Extraction of Boron from Ludwigite Ore: Mechanism of Soda-Ash Roasting of Lizardite and Szaibelyite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Soda-Ash Roasting
2.3. Characterization
3. Results and Discussion
3.1. Thermodynamic Analysis
3.1.1. Na2CO3-MgSiO3/Mg2SiO4 System
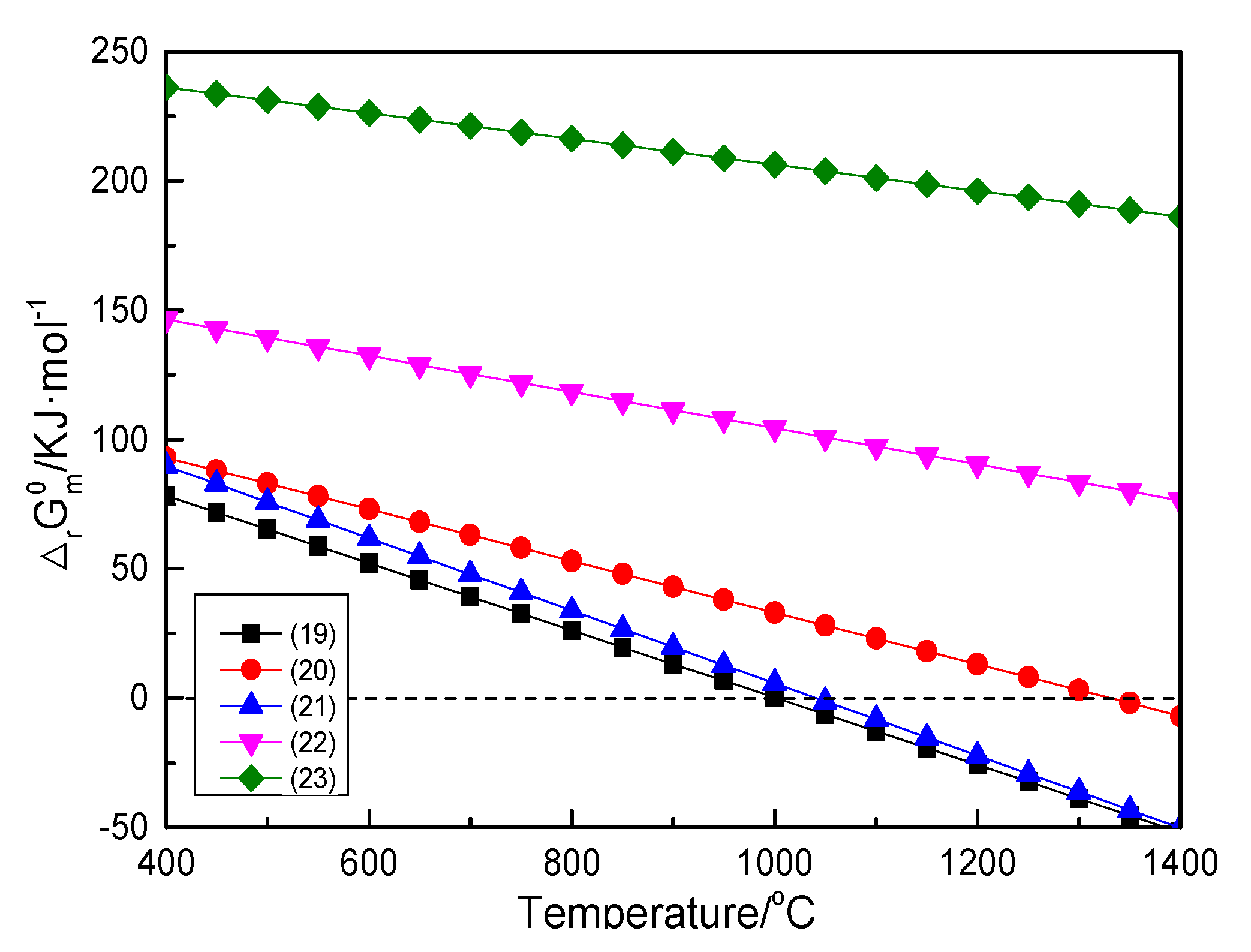
3.1.2. Na2CO3-Mg2B2O5 System
3.2. TG-DSC Analysis
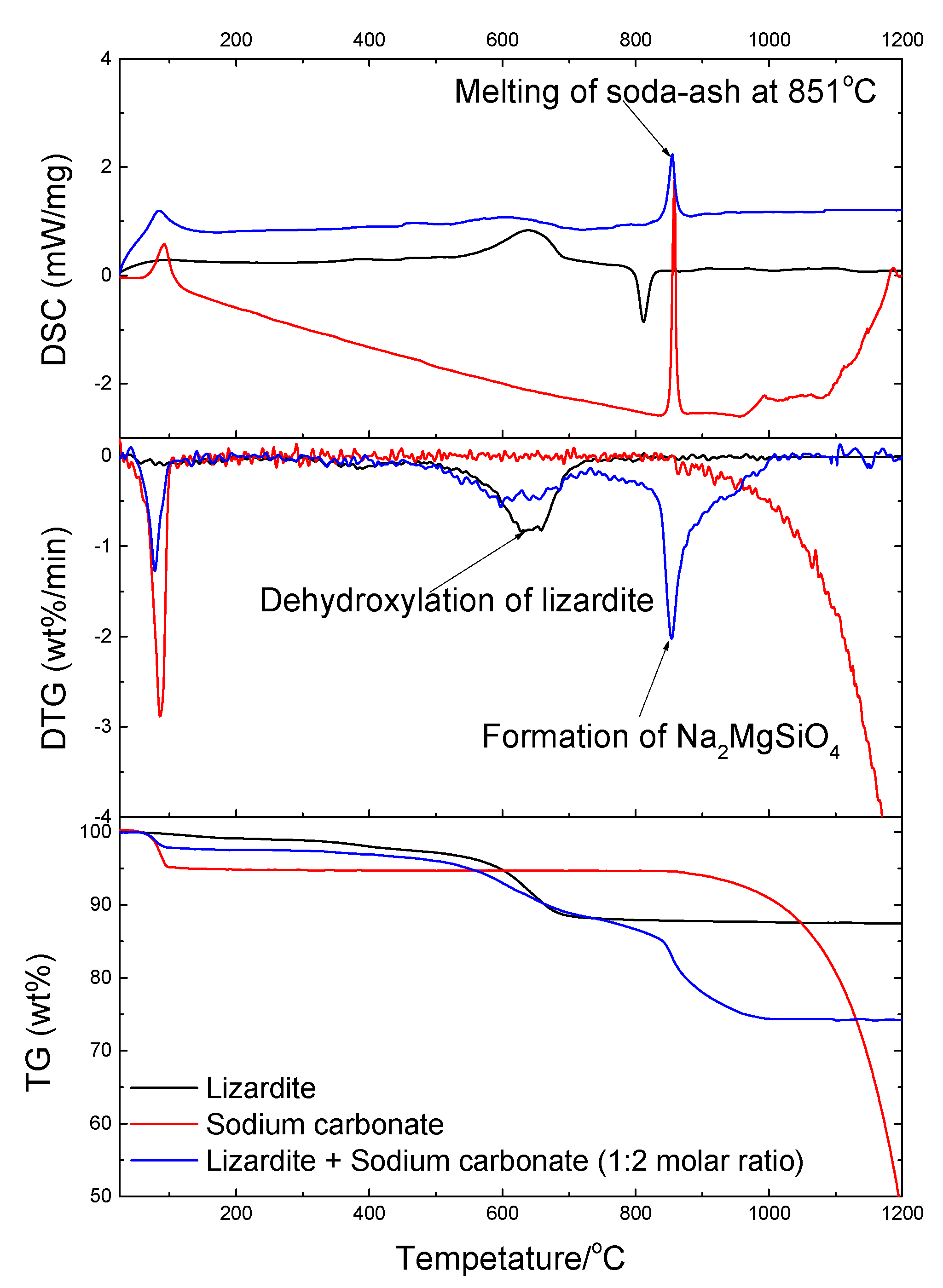
3.2.1. Lizardite with Soda Ash
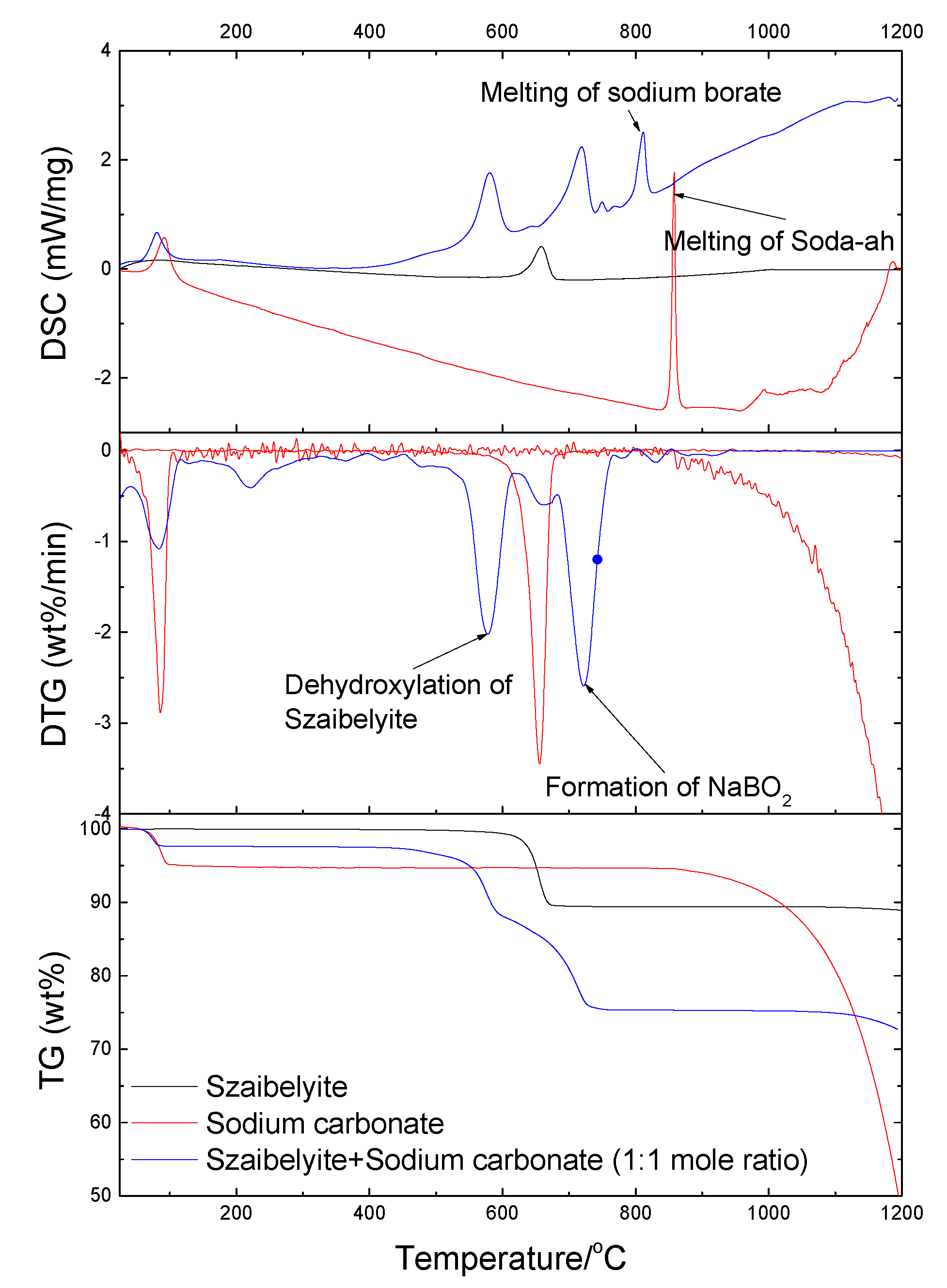
3.2.2. Szaibelyite with Soda Ash
3.3. Phase Transformation and Microstructure
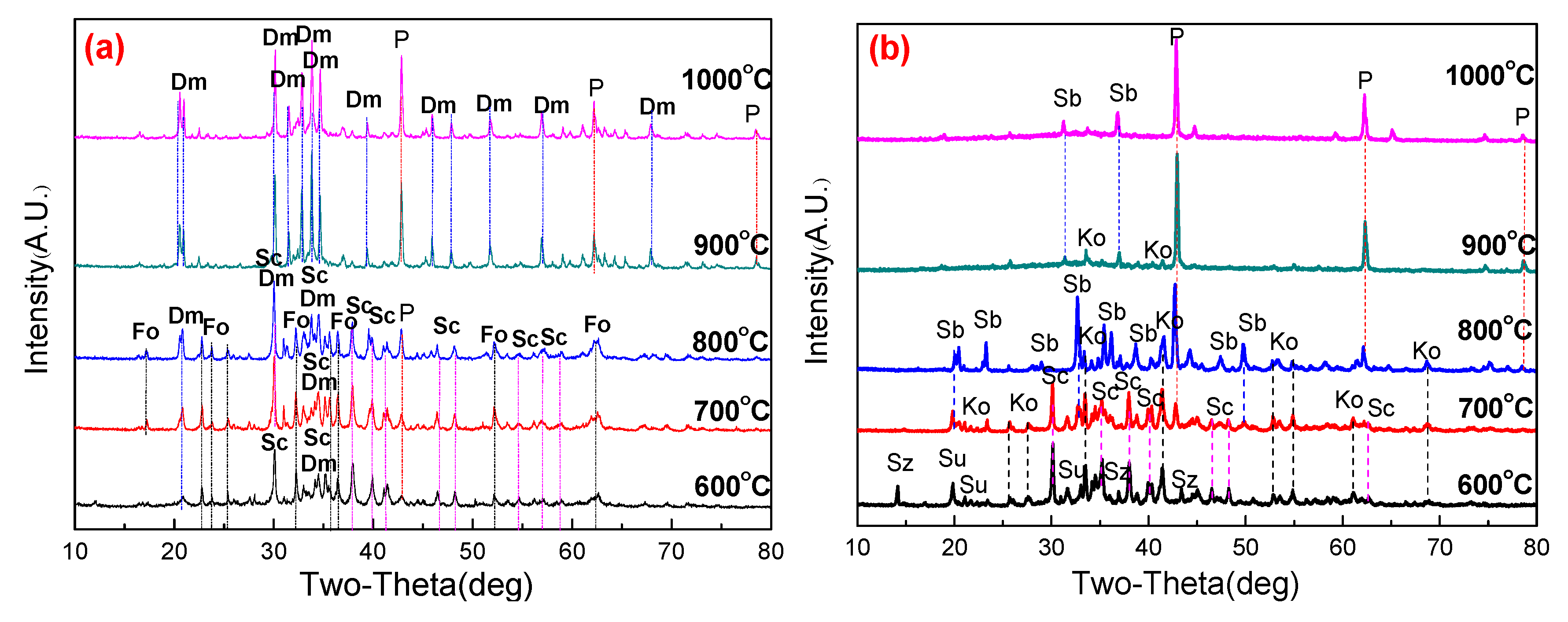
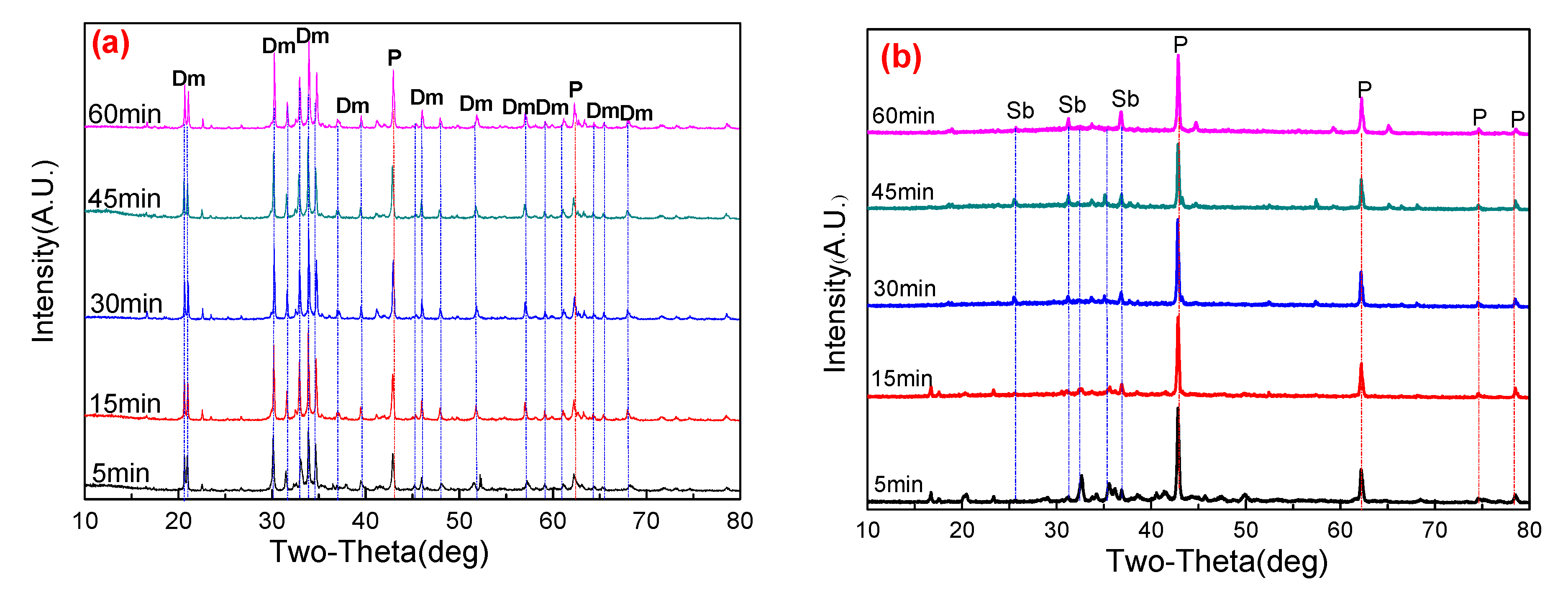
3.3.1. Effect of Roasting Temperature
3.3.2. Effect of Roasting Time
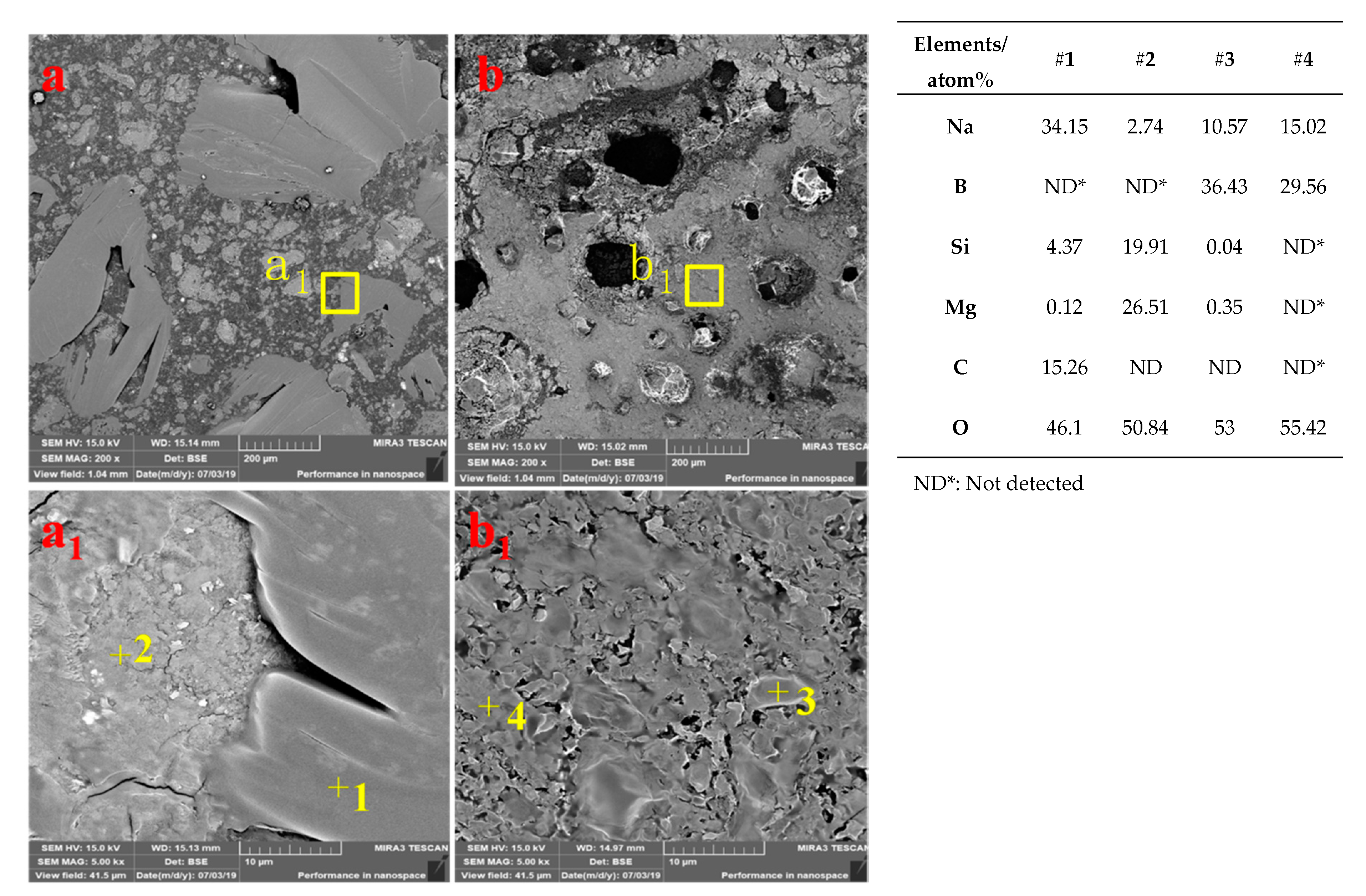
3.3.3. Microstructure
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- An, J.; Xue, X. Life cycle environmental impact assessment of borax and boric acid production in China. J. Clean. Prod. 2014, 66, 121–127. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Palmer, M.R.; Peng, Q.-M.; Yang, J.-H. Chemical and stable isotopic compositions of Proterozoic metamorphosed evaporites and associated tourmalines from the Houxianyu borate deposit, eastern Liaoning, China. Chem. Geol. 1997, 135, 189–211. [Google Scholar] [CrossRef]
- Brioche, A.S. Mining Engineering—Research and Technology. In Mineral Commodity Summaries 2019; Nova Science Publishers: New York, NY, USA, 2019; 36p. [Google Scholar]
- Palmer, M.R.; Helvaci, C. The boron isotope geochemistry of the Kirka borate deposit, western Turkey. Geochim. Cosmochim. Acta 1995, 59, 3599–3605. [Google Scholar] [CrossRef]
- Peng, Q.M.; Palmer, M.R. The paleoproterozoic Mg and Mg-Fe borate deposits of Liaoning and Jilin Provinces, northeast China. Econ. Geol. Bull. Soc. Econ. Geol. 2002, 97, 93–108. [Google Scholar] [CrossRef]
- Li, Y.-J.; Gao, T.; Han, Y.-X. Research on processing mineralogy of paigeite. Non Ferr. Min. Metall. 2006, 22, 3. [Google Scholar]
- Liu, R.; Wang, X.J.; Gao, Y.L.; Lu, Q.; Xue, X.X. Reaction Synthesis and Oxidation Behaviours of Ludwigite. Adv. Mater. Res. 2011, 146, 6. [Google Scholar] [CrossRef]
- Li, Z.H.; Han, Y.X.; Gao, P.; Ying, P. Research on Processing Mineralogical Characterization of the Paigeite Ore. J. Northeast. Univ. 2016, 37, 258–262. [Google Scholar]
- Wang, G.; Xue, Q.; She, X.; Wang, J. Carbothermal Reduction of Boron-bearing Iron Concentrate and Melting Separation of the Reduced Pellet. ISIJ Int. 2015, 55, 751–757. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Jiang, T.; Zhou, M.; Wen, J.; Chen, W.; Xue, X. Effects of mechanical activation on physicochemical properties and alkaline leaching of boron concentrate. Hydrometallurgy 2017, 173, 32–42. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Ding, Y.; Ma, S.; Xue, Q. New Separation Method of Boron and Iron from Ludwigite Based on Carbon Bearing Pellet Reduction and Melting Technology. ISIJ Int. 2012, 52, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Duan, H.M.; Lü, X.; Ning, Z.; Zhai, Y. Boron extraction from boron-concentrate ore by ammonium sulfate roasting method. J. Northeast. Univ. 2011, 32, 1724–1728. [Google Scholar]
- Liu, H.W.; Zhong, J.; Yu, T. Study on the technology of improving reactive activity of ludwigite from Wengquangou. Inorg. Chem. Ind. 2008, 40, 21–24. [Google Scholar]
- Jiang, T.; Yin, L.; Zhu, L.; Xue, X.X. Preparation of borax from boron concentrate by sodium- roasting and leaching. CIESC J. 2014, 65, 737–743. [Google Scholar]
- Li, G.; Liang, B.; Rao, M.; Zhang, Y.; Jiang, T. An innovative process for extracting boron and simultaneous recovering metallic iron from ludwigite ore. Miner. Eng. 2014, 56, 57–60. [Google Scholar] [CrossRef]
- Qin, S.; Yin, B.; Zhang, Y.; Zhang, Y. Leaching kinetics of szaibelyite ore in NaOH solution. Hydrometallurgy 2015, 157, 333–339. [Google Scholar] [CrossRef]
- Qin, S.; Zhang, Y.; Zhang, Y. Nucleation and morphology of sodium metaborate dihydrate from NaOH solution. J. Cryst. Growth 2016, 433, 143–147. [Google Scholar] [CrossRef]
- Ning, Z.-Q.; Zhai, Y.-C.; Song, Q.-S. Extracting B2O3 from calcined boron mud using molten sodium hydroxide. Rare Met. 2015, 34, 744–751. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, H.G. Thermal and carbothermic decomposition of Na2CO3 and Li(2)CO3. Metall. Mater. Trans. B Process Metall. Mater. Process. Sci. 2001, 32, 17–24. [Google Scholar] [CrossRef]
- Hu, B.S.; Xiong, F.W. The Influence of Serpentine Kind and Size on Sintering Process. Sinter. Pelletizing 2010, 35, 23–26. [Google Scholar]
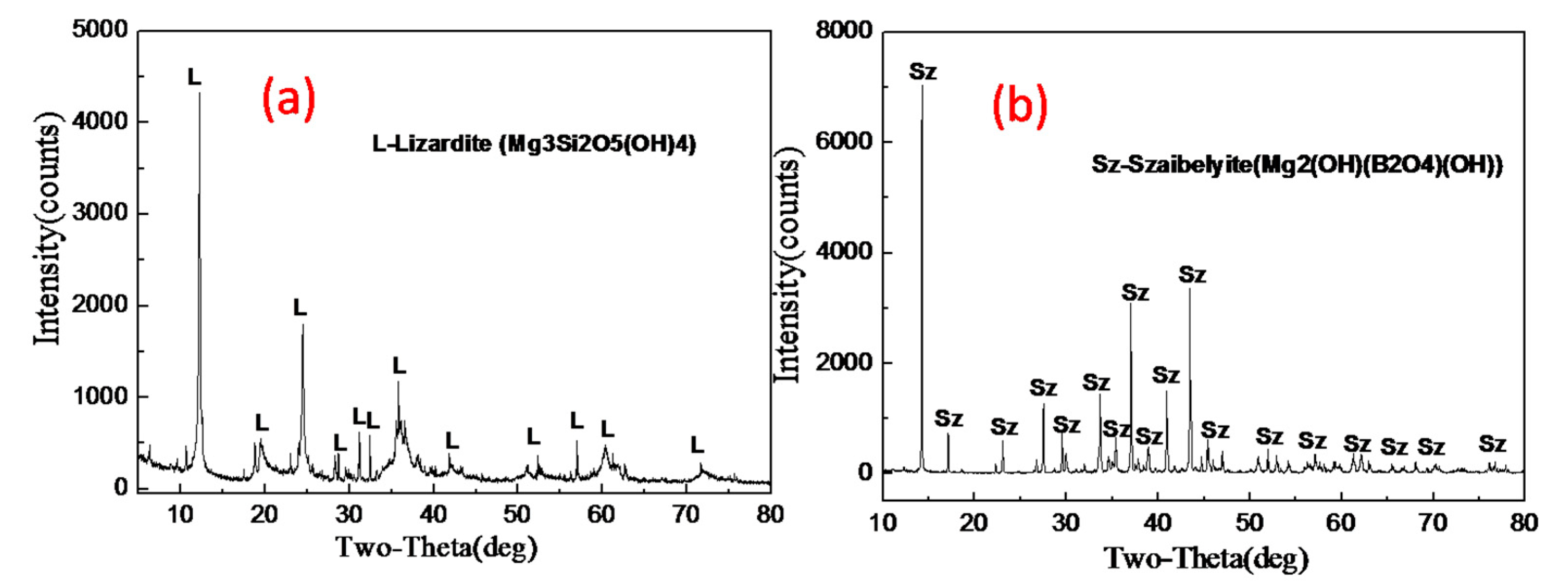
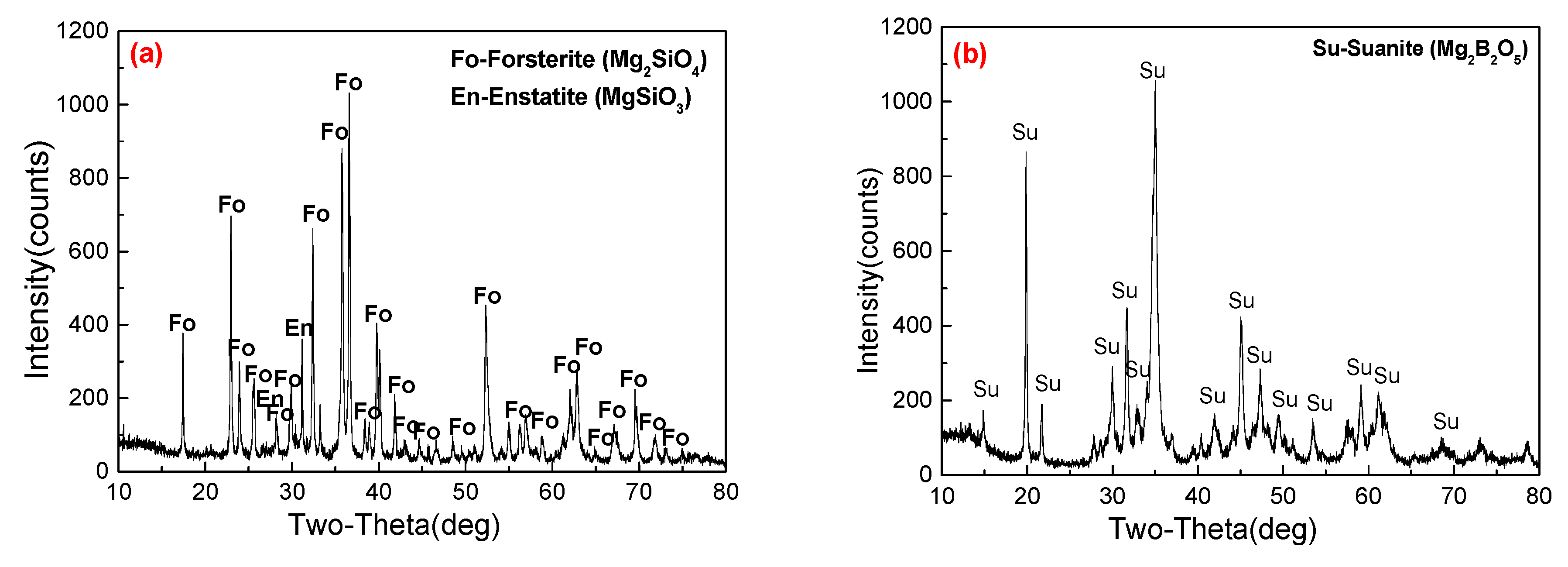
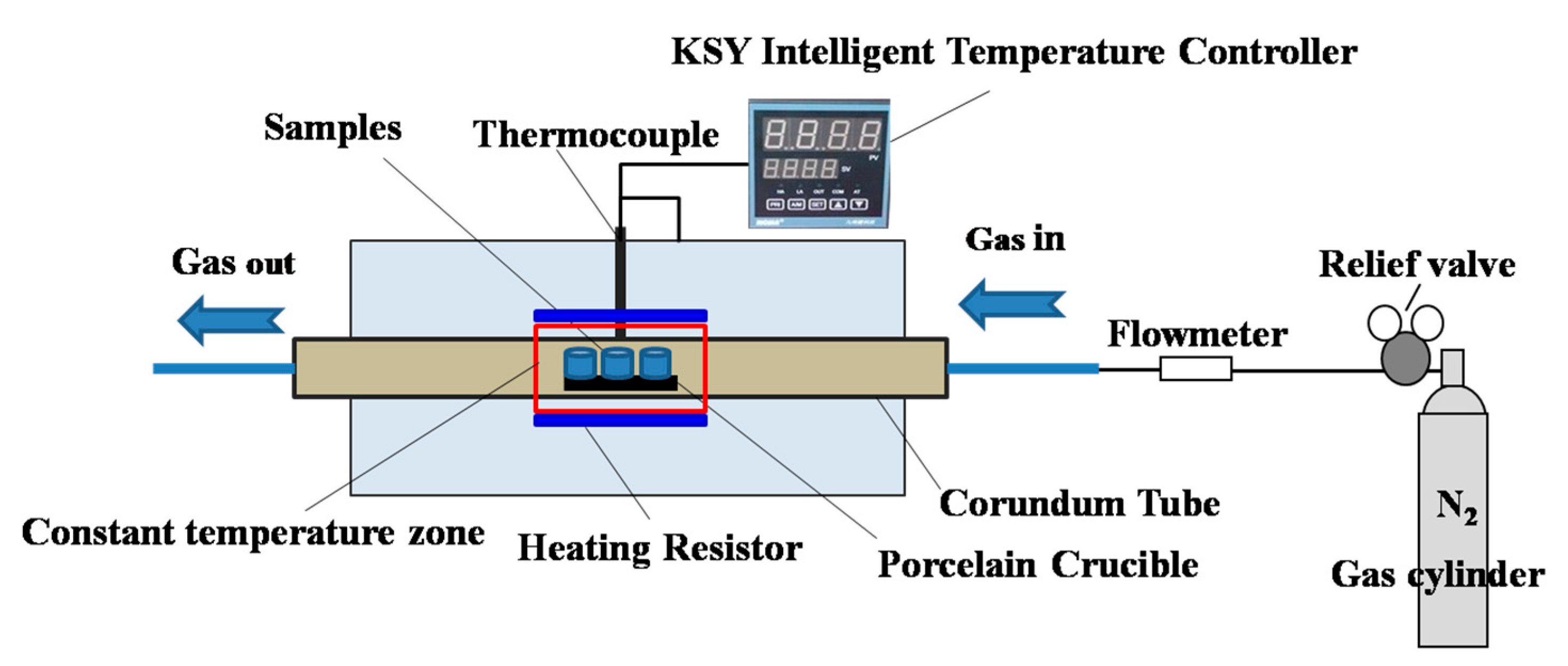
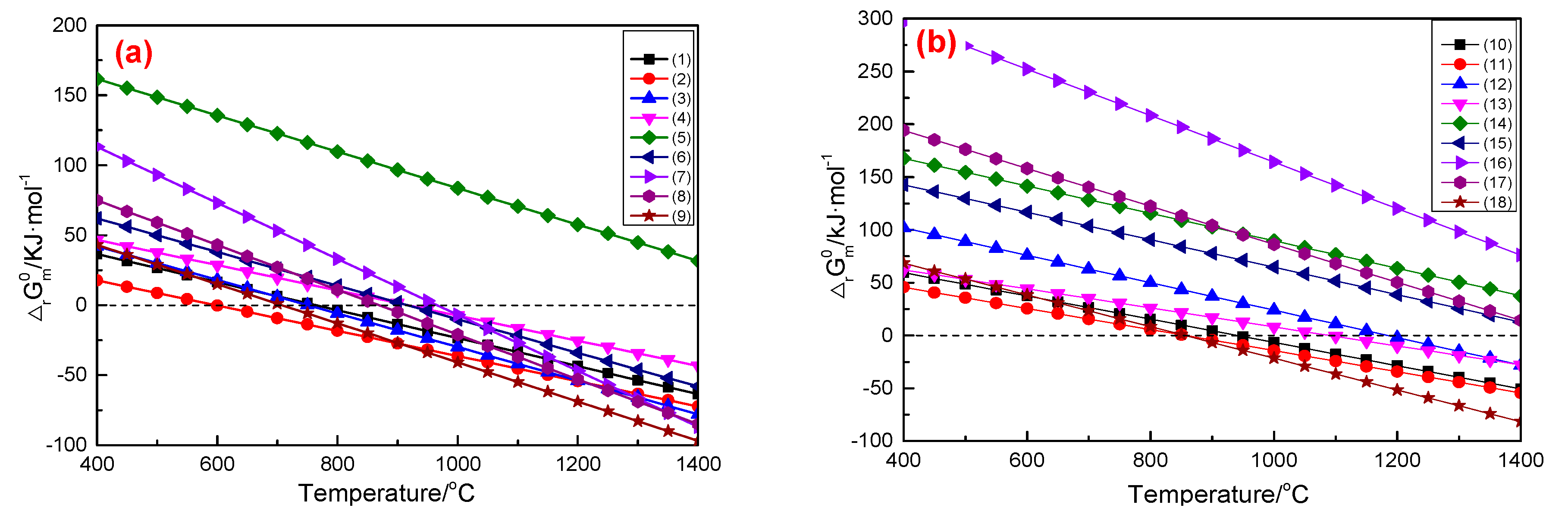
| Samples | TFe | B2O3 | SiO2 | CaO | MgO | K2O |
|---|---|---|---|---|---|---|
| Lizardite | 5.50 | ND* | 37.36 | 1.24 | 41.82 | 0.04 |
| Szaibelyite | 1.87 | 39.16 | 0.74 | 0.88 | 46.21 | 0.15 |
