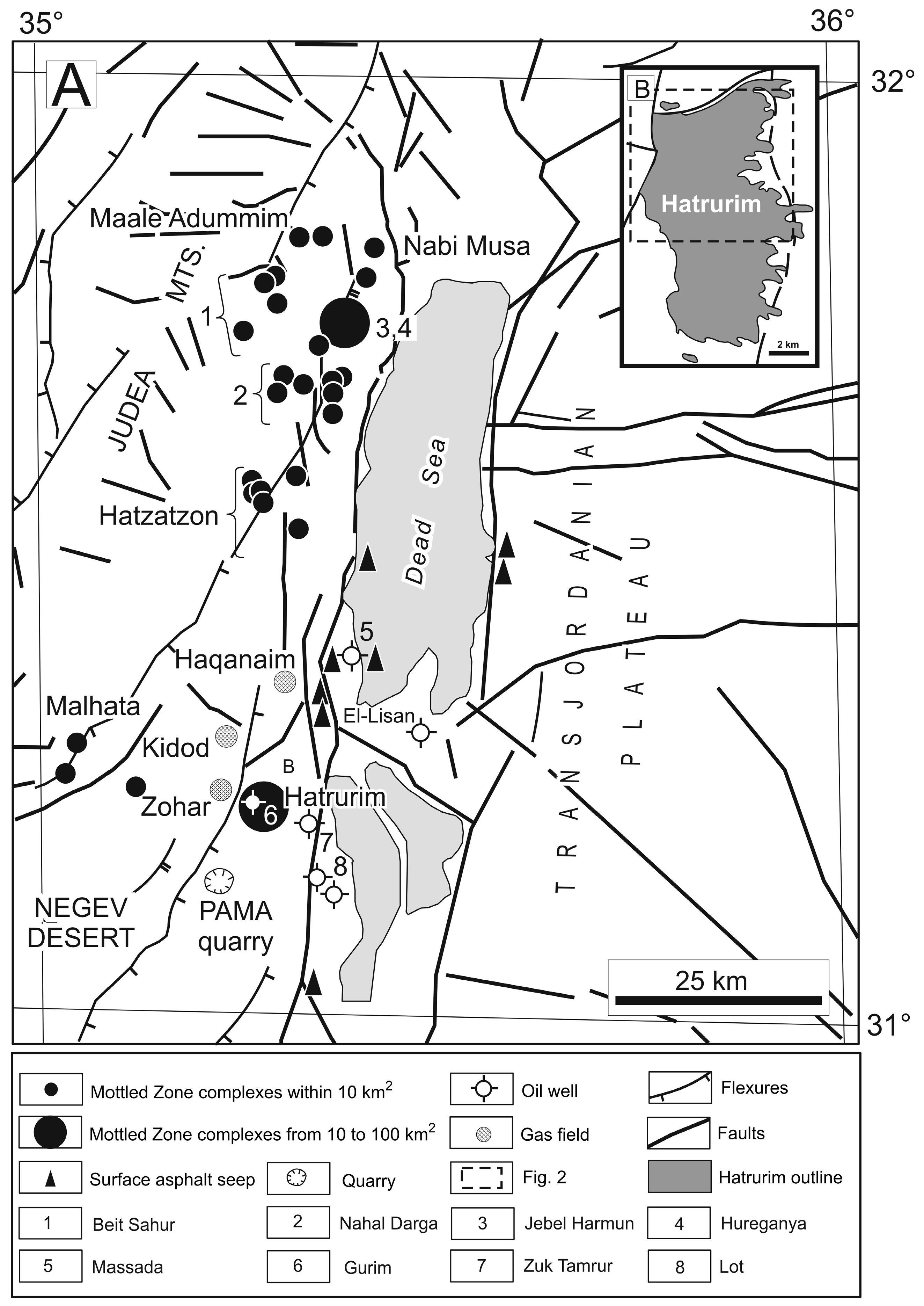
Figure 1.
(
A) Generalized geology of the Dead Sea Transform area, with locations of Mottled Zone complexes, gas fields, asphalt shows, and oil wells, after [
71,
73,
74]. (
B) Hatrurim Basin, simplified after [
74].
Figure 1.
(
A) Generalized geology of the Dead Sea Transform area, with locations of Mottled Zone complexes, gas fields, asphalt shows, and oil wells, after [
71,
73,
74]. (
B) Hatrurim Basin, simplified after [
74].
Figure 2.
Larnite-bearing CM rocks from the Hatrurim Basin. Field images. (a) Panoramic view of the Hatrurim Basin. (b) Outcrop of larnite CM rocks on a hilltop. (c) Massive fresh larnite rocks. (d–g) Typical appearance of pseudo-conglomerates consisting of larnite- and/or gehlenite-bearing “pebbles” in a light-colored matrix of secondary minerals (mainly calcite, aragonite, gypsum, and ettringite, with lesser percentages of tobermorite, jennite, afwillite, and hydrogarnets). Photographs are ordered according to increasing alteration degrees of rocks. Photographs of 2005 and 2007.
Figure 2.
Larnite-bearing CM rocks from the Hatrurim Basin. Field images. (a) Panoramic view of the Hatrurim Basin. (b) Outcrop of larnite CM rocks on a hilltop. (c) Massive fresh larnite rocks. (d–g) Typical appearance of pseudo-conglomerates consisting of larnite- and/or gehlenite-bearing “pebbles” in a light-colored matrix of secondary minerals (mainly calcite, aragonite, gypsum, and ettringite, with lesser percentages of tobermorite, jennite, afwillite, and hydrogarnets). Photographs are ordered according to increasing alteration degrees of rocks. Photographs of 2005 and 2007.
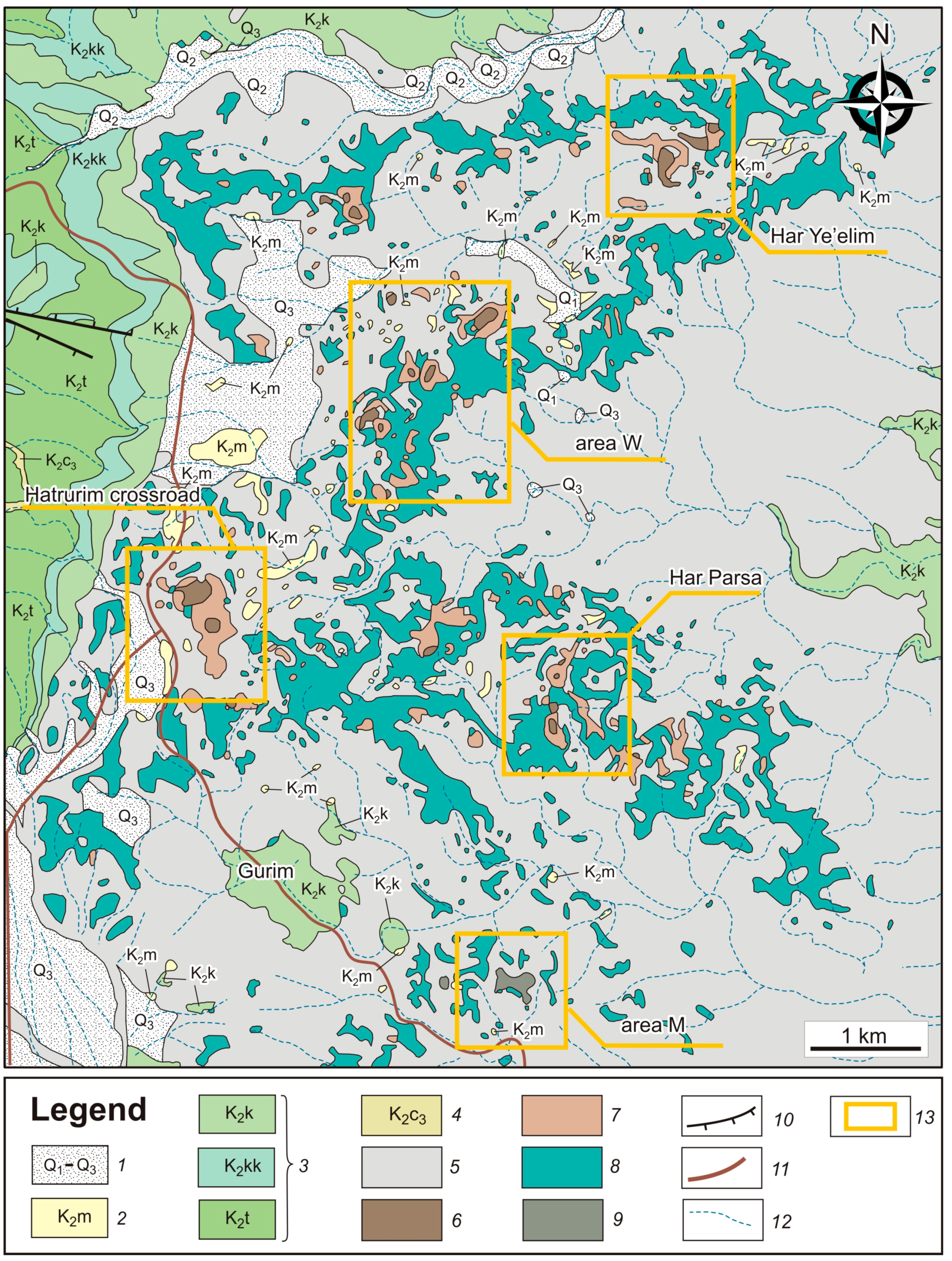
Figure 3.
Geological map of the northwestern Hatrurim Basin, modified after the 1:50,000 Geological Map of Israel [
75]. 1 = Pleistocene terrace conglomerates (Q
1, Q
2, Q
3); 2 = Maastrichtian organic-rich marine chalk (K
2m); 3 = Campanian (K
2k), Santonian (K
2kk), and Turonian (K
2t) limestone, chalk, and dolomite with chert and phosphorite intercalations; 4 = Cenomanian (K
2c
3) limestone, dolomite, and chalk; 5 = Low-grade Hatrurim Fm rocks; 6 = Larnite rocks (High-grade Hatrurim Fm rocks); 7 = “Olive rocks” (Hatrurim Fm); 8 = Spurrite marbles (medium-grade Hatrurim Fm rocks); 9 = Pseudo-conglomerates; 10 = Faults; 11 = Road;12 = Wadi; 13 = Sampling sites. Areas W and M are sampling sites of W and M series, respectively.
Figure 3.
Geological map of the northwestern Hatrurim Basin, modified after the 1:50,000 Geological Map of Israel [
75]. 1 = Pleistocene terrace conglomerates (Q
1, Q
2, Q
3); 2 = Maastrichtian organic-rich marine chalk (K
2m); 3 = Campanian (K
2k), Santonian (K
2kk), and Turonian (K
2t) limestone, chalk, and dolomite with chert and phosphorite intercalations; 4 = Cenomanian (K
2c
3) limestone, dolomite, and chalk; 5 = Low-grade Hatrurim Fm rocks; 6 = Larnite rocks (High-grade Hatrurim Fm rocks); 7 = “Olive rocks” (Hatrurim Fm); 8 = Spurrite marbles (medium-grade Hatrurim Fm rocks); 9 = Pseudo-conglomerates; 10 = Faults; 11 = Road;12 = Wadi; 13 = Sampling sites. Areas W and M are sampling sites of W and M series, respectively.
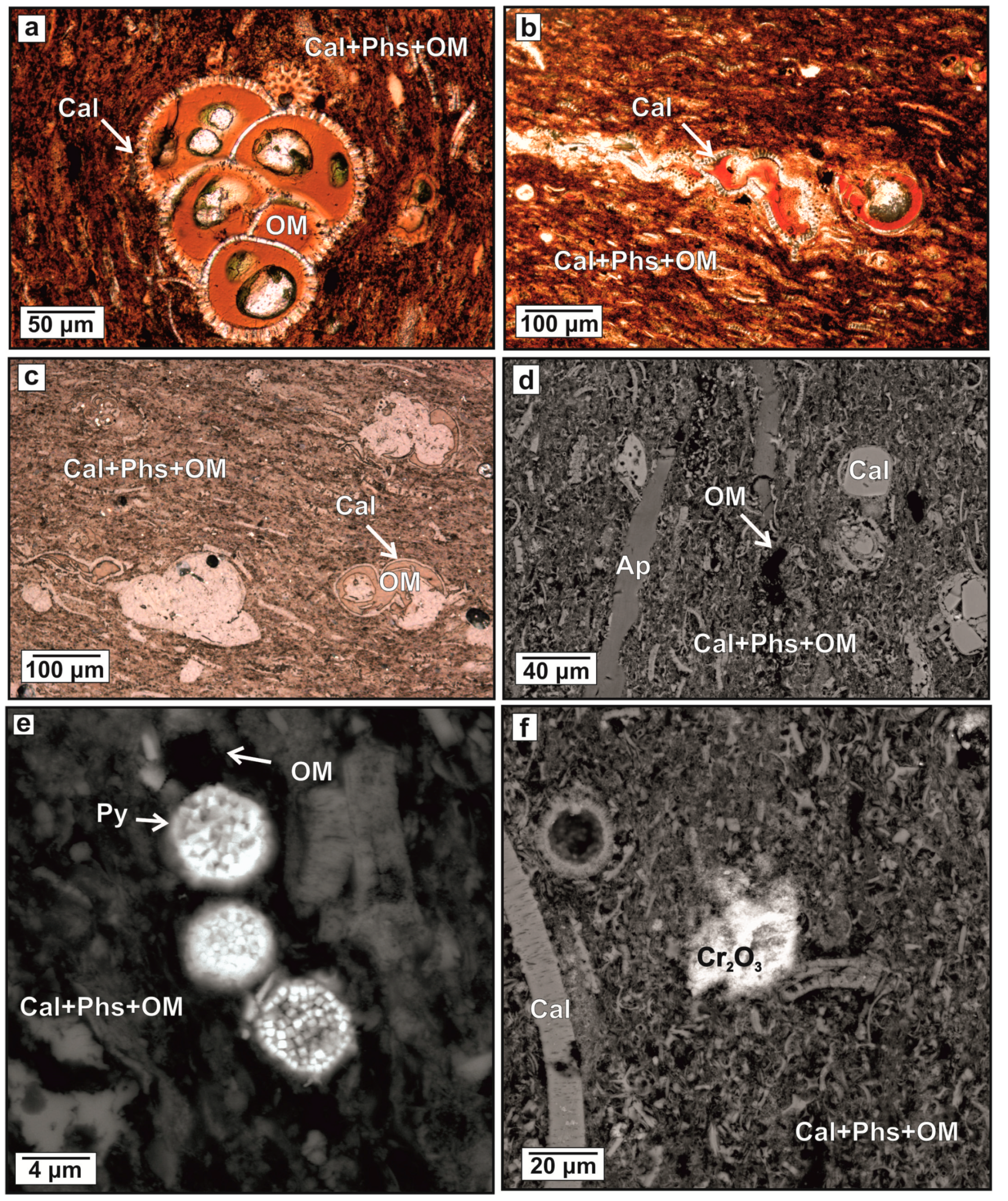
Figure 4.
(a–c) Appearance of Maastrichtian organic-rich marine chalky sediments (‘oil shales’): transmitted light images, (d–f) back-scattered electron (BSE) images. Mineral names are abbreviated as Ap = francolite; Cal = calcite; Cr2O3 = Cr (oxy)hydroxides; OM = organic matter; Phs = phosphatic material; Py = pyrite.
Figure 4.
(a–c) Appearance of Maastrichtian organic-rich marine chalky sediments (‘oil shales’): transmitted light images, (d–f) back-scattered electron (BSE) images. Mineral names are abbreviated as Ap = francolite; Cal = calcite; Cr2O3 = Cr (oxy)hydroxides; OM = organic matter; Phs = phosphatic material; Py = pyrite.
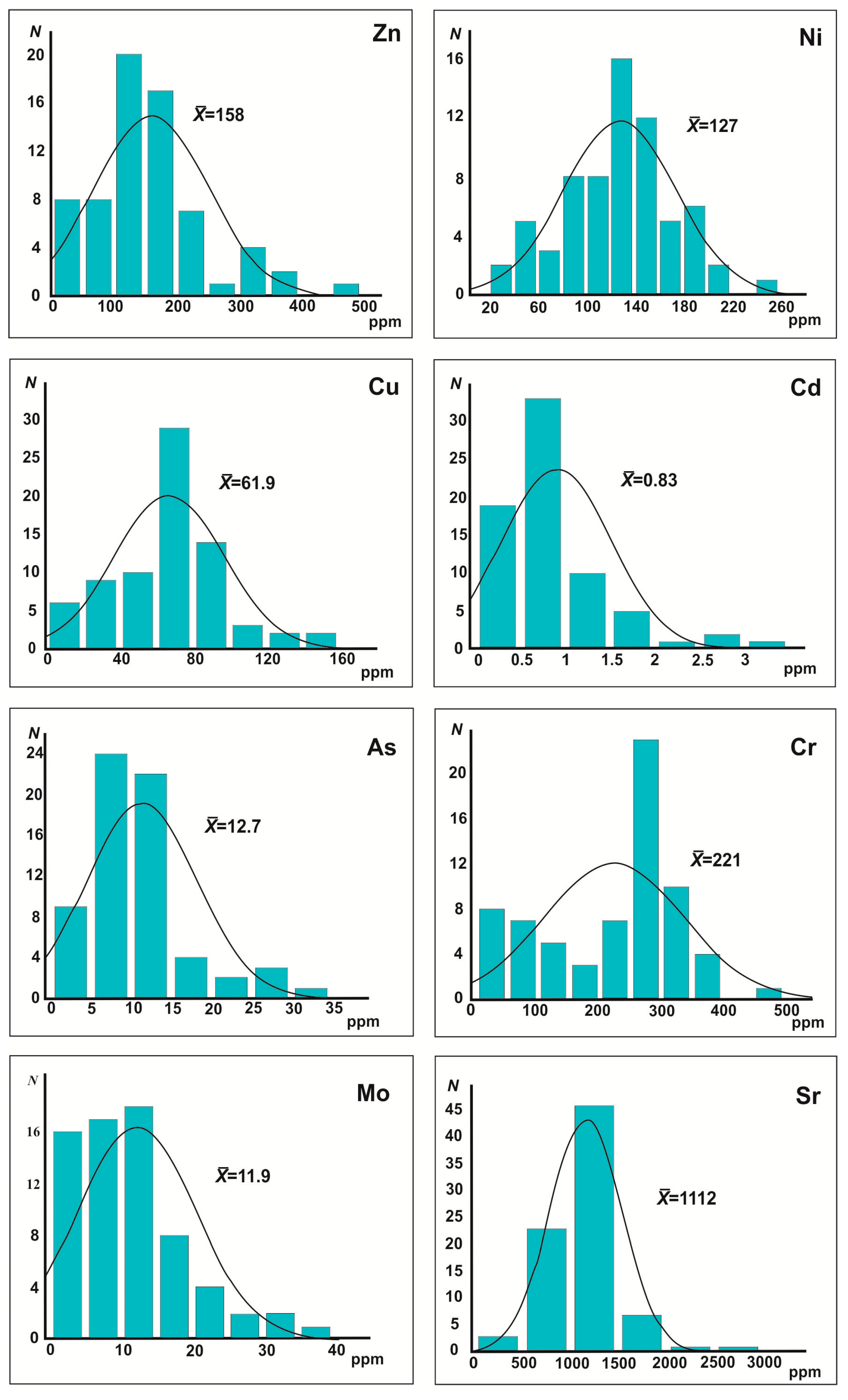
Figure 5.
Trace-element compositions of the Ghareb Fm bituminous calcareous rocks, compared.
N = number of samples;
= mean value (See data set in
Table 4).
Figure 5.
Trace-element compositions of the Ghareb Fm bituminous calcareous rocks, compared.
N = number of samples;
= mean value (See data set in
Table 4).
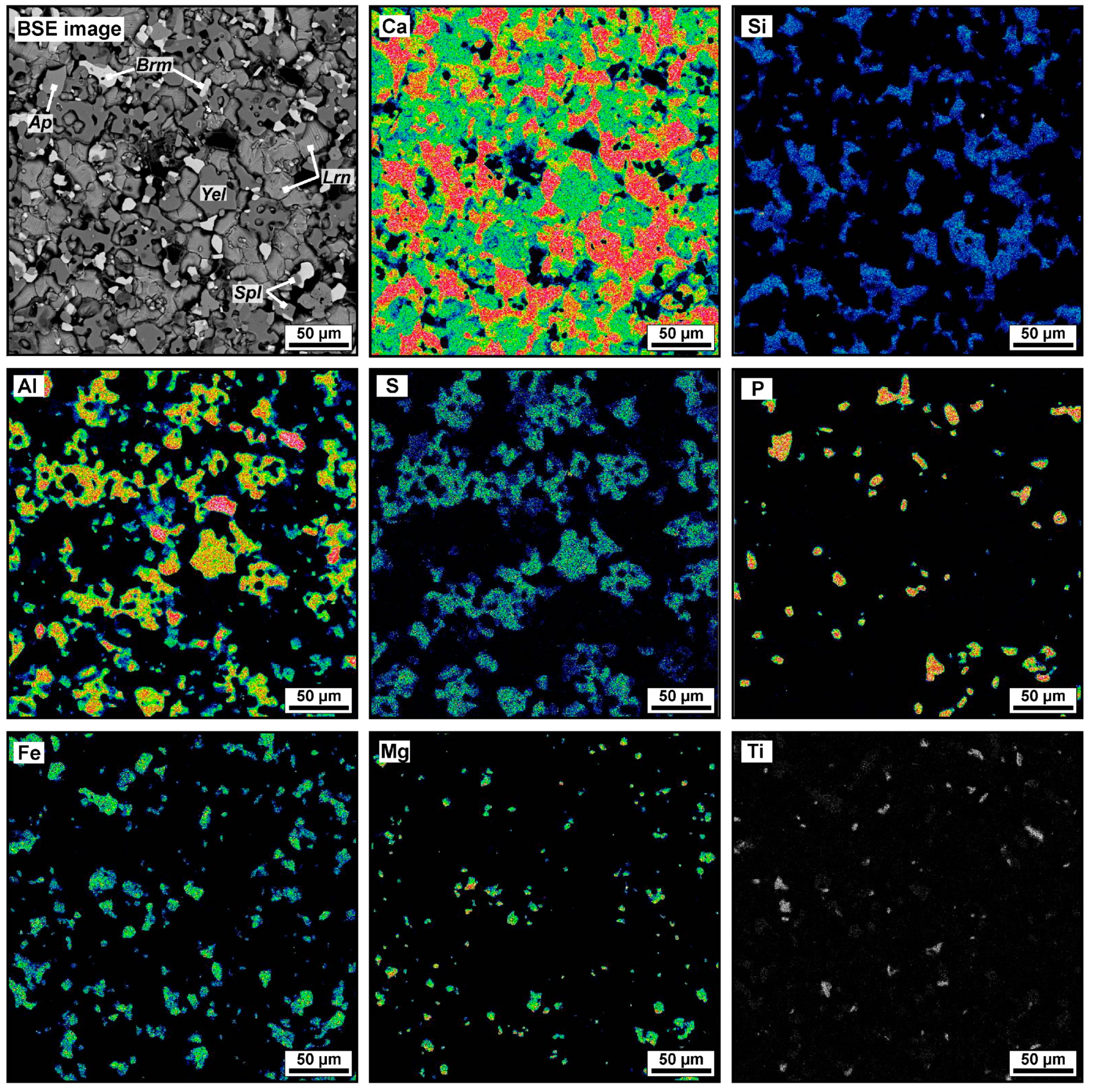
Figure 6.
BSE image and elemental maps (Ca, Si, Al, S, P, Fe, Mg, Ti) of typical ye’elimite–larnite CM rock: uniformly distributed main, minor, and accessory phases. Sample MP-10-1. Mineral names are abbreviated as Ap = fluorapatite, Brm = brownmillerite, Lrn = larnite, Spl = spinel, Yel = ye’elimite.
Figure 6.
BSE image and elemental maps (Ca, Si, Al, S, P, Fe, Mg, Ti) of typical ye’elimite–larnite CM rock: uniformly distributed main, minor, and accessory phases. Sample MP-10-1. Mineral names are abbreviated as Ap = fluorapatite, Brm = brownmillerite, Lrn = larnite, Spl = spinel, Yel = ye’elimite.
