Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores
Abstract
:1. Introduction
- REE-bearing minerals occur in many mineral classes, including REE-phosphates, REE-carbonates, REE-halogenides, REE-oxides, REE-silicates, REE-arsenates, as well as combinations of it as REE-fluoro-carbonates [24].
- Many REE minerals have a complex mineral chemistry with light REE (LREE, elements La to Eu), heavy REE (HREE, elements Gd to Lu), Th, U, Si, Al, Ca, F, CO3, PO4, Nb, Y, As, S and others.
- Most of the REE minerals are solid solutions with single and coupled substitution involving LREE, HREE, Y, Si, Al, Ca, F, P, Nb, Th, U and others. Compositional variations are widespread.
- Many REE minerals are hydrated phases. This considerably hinders their identification by chemical analysis.
- The often complex and multistage geological processes of REE enrichment lead to mineral intergrowths, pseudomorphs, partial and complete replacement, hydration and dehydration. This often results in a complex REE mineral assemblage.
2. Approach and Analytical Methods
3. Energy-Dispersive X-ray (EDX) Spectra of Rare Earth Element (REE)-Bearing Minerals
- During a first automated SEM-MLA measurement, all EDX spectra in a given sample are captured (XBSE-STD measurement mode). The discrimination of these spectra is provided by a high reliability value of 1℮−10 which means a high degree of conformance. The spectra that fall within this limit of conformance receive consecutive numbers (Mineral 1, Mineral 2, …). Dependent on the mineralogical diversity of the ore, ca. 100–150 different EDX spectra can be collected from REE-bearing and gangue minerals (e.g., carbonates and silicates). A certain fraction of these spectra will be from grain boundaries or artefacts of preparation effects. These can be ignored during further assessment by a tentative classification of the measurement.
- The MLA software functions allow driving the SEM stage to the mineral grains (e.g., Mineral 1, Mineral 2, …) where the consecutively numbered spectra were obtained for the first time during the measurement. A quantitative element analysis by EDS is performed from these grains. The EDX spectra from gangue minerals can then be labelled by corresponding mineral names (e.g., calcite, dolomite, fluorite, …). It is recommended labelling several slightly differing spectra from the same gangue minerals (e.g., calcite1, calcite-mix).
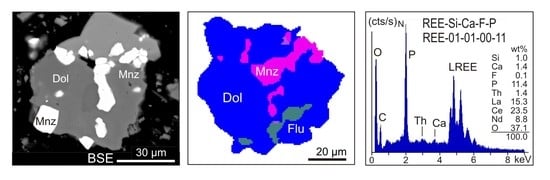
- The EDX spectra from REE-bearing mineral grains receive a generic label that matches the normalised results of EDS element analysis. An EDX spectrum from an REE-bearing mineral suggesting e.g., 3.9 wt % Si, 4.7 wt % Ca, 14.3 wt % F and 2.4 wt % P (when totals are normalised to 100) will be labelled as REE-04-05-14-02 (Figure 1g–i). The range of the labelled elements should be the same for all REE-bearing minerals in a particular study, e.g., REE-Si-Ca-F-P, to assure consistency and comparability. When P and F are purposefully positioned at the end of the label, this will facilitate the subsequent step of spectra grouping (see below). Due to the carbon coating and the analytical uncertainty related to peak interferences, the elements C and the REE are not used in this generic labelling process. In a similar manner EDX spectra from REE-bearing minerals with Y, Nb or further elements such as As can also be labelled. A similar approach has previously been applied to automated SEM-MLA measurements of sewage sludge ashes [29], soils [30] or to zoned metamorphic garnets [31].
- A labelling of the EDX spectra based on quantitative EDS analysis of the REE is not reasonable because the relative REE concentrations have only secondary relevance for the distinction of mineral classes. Also, a considerable analytical uncertainty is caused by the REE peak interferences, which could yield erroneous absolute concentrations of REE. Therefore, when the totals are normalised to 100 wt %, the analysis of Si, Ca, F and P will provide at best the relative proportions of these elements in a REE bearing mineral grain.
