Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau
Abstract
:1. Introduction
2. Overview of Analytical Methods
3. The Hydrochemistry and Distribution of Li-rich Brines in Salt Lakes on the QTP
4. The Formation of Li Brine Deposits in Salt Lakes on the QTP
4.1. The Sources of Li in Brines in Salt Lakes
4.2. The Formation Model of Li Brine Deposits on the QTP
5. Perspectives for Future Work
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Grosjean, C.; Miranda, P.H.; Perrin, M.; Poggi, P. Assessment of world lithium resources and consequences of their geographic distribution on the expected development of the electric vehicle industry. Renew. Sust. Energ. Rev. 2012, 16, 1735–1744. [Google Scholar] [CrossRef]
- Kesler, S.E.; Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Everson, M.P.; Wallington, T.J. Global lithium resources: Relative importance of pegmatite, brine and other deposits. Ore Geol. Rev. 2012, 48, 55–69. [Google Scholar] [CrossRef]
- Tadesse, B.; Makuei, F.; Albijanic, B.; Dyer, L. The beneficiation of lithium minerals from hard rock ores: A review. Miner. Eng. 2019, 131, 170–184. [Google Scholar] [CrossRef]
- Ebensperger, A.; Maxwell, P.; Moscoso, C. The lithium industry: Its recent evolution and future prospect. Resour. Policy 2005, 30, 218–231. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Jaskula, B.W. 2016 Minerals Yearbook. Lithium; U.S. Geological Survey: Reston, VI, USA, 2018.
- Zheng, M.P.; Liu, X.F. Lithium resources in China. Adv. Mater. Ind. 2007, 8, 13–16. [Google Scholar]
- Wang, Q.S.; Qiu, J.Z.; Shao, H.N.; Xu, H. Analysis on metallogenic characteristic and resource potential of salt lake brine lithium deposits in the global. China Min. Mag. 2015, 24, 82–88. [Google Scholar]
- Zheng, M.P.; Liu, X.F. Hydrochemistry and Minerals Assemblages of Salt Lakes in the Qinghai-Tibet Plateau, China. Acta Geol. Sin. 2010, 84, 1585–1600. [Google Scholar]
- Flexer, V.; Femando, B.C.; Inés, G.C. Lithium recovery from brines: A vital raw material for green energies with a potential environmental impact in its mining and processing. Sci. Total Environ. 2018, 639, 1188–1204. [Google Scholar] [CrossRef]
- Li, R.Q.; Liu, C.L.; Jiao, P.C.; Wang, J.Y. The tempo-spatial characteristics and forming mechanism of Lithium-rich brines in China. China Geol. 2018, 1, 72–83. [Google Scholar] [CrossRef]
- Tong, W.; Mu, Z.G.; Liu, S.B. The Late-Cenozoic volcanoes and active high-temperature hydrothermal systems in China. Acta Geophys. Sin. 1990, 33, 329–335. [Google Scholar]
- Pan, G.T.; Wang, L.Q.; Li, R.S.; Yuan, S.H.; Ji, W.H.; Yin, F.G.; Zhang, W.P.; Wang, B.D. Tectonic evolution of the Qinghai-Tibet Plateau. J. Asian Earth Sci. 2012, 53, 3–14. [Google Scholar] [CrossRef]
- Zheng, X.Y. Salt Lakes in the Tibet; Science Press: Beijing, China, 1988; pp. 1–80. [Google Scholar]
- Zheng, M.P.; Xiang, J. Salt Lakes in the Tibetan Plateau; Science Press: Beijing, China, 1989; pp. 1–219. [Google Scholar]
- Zheng, X.Y.; Zhang, M.G.; Xu, C.; Li, B.X. Salt Lakes in China; Science Press: Beijing, China, 2002. [Google Scholar]
- Zheng, M.P.; Liu, X.F. Hydrochemistry of salt lakes of the Qinghai-Tibet Plateau, China. Aquat. Geochem. 2009, 15, 293–320. [Google Scholar] [CrossRef]
- Dong, T.; Tan, H.B.; Zhang, W.J.; Zhang, Y.F. The geochemical pattern of Li in salt lakes in the Tibet. J. Hohai Uni. 2015, 43, 230–235. [Google Scholar]
- Wang, Z.; Li, M.L.; Shao, B.; Wu, G.D.; Wu, X.W. Research of natural evaporation of Eyacuo salt lake brine in Tibet. J. Salt Sci. Chem. Ind. 2018, 47, 28–30. [Google Scholar]