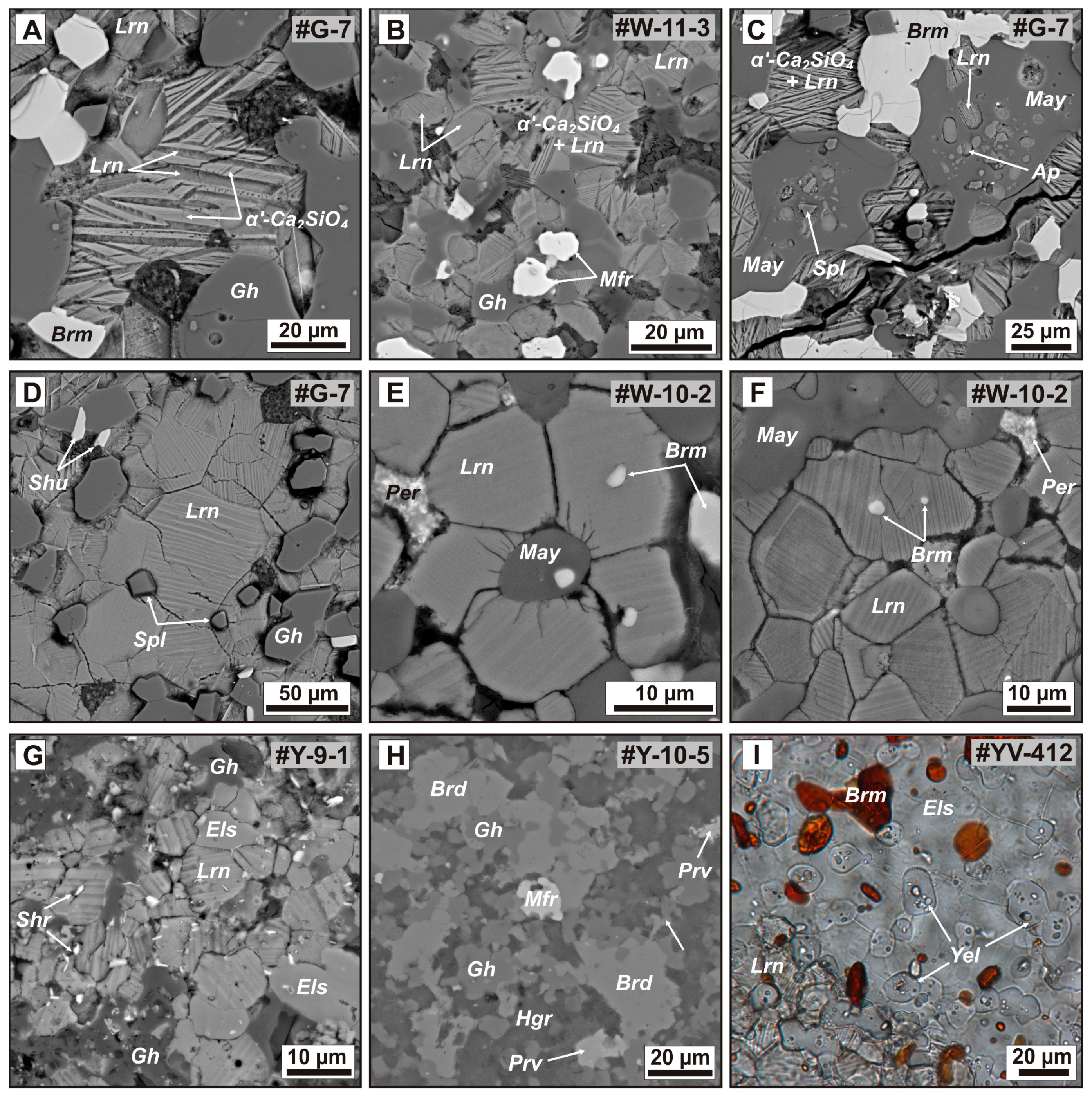
Figure 7.
Morphological diversity of the main phases in ye’elimite– and mayenite-bearing larnite CM rocks. (A) Close association of round larnite grains with {100} twinning and aggregates of larnite (β-Ca2SiO4) lamelli in the flamite (α′-Ca2SiO4) matrix. The α′-Ca2SiO4 and β-Ca2SiO4 lamelli are oriented as {} α′ ‖ {100}β and <0001>α′ ‖ <010>β. (B) Typical assemblage of Ca2SiO4-bearing rocks consisting of larnite, larnite (β-Ca2SiO4) and flamite (α′-Ca2SiO4) aggregates, gehlenite, and magnesioferrite. (C) Mayenite grains filled with larnite, fluorapatite, and spinel inclusions. Mayenite associated with brownmillerite and larnite-flamite aggregates. (D) Large grains of larnite associated with gehlenite, spinel and scarce shulamitite. (E) Mayenite grain surrounded by larnite with radiating cracks. (F) Mosaic of anhedral larnite in association with mayenite, brownmillerite, and periclase. (G) Intergrown rock-forming gehlenite, larnite, and fluorellestadite with numerous fine grains of accessory sharyginite. (H) Local area of bredigite-enriched larnite CM rock. (I) Large fluorellestadite crystals filled with ye’elimite and brownmillerite inclusions. A-H = back-scattered electron (BSE) images; I = photomicrograph in polarized transmitted light. Mineral names are abbreviated as Ap = fluorapatite, Brd = bredigite, Brm = brownmillerite, Els = fluorellestadite, Hgr = hydrogarnet, Gh = gehlenite, Lrn = larnite, May = mayenite supergroup minerals, Mfr = magnesioferrite, Per = periclase, Prv = perovskite, Shr = sharyginite, Spl = spinel, Yel = ye’elimite.
Figure 7.
Morphological diversity of the main phases in ye’elimite– and mayenite-bearing larnite CM rocks. (A) Close association of round larnite grains with {100} twinning and aggregates of larnite (β-Ca2SiO4) lamelli in the flamite (α′-Ca2SiO4) matrix. The α′-Ca2SiO4 and β-Ca2SiO4 lamelli are oriented as {} α′ ‖ {100}β and <0001>α′ ‖ <010>β. (B) Typical assemblage of Ca2SiO4-bearing rocks consisting of larnite, larnite (β-Ca2SiO4) and flamite (α′-Ca2SiO4) aggregates, gehlenite, and magnesioferrite. (C) Mayenite grains filled with larnite, fluorapatite, and spinel inclusions. Mayenite associated with brownmillerite and larnite-flamite aggregates. (D) Large grains of larnite associated with gehlenite, spinel and scarce shulamitite. (E) Mayenite grain surrounded by larnite with radiating cracks. (F) Mosaic of anhedral larnite in association with mayenite, brownmillerite, and periclase. (G) Intergrown rock-forming gehlenite, larnite, and fluorellestadite with numerous fine grains of accessory sharyginite. (H) Local area of bredigite-enriched larnite CM rock. (I) Large fluorellestadite crystals filled with ye’elimite and brownmillerite inclusions. A-H = back-scattered electron (BSE) images; I = photomicrograph in polarized transmitted light. Mineral names are abbreviated as Ap = fluorapatite, Brd = bredigite, Brm = brownmillerite, Els = fluorellestadite, Hgr = hydrogarnet, Gh = gehlenite, Lrn = larnite, May = mayenite supergroup minerals, Mfr = magnesioferrite, Per = periclase, Prv = perovskite, Shr = sharyginite, Spl = spinel, Yel = ye’elimite.
![Minerals 09 00465 g007 Minerals 09 00465 g007]()
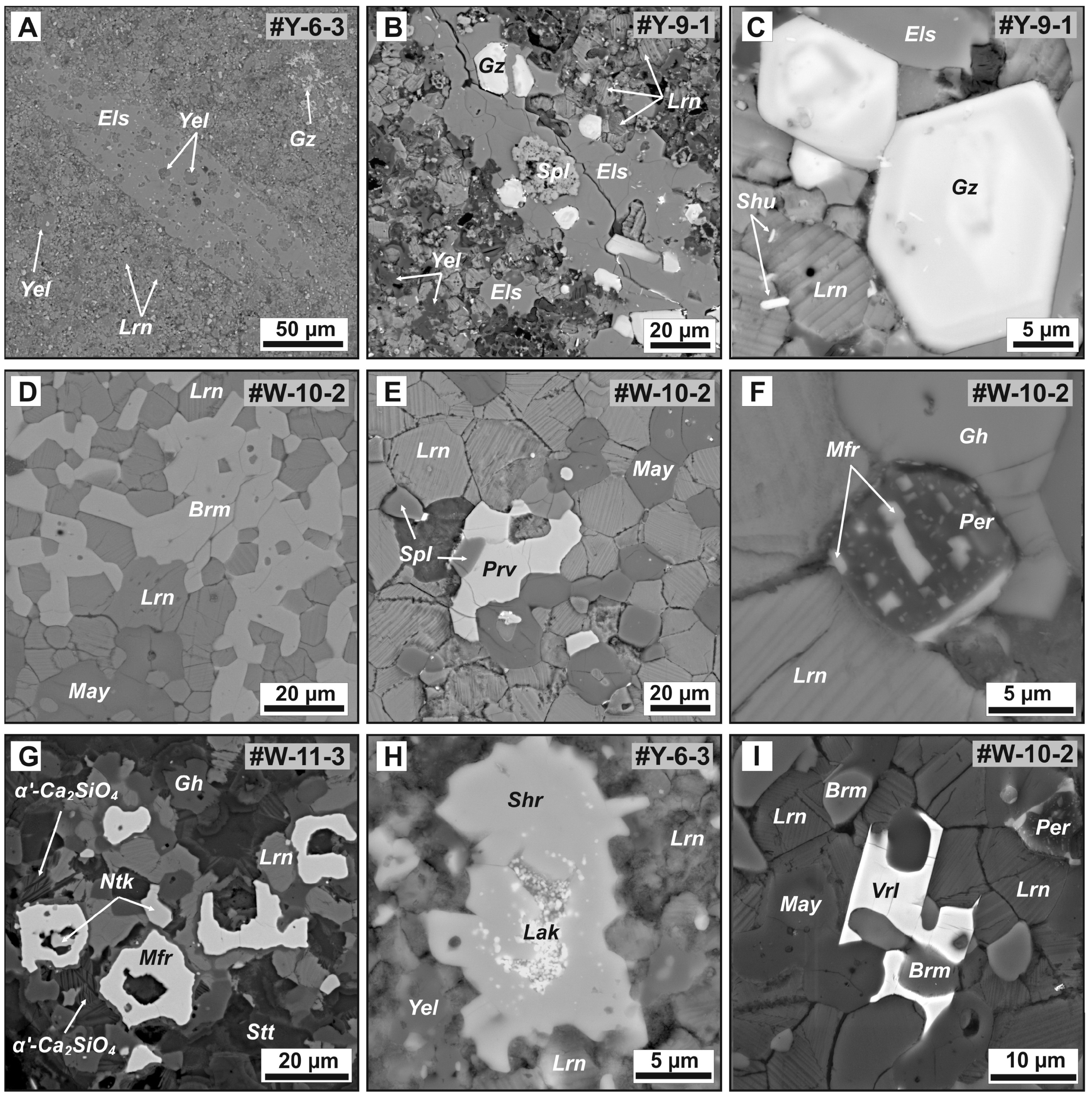
Figure 8.
BSE images of accessory minerals in ye’elimite- and mayenite-bearing larnite CM rocks. Nest of anhedral grains (A) and small faceted crystals (B,C) of gazeevite associated with larnite, fluorellestadite, ye’elimite, shulamitite, and spinel. (D) Mosaic intergrowth of larnite and brownmillerite. (E) Anhedral segregation of perovskite and spinel grains in mayenite–larnite rock. (F) Magnesioferrite microinclusions in periclase. (G) Close association of magnesioferrite and nataliakulikite in gehlenite–larnite rock. (H) Ultrafine lakargiite inclusions in sharyginite. (I) Grains of vorlanite in interstitials between larnite, mayenite and brownmillerite. Mineral names are abbreviated as Brm = brownmillerite, Els = fluorellestadite, Gh = gehlenite, Gz = gazeevite, Lak = lakargiite, Lrn = larnite, May = mayenite supergroup minerals, Mfr = magnesioferrite, Ntk = nataliakulikite, Per = periclase, Prv = perovskite, Shr = sharyginite, Spl = spinel, Vrl = vorlanite, Yel = ye’elimite.
Figure 8.
BSE images of accessory minerals in ye’elimite- and mayenite-bearing larnite CM rocks. Nest of anhedral grains (A) and small faceted crystals (B,C) of gazeevite associated with larnite, fluorellestadite, ye’elimite, shulamitite, and spinel. (D) Mosaic intergrowth of larnite and brownmillerite. (E) Anhedral segregation of perovskite and spinel grains in mayenite–larnite rock. (F) Magnesioferrite microinclusions in periclase. (G) Close association of magnesioferrite and nataliakulikite in gehlenite–larnite rock. (H) Ultrafine lakargiite inclusions in sharyginite. (I) Grains of vorlanite in interstitials between larnite, mayenite and brownmillerite. Mineral names are abbreviated as Brm = brownmillerite, Els = fluorellestadite, Gh = gehlenite, Gz = gazeevite, Lak = lakargiite, Lrn = larnite, May = mayenite supergroup minerals, Mfr = magnesioferrite, Ntk = nataliakulikite, Per = periclase, Prv = perovskite, Shr = sharyginite, Spl = spinel, Vrl = vorlanite, Yel = ye’elimite.
Figure 9.
Morphology and distribution of secondary minerals in larnite CM rocks. BSE images. (A–D) Microstructure of slightly hydrated (etched) larnite with a single set of polysynthetic twins and a lamellar aggregate of larnite and flamite; pore space filled with hillebrandite and chlorkyuygenite; gehlenite grains partially replaced by hydrogarnet. (E) Aggregate of chlorkyuygenite and hydrocalumite after mayenite. (F) Vugs filled with hillebrandite. (G) Aggregate of hillebrandite fibers with fresh fluorapatite, spinel, and perovskite. (H) Ettringite and afwillite filling a crack in larnite rock. (I) Small particle of supergene chromatite. Mineral names are abbreviated as Ap = fluorapatite, Afw = afwillite, Brm = brownmillerite, Brt = barite, Chm = chromatite, Cl-Kgn = chlorkyuygenite, Ett = ettringite, Gh = gehlenite, Gz = gazeevite, Hcl = hydrocalumite, Hgr = hydrogarnet, Hlb = hillebrandite, Lrn = larnite, May = mayenite supergroup minerals, Prv = perovskite, Spl = spinel.
Figure 9.
Morphology and distribution of secondary minerals in larnite CM rocks. BSE images. (A–D) Microstructure of slightly hydrated (etched) larnite with a single set of polysynthetic twins and a lamellar aggregate of larnite and flamite; pore space filled with hillebrandite and chlorkyuygenite; gehlenite grains partially replaced by hydrogarnet. (E) Aggregate of chlorkyuygenite and hydrocalumite after mayenite. (F) Vugs filled with hillebrandite. (G) Aggregate of hillebrandite fibers with fresh fluorapatite, spinel, and perovskite. (H) Ettringite and afwillite filling a crack in larnite rock. (I) Small particle of supergene chromatite. Mineral names are abbreviated as Ap = fluorapatite, Afw = afwillite, Brm = brownmillerite, Brt = barite, Chm = chromatite, Cl-Kgn = chlorkyuygenite, Ett = ettringite, Gh = gehlenite, Gz = gazeevite, Hcl = hydrocalumite, Hgr = hydrogarnet, Hlb = hillebrandite, Lrn = larnite, May = mayenite supergroup minerals, Prv = perovskite, Spl = spinel.
Figure 10.
Compositional variations (wt %) of CaSiO
4 polymorphs from the Hatrurim larnite CM rocks. Symbols stand for: circles = larnite; squares = α′-Ca
2SiO
4 polymorphs; triangles = α-Ca
2SiO
4 ((Na,K)Ca
7(SiO
4)
3(PO
4)), from garnet-rich paralava, Gurim, Hatrurim Basin; rhombs = average flamite composition (holotype, (Na,K)Ca
9(SiO
4)
4(PO
4), α′
L-Ca
2SiO
4) from larnite–gehlenite–rankinite paralava, Hatrurim Basin [
52].
Figure 10.
Compositional variations (wt %) of CaSiO
4 polymorphs from the Hatrurim larnite CM rocks. Symbols stand for: circles = larnite; squares = α′-Ca
2SiO
4 polymorphs; triangles = α-Ca
2SiO
4 ((Na,K)Ca
7(SiO
4)
3(PO
4)), from garnet-rich paralava, Gurim, Hatrurim Basin; rhombs = average flamite composition (holotype, (Na,K)Ca
9(SiO
4)
4(PO
4), α′
L-Ca
2SiO
4) from larnite–gehlenite–rankinite paralava, Hatrurim Basin [
52].
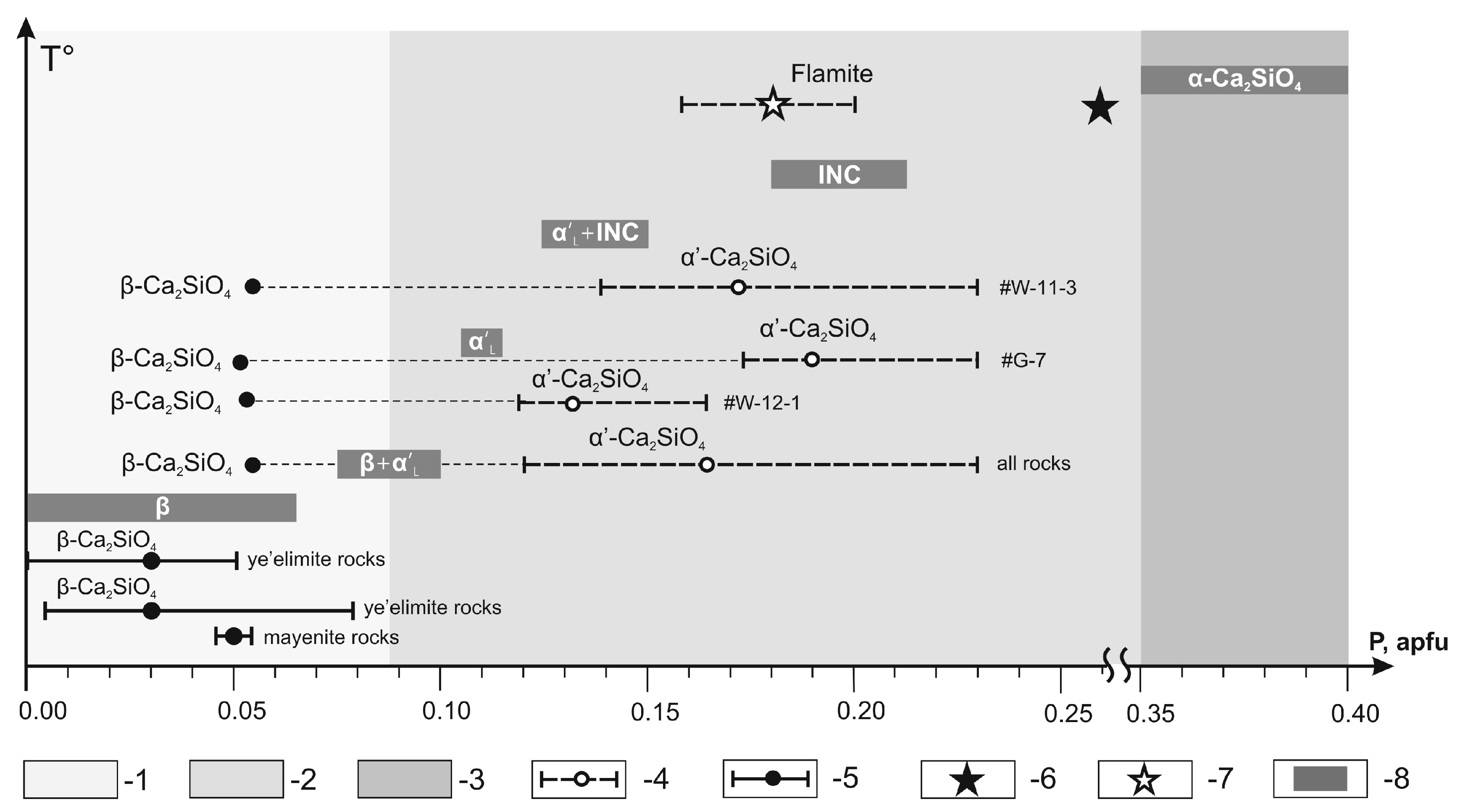
Figure 11.
Compositional ranges of pure and P-doped synthetic β-Ca
2SiO
4, α′-Ca
2SiO
4 and α-Ca
2SiO
4 modifications and incommensurate phase (INC) [
91] and their natural analogs from the Hatrurim larnite CM rocks [
4,
52,
88], compared. 1 = field of complete α′-Ca
2SiO
4-to-β-Ca
2SiO
4 transition; 2 = field of quenched of α′-Ca
2SiO
4 modifications and incommensurate phase (INC); 3 = field of quenched α-Ca
2SiO
4 modification; 4 = range (dash line) and average composition (open circle) of natural flamite (α′-Ca
2SiO
4 modification), this study; 5 = range (solid line) and average composition (black circle) of natural larnite (β-Ca
2SiO
4 modification), this study; 6, 7 = flamite compositions after [
88] (6) and [
52] (7). 8 = ranges of pure and P-doped synthetic β-Ca
2SiO
4, α′-Ca
2SiO
4 and α-Ca
2SiO
4 modifications and incommensurate phase (INC), after [
91].
Figure 11.
Compositional ranges of pure and P-doped synthetic β-Ca
2SiO
4, α′-Ca
2SiO
4 and α-Ca
2SiO
4 modifications and incommensurate phase (INC) [
91] and their natural analogs from the Hatrurim larnite CM rocks [
4,
52,
88], compared. 1 = field of complete α′-Ca
2SiO
4-to-β-Ca
2SiO
4 transition; 2 = field of quenched of α′-Ca
2SiO
4 modifications and incommensurate phase (INC); 3 = field of quenched α-Ca
2SiO
4 modification; 4 = range (dash line) and average composition (open circle) of natural flamite (α′-Ca
2SiO
4 modification), this study; 5 = range (solid line) and average composition (black circle) of natural larnite (β-Ca
2SiO
4 modification), this study; 6, 7 = flamite compositions after [
88] (6) and [
52] (7). 8 = ranges of pure and P-doped synthetic β-Ca
2SiO
4, α′-Ca
2SiO
4 and α-Ca
2SiO
4 modifications and incommensurate phase (INC), after [
91].
