Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications
Abstract
:1. Introduction
2. Brief Review of Early Paleozoic Climate and Glendonite Findings
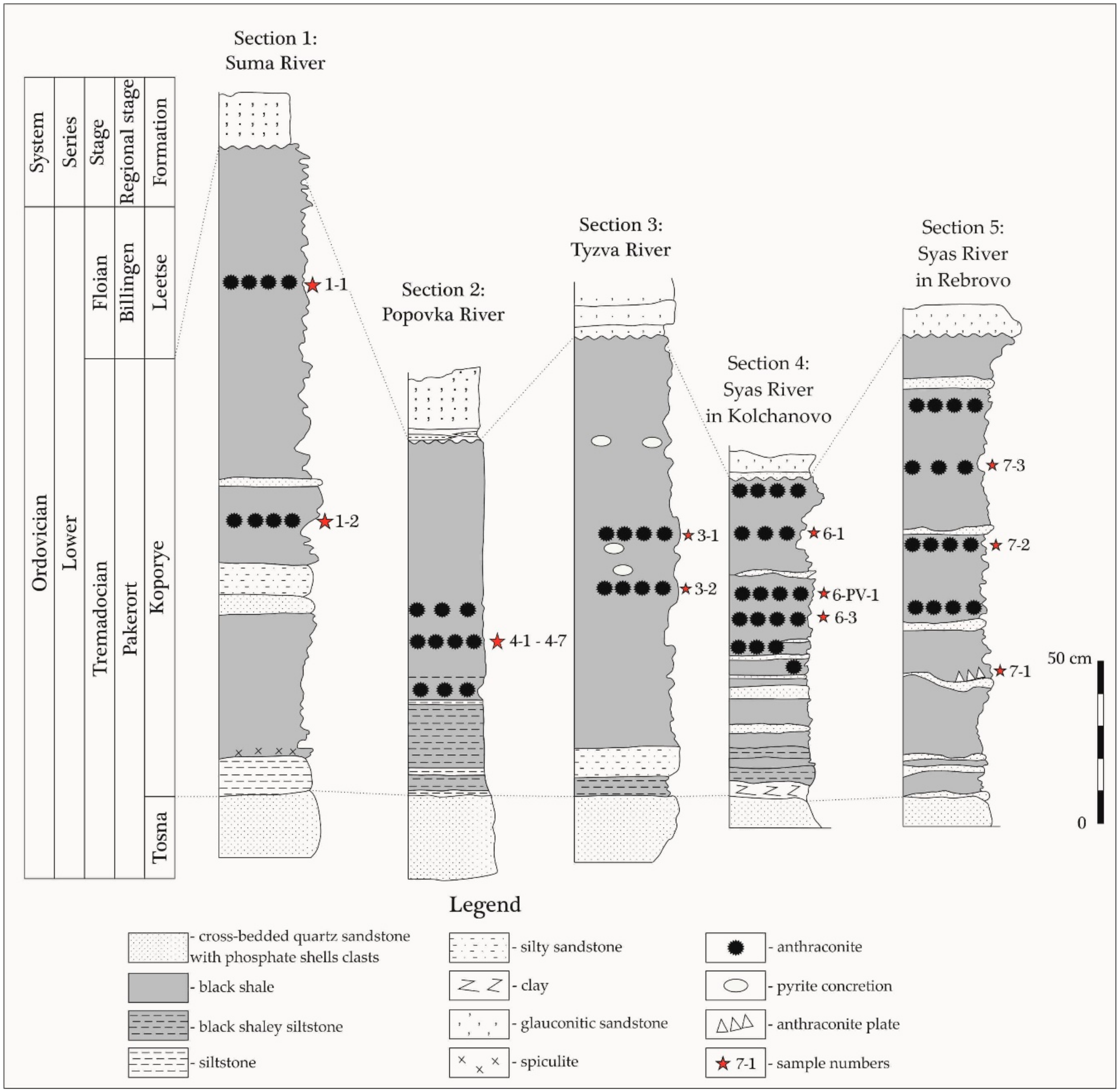
3. Geological Background
4. Overview of Previous Studies of Anthraconites
5. Materials and Methods
6. Results
6.1. Morphologies of Studied Anthraconites
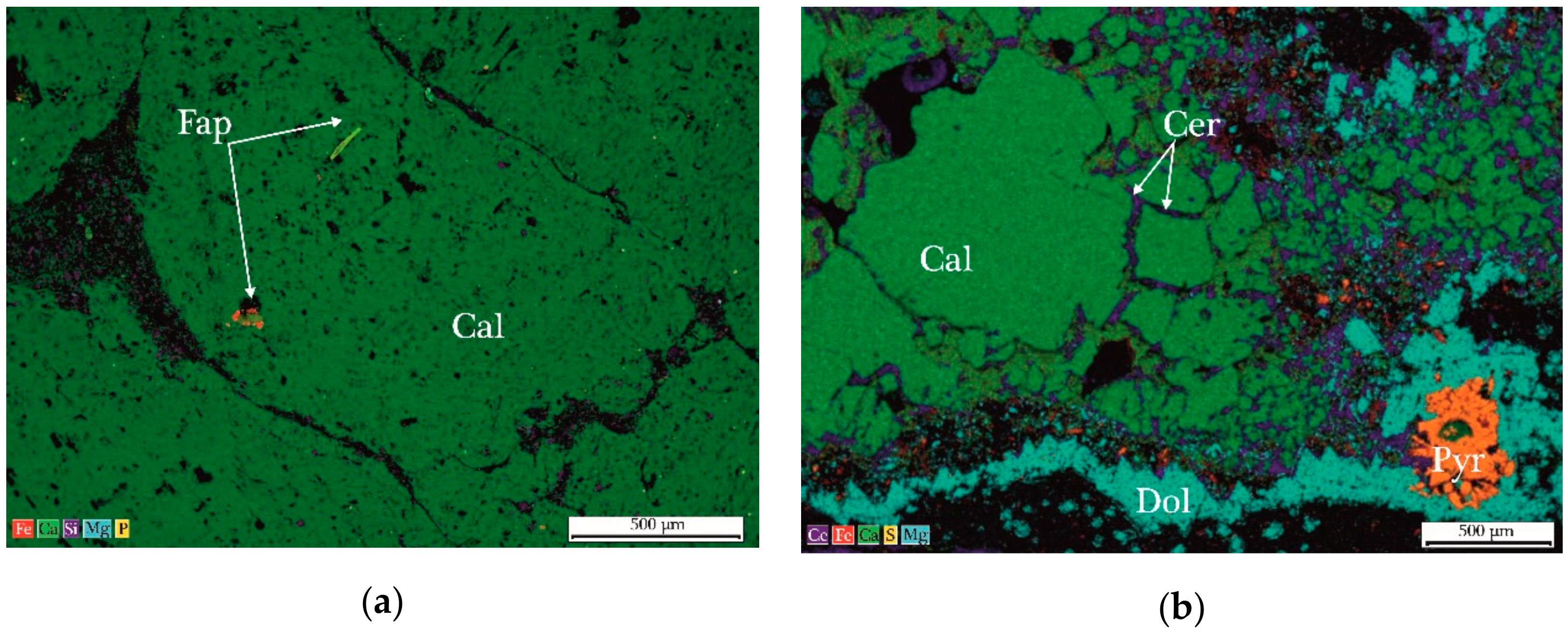
6.2. Mineralalogy of Anthraconites Revealed by X-ray Diffraction and Microprobe Analysis
6.3. Petrography of Anthraconites
6.4. Stable Isotope Analysis
7. Discussion
8. Conclusions
- The morphology, internal structure, petrographic and cathodoluminescence characteristics suggest that the anthraconites were precipitated as primary ikaite, which was subsequently replaced by calcite and later partly by dolomite.