- Wu, J.L.; Wang, X.K.; Dong, J.G.; Sha, Z.L. Salting-out law of the brine from the Laguocuo salt lake through isothermal evaporation at 15 °C. J. Tianjin Uni. Sci. Tech. 2014, 29, 55–57. [Google Scholar]
- Wu, Q.; Zheng, M.P.; Nie, Z.; Bu, L.Z. Natural evaporation and crystallization regularity of Dangxiongcuo carbonate-type salt lake brine in Tibet. Chinese J. Inorg. Chem. 2012, 28, 1895–1903. [Google Scholar]
- Qing, D.L.; Ma, H.Z.; Li, B.K. Boron concentration and isotopic fractionation research in BangkogCo intercrystal brine evaporation process. J. Salt Lake Res. 2012, 20, 15–20. [Google Scholar]
- Yu, J.J.; Zheng, M.P.; Wu, Q.; Wang, Y.S.; Nie, Z.; Bu, L.Z. Natural evaporation and crystallization of Dujiali salt lake water in Tibet. Chem. Ind. Eng. Prog. 2015, 34, 4172–4178. [Google Scholar]
- Zhang, N.; Yuan, J.J.; Dong, J.G.; Sha, Z.L. Laws of crystallization of the brine in Jiezechaka Lake in Tibet at 15 oC through evaporation and concentration. J. Tianjin Uni. Sci. Tech. 2013, 28, 44–48. [Google Scholar]
- Zhao, Y.Y. Comprehensive utilization of brines from Mami Co. Ind. Miner. Proc. 2013, 11, 48. [Google Scholar]
- Wu, Z.M.; Zheng, M.P.; Liu, X.F.; Nie, Z. Concentration of brines from the Dogai Coring Lake, northern Tibet, by using the two-step process: Freezing and solar evaporation. Chine. J. Inorg. Chem. 2012, 28, 995–1000. [Google Scholar]
- Wang, X.K.; Zhang, Z.Z.; Sha, Z.L.; Dong, J.G. Study on the salt crystallization law of brine from Chabocuo salt lake in Tibet through evaporation under the temperature of 15 °C. J. Tianjin Uni. Sci. Tech. 2013, 28, 34–38. [Google Scholar]
- Xia, S.; Li, Y.Y.; Tang, J.L.; Ning, W.Y.; Zheng, X.F. Experimental research on the isothermal evaporation of the brine in Baqiancuo salt lake. J. Salt Lake Res. 2013, 21, 29–31. [Google Scholar]
- Yang, M.J.; Dong, J.G.; Yuan, J.J.; Sha, Z.L. Investigation on the salting-out law of Longmucuo brine under 5 °C evaporation. J. Tianjin Uni. Sci. Tech. 2013, 28, 47–50. [Google Scholar]
- Liu, Y.; Wang, Y.S.; Nie, Z.; Wu, Q. Research on 15 °C-isothermal evaporation experiment of carbonate-type brines from Pengyan Co salt lake in Tibet. Inorg. Chem. Ind. 2017, 49, 21–25. [Google Scholar]
- Li, J.D.; Shi, T.C.; Wang, Y.X.; Wu, C.; Fu, J.L. Research on natural evaporation of brine in Yiliping Mining area. J. Salt Lake Res. 2008, 16, 32–36, 65. [Google Scholar]
- Liu, X.F.; Zheng, M.P.; Qi, W. Sources of ore-forming materials of the superlarge B and Li deposit in Zabuye Salt Lake, Tibet, China. Acta Geol. Sin. 2007, 81, 1709–1715. [Google Scholar]
- Tan, H.; Chen, J.; Rao, W.; Zhang, W.; Zhou, H. Geothermal constraints on enrichment of boron and lithium in salt lakes: An example from a river-salt lake system on the northern slope of the eastern Kunlun Mountains, China. J. Asian Earth Sci. 2012, 51, 21–29. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gao, C.L.; Cheng, A.Y.; Liu, Y.; Zhang, L.S.; He, X.H. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan Plateau, China. Ore Geol. Rev. 2013, 50, 171–183. [Google Scholar] [CrossRef]
- Wei, H.Z.; Jiang, S.Y.; Tan, H.B.; Zhang, W.J.; Li, B.K.; Yang, T.L. Boron isotope geochemistry of salt sediments from the Dongtai salt lake in Qaidam Basin: Boron budget and sources. Chem. Geol. 2014, 380, 74–83. [Google Scholar] [CrossRef]
- Gao, C.L. Sedimentary Evolution of Da Qaidam and Taijinaier Salt Lakes and a Case Study on the Genesis of Borate ore and Lithium Brine Deposits. Ph.D. Thesis, Chinese Academy of Sciences, Beijing, China, 2012. [Google Scholar]
- Stober, I.; Zhong, J.; Zhang, L.; Bucher, K. Deep hydrothermal fluid-rock interaction: The thermal springs of Da Qaidam, China. Geofluids 2016, 16, 711–728. [Google Scholar] [CrossRef]
