geochemistry of the reduced fluid in alkali magmas. Geochem. Int. 1987, 24, 20–27. [Google Scholar]
- Markl, G.; Marks, M.; Schwinn, G.; Sommer, H. Phase equilibrium constraints on intensive crystallization parameters of the Ilimaussaq complex, south Greenland. J. Petrol. 2001, 42, 2231–2257. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Magnetite compositions and oxygen fugacities of the Khibina magmatic system. Lithos 2006, 91, 35–45. [Google Scholar] [CrossRef]
- Marks, M.A.; Markl, G. The Ilímaussaq alkaline complex, South Greenland. In Layered Intrusions; Charlier, B., Ed.; Springer Geology: Dordrecht, The Netherlands, 2015; pp. 649–691. [Google Scholar]
- Konnerup-Madsen, J.; Kreulen, R.; Rose-Hansen, J. Stable isotope characteristics of hydrocarbon gases in the alkaline Ilimaussaq complex, South Green. Bull. Mineral. 1988, 111, 567–576. [Google Scholar]
- Taran, Y.A.; Varley, N.R.; Inguaggiato, S.; Cienfuegos, E. Geochemistry of H2-and CH4-enriched hydrothermal fluids of Socorro Island, Revillagigedo Archipelago, Mexico. Evidence for serpentinization and abiogenic methane. Geofluids 2010, 10, 542–555. [Google Scholar] [CrossRef]
- Burruss, R.C.; Laughrey, C.D. Carbon and hydrogen isotopic reversals in deep basin gas: Evidence for limits to the stability of hydrocarbons. Org. Geochem. 2010, 41, 1285–1296. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Dai, J.X.; Li, J.; Zhou, O.H. Hydrogen isotope composition of natural gases from the Tarim Basin and its indications of depositional environments of the source rocks. Sci. China D Earth Sci. 2008, 51, 300–311. [Google Scholar] [CrossRef]
- Kiyosu, Y.; Krouse, H.R. Carbon isotope effect during abiogenic oxidation of methane. Earth Planet. Sci. Lett. 1989, 95, 302–306. [Google Scholar] [CrossRef]