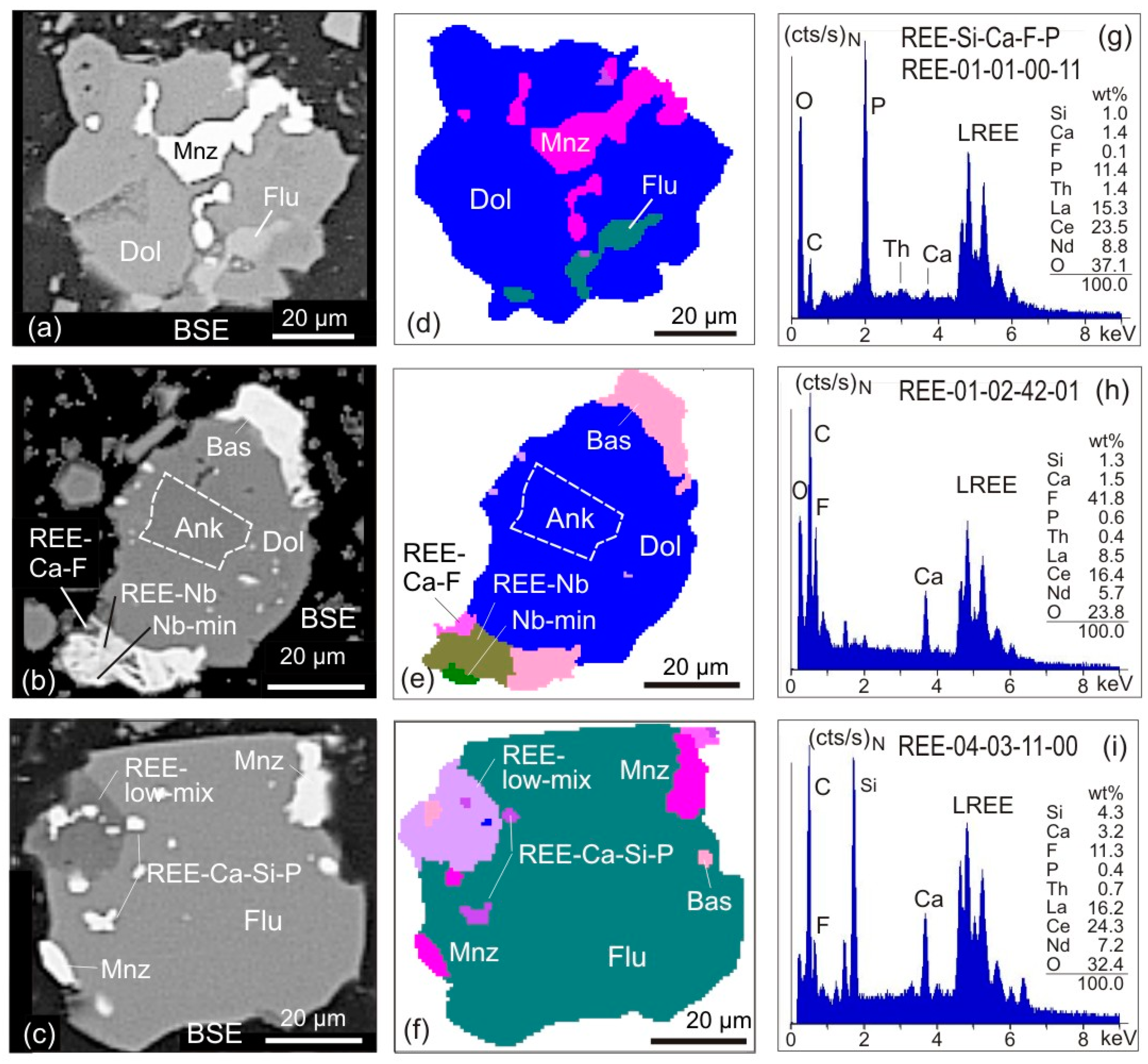
- EDX spectra from REE-bearing minerals with high content of P are summarised under the group REE-P-monazite. A typical mineral of this group would be monazite.
- EDX spectra from REE-bearing minerals with low content of P but elevated contents of Si and Ca are summarised as the REE-Ca-Si-P group. A typical mineral of this group would be britholite.
- The EDX spectra from REE-bearing minerals without P but high contents of Ca and F, and intermediate to low contents of Si are summarised as the REE-Ca-F group. A typical mineral of this group would be synchysite.
- The EDX spectra from REE-bearing minerals with dominant F at low Ca and Si concentrations are combined as the REE-F group. Typical minerals of this group are bastnaesite and parisite. The element carbon cannot be used for labelling due to the carbon-coating of the samples.
- EDX spectra from grains with detectable but low REE contents are merged to form the REE-Low-Mix group. In contrast to the groups 1–4 with high cts/s on the numerous lines of REE and corresponding elevated element contents, the REE-Low-Mix group integrates spectra with low counts on the REE lines and apparently low REE contents. Minerals containing low REE concentrations (for example allanite) can generate such spectra, but similar may be true for small acicular and fibrous crystals of REE minerals enclosed in gangue minerals. In the latter case, the excitation bulb of the electron beam captures gangue minerals beside and below, which will led to such mixed spectra, with low counts on the REE.