- Shang, B. Sources of Li and K in the Duogecuoren Salt Lake in Tibet. Master Thesis, China University of Geosciences (Beijing), Beijing, China, 2013. [Google Scholar]
- Sun, Y.W. Hydrochemical Characteristics and Forming Mechanism of Eya Co Salt Lake in Tibet; Chendu University of Technology: Sichuan, China, 2017. [Google Scholar]
- Qu, L.H.; Zhao, F.; Zhou, X.Y.; Zhao, T.S.; Meng, S.X. The Geological Characteristics and Metallogenic Model of the Kangruchaka Salt Lake, North Qiangtang Basin (Tibet). Xinjiang Geol. 2018, 36, 469–475. [Google Scholar]
- Qu, L.H. The Geological Geochemical Features and Genesis of the Rejuecaka Salt Lake, North Qiangtang Basin (Tibet). Masters Thesis, China University of Geosciences (Beijing), Beijing, China, 2015. [Google Scholar]
- Risacher, F.; Fritz, B. Geochemistry of Bolivian salars, Lipez, southern Altiplano: Origin of solutes and brine evolution. Geochim. Cosmochim. Acta 1991, 55, 687–705. [Google Scholar] [CrossRef]
- Risacher, F.; Fritz, B. Origin of salt and brine evolution of Bolivian and Chilean Salars. Aquat. Geochem. 2009, 15, 123–157. [Google Scholar] [CrossRef]
- Carmona, V.; Pueyo, J.J.; Taberner, C.; Chong, G.; Thirlwall, M. Solute inputs in the Salar de Atacama (N. Chile). J. Geochem. Explor. 2000, 69, 449–452. [Google Scholar] [CrossRef]
- Risacher, F.; Alonso, H.; Salazar, C. The origin of brines and salts in Chilean Salars: A hydrochemical review. Earth Sci. Rev. 2003, 63, 249–292. [Google Scholar] [CrossRef]
- Munk, L.A.; Boutt, D.F.; Hynek, S.A.; Moran, B.J. Hydrogeochemical fluxes and processes contributing to the formation of lithium-enriched brines in a hyper-arid continental basin. Chem. Geol. 2018, 493, 37–57. [Google Scholar] [CrossRef]
- Steinmetz, R.L.L.; Salvi, S.; García, M.G.; Arnold, Y.P.; Béziat, D.; Franco, G.; Constantini, O.; Córdoba, F.E.; Caffe, P.J. Northern Puna Plateau-scale survey of Li brine-type deposits in the Andes of NW Argentina. J. Geochem. Explor. 2018, 190, 26–38. [Google Scholar] [CrossRef]
- Qinghai Institute of Salt Lakes. The Introduction to Analyzing Methods of Brines and Salt Deposits, 2nd ed.; Science Press: Beijing, China, 1988; pp. 29–71. [Google Scholar]
- Sun, H.; Liao, K.; Pan, Y.; Wang, J. Atlas of the Qinghai-Tibet Plateau; Science Press: Beijing, China, 1990. [Google Scholar]
- Gao, F.; Zheng, M.P.; Nie, Z.; Liu, J.H.; Song, P.S. Brine lithium resource in the salt lake and advances in its exploitation. Acta Geosci. Sin. 2011, 32, 483–492. [Google Scholar]
- Zheng, M.P.; Deng, Y.J.; Nie, Z.; Bu, L.Z.; Shi, S.Y. 25 °C-Isothermal evaporation of autumn brines from the Zabuye Salt Lake, Tibet, China. Acta Geol. Sin. 2007, 12, 1742–1749. [Google Scholar]
- China Salty Lakes Resources and Environment Database. Qinghai Institute of Salt Lakes; Chinese Academy of Sciences: Xining, China, 2019. [Google Scholar]
- Hu, D.S. Geochemical characteristics of the water body in the Kekexili region lakes. Oceanol. Limnol. Sin. 1997, 28, 153–164. [Google Scholar]
- Bai, Y.M.; Wang, X.L.; Yang, L.C.; Wang, Z.T.; Ye, C.Y. Hydrochemical characteristics of Duoxiu Lake and Yanhu Lake in northeastern Hoh Xil region, Qinghai. J. Salt Lake Res. 2018, 26, 27–33. [Google Scholar]
- He, L.; Han, F.Q.; Han, W.X.; Yan, J.P.; Li, B.K.; Han, Y.Z.; Nian, X.Q.; Chen, Y.J.; Han, J.L. Hydrochemical characteristics of Lexiewudan Lake in Hoh Xil, Qinghai. J. Salt Lake Res. 2015, 23, 28–33. [Google Scholar]
- Yu, J.J.; Zheng, M.P.; Wu, Q. Research progress of lithium extraction process in lithium-containing salt lake. Chem. Ind. Eng. Prog. 2013, 32, 13–21. [Google Scholar]