- Proskurowski, G.; Lilley, M.D.; Kelley, D.S.; Olson, E.J. Low temperature volatile production at the Lost City Hydrothermal Field, evidence from a hydrogen stable isotope geothermometer. Chem. Geol. 2006, 229, 331–343. [Google Scholar] [CrossRef]
- Suda, K.; Ueno, Y.; Yoshizaki, M.; Nakamura, H.; Kurokawa, K.; Nishiyama, E.; Maruyama, S. Origin of methane in serpentinite-hosted hydrothermal systems: The CH4-H2-H2O hydrogen isotope systematics of the Hakuba Happo hot spring. Earth Planet. Sci. Lett. 2014, 386, 112–125. [Google Scholar] [CrossRef]
- Tsunogai, U.; Kamimura, K.; Anzai, S.; Nakagawa, F.; Komatsu, D.D. Hydrogen isotopes in volcanic plumes: Tracers for remote temperature sensing of fumaroles. Geochim. Cosmochim. Acta 2011, 75, 4531–4546. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Voglesonger, K.; Lin, L.H.; Lacrampe-Couloume, G.; Telling, J.; Abrajano, T.A.; Pratt, L.M. Hydrogeologic controls on episodic H2 release from Precambrian fractured rocks e Energy for deep subsurface life on Earth and Mars. Astrobiology 2007, 7, 971–986. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Meng, Q.; Wua, X.; Jia, H. Genetic types of natural gas and filling patterns in Daniudi gas field, Ordos Basin. China. J. Asian Earth Sci. 2015, 107, 1–11. [Google Scholar] [CrossRef]
- Kita, J.; Matsuo, S.; Wakita, H. H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. Geophys. Res. 1982, 87, 10789–10795. [Google Scholar] [CrossRef]
- Wiersberg, T.; Erzinger, J. Origin and spatial distribution of gas at seismogenic depths of the San Andreas Fault from drill-mud gas analysis. Appl. Geochem. 2008, 23, 1675–1690. [Google Scholar] [CrossRef]
- Mavrogenes, J.A.; Bodnar, R.J. Hydrogen movement into and out of fluid inclusions in quartz: Experimental evidence and geologic implications. Geochim. Cosmochim. Acta. 1994, 58, 141–148. [Google Scholar] [CrossRef]
- Taylor, H.P.; Sheppard, S.M.F. Igneous rocks I: Processes of isotopic fractionation and isotopic systematics. Rev. Mineral. 1986, 16, 227–271. [Google Scholar]