| Reaction | Equation | ∆rGmΘ − T (kJ·mol−1) |
|---|---|---|
| (1) | 2/3MgSiO3 + Na2CO3 = 1/3Na6Si2O7 + 2/3MgO + CO2(g) | 76.46 − 0.10T |
| (2) | MgSiO3 + Na2CO3 = Na2SiO3 + MgO + CO2(g) | 53.69 − 0.09T |
| (3) | 2MgSiO3 + Na2CO3 = Na2Si2O5 + 2MgO + CO2(g) | 90.08 − 0.12T |
| (4) | 1/2MgSiO3 + Na2CO3 = 1/2Na4SiO4 + 1/2MgO + CO2(g) | 82.59 − 0.09T |
| (5) | 1/5MgSiO3 + Na2CO3 = 1/5Na10SiO7 + 1/5MgO + CO2(g) | 213.66 − 0.13T |
| (6) | 8/3MgSiO3 + Na2CO3 = 1/3Na6Si8O19 + 8/3MgO + CO2(g) | 109.95 − 0.12T |
| (7) | 6MgSiO3 + Na2CO3 = Na2Mg2Si6O15 + 4MgO + CO2(g) | 193.07 − 0.20T |
| (8) | 4MgSiO3 + Na2CO3 = Na2MgSi4O10 + 3MgO + CO2(g) | 139.01 − 0.16T |
| (9) | MgSiO3 + Na2CO3 = Na2MgSiO4 + CO2(g) | 99.06 − 0.14T |
| (10) | 2/3Mg2SiO4 + Na2CO3 = 1/3Na6Si2O7 + 4/3MgO + CO2(g) | 103.42 − 0.11T |
| (11) | Mg2SiO4 + Na2CO3 = Na2SiO3 + 2MgO + CO2(g) | 85.62 − 0.10T |
| (12) | 2Mg2SiO4 + Na2CO3 = Na2Si2O5 + 4MgO + CO2(g) | 153.93 − 0.13T |
| (13) | 1/2Mg2SiO4 + Na2CO3 = 1/2Na4SiO4 + MgO + CO2(g) | 98.09 − 0.09T |