- The negative stable carbon isotopic ratios show that ikaite-glendonite transformation was influenced by methane and/or organic matter degradation. Thus, there were three intermixed sources of carbon isotopes—dissolved inorganic carbon from seawater, degradation of organic matter, and/or methanotrophy. The oxygen isotope ratios do not reflect isotopic composition of the ikaite and probably was changed during diagenesis by meteoric water.
- Based on our mineralogical, petrographic, cathodoluminescence, and isotopic data, along with the stratigraphic position of the studied samples, we propose a new model for the formation of anthraconites in the Baltic paleobasin. Our model invokes the precipitation of ikaite in an upwelling zone along the western margin of Baltica (modern co-ordinates), which brought deep cold phosphate-rich waters up to shallower water depths, providing favorable conditions for ikaite precipitation within the relatively shallow Baltic paleobasin. The unstable ikaite was subsequently transformed to calcite and/or dolomite during diagenesis under reducing conditions that were probably favored by degradation of organic matter and/or methanotrophy.
- The complex mineralogical composition of the studied anthraconite samples was formed during early diagenesis under reducing conditions (calcite + pyrite), and by oxidative processes during diagenesis, when calcite and pyrite were transformed to gypsum and dolomite. The source of cerianite observed in the fractures remains unclear.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Torsvik, T.H.; Cocks, L.R. Earth History and Palaegeography; Cambridge University Press: Cambridge, UK, 2016; pp. 1–332. [Google Scholar]
- Thompson, C.K.; Kah, L.C. Sulfur isotope evidence for widespread euxinia and a fluctuating oxycline in Early to Middle Ordovician greenhouse oceans. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 313–314, 189–214. [Google Scholar] [CrossRef]
- Saltzman, M.R.; Edwards, C.T.; Adrain, J.M.; Westrop, S.R. Persistent oceanic anoxia and elevated extinction rates separate the Cambrian and Ordovician radiations. Geology 2015, 43, 807–811. [Google Scholar] [CrossRef]
- Edwards, C.T.; Fike, D.A.; Saltzman, M.R.; Lu, W.; Lu, Z. Evidence for local and global redox conditions at an Early Ordovician (Tremadocian) mass extinction. Earth Planet. Sci. Lett. 2018, 481, 125–135. [Google Scholar] [CrossRef]
- Buchardt, B.; Nielsen, A.T. Carbon and oxygen isotope composition of Cambro-Silurian limestone and anthraconite from Bornholm: Evidence for deep burial diagenesis. Bull. Geol. Soc. Den. 1985, 33, 415–435. [Google Scholar]
- Schmidt, F. Revision der ostbaltischen silurischen Trilobiten nebst geognostischer Übersicht des ostbaltischen Silurgebiets. Abtheilung I.; Phacopiden, Cheiruriden und Encrinuriden. Mémoires De L’académie Impériale Des Sci. De St.-Pétersbourg. VIIe Série 1882, 30, 1–255. [Google Scholar]
- Hadding, A. The Pre-Quaternary Sedimentary Rocks of Sweden. VII. Cambrian and Ordovician Limestones; Lund CWK Gleerup: Lund, Sweden, 1958. [Google Scholar]