- Sheppard, S.M.F. Characterization and isotopic variations in natural waters. Rev. Mineral. 1986, 16, 165–181. [Google Scholar]
- Luo, M.; Huang, H.; Zhang, P.; Wu, Q.; Chen, D. Origins of gas discharging from the Qiangtang Basin in the northern Qinghai–Tibet Plateau, China: Evidence from gas compositions, helium, and carbon isotopes. JGE 2014, 146, 119–126. [Google Scholar] [CrossRef]
- Sherwood Lollar, B.; Lacrampe-Couloume, G.; Voglesonger, K.; Onstott, T.C.; Pratt, L.M.; Slater, G.F. Isotopic signatures of CH4 and higher hydrocarbon gases from Precambrian Shield sites: A model for abiogenic polymerization of hydrocarbons. Geochim. Cosmochim. Acta 2008, 72, 4778–4795. [Google Scholar] [CrossRef]
- Suda, K.; Gilbert, A.; Yamada, K.; Yoshida, N.; Ueno, Y. Compound– and position–specific carbon isotopic signatures of abiogenic hydrocarbons from on–land serpentinite–hosted Hakuba Happo hot spring in Japan. Geochim. Cosmochim. Acta 2017, 206, 201–215. [Google Scholar] [CrossRef]
- Lapidus, A.L.; Loktev, S.M. Present-day catalytic syntheses of hydrocarbons from carbon dioxide and hydrogen. Zh. Vses. Khim. O-va im. D.I. Mendeleeva 1986, 31, 527–532. (In Russian) [Google Scholar]
- Ione, K.G.; Mysov, V.M.; Stepanov, V.G.; Parmon, V.N. New data on the possibility of catalytic abiogenic synthesis of hydrocarbons in the earth’s crust. Petrol. Chem. 2001, 41, 159–165. [Google Scholar]
- Chukanov, N.V.; Pekov, I.V.; Sokolov, S.V.; Nekrasov, A.N.; Ermolaeva, V.N.; Naumova, I.S. On the problem of the formation and geochemical role of bituminous matter in pegmatites of the Khibiny and Lovozero alkaline massifs, Kola Peninsula, Russia. Geochem. Int. 2006, 44, 715–728. [Google Scholar] [CrossRef]