- Bian, S.; Li, D.; Gao, D.; Peng, J.; Dong, Y.; Li, W. Hydrometallurgical processing of lithium, potassium, and boron for the comprehensive utilization of Da Qaidam lake brine via natural evaporation and freezing. Hydrometallurgy 2017, 173, 80–83. [Google Scholar] [CrossRef]
- Nie, X.Y.; Sun, S.Y.; Song, X.; Yu, J.G. Further investigation into lithium recovery from salt lake brines with different feed characteristics by electrodialysis. J. Membr. Sci. 2017, 530, 185–191. [Google Scholar] [CrossRef]
- Guo, Z.Y.; Ji, Z.Y.; Chen, Q.B.; Liu, J.; Zhao, Y.Y.; Li, F.; Liu, Z.Y.; Yuan, J.S. Prefractionation of LiCl from concentrated seawater/salt lake brines by electrodialysis with monovalent selective ion exchange membranes. J. Clean. Prod. 2018, 193, 338–350. [Google Scholar] [CrossRef]
- Shi, D.; Cui, B.; Li, L.; Peng, X.; Zhang, L.; Zhang, Y. Lithium extraction from lowgrade salt lake brine with ultrahigh Mg/Li ratio using TBP-kerosene-FeCl3 system. Sep. Purif. Technol. 2019, 211, 303–309. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Z.W.; Ghahreman, A. Novel approaches for lithium extraction from salt-lake brines: A review. Hydrometallurgy 2019, 187, 81–100. [Google Scholar] [CrossRef]
- Ide, Y.F.; Kunasz, I.A. Origin of Lithium ion Salar de Atacama, Northern Chile. In Geology of the Andes and Its Relation to Hydrocarbon and Mineral Resources; Erickson, G.E., Cãnas Pinochet, M.T., Reinemund, J.A., Eds.; Circum-Pacific Council for Energy and Mineral Resources, Earth Science Series: Houston, TX, USA, 1989; Volume 11. [Google Scholar]
- Alonso, H.; Risacher, F. Geoquímica del Salar de Atacama, Parte 1: Origen de los componentes y balance salino. Rev. Geol. Chile 1996, 23, 113–122. [Google Scholar]
- Garrett, D.E. Lithium Handbook of Deposits, Processing, Properties, and Use; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Lowenstein, T.; Risacher, F. Closed basin brine evolution and the influence of Ca–Cl inflow waters. Death Valley and Bristol Dry Lake, California, Qaidam Basin, China, and Salar de Atacama, Chile. Aquat. Geochem. 2009, 15, 71–94. [Google Scholar] [CrossRef]
- Munk, L.A.; Jennings, M.; Bradley, D.; Hynek, S.; Godfrey, L. Geochemistry of Lithium-Rich Brines in Clayton Valley, Nevada, USA. In Proceedings of the 11th SGA Biennial Meeting, Antofagasta, Chile, 26–29 September 2011; pp. 217–219. [Google Scholar]
- Tan, H.; Su, J.; Peng, X.; Tao, D.; Elenga, H.I. Enrichment mechanism of Li, B and K in the geothermal water and associated deposits from the Kawu area of the Tibetan Plateau: Constraints from geochemical experimental data. Appl. Geochem. 2018, 93, 60–68. [Google Scholar] [CrossRef]
- Godfrey, L.V.; Chan, L.H.; Alonso, R.N.; Lowenstein, T.K.; Mcdonough, W.F.; Houston, J.; Li, J.; Bobst, A.; Jordan, T.E. The role of climate in the accumulation of lithium-rich brine in the Central Andes. Appl. Geochem. 2013, 38, 92–102. [Google Scholar] [CrossRef]
- Hofstra, A.H.; Todorov, T.I.; Mercer, C.N.; Adams, D.T.; Marsh, E.E. Silicate Melt Inclusion Evidence for Extreme Pre-eruptive Enrichment and Post-eruptive Depletion of Lithium in Silicic Volcanic Rocks of the Western United States: Implications for the Origin of Lithium-Rich Brines. Econ. Geol. 2013, 108, 1691–1701. [Google Scholar] [CrossRef]
- Steinmetz, R.L.L. Lithium and boron-bearing brines in the Central Andes: Exploring hydrofacies on the eastern Puna plateau between 23 and 23 30’S. Miner. Deposita 2017, 52, 35–50. [Google Scholar] [CrossRef]
- Campbell, M.G. Battery lithium could come from geothermal waters. The New Scientist, 9 December 2009; Volume 204, 23. [Google Scholar]