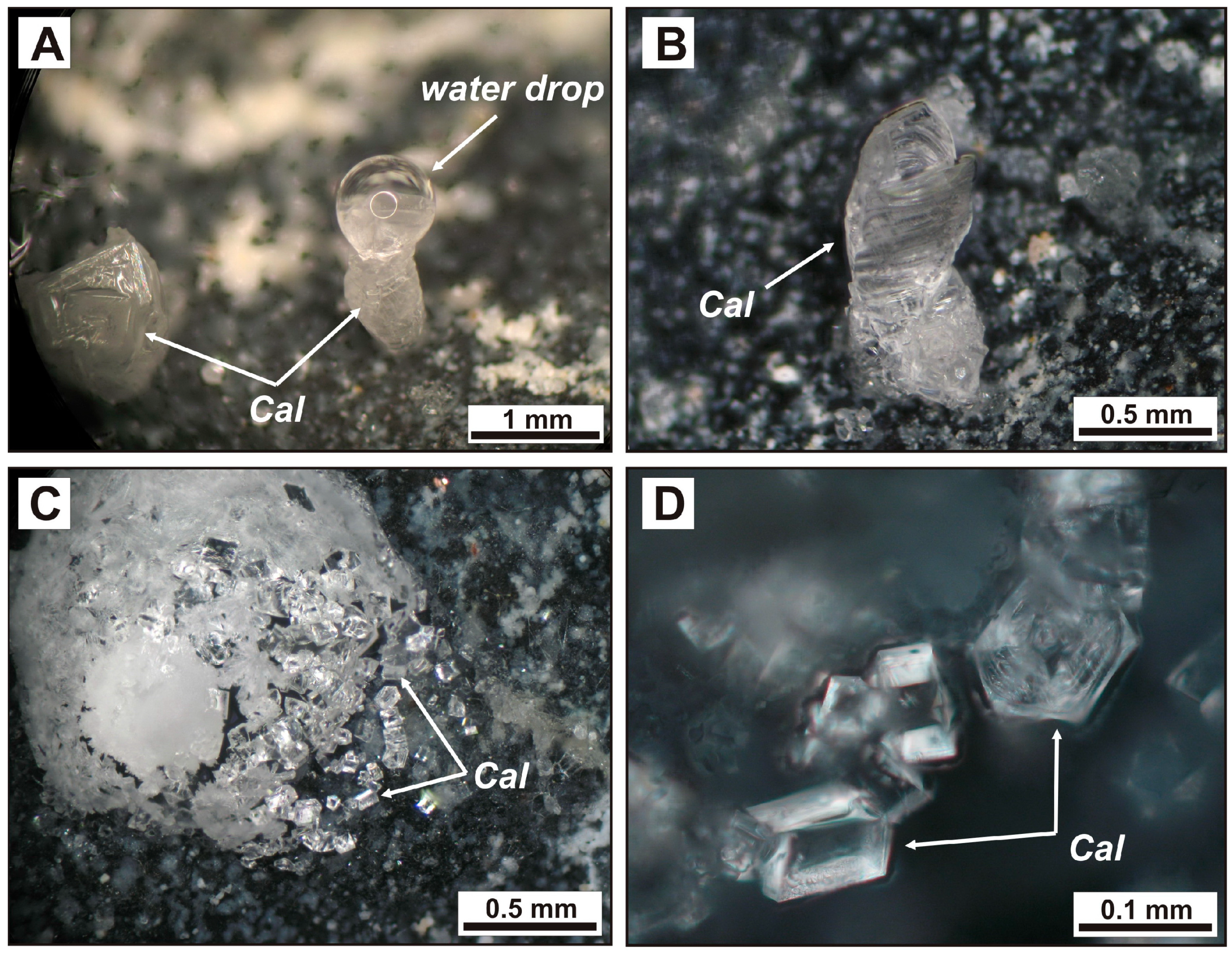
Figure 12.
Products of experimental hydration of larnite CM rocks placed in a closed desiccator together with a water-filled vessel. A, B: calcite tube with a drop of water condensed (A) on its top and evaporated (B). (C,D) crust and individual crystals of fine calcite on the surface of hydrated larnite rocks kept for three to seven months in the desiccator. Cal = calcite.
Figure 12.
Products of experimental hydration of larnite CM rocks placed in a closed desiccator together with a water-filled vessel. A, B: calcite tube with a drop of water condensed (A) on its top and evaporated (B). (C,D) crust and individual crystals of fine calcite on the surface of hydrated larnite rocks kept for three to seven months in the desiccator. Cal = calcite.
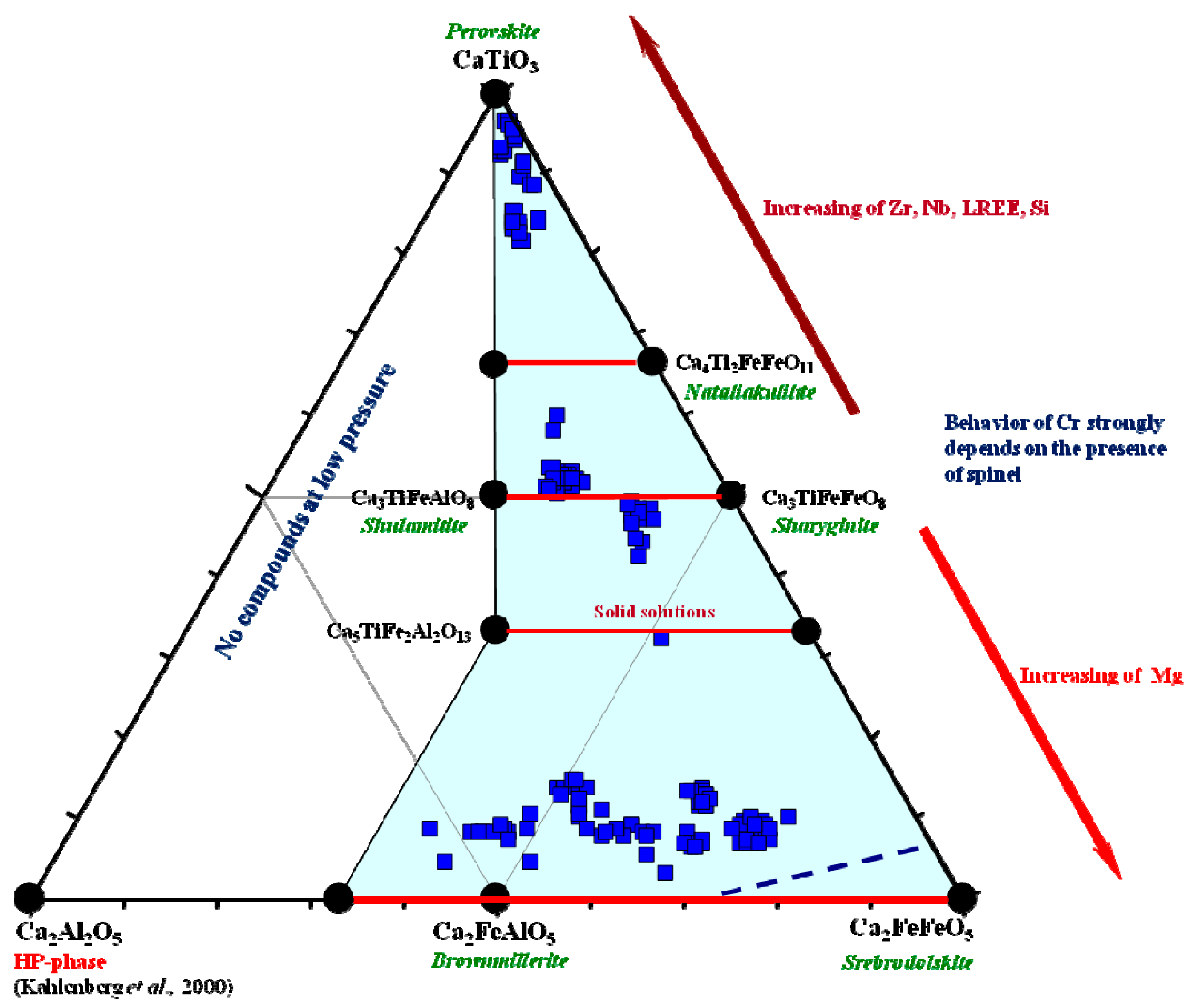
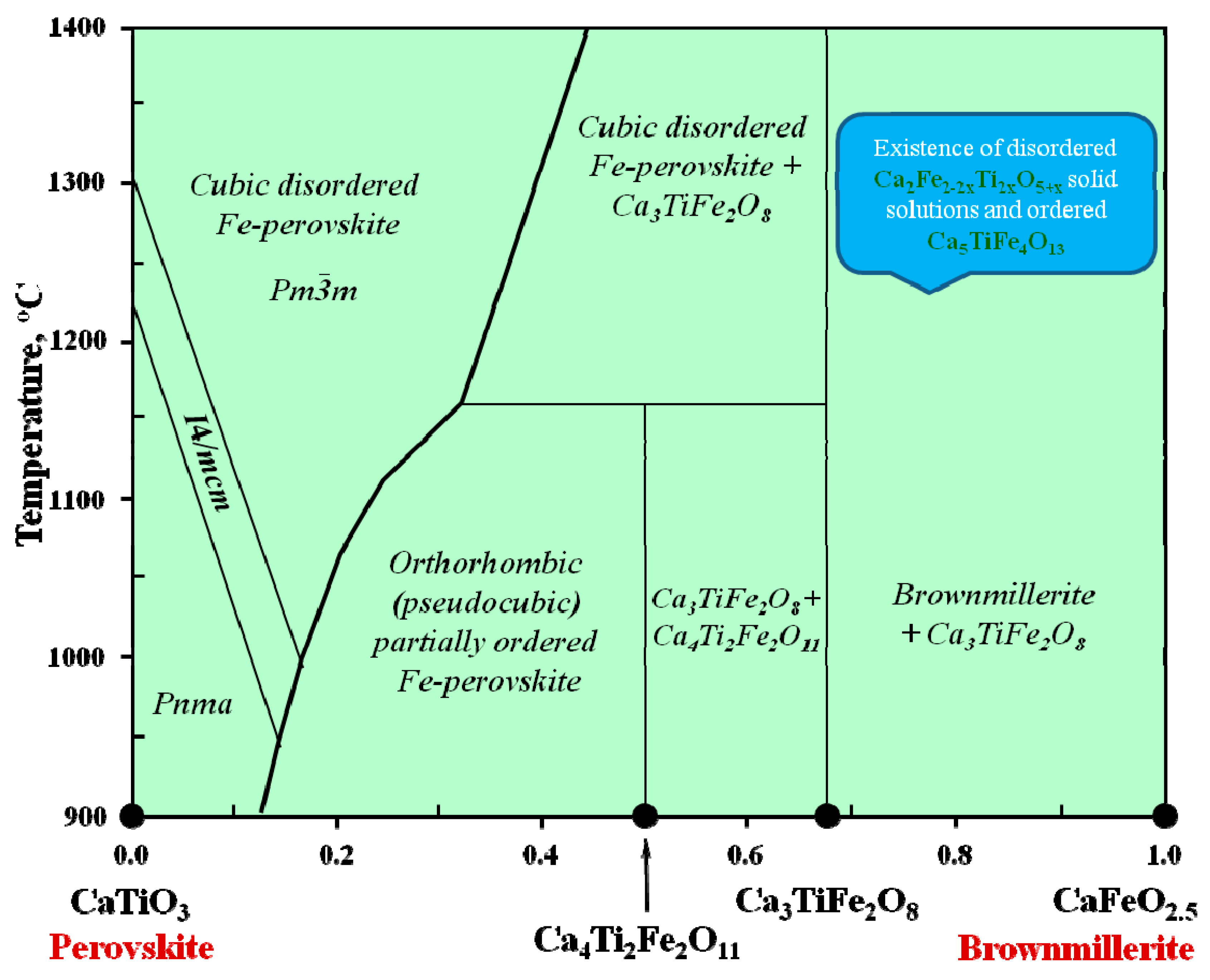
Figure 13.
Phase diagram for the CaTiO
3–CaFeO
2.5 system, modified after [
21,
122,
123,
124].
Figure 13.
Phase diagram for the CaTiO
3–CaFeO
2.5 system, modified after [
21,
122,
123,
124].
Figure 14.
Mineral chemistry for perovskite–supergroup minerals from the Hatrurim larnite CM rocks in CaTiO
3–Ca
2Fe
2O
5–Ca
2Al
2O
5 coordinates, modified after [
25]. Blue field shows natural compositions. Data are from this study (
Table 18) and previous publications [
4,
25,
26,
32].
Figure 14.
Mineral chemistry for perovskite–supergroup minerals from the Hatrurim larnite CM rocks in CaTiO
3–Ca
2Fe
2O
5–Ca
2Al
2O
5 coordinates, modified after [
25]. Blue field shows natural compositions. Data are from this study (
Table 18) and previous publications [
4,
25,
26,
32].
Figure 15.
Composition variations of spinel-group minerals from the Hatrurim larnite CM rocks. Circles, squares, and triangles are ye’elimite–, mayenite–, and gehlenite-bearing larnite rocks, respectively.
Figure 15.
Composition variations of spinel-group minerals from the Hatrurim larnite CM rocks. Circles, squares, and triangles are ye’elimite–, mayenite–, and gehlenite-bearing larnite rocks, respectively.
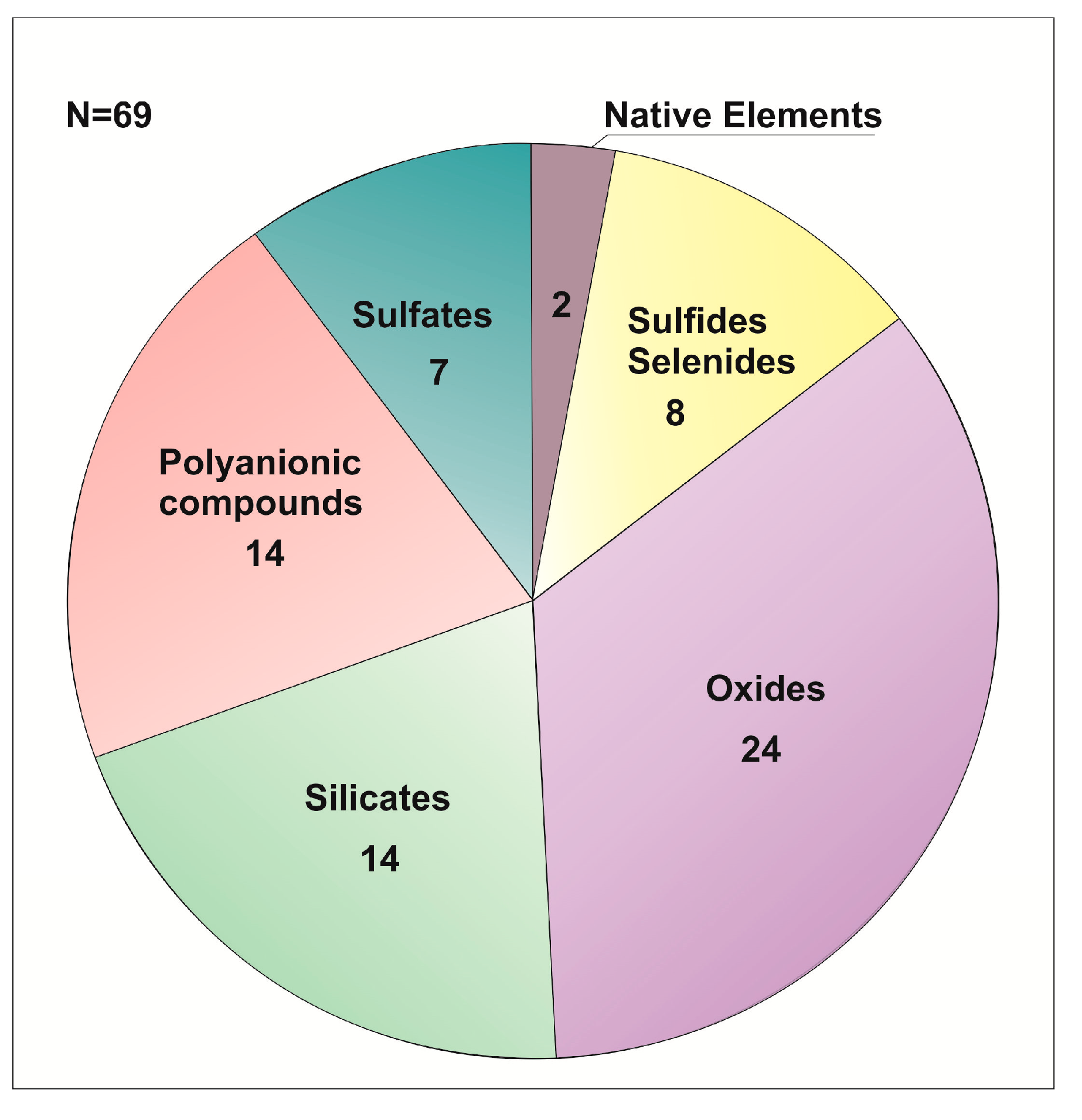
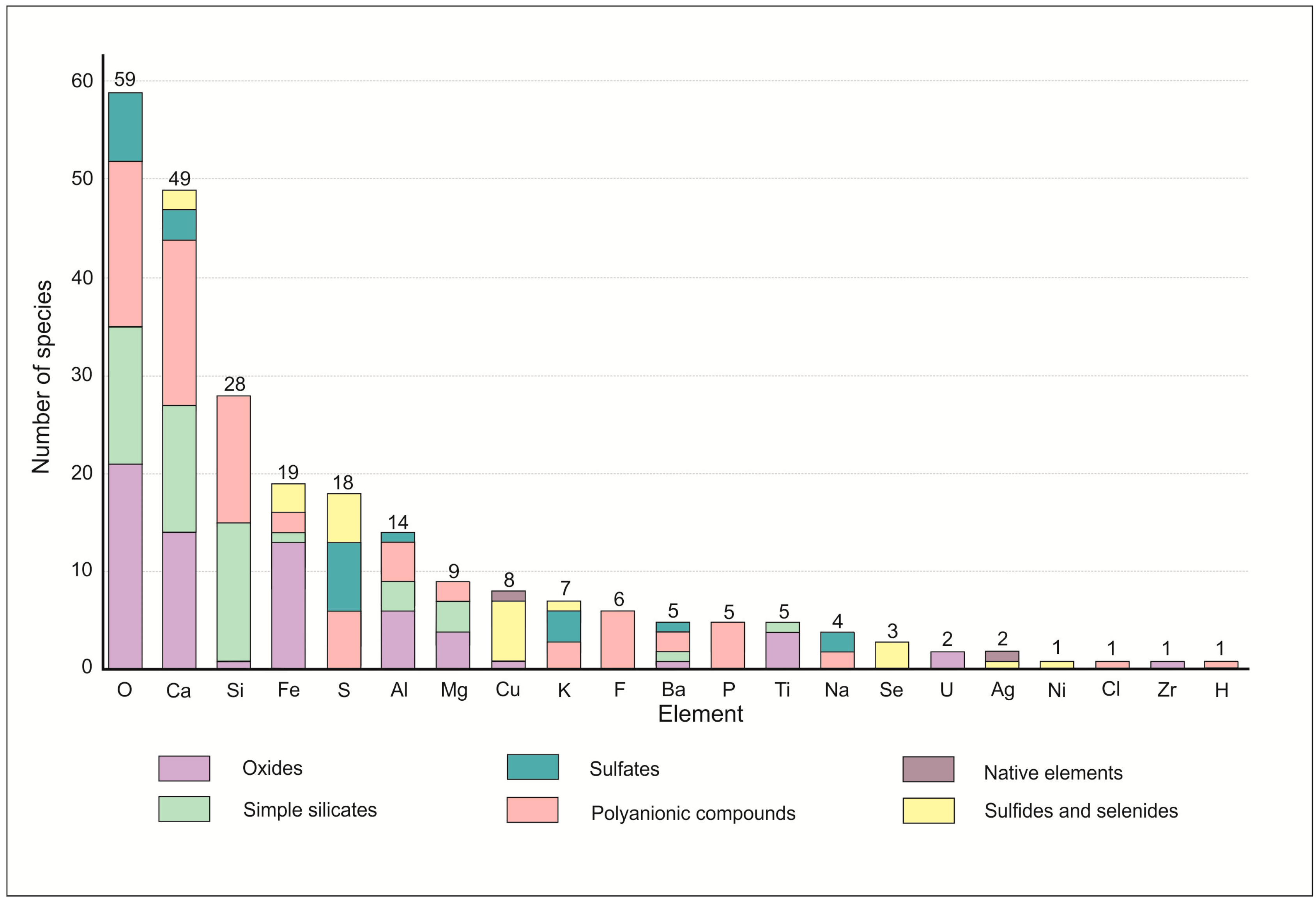
Figure 16.
Distribution of chemical classes of minerals in the Hatrurim Fm larnite CM rocks. N is the number of species.
Figure 16.
Distribution of chemical classes of minerals in the Hatrurim Fm larnite CM rocks. N is the number of species.
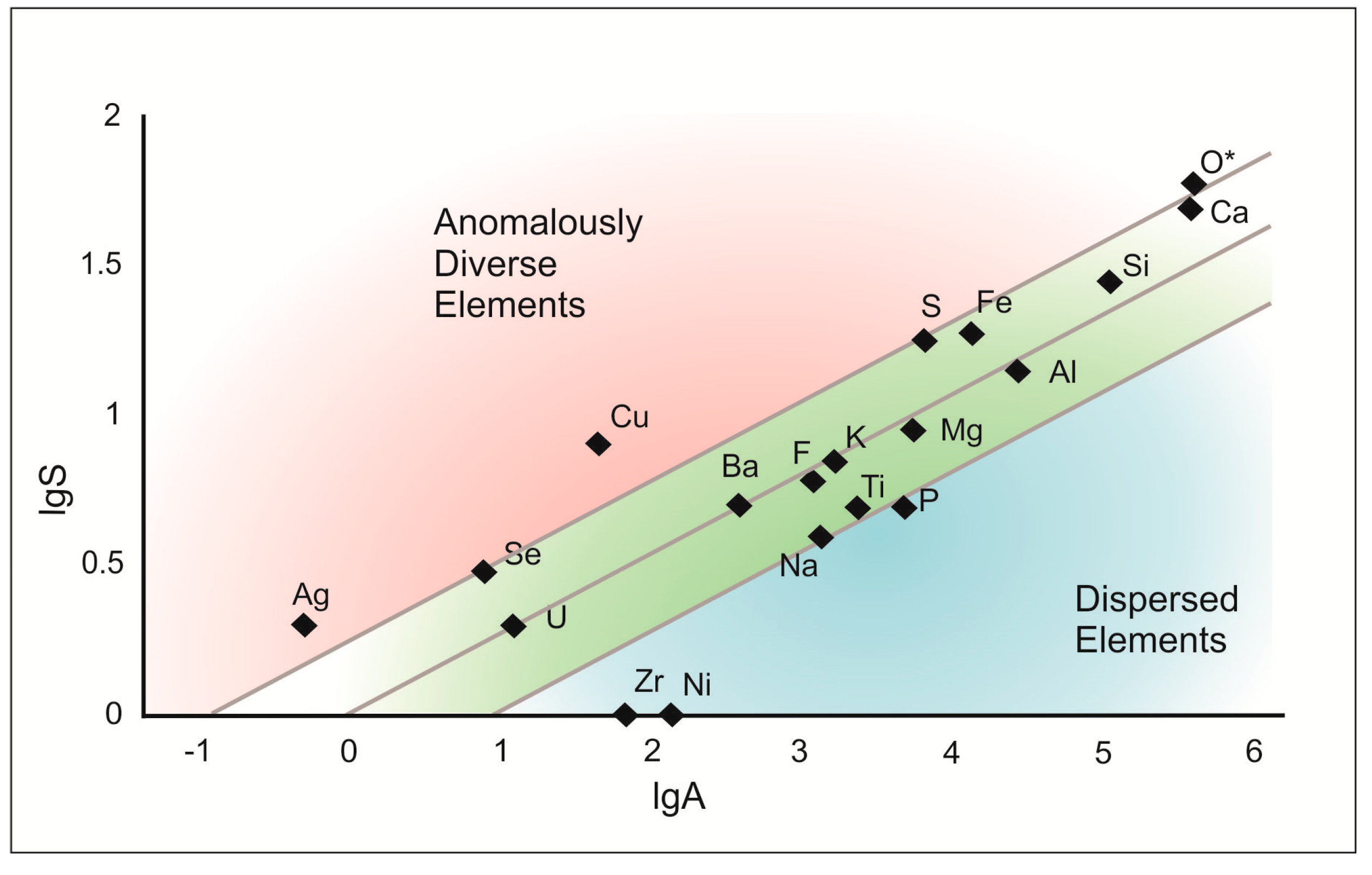
Figure 17.
Number of mineral species (lgS) formed by essential constituent elements versus abundances of these elements in atomic ppm (lgA) in Hatrurim Fm larnite CM rocks. Lines on either side of the central trend present 95% confidence limits for the fit. Based on idea of A. Christy [
132,
133].
Figure 17.
Number of mineral species (lgS) formed by essential constituent elements versus abundances of these elements in atomic ppm (lgA) in Hatrurim Fm larnite CM rocks. Lines on either side of the central trend present 95% confidence limits for the fit. Based on idea of A. Christy [
132,
133].
Figure 18.
Mineralogical diversity of natural Ca2SiO4-bearing rocks and productivity of essential constituent elements.
Figure 18.
Mineralogical diversity of natural Ca2SiO4-bearing rocks and productivity of essential constituent elements.