- Taran, Y.A.; Kliger, G.A.; Cienfuegos, E.; Shuykin, A.N. Carbon and hydrogen isotopic compositions of products of open-system catalytic hydrogenation of CO2: Implications for abiogenic hydrocarbons in Earth’s crust. Geochim. Cosmochim. Acta 2010, 74, 6112–6125. [Google Scholar] [CrossRef]
- Sinev, M.Y.; Fattakhova, Z.T.; Lomonosov, V.I.; Gordienko, Y.A. Kinetics of oxidative coupling of methane: Bridging the gap between comprehension and description. J. Natural Gas Chem. 2009, 18, 273–287. [Google Scholar] [CrossRef]
- Nivin, V.A.; Mel’nik, N.A. Effects of radioactivity on gas-component contents in alkali igneous rocks. Geochem. Int. 1990, 27, 94–97. [Google Scholar]
- Lin, L.-H.; Slater, G.F.; Sherwood Lollar, B.; Lacrampe-Couloume, G.; Onstott, T.C. The yield and isotopic composition of radiolytic H2, a potential energy source for the deep subsurface biosphere. Geochim. Cosmochim. Acta 2005, 69, 893–903. [Google Scholar] [CrossRef]
- Dzaugis, M.E.; Spivack, A.J.; Dunlea, A.G.; Murray, R.W.; D’Hondt, S. Radiolytic hydrogen hroduction in the subseafloor basaltic aquifer. Front. Microbiol. 2016, 7, 76. [Google Scholar] [CrossRef]
- Levashkevich, V.G. Principles of Geothermal Field Distribution at the Marginal East European Platform. Doctor of Sciences (Geol.-Miner.) Dissertation, Institute of Geochemistry and Geophisics, Minsk, Belarus, 2006; p. 332. (In Russian). [Google Scholar]
- Kozyrev, A.A.; Semenova, I.E.; Zemtsovskiy, A.V. Investigation of geomechanical features of the rock mass in mining of two contiguous deposits under tectonic stresses. Proc. Engin. 2017, 191, 324–331. [Google Scholar] [CrossRef]
- Cherskii, N.V.; Tsarev, V.P.; Soroko, T.I.; Kuznetsov, O.L. Influence of Tectonic Seismic Processes on the Formation and Accumulation of Hydrocarbons; Nauka: Novosibirsk, Russia, 1985. (In Russian) [Google Scholar]
- Hirose, T.; Kawagucci, S.; Suzuki, K. Mechanoradical H2 generation during simulated faulting: Implications for an earthquake-driven subsurface biosphere. Geophys. Res. Let. 2011, 38, L17303. [Google Scholar] [CrossRef]