- Giordano, G.; Ahumada, F.; Aldega, L.; Becchio, R.; Bigi, S.; Caricchi, C.; Chiodi, A.; Corrado, S.; De Benedetti, A.A.; Favetto, A.; et al. Preliminary data on the structure and potential of the Tocomar geothermal field (Puna plateau, Argentina). Energy Procedia 2016, 97, 202–209. [Google Scholar] [CrossRef]
- Xia, L.Q.; Li, X.M.; Ma, Z.P.; Xu, X.Y.; Xia, Z.C. Cenozoic volcanism and tectonic evolution of the Tibetan Plateau. Gondwana Res. 2011, 19, 850–866. [Google Scholar] [CrossRef]
- Grimaud, D.; Huang, S.; Michard, G.; Zheng, K. Chemical study of geothermal waters of Central Tibet (China). Geothermics 1985, 14, 35–48. [Google Scholar] [CrossRef]
- Jiang, H.C.; Zhou, R.L. Distribution of Geothermal Water in Structure System of Qinghai-Xizang Plateau and its Prospecting; Bulletin of the 562 Comprehensive Geological Brigade; Chinese Academy of Geological Sciences: Beijing, China, 1994; pp. 243–258. [Google Scholar]
- Li, Z.Q. Present Hydrothermal Activities During Collisional Orogenics of the Tibetan Plateau. Ph.D. Thesis, Chinese Academy of Geological Sciences, Beijing, China, 2002. [Google Scholar]
- Tan, H.B.; Zhang, Y.F.; Zhang, W.J.; Kong, N.; Zhang, Q.; Huang, J.Z. Understanding the circulation of geothermal waters in the Tibetan Plateau using oxygen and hydrogen stable isotopes. Appl. Geochem. 2014, 51, 23–32. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Tan, H.B.; Zhang, W.J.; Huang, J.Z.; Zhang, Q. A New Geochemical Perspective on Hydrochemical Evolution of the Tibetan Geothermal System. Geochem. Inter. 2015, 53, 1090–1106. [Google Scholar] [CrossRef]
- Xu, P.; Tan, H.B.; Zhang, Y.F.; Zhang, W. Geochemical characteristics and source mechanism of geothermal water in Tethys Himalaya belt. Geol. China 2018, 45, 1142–1154. [Google Scholar]
- Zhang, P.X. Salt Lakes in the Qaidam Basin; Science Press: Beijing, China, 1987. [Google Scholar]
- Guo, Q.H. Hydrogeochemistry of high-temperature geothermal systems in China: A review. Appl. Geochem. 2012, 27, 1887–1898. [Google Scholar] [CrossRef]
- Niu, X.S.; Liu, X.F.; Chen, W.X. Travertine in south bank of Dogai Coring, Tibet: Geochemical characteristics and potash geological significance. Acta Sedimentol. Sin. 2013, 31, 1031–1040. [Google Scholar]
- Niu, X.S.; Zheng, M.P.; Liu, X.F.; Qi, L.J. Sedimentary property and the geological significance of travertines in Qinghai-Tibetan Plateau. Sci. Tech. Rev. 2017, 35, 59–64. [Google Scholar]
- Lv, G.R. The Tibet Autonomous region in Gaize County Analysis of Metallogenic Mode and Prospecting Prospect Saline Lake Laguocuo. Master Thesis, China University of Geoscience, Beijing, China, 2013. [Google Scholar]
- Fan, Q.S.; Ma, H.Z.; Tan, H.B.; Xu, J.X.; Li, T.W. Characteristics and origin of brines in western Qaidam Basin. Geochemica 2007, 36, 633–637. [Google Scholar]
- Li, H.P.; Zheng, M.P.; Hou, X.H.; Yan, L.J. Control Factors and Water Chemical Characteristics of Potassium-rich Deep Brine in Nanyishan Structure of Western Qaidam Basin. Acta Geosci. Sin. 2015, 36, 41–50. [Google Scholar] [CrossRef]
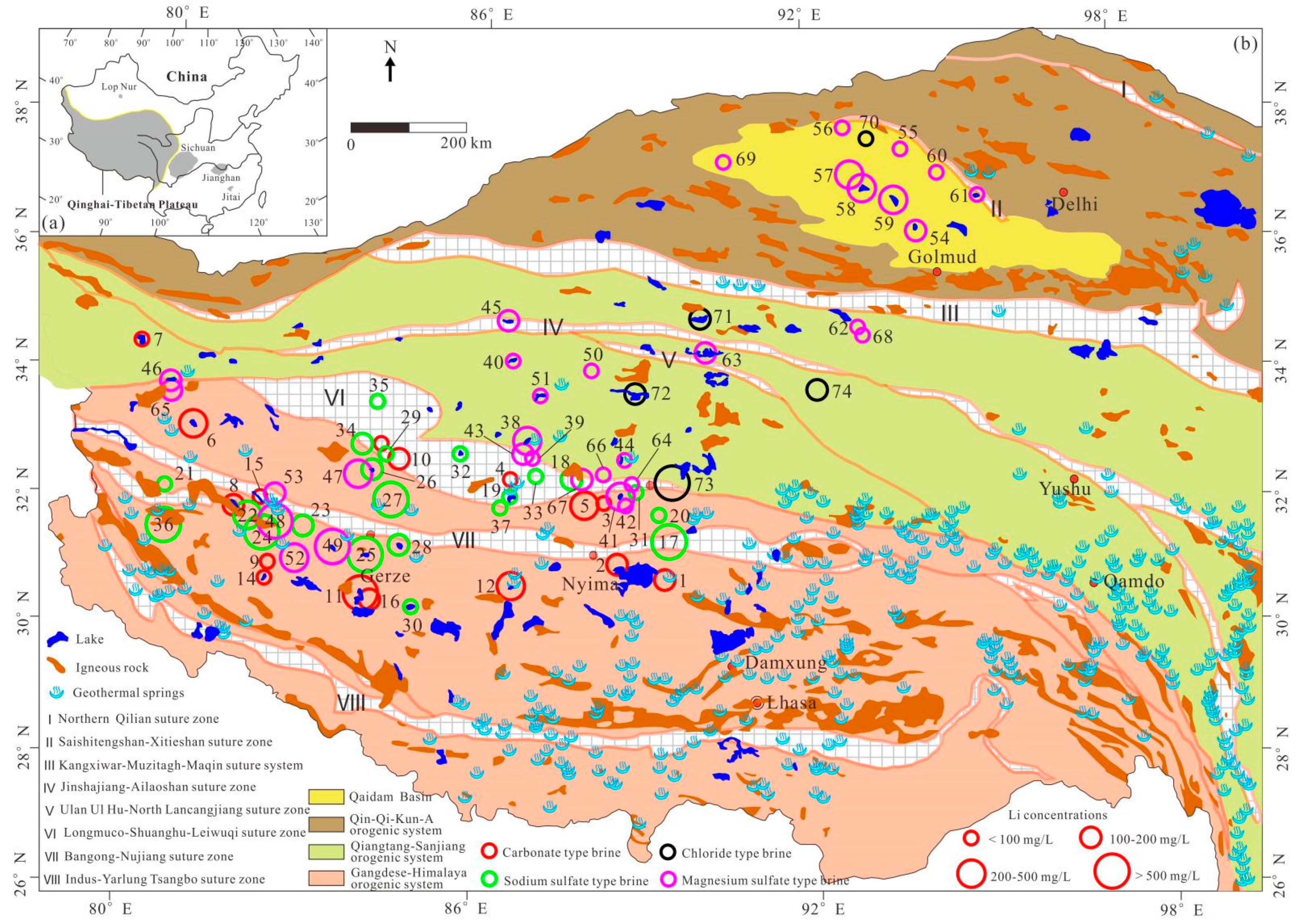
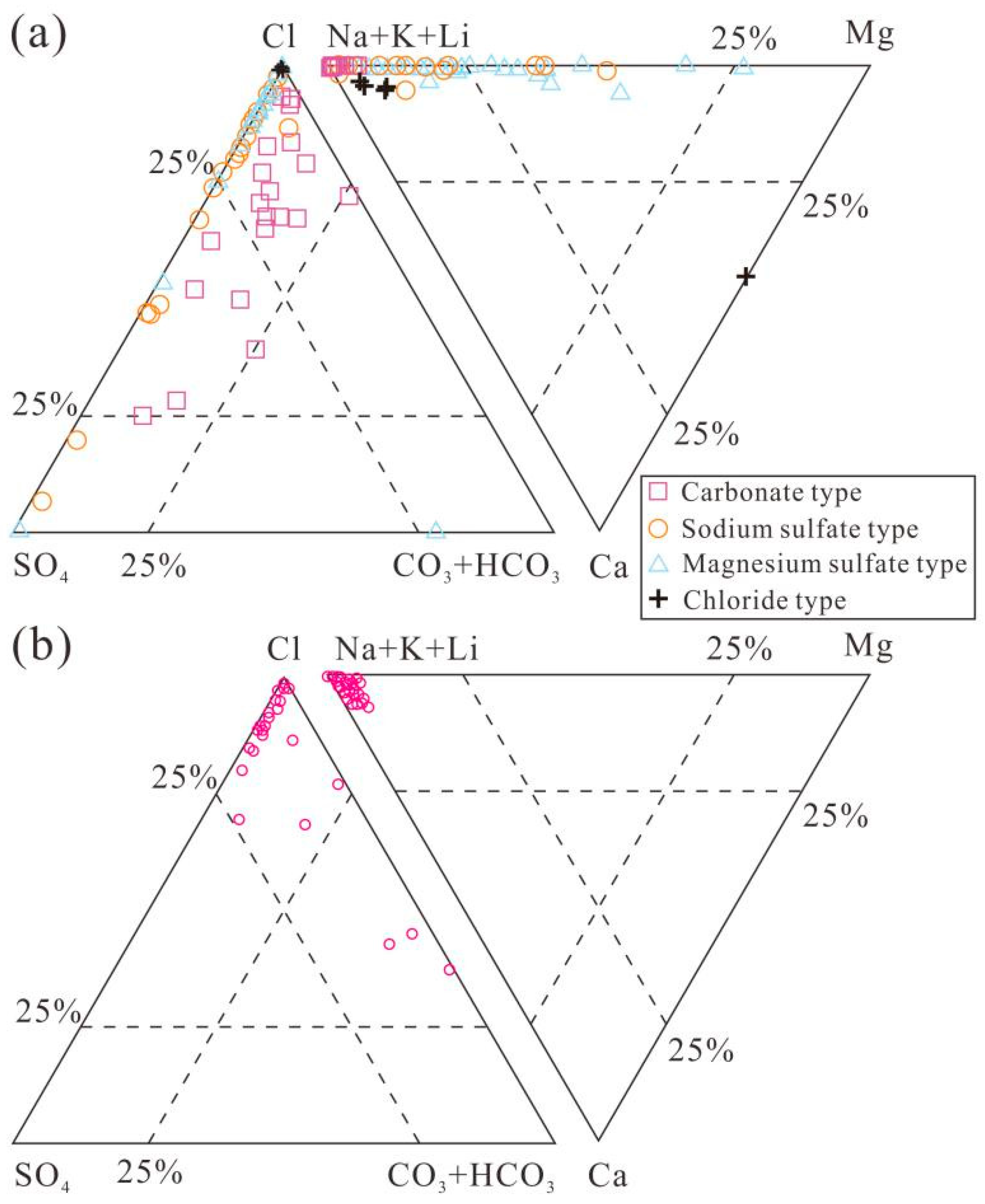
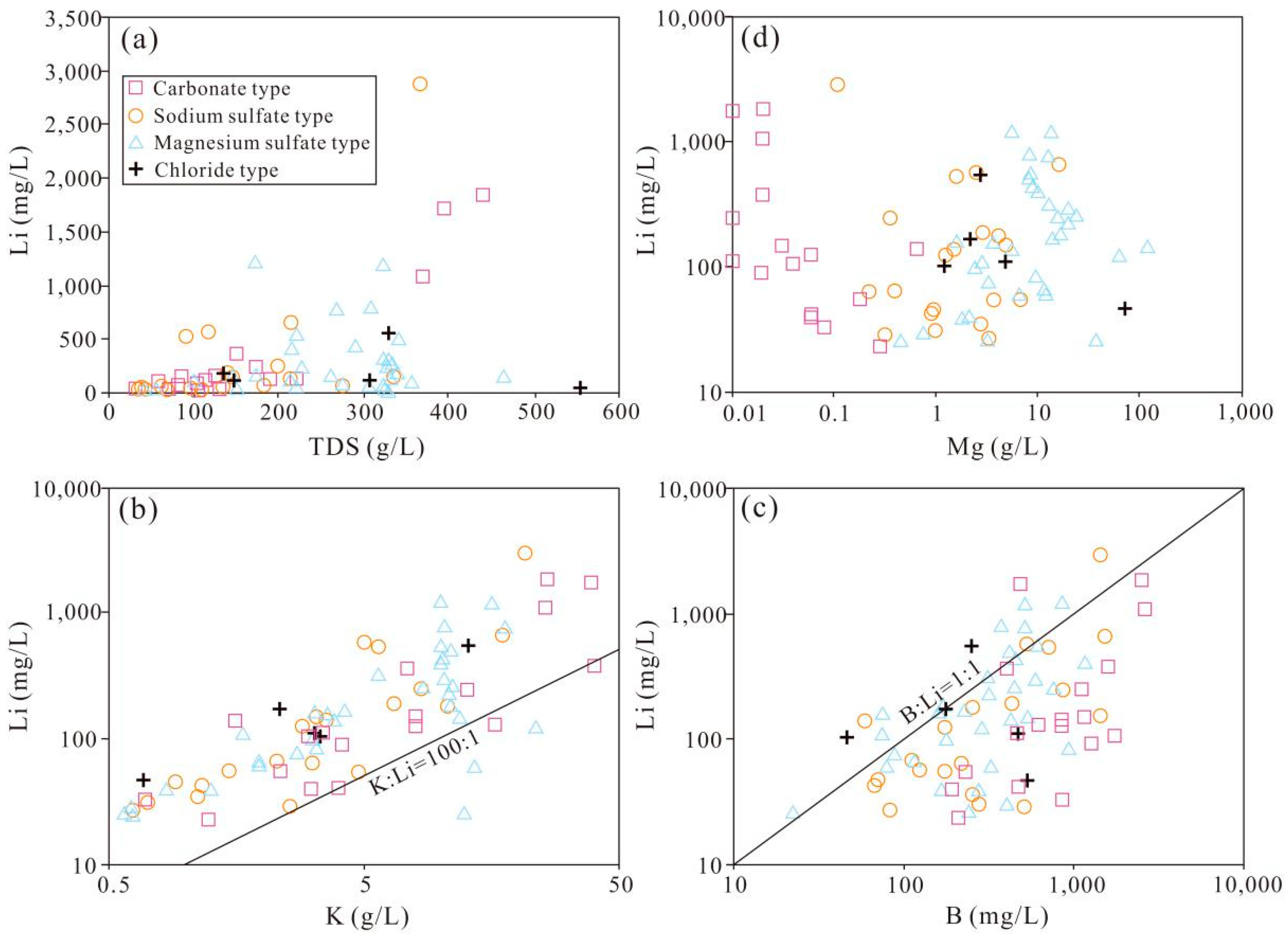
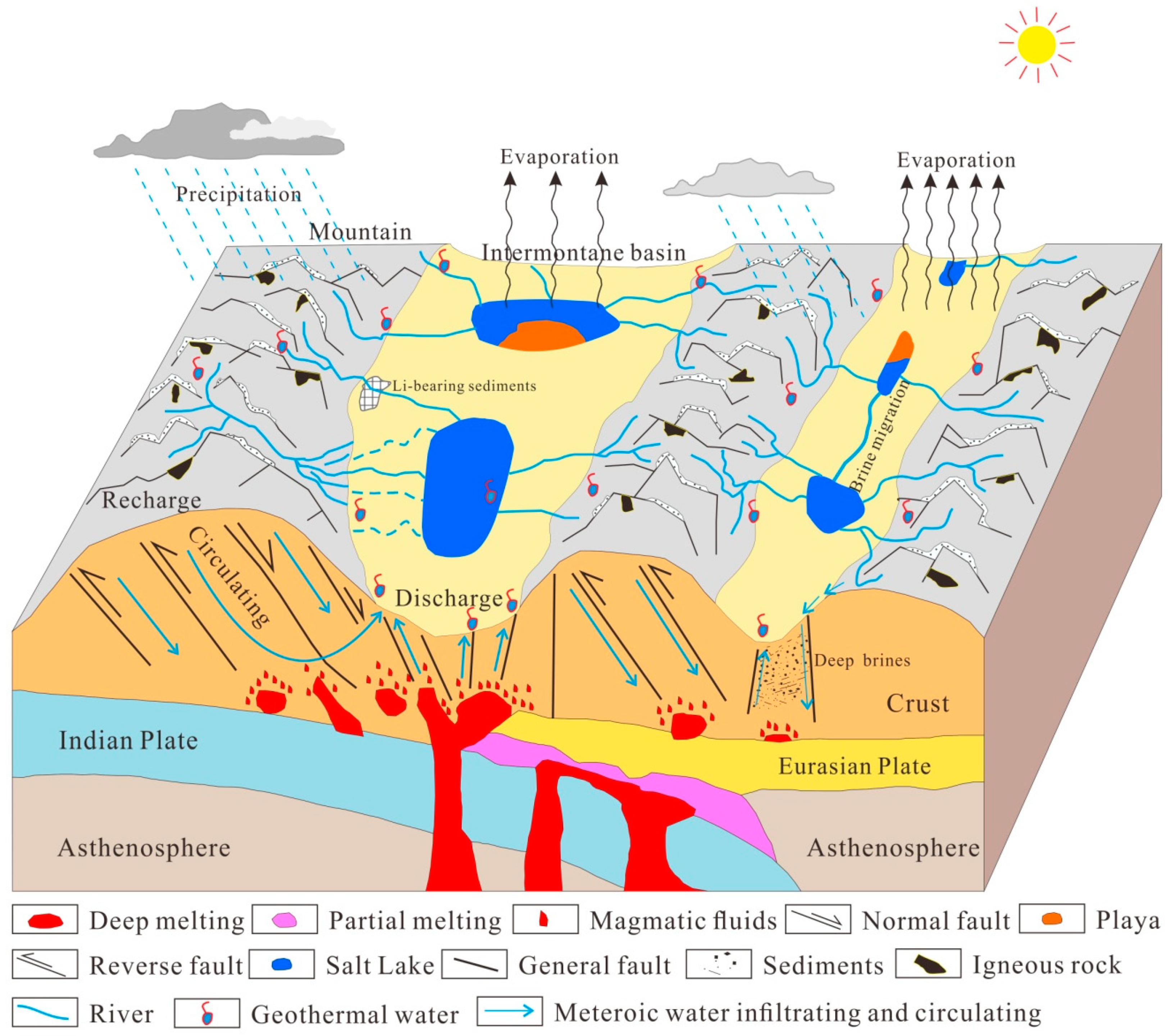
| No. | Name | Water Type | Sample Type 1 | Area | TDS | Na+ | K+ | Mg2+ | Ca2+ | Cl− | SO42− | HCO3− | CO32− | B3+ | Li+ | Mg/Li Ratio | Data Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (km2) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (g/L) | (mg/L) | (mg/L) | ||||||