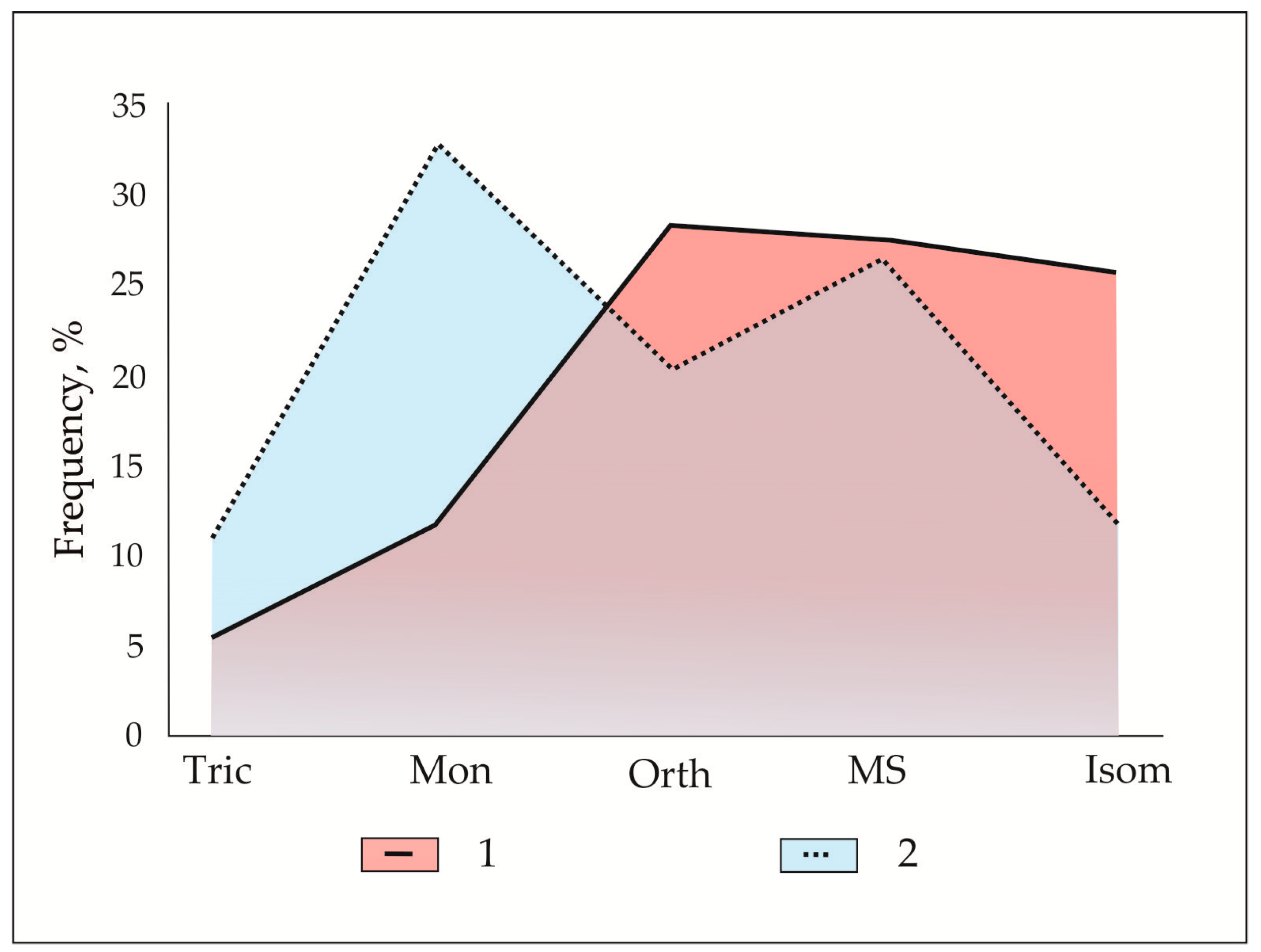
Figure 19.
Symmetry distribution of mineral assemblages in the Hatrurim Fm larnite CM rocks (1) and the Earth lithosphere minerals (2), after [
134], compared. Isom = isometric; Orth = orthorhombic; MS = medium symmetric phases (hexagonal, trigonal, tetragonal); Mon = monoclinic; Tric = triclinic.
Figure 19.
Symmetry distribution of mineral assemblages in the Hatrurim Fm larnite CM rocks (1) and the Earth lithosphere minerals (2), after [
134], compared. Isom = isometric; Orth = orthorhombic; MS = medium symmetric phases (hexagonal, trigonal, tetragonal); Mon = monoclinic; Tric = triclinic.
Table 1.
Minerals in Hatrurim Fm Ca-rich combustion metamorphic (CM) rocks (Israel) and their cement chemist notation (CCN) notations.
Table 1.
Minerals in Hatrurim Fm Ca-rich combustion metamorphic (CM) rocks (Israel) and their cement chemist notation (CCN) notations.
| Mineral | Chemical Formula | Symmetry | CCN Notation |
|---|
| Silicates |
| Hatrurite | Ca3SiO5 | trigonal | C3S (Alite) |
| Flamite | α′-Ca2SiO4 | orthorhombic | C2S (Type I belite) |
| Larnite | β-Ca2SiO4 | monoclinic | C2S (Type II belite) |
| Calcioolivine | γ-Ca2SiO4 | orthorhombic | γ-Ca2SiO4 |
| Rankinite | Ca3Si2O7 | monoclinic | C3S2 |
| Pseudowollastonite | α-Ca3Si3O9 | monoclinic | α-CS |
| Wollastonite | β-Ca3Si3O9 | triclinic | β-CS |
| Parawollastonite | Ca3Si3O9 | monoclinic | CS |
| Bredigite | Ca7Mg(SiO4)4 | orthorhombic | C7MS4 |
| Merwinite | Ca3Mg(SiO4)2 | monoclinic | C3MS2 |
| Gehlenite | Ca2Al2SiO7 | tetragonal | C2AS |
| Polyanionic minerals |
| Nagelschmidtite a | Ca7Si2P2O16 | hexagonal | C7S2P2 |
| Spurrite | Ca5(SiO4)2(CO3) | monoclinic | |
| Ye’elimite b | Ca4Al6O12(SO4) | isometric | C4A3 |
| Cuspidine | Ca4(Si2O7)F2 | monoclinic | C4S22 |
| Fluorapatite | Ca10(PO4)6F2 | hexagonal | C10P62 |
| Fluorellestadite | Ca10[(SiO4)(PO4),(SO4)]6F2 | hexagonal | C10S332 |
| Ternesite (sulfospurrite) | Ca5(SiO4)2(SO4) | orthorhombic | C5S2 |
| Silicocarnotite | Ca5[(SiO4),(PO4)](PO4) | orthorhombic | C5SP2 |
| Fluormayenite–Fluorkyuygenite | Ca12Al14O32[(H2O)0–4F2] | isometric | C12A7Hn2 |
| Aluminate |
| Grossite | CaAl4O7 | monoclinic | CA2 |
| Multiple oxides |
| Srebrodolskite | Ca2Fe2O5 | orthorhombic | CF |
| Brownmillerite | Ca2(Fe2−xAlx)O5 | orthorhombic | C4AF |
| Shulamitite | Ca3TiFe3+AlO8 | orthorhombic | C3FTA |
| Sharyginite | Ca3TiFe2O8 | orthorhombic | C3F2T |
| Perovskite | CaTiO3 | orthorhombic | CT |
| Magnesioferrite | MgFe3+2O4 | isometric | MF |
| Spinel | (Mg,Fe)Al2O4 | isometric | MA |
| Simple oxide |
| Periclase | MgO | isometric | M |
Table 2.
Assemblages of primary minerals found in Hatrurim larnite-bearing CM rocks (Israel).
Table 2.
Assemblages of primary minerals found in Hatrurim larnite-bearing CM rocks (Israel).
| Mineral/Sample | Larnite β-Ca2SiO4 | Flamite α′L-Ca2SiO4 | α′H-Ca2SiO4 | Gehlenite | Mayenite Group | Ye’elimite | Fluorellestadite | Fluorapatite | Silicocarnotite Ternesite | Barite Hashemite | Gazeevite | Brownmillerite Srebrodolskite | Shulamitite Sharyginite | Perovskite Nataliakulikite | Fe–Mg Spinel | Magnesioferrite Magnetite | Periclase | Bredigite | Other Primary Minor and Accessory Minerals | Secondary Phases |
|---|
| Y-5-1 | ▲57.8 | □0.3 | □1.0 | ●4.4 | | ●6.8 | ●3.2 | ▲21.4 | | □tr | □0.4 | | | | ●4.2 | | | | Vrl | □0.3 |
| Y-6-3 | ▲68.7 | | | | | ●8.5 | □1.9 | | | □0.1 | □1.2 | □1.3 | □1.6 | | □tr | ●9.6 | | | Lak, Hem, Arc, Anh | ●7.2 |
| Y-8 | ▲ | | | | | ▲ | ● | ● | | □ | □ | ● | □ | | | ● | | | Mer, Hem | □ |
| M5-30 | ▲ | | | | ● | ▲ | ● | | ▲ | | | ▲ | | | | | □ | | | □ |
| M5-31 | ▲ | | | | ▲ | ● | | ▲ | ▲ | □ | | ▲ | □ | | ▲ | ▲ | | | Vrl | □ |
| M4-217 | ▲ | | | | | ▲ | ▲ | | | | | ▲ | | | | | ● | | | □ |
| YV-412 | ▲ | | | | | ▲ | ▲ | | | | | ▲ | | | | | ● | | Berz/Bell, Euc, Vrl | □tr |
| YV-411 | ▲ | | | | | ▲ | | ▲ | | ● | | | ● | | | ● | | | | □tr |
| YV-410 | ▲ | | | ▲ | | ● | | ▲ | | | | ▲ | □ | | | □ | | | Hat, Po | □tr |
| H-201 | ▲ | | | | | ▲ | ● | | | | | ▲ | ● | | | ▲ | | | | □ |
| MP-10-1 | ▲ | | | | | ▲ | | ▲ | | | | ▲ | ● | | ● | | | | | □tr |
| Y-10-5 | ▲23.2 | | | ▲53.0 | | | | □2.9 | | | | | | □0.2 | | ●3.0 | | ▲11.5 | Po, Andr | ●6.2 |
| W-11-3 | ▲32.4 | □1.6 | □2.4 | ▲45.1 | | | | ●6.0 | | □0.1 | | | | □0.6 | | ●5.6 | | | | ●6.5 |
| Y-7 | ▲43.2 | ▲15.2 | □1.3 | □0.7 | ●6.0 | □tr | ▲10.6 | | | | | ▲10.5 | | | | | | | | ▲11.8 |
| Y-9-1 | ▲73.3 | | | □1.5 | □1.2 | □0.5 | ●8.6 | □1.8 | | □0.1 | □0.3 | | □1.4 | | □tr | □0.3 | | | | ▲11.4 |
| G-7 | ▲51.4 | ●4.7 | ●3.0 | ▲17.7 | □2.5 | | | ●3.0 | | | | □4.7 | ●6.5 | | ●7.1 | | | | Vrl, Cpp | □tr |
| W-10-2 | ▲73.6 | | | ●8.7 | ●9.9 | | | □1.5 | | □tr | | □2.9 | □tr | □0.1 | □1.3 | □tr | □tr | | Vrl | □1.9 |
| W-12-1 | ▲ | | | | ▲ | | | | | □ | □ | ▲ | | | | □ | | | | □tr |
| CONCR | ▲ | □ | □ | ▲ | ▲ | | | □ | | | | ▲ | | | ▲ | ▲ | | | | □ |
| M5-32 | ▲ | □ | □ | | ▲ | | | □ | | | | ▲ | □ | | | | ● | | Vrl | □ |
| M4-215 | ▲ | | | | ▲ | | | □ | | | | | □ | ● | ▲ | | ● | | | □ |
| M4-218 | ▲ | | | | ▲ | □ | | □ | | | | | ▲ | | ▲ | ▲ | ● | | Old, Po, K-Fe-sulf | □ |
| M4-251 | ▲ | □ | □ | ▲ | ▲ | | | | | | | | □ | ▲ | ▲ | | | | | □ |
| Y-2-1 | ▲ | | | □ | ▲ | | | | | | | ▲ | | | | □ | ● | | Cus | □tr |
| H-401 | ▲ | ▲ | □ | ▲ | ▲ | | | □ | | | | | | ▲ | | ▲ | | □ | Hem | □ |
Table 3.
Secondary mineral assemblages in Ca2SiO4-bearing CM rocks (Hatrurim Basin, Israel).
Table 3.
Secondary mineral assemblages in Ca2SiO4-bearing CM rocks (Hatrurim Basin, Israel).
| Mineral/Sample | Calcite | Portlandite | Hydrogarnet | Hydrocalumite | Hillebrandite | Straetlingite | Afwillite | Ettringite | Apophyllite-(OH) | Chlorkyuygenite | Gibbsite | Other |
|---|
| Y-5-1 | | | □0.3 | | | | | | | | | |
| Y-6-3 | □0.8 | | □2.6 | | □0.8 | | ●3.0 | □(Cr) | | | | Chromatite |
| Y-8 | | | | | | □ | | | | | | |
| YV-412 | | | | | | | | | | | □tr | |
| YV-411 | | | | | | | | | | | □tr | Tobermorite |
| YV-410 | | | | | | | | | | | □tr | Tobermorite |
| | | | | | | | | | | | | |
| Y-7 | | | ●7.8 | | □2.2 | □1.8 | | | | | | |
| Y-9-1 | | | ●8.3 | □1.3 | | | | | □1.8 | □tr | | |
| | | | | | | | | | | | | |
| G-7 | | | | | □tr | □tr | | | | | | |
| W-10-2 | □0.2 | □1.0 | □0.4 | | □0.3 | □tr | | | | | | |
| W-12-1 | | | | | □ | | | | | | | |
| M4-218 | | □ | □ | | □ | □ | | | | | | Foshagite, Hem |
| Y-2-1 | | | | | □ | □ | | | | □tr | | |
| | | | | | | | | | | | | |
| Y-10-5 | | | ●5.2 | | □0.7 | | | | | | | Hem□0.3 |
| W-11-3 | □0.7 | □0.7 | ●3.0 | | □1.9 | | □0.2 | □tr | | | | |
Table 4.
Major-element (in wt %) compositions of sedimentary protoliths (Ghareb Fm “oil shales”).
Table 4.
Major-element (in wt %) compositions of sedimentary protoliths (Ghareb Fm “oil shales”).
| Locality | Sample | SiO2 | TiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | P2O5 | SO3 | LOI | Total | CO2 | Corg |
|---|
| Pama | 1 | YG-1 | 2.40 | 0.04 | 0.60 | 0.30 | 2.30 | 45.0 | n.a. | n.a. | 11.2 | 3.60 | 33.0 | 98.4 | n.a. | 6.00 |
| 2 | YG-3 | 4.60 | 0.07 | 1.20 | 0.50 | 0.50 | 32.0 | n.a. | n.a. | 0.90 | 8.00 | n.a. | | n.a. | 21.0 |
| 3 | YG-13 | 8.90 | 0.15 | 3.80 | 2.00 | 0.30 | 36.0 | n.a. | n.a. | 0.80 | 4.60 | n.a. | | n.a. | 7.00 |
| 4 | YG-49 | 12.2 | 0.21 | 5.20 | 2.40 | 0.40 | 33.0 | n.a. | n.a. | 1.00 | 5.70 | n.a. | | n.a. | 7.00 |
| 5 | YG-72p | 16.6 | 0.31 | 7.00 | 2.40 | 0.40 | 36.0 | n.a. | n.a. | 1.00 | 3.00 | n.a. | | n.a. | 8.00 |
| | 6 | A | 9.30 | 0.16 | 3.80 | 1.70 | 0.94 | 34.2 | 0.24 | 0.34 | 2.70 | 6.80 | 35.7 | 95.9 | n.a. | n.a. |
| 7 | B | 11.1 | 0.21 | 5.10 | 2.30 | 0.93 | 34.1 | 0.21 | 0.35 | 1.80 | 6.20 | 33.6 | 95.9 | n.a. | n.a. |
| 8 | C | 7.40 | 0.11 | 2.40 | 1.10 | 0.96 | 34.1 | 0.29 | 0.31 | 3.60 | 7.30 | 37.3 | 94.9 | n.a. | n.a. |
| Rotem borehole | 9 | 401/40–41 | 14.0 | n.a. | 5.53 | 2.70 | 0.61 | 31.7 | 0.24 | 0.37 | 0.73 | 6.90 | 38.2 | 94.1 | n.a. | 9.51 |
| 10 | 423/42–43 | 11.7 | n.a. | 4.53 | 2.26 | 0.52 | 33.2 | 0.22 | 0.32 | 1.95 | 6.65 | 39.6 | 94.3 | n.a. | 10.1 |
| 11 | 478/47–48 | 9.44 | n.a. | 3.53 | 1.96 | 0.50 | 37.6 | 0.21 | 0.29 | 1.75 | 5.93 | 39.7 | 94.9 | n.a. | 8.53 |
| 12 | 523/52–53 | 7.63 | n.a. | 2.46 | 1.53 | 0.54 | 38.6 | 0.24 | 0.29 | 2.22 | 6.71 | 41.8 | 95.3 | n.a. | 10.3 |
| 13 | 534/53–54 | 8.30 | n.a. | 2.87 | 1.74 | 0.58 | 36.0 | 0.24 | 0.28 | 3.07 | 7.48 | 42.0 | 95.1 | n.a. | 11.9 |
| 14 | 556/55–56 | 9.94 | n.a. | 3.23 | 1.98 | 0.68 | 34.4 | 0.26 | 0.32 | 2.76 | 7.92 | 41.4 | 95.0 | n.a. | 11.8 |
| 15 | 589/58–59 | 8.08 | n.a. | 1.41 | 0.70 | 0.53 | 35.6 | 0.24 | 0.30 | 4.66 | 7.65 | 44.3 | 95.7 | n.a. | 13.3 |
| 16 | 601/60–61 | 5.91 | n.a. | 1.14 | 0.54 | 0.45 | 35.5 | 0.24 | 0.28 | 3.28 | 8.26 | 48.3 | 95.6 | n.a. | 15.6 |
| 17 | 612/61–62 | 6.37 | n.a. | 1.27 | 0.50 | 0.48 | 35.0 | 0.24 | 0.29 | 3.42 | 8.50 | 48.2 | 95.8 | n.a. | 15.8 |
| Ta’alat Hayamim borehole | 18 | AG-47 | 11.7 | 0.10 | 4.30 | 2.10 | 0.70 | 43.0 | 0.30 | 0.50 | 0.19 | 1.20 | 35.1 | 99.2 | n.a. | n.a. |
| 19 | AG-48 | 11.4 | 0.20 | 4.40 | 2.10 | 0.60 | 36.4 | 0.40 | 0.50 | 2.40 | 3.60 | 36.5 | 98.5 | n.a. | n.a. |
| Hatrurim Basin | 20 | YV-114 | 7.90 | 0.10 | 2.80 | 1.80 | 0.50 | 47.0 | 0.30 | 0.30 | 0.70 | n.a. | 37.9 | 99.3 | n.a. | n.a. |
| 21 | 4 | 6.90 | 0.10 | 2.00 | 1.00 | 1.00 | 46.7 | 0.50 | <0.20 | 2.50 | n.a. | 38.8 | 99.7 | 24.6 | n.a. |
| Hatrurim Basin | 22 | 11 | 5.60 | <0.1 | 0.90 | 0.60 | 0.50 | 49.0 | 1.20 | 0.30 | 2.80 | n.a. | 39.6 | 100.8 | 31.7 | n.a. |
| 23 | 26 | 18.5 | 0.30 | 3.60 | 1.80 | 2.20 | 37.9 | 1.40 | 0.30 | 2.80 | n.a. | 31.5 | 100 | n.a. | n.a. |
| 24 | 28 | 16.9 | 0.20 | 5.30 | 2.20 | 1.60 | 33.5 | 1.70 | 0.30 | 1.40 | n.a. | 36.9 | 100 | n.a. | n.a. |
| 25 | AB-624 | 8.50 | 0.12 | 3.20 | 3.20 | 0.80 | 42.0 | n.a. | n.a. | <0.50 | <0.50 | n.a. | | n.a. | n.a. |
| 26 | AB-651 | 13.8 | 0.22 | 5.70 | 2.60 | 1.40 | 36.0 | n.a. | n.a. | 1.20 | <0.50 | n.a. | | n.a. | n.a. |
| 27 | AB-657 | 2.40 | 0.02 | 0.60 | 1.30 | 2.20 | 43.0 | n.a. | n.a. | <0.50 | <0.50 | n.a. | | n.a. | n.a. |
| 28 | AB-730 | 3.70 | 0.04 | 1.20 | 1.50 | 0.60 | 51.0 | n.a. | n.a. | <0.50 | <0.50 | n.a. | | n.a. | n.a. |
| Nabi Musa | 29 | 10 | 5.20 | 0.10 | 1.00 | 0.80 | 0.50 | 49.4 | 0.80 | 0.20 | 2.20 | n.a. | 40.3 | 100 | n.a. | n.a. |
| 30 | AG-27b | 3.10 | <0.10 | 0.90 | 0.40 | 0.40 | 41.8 | 0.30 | 0.20 | 1.70 | 0.40 | 48.7 | 97.4 | n.a. | n.a. |
| 31 | AG-19b | 6.30 | <0.10 | 1.00 | 0.40 | 0.30 | 51.0 | 0.30 | 0.10 | 3.60 | 0.90 | 34.6 | 99.4 | n.a. | n.a. |
| Nahal Ayalon | 32 | 438 | 8.10 | 0.20 | 3.50 | 0.60 | 0.90 | 46.5 | 0.20 | <0.20 | 0.70 | 0.8 * | 38.8 | 100 | 34.7 | 0.30 |
| N | 78 | 69 | 78 | 78 | 78 | 78 | 23 | 23 | 78 | 72 | | | 3 | 41 |
| Mean | 8.84 | 0.14 | 3.37 | 1.77 | 0.60 | 37.3 | 0.40 | 0.28 | 1.34 | 3.77 | | | 30.3 | 8.52 |
| S | 3.77 | 0.08 | 1.84 | 0.77 | 0.42 | 6.70 | 0.33 | 0.11 | 1.02 | 2.76 | | | 5.19 | 3.05 |
| Min | 2.40 | 0.02 | 0.60 | 0.30 | 0.30 | 25.0 | 0.20 | 0.10 | 0.19 | 0.10 | | | 24.6 | 0.30 |
| Max | 18.5 | 0.31 | 7.00 | 3.50 | 5.10 | 51.0 | 1.70 | 0.50 | 11.2 | 8.50 | | | 34.7 | 21.0 |
| Post-Archean carbonate ** | 13.7 | 0.10 | 1.76 | 0.91 | 10.6 | 34.5 | 0.17 | 0.62 | 0.05 | n.a. | 37.3 | 99.7 | n.a. | n.a. |
Table 5.
Trace-element compositions (in ppm) of sedimentary protoliths (Ghareb Fm “oil shales”).
Table 5.