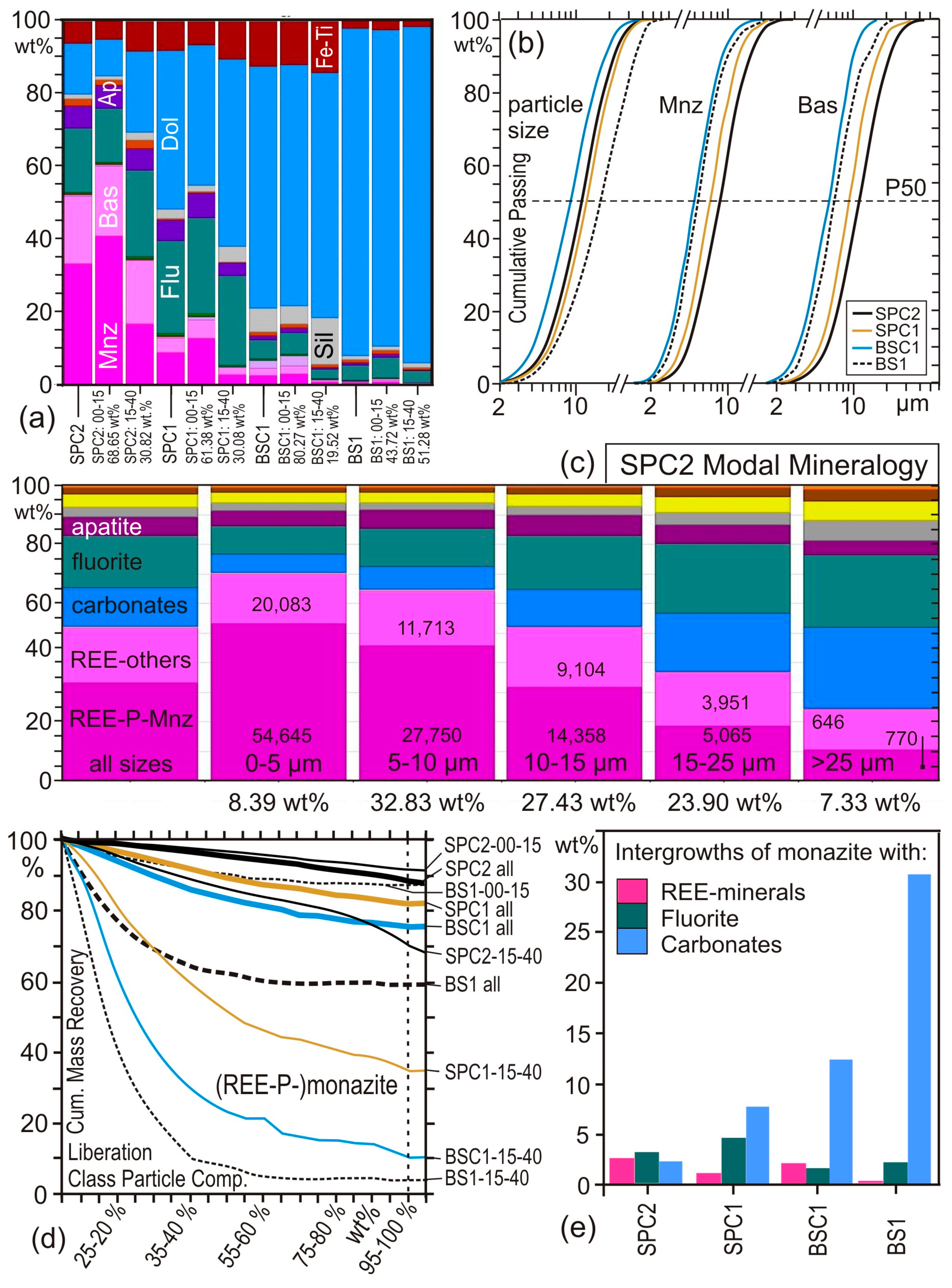
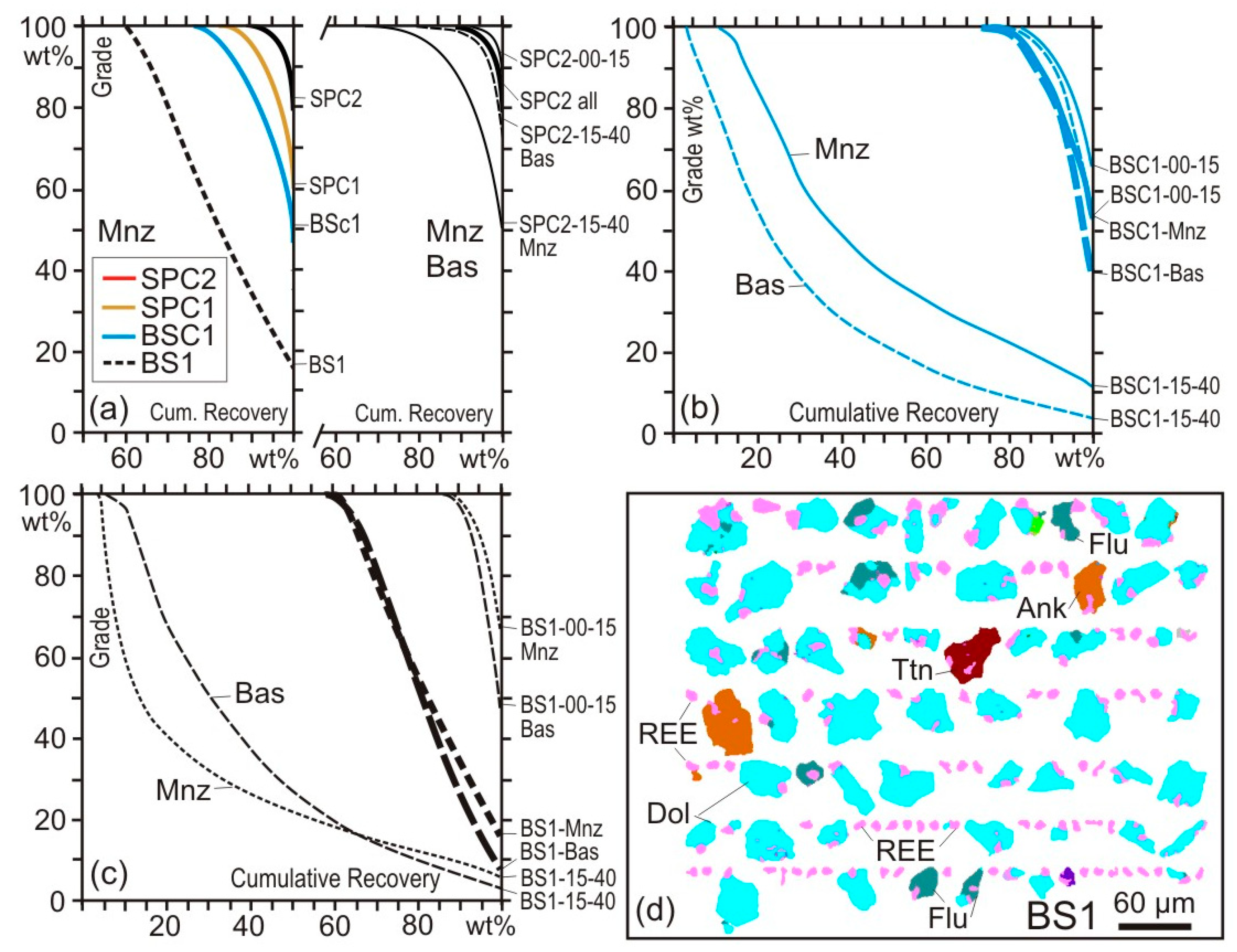
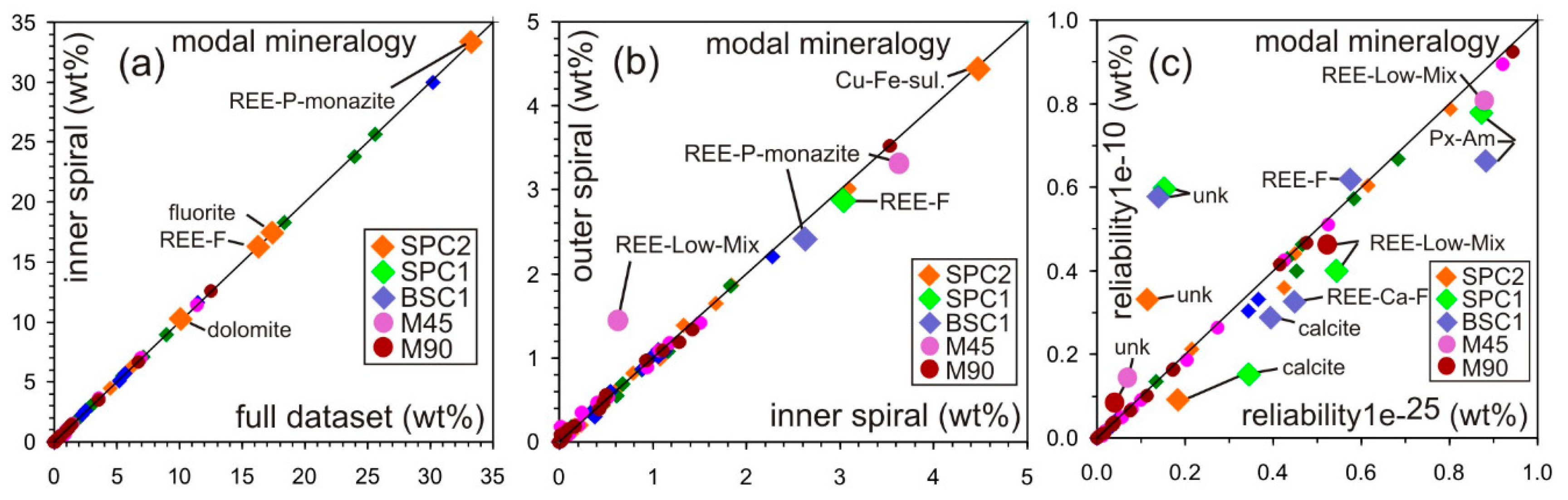
4. Case Studies
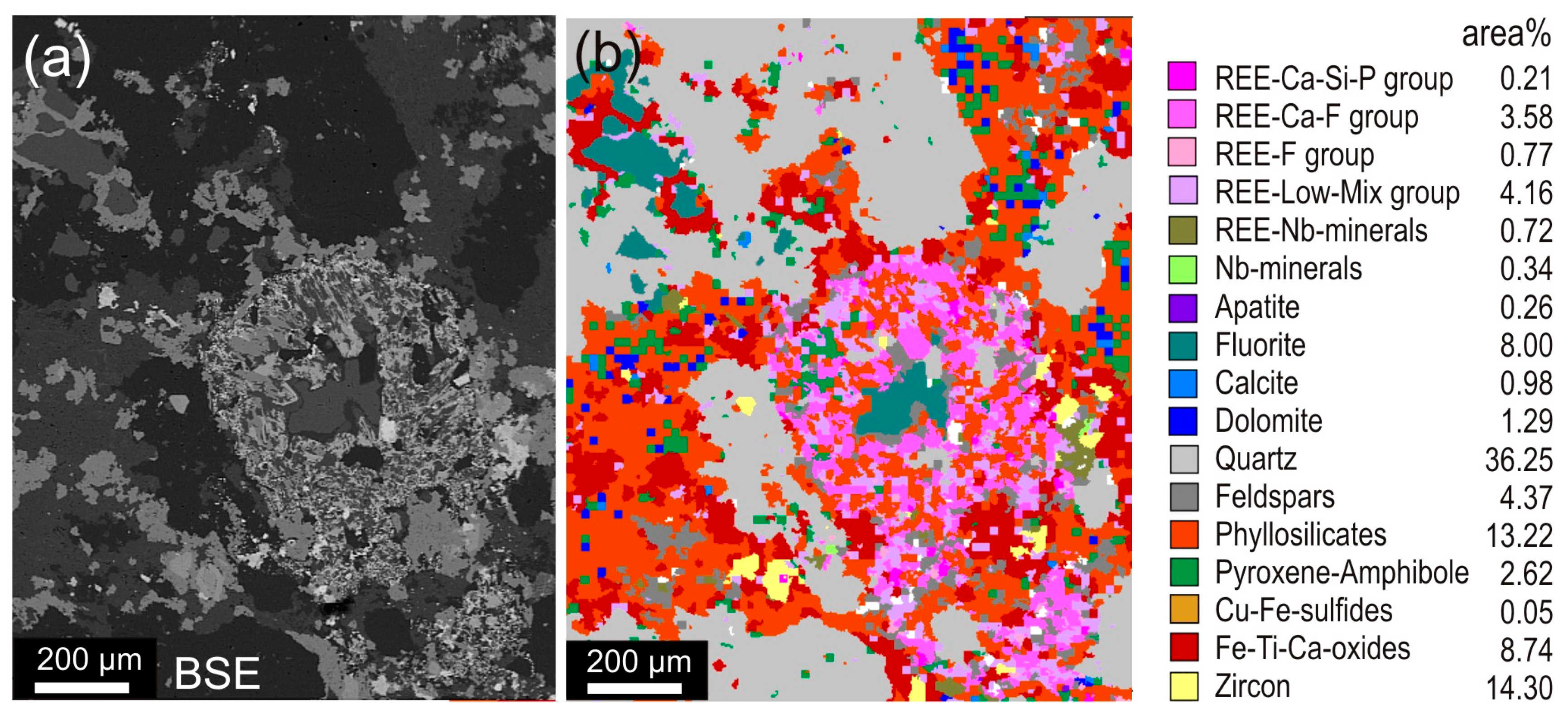
4.1. Case Study 1: Run-of-Mine Ore
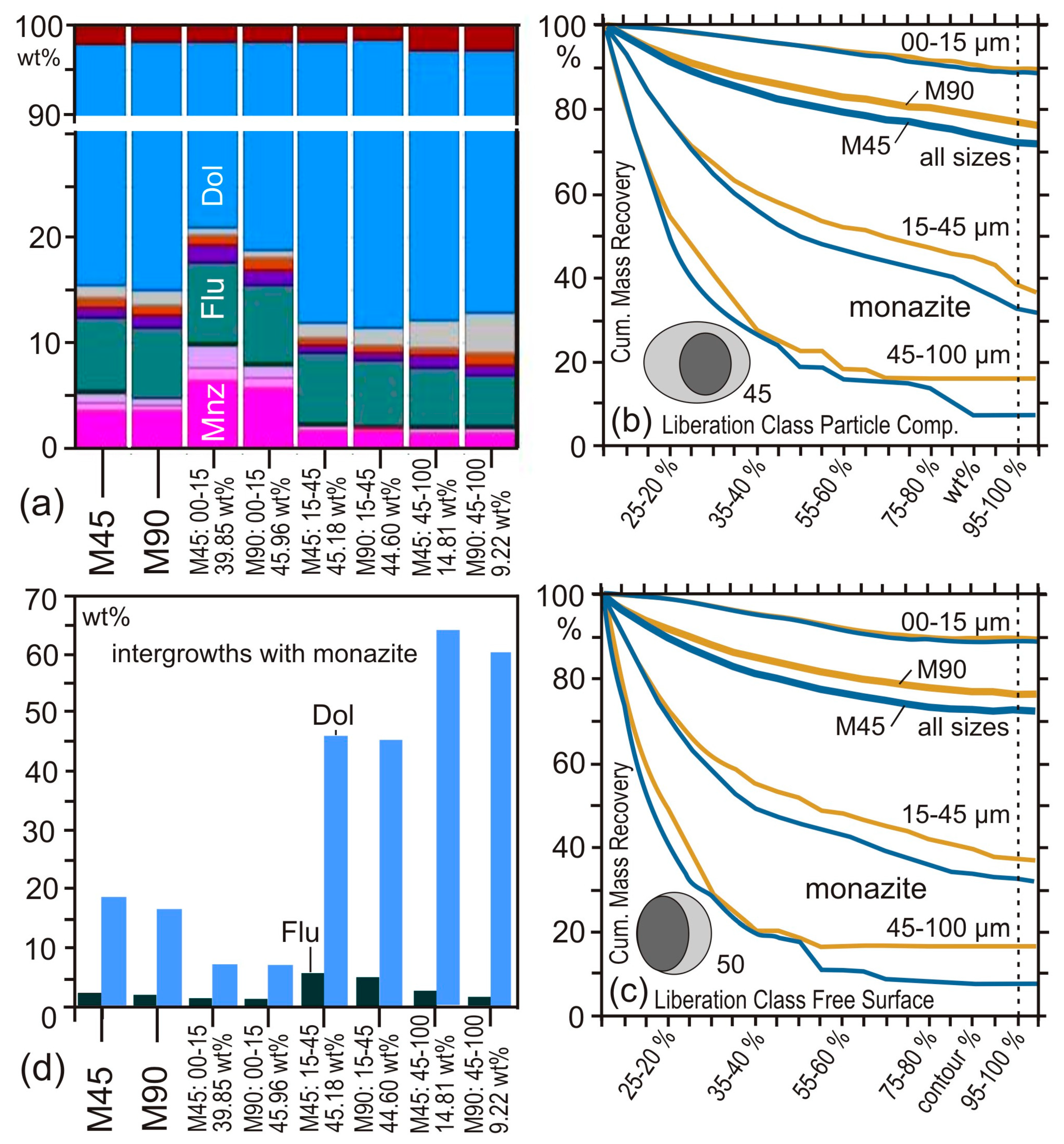
4.2. Case Study 2: Comminution
4.3. Case Study 3: Flotation
5. Analytical Uncertainties
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chakhmouradian, A.R.; Wall, F. Rare earth elements: Minerals, mines, magnets (and more). Elements 2012, 8, 333–340. [Google Scholar] [CrossRef]
- Guarino, V.; de’ Gennaro, R.; Melluso, L.; Ruberti, E.; Azzone, R.G. The transition from miaskitic to agpaitic rocks as marked by the accessory mineral assemblages, in the Passa Quatro alkaline complex (southeastern Brazil). Can. Mineral. 2019, 57, 1–23. [Google Scholar] [CrossRef]