| 1 | Bange Co | Carbonate | Inter-brine | 140 | 174 | 54 | 13 | 0 | 0 | 42 | 43 | 5 | 14 | 1110 | 245 | 0.0 | [14] |
| Surface brine | 222 | 62 | 8 | 0 | 0 | 40 | 1 | 12 | 7 | 849 | 127 | 0.4 | |||||
| 2 | Dujiali Lake | Carbonate | Surface brine | 80 | 114 | 41 | 3 | 0 | 0 | 16 | 43 | 3 | 6 | 461 | 112 | 0.0 | [14] |
| Inter-brine | 126 | 51 | 8 | 0 | 0 | 41 | 37 | 0 | 12 | 1150 | 150 | 0.2 | |||||
| 3 | Beilei Co | Carbonate | Surface brine | 20 | 131 | 45 | 3 | 0 | 0 | 59 | 16 | 4 | 3 | 195 | 40 | 1.6 | [14] |
| 4 | Gangtang Co | Carbonate | Surface brine | 11 | 72 | 25 | 4 | 0 | 0 | 39 | 2 | 0 | 2 | 480 | 41 | 1.5 | [14] |
| 5 | Pengyan Co | Carbonate | Surface brine | 40 | 96 | 35 | 2 | 0 | 0 | 48 | 8 | 3 | 1 | 75 | 254 | 0.1 | [30] |
| 6 | Jieze Chaka | Carbonate | Surface brine | 104 | 146 | 44 | 3 | 0 | 0 | 67 | 3 | 0 | 3 | 258 | 192 | 2.1 | [24] |
| 7 | Akesayi Lake | Carbonate | Surface brine | 164 | 56 | 20 | 1 | 1 | 0 | 32 | 2 | 2 | 0 | - | 94 | 5.9 | [14] |
| 8 | Aweng Co | Carbonate | Surface brine | 55 | 87 | 37 | 2 | 1 | 0 | 25 | 18 | 0 | 2 | 863 | 140 | 4.5 | [14] |
| 9 | Chalaka Co | Carbonate | Surface brine | 10 | 105 | 40 | 4 | 0 | 0 | 26 | 27 | 0 | 3 | 1270 | 90 | 0.3 | [14] |
| 10 | Caimaer Co | Carbonate | Surface brine | 32 | 191 | 61 | 16 | 0 | 0 | 90 | 17 | 0 | 6 | 625 | 130 | 0.0 | [14] |
| 11 | Zhabuye Lake | Carbonate | Surface brine | 250 | 414 | 130 | 41 | 0 | 0 | 156 | 39 | 0 | 44 | 3377 | 1048 | 0.0 | [51] |
| Inter-brine | 369 | 119 | 25 | 0 | 0 | 138 | 47 | 1 | 27 | 2616 | 1085 | 0.0 | [14] | ||||
| 12 | Dangxiong Co | Carbonate | Surface brine | 56 | 151 | 54 | 7 | 0 | 0 | 68 | 7 | 0 | 11 | 405 | 360 | 0.0 | [21] |
| 13 | Cona Co | Carbonate | Surface brine | 48 | 33 | 12 | 1 | 0 | 0 | 6.2 | 8 | 1 | 3 | 851 | 33 | 2.5 | [14] |
| 14 | Gangma Co | Carbonate | Surface brine | 13 | 81 | 29 | 2 | 0 | 0 | 34 | 10 | 0 | 4 | 230 | 56 | 3.3 | [14] |
| 15 | Nawu Co | Carbonate | Surface brine | 46 | 106 | 35 | 1 | 0 | 0 | 14 | 48 | 1 | 5 | 213 | 23 | 12.1 | [14] |
| 16 | Leen Co | Carbonate | Surface brine | 1 | 58 | 18 | 3 | 0 | 0 | 19 | 8 | 0 | 3 | 1762 | 106 | 0.4 | [14] |
| 17 | Zanzong Co | Sodium Sulfate | Surface brine | 8 | 413 | 95 | 17 | 3 | 0 | 162 | 106 | 0 | 2 | 952 | 2756 | 0.1 | [14] |
| 18 | Kongkong Chaka | Sodium Sulfate | Surface brine | 36 | 334 | 124 | 4 | 2 | 0 | 189 | 14 | 0 | 0 | 59 | 140 | 10.9 | [14] |
| 19 | Yibu Chaka | Sodium Sulfate | Surface brine | 100 | 97 | 34 | 1 | 1 | 0 | 43 | 17 | 0 | 0 | 68 | 43 | 21.0 | [14] |
| 20 | Angdaer Co | Sodium Sulfate | Surface brine | 45 | 181 | 69 | 3 | 0 | 0 | 92 | 8 | 1 | 6 | 219 | 64 | 3.4 | [14] |
| 21 | Rebang Co | Sodium Sulfate | Surface brine | 27 | 70 | 21 | 3 | 0 | 0 | 19 | 25 | 0 | 1 | 506 | 29 | 11.1 | [14] |
| 22 | Qia Chaka | Sodium Sulfate | Surface brine | 3 | 199 | 70 | 8 | 0 | 0 | 97 | 20 | 0 | 0 | 869 | 250 | 1.4 | [14] |
| 23 | Bieruoze Co | Sodium Sulfate | Surface brine | 40 | 145 | 32 | 3 | 5 | 0 | 4 | 65 | 2 | 0 | 1436 | 150 | 33.0 | [14] |
| 24 | Nieer Co | Sodium Sulfate | Surface brine | 33 | 215 | 40 | 17 | 16 | 0 | 92 | 43 | 0 | 0 | 1507 | 655 | 24.8 | [14] |
| 25 | Laguo Co | Sodium Sulfate | Surface brine | 86 | 91 | 27 | 6 | 2 | 0 | 42 | 12 | 0 | 1 | 714 | 530 | 3.0 | [14] |
| 26 | Chabo Co | Sodium Sulfate | Surface brine | 32 | 199 | 63 | 10 | 4 | 0 | 110 | 12 | 0 | 0 | 241 | 165 | 24.2 | [27] |
| 27 | Duoma Co | Sodium Sulfate | Surface brine | 18 | 117 | 33 | 5 | 3 | 0 | 72 | 2 | 0 | 0 | 529 | 570 | 4.4 | [14] |
| 28 | Dong Co | Sodium Sulfate | Surface brine | 100 | 140 | 24 | 6 | 3 | 0 | 25 | 37 | 0 | 1 | 433 | 190 | 15.4 | [14] |