Trace-element compositions (in ppm) of sedimentary protoliths (Ghareb Fm “oil shales”).
| Locality | Sample | V | Cr | Mn | Co | Ni | Cu | Zn | As | Rb | Sr | Mo | Cd | Sb | Ba | Pb | Th | U |
|---|
| Pama | 1 | PAMA | 67.3 | 245 | n.a. | n.a. | 139 | 74.9 | 245 | 6.21 | 14.7 | 1370 | 11.9 | n.a. | n.a. | 125 | n.a. | 2.61 | 15.1 |
| 2 | PAMA | 57.0 | 234 | 49.0 | n.a. | 142 | 67.0 | 220 | 6.00 | 14.0 | 1226 | 8.00 | n.a. | n.a. | 55.0 | n.a. | <2.00 | 27.0 |
| 3 | YV-195 | 85.0 | 240 | n.a. | n.a. | 115 | 55.0 | 165 | 9.00 | 14.0 | n.a. | 22.0 | n.a. | n.a. | 50.0 | 14.0 | 2.00 | 11.0 |
| 4 | YG-1 | 83.0 | 190 | 60.00 | 1.70 | 117 | 64.0 | 206 | 54.0 | n.a. | 2079 | 19.0 | 3.10 | 1.90 | 66.0 | 3.30 | 1.50 | 100 |
| 5 | YG-3 | 140 | 465 | 53.00 | 2.40 | 244 | 129 | 380 | 89.0 | n.a. | 1442 | 30.0 | 2.10 | 4.30 | 59.0 | 4.70 | 1.80 | 42.0 |
| 6 | YG-13 | 89.0 | 232 | 45.00 | 4.40 | 126 | 61.0 | 126 | 11.0 | n.a. | 1331 | 11.0 | 0.50 | 0.80 | 45.0 | 4.00 | 2.00 | 13.0 |
| 7 | YG-49 | 99.0 | 259 | 50.00 | 5.80 | 128 | 65.0 | 131 | 13.0 | n.a. | 1051 | 13.0 | 0.60 | 1.10 | 54.0 | 5.30 | 2.30 | 16.0 |
| 8 | YG-72p | 104 | 321 | 44.0 | 4.90 | 79.0 | 60.0 | 109 | 7.00 | n.a. | 1183 | 6.00 | 0.50 | 0.80 | 74.0 | 6.40 | 2.40 | 16.0 |
| Ta’alat Hayamim borehole | 9 | AG-47c | 90.0 | 80.0 | 1300 | 25.0 | 220 | 30.0 | 200 | 3.50 | 20.0 | 930 | 1.10 | 0.90 | 1.80 | 45.0 | 15.0 | 3.00 | 3.50 |
| 10 | AG-48c | 130 | 300 | 80.0 | 15.0 | 150 | 115 | 200 | 15.0 | 19.0 | 1200 | 13.0 | 0.60 | 1.20 | 110 | 7.50 | 2.00 | 15.5 |
| Hatrurim Basin | 11 | YV-114 | 54.0 | 115 | 200 | n.a. | 47.0 | n.a. | 50.0 | n.a. | 11.0 | 1100 | 5.70 | n.a. | n.a. | 46.0 | n.a. | 1.40 | 5.40 |
| 12 | 4 | 115 | 400 | <100 | n.a. | 190 | n.a. | 175 | n.a. | 1.00 | 500 | 20.0 | n.a. | n.a. | 490 | n.a. | 1.20 | 29.0 |
| 13 | 11 | 36.0 | 25.0 | <100 | n.a. | 95.0 | n.a. | 45.0 | n.a. | 7.00 | 680 | 10.0 | n.a. | n.a. | 40.0 | n.a. | 0.40 | 9.00 |
| 14 | 26 | 90.0 | 210 | 100 | n.a. | 150 | n.a. | 360 | n.a. | 13.0 | 1266 | 4.00 | n.a. | n.a. | 755 | n.a. | 2.20 | 17.0 |
| 15 | 28 | 82.0 | 200 | 95.0 | n.a. | 175 | n.a. | 460 | n.a. | 18.0 | 1389 | 9.00 | n.a. | n.a. | 2200 | n.a. | 1.90 | 16.0 |
| 16 | AB-624 | 168 | 52.0 | 827 | 18.0 | 152 | 37.0 | 133 | 11.00 | n.a. | 970 | 2.70 | 1.60 | 0.70 | 57.0 | 12.0 | 1.40 | 7.00 |
| 17 | AB-651 | 111 | 155 | 109 | 8.00 | 153 | 68.0 | 171 | 13.0 | n.a. | 780 | 4.80 | 0.60 | 1.00 | 47.0 | 8.00 | 1.90 | 16.0 |
| | 18 | AB-657 | 34.0 | 4.00 | 131 | 2.00 | 31.0 | 9.00 | 54.0 | 4.00 | n.a. | 890 | 1.50 | 0.20 | 0.30 | 68.0 | 4.00 | 0.40 | 6.00 |
| 19 | AB-730 | 37.0 | 15.0 | 218 | 5.00 | 48.0 | 16.0 | 24.0 | 4.00 | n.a. | 966 | 1.20 | 0.20 | 0.20 | 72.0 | 5.00 | 0.70 | 3.00 |
| Nabi Musa | 20 | 10 | 72.0 | 250 | <100 | n.a. | 155 | n.a. | 338 | n.a. | 6.00 | 1300 | 22.0 | n.a. | n.a. | 215 | n.a. | 0.70 | 23.0 |
| 21 | AG-27b | 50.0 | 228 | 15.0 | 4.00 | 84.0 | 74.0 | 340 | 9.00 | 4.00 | 1021 | 13.0 | 1.90 | 1.00 | 86.0 | 3.00 | 25.5 | 0.70 |
| 22 | AG-19b | 83.0 | 138 | 15.0 | 2.00 | 73.0 | 83.0 | 330 | 7.00 | 1.10 | 1220 | 12.4 | 1.40 | 0.60 | 174 | 1.00 | 33.0 | 1.60 |
| N | 68 | 68 | 66 | 59 | 68 | 62 | 68 | 62 | 13 | 67 | 68 | 59 | 59 | 68 | 60 | 68 | 68 |
| Mean | 95.2 | 221 | 107 | 5.37 | 127 | 61.9 | 158 | 12.7 | 11.1 | 1112 | 11.9 | 0.83 | 1.21 | 88.8 | 5.61 | 2.14 | 16.5 |
| S | 30.5 | 107 | 140 | 2.77 | 42.9 | 23.6 | 82.4 | 8.54 | 5.80 | 261 | 7.71 | 0.48 | 0.64 | 112 | 2.40 | 3.05 | 9.11 |
| Min | 33.0 | 4.00 | 15.0 | 1.30 | 31.0 | 9.00 | 15.0 | 2.00 | 1.00 | 400 | 0.90 | 0.20 | <0.1 | 24.0 | 1.00 | 0.40 | 0.70 |
| Max | 168 | 465 | 1300 | 25.0 | 244 | 129 | 460 | 89.0 | 20.0 | 3000 | 37.0 | 3.10 | 4.30 | 2200 | 15.0 | 33.0 | 100 |
| Post-Archean carbonate ** | 20.0 | 9.00 | 464 | 3.00 | 6.00 | 8.00 | 22.0 | 4.80 | 17.0 | 245 | 0.56 | 0.11 | n.a. | 178 | 13.3 | 1.99 | 1.10 |
Table 6.
Effective correlation factor for bitumen-bearing calcareous rocks from Hatrurim Basin and Nabi Musa MZ areas, Pama career, Ta’alat Hayamim borehole, Ghareb Fm, Israel.
Table 6.
Effective correlation factor for bitumen-bearing calcareous rocks from Hatrurim Basin and Nabi Musa MZ areas, Pama career, Ta’alat Hayamim borehole, Ghareb Fm, Israel.
| Component | Factor | Communality |
|---|
| 1 | 2 | 3 | 4 | 5 |
|---|
| SiO2 | −0.94 | 0.16 | −0.16 | −0.01 | −0.07 | 0.94 |
| TiO2 | −0.93 | 0.11 | −0.17 | −0.04 | −0.10 | 0.92 |
| Al2O3 | −0.95 | 0.15 | −0.19 | 0.03 | −0.04 | 0.97 |
| Fe2O3 | −0.89 | 0.31 | −0.19 | −0.02 | −0.04 | 0.93 |
| MgO | 0.62 | −0.24 | −0.63 | 0.35 | −0.04 | 0.96 |
| CaO | 0.57 | −0.41 | −0.38 | −0.54 | 0.01 | 0.94 |
| P2O5 | 0.62 | −0.29 | −0.65 | 0.27 | −0.04 | 0.97 |
| SO3 | 0.31 | 0.31 | 0.55 | 0.62 | 0.25 | 0.94 |
| TOC | 0.26 | 0.61 | 0.20 | 0.33 | 0.23 | 0.65 |
| V | 0.08 | 0.91 | −0.09 | −0.06 | −0.02 | 0.85 |
| Cr | 0.12 | 0.92 | 0.11 | −0.20 | −0.09 | 0.93 |
| Mn | −0.15 | 0.69 | −0.56 | 0.21 | 0.21 | 0.91 |
| Co | −0.74 | 0.53 | −0.28 | 0.09 | 0.10 | 0.92 |
| Ni | 0.40 | 0.82 | 0.16 | −0.04 | −0.02 | 0.86 |
| Cu | 0.38 | 0.77 | −0.01 | −0.41 | −0.07 | 0.91 |
| Zn | 0.73 | 0.60 | 0.13 | −0.03 | −0.09 | 0.92 |
| As | 0.75 | 0.46 | −0.17 | 0.01 | 0.06 | 0.81 |
| Sr | 0.69 | 0.08 | −0.61 | −0.23 | 0.02 | 0.91 |
| Mo | 0.73 | 0.38 | 0.20 | −0.03 | −0.14 | 0.74 |
| Cd | 0.92 | 0.00 | −0.27 | 0.17 | −0.03 | 0.95 |
| Sb | 0.83 | 0.36 | 0.27 | −0.17 | −0.08 | 0.93 |
| Ba | −0.08 | 0.15 | 0.08 | 0.32 | −0.89 | 0.94 |
| Pb | −0.58 | 0.64 | −0.44 | −0.10 | 0.03 | 0.95 |
| Th | −0.73 | 0.44 | −0.35 | 0.19 | −0.03 | 0.89 |
| U | 0.83 | −0.06 | −0.48 | 0.24 | −0.07 | 0.98 |
| Factor loading, % | 43.20 | 24.52 | 12.20 | 6.32 | 4.20 | |
Table 7.
Bulk (in wt %) and trace element (in ppm) compositions of Hatrurim ye’elimite–larnite CM rocks.
Table 7.
Bulk (in wt %) and trace element (in ppm) compositions of Hatrurim ye’elimite–larnite CM rocks.
| Sample | LLD | YV-412 | Y-5-1 | YV-411 | Y-6-3 | Y-8 | M5-30 | YV-410 | M5-31 | M4-217 | MP-10-1 | MP-10-2 | n | Mean | S | Min | Max |
|---|
| Bulk composition (in wt %) |
| SiO2 | 0.25 | 21.0 | 21.3 | 21.9 | 24.1 | 22.5 | 24.6 | 23.0 | 25.6 | 25.9 | 21.2 | 23.2 | 11 | 23.1 | 1.51 | 21.0 | 25.9 |
| TiO2 | 0.10 | 0.41 | 0.35 | 0.41 | 0.36 | 0.32 | 0.39 | 0.42 | 0.43 | 0.38 | 0.42 | 0.45 | 11 | 0.40 | 0.03 | 0.32 | 0.45 |
| Al2O3 | 0.25 | 9.88 | 9.27 | 10.4 | 9.73 | 7.92 | 11.7 | 11.9 | 12.1 | 11.7 | 10.7 | 10.8 | 11 | 10.7 | 0.95 | 7.92 | 12.1 |
| Fe2O3 | 0.20 | 4.32 | 3.78 | 5.00 | 2.73 | 2.42 | 4.55 * | 4.80 | 5.81 * | 4.49 * | 5.70 * | 4.70 * | 11 | 4.45 | 0.83 | 2.42 | 5.81 |
| FeO | 0.10 | bdl | 0.48 | bdl | 0.48 | 0.42 | n.a. | bdl | n.a. | n.a. | n.a. | n.a. | 6 | 0.28 | 0.20 | <0.10 | 0.48 |
| MnO | 0.01 | bdl | 0.21 | 0.05 | 0.04 | 0.03 | 0.14 | 0.26 | 0.14 | 0.15 | 0.06 | 0.01 | 11 | 0.10 | 0.07 | <0.01 | 0.26 |
| MgO | 0.20 | 0.81 | 1.15 | 1.37 | 1.78 | 1.66 | 0.82 | 1.13 | 0.82 | 0.93 | 1.70 | 1.01 | 11 | 1.18 | 0.33 | 0.81 | 1.78 |
| CaO | 0.25 | 56.0 | 51.9 | 53.0 | 51.6 | 52.9 | 53.6 | 51.1 | 50.8 | 51.9 | 51.3 | 51.3 | 11 | 52.1 | 0.90 | 50.8 | 56.0 |
| N.a.2O | 0.05 | 0.25 | 0.29 | 0.47 | 0.45 | 0.16 | 0.15 | 0.18 | 0.72 | 0.30 | 0.56 | 0.60 | 11 | 0.36 | 0.16 | 0.15 | 0.72 |
| K2O | 0.05 | 0.13 | 0.60 | 0.35 | 1.74 | 0.19 | 0.09 | 2.43 | 0.48 | 0.45 | 0.13 | 0.15 | 11 | 0.47 | 0.51 | 0.09 | 2.43 |
| P2O5 | 0.03 | 1.34 | 3.84 | 1.92 | 0.78 | 0.79 | 2.31 | 1.82 | 2.07 | 2.25 | 2.41 | 1.98 | 11 | 1.88 | 0.52 | 0.78 | 3.84 |
| CO2 | 0.06 | 0.76 | 0.67 | 0.86 | 0.76 | 0.69 | 0.89 | 0.49 | 0.08 | 0.62 | n.a. | n.a. | 9 | 0.69 | 0.12 | 0.08 | 0.89 |
| F | 0.03 | n.a. | bdl | n.a. | bdl | 0.09 | 0.07 | n.a. | n.a. | n.a. | n.a. | n.a. | 4 | 0.14 | 0.09 | <0.03 | 0.26 |
| SO3 | 0.05 | 4.97 | 4.44 | 3.29 | 2.83 | 2.70 | 2.20 | 2.17 | 1.39 | 1.35 | 2.31 | 3.10 | 11 | 2.71 | 0.86 | 1.35 | 4.97 |
| H2O | 0.05 | 0.10 | 0.99 | 0.96 | 2.50 | 7.54 | 2.43 | 0.54 | 0.42 | n.a. | n.a. | n.a. | 8 | 1.31 | 0.93 | 0.10 | 7.54 |
| Total | - | 99.9 | 99.5 | 99.9 | 99.9 | 100.3 | 99.4 | 100.2 | 100.9 | 100.4 | 96.5 | 97.3 | - | - | - | - | - |
| Trace element composition (in ppm) |
| B | 1.00 | 26.9 | 42.2 | 43.7 | 45.3 | 37.3 | n.a. | 44.5 | n.a. | n.a. | n.a. | n.a. | 6 | 41.9 | 22.5 | 26.9 | 45.3 |
| V | 2.00 | 40.0 | 87.0 | 112 | 82.0 | 141 | 39.9 | 82.0 | 53.3 | 49.2 | n.a. | n.a. | 9 | 72.2 | 25.6 | 39.9 | 141 |
| Cr | 1.50 | 224 | 178 | 243 | 119 | 40.6 | 182 | 175 | 377 | 95.0 | n.a. | n.a. | 9 | 174 | 52.6 | 40.6 | 377 |
| Ni | 1.00 | 240 | 182 | 151 | 65.0 | 44.2 | 152 | 214 | 205 | 129 | n.a. | n.a. | 9 | 157 | 50.8 | 44.2 | 240 |
| Cu | 1.00 | 96.4 | 50.3 | 57.5 | 17.7 | 24.6 | 103 | 40.4 | 66.2 | 34.8 | n.a. | n.a. | 9 | 51.9 | 25.2 | 17.7 | 103 |
| Zn | 1.00 | 244 | 376 | 343 | 73.0 | 60.5 | 58.7 | 333 | 426 | 249 | n.a. | n.a. | 9 | 240 | 128 | 58.7 | 426 |
| Ga | 0.70 | 8.00 | 7.00 | 9.45 | 8.37 | 6.19 | 9.28 | 11.5 | 8.71 | 8.45 | n.a. | n.a. | 9 | 8.47 | 0.82 | 6.19 | 11.5 |
| Ge | 0.20 | 1.06 | bdl | 1.30 | 1.26 | 0.52 | 2.10 | 3.02 | 1.29 | 2.38 | n.a. | n.a. | 9 | 1.42 | 0.77 | <0.2 | 3.02 |
| As | 0.80 | 13.2 | 15.8 | 14.5 | bdl | 0.90 | 22.1 | 18.6 | 13.0 | 8.72 | n.a. | n.a. | 9 | 12.1 | 6.85 | <0.8 | 22.1 |
| Se | 0.20 | 96.2 | 2.93 | 9.77 | 39.7 | 19.9 | bdl | bdl | bdl | 23.4 | n.a. | n.a. | 9 | 13.7 | 14.7 | <0.2 | 96.2 |
| Rb | 0.20 | 4.92 | 11.7 | 7.72 | 31.9 | 5.00 | 3.45 | 2.65 | 10.4 | 12.0 | n.a. | n.a. | 9 | 7.88 | 3.53 | 2.65 | 31.9 |
| Sr | 1.00 | 2009 | 1453 | 1502 | 1407 | 1243 | 2903 | 1407 | 2050 | 2203 | n.a. | n.a. | 9 | 1719 | 351 | 1243 | 2903 |
| Y | 0.20 | 36.9 | 44.1 | 52.4 | 41.2 | 47.9 | 35.4 | 53.2 | 34.5 | 69.2 | n.a. | n.a. | 9 | 44.4 | 7.09 | 34.5 | 69.2 |
| Zr | 0.50 | 69.4 | 65.7 | 67.0 | 73.7 | 72.0 | 68.5 | 69.0 | 80.4 | 77.0 | n.a. | n.a. | 9 | 70.9 | 3.49 | 65.7 | 80.4 |
| Nb | 0.20 | 9.08 | 6.34 | 9.32 | 7.63 | 6.62 | 5.51 | 10.4 | 8.31 | 10.5 | n.a. | n.a. | 9 | 8.24 | 1.48 | 5.51 | 10.5 |
| Mo | 0.20 | 16.0 | 12.1 | 9.69 | 0.48 | 0.69 | 8.82 | 2.91 | 18.9 | 6.56 | n.a. | n.a. | 9 | 8.11 | 5.25 | 0.48 | 18.9 |
| Ag | 0.01 | 1.81 | 0.50 | 0.45 | 0.22 | 0.39 | n.a. | 0.57 | n.a. | n.a. | n.a. | n.a. | 6 | 0.48 | 0.26 | 0.22 | 1.81 |
| Ba | 1.00 | 210 | 1629 | 1459 | 1994 | 5025 | 19.0 | 451 | 481 | 135 | n.a. | n.a. | 9 | 908 | 761 | 19.0 | 5025 |
| Pb | 0.80 | 18.5 | 3.60 | 46.7 | 14.7 | 9.30 | bdl | 9.28 | 8.58 | 2.77 | n.a. | n.a. | 9 | 9.53 | 6.19 | <0.8 | 46.7 |
| Th | 1.00 | 11.2 | 9.40 | 9.20 | 7.10 | 5.20 | bdl | 8.60 | 11.9 | 8.30 | n.a. | n.a. | 9 | 8.43 | 3.46 | <1.0 | 11.9 |
| U | 1.00 | 23.5 | 14.3 | 24.5 | bdl | bdl | 12.0 | 13.7 | 26.7 | 2.39 | n.a. | n.a. | 9 | 13.1 | 9.15 | <1.0 | 26.7 |
Table 8.
Bulk (in wt %) and trace element (in ppm) compositions of Hatrurim mayenite–larnite and gehlenite-larnite CM rocks.
Table 8.
Bulk (in wt %) and trace element (in ppm) compositions of Hatrurim mayenite–larnite and gehlenite-larnite CM rocks.
| Sample | LLD | Y-7 | CONCR | Y-2-1 | W-10-2 | W-12-1 | M4-218 | G-7 | M4-215 | M5-32 | n | Mean | S | Min | Max | Y-10-5 | W-11-3 |
|---|
| Bulk composition (in wt %) |
| SiO2 | 0.25 | 19.8 | 25.5 | 22.5 | 24.9 | 22.4 | 27.1 | 24.4 | 26.8 | 24.5 | 9 | 24.4 | 1.60 | 19.8 | 27.1 | 26.4 | 25.1 |
| TiO2 | 0.10 | 0.35 | 0.47 | 0.38 | 0.44 | 0.42 | 0.45 | 0.46 | 0.38 | 0.41 | 9 | 0.42 | 0.03 | 0.35 | 0.47 | 0.37 | 0.47 |
| Al2O3 | 0.25 | 8.17 | 11.6 | 9.23 | 11.0 | 9.59 | 12.5 | 8.67 | 12.5 | 11.7 | 9 | 10.6 | 1.45 | 8.17 | 12.5 | 9.86 | 10.8 |
| Fe2O3 | 0.20 | 2.94 | 4.51 | 3.29 | 2.08 | 2.55 | 3.40 | 3.26 | 2.21 | 4.56 | 9 | 3.17 | 0.73 | 2.08 | 4.56 | 2.92 | 4.68 |
| FeO | 0.10 | 0.47 | n.a. | 1.03 | 0.67 | 0.89 | n.a. | 0.94 | n.a. | n.a. | 5 | 0.83 | 0.45 | 0.47 | 1.03 | 0.47 | 0.62 |
| MnO | 0.01 | 0.03 | 0.14 | bdl | bdl | bdl | 0.13 | bdl | 0.13 | 0.13 | 9 | 0.06 | 0.06 | <0.01 | 0.14 | 0.04 | 0.02 |
| MgO | 0.20 | 0.94 | 0.71 | 0.80 | 0.78 | 0.66 | 0.75 | 0.86 | 0.70 | 0.51 | 9 | 0.75 | 0.07 | 0.51 | 0.94 | 1.77 | 0.68 |
| CaO | 0.25 | 51.2 | 51.1 | 56.7 | 54.0 | 55.8 | 51.1 | 55.6 | 52.2 | 52.8 | 9 | 53.3 | 1.93 | 51.1 | 56.7 | 47.9 | 48.6 |
| N.a.2O | 0.05 | 0.13 | 0.75 | 0.24 | 0.45 | 0.44 | 0.27 | 0.35 | 0.15 | 0.43 | 9 | 0.33 | 0.12 | 0.13 | 0.75 | 0.22 | 0.33 |
| K2O | 0.05 | 0.07 | 0.49 | 0.13 | 0.54 | 0.22 | 0.27 | 0.97 | 0.17 | 0.86 | 9 | 0.38 | 0.26 | 0.07 | 0.97 | 0.20 | 0.69 |
| P2O5 | 0.03 | 2.19 | 2.58 | 2.08 | 2.20 | 2.28 | 2.27 | 2.30 | 2.49 | 2.62 | 9 | 2.33 | 0.15 | 2.08 | 2.62 | 0.98 | 2.22 |
| CO2 | 0.06 | 0.98 | 1.15 | 1.01 | 0.65 | 1.06 | 0.89 | 0.73 | 0.89 | 0.62 | 9 | 0.89 | 0.15 | 0.62 | 1.15 | 0.83 | 0.13 |
| F | 0.03 | 0.19 | n.a. | 0.24 | 0.19 | 0.21 | n.a. | 0.09 | n.a. | n.a. | 5 | 0.20 | 0.11 | 0.09 | 0.24 | bdl | bdl |
| SO3 | 0.05 | 0.73 | 0.60 | 0.38 | 0.20 | 0.18 | 0.11 | 0.05 | 0.03 | 0.02 | 9 | 0.22 | 0.20 | <0.05 | 0.73 | 0.25 | 0.13 |
| H2O | 0.05 | 12.1 | 0.67 | 2.19 | 1.69 | 2.95 | 1.16 | 1.25 | 1.47 | 1.23 | 9 | 1.71 | 0.65 | 0.67 | 12.1 | 8.10 | 5.50 |
| Total | - | 100.3 | 100.3 | 100.2 | 99.8 | 99.6 | 100.4 | 99.9 | 100.1 | 100.5 | - | - | - | - | - | 100.3 | 100 |
| Trace element composition (in ppm) |
| B | 1.00 | 16.8 | 75.8 | 15.4 | 28.8 | 11.9 | 31.4 | 17.5 | n.a. | 103 | 8 | 31.0 | 24.0 | 11.9 | 103 | 38.8 | 60.2 |
| V | 2.00 | 24.2 | 55.9 | 41.8 | 42.7 | 52.8 | 46.6 | 47.5 | 64.9 | 60.3 | 9 | 49.7 | 6.91 | 24.2 | 64.9 | 63.7 | 51.0 |
| Cr | 1.50 | 135 | 361 | 234 | 220 | 198 | 250 | 284 | 202 | 284 | 9 | 239 | 35.6 | 135 | 361 | 44.9 | 123 |
| Ni | 1.00 | 93.0 | 230 | 95.0 | 60.8 | 163 | 58.0 | 175 | 64.4 | 263 | 9 | 126 | 64.1 | 58.0 | 263 | 69.0 | 139 |
| Cu | 1.00 | 23.9 | 86.0 | 18.0 | 11.7 | 26.8 | 23.8 | 100 | 16.1 | 41.9 | 9 | 33.8 | 24.5 | 11.7 | 100 | 24.4 | 80.0 |
| Zn | 1.00 | 100 | 445 | 71.3 | 191 | 26.5 | 161 | 55.1 | 42.0 | 210 | 9 | 119 | 68.2 | 26.5 | 445 | 44.6 | 134 |
| Ga | 0.70 | 6.20 | 8.70 | 6.44 | 8.40 | 7.55 | 10.3 | 4.79 | 8.17 | 8.64 | - | 9 | 7.73 | 1.04 | 4.79 | 10.3 | - |
| Ge | 0.20 | bdl | 1.84 | 0.72 | bdl | 0.54 | 2.30 | 0.96 | 2.61 | 1.58 | - | 9 | 1.16 | 0.82 | <0.2 | 2.61 | - |
| As | 0.80 | 9.90 | 10.7 | 31.3 | 12.5 | 17.7 | 22.8 | 7.00 | 33.9 | 24.1 | - | 9 | 18.4 | 8.01 | 7.00 | 33.9 | - |
| Se | 0.20 | 7.55 | 5.71 | 2.82 | bdl | 3.87 | bdl | 1.24 | 9.71 | bdl | - | 9 | 3.08 | 2.83 | <0.2 | 9.71 | - |
| Rb | 0.20 | 2.57 | 8.59 | 2.74 | 4.44 | 2.30 | 2.00 | 4.73 | 1.85 | 9.86 | - | 9 | 3.91 | 2.32 | 1.85 | 9.86 | - |
| Sr | 1.00 | 1146 | 1880 | 1454 | 1547 | 1453 | 1830 | 1550 | 1465 | 1843 | - | 9 | 1592 | 172 | 1146 | 1880 | - |
| Y | 0.20 | 55.7 | 42.5 | 30.7 | 32.0 | 36.6 | 39.1 | 31.2 | 43.0 | 41.0 | - | 9 | 37.9 | 4.82 | 30.7 | 55.7 | - |
| Zr | 0.50 | 57.9 | 78.0 | 57.9 | 59.0 | 58.0 | 69.4 | 75.0 | 51.9 | 72.2 | - | 9 | 64.2 | 7.67 | 51.9 | 78.0 | - |
| Nb | 0.20 | 6.32 | 7.64 | 3.10 | 6.54 | 4.49 | 6.48 | 10.7 | 6.05 | 7.71 | - | 9 | 6.46 | 1.08 | 3.10 | 10.7 | - |
| Mo | 0.20 | 1.27 | 23.2 | 3.16 | 5.63 | 15.0 | 8.22 | 6.22 | 13.4 | 20.2 | - | 9 | 10.3 | 6.10 | 1.27 | 23.2 | - |
| Ag | 0.01 | 0.65 | 0.67 | 0.05 | 0.03 | 0.02 | 2.89 | 0.09 | n.a. | 0.43 | - | 8 | 0.32 | 0.30 | 0.02 | 2.89 | - |
| Ba | 1.00 | 26.5 | 290 | 101 | 225 | 428 | 56.0 | 103 | 47.2 | 350 | - | 9 | 168 | 121 | 26.5 | 428 | - |
| Pb | 0.80 | 1.50 | 2.30 | 4.20 | 2.10 | bdl | bdl | 5.80 | bdl | bdl | - | 9 | 1.79 | 1.31 | <0.8 | 5.80 | - |
| Th | 1.00 | 5.00 | bdl | 7.10 | 8.70 | 6.70 | 7.50 | 12.6 | 6.78 | 9.80 | - | 9 | 7.37 | 2.97 | <1.0 | 12.60 | - |
| U | 1.00 | 7.20 | 22.0 | bdl | 4.80 | 8.40 | 6.00 | 62.4 | 6.00 | 19.1 | - | 9 | 10.5 | 7.47 | <1.0 | 62.4 | - |
Table 9.