- Guarino, V.; Wu, F.Y.; Melluso, L.; Gomes, C.B.; Tassinari, C.C.G.; Ruberti, E.; Brilli, M. U-Pb ages, geochemistry, C-O-Nd-Sr-Hf isotopes and petrogenesis of the Catalão II carbonatitic complex (Alto Paranaíba Igneous Province, Brazil): Implications for regional-scale heterogeneities in the Brazilian carbonatite associations. Int. J. Earth Sci. 2017, 106, 1963–1989. [Google Scholar] [CrossRef]
- Melluso, L.; Guarino, V.; Lustrino, M.; Morra, V.; de’ Gennaro, R. The REE- and HFSE-bearing phases in the Itatiaia alkaline complex (Brazil), and geochemical evolution of feldspar-rich felsic melts. Mineral. Mag. 2017, 81, 217–250. [Google Scholar] [CrossRef]
- Marks, M.A.W.; Markl, G. A global review of agpaitic rocks. Earth-Sci. Rev. 2017, 173, 229–258. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Zaitsev, A.N. Rare earth mineralization in igneous rocks: Sources and processes. Elements 2012, 8, 347–353. [Google Scholar] [CrossRef]
- Williams-Jones, A.E.; Migdisov, A.A.; Samson, I.M. Hydrothermal mobilisation of the rare earth elements—a tale of “Ceria” and “Yttria”. Elements 2012, 8, 355–360. [Google Scholar] [CrossRef]
- Richter, L.; Diamond, L.W.; Atanasova, P.; Banks, D.A.; Gutzmer, J. Hydrothermal formation of heavy rare earth element (HREE)–xenotime deposits at 100 °C in a sedimentary basin. Geology 2018, 46, 263–266. [Google Scholar] [CrossRef]
- Jones, A.P.; Wall, F.; Williams, C.T. Rare Earth Minerals: Chemistry, Origin and Ore Deposits; The Mineralogical Society Series 7; Chapmann & Hall: London, UK, 1996; p. 372. [Google Scholar]
- Yang, K.F.; Fan, H.R.; Santosh, M.; Hu, F.F.; Wang, K.Y. Mesoproterozoic carbonatitic magmatism in the Bayan Obo deposit, Inner Mongolia, North China: Constraints for the mechanism of super accumulation of rare earth elements. Ore Geol. Rev. 2011, 40, 122–131. [Google Scholar] [CrossRef]
- Long, K.R.; Van Gosen, B.R.; Foley, N.K.; Cordier, D. The Principal Rare Earth Elements Deposits of The UNITED STATES—A Summary of Domestic Deposits and a Global Perspective; USGS Scientific Investigation Report 2010–5220; USGS: Reston, VA, USA, 2010; p. 96. Available online: https://pubs.usgs.gov/sir/2010/5220/ (accessed on 30 August 2019).