| 29 | Buerga Co | Sodium Sulfate | Surface brine | 12 | 136 | 42 | 5 | 4 | 0 | 65 | 18 | 1 | 0 | 177 | 55 | 67.6 | [14] |
| 30 | Dawa Co | Sodium Sulfate | Surface brine | 110 | 36 | 8 | 1 | 1 | 0 | 4 | 20 | 0 | 0 | 272 | 31 | 32.2 | [14] |
| 31 | Dirangbi Co | Sodium Sulfate | Surface brine | 26 | 110 | 19 | 1 | 3 | 0 | 19 | 28 | 0 | 0 | 252 | 35 | 79.6 | [12] |
| 32 | Gemu Co | Sodium Sulfate | Surface brine | 76 | 275 | 105 | 2 | 0 | 0 | 160 | 6 | 0 | 0 | 111 | 65 | 6.0 | [52] |
| 33 | Jiaomu Chaka | Sodium Sulfate | Surface brine | 12 | 40 | 13 | 1 | 1 | 1 | 23 | 2 | 0 | 0 | 71 | 46 | 20.3 | [52] |
| 34 | Taoxing Co | Sodium Sulfate | Surface brine | 5 | 213 | 77 | 3 | 1 | 0 | 106 | 24 | 1 | 0 | 175 | 125 | 9.9 | [52] |
| 35 | Xin Co | Sodium Sulfate | Surface brine | 25 | 63 | 12 | 2 | 7 | 0 | 31 | 10 | 1 | 0 | 122 | 55 | 124.0 | [52] |
| 36 | Gaerkunsha Lake | Sodium Sulfate | Inter-brine | 2 | 365 | 118 | 21 | 0 | 3 | 187 | 28 | 0 | 0 | 1436 | 2895 | 0.0 | [14] |
| 37 | Yanjian Co | Sodium Sulfate | Surface brine | 5 | 42 | 9 | 1 | 3 | 1 | 13 | 15 | 1 | 0 | 83 | 27 | 123.6 | [14] |
| 38 | Maerguo Chaka | Magnesium Sulfate | Surface brine | 80 | 323 | 100 | 6 | 13 | 1 | 189 | 13 | 0 | 0 | 313 | 320 | 40.7 | [14] |
| 39 | Kangru Chaka | Magnesium Sulfate | Surface brine | 10 | 323 | 117 | 3 | 3 | 0 | 186 | 12 | 0 | 0 | 89 | 77 | 43.5 | [14] |
| 40 | Maergai Chaka | Magnesium Sulfate | Surface brine | 80 | 324 | 28 | 1 | 1 | 0 | 45 | 3 | 0 | 0 | 406 | 30 | 25.5 | [14] |
| 41 | Eya Co | Magnesium Sulfate | Surface brine | 4 | 150 | 35 | 7 | 12 | 0 | 82 | 7 | 0 | 0 | 205 | 180 | 67.0 | [19] |
| 42 | Biluo Co | Magnesium Sulfate | Surface brine | 24 | 323 | 90 | 14 | 12 | 0 | 194 | 11 | 0 | 0 | 328 | 61 | 199.1 | [14] |
| 43 | Rejue Chaka | Magnesium Sulfate | Surface brine | 19 | 146 | 49 | 4 | 4 | 0 | 85 | 5 | 0 | 0 | 75 | 160 | 23.4 | [14] |
| 44 | Daerwocuowen Co | Magnesium Sulfate | Surface brine | 45 | 136 | 45 | 2 | 4 | 1 | 80 | 6 | 0 | 0 | - | 64 | 55.2 | [14] |
| 45 | Yongbo Co | Magnesium Sulfate | Surface brine | 40 | 314 | 107 | 5 | 5 | 1 | 187 | 8 | 1 | 0 | 0 | 166 | 29.8 | [14] |
| 46 | Longmu Co | Magnesium Sulfate | Surface brine | 97 | 143 | 33 | 4 | 12 | 1 | 86 | 7 | 0 | 0 | 273 | 110 | 107.3 | [29] |
| 47 | Chana Co | Magnesium Sulfate | Surface brine | 4 | 329 | 85 | 10 | 20 | 0 | 183 | 26 | 0 | 0 | 600 | 300 | 67.1 | [14] |
| 48 | Zhacang Chaka | Magnesium Sulfate | Surface brine | 35 | 341 | 106 | 11 | 8 | 0 | 178 | 35 | 0 | 0 | 425 | 505 | 16.6 | [14] |
| Inter-brine | 221 | 42 | 10 | 9 | 0 | 86 | 18 | 1 | 0 | 615 | 553 | 15.4 | |||||
| Magnesium Sulfate | Surface brine | 60 | 290 | 61 | 10 | 9 | 0 | 111 | 19 | 0 | 0 | 453 | 436 | 21.1 | |||
| Inter-brine | 268 | 17 | 18 | 13 | 0 | 169 | 16 | 0 | 0 | 522 | 780 | 16.4 | |||||
| Magnesium Sulfate | Surface brine | 33 | 308 | 94 | 10 | 8 | 0 | 169 | 24 | 0 | 0 | 375 | 800 | 10.5 | |||
| Inter-brine | 323 | 88 | 16 | 14 | 0 | 172 | 29 | 0 | 0 | 522 | 1207 | 11.5 | |||||
| 49 | Mami Co | Magnesium Sulfate | Surface brine | 94 | 174 | 49 | 10 | 6 | 0 | 93 | 17 | 0 | 1 | 859 | 1227 | 4.6 | [14] |
| 50 | Yupan Co | Magnesium Sulfate | Surface brine | 20 | - | 120 | 2 | 7 | 0 | - | 8 | 1 | 0 | 1323 | 62 | 115.9 | [14] |
| 51 | Coni Co | Magnesium Sulfate | Surface brine | 67 | 57 | 19 | 1 | 2 | 1 | 31 | 8 | 0 | 0 | 276 | 40 | 52.5 | [12] |
| 52 | Baqian Co | Magnesium Sulfate | Surface brine | 15 | 216 | 58 | 10 | 10 | 1 | 123 | 7 | 0 | 0 | 1169 | 410 | 24.9 | [28] |
| 53 | Purang Chaka | Magnesium Sulfate | Surface brine | 35 | 340 | 96 | 11 | 17 | 0 | 186 | 28 | 2 | 0 | 170 | 187 | 91.9 | [52] |