Mineral chemistry (WDS-EDS, wt %) of larnite and α′-Ca2SiO4 phase from Hatrurim larnite CM rocks, compared with other Ca2SiO4 polymorphs.
Table 9.
Mineral chemistry (WDS-EDS, wt %) of larnite and α′-Ca2SiO4 phase from Hatrurim larnite CM rocks, compared with other Ca2SiO4 polymorphs.
| Type | Sample | Polymorph | n | SO3 | P2O5 | SiO2 | TiO2 | Al2O3 | FeO | MgO | CaO | SrO | Na2O | K2O | Total | S | P | Si | Ti | Al | Fe | Mg | Ca | Sr | Na | K |
|---|
| Y | Y-5-1 | Larnite | 2 | n.a. | 0.46 | 34.58 | n.a. | 0.37 | 0.64 | n.a. | 63.62 | n.a. | 0.39 | n.a. | 100.05 | | 0.01 | 0.99 | | 0.01 | 0.02 | | 1.95 | | 0.02 | 0.00 |
| Y | Y-6-3 | Larnite | 3 | n.a. | 0.82 | 34.49 | n.a. | 0.32 | 0.47 | <0.02 | 63.29 | n.a. | 0.39 | 0.11 | 99.89 | | 0.02 | 0.99 | | 0.01 | 0.01 | 0.00 | 1.94 | | 0.02 | 0.00 |
| Y | M5-30 | Larnite | 3 | 0.10 | 0.98 | 32.98 | 0.11 | 0.63 | 0.11 | <0.02 | 64.30 | 0.30 | 0.19 | 0.04 | 99.75 | 0.00 | 0.02 | 0.95 | 0.00 | 0.02 | 0.00 | 0.00 | 1.98 | 0.00 | 0.01 | 0.00 |
| Y | M5-31 | Larnite | 6 | 0.10 | 1.88 | 32.59 | 0.07 | 0.13 | 0.06 | <0.02 | 63.75 | 0.04 | 0.78 | 0.08 | 99.49 | 0.00 | 0.05 | 0.94 | 0.00 | 0.00 | 0.00 | 0.00 | 1.97 | 0.00 | 0.04 | 0.00 |
| Y | M4-217 | Larnite | 4 | 0.04 | 0.97 | 33.41 | 0.09 | 0.31 | 0.07 | <0.02 | 64.30 | 0.21 | 0.28 | 0.09 | 99.76 | 0.00 | 0.02 | 0.96 | 0.00 | 0.01 | 0.00 | 0.00 | 1.98 | 0.00 | 0.02 | 0.00 |
| M | Y-9-1 | Larnite | 3 | n.a. | 0.32 | 34.95 | n.a. | 0.46 | 0.37 | 0.24 | 63.14 | n.a. | 0.34 | 0.23 | 100.05 | | 0.01 | 1.00 | | 0.02 | 0.01 | 0.01 | 1.93 | | 0.02 | 0.01 |
| M | G-7 | Larnite | 4 | n.a. | 2.12 | 31.68 | n.a. | 0.20 | 0.08 | 0.04 | 64.22 | 0.25 | 0.41 | 0.79 | 99.77 | | 0.05 | 0.92 | | 0.01 | 0.00 | 0.00 | 1.99 | 0.00 | 0.02 | 0.03 |
| M | G-7 | α′-Ca2SiO4 | 5 | n.a. | 7.85 | 27.26 | n.a. | 0.11 | 0.03 | 0.10 | 59.90 | 0.29 | 0.65 | 3.08 | 99.26 | | 0.19 | 0.79 | | 0.00 | 0.00 | 0.00 | 1.85 | 0.00 | 0.04 | 0.11 |
| M | W-10-2 | Larnite | 5 | n.a. | 1.43 | 33.80 | n.a. | 0.32 | n.a. | n.a. | 63.52 | n.a. | 0.48 | 0.63 | 100.19 | | 0.03 | 0.96 | | 0.01 | | | 1.94 | | 0.03 | 0.02 |
| M | W-12-1 | Larnite | 5 | n.a. | 2.15 | 32.29 | n.a. | 0.61 | 0.13 | 0.05 | 63.81 | 0.26 | 0.51 | 0.19 | 99.99 | | 0.05 | 0.93 | | 0.02 | 0.00 | 0.00 | 1.96 | 0.00 | 0.03 | 0.01 |
| M | W-12-1 | α′-Ca2SiO4 | 2 | n.a. | 5.05 | 30.23 | n.a. | 0.25 | <0.02 | 0.06 | 62.97 | 0.30 | 0.63 | 0.18 | 99.65 | | 0.12 | 0.86 | | 0.01 | 0.00 | 0.00 | 1.93 | 0.00 | 0.03 | 0.01 |
| M | CONCR | Larnite | 24 | <0.03 | 2.09 | 33.03 | 0.08 | 0.11 | 0.02 | 0.05 | 63.11 | 0.19 | 1.07 | 0.10 | 99.84 | 0.00 | 0.05 | 0.95 | 0.00 | 0.00 | 0.00 | 0.00 | 1.94 | 0.00 | 0.06 | 0.00 |
| M | M5-32 | Larnite | 11 | 0.04 | 2.47 | 32.20 | 0.18 | 0.27 | 0.06 | <0.02 | 62.79 | 0.32 | 0.57 | 0.83 | 99.73 | 0.00 | 0.06 | 0.93 | 0.00 | 0.01 | 0.00 | 0.00 | 1.93 | 0.01 | 0.03 | 0.03 |
| M | M5-32 | α′-Ca2SiO4 | 2 | <0.03 | 7.51 | 27.99 | 0.07 | 0.09 | 0.04 | <0.02 | 59.05 | 0.36 | 0.95 | 3.58 | 99.62 | 0.00 | 0.18 | 0.81 | 0.00 | 0.00 | 0.00 | 0.00 | 1.82 | 0.01 | 0.05 | 0.13 |
| M | M4-215 | Larnite | 4 | <0.03 | 0.91 | 33.10 | 0.09 | 0.19 | 0.02 | <0.02 | 65.19 | 0.01 | 0.23 | 0.19 | 99.92 | 0.00 | 0.02 | 0.95 | 0.00 | 0.01 | 0.00 | 0.00 | 2.01 | 0.00 | 0.01 | 0.01 |
| M | M4-218 | Larnite | 5 | <0.03 | 1.69 | 32.70 | 0.16 | 0.25 | 0.07 | <0.02 | 64.19 | 0.22 | 0.41 | 0.29 | 99.99 | 0.00 | 0.04 | 0.94 | 0.00 | 0.01 | 0.00 | 0.00 | 1.98 | 0.00 | 0.02 | 0.01 |
| M | M4-251 | Larnite | 4 | <0.03 | 1.80 | 32.75 | 0.19 | 0.22 | 0.05 | <0.02 | 63.64 | 0.27 | 0.31 | 0.75 | 99.96 | 0.00 | 0.04 | 0.94 | 0.00 | 0.01 | 0.00 | 0.00 | 1.96 | 0.00 | 0.02 | 0.03 |
| M | M4-251 | α′-Ca2SiO4 | 1 | 0.10 | 4.83 | 31.54 | 0.02 | 0.17 | <0.02 | <0.02 | 61.37 | 0.28 | 0.35 | 1.15 | 99.84 | 0.00 | 0.12 | 0.90 | 0.00 | 0.01 | 0.00 | 0.00 | 1.87 | 0.00 | 0.02 | 0.04 |
| M | Y-2-1 | Larnite | 3 | n.a. | 0.96 | 33.77 | n.a. | <0.02 | <0.02 | n.a. | 64.67 | n.a. | 0.40 | <0.01 | 99.80 | | 0.02 | 0.97 | | 0.00 | 0.00 | | 1.99 | | 0.02 | 0.00 |
| M | H-401 | α′-Ca2SiO4 | 22 | <0.03 | 4.64 | 31.05 | 0.02 | <0.02 | 0.07 | 0.03 | 61.83 | 0.21 | 0.41 | 1.46 | 99.73 | 0.00 | 0.11 | 0.89 | 0.00 | 0.00 | 0.00 | 0.00 | 1.90 | 0.00 | 0.02 | 0.05 |
| Gh | Y-10-5 | Larnite | 1 | n.a. | 0.05 | 34.55 | n.a. | n.a. | n.a. | n.a. | 64.54 | n.a. | n.a. | n.a. | 99.14 | | 0.00 | 1.00 | | | | | 2.00 | | | |
| Gh | W-11-3 | Larnite | 26 | n.a. | 2.26 | 33.03 | n.a. | 0.11 | 0.10 | <0.02 | 63.22 | 0.27 | 0.65 | 0.65 | 100.31 | | 0.05 | 0.94 | | 0.00 | 0.00 | 0.00 | 1.93 | 0.00 | 0.04 | 0.02 |
| Gh | W-11-3 | α′-Ca2SiO4 | 18 | n.a. | 6.98 | 29.14 | n.a. | 0.14 | 0.24 | <0.02 | 59.54 | 0.37 | 0.94 | 2.49 | 99.85 | | 0.17 | 0.83 | | 0.00 | 0.01 | 0.00 | 1.82 | 0.01 | 0.05 | 0.09 |
| PL-1 | YV-402 | Flamite | 21 | n.a. | 7.38 | 28.87 | n.a. | 0.04 | 0.15 | 0.16 | 59.76 | 0.24 | 1.55 | 1.73 | 100.03 | | 0.18 | 0.82 | | 0.00 | 0.00 | 0.01 | 1.82 | 0.00 | 0.09 | 0.06 |
| PL-2 | G2-G4 | α-Ca2SiO4 | 82 | n.a. | 10.43 | 26.44 | 0.01 | 0.02 | 0.27 | 0.14 | 58.29 | 0.29 | 1.60 | 2.40 | 99.90 | | 0.25 | 0.75 | 0.00 | 0.00 | 0.01 | 0.01 | 1.77 | 0.00 | 0.09 | 0.09 |
Table 10.
Average composition of larnite, α′-Ca2SiO4(ss) and bredigite from the Hatrurim larnite rocks (WDS-EDS, wt %).
Table 10.
Average composition of larnite, α′-Ca2SiO4(ss) and bredigite from the Hatrurim larnite rocks (WDS-EDS, wt %).
| Mineral | Larnite (n = 41) | Larnite (n = 46) | α′-Ca2SiO4(ss) (n = 50) | Bredigite (n = 15) |
|---|
| Rock | Ye’elimite Rocks | Mayenite Rocks | Ye’elimite and Mayenite Rocks | Gehlenite Rocks |
|---|
| | Mean | S | Min | Max | Mean | S | Min | Max | Mean | S | Min | Max | Mean | S | Min | Max |
|---|
| SiO2 | 33.36 | 0.77 | 31.92 | 35.13 | 32.70 | 0.50 | 31.68 | 34.95 | 29.84 | 1.35 | 27.34 | 32.45 | 34.00 | 0.83 | 32.4 | 35.65 |
| TiO2 | 0.09 | 0.06 | 0.04 | 0.39 | 0.12 | 0.07 | <0.02 | 0.23 | 0.08 | 0.07 | <0.02 | 0.25 | 0.11 | 0.12 | <0.02 | 0.33 |
| Cr2O3 | <0.02 | 0.03 | <0.02 | 0.12 | 0.04 | 0.05 | <0.02 | 0.15 | 0.14 | 0.26 | <0.02 | 0.70 | <0.02 | 0.01 | <0.02 | 0.03 |
| Al2O3 | 0.21 | 0.17 | <0.02 | 0.80 | 0.16 | 0.12 | <0.02 | 0.61 | 0.08 | 0.33 | <0.02 | 2.10 | 0.58 | 0.73 | <0.02 | 2.19 |
| FeO | 0.09 | 0.11 | <0.02 | 0.71 | 0.06 | 0.13 | <0.02 | 0.69 | 0.05 | 0.12 | <0.02 | 0.63 | 0.21 | 0.20 | <0.02 | 0.54 |
| MgO | 0.05 | 0.06 | <0.02 | 0.31 | 0.04 | 0.08 | <0.02 | 0.24 | 0.04 | 0.05 | <0.02 | 0.10 | 5.59 | 0.27 | 5.06 | 6.12 |
| CaO | 64.13 | 0.72 | 62.23 | 65.71 | 63.23 | 0.88 | 61.82 | 65.50 | 59.86 | 1.78 | 56.36 | 62.97 | 58.26 | 0.55 | 57.14 | 58.98 |
| SrO | 0.07 | 0.11 | <0.04 | 0.30 | 0.22 | 0.10 | <0.04 | 0.34 | 0.09 | 0.10 | <0.04 | 0.36 | <0.04 | | | |
| Na2O | 0.44 | 0.22 | 0.08 | 0.94 | 0.62 | 0.33 | <0.03 | 1.25 | 0.69 | 0.29 | 0.35 | 1.29 | 0.18 | 0.19 | <0.03 | 0.46 |
| K2O | 0.09 | 0.04 | 0.02 | 0.18 | 0.44 | 0.33 | 0.06 | 1.35 | 2.12 | 0.62 | 1.23 | 3.58 | <0.01 | 0.01 | <0.01 | 0.04 |
| P2O5 | 1.16 | 0.46 | 0.38 | 2.28 | 2.17 | 0.60 | 0.50 | 3.33 | 6.54 | 1.73 | 4.26 | 9.46 | 0.56 | 0.09 | <0.02 | 0.62 |
| SO3 | 0.05 | 0.05 | <0.03 | 0.17 | <0.03 | 0.06 | <0.03 | 0.39 | <0.03 | | | | 0.03 | 0.02 | <0.03 | 0.06 |
| V2O5 | <0.03 | 0.03 | <0.03 | 0.09 | <0.03 | | | | <0.03 | 0.06 | <0.03 | 0.32 | <0.03 | | | |
| BaO | <0.05 | 0.07 | <0.05 | 0.26 | <0.05 | | | | <0.05 | | | | <0.05 | 0.03 | <0.05 | 0.10 |
| Total | 99.74 | | | | 99.80 | | | | 99.53 | | | | 99.52 | | | |
| | 4 oxygen atoms, apfu | 16 oxygen atoms, apfu |
| Si | 0.96 | | | | 0.94 | | | | 0.85 | | | | 3.82 | | | |
| Al | 0.01 | | | | 0.01 | | | | 0.00 | | | | 0.09 | | | |
| Fe | 0.00 | | | | 0.00 | | | | 0.00 | | | | 0.03 | | | |
| Mg | 0.00 | | | | 0.00 | | | | 0.00 | | | | 0.94 | | | |
| Ca | 1.97 | | | | 1.94 | | | | 1.83 | | | | 7.00 | | | |
| Na | 0.02 | | | | 0.04 | | | | 0.04 | | | | 0.04 | | | |
| K | 0.00 | | | | 0.02 | | | | 0.08 | | | | 0.00 | | | |
| P | 0.03 | | | | 0.05 | | | | 0.16 | | | | 0.06 | | | |
| S | 0.00 | | | | 0.00 | | | | 0.00 | | | | 0.02 | | | |
Table 11.
Mineral chemistry (WDS-EDS, wt %) of ye’elimite from Hatrurim larnite CM rocks.
Table 11.
Mineral chemistry (WDS-EDS, wt %) of ye’elimite from Hatrurim larnite CM rocks.
| Sample | Y-5-1 | Y-6-3 | Y-8 | M5-30 | M5-31 | M4-217 | H-201 |
|---|
| n | 10 | 7 | 6 | 5 | 14 | 7 | 2 |
| P2O5 | n.a. | 0.26 | 0.32 | <0.02 | 0.21 | 0.21 | n.a. |
| SiO2 | 0.83 | 1.91 | 1.45 | 0.21 | 0.94 | 0.64 | 0.68 |
| Al2O3 | 48.40 | 46.88 | 47.79 | 49.10 | 47.18 | 48.38 | 47.57 |
| Fe2O3 | 0.87 | 1.10 | 1.10 | 0.80 | 2.33 | 0.93 | 2.24 |
| MgO | n.a. | 0.17 | <0.02 | <0.02 | 0.07 | 0.04 | 0.12 |
| CaO | 36.03 | 35.27 | 36.20 | 36.15 | 35.77 | 35.83 | 36.16 |
| BaO | 0.53 | 0.91 | n.a. | n.a. | n.a. | n.a. | 0.14 |
| SrO | 0.26 | 0.35 | 0.31 | 0.52 | 0.33 | 0.65 | n.a. |
| Na2O | 0.16 | 0.05 | 0.08 | 0.03 | 0.23 | 0.09 | n.a. |
| K2O | n.a. | 0.13 | 0.17 | 0.02 | 0.08 | 0.05 | n.a. |
| SO3 | 13.03 | 12.91 | 13.00 | 13.06 | 12.98 | 13.01 | 12.98 |
| Total | 100.12 | 99.95 | 100.41 | 99.89 | 100.11 | 99.82 | 99.87 |
| Formula based on 11 cations M4[T6O12][XO4] |
| P | | 0.023 | 0.028 | 0.000 | 0.018 | 0.018 | |
| Si | 0.085 | 0.197 | 0.148 | 0.021 | 0.097 | 0.066 | 0.069 |
| Al | 5.833 | 5.693 | 5.740 | 5.916 | 5.698 | 5.842 | 5.756 |
| Fe | 0.067 | 0.085 | 0.084 | 0.062 | 0.179 | 0.072 | 0.173 |
| Σ[T] | 5.985 | 5.998 | 6.000 | 5.999 | 5.992 | 5.998 | 5.998 |
| Mg | | 0.026 | 0.000 | 0.000 | 0.010 | 0.006 | 0.018 |
| Ca | 3.948 | 3.894 | 3.952 | 3.960 | 3.927 | 3.934 | 3.977 |
| Ba | 0.021 | 0.037 | | | | | 0.006 |
| Sr | 0.016 | 0.021 | 0.018 | 0.031 | 0.020 | 0.039 | |
| Na | 0.030 | 0.009 | 0.014 | 0.005 | 0.042 | 0.016 | |
| K | | 0.017 | 0.022 | 0.003 | 0.010 | 0.007 | |
| Σ[M] | 4.014 | 4.004 | 4.006 | 3.999 | 4.010 | 4.002 | 4.002 |
| S | 1.000 | 0.998 | 0.994 | 1.002 | 0.998 | 1.001 | 1.000 |
Table 12.
Average (n = 60) mineral chemistry (WDS-EDS, in wt %) of ye’elimite from Hatrurim larnite CM rocks.
Table 12.
Average (n = 60) mineral chemistry (WDS-EDS, in wt %) of ye’elimite from Hatrurim larnite CM rocks.
| Component | Mean | S | Min | Max |
|---|
| P2O5 | 0.19 | 0.13 | <0.02 | 0.47 |
| SiO2 | 0.89 | 0.71 | 0.12 | 3.90 |
| Al2O3 | 47.61 | 1.41 | 46.16 | 49.12 |
| Fe2O3 | 2.03 | 1.04 | 0.77 | 6.60 |
| CaO | 36.01 | 0.64 | 34.98 | 38.49 |
| BaO | 0.29 | 0.04 | 0.14 | 0.91 |
| SrO | 0.40 | 0.22 | <0.04 | 0.70 |
| Na2O | 0.14 | 0.09 | <0.03 | 0.32 |
| K2O | 0.07 | 0.03 | <0.01 | 0.17 |
| SO3 | 12.80 | 0.67 | 10.93 | 13.69 |
| Total | 100.43 | | | |
Table 13.
Mineral chemistry (WDS-EDS, wt %) of mayenite-supergroup minerals from Hatrurim CM larnite rocks.
Table 13.