| (14) | 1/5Mg2SiO4 + Na2CO3 = 1/5Na10SiO7 + 2/5MgO + CO2(g) | 219.61 − 0.13T |
| (15) | 8/3Mg2SiO4 + Na2CO3 = 1/3Na6Si8O19 + 16/3MgO + CO2(g) | 194.65 − 0.13T |
| (16) | 6Mg2SiO4 + Na2CO3 = Na2Mg2Si6O15 + 10MgO + CO2(g) | 384.16 − 0.22T |
| (17) | 4Mg2SiO4 + Na2CO3 = Na2MgSi4O10 + 7MgO + CO2(g) | 266.27 − 0.18T |
| (18) | Mg2SiO4 + Na2CO3 = Na2MgSiO4 + MgO + CO2(g) | 128.39 − 0.15T |
| Reaction | Equation | ∆rGmΘ − T (kJ·mol−1) |
|---|---|---|
| (19) | Mg2B2O5 + Na2CO3 = 2NaBO2 +2MgO + CO2(g) | 130.16 − 0.13T |
| (20) | 1/3Mg2B2O5 + Na2CO3 = 2/3Na3BO3 +2/3MgO + CO2(g) | 133.03 − 0.10T |
| (21) | 1/2Mg2B2O5 + Na2CO3 = 1/2Na4B2O5 + MgO + CO2(g) | 145.77 − 0.14T |
| (22) | 2Mg2B2O5 + Na2CO3 = Na2B4O7 + 4MgO + CO2(g) | 174.42 − 0.07T |
| (23) | 3Mg2B2O5 + Na2CO3 = 2NaB3O5 + 6MgO + CO2(g) | 256.21 − 0.05T |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Li, G.; You, J.; Wang, J.; Luo, J.; Duan, J.; Zhang, T.; Peng, Z.; Rao, M.; Jiang, T. Extraction