| 54 | Bieletan playa | Magnesium Sulfate | Inter-brine | 1120 | 213 | 23 | 23 | 64 | 0 | 240 | 7 | 0 | 0 | 292 | 124 | 517.3 | [14] |
| 55 | Mahai Lake | Magnesium Sulfate | Inter-brine | 2000 | 465 | 112 | 12 | 122 | 1 | 304 | 30 | 0 | 0 | 535 | 150 | 810.5 | [14] |
| 56 | Kunteyi playa | Magnesium Sulfate | Inter-brine | 1680 | 329 | 54 | 12 | 38 | 7 | 217 | 1 | 0 | 0 | 243 | 27 | 1414.8 | [14] |
| 57 | Yiliping playa | Magnesium Sulfate | Inter-brine | 360 | 327 | 86 | 11 | 22 | 0 | 193 | 20 | 0 | 0 | 303 | 244 | 89.7 | [31] |
| 58 | Xitai Lake | Magnesium Sulfate | Inter-brine | 570 | 335 | 101 | 8 | 16 | 0 | 184 | 35 | 0 | 0 | 757 | 256 | 61.5 | [14] |
| 59 | Dongtai Lake | Magnesium Sulfate | Surface brine | 201 | 332 | 117 | 4 | 6 | 0 | 187 | 18 | 0 | 0 | 428 | 141 | 40.3 | [14] |
| 60 | Da Qaidam Lake | Magnesium Sulfate | Surface brine | 240 | 274 | 88 | 3 | 10 | 1 | 156 | 17 | 0 | 0 | 939 | 85 | 114.2 | [14] |
| 61 | Xiaoqaidam Lake | Magnesium Sulfate | Surface brine | 152 | 350 | 106 | 3 | 13 | 0 | 184 | 32 | 0 | 0 | - | 36 | 374.4 | [14] |
| 62 | Yanhu Lake | Magnesium Sulfate | Surface brine | 32 | 221 | 72 | 2 | 7 | 1 | 123 | 16 | 1 | 0 | 80 | 62 | 107.7 | [53] |
| 63 | Xijinwulan Lake | Magnesium Sulfate | Surface brine | 346 | 357 | 93 | 3 | 3 | 0 | 152 | 4 | 0 | 0 | 180 | 101 | 24.6 | [14] |
| 64 | Caiduo Chaka | Magnesium Sulfate | Surface brine | 44 | 151 | 57 | 1 | 2 | 1 | 76 | 13 | 1 | 0 | 168 | 40 | 46.4 | [12] |
| 65 | Mang Co | Magnesium Sulfate | Surface brine | 12 | 101 | 33 | 2 | 3 | 0 | 56 | 7 | 0 | 1 | 75 | 111 | 26.5 | [52] |
| 66 | Qiagang Co | Magnesium Sulfate | Surface brine | 20 | 42 | 15 | 1 | 1 | 0 | 24 | 2 | 0 | 0 | 22 | 26 | 17.3 | [52] |
| 67 | Zhaqiongema Co | Magnesium Sulfate | Surface brine | 10 | 262 | 97 | 3 | 2 | 0 | 146 | 14 | 1 | 0 | 161 | 164 | 9.9 | [52] |
| 68 | Duoxiu Lake | Magnesium Sulfate | Surface brine | 5 | 306 | 102 | 2 | 12 | 1 | 182 | 8 | 0 | 0 | 116 | 67 | 173.9 | [54] |
| 69 | Gas Hur Lake | Magnesium Sulfate | Surface brine | 103 | 333 | 77 | 5 | 30 | 0 | 176 | 45 | 0 | 0 | 108 | 25 | 1190.8 | [14] |
| 70 | Niulangzhinv Lake | Chloride | Surface brine | 30 | 555 | 0 | 1 | 72 | 100 | 382 | 0 | 0 | 0 | 535 | 47 | 1549.4 | [14] |
| 71 | Lexiewudan Lake | Chloride | Surface brine | 227 | 90 | 30 | 2 | 1 | 2 | 54 | 1 | 0 | 0 | 79 | 103 | 11.2 | [55] |
| 72 | Duogecuoren Co | Chloride | Surface brine | 260 | 148 | 53 | 3 | 1 | 12 | 86 | 2 | 0 | 0 | 47 | 104 | 11.9 | [38] |
| 73 | Queer Chaka | Chloride | Surface brine | 8 | 330 | 110 | 13 | 3 | 5 | 195 | 1 | 1 | 0 | 251 | 548 | 5.0 | [52] |
| 74 | Goulu Co | Chloride | Surface brine | 35 | 308 | 105 | 3 | 5 | 6 | 188 | 1 | 0 | 0 | 475 | 111 | 43.4 | [53] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Fan, Q.; Wang, J.; Qin, Z.; Zhang, X.; Wei, H.; Du, Y.; Shan, F. Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau. Minerals 2019, 9, 528. https://doi.org/10.3390/min9090528
Li Q, Fan Q, Wang J, Qin Z, Zhang X, Wei H, Du Y, Shan F. Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau. Minerals. 2019; 9(9):528. https://doi.org/10.3390/min9090528
Chicago/Turabian StyleLi, Qingkuan, Qishun Fan, Jianping Wang, Zhanjie Qin, Xiangru Zhang, Haicheng Wei, Yongsheng Du, and Fashou Shan. 2019. "Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau" Minerals 9, no. 9: 528. https://doi.org/10.3390/min9090528
APA StyleLi, Q., Fan, Q., Wang, J., Qin, Z., Zhang, X., Wei, H., Du, Y., & Shan, F. (2019). Hydrochemistry, Distribution and Formation of Lithium-Rich Brines in Salt Lakes on the Qinghai-Tibetan Plateau. Minerals, 9(9), 528. https://doi.org/10.3390/min9090528