Mineral chemistry (WDS-EDS, wt %) of mayenite-supergroup minerals from Hatrurim CM larnite rocks.
| Sample | M5-30 | W-10-2 | M4-218 | M4-251 | M5-32 | M4-215 | M5-30 | W-10-2 | Y-2-1 | W-12-1 | G-7 | Y-9-1 |
|---|
| Mineral | Fmay | Fmay | Fky | Fky | Fky | Fky | Fky | Fky | Fky | Fky | Fky | Clky |
|---|
| n | 1 | 13 | 16 | 10 | 22 | 11 | 4 | 9 | 12 | 5 | 15 | 22 |
|---|
| P2O5 | 0.16 | 0.05 | 0.03 | 0.01 | 0.01 | 0.04 | 0.06 | 0.03 | 0.04 | n.a. | n.a. | 0.04 |
| SO3 | 0.15 | 0.04 | <0.02 | 0.19 | 0.12 | 0.03 | 0.18 | 0.03 | 0.03 | n.a. | <0.02 | 0.04 |
| SiO2 | 0.05 | 0.31 | 0.54 | 0.14 | 0.25 | 1.31 | 0.18 | 0.40 | 0.49 | 0.20 | 0.79 | 0.53 |
| Al2O3 | 49.04 | 47.79 | 45.50 | 46.72 | 46.38 | 46.27 | 47.23 | 46.09 | 43.98 | 45.76 | 45.55 | 42.53 |
| Fe2O3 | 1.78 | 2.75 | 2.77 | 2.01 | 2.21 | 1.72 | 1.56 | 2.58 | 3.63 | 2.87 | 3.17 | 5.63 |
| CaO | 47.35 | 46.62 | 44.58 | 44.77 | 44.48 | 44.90 | 45.03 | 44.90 | 44.08 | 45.31 | 44.77 | 43.59 |
| SrO | 0.28 | 0.15 | 0.11 | 0.10 | 0.12 | <0.02 | 0.14 | 0.11 | 0.06 | n.a. | <0.02 | 0.12 |
| Na2O | 0.07 | 0.07 | 0.04 | 0.12 | 0.24 | 0.05 | 0.10 | 0.07 | <0.02 | n.a. | <0.02 | 0.06 |
| K2O | 0.01 | 0.02 | 0.01 | 0.12 | 0.14 | 0.02 | 0.04 | 0.02 | 0.01 | n.a. | 0.20 | 0.02 |
| F | 1.90 | 1.56 | 2.73 | 2.13 | 1.99 | 2.72 | 1.70 | 1.58 | 1.52 | 1.84 | 1.93 | 0.25 |
| Cl | 0.23 | 0.69 | 0.08 | 0.39 | 0.30 | 0.04 | 0.18 | 0.67 | 0.99 | 0.29 | n.a. | 5.24 |
| H2O | 0.38 | 0.40 | 4.59 | 4.91 | 5.03 | 4.52 | 5.15 | 5.06 | 4.86 | 5.09 | 5.02 | 3.97 |
| Total | 101.40 | 100.45 | 100.98 | 101.60 | 101.26 | 101.63 | 101.54 | 101.53 | 99.71 | 101.36 | 101.42 | 102.02 |
| O-(F,Cl)2 | 0.85 | 0.81 | 1.17 | 0.98 | 0.91 | 1.15 | 0.76 | 0.82 | 0.86 | 0.84 | 0.81 | 1.29 |
| Total | 100.55 | 99.63 | 99.82 | 100.62 | 100.36 | 100.48 | 100.78 | 100.72 | 98.84 | 100.53 | 100.61 | 100.73 |
| Formula based on 26 cations X12T14O32−x(OH)3x[W6−3x] |
| P | 0.032 | 0.010 | 0.006 | 0.002 | 0.003 | 0.008 | 0.012 | 0.006 | 0.008 | | | 0.009 |
| S | 0.027 | 0.007 | 0.000 | 0.035 | 0.022 | 0.005 | 0.033 | 0.006 | 0.005 | | 0.000 | 0.008 |
| Si | 0.012 | 0.074 | 0.135 | 0.034 | 0.062 | 0.324 | 0.044 | 0.098 | 0.126 | 0.050 | 0.194 | 0.135 |
| Al | 13.601 | 13.438 | 13.380 | 13.598 | 13.531 | 13.449 | 13.682 | 13.446 | 13.156 | 13.375 | 13.283 | 12.793 |
| Fe | 0.315 | 0.494 | 0.520 | 0.374 | 0.412 | 0.320 | 0.289 | 0.480 | 0.693 | 0.536 | 0.589 | 1.081 |
| Σ[T] | 13.986 | 14.022 | 14.041 | 14.042 | 14.030 | 14.105 | 14.059 | 14.036 | 13.987 | 13.961 | 14.067 | 14.026 |
| Ca | 11.938 | 11.917 | 11.917 | 11.846 | 11.797 | 11.865 | 11.859 | 11.910 | 11.988 | 12.039 | 11.870 | 11.920 |
| Sr | 0.038 | 0.020 | 0.016 | 0.014 | 0.017 | 0.000 | 0.021 | 0.015 | 0.009 | | 0.000 | 0.018 |
| Na | 0.034 | 0.035 | 0.021 | 0.058 | 0.113 | 0.022 | 0.049 | 0.032 | 0.012 | | 0.000 | 0.030 |
| K | 0.004 | 0.006 | 0.004 | 0.039 | 0.043 | 0.005 | 0.012 | 0.007 | 0.003 | | 0.063 | 0.007 |
| Σ[X] | 12.014 | 11.978 | 11.958 | 11.958 | 11.970 | 11.892 | 11.941 | 11.964 | 12.013 | 12.039 | 11.933 | 11.974 |
| F | 1.414 | 1.178 | 2.152 | 1.660 | 1.560 | 2.123 | 1.320 | 1.238 | 1.222 | 1.440 | 1.508 | 0.202 |
| Cl | 0.090 | 0.280 | 0.033 | 0.163 | 0.124 | 0.016 | 0.076 | 0.282 | 0.425 | 0.123 | | 2.267 |
| OH | 0.602 | 0.635 | 0.014 | 0.266 | 0.324 | 0.293 | 0.768 | 0.605 | 0.480 | 0.447 | 0.690 | 0.303 |
| H2O | | | 3.814 | 3.911 | 3.992 | 3.568 | 3.836 | 3.875 | 3.872 | 3.990 | 3.802 | 3.229 |
| Σ[W] | 2.105 | 2.094 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 | 6.000 |
Table 14.
Average mineral chemistry (WDS-EDS, wt %) of mayenite-supergroup minerals from Hatrurim larnite CM rocks.
Table 14.
Average mineral chemistry (WDS-EDS, wt %) of mayenite-supergroup minerals from Hatrurim larnite CM rocks.
| Mineral | Fluormayenite (n = 14) | Fluorkyuygenite (n = 104) |
|---|
| n | Mean | S | Min | Max | Mean | S | Min | Max |
|---|
| P2O5 | 0.06 | 0.06 | <0.02 | 0.16 | 0.03 | 0.03 | <0.02 | 0.12 |
| SO3 | 0.05 | 0.06 | <0.03 | 0.22 | 0.06 | 0.08 | <0.03 | 0.31 |
| SiO2 | 0.29 | 0.28 | 0.05 | 1.26 | 0.51 | 0.42 | 0.05 | 1.58 |
| Al2O3 | 47.88 | 0.73 | 46.35 | 49.04 | 45.84 | 1.01 | 41.95 | 47.69 |
| Fe2O3 | 2.68 | 0.32 | 1.78 | 2.98 | 2.57 | 0.66 | 1.53 | 4.41 |
| CaO | 46.67 | 0.32 | 46.29 | 47.35 | 44.66 | 0.58 | 43.36 | 47.18 |
| SrO | 0.16 | 0.09 | 0.02 | 0.29 | 0.08 | 0.09 | <0.04 | 0.36 |
| Na2O | 0.07 | 0.03 | <0.03 | 0.12 | 0.09 | 0.10 | <0.03 | 0.36 |
| K2O | 0.02 | 0.02 | <0.01 | 0.05 | 0.07 | 0.07 | <0.01 | 0.20 |
| F | 1.59 | 0.18 | 1.37 | 1.90 | 2.07 | 0.49 | 1.13 | 3.11 |
| Cl | 0.66 | 0.14 | 0.23 | 0.78 | 0.37 | 0.33 | 0.01 | 1.03 |
| H2O | 0.40 | 0.11 | 0.21 | 0.57 | 4.95 | 1.12 | 4.28 | 8.76 |
| Total | 100.52 | | | | 101.30 | | | |
| O-(F,Cl)2 | 0.82 | 0.07 | 0.73 | 0.96 | 0.84 | 0.33 | 0.00 | 1.33 |
| Total | 99.70 | | | | 100.46 | | | |
Table 15.
Mineral chemistry (WDS-EDS, wt %) of fluorapatite and fluorellestadite from Hatrurim larnite CM rocks.
Table 15.
Mineral chemistry (WDS-EDS, wt %) of fluorapatite and fluorellestadite from Hatrurim larnite CM rocks.
| Type | Y | Y | Y | Y | M | M | M | M | M | M | Gh | Gh |
|---|
| Sample | Y-5-1 | Y-6-3 | Y-8 | M5-30 | Y-9-1 | W-10-2 | CONCR | CONCR | M4-215 | Y-2-1 | W-11-3 | Y-10-5 |
|---|
| Mineral | Ap | Ap | Els | Els | Ap | Ap | Ap | Ap | Ap | Ap | Ap | Ap |
|---|
| n | 9 | 10 | 7 | 2 | 4 | 24 | 4 | 1 | 1 | 7 | 3 | 2 |
|---|
| P2O5 | 19.17 | 24.27 | 13.39 | 6.15 | 14.83 | 30.98 | 35.76 | 23.72 | 40.81 | 33.58 | 37.53 | 29.31 |
| V2O5 | 0.31 | 0.47 | 0.33 | n.a. | 0.35 | 0.44 | n.a. | n.a. | n.a. | 0.35 | 0.52 | 0.50 |
| SO3 | 12.60 | 9.46 | 16.01 | 21.32 | 15.60 | 5.62 | 2.71 | 17.83 | 0.54 | 4.12 | 0.82 | 6.27 |
| SiO2 | 10.00 | 7.73 | 12.31 | 15.17 | 11.57 | 5.35 | 3.59 | 2.07 | 0.65 | 3.89 | 3.29 | 6.28 |
| FeO | 0.40 | 0.47 | 0.31 | n.a. | 0.36 | 0.10 | 0.04 | 0.02 | <0.02 | 0.12 | 0.19 | 0.33 |
| CaO | 55.58 | 55.49 | 55.44 | 55.63 | 55.74 | 55.50 | 55.95 | 55.37 | 56.04 | 55.59 | 55.23 | 55.91 |
| SrO | n.a. | 0.67 | 0.50 | 0.83 | n.a. | 0.46 | 0.35 | 0.27 | 0.13 | 0.19 | 0.83 | n.a. |
| Na2O | n.a. | <0.02 | 0.03 | 0.13 | n.a. | 0.02 | 0.03 | 0.02 | <0.02 | 0.02 | n.a. | n.a. |
| K2O | n.a. | n.a. | n.a. | n.a. | n.a. | 0.05 | <0.01 | 0.03 | <0.01 | <0.01 | n.a. | n.a. |
| F | 3.63 | 2.57 | 2.67 | 1.92 | 2.89 | 2.76 | 3.00 | 2.95 | 3.20 | 3.05 | 2.39 | 3.16 |
| Cl | n.a. | n.a. | 0.10 | n.a. | 0.23 | 0.01 | n.a. | n.a. | n.a. | 0.01 | n.a. | n.a. |
| Total | 101.67 | 101.12 | 101.09 | 101.13 | 101.58 | 101.27 | 101.44 | 102.28 | 101.36 | 100.91 | 100.82 | 101.75 |
| O-(F,Cl)2 | 1.53 | 1.08 | 1.15 | 0.81 | 1.27 | 1.16 | 1.26 | 1.24 | 1.35 | 1.29 | 1.01 | 1.33 |
| Total | 100.14 | 100.04 | 99.94 | 100.32 | 100.31 | 100.11 | 100.18 | 101.04 | 100.02 | 99.63 | 99.81 | 100.43 |
| Formula based on 10 cations in the Ca atoms M10[ZO4]6 × 2 |
| P | 2.71 | 3.41 | 1.89 | 0.86 | 2.09 | 4.37 | 5.02 | 3.37 | 5.74 | 4.75 | 5.31 | 4.12 |
| V | 0.03 | 0.05 | 0.04 | 0.00 | 0.04 | 0.05 | | | | 0.04 | 0.06 | 0.06 |
| S | 1.58 | 1.18 | 2.00 | 2.65 | 1.95 | 0.70 | 0.34 | 2.24 | 0.07 | 0.52 | 0.10 | 0.78 |
| Si | 1.67 | 1.28 | 2.05 | 2.51 | 1.93 | 0.89 | 0.60 | 0.35 | 0.11 | 0.65 | 0.55 | 1.04 |
| Σ[Z] | 5.99 | 5.92 | 5.97 | 6.02 | 6.00 | 6.02 | 5.95 | 5.96 | 5.92 | 5.95 | 6.02 | 6.00 |
| Fe | 0.06 | 0.07 | 0.04 | | 0.05 | 0.01 | 0.01 | 0.00 | 0.00 | 0.02 | 0.03 | 0.05 |
| Ca | 9.94 | 9.87 | 9.90 | 9.88 | 9.95 | 9.93 | 9.95 | 9.96 | 9.99 | 9.96 | 9.89 | 9.95 |
| Sr | | 0.06 | 0.05 | 0.08 | | 0.04 | 0.03 | 0.03 | 0.01 | 0.02 | 0.08 | |
| Na | | 0.00 | 0.01 | 0.04 | | 0.01 | 0.01 | 0.01 | 0.00 | 0.00 | | |
| K | | | | | | 0.01 | 0.00 | 0.01 | 0.00 | 0.00 | | |
| Σ[M] | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| F | 1.91 | 1.35 | 1.40 | 1.00 | 1.52 | 1.46 | 1.57 | 1.56 | 1.68 | 1.61 | 1.26 | 1.66 |
| Cl | | | 0.03 | | 0.07 | 0.00 | | | | 0.00 | | |
Table 16.
Average mineral chemistry (WDS-EDS, wt %) of fluorapatite and fluorellestadite from Hatrurim ye’elimite– and mayenite-bearing larnite CM rocks.
Table 16.
Average mineral chemistry (WDS-EDS, wt %) of fluorapatite and fluorellestadite from Hatrurim ye’elimite– and mayenite-bearing larnite CM rocks.
| Mineral | Fluorellestadite (n = 25) | Fluorapatite (n = 60) |
|---|
| Component | Mean | S | Min | Max | Mean | S | Min | Max |
|---|
| P2O5 | 11.43 | 3.84 | 6.12 | 15.60 | 28.37 | 6.37 | 15.11 | 39.68 |
| V2O5 | 0.29 | 0.14 | <0.03 | 0.49 | 0.39 | 0.14 | <0.03 | 0.62 |
| SO3 | 17.41 | 2.64 | 13.63 | 21.45 | 7.13 | 3.87 | 0.65 | 15.53 |
| SiO2 | 13.04 | 1.47 | 11.51 | 15.21 | 6.32 | 2.57 | 1.73 | 11.94 |
| FeO | 0.32 | 0.25 | <0.02 | 0.69 | 0.20 | 0.21 | <0.02 | 0.75 |
| CaO | 55.49 | 0.20 | 55.12 | 55.76 | 55.56 | 0.26 | 55.07 | 56.27 |
| SrO | 0.58 | 0.16 | 0.37 | 0.86 | 0.47 | 0.22 | 0.03 | 0.85 |
| Na2O | 0.06 | 0.11 | <0.03 | 0.26 | <0.03 | 0.02 | <0.03 | 0.07 |
| K2O | <0.01 | | | | 0.03 | 0.05 | <0.01 | 0.21 |
| F | 2.56 | 0.40 | 1.75 | 3.12 | 2.90 | 0.47 | 2.20 | 4.02 |
| Cl | 0.14 | 0.10 | <0.01 | 0.33 | 0.02 | 0.05 | <0.01 | 0.21 |
| Total | 101.31 | | | | 101.41 | | | |
| O-(F,Cl)2 | 1.10 | 0.19 | 0.74 | 1.39 | 1.22 | 0.20 | 0.93 | 1.69 |
| Total | 100.21 | | | | 100.19 | | | |
Table 17.
Mineral chemistry (WDS, wt %) of intermediate phases of the silicocarnotite–ternesite series from Hatrurim larnite CM rocks, compared with holotype silicocarnotite and ternesite.
Table 17.
Mineral chemistry (WDS, wt %) of intermediate phases of the silicocarnotite–ternesite series from Hatrurim larnite CM rocks, compared with holotype silicocarnotite and ternesite.
| Number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|
| Sample | M5-30 | M5-31 | YV-567 | YV-415 | Ideal | Ideal | Ideal | Ideal |
|---|
| n | 10 | 9 | 16 | 6 | | | | |
|---|
| P2O5 | 2.95 | 14.33 | 27.94 | 25.81 | 29.42 | 0.00 | 14.74 | 2.95 |
| V2O5 | 0.09 | 0.33 | 0.51 | n.a. | n.a. | n.a. | n.a. | n.a. |
| SO3 | 14.94 | 8.07 | 0.32 | 1.85 | 0.00 | 16.66 | 8.31 | 14.99 |
| SiO2 | 23.09 | 18.21 | 12.70 | 13.62 | 12.45 | 25.00 | 18.72 | 23.74 |
| TiO2 | 0.28 | 0.12 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Cr2O3 | 0.08 | <0.02 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Al2O3 | 0.07 | <0.02 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| FeO | 0.07 | 0.07 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| CaO | 58.24 | 57.92 | 57.24 | 57.61 | 58.13 | 58.34 | 58.23 | 58.32 |
| SrO | 0.16 | 0.06 | 0.17 | 0.13 | n.a. | n.a. | n.a. | n.a. |
| Na2O | 0.07 | 0.11 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Total | 100.04 | 99.31 | 98.88 | 99.02 | 100.00 | 100.00 | 100.00 | 100.00 |
| Formula based on 12 oxygens M5[TO4]2[TO4] |
| P | 0.20 | 0.98 | 1.92 | 1.77 | 2.00 | 0.00 | 1.00 | 0.20 |
| V | 0.00 | 0.02 | 0.03 | | | | | |
| S | 0.90 | 0.49 | 0.02 | 0.11 | 0.00 | 1.00 | 0.50 | 0.90 |
| Si | 1.85 | 1.48 | 1.03 | 1.11 | 1.00 | 2.00 | 1.50 | 1.90 |
| Al | 0.01 | 0.00 | | | | | | |
| Ti | 0.02 | 0.01 | | | | | | |
| Σ[T] | 2.99 | 2.98 | 3.00 | 2.99 | 3.00 | 3.00 | 3.00 | 3.00 |
| Cr | 0.00 | 0.00 | | | | | | |
| Fe | 0.00 | 0.00 | | | | | | |
| Ca | 5.01 | 5.03 | 4.99 | 5.01 | 5.00 | 5.00 | 5.00 | 5.00 |
| Sr | 0.01 | 0.00 | 0.01 | 0.01 | | | | |
| Na | 0.01 | 0.02 | | | | | | |
Table 18.
Mineral chemistry (wt %) of perovskite–supergroup minerals (anion deficient perovskites, brownmillerite subgroup) in Hatrurim larnite CM rocks, Israel.
Table 18.
Mineral chemistry (wt %) of perovskite–supergroup minerals (anion deficient perovskites, brownmillerite subgroup) in Hatrurim larnite CM rocks, Israel.
| Type | Y | Y | Y | M | M | M | M | Y | Y | M | M | Gh | Gh | M |
|---|
| Sample | Y-6-3 | Y-6-3 | Y-8 | W-10-2 | Y-2-1 | G-7 | W-12-1 | Y-6-3 | Y-6-3 | W-10-2 | G-7 | W-11-3 | W-11-3 | W-10-2 |
|---|
| Mineral | Srb | Brm | Srb | Brm | Brm | Brm | Brm | Shr | Shr | Shu | Shu | Ntk | Prv | Prv |
|---|
| n | 18 | 4 | 8 | 22 | 7 | 11 | 13 | 5 | 2 | 1 | 7 | 49 | 6 | 7 |
|---|
| SiO2 | 0.58 | 0.99 | 0.68 | 0.67 | 0.64 | 1.05 | 0.72 | 1.08 | 0.60 | 0.98 | 1.04 | 5.05 | 3.81 | 2.86 |
| TiO2 | 0.31 | 0.65 | 0.30 | 2.64 | 3.07 | 2.97 | 3.41 | 19.35 | 16.59 | 20.97 | 19.47 | 29.04 | 33.36 | 39.31 |
| ZrO2 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 2.52 | n.a. | 0.49 | 0.68 | 0.74 | 0.55 |
| Nb2O5 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.04 | 0.06 | n.a. |
| Cr2O3 | 0.25 | 0.60 | <0.02 | 0.66 | 1.14 | 0.45 | 2.34 | <0.02 | n.a. | n.a. | 0.48 | 0.08 | 0.07 | 0.41 |
| Al2O3 | 4.39 | 11.04 | 6.72 | 13.48 | 10.85 | 13.78 | 15.40 | 5.93 | 5.93 | 7.88 | 9.33 | 2.07 | 1.92 | 3.13 |
| Fe2O3 | 51.61 | 41.89 | 48.51 | 36.03 | 38.46 | 34.76 | 30.88 | 30.13 | 30.69 | 25.47 | 25.46 | 14.23 | 17.66 | 11.68 |
| FeO* | 0.24 | 0.48 | 0.11 | 1.03 | 1.24 | 1.54 | 1.31 | n.a. | n.a. | 1.00 | 0.10 | 5.47 | | |
| MnO | 0.31 | 0.52 | 0.32 | n.a. | n.a. | n.a. | n.a. | 0.65 | 0.84 | n.a. | n.a. | 0.07 | 0.03 | n.a. |
| MgO | 0.26 | 0.50 | 0.61 | 0.68 | 0.66 | 0.58 | 0.84 | <0.02 | 0.45 | n.a. | 0.30 | <0.02 | <0.02 | n.a. |
| CaO | 42.33 | 43.89 | 42.63 | 44.64 | 44.19 | 44.66 | 45.27 | 42.79 | 42.63 | 42.55 | 43.33 | 42.10 | 41.98 | 42.44 |
| SrO | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | 0.27 | 0.27 | n.a. |
| Total | 100.30 | 100.59 | 99.89 | 99.95 | 100.39 | 99.98 | 100.31 | 99.93 | 100.24 | 98.97 | 100.01 | 99.10 | 99.76 | 100.38 |
| Formula based on | 4 cations and 5 oxygens | 6 cations and 8 oxygens | 8 cations 11 oxygens | 2 cations |
| Si | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.04 | 0.03 | 0.07 | 0.04 | 0.06 | 0.07 | 0.45 | 0.08 | 0.06 |
| Al | 0.23 | 0.55 | 0.34 | 0.66 | 0.54 | 0.68 | 0.75 | 0.46 | 0.46 | 0.61 | 0.71 | 0.22 | 0.05 | 0.08 |
| Fe3+ | 1.70 | 1.33 | 1.58 | 1.13 | 1.22 | 1.09 | 0.95 | 1.48 | 1.51 | 1.25 | 1.23 | 0.95 | 0.30 | 0.19 |
| Fe2+ | 0.01 | 0.02 | 0.00 | 0.04 | 0.04 | 0.05 | 0.05 | | | 0.05 | 0.01 | 0.41 | | |
| Ti | 0.01 | 0.02 | 0.01 | 0.08 | 0.10 | 0.09 | 0.11 | 0.95 | 0.82 | 1.03 | 0.94 | 1.93 | 0.56 | 0.65 |
| Zr+Nb | | | | | | | | | 0.08 | | 0.02 | 0.03 | 0.01 | 0.01 |
| Cr | 0.01 | 0.02 | 0.00 | 0.02 | 0.04 | 0.01 | 0.08 | 0.00 | 0.00 | | 0.02 | 0.01 | 0.00 | 0.01 |
| Mn | 0.01 | 0.02 | 0.01 | | | | | 0.04 | 0.05 | | | 0.01 | 0.00 | |
| Mg | 0.02 | 0.03 | 0.04 | 0.04 | 0.04 | 0.04 | 0.05 | 0.00 | 0.04 | | 0.03 | 0.00 | 0.00 | |
| Ca + Sr | 1.99 | 1.98 | 1.98 | 2.00 | 2.00 | 1.99 | 1.99 | 3.00 | 3.00 | 2.99 | 2.98 | 4.01 | 1.00 | 1.00 |
Table 19.
Average mineral chemistry (WDS-EDS, wt %) of brownmillerite subgroup minerals from Hatrurim larnite CM rocks.
Table 19.