- Berg-Madsen, V. Origin and usage of the geological terms orsten, stinkstone, and anthraconite. Arch. Nat. Hist. 1989, 16, 191–208. [Google Scholar] [CrossRef]
- Lindström, M. White sand crosses in turbated siltstone bed, basal Cambrian, Kinnekulle, Sweden. Geol. Palaeontol. 1977, 11, 1–8. [Google Scholar]
- Lindström, M. Cold age sediment in Lower Cambrian of South Sweden. Geol. Palaeontol. 1972, 6, 9–23. [Google Scholar]
- Lindström, M. Do “white sand crosses” from the Lower Cambrian of Kinnekulle have glendonite affinity? Geol. Föreningen i Stockh. Förhandlingar 1984, 106, 32. [Google Scholar] [CrossRef]
- Stolley, E. Pseudo-Gaylussit, Pseudo-Pirssonit und Protospongia im cambrischen Alaunschiefer Bornholms. Medd. fra Dan. Geol. Foren. 1909, 15, 351–368. [Google Scholar] [CrossRef]
- Callisen, K. Tenformede Tungspatkrystaller (”Pseudo-Gaylussit” og ”Pseudo-Pirssonit”) i Alunskiferen. Medd. fra Dan. Geol. Foren. 1914, 4, 245–258. [Google Scholar]
- Choh, S.J. First report of glendonite in the uppermost Cambrian. Proceedings of GSA Annual Meeting in Indianapolis, Indiana, IN, USA, 4–7 November 2018. [Google Scholar]
- Brøgger, W.C. Die Silurischen Etagen 2 und 3 im Kristianiagebiet und auf Eker, ihre Gliederung, Fossilien, Schichtenstörungen und Contactmetamorphosen; A.W. Brögger: Oslo, Norway, 1882. [Google Scholar]
- Calner, M.; Erlström, M.; Lehnert, O.; Ahlberg, P. Lower Palaeozoic geology of southern Sweden. In The Lower Palaeozoic of Southern Sweden and the Oslo Region, Norway; Calner, M., Ahlberg, P., Eds.; Geological Survey of Sweden: Stockholm, Sweden, 2013; pp. 6–9. [Google Scholar]
- Trotter, J.A.; Williams, I.S.; Barnes, C.R.; Lécuyer, C.; Nicoll, R.S. Did cooling oceans trigger Ordovician biodiversification? Evidence from conodont thermometry. Science 2008, 321, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Hearing, T.W.; Harvey, T.H.; Williams, M.; Leng, M.J.; Lamb, A.L.; Wilby, P.R.; Gabbott, S.E.; Pohl, A.; Donnadieu, Y. An early Cambrian greenhouse climate. Sci. Adv. 2018, 4, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Babcock, L.E.; Peng, S.C.; Brett, C.E.; Zhu, M.Y.; Ahlberg, P.; Bevis, M.; Robison, R.A. Global climate, sea level cycles, and biotic events in the Cambrian Period. Palaeoworld 2015, 24, 5–15. [Google Scholar] [CrossRef]
- Landing, E.; MacGabhann, B.A. First evidence for Cambrian glaciation provided by sections in Avalonian New Brunswick and Ireland: Additional data for Avalon–Gondwana separation by the earliest Palaeozoic. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 285, 174–185. [Google Scholar] [CrossRef]
- Le Heron, D.P.; Vandyk, T.M.; Wu, G.; Li, M. New perspectives on the Luoquan Glaciation (Ediacaran-Cambrian) of North China. Depos. Rec. 2018, 4, 274–292. [Google Scholar] [CrossRef]
- Runkel, A.C.; Mackey, T.J.; Cowan, C.A.; Fox, D.L. Tropical shoreline ice in the late Cambrian: Implications for Earth’s climate between the Cambrian Explosion and the Great Ordovician Biodiversification Event. Gsa Today 2010, 20, 4–10. [Google Scholar] [CrossRef]