- McMahon, S.; Parnell, J.; Blamey, N.J.F. Evidence for seismogenic hydrogen gas, a potential microbial energy source on Earth and Mars. Astrobiology 2016, 16, 690–702. [Google Scholar] [CrossRef]
- Ikorsky, S.V.; Nivin, V.A. Study of the Localization, Setting and Scales of Natural Combustible Gas Emissions at the Mines of Lovozero GOK; Unpublished report; Archives of KSC RAS: Apatity, Russia, 1985. (In Russian) [Google Scholar]
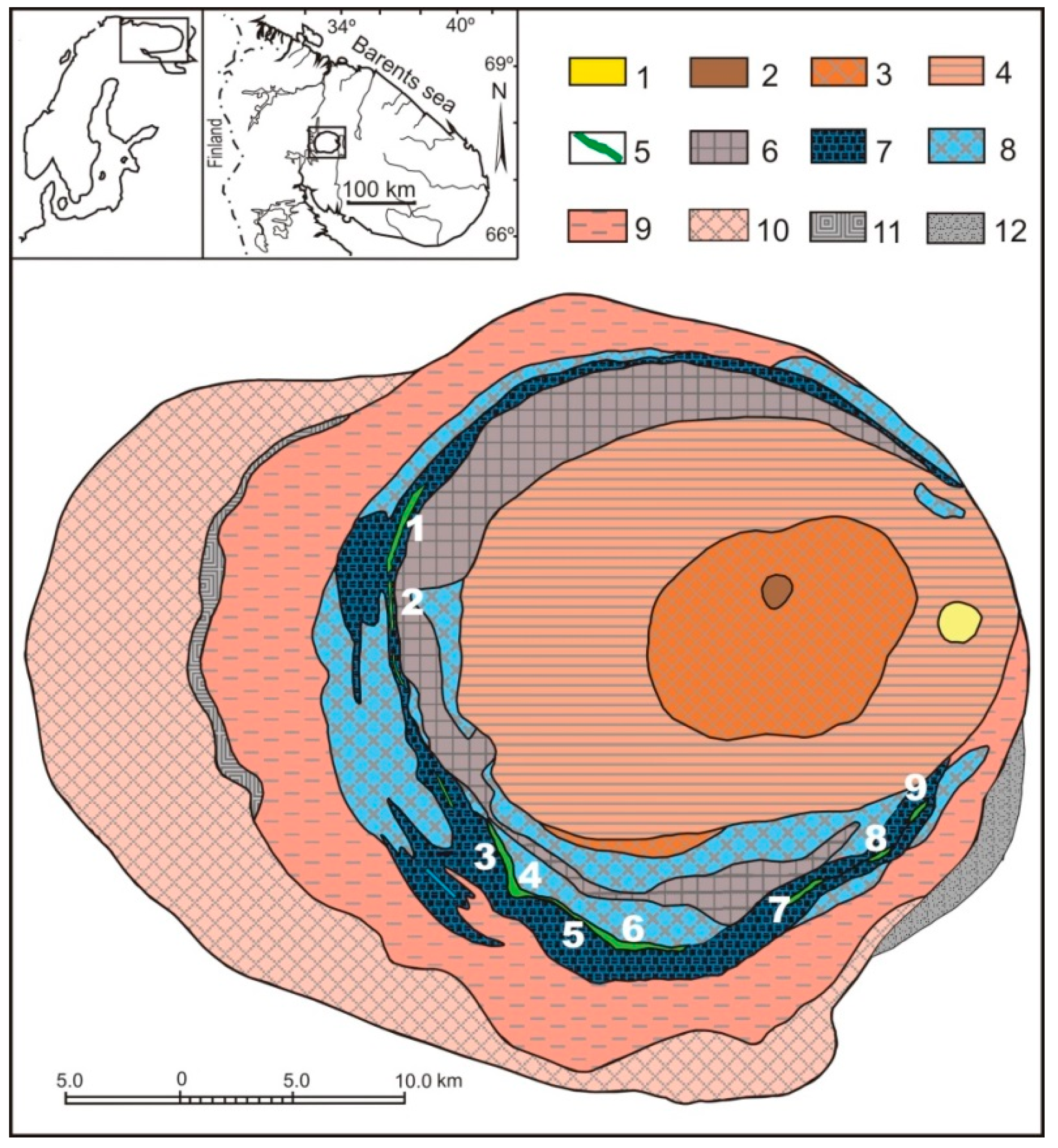
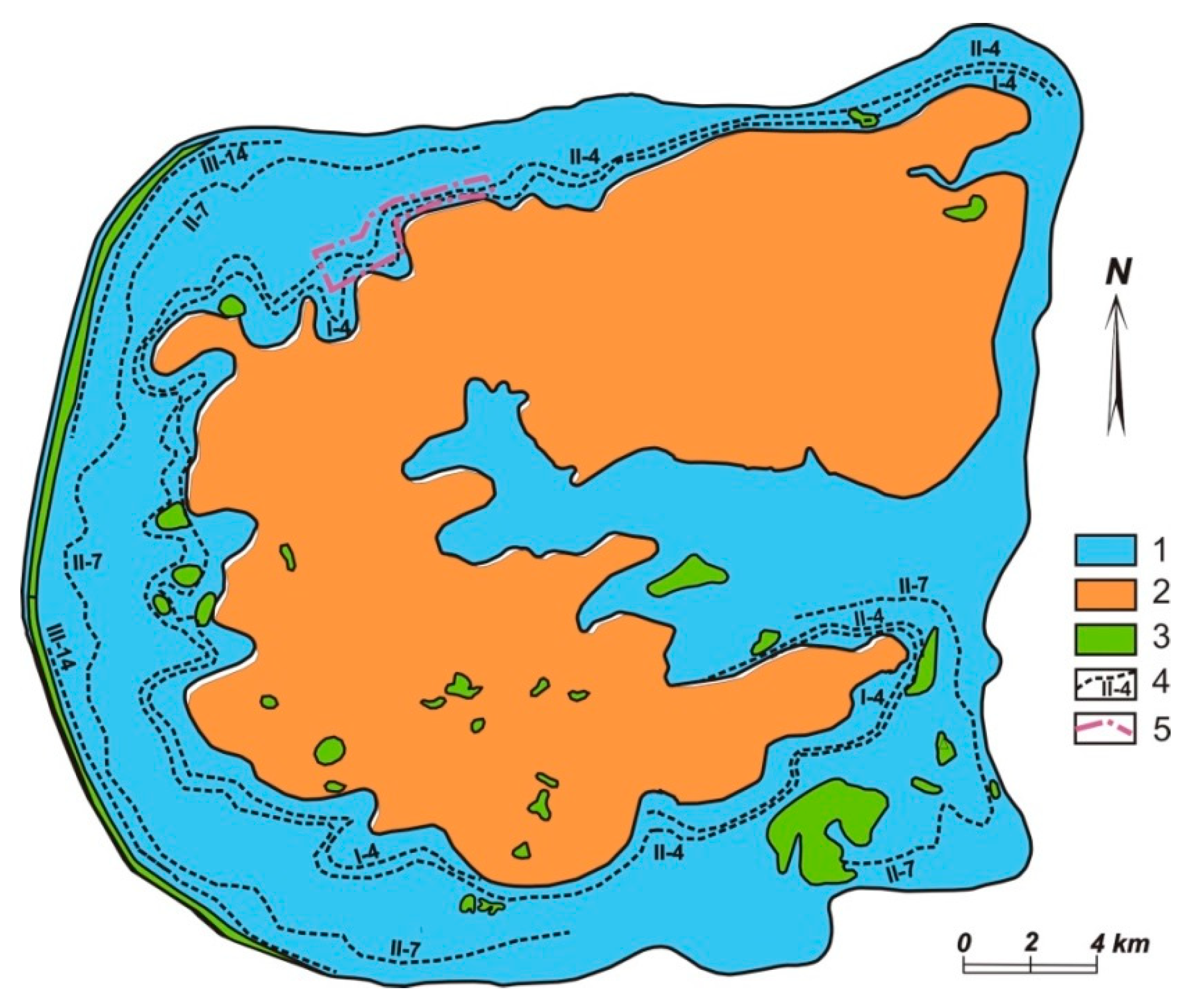
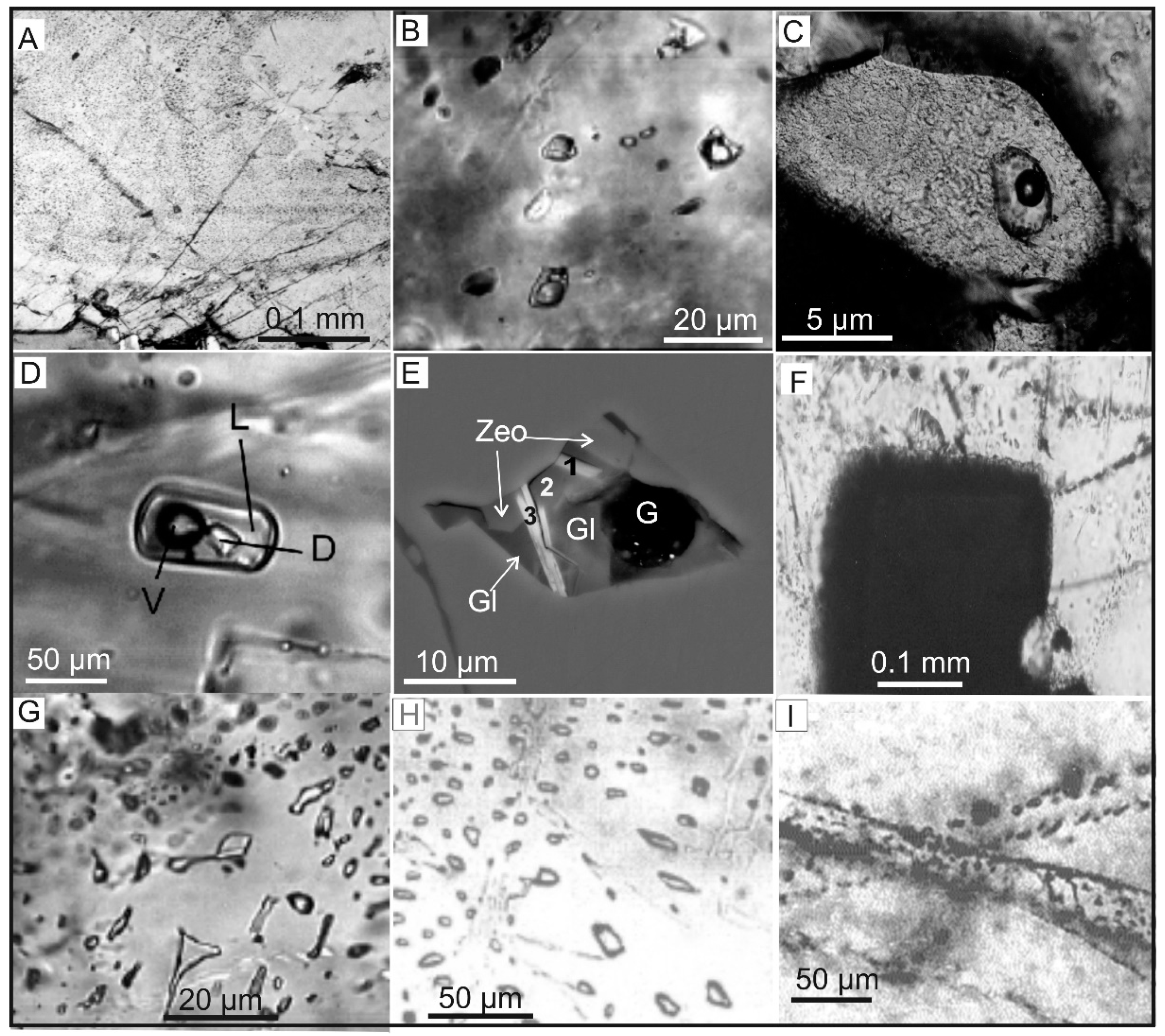
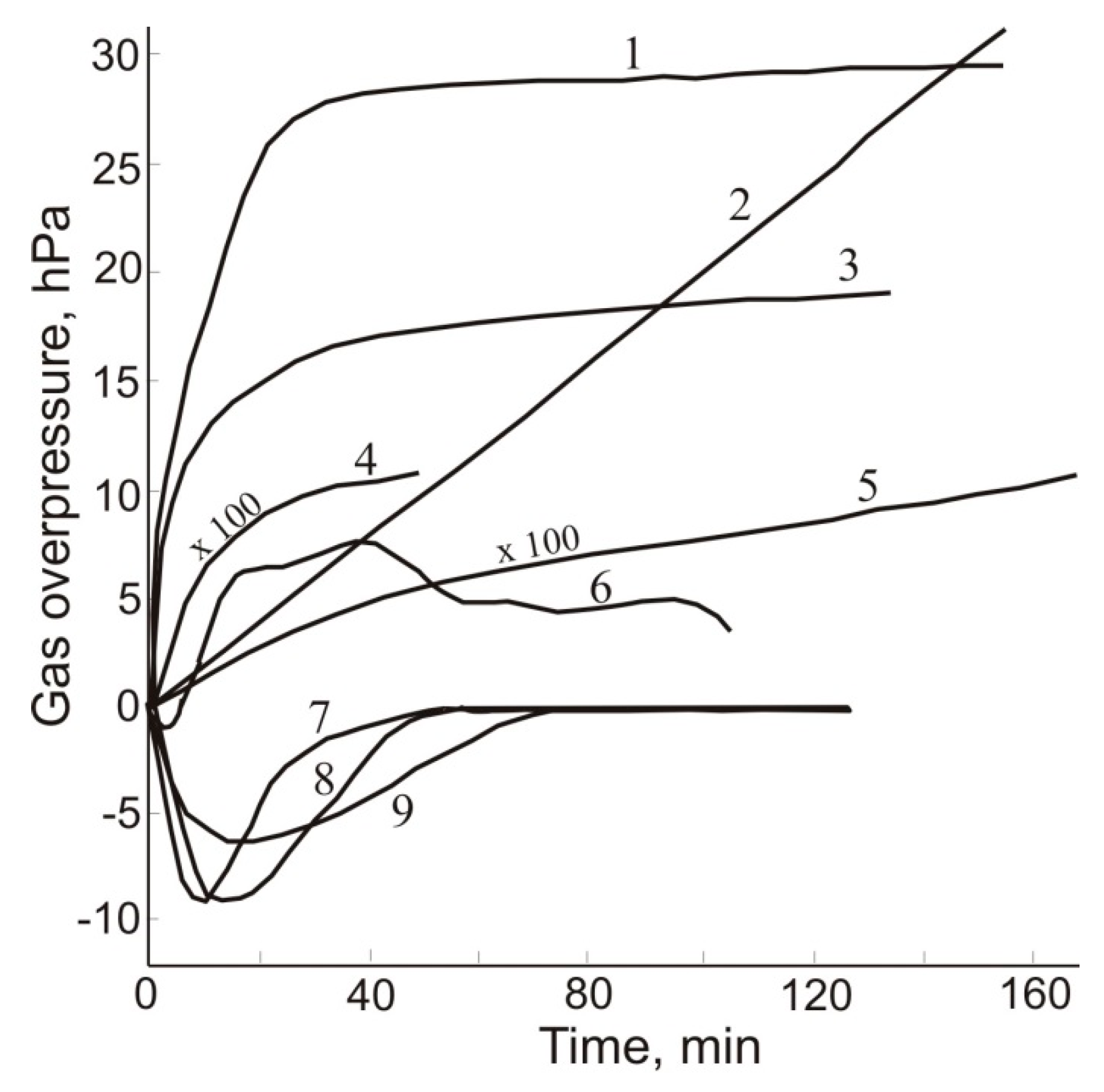
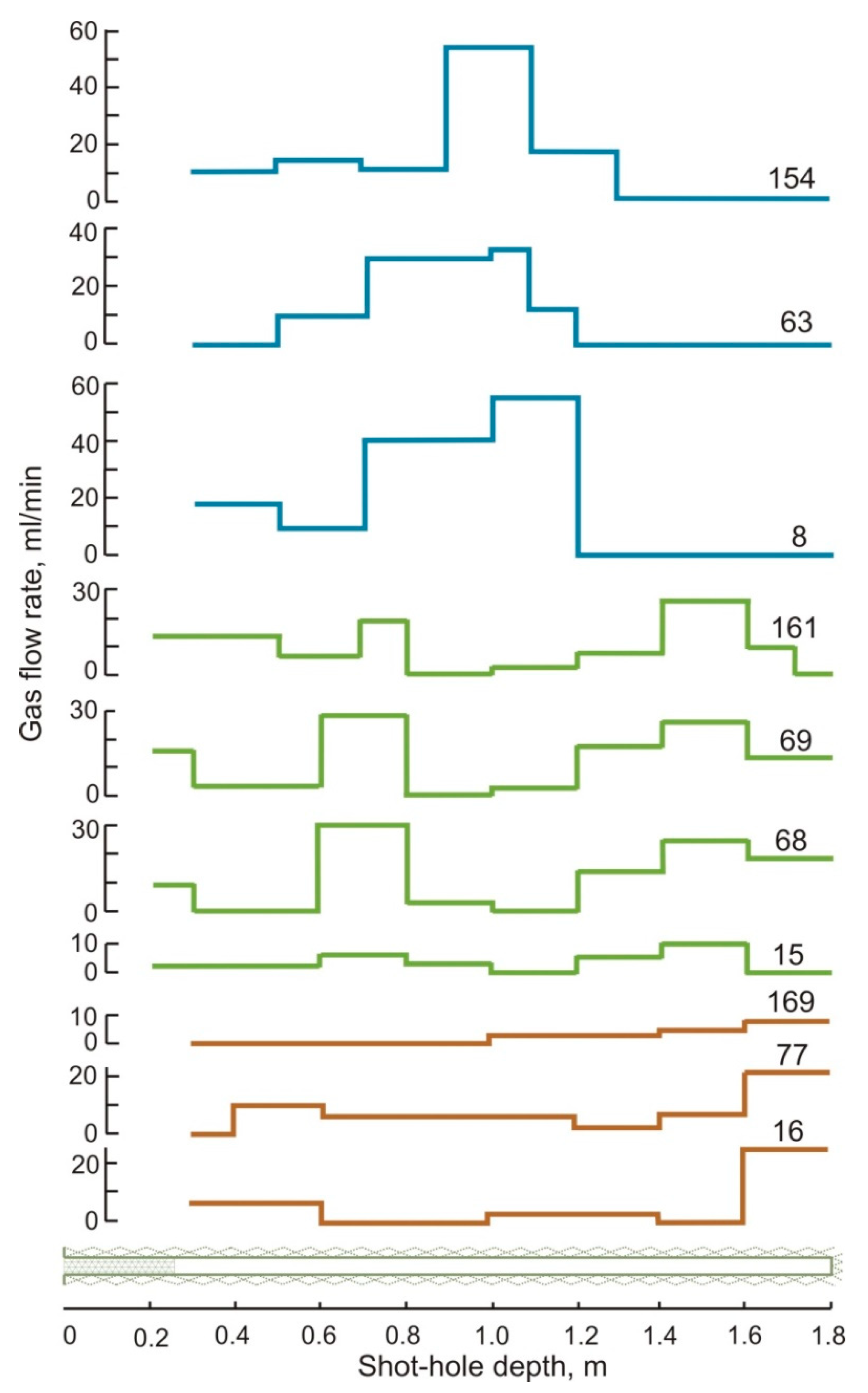
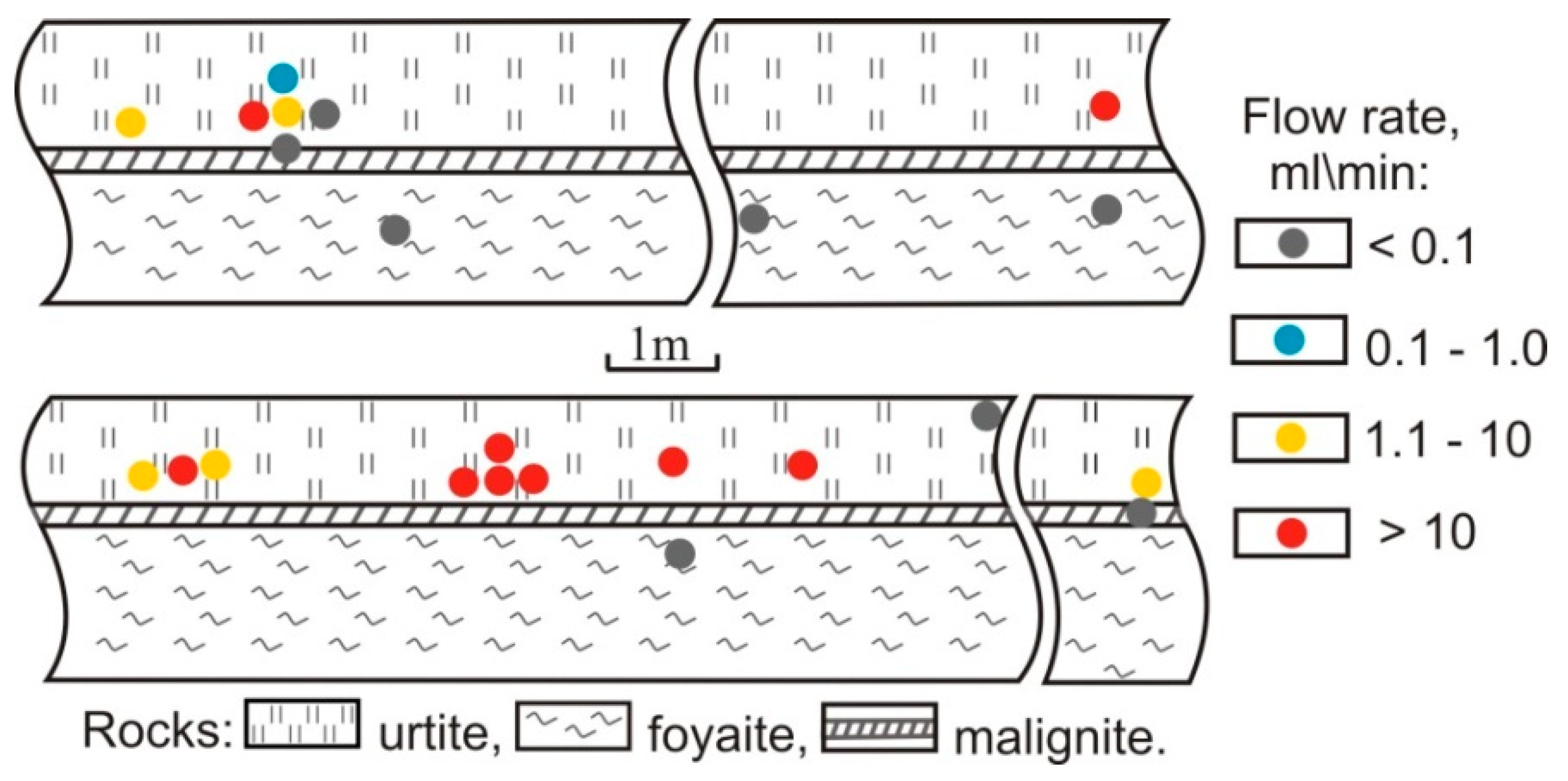
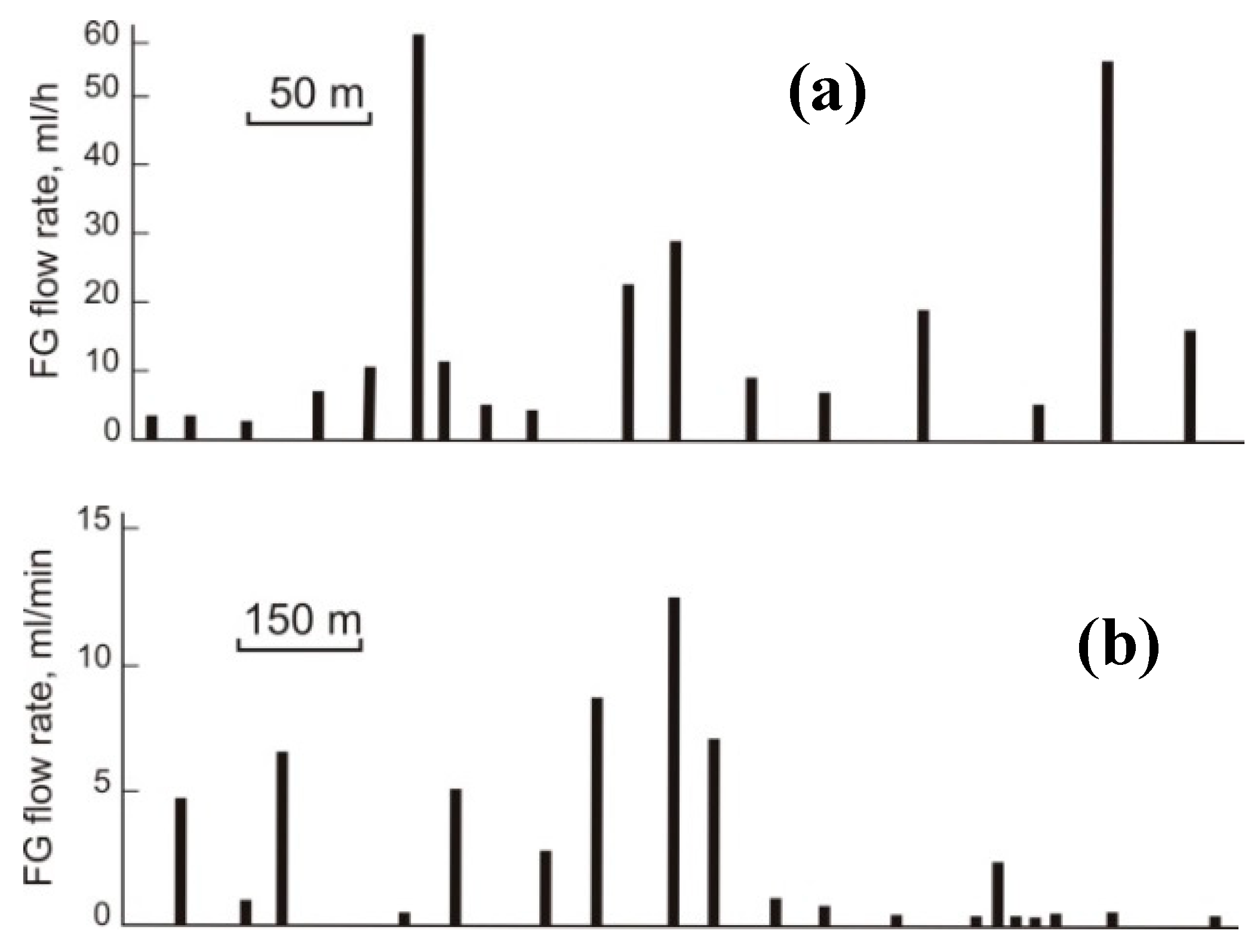
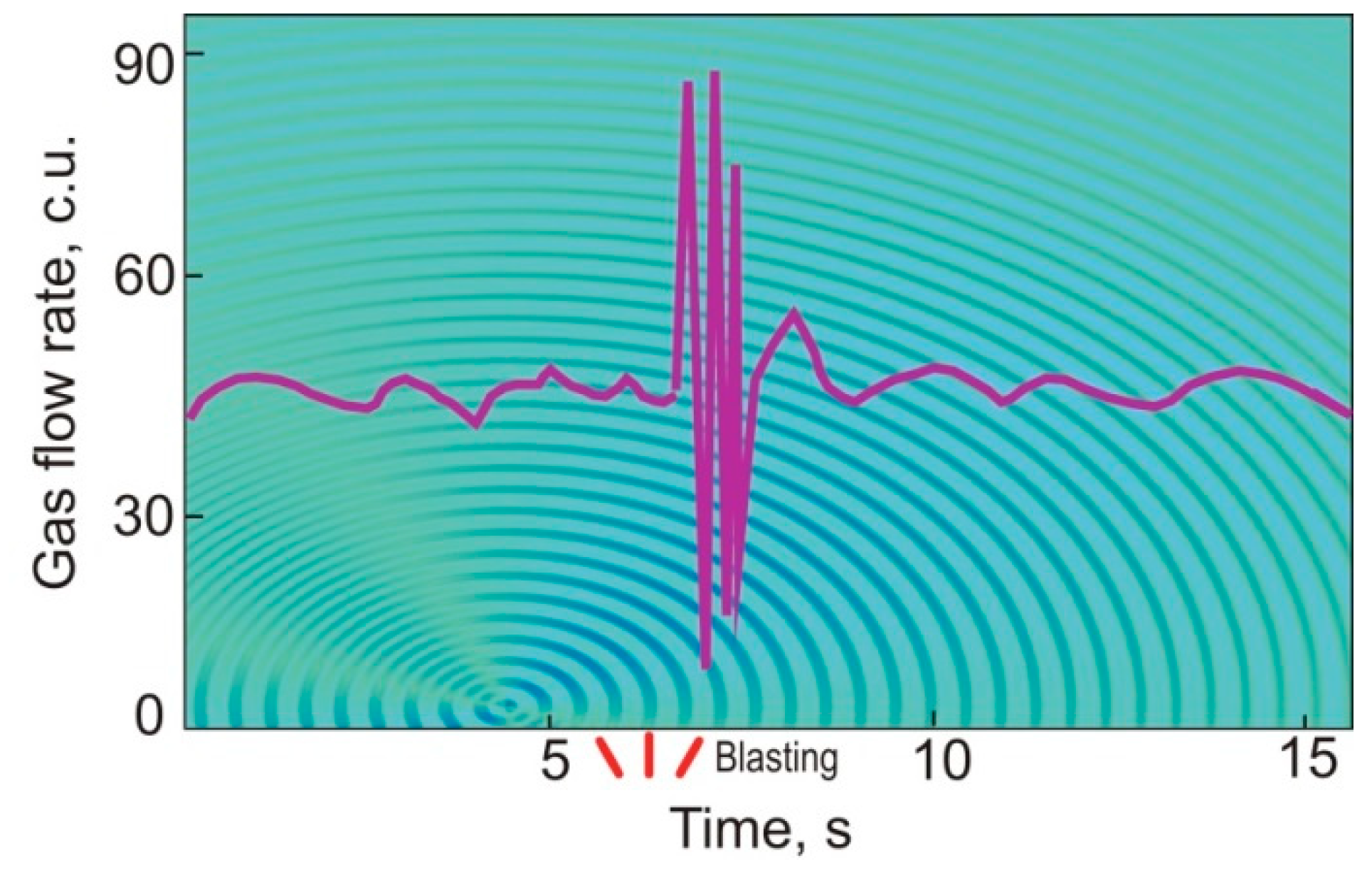
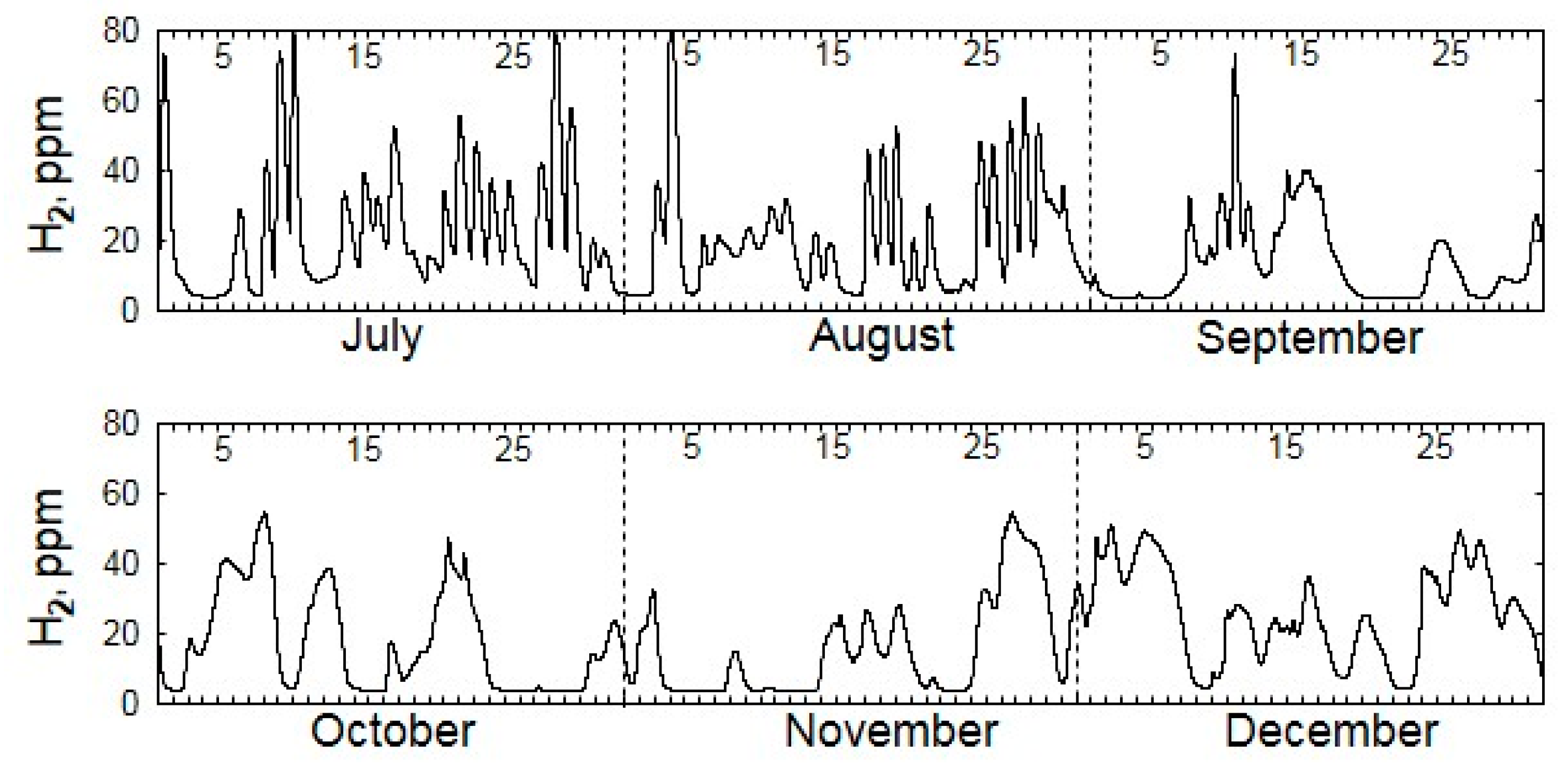
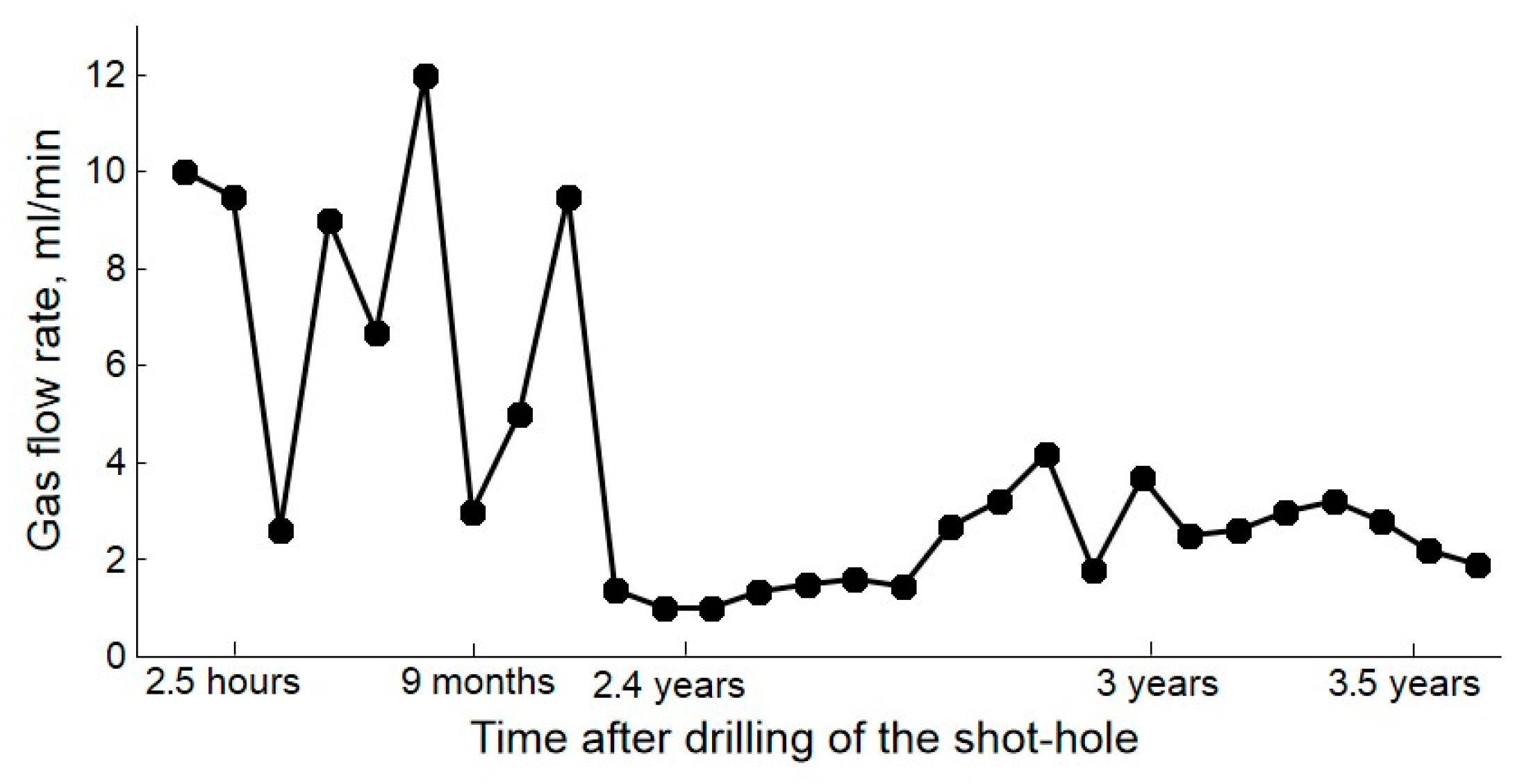
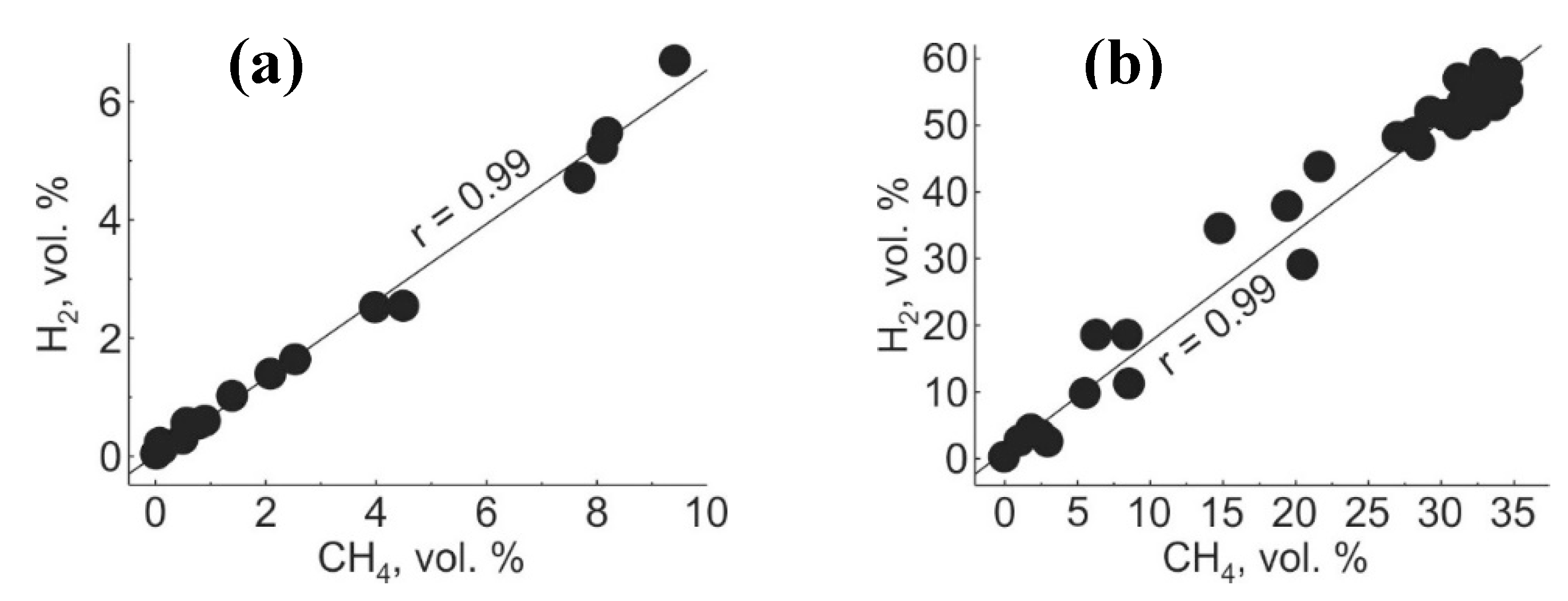
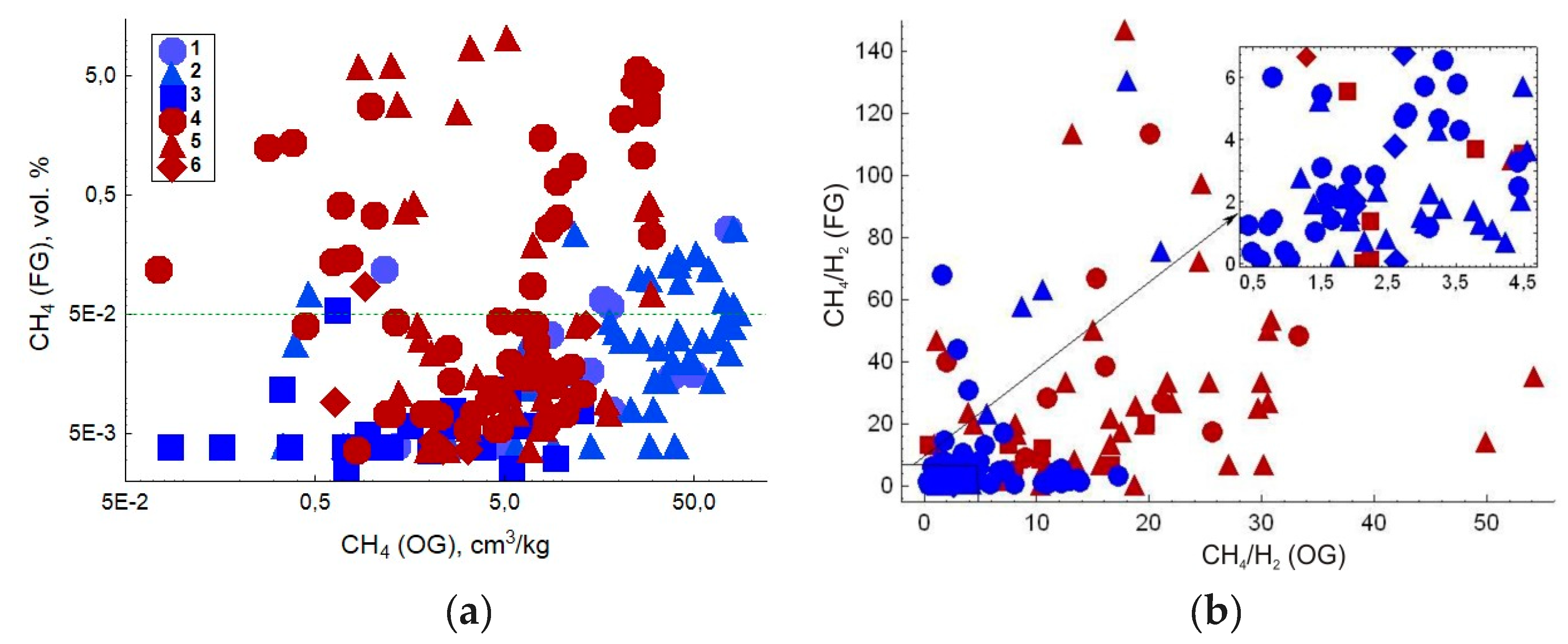
| Gas | Number of Analyses | Minimum | Maximum | Median |
|---|---|---|---|---|
| The Khibiny apatite-nepheline deposits | ||||
| CH4 | 5029 | 0.006 | 238 | 8.28 |
| C2H6 | 3037 | 0.00003 | 8.61 | 0.44 |
| 1 С3–С5 | 1233 | 0.00005 | 1.70 | 0.05 |
| H2 | 4989 | 0.003 | 18.11 | 0.5 |
| He | 2627 | 0.00005 | 0.43 | 0.01 |
| N2 | 1508 | 0.04 | 14.3 | 0.89 |
| O2 | 1494 | 0.0015 | 1.84 | 0.08 |
| CO | 168 | 0.00007 | 3.03 | 0.005 |
| CO2 | 907 | 0.00021 | 4.03 | 0.06 |
| The Lovozero loparite deposit | ||||
| CH4 | 689 | 0.011 | 82.2 | 3.7 |
| C2H6 | 676 | 0.0014 | 5.58 | 0.29 |
| 1 С3–С5 | 68 | 0.00032 | 0.872 | 0.043 |
| H2 | 664 | 0.057 | 25.15 | 1.34 |
| He | 664 | 0.00011 | 0.26 | 0.005 |
| N2 | 665 | 0.013 | 6.53 | 0.43 |
| O2 | 663 | 0.0034 | 1.2 | 0.05 |
| CO | 51 | 0.00013 | 0.014 | 0.0015 |
| CO2 | 3 | 0.013 | 1.05 | 0.05 |
| Variable Min–Max/Median (Number of Analyses), cm3/kg | |||
|---|---|---|---|