- Hatch, G.P. Rare Earth Elements: Dynamics in the Global Market for Rare Earths. Elements 2012, 8, 341–346. [Google Scholar] [CrossRef]
- Mariano, A.N.; Mariano, A., Jr. Rare earth elements: Rare earth mining and exploration in North America. Elements 2012, 8, 369–376. [Google Scholar] [CrossRef]
- Kynicky, J.; Smith, M.P.; Xu, C. Rare earth elements: Diversity of rare earth deposits: The key example of China. Elements 2012, 8, 361–367. [Google Scholar] [CrossRef]
- Reisman, D.; Weber, R.; McKernan, J.; Northeim, C. Rare Earth Elements: A Review of Production, Processing, Recycling, and Associated Environmental Issues; EPA Report; EPA/600/R-12/572; U.S. Environmental Protection Agency (EPA): Washington, DC, USA, 2013. Available online: https://nepis.epa.gov/Adobe/PDF/P100EUBC.pdf (accessed on 30 August 2019).
- Weng, Z.H.; Jowitt, S.M.; Mudd, G.M.; Haque, N. Assessing rare earth element mineral deposit types and links to environmental impacts. Appl. Earth Sci. 2013, 122, 83–96. [Google Scholar] [CrossRef]
- Dutta, T.; Kim, K.-H.; Uchimiya, M.; Kwonc, E.E.; Jeon, B.-H.; Deep, A.; Yun, S.-T. Global demand for rare earth resources and strategies for green mining. Environ. Res. 2016, 150, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Wall, F.; Rollat, A.; Pell, R.S. Responsible sourcing of critical metals. Elements 2017, 13, 313–318. [Google Scholar] [CrossRef]
- Rollinson, H. Using Geochemical Data: Evaluation, Presentation Interpretation; Longman Group: Harlow, UK, 1993; p. 354. [Google Scholar]
- Gu, Y. Automated scanning electron microscope based mineral liberation analysis. An introduction to JKMRC/FEI Mineral Liberation Analyser. J. Miner. Mater. Character. Eng. 2003, 2, 33–41. [Google Scholar] [CrossRef]
- Fandrich, R.; Gu, Y.; Burrows, D.; Moeller, K. Modern SEM-based mineral liberation analysis. Int. J. Miner. Process. 2007, 84, 310–320. [Google Scholar] [CrossRef]
- Lastra, R. Seven practical application cases of liberation analysis. Int. J. Miner. Process. 2007, 84, 337–347. [Google Scholar] [CrossRef]
- Petruk, W. Applied Mineralogy in the Mining Industry, 1st ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; p. 288. [Google Scholar]
- Chang, L.L.A.; Howie, R.A.; Zussman, J. Rock-Forming Minerals, 2nd ed.; Non-silicates: Sulphates, carbonates, phosphates, halides; Geological Society London: London, UK, 1998; Volume 5B, p. 383. [Google Scholar]
- Thompson, W.; Lombard, A.; Santiago, E.; Singh, A. Mineralogical studies in assisting benefication of rare earth element minerals from carbonatite deposits. In Proceedings of the 10th International Congress for Applied Mineralogy (ICAM), Trondheim, Norway, 1–5 August 2011; Springer: Berlin/Heidelberg, Germany, 2012; pp. 665–672, ISBN 9788273851390. [Google Scholar]
- Smythe, D.M.; Lombard, A.; Coetzee, L.L. Rare earth element deportment studies utilising QEMSCAN technology. Miner. Eng. 2013, 52, 52–61. [Google Scholar] [CrossRef]
- Smith, D.G.W. Identification of rare earth and yttrium minerals using the minident database. In Russel, P.E. Microbeam Analysis. In Proceedings of the 24th Annual Conference of the Microbeam Analysis Society, Asheville, NC, USA, 16–21 July 1989; pp. 559–562. [Google Scholar]
- Smith, D.G.W.; de St. Jorre, L. The MinIdent Data Base-examples of applications to the Thor Lake rare metal deposits. In Proceedings of the 14th General meeting of International Mineralogical Association, Stanford, CA, USA, 13–18 July 1986; p. 234. [Google Scholar]
- Greb, V.G.; Guhl, A.; Weigand, H.; Schulz, B.; Bertau, M. Understanding phosphorous phases in sewage sludge ashes: A wet-process investigation coupled with automated mineralogy analysis. Miner. Eng. 2016, 99, 30–39. [Google Scholar] [CrossRef]
- Pietranik, A.; Kierczak, J.; Tyszka, R.; Schulz, B. Understanding heterogeneity of a slag derived technosol: The role of automated SEM-EDS analyses. Minerals 2018, 8, 513. [Google Scholar] [CrossRef]
- Schulz, B. Polymetamorphism in garnet micaschists of the Saualpe Eclogite Unit (Eastern Alps, Austria), resolved by automated SEM methods and EMP-Th-U-Pb monazite dating. J. Metamorph. Geol. 2017, 35, 141–163. [Google Scholar] [CrossRef]