- Verbitskii, V.R.; Verbitskii, I.V.; Vasilyeva, O.V.; Savanin, V.V.; Kyamyarya, V.V.; Mazurkevich, K.N.; Krotova-Putintzeva, A.E.; Semyenova, L.R.; Bogdanov, Y.B.; Petrov, B.V. State Geological Map of Russian Federation. Map Scale 1:1 000 000. Sheets O-35—Pskov, (N-35), O-36—Saint-Petersburg. Explanatory Notes; Cartographic Factory VSEGEI: Saint Petersburg, Russia, 2012; 510p. (In Russian) [Google Scholar]
- Geology of USSR. Leningrad, Pskov, Novgorod regions. Geological description. North-West local GU.; Nedra: Moscow, Russia, 1971; p. 504. (In Russian)
- State geological map of USSR. Map scale 1:1 000 000. Sheets O-(35), 36 Leningrad. Explanatory notes.; Cartographic Factory VSEGEI: Leningrad, Russia, 1989; p. 212. (In Russian)
- Popov, L.E.; Khazanovich, K.K. Key Sections and Stratigraphy of Cambrian-Ordovician Phosphate-Bearing Obolus Sandstone on the North-Western of the Russian Platform; Nauka: Leningrad, Russia, 1989; p. 222. (In Russian) [Google Scholar]
- Loog, A. On the geochemistry of postsedimentary mineral formation in the Tremadoc graptolitic argillites of North Estonia. Acta et Comment. Univ. Tartu. 1980, 527, 44–50. [Google Scholar]
- Tugarova, M.A.; Platonov, M.V. Carbonate microbioliths in the Koporye Formation argillites of north-west flank of the Moscow symiclise. Lithosphere 2014, 4, 36–49. (In Russian) [Google Scholar]
- Popov, L.E.; Alvaro, J.J.; Holmer, L.E.; Bauert, H.; Pour, M.G.; Dronov, A.V.; Lehnert, O.; Hints, O.; Mannik, P.; Zhang, Z.; et al. Glendonite occurences in the Tremadocian of Baltica: First Early Palaeozoic evidence of massive ikaite precipitation at temperature latitudes. Sci. Rep. 2019, 9, 7205. [Google Scholar] [CrossRef]
- Zaitsev, A.V.; Pokrovsky, B.G. Carbon and oxygen isotope compositions of Lower-Middle Ordovician carbonate rocks in the northwestern Russian platform. Lithol. Miner. Resour. 2014, 49, 283–291. [Google Scholar] [CrossRef]
- Teichert, B.M.A.; Luppold, F.W. Glendonites from an Early Jurassic methane seep-climate or methane indicators? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2013, 390, 81–93. [Google Scholar] [CrossRef]
- Morales, C.; Rogov, M.; Wierzbowski, H.; Ershova, V.; Suan, G.; Adatte, T.; Föllmi, K.B.; Tegelaar, E.; Reichart, G.J.; de Lange, G.J.; et al. Glendonites track methane seepage in Mesozoic polar seas. Geology 2017, 45, 503–506. [Google Scholar] [CrossRef]
- Rogov, M.A.; Ershova, V.B.; Shchepetova, E.V.; Zakharov, V.A.; Pokrovsky, B.G.; Khudoley, A.K. Earliest Cretaceous (late Berriasian) glendonites from Northeast Siberia revise the timing of initiation of transient Early Cretaceous cooling in the high latitudes. Cretac. Res. 2017, 71, 102–112. [Google Scholar] [CrossRef]
- Vickers, M.; Watkinson, M.; Price, G.D.; Jerret, R. An improved model for the ikaite-glendonite transformation: Evidence from the Lower Cretaceous of Spitsbergen, Svalbard. Norw. J. Geol. 2018, 98, 1–15. [Google Scholar] [CrossRef]
- Derkachev, A.N.; Nikolaeva, N.A.; Mozherovsky, A.V.; Grigor’eva, T.N.; Ivanova, E.D.; Pletnev, S.P.; Barinov, N.N.; Chubarov, V.M. Mineralogical and geochemical indicators of anoxic sedimentation conditions in local depressions within the Sea of Okhotsk in the Late Pleistocene-Holocene. Russ. J. Pac. Geol. 2007, 1, 203–229. [Google Scholar] [CrossRef]