| The Khibiny apatite-nepheline deposits | |||
| Extraction manner 1 | UR | LV | LVH |
| CH4 | 0.004–7.64 | 0.0007–11.5 | 0.0001–13.92 |
| 0.07 (32) | 0.12 (134) | 0.21 (454) | |
| C2H6 | 0.0002–2.2 | 0.0001–0.49 | 0.00002–1.96 |
| 0.014 (20) | 0.08 (97) | 0.01 (247) | |
| H2 | 0.0001–0.058 | 0.0004–2.40 | 0.001–4.29 |
| 0.004 (33) | 0.063 (129) | 0.028 (294) | |
| He | 0.0002–0.09 | 0.0006–0.13 | 0.0008–0.17 |
| 0.004 (36) | 0.004 (140) | 0.004 (283) | |
| CO2 | 0.07–3.57 | 0.015–3.6 | 0.013–29.7 |
| 0.47 (17) | 0.22 (77) | 0.88 (134) | |
| The Lovozero loparite deposit | |||
| Extraction manner 2 | LV | HV | HVH |
| CH4 | 0.0039–11.0 | 0.0004–0.83 | 0.017–2.01 |
| 0.61 (24) | 0.04 (12) | 0.10 (23) | |
| C2H6 | 0.002–0.45 | 0.00004–0.04 | 0.0008–0.12 |
| 0.05 (15) | 0.07 (8) | 0.01 (21) | |
| H2 | 0.0018–0.99 | 0.00002–0.11 | 0.001–0.49 |
| 0.19 (22) | 0.0016 (12) | 0.011 (23) | |
| He | 0.003–0.026 | 0.00002–0.004 | 0.00001–0.006 |
| 0.009 (14) | 0.0002 (12) | 0.0002 (22) | |
| CO2 | 0.05–1.7 | 0.54–0.92 | 0.008–2.48 |
| 0.3 (5) | 0.73 (2) | 0.82 (6) | |
| Sample | CH4 | C2H6 | C3–C5 | H2 | He | N2 | O2 |
|---|---|---|---|---|---|---|---|
| The Khibiny apatite-nepheline deposits [18] | |||||||
| 9-б | 75.0 | 8.1 | 0.86 | 17.6 | 0.31 | 1.1 | 0.1 |
| 36 | 76.6 | 5.9 | 0.52 | 19.0 | 0.42 | 8.8 | 1.3 |
| Nn | 79.7 | 5.2 | 0.7 | 2.9 | 0.22 | 9.1 | 2.1 |
| IHS-12 | 64.6 | 3.6 | 1 n.a. | 17.9 | 0.64 | 8.9 | 2.2 |
| 360/1190 | 66.8 | 3.2 | 0.32 | 18.9 | 0.65 | 8.2 | 2.3 |
| 721-2 | 62.3 | 2.6 | 0.81 | 26.5 | 0.26 | 7.5 | 0.8 |
| The Lovozero loparite deposit [19] | |||||||
| 1/51 | 49.6 | 2.75 | 0.14 | 41.6 | 0.87 | 1.4 | 0.43 |
| 14/26 | 51.4 | 2.07 | 0.10 | 33.0 | 0.95 | 9.7 | 3.0 |
| 25/26 | 57.2 | 3.23 | 0.13 | 29.0 | 0.75 | 6.6 | 1.8 |
| 45/21 | 65.8 | 6.32 | 0.37 | 10.9 | 1.87 | 11.7 | 2.5 |
| 110/19 | 52.6 | 3.15 | 0.11 | 37.0 | 1.0 | 4.2 | 1.15 |
| 173/37 | 34.6 | 1.28 | 0.06 | 57.8 | 0.89 | 4.0 | 0.68 |