- Merker, R.G.; Leissner, T.; Schulz, B. Use of virtual fractions for MLA of Y-bearing REE ores. In Proceedings of the 28th International Mineral Processing Congress 2016, Québec, QC, Canada, 11–15 September 2016. Conference Proceedings Paper ID 894. [Google Scholar]
- Merker, R.G.; Smith, D.L. Rare earths—espects of market and beneficiation. World Metall.-Erzmetall. 2018, 71, 189–197. [Google Scholar]
- Pinckston, D.R.; Smith, D.G.W. Mineralogy of the Lake zone, Thor Lake rare-metals deposit, N.W.T., Canada. Can. J. Earth Sci. 1995, 32, 516–532. [Google Scholar] [CrossRef]
- Sheard, E.R.; Williams-Jones, A.E.; Heiligmann, M.; Pederson, C.; Trueman, D.L. Controls on the concentration of zirconium, niobium, and the rare earth elements in the Thor Lake rare metal deposit, Northwest Territories, Canada. Econ. Geol. 2012, 107, 81–104. [Google Scholar] [CrossRef]
- Jackson, B.R.; Reid, A.F.; Wittemberg, J.C. Rapid production of high quality polished sections for automated image analysis of minerals. Proc. Australas. Inst. Miner. Metall. 1984, 289, 93–97. [Google Scholar]
- Echlin, P. Handbook of Sample Preparation for Scanning Electron Microscopy and X-ray Microanalysis, 1st ed.; Springer Science: Berlin/Heidelberg, Germany, 2009; p. 326. [Google Scholar] [CrossRef]
- Finch, J.A.; Gomez, C.O. Separability curves from image analysis data (Technical Note). Miner. Eng. 1989, 2, 565–568. [Google Scholar] [CrossRef]
- Leistner, T.; Peuker, U.A.; Rudolph, M. How gangue particle size can affect the recovery of ultrafine particles during froth flotation. Miner. Eng. 2017, 9, 1–9. [Google Scholar] [CrossRef]
- Lastra, R.; Petruk, W. Mineralogical characterization of sieved and un-sieved samples. J. Miner. Mater. Charact. Eng. 2014, 2, 40–48. [Google Scholar] [CrossRef]
- Neethling, S.J.; Cilliers, J.J. Grade-recovery curves: A new approach for analysis of and predicting from plant data. Miner. Eng. 2012, 36, 105–110. [Google Scholar] [CrossRef]
- Sandmann, D.; Schulz, B.; Gutzmer, J.; Eickhoff, A. Controls on the Industrial Regrind Milling Process of Coarse-Grained Ores by SEM-Based Image Analysis; Abstracts 89. In Proceedings of the DGK, DMG & +MG, Salzburg, Austria, 20–24 September 2011; p. 131. [Google Scholar]
- Leigh, G.M.; Sutherland, D.N.; Gottlieb, P. Confidence limits for liberation measurements. Miner. Eng. 1993, 2, 155–161. [Google Scholar] [CrossRef]

| MLA-Group | Mineral | General Formula | Dens. | ∑REE | P | Ca | Si | C | F | O |
|---|---|---|---|---|---|---|---|---|---|---|
| REE-P-monazite | monazite | (LREE,Y,Th,Si,Ca)PO4 | 5.10 | 59.49 | 13.21 | 0.00 | 0.00 | 0.00 | 0.00 | 27.29 |
| REE-Al-P-phases | florencite | (LREE)Al3(PO4)2(OH)6 | 3.58 | 27.31 | 12.07 | 0.00 | 0.00 | 0.00 | 0.00 | 43.66 |
| REE-Ca-Si-P-phases | britholite | (LREE,Ca)5(SiO4,PO4)3(OH,F) | 4.45 | 46.44 | 2.00 | 14.70 | 9.40 | 0.00 | 0.50 | 26.85 |
| REE-Ca-F-phases | synchysite | (LREE,Ca)(CO3)2F | 4.02 | 43.89 | 0.00 | 12.56 | 0.00 | 7.53 | 5.95 | 30.07 |
| REE-F-phases | bastnaesite | (LREE)(CO3)F | 4.97 | 63.94 | 0.00 | 0.00 | 0.00 | 5.48 | 8.67 | 21.90 |
| REE-F-phases | fluocerite | (LREE)F3 | 6.13 | 71.07 | 0.00 | 0.00 | 0.00 | 0.00 | 28.93 | 0.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, B.; Merker, G.; Gutzmer, J. Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores. Minerals 2019, 9, 527. https://doi.org/10.3390/min9090527
Schulz B, Merker G, Gutzmer J. Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores. Minerals. 2019; 9(9):527. https://doi.org/10.3390/min9090527
Chicago/Turabian StyleSchulz, Bernhard, Gerhard Merker, and Jens Gutzmer. 2019. "Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores" Minerals 9, no. 9: 527. https://doi.org/10.3390/min9090527
APA StyleSchulz, B., Merker, G., & Gutzmer, J. (2019). Automated SEM Mineral Liberation Analysis (MLA) with Generically Labelled EDX Spectra in the Mineral Processing of Rare Earth Element Ores. Minerals, 9(9), 527. https://doi.org/10.3390/min9090527