- Suess, E.; Balzer, W.; Hesse, K.F.; Muller, P.J.; Ungerer, C.A.; Wefer, G. Calcium carbonate hexahydrate from organic-rich sediments of the Antarctic Shelf: Precursors of glendonites. Science 1982, 216, 1128–1131. [Google Scholar] [CrossRef]
- Kodina, L.A.; Tokarev, V.G.; Vlasova, L.N.; Korobeinik, G.S. Contribution of biogenic methane to ikaite formation in the Kara Sea; evidence from the stable carbon isotope geochemistry. Proc. Mar. Sci. 2003, 6, 349–374. [Google Scholar]
- Frank, T.D.; Tomas, S.G.; Fielding, C.R. On using carbon and oxygen isotope data from glendonites as paleoenvironmental proxies: A case study from the Permian system of eastern Australia. J. Sediment. Res. 2008, 78, 713–723. [Google Scholar] [CrossRef]
- James, N.P.; Narbonne, G.M.; Dalrymple, R.W.; Kyser, T.K. Glendonites in Neoproterozoic low latitude, interglacial, sedimentary rocks, northwest Canada: Insights into the Cryogenian ocean and Precambrian cold-water carbonates. Geology 2005, 33, 9–12. [Google Scholar] [CrossRef]
- Joeckel, R.M.; Ang Clement, B.J.; VanFleet Bates, L.R. Sulfate-mineral crusts from pyrite weathering and acid rock drainage in the Dakota Formation and Graneros Shale, Jefferson Country, Nebraska. Chem. Geol. 2005, 15, 433–452. [Google Scholar] [CrossRef]
- Ritsema, C.J.; Groenenberg, J.E. Pyrite oxidation, carbonate weathering, and gypsum formation in a drained potential acid sulfate soil. Soil Sci. Soc. Am. J. 1993, 57, 968–976. [Google Scholar] [CrossRef]
- Machel, H.G. Concepts and models of dolomitization: A critical reappraisal. In The Geometry and Petrogenesis of Dolomite Hydrocarbon Reservoirs; Braothwaite, C.J.R., Rizzi, G., Eds.; Geological Society, London, Special Publications: London, UK, 2004; Volume 235, pp. 7–63. [Google Scholar]
- Greinert, J.; Derkachev, A. Glendonites and methane-derived Mg-calcites in the Sea of Okhotsk, Eastern Siberia: Implications of a venting-related ikaite/glendonite formation. Mar. Geol. 2004, 204, 129–144. [Google Scholar] [CrossRef]
- Campbell, K.A. Hydrocarbon seep and hydrothermal vent paleoenvironments and paleontology: Past developments and future research directions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 232, 362–407. [Google Scholar] [CrossRef]
- Coleman, M.L. Microbial processes: Controls on the shape and composition of carbonate concretions. Mar. Geol. 1993, 113, 127–140. [Google Scholar] [CrossRef]
- Kaplan, M.E. Calcite pseudomorph in Jurassic and Lower Cretaceous deposit of the north part of East Siberia. Geol. Geofiz. 1978, 12, 62–70. (In Russian) [Google Scholar]
- De Lurio, J.L.; Frakes, L.A. Glendonites as a paleoenvironmental tool: Implications for early Cretaceous high latitude climates in Australia. Geochim. Cosmochim. Acta 1999, 63, 1039–1048. [Google Scholar] [CrossRef]
- Selleck, B.W.; Carr, P.F.; Jones, B.G. A review and synthesis of glendonites (pseudomorphs after ikaite) with new data: Assessing applicability as recorders of ancient coldwater conditions. J. Sediment. Res. 2007, 77, 980–991. [Google Scholar] [CrossRef]