| CH4 in AGM, vol. % | Variable Min–Max/Median (Number of Analyses), vol. % | ||||
|---|---|---|---|---|---|
| CH4 | C2H6 | C3–C5 | H2 | He | |
| The Khibiny apatite-nepheline deposits | |||||
| >50 | 68–99 | 0.1–9.5 | 0.2–0.8 | 0.5–29 | 0.3–2.1 |
| 87 (12) | 3.9 (9) | 0.5 (5) | 9 (12) | 0.7 (6) | |
| <50, >10 | 56–98 | 1.2–8.3 | 0.4–2.2 | 0.7–40 | 0.1–1.6 |
| 84 (23) | 5.3 (18) | 0.7 (9) | 11 (23) | 0.8 (20) | |
| <10, >1 | 83–94 | 3.2–7.2 | 0.5–0.5 | 2.8–13.4 | 0.1–0.7 |
| 88 (8) | 4.4 (4) | 0.5 (1) | 9.7 (8) | 0.6 (5) | |
| <1, >0.1 | 2.5–99 | 0.7–22 | 0.2–13 | 0.2–97 | 0.04–17 |
| 87 (51) | 4.5 (18) | 0.6 (17) | 9 (8) | 0.6 (42) | |
| <0.1 | 4.9–99 | 0.5–33 | 0.2–4.3 | 0.9–92 | 0.6–32 |
| 82 (165) | 6 (49) | 0.8 (25) | 9 (164) | 4 (133) | |
| The Lovozero loparite deposit | |||||
| >50 | 60–73 | 2.4–6.3 | 0.1–0.3 | 18–36 | 0.8–2.6 |
| 64 (4) | 3 (4) | 0.2 (3) | 32 (4) | 0.9 (4) | |
| <50, >10 | 37–92 | 1.0–3.6 | 0.02–0.2 | 2–61 | 0.8–3.1 |
| 60 (14) | 2.4 (11) | 0.12 (6) | 38 (14) | 1.0 (13) | |
| <10, >1 | 18–96 | 0.2–4.5 | 0.04–2.3 | 4–81 | 0.1–12 |
| 57 (51) | 1.7(26) | 0.14 (10) | 40 (51) | 1.0 (46) | |
| <1, >0.1 | 9–99 | 0.5–6 | 0.02–0.5 | 0.2–90 | 0.1–3.5 |
| 59 (59) | 1.2 (5) | 0.06 (3) | 40 (59) | 1.0 (57) | |
| <0.1 | 8–97 | 0.9–42 | 1.0 | 1–91 | 0.3–79 |
| 65 (285) | 15 (26) | 1.0 (1) | 24 (265) | 9 (264) | |
| Component | Gas Type | Concentration 1 | Reference |
|---|---|---|---|
| Khibiny | |||
| δ 13СC1-C5 | OG | −13.2–−4.3 | [106,107] |
| δ 13СCH4 | OG | −14.6–−3.2 | [106] |
| δ 13СCH4 | OG | −25.3–−3.3 | [81] |
| δ 13СCH4 | OG | −22.4–−5.5 | [36] |
| δ 13СCH4 | OG | −13.3–−7.6 | [38] |
| δ 13СC2H6 | OG | −24.5–−9.1 | [106] |
| δ 13СC2H6 | OG | −19.2–−14.3 | [36] |
| δ 13СC2H6 | OG | −23.3–−16.1 | [38] |
| δ 13СC3H8 | OG | −26.2–−25.7 | [107] |
| δ 13СC3H8 | OG | −19.6–−13.0 | [36] |
| δ 13СC3H8 | OG | −21.3–−14.1 | [38] |