of Boron from Ludwigite Ore: Mechanism of Soda-Ash Roasting of Lizardite and Szaibelyite. Minerals 2019, 9, 533. https://doi.org/10.3390/min9090533
Zhang X, Li G, You J, Wang J, Luo J, Duan J, Zhang T, Peng Z, Rao M, Jiang T. Extraction of Boron from Ludwigite Ore: Mechanism of Soda-Ash Roasting of Lizardite and Szaibelyite. Minerals. 2019; 9(9):533. https://doi.org/10.3390/min9090533
Chicago/Turabian StyleZhang, Xin, Guanghui Li, Jinxiang You, Jian Wang, Jun Luo, Jiaoyang Duan, Tao Zhang, Zhiwei Peng, Mingjun Rao, and Tao Jiang. 2019. "Extraction of Boron from Ludwigite Ore: Mechanism of Soda-Ash Roasting of Lizardite and Szaibelyite" Minerals 9, no. 9: 533. https://doi.org/10.3390/min9090533
APA StyleZhang, X., Li, G., You, J., Wang, J., Luo, J., Duan, J., Zhang, T., Peng, Z., Rao, M., & Jiang, T. (2019). Extraction of Boron from Ludwigite Ore: Mechanism of Soda-Ash Roasting of Lizardite and Szaibelyite. Minerals, 9(9), 533. https://doi.org/10.3390/min9090533