- Price, G.D.; Nunn, E.V. Valanginian isotope variation in glendonites and belemnites from Arctic Svalbard: Transient glacial temperatures during the Cretaceous greenhouse. Geology 2010, 38, 251–254. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, Z.; Rickaby, R.E.M.; Domack, E.W.; Wellner, J.S.; Kennedy, H.A. Ikaite abundance controlled by porewater phosphorous level: Potential links to dust and productivity. J. Geol. 2015, 123, 269–281. [Google Scholar] [CrossRef]
- Stockmann, G.J.; Ranta, E.; Trampe, E.; Sturkell, E.; Seaman, P. Carbon mineral storage in seawater: Ikaite (CaCO3·6H2O) columns in Greenland. Energy Procedia 2018, 146, 59–67. [Google Scholar] [CrossRef]
- Qu, Y.; Teichert, B.M.A.; Birgel, D.; Goedert, J.L.; Peckmann, J. The prominent role of bacterial sulfate reduction in the formation of glendonite: A case study from Paleogene marine strata of western Washington State. Facies 2017, 63, 10. [Google Scholar] [CrossRef]
- Buchardt, B.; Nielsen, A.T.; Schovsbo, N.H. Alun Skiferen i Skandinavien. Geol. Tidsskr. 1997, 3, 1–30. (In Danish) [Google Scholar]
- Dronov, A.; Holmer, L. Ordovician sea-level curve: Baltoscandian view. In Proceedings of the Basin Stratigraphy—Modern Methods and Problems, 5th Baltic Stratigraphical Congress, Vilnius, Lithuania, 22–27 September 2002; pp. 33–35. [Google Scholar]
- Haq, B.U.; Schutter, S.R. A chronology of Paleozoic sea-level changes. Science 2008, 322, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Vyalov, V.I.; Larichev, A.I.; Balakhonova, A.S. Ore genesis of Dictionema shale and Obolus sandstones of the Baltic basin. Reg. Geol. Metallog. 2013, 55, 87–98. [Google Scholar]
- Kelly, J.R.; Doering, P.H. Seasonal deepening of the pycnocline in a shallow shelf ecosystem and its influence on near-bottom dissolved oxygen. Mar. Ecol. Prog. Ser. 1999, 178, 151–168. [Google Scholar] [CrossRef]
- Trabucho-Alexandre, J.; Hay, W.W.; De Boer, P.L. Phanerozoic environments of black shale deposition and the Wilson Cycle. Solid Earth 2011, 3, 29–42. [Google Scholar] [CrossRef]
- Cooper, R.A.; Rigby, S.; Loydell, D.K.; Bates, D.E.D. Palaeoecology of the Graptoloidea. Earth-Sci. Rev. 2012, 112, 23–41. [Google Scholar] [CrossRef]
- Modliński, Z.; Szymański, B. The Ordovician lithostratigraphy of the Peribaltic Depression (NE Poland). Kwart. Geol. 1997, 41, 273–288. [Google Scholar]





| No | Sample | Location | Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cal-1 | Cal-2 | Dol | Qtz | Gyp | Pyr | Zeo | Fap | Kfsp | |||
| 1 | 1-1-2 | Suma River | ■ | ± | |||||||
| 2 | 1-2-2 | ■ | ± | ||||||||
| 3 | 3-1-1 | Tyzva River | ■ | ± | ± | ||||||
| 4 | 3-1-2 | ■ | ± | ± | |||||||
| 5 | 3-1-3 | ■ | ± | ± | |||||||
| 6 | 3-1-4 | ■ | ± | ± | |||||||
| 7 | 3-1-5 | ■ | ± | ± | |||||||
| 8 | 3-2-1 | ■ | ± | □ | □ | ± | |||||
| 9 | 3-2-8 | □ | ■ | ± | □ | ± | |||||
| 10 | 4-1 | Popovka River | ± | ■ | ■ | ||||||
| 11 | 4-2 | ■ | ± | ± | □ | ||||||
| 12 | 4-3 | ■ | ± | ± | □ | ||||||
| 13 | 4-4 | ± | ■ | ■ | ± | ||||||
| 14 | 4-5 | ■ | ■ | ± | □ | ||||||
| 15 | 4-6 | □ | ■ | ± | □ | ||||||