| δ 13СC4H10 | OG | −19.7–−13.2 | [36] |
| δ 13СC4H10 | OG | −17.4–−16.8 | [38] |
| δ 13СC5H12 | OG | −14.0 | [36] |
| δ 13СC5H12 | OG | −20.6–−19.6 | [38] |
| δ 13ССО2 | OG | −8.5–+10.6 | [107] |
| δ 13ССО2 | OG | −7.3–−3.8 | [81] |
| δ 13ССО2 | OG | −16.8–−13.6 | [36] |
| δ 13ССО2 | OG | −18.6–−14.6 | [38] |
| δ DСН4 | OG | −118–−50 | [38] |
| δ DС2Н6 | OG | −175–−120 | [38] |
| δ 13СC1-C5 | FG | −19.3–−11.8 | [106,107] |
| δ 13СCH4 | FG | −16.5–−6.5 | [107,109] |
| δ 13СC2H6 | FG | −24.0–−11.7 | [107,109] |
| δ DСН4 | FG | −82–−56 | [108] |
| δ DС2Н6 | FG | −173–−144 | [108] |
| Lovozero | |||
| δ 13СC1-C5 | OG | −5.3 | [106] |
| δ 13СCH4 | OG | −11.6–−4.7 | [45] |
| δ 13СC2H6 | OG | −17.0–−9.6 | [45] |
| δ 13СC3H8 | OG | −15.9–−7.8 | [45] |
| δ 13ССО2 | OG | −29.9–−16.3 | [45] |
| δ DСН4 | OG | −164–−132 | [110] |
| δ DСН4 | OG | −143–−93 | [45] |
| δ DС2Н6 | OG | −211–−147 | [45] |
| δ DН2 | OG | −629–−198 | [110] |
| δ 13СC1-C5 | FG | −15.7–−7.1 | [110] |
| δ 13СCH4 | FG | −11.8 | [110] |
| δ 13СC2H6 | FG | −15.2 | [110] |
| δ DСН4 | FG | −167–−162 | [110] |
| δ DС2Н6 | FG | −202 | [110] |
| δ DН2 | FG | −644–−359 | [110] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nivin, V.A. Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review. Minerals 2019, 9, 535. https://doi.org/10.3390/min9090535
Nivin VA. Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review. Minerals. 2019; 9(9):535. https://doi.org/10.3390/min9090535
Chicago/Turabian StyleNivin, Valentin A. 2019. "Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review" Minerals 9, no. 9: 535. https://doi.org/10.3390/min9090535
APA StyleNivin, V. A. (2019). Occurrence Forms, Composition, Distribution, Origin and Potential Hazard of Natural Hydrogen–Hydrocarbon Gases in Ore Deposits of the Khibiny and Lovozero Massifs: A Review. Minerals, 9(9), 535. https://doi.org/10.3390/min9090535