| 16 | 4-7 | □ | □ | ± | ■ | ||||||
| 17 | 6-1-1 | Syas River in Rebrovo | ■ | ■ | ± | ||||||
| 18 | 6-1-2 | ■ | ± | ||||||||
| 19 | 6-PV-1 | ■ | ± | ||||||||
| 20 | 6-3-2 | ■ | ± | ± | |||||||
| 21 | 7-1 | Syas River in Kolchanovo | ■ | □ | ± | ||||||
| 22 | 7-2-1 | ■ | ± | ± | |||||||
| 23 | 7-2-2 | ■ | ± | ± | |||||||
| 24 | 7-3-1 | ■ | ± | ± | |||||||
| 25 | 7-3-2 | ■ | ± | ± | |||||||
| 26 | 7-3-3 | ■ | ± | ± | |||||||
| No | Sample | Phase | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cal | Dol | Qtz | Gyp | Pyr | Mic | Cer | Fap | ||
| 1 | 1-1-2 | ■ | ± | ± | ± | ||||
| 3 | 3-1-1 | ■ | ± | ± | ± | ||||
| 15 | 4-6 | ■ | ■ | □ | ± | ± | □ | ||
| 18 | 6-1-2 | ■ | ± | ± | |||||
| No. | Sample | Location | Mineral Composition of Anthraconite Sample | δ18O ‰ V-PDB | δ13C ‰ V-PDB |
|---|---|---|---|---|---|
| 1 | 1-1 | Suma River | Dolomite | −3.5 | −3.1 |
| 2 | 1-2 | Dolomite | −3.5 | −3.5 | |
| 3 | 3-1-1 | Tyzva River | Calcite | −7.1 | −8.5 |
| 4 | 3-1-2 | Calcite | −7.1 | −7.9 | |
| 5 | 3-1-3 | Calcite | −7.2 | −7.3 | |
| 6 | 3-1-4 | Calcite | −6.9 | −9.5 | |
| 7 | 3-1-5 | Calcite | −7.0 | −6.7 | |
| 8 | 3-2-1 | calcite + gypsum + pyrite | −6.6 | −7.4 | |
| 9 | 3-2-8 | dolomite + calcite + gypsum | −6.3 | −7.9 | |
| 10 | 4-2 | Popovka River | calcite + gypsum | −4.9 | −8.4 |
| 11 | 4-3 | calcite + gypsum | −5.1 | −7.0 | |
| 12 | 4-5 | calcite + dolomite + gypsum | −5.1 | −6.3 | |
| 13 | 4-6 | dolomite + calcite + gypsum | −5.8 | −9.2 | |
| 15 | 6-1-1 | Syas River in Kolchanovo | Calcite | −6.3 | −0.9 |
| 16 | 6-1-2 | Calcite | −6.2 | −1.5 | |
| 17 | 6-3-2 | Calcite | −6.0 | −2.3 | |
| 18 | 6-PV-1 | Calcite | −6.5 | −2.4 | |
| 19 | 6-PV-2 | Calcite | −6.7 | −2.3 | |
| 20 | 7-1 | Syas River in Rebrovo | Calcite | −6.4 | −3.7 |
| 21 | 7-2-1 | Calcite | −6.3 | −2.2 | |
| 22 | 7-2-2 | Calcite | −6.3 | −0.9 | |
| 23 | 7-3-1 | Calcite | −6.0 | −2.2 | |
| 24 | 7-3-2 | Calcite | −5.6 | −0.9 | |
| 25 | 7-3-3 | Calcite | −6.2 | −3.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikhailova, K.; Vasileva, K.; Fedorov, P.; Ershova, V.; Vereshchagin, O.; Rogov, M.; Pokrovsky, B. Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications. Minerals 2019, 9, 524. https://doi.org/10.3390/min9090524
Mikhailova K, Vasileva K, Fedorov P, Ershova V, Vereshchagin O, Rogov M, Pokrovsky B. Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications. Minerals. 2019; 9(9):524. https://doi.org/10.3390/min9090524
Chicago/Turabian StyleMikhailova, Kseniia, Kseniia Vasileva, Petr Fedorov, Victoria Ershova, Oleg Vereshchagin, Mikhail Rogov, and Boris Pokrovsky. 2019. "Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications" Minerals 9, no. 9: 524. https://doi.org/10.3390/min9090524
APA StyleMikhailova, K., Vasileva, K., Fedorov, P., Ershova, V., Vereshchagin, O., Rogov, M., & Pokrovsky, B. (2019). Glendonite-Like Carbonate Aggregates from the Lower Ordovician Koporye Formation (Russian Part of the Baltic Klint): Detailed Mineralogical and Geochemical Data and Paleogeographic Implications. Minerals, 9(9), 524. https://doi.org/10.3390/min9090524