Average mineral chemistry (WDS-EDS, wt %) of brownmillerite subgroup minerals from Hatrurim larnite CM rocks.
| Rock | Ye’elimite–Larnite Rocks | Mayenite–Larnite Rocks |
|---|
| Mineral | Srebrodolskite (n = 30) | Brownmillerite (n = 4) | Brownmillerite (n = 65) |
|---|
| | Mean | S | Min | Max | Mean | S | Min | Max | Mean | S | Min | Max |
|---|
| SiO2 | 0.61 | 0.18 | 0.28 | 0.96 | 0.99 | 0.20 | 0.71 | 1.16 | 0.75 | 0.22 | 0.43 | 1.71 |
| TiO2 | 0.30 | 0.18 | <0.02 | 0.69 | 0.65 | 0.04 | 0.62 | 0.67 | 2.93 | 0.43 | 2.17 | 4.07 |
| Cr2O3 | 0.04 | 0.06 | <0.02 | 0.25 | 0.60 | 0.33 | 0.37 | 0.83 | 1.07 | 0.79 | 0.38 | 3.58 |
| Al2O3 | 5.11 | 2.36 | 0.49 | 10.30 | 11.04 | 1.59 | 9.52 | 13.05 | 13.64 | 1.66 | 10.56 | 17.59 |
| Fe2O3 | 50.66 | 3.05 | 44.19 | 56.64 | 41.89 | 1.27 | 40.82 | 43.42 | 34.97 | 3.07 | 26.46 | 39.43 |
| FeO* | 0.20 | 0.30 | <0.02 | 1.13 | 0.48 | 0.27 | 0.15 | 0.55 | 1.15 | 0.33 | 0.18 | 1.99 |
| MnO | 0.31 | 0.05 | 0.21 | 0.44 | 0.52 | 0.04 | 0.49 | 0.54 | <0.02 | | | |
| MgO | 0.33 | 0.17 | 0.20 | 0.74 | 0.50 | 0.16 | 0.40 | 0.73 | 0.70 | 0.15 | 0.46 | 1.09 |
| CaO | 42.42 | 0.38 | 41.51 | 43.00 | 43.89 | 0.28 | 43.67 | 44.30 | 44.72 | 0.45 | 43.71 | 45.69 |
| Total | 99.98 | | | | 100.54 | | | | 99.94 | | | |
Table 20.
Average mineral chemistry (WDS-EDS, wt %) of perovskite–supergroup minerals from Hatrurim larnite CM rocks.
Table 20.
Average mineral chemistry (WDS-EDS, wt %) of perovskite–supergroup minerals from Hatrurim larnite CM rocks.
| Rock | Mayenite–Larnite Rocks |
|---|
| Mineral | Sharyginite (n = 7) | Shulamitite (n = 8) | Perovskite (n = 13) |
|---|
| | Mean | S | Min | Max | Mean | S | Min | Max | Mean | S | Min | Max |
|---|
| SiO2 | 0.96 | 0.30 | 0.56 | 1.33 | 1.04 | 0.10 | 0.90 | 1.20 | 2.86 | 0.17 | 2.59 | 3.14 |
| TiO2 | 18.66 | 1.31 | 16.45 | 19.95 | 19.47 | 0.15 | 19.32 | 20.97 | 39.31 | 0.84 | 37.95 | 40.83 |
| ZrO2 | 0.70 | 1.30 | <0.02 | 3.10 | <0.02 | | | | 0.55 | 0.11 | 0.43 | 0.69 |
| Cr2O3 | <0.02 | | | | 0.48 | 0.06 | 0.38 | 0.57 | 0.41 | 0.09 | 0.35 | 0.51 |
| Al2O3 | 5.93 | 0.33 | 5.42 | 6.35 | 9.33 | 0.37 | 7.88 | 9.92 | 3.13 | 0.22 | 2.82 | 3.51 |
| Fe2O3 | 30.27 | 0.30 | 29.93 | 30.85 | 25.57 | 0.45 | 25.15 | 26.59 | 11.68 | 0.45 | 10.84 | 12.22 |
| FeO* | | | | | 0.04 | 0.35 | 0.01 | 1.00 | | | | |
| MnO | 0.70 | 0.15 | 0.49 | 0.87 | <0.02 | | | | <0.02 | | | |
| MgO | 0.09 | 0.20 | 0.00 | 0.45 | 0.04 | 0.11 | <0.02 | 0.30 | <0.02 | | | |
| CaO | 42.75 | 0.16 | 42.55 | 43.00 | 43.33 | 0.11 | 42.55 | 43.48 | 42.44 | 0.20 | 42.17 | 42.66 |
| Total | 100.06 | | | | 99.30 | | | | 100.38 | | | |
Table 21.
Mineral chemistry (wt %) of spinel-group minerals in Hatrurim larnite CM rocks, Israel.
Table 21.
Mineral chemistry (wt %) of spinel-group minerals in Hatrurim larnite CM rocks, Israel.
| Type | Sample | Phase | n | TiO2 | Cr2O3 | V2O3 | Al2O3 | Fe2O3 | FeO | Mn2O3 | MnO | MgO | CaO | NiO | ZnO | CuO | Total | Ti | Cr+V | Al | Fe3+ | Fe2+ | Mn3+ | Mn2+ | Mg | Ca | Ni+Zn+Cu |
|---|
| Y | H-201 | Mfr | 18 | 0.21 | 1.05 | n.a. | 16.73 | 58.48 | 0.06 | | 0.14 | 19.75 | 0.79 | 1.68 | 1.07 | n.a. | 99.96 | 0.01 | 0.03 | 0.61 | 1.36 | 0.00 | | 0.00 | 0.91 | 0.03 | 0.07 |
| | | | S | 0.05 | 0.18 | | 0.93 | 1.09 | 0.06 | | 0.02 | 0.14 | 0.15 | 0.15 | 0.19 | n.a. | | | | | | | | | | | |
| Y | M5-31 | Mfr | 9 | 0.24 | 2.65 | <0.03 | 19.59 | 53.76 | 0.19 | | 0.11 | 20.16 | 0.93 | 0.67 | 1.72 | 0.08 | 100.11 | 0.01 | 0.06 | 0.70 | 1.23 | 0.01 | | 0.00 | 0.91 | 0.03 | 0.06 |
| | | | S | 0.03 | 0.45 | 0.00 | 1.69 | 2.44 | 0.26 | | 0.01 | 0.15 | 0.18 | 0.05 | 0.26 | 0.03 | | | | | | | | | | | |
| Y | Y-8 | Mfr | 1 | <0.02 | 0.18 | n.a. | 10.56 | 67.14 | 0.09 | | 0.40 | 19.67 | 1.41 | n.a. | 0.42 | n.a. | 99.87 | 0.00 | 0.01 | 0.39 | 1.60 | 0.00 | | 0.01 | 0.93 | 0.05 | 0.01 |
| Y | YV-411 | Mfr | 6 | 0.26 | 0.74 | n.a. | 15.64 | 60.07 | 0.16 | | 0.62 | 19.91 | 1.04 | 0.48 | 0.91 | n.a. | 99.81 | 0.01 | 0.02 | 0.57 | 1.40 | 0.00 | | 0.02 | 0.92 | 0.03 | 0.03 |
| | | | S | 0.05 | 0.06 | | 0.24 | 0.36 | 0.21 | | 0.10 | 0.14 | 0.23 | 0.06 | 0.14 | | | | | | | | | | | | |
| Y | Y-6-3 | Mfr | 2 | <0.02 | 0.70 | n.a. | 17.86 | 58.49 | 1.83 | | n.a. | 20.17 | 1.14 | n.a. | n.a. | n.a. | 100.18 | 0.00 | 0.02 | 0.64 | 1.34 | 0.05 | | | 0.92 | 0.04 | |
| Y | Y-5-1 | Mfr | 7 | <0.02 | 0.27 | n.a. | 14.32 | 60.23 | 0.06 | 2.54 | | 19.68 | 1.21 | 0.59 | 1.39 | n.a. | 100.29 | 0.00 | 0.01 | 0.52 | 1.41 | 0.00 | 0.06 | | 0.91 | 0.04 | 0.05 |
| | | | S | 0.00 | 0.07 | | 0.33 | 0.48 | 0.04 | 0.10 | | 0.09 | 0.11 | 0.15 | 0.18 | | | | | | | | | | | | |
| M | H-401 | Mag | 31 | 1.05 | 0.61 | 0.12 | 2.39 | 64.14 | 29.98 | | 0.14 | 1.16 | 0.46 | n.a. | n.a. | n.a. | 100.04 | 0.03 | 0.02 | 0.11 | 1.81 | 0.94 | | 0.00 | 0.07 | 0.02 | |
| | | | S | 0.45 | 0.14 | 0.05 | 0.81 | 1.37 | 1.87 | | 0.05 | 0.91 | 0.18 | | | | | | | | | | | | | | |
| M | M4-251 | Spl | 9 | 0.10 | 6.10 | <0.03 | 53.03 | 12.81 | 4.23 | | 0.10 | 22.66 | 0.47 | 0.18 | 0.71 | 0.05 | 100.43 | 0.00 | 0.13 | 1.62 | 0.25 | 0.09 | | 0.00 | 0.88 | 0.01 | 0.02 |
| | | | S | 0.04 | 7.52 | 0.00 | 8.13 | 1.49 | 0.90 | | 0.01 | 1.47 | 0.18 | 0.12 | 0.04 | 0.08 | | | | | | | | | | | |
| M | M4-218 | Spl | 13 | 0.13 | 5.44 | <0.03 | 44.56 | 22.59 | 5.16 | | 0.10 | 21.37 | 0.50 | 0.08 | 0.33 | n.a. | 100.26 | 0.00 | 0.12 | 1.42 | 0.46 | 0.12 | | 0.00 | 0.86 | 0.01 | 0.01 |
| | | | S | 0.03 | 3.80 | 0.01 | 3.64 | 1.74 | 1.06 | | 0.01 | 0.77 | 0.09 | 0.01 | 0.07 | | | | | | | | | | | | |
| | | Mfr | 1 | 0.05 | n.a. | n.a. | 7.04 | 70.13 | 4.90 | | 0.59 | 16.60 | 1.18 | n.a. | n.a. | n.a. | 100.49 | 0.00 | | 0.27 | 1.73 | 0.13 | | 0.02 | 0.81 | 0.04 | |
| M | M4-215 | Spl | 12 | 0.15 | 13.19 | <0.03 | 49.64 | 9.77 | 3.30 | | 0.03 | 23.52 | 0.41 | <0.02 | 0.10 | <0.03 | 100.11 | 0.00 | 0.27 | 1.53 | 0.19 | 0.07 | | 0.00 | 0.92 | 0.01 | 0.00 |
| | | | S | 0.06 | 8.10 | 0.00 | 8.55 | 3.05 | 0.64 | | 0.02 | 0.84 | 0.11 | 0.00 | 0.04 | 0.00 | | | | | | | | | | | |
| M | G-7 | Spl | 3 | n.a. | 1.88 | n.a. | 44.42 | 26.43 | 4.44 | | n.a. | 21.14 | 0.87 | 0.86 | n.a. | n.a. | 100.04 | | 0.04 | 1.42 | 0.54 | 0.10 | | | 0.86 | 0.03 | 0.02 |
| | | Mfr | 1 | n.a. | <0.02 | n.a. | 17.35 | 60.24 | 0.02 | | 0.40 | 20.89 | 1.30 | n.a. | n.a. | n.a. | 100.19 | | 0.00 | 0.62 | 1.38 | 0.00 | | 0.01 | 0.95 | 0.04 | |
| M | W-10-2 | Spl | 5 | 0.21 | 2.19 | n.a. | 44.70 | 25.22 | 4.96 | | n.a. | 20.61 | 0.79 | 0.93 | 0.75 | n.a. | 100.37 | 0.01 | 0.05 | 1.43 | 0.52 | 0.11 | | | 0.83 | 0.02 | 0.04 |
| | | | S | 0.48 | 0.68 | | 0.44 | 1.10 | 1.22 | | | 0.63 | 0.17 | 0.06 | 0.18 | | | | | | | | | | | | |
| M | Y-9-1 | Mfr | 2 | <0.02 | <0.02 | n.a. | 11.11 | 67.33 | 0.11 | | <0.02 | 20.85 | 0.67 | n.a. | n.a. | n.a. | 100.06 | 0.00 | 0.00 | 0.41 | 1.59 | 0.00 | | 0.00 | 0.98 | 0.02 | |
| M | CONCR | Spl | 1 | n.a. | 0.11 | n.a. | 34.33 | 41.10 | 0.03 | | 0.11 | 23.55 | 0.48 | n.a. | n.a. | n.a. | 99.71 | | 0.00 | 1.13 | 0.87 | 0.00 | | 0.00 | 0.98 | 0.02 | |
| Geh | Y-10-5 | Mfr | 4 | <0.02 | 0.32 | n.a. | 2.33 | 75.69 | 1.77 | | 1.15 | 17.64 | 1.14 | <0.02 | <0.03 | n.a. | 100.05 | 0.00 | 0.01 | 0.09 | 1.90 | 0.05 | | 0.03 | 0.88 | 0.04 | 0.00 |
| Geh | W-11-3 | Mfr | 45 | <0.02 | 0.41 | n.a. | 3.55 | 74.01 | 1.30 | | 0.27 | 17.25 | 1.50 | 0.81 | 0.66 | 0.44 | 100.23 | 0.00 | 0.01 | 0.14 | 1.85 | 0.04 | | 0.01 | 0.85 | 0.05 | 0.04 |
| | | | S | 0.04 | 0.09 | | 0.41 | 0.49 | 0.62 | | 0.05 | 0.40 | 0.16 | 0.10 | 0.14 | 0.09 | | | | | | | | | | | |
Table 22.
Mineral chemistry (WDS-EDS, wt %) of gehlenite from Hatrurim larnite CM rocks.
Table 22.
Mineral chemistry (WDS-EDS, wt %) of gehlenite from Hatrurim larnite CM rocks.
| Type | Gh | Gh | Gh | Gh | M | M | M | M | Y |
|---|
| Sample | W-11-3 | W-11-3 | Y-10-5 | W-10-2 | G-7 | H-401 | CONCR | M4-251 | YV-410 |
|---|
| Position | core | rim | | | | | | | |
|---|
| n | 36 | 7 | 5 | 1 | 9 | 7 | 1 | 4 | 7 |
|---|
| SiO2 | 22.17 | 21.60 | 25.05 | 21.97 | 22.11 | 26.16 | 22.28 | 21.71 | 25.92 |
| TiO2 | 0.04 | <0.01 | <0.01 | <0.01 | <0.01 | 0.09 | 0.11 | 0.28 | 0.09 |
| Al2O3 | 32.91 | 28.05 | 29.54 | 34.89 | 34.55 | 26.68 | 34.32 | 35.58 | 26.68 |
| Fe2O3 | 4.25 | 10.48 | 2.12 | 2.50 | 2.37 | 4.47 | 2.13 | 1.87 | 5.30 |
| FeO | 0.28 | 0.18 | 1.09 | 0.28 | 0.69 | 1.34 | 0.51 | 0.17 | 0.59 |
| MgO | 0.28 | 0.35 | 1.78 | 0.10 | <0.02 | 1.41 | 0.08 | 0.00 | 1.41 |
| CaO | 40.26 | 39.17 | 40.24 | 40.43 | 40.33 | 39.07 | 40.15 | 40.53 | 39.44 |
| SrO | n.a. | n.a. | n.a. | n.a. | n.a. | 0.18 | 0.21 | 0.24 | 0.18 |
| Na2O | <0.02 | <0.02 | n.a. | n.a. | n.a. | 0.49 | 0.07 | <0.02 | 0.49 |
| K2O | <0.01 | <0.01 | n.a. | n.a. | n.a. | 0.24 | 0.03 | <0.01 | 0.26 |
| Sum | 100.20 | 98.79 | 99.61 | 99.92 | 99.81 | 99.69 | 99.66 | 100.18 | 100.44 |
| Formula based on 5 cations and 7 oxygens | A2B[T2O7] | | | | |
| Si | 1.026 | 1.027 | 1.158 | 1.011 | 1.020 | 1.217 | 1.029 | 0.996 | 1.204 |
| AlIV | 0.974 | 0.973 | 0.842 | 0.989 | 0.980 | 0.783 | 0.971 | 1.004 | 0.796 |
| Σ[T] | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 | 2.000 |
| AlVI | 0.822 | 0.598 | 0.768 | 0.903 | 0.898 | 0.679 | 0.897 | 0.920 | 0.664 |
| Ti | 0.001 | | | | | 0.003 | 0.004 | 0.009 | 0.003 |
| Fe3+ | 0.148 | 0.375 | 0.074 | 0.086 | 0.082 | 0.157 | 0.074 | 0.064 | 0.185 |
| Fe2+ | 0.011 | 0.007 | 0.042 | 0.011 | 0.027 | 0.052 | 0.020 | 0.007 | 0.023 |
| Mg | 0.019 | 0.025 | 0.123 | 0.007 | | 0.098 | 0.006 | | 0.098 |
| Σ[B] | 1.002 | 1.005 | 1.007 | 1.007 | 1.007 | 0.990 | 1.000 | 1.001 | 0.973 |
| Ca | 1.998 | 1.995 | 1.993 | 1.993 | 1.993 | 1.947 | 1.987 | 1.993 | 1.963 |
| Sr | | | | | | 0.005 | 0.006 | 0.006 | 0.005 |
| Na | | | | | | 0.044 | 0.006 | | 0.044 |
| K | | | | | | 0.015 | 0.002 | | 0.015 |
| Σ[A] | 1.998 | 1.995 | 1.993 | 1.993 | 1.993 | 2.010 | 2.000 | 1.999 | 2.027 |
| End members |
| Ca2AlAlSiO7 | 82.04 | 59.53 | 76.32 | 89.65 | 89.18 | 62.78 | 88.95 | 91.95 | 62.37 |
| Ca2Fe3+AlSiO7 | 14.78 | 37.31 | 7.31 | 8.59 | 8.18 | 15.82 | 7.40 | 6.44 | 19.05 |
| Ca2MgSi2O7 | 1.94 | 2.47 | 12.20 | 0.68 | 0.00 | 9.91 | 0.55 | 0.00 | 10.03 |
| Ca2Fe2+Si2O7 | 1.23 | 0.70 | 4.17 | 1.08 | 2.64 | 5.62 | 2.33 | 1.60 | 2.67 |
| NaCaAlSi2O7 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 5.87 | 0.77 | 0.01 | 5.89 |
Table 23.
Average mineral chemistry (WDS-EDS, wt %) of periclase in Hatrurim larnite CM rocks and periclase from Jordanian CM marbles, compared.
Table 23.
Average mineral chemistry (WDS-EDS, wt %) of periclase in Hatrurim larnite CM rocks and periclase from Jordanian CM marbles, compared.
| Locality | Hatrurim Basin | Jordan |
|---|
| Type | M | M | Y | Gh | Gh | Gh | Spurrite marble |
|---|
| Sample | M5-32 | M4-215 | YV-412 | W-10-2 | W-10-2 | W-10-2 | Mean | S | Min | Max |
|---|
| n | 10 | 7 | 4 | 2 | 1 | 2 | 15 | | | |
|---|
| Cr2O3 | 0.21 | <0.02 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| Al2O3 | 0.20 | <0.02 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| FeO | 20.10 | 23.93 | 2.55 | 7.66 | 14.77 | 23.84 | 0.43 | 0.04 | 0.37 | 0.46 |
| MnO | 0.18 | 0.10 | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. | n.a. |
| MgO | 75.38 | 75.22 | 90.69 | 87.85 | 80.67 | 74.07 | 70.00 | 2.02 | 66.45 | 71.51 |
| CaO | 0.44 | 0.45 | 0.78 | 0.45 | 0.60 | 0.78 | <0.01 | | | |
| NiO | 1.98 | 0.22 | 3.50 | 2.50 | 2.32 | n.a. | 5.13 | 0.35 | 4.80 | 5.59 |
| ZnO | 1.45 | <0.03 | 2.17 | 1.00 | 1.01 | n.a. | 19.77 | 2.84 | 15.24 | 23.13 |
| CuO | <0.03 | <0.03 | n.a. | n.a. | n.a. | n.a. | 3.34 | 0.54 | 2.54 | 4.06 |
| Total | 99.93 | 99.92 | 99.68 | 99.46 | 99.37 | 98.68 | 98.67 | | | |
| Formula based on 1 cation |
| Cr | 0.00 | 0.00 | | | | | | | | |
| Al | 0.00 | 0.00 | | | | | | | | |
| Fe | 0.13 | 0.15 | 0.01 | 0.05 | 0.09 | 0.15 | 0.00 | | | |
| Mn | 0.00 | 0.00 | | | | | | | | |
| Mg | 0.85 | 0.84 | 0.95 | 0.93 | 0.89 | 0.84 | 0.85 | | | |
| Ca | 0.00 | 0.00 | 0.01 | 0.00 | 0.00 | 0.01 | | | | |
| Ni | 0.01 | 0.00 | 0.02 | 0.01 | 0.01 | | 0.03 | | | |
| Zn | 0.01 | 0.00 | 0.01 | 0.01 | 0.01 | | 0.12 | | | |
| Cu | 0.00 | 0.00 | | | | | 0.02 | | | |
Table 24.
Representative WDS-EDS analyses (wt %) of minerals of barite–hashemite series in Hatrurim larnite CM rocks.
Table 24.
Representative WDS-EDS analyses (wt %) of minerals of barite–hashemite series in Hatrurim larnite CM rocks.
| Type | Y | Y | Y | Y | Y | Y | Gh | Gh |
|---|
| Sample | YV-411 | Y-6-3 | Y-6-3 | Y-8-1 | Y-5-1 | M5-31 | W-11-3 | W-11-3 |
|---|
| Mineral | Barite | Barite | Barite | Barite | Barite | Barite | Barite | Hashemite |
|---|
| n | 7 | 3 | 2 | 2 | 5 | 3 | 7 | 6 |
| BaO | 64.00 | 62.98 | 61.85 | 60.23 | 63.43 | 61.58 | 62.76 | 61.45 |
| SrO | 0.59 | 0.79 | 0.90 | 1.91 | 0.65 | 2.06 | 0.75 | 0.44 |
| CaO | 0.76 | 0.75 | 0.92 | 1.59 | 0.84 | 0.86 | 0.67 | 0.53 |
| FeO | 0.04 | 0.67 | n.a. | 0.50 | 0.67 | n.a. | n.a. | n.a. |
| CrO3 | 0.87 | 3.28 | 18.64 | 8.38 | 3.14 | 0.48 | 16.77 | 28.20 |
| SO3 | 33.23 | 31.71 | 17.37 | 26.80 | 31.47 | 34.30 | 18.91 | 9.40 |
| Total | 99.49 | 100.17 | 99.67 | 99.40 | 100.20 | 99.28 | 99.86 | 100.03 |
| Formula based on 4 oxygens |
| Ba | 0.98 | 0.95 | 0.98 | 0.92 | 0.96 | 0.93 | 1.00 | 0.99 |
| Sr | 0.01 | 0.02 | 0.02 | 0.04 | 0.01 | 0.05 | 0.02 | 0.01 |
| Ca | 0.03 | 0.03 | 0.04 | 0.07 | 0.03 | 0.04 | 0.03 | 0.02 |
| Fe | 0.00 | 0.02 | | 0.02 | 0.02 | | | |
| Cr | 0.02 | 0.08 | 0.46 | 0.20 | 0.07 | 0.01 | 0.41 | 0.70 |
| S | 0.97 | 0.92 | 0.53 | 0.79 | 0.92 | 0.99 | 0.58 | 0.29 |
Table 25.
Partitioning of major and trace elements among rock-forming minerals identified in Hatrurim larnite CM rocks.
Table 25.
Partitioning of major and trace elements among rock-forming minerals identified in Hatrurim larnite CM rocks.
| Mineral | Element Incorporation Ratio (KEL) |
|---|
| | Al | Si | P | Ca | Na | K | Fe | S | F | Sr | Cr | Ba |
|---|
| Larnite (Y) | 0.0 | 1.4 | 0.6 | 1.2 | 1.2 | 0.1 | 0.2 | 0.0 | 0.0 | 0.3 | 0.7 | 0.0 |
| Larnite (M) | 0.0 | 1.4 | 0.9 | 1.2 | 1.7 | 1.1 | 0.1 | n.d. | 0.0 | 1.2 | 0.7 | 0.0 |
| α′-Ca2SiO4(ss) | 0.0 | 1.3 | 3.1 | 1.1 | 1.9 | 4.1 | 0.1 | n.d. | 0.0 | 0.5 | 2.7 | 0.0 |
| Fluorellestadite | 0.0 | 0.6 | 5.4 | 1.1 | 0.2 | n.d. | 0.5 | 9.0 | 16.6 | 2.9 | 0.0 | 0.0 |
| Fluorapatite | 0.0 | 0.3 | 13.3 | 1.1 | 0.0 | 0.1 | 0.3 | 3.7 | 18.8 | 2.4 | 0.0 | 0.0 |
| Fluormayenite | 4.5 | 0.0 | 0.0 | 0.9 | 0.2 | 0.0 | 0.8 | 0.1 | 8.6 | 0.9 | 0.0 | 0.0 |
| Fluorkyuygenite | 4.3 | 0.0 | 0.0 | 0.8 | 0.0 | 0.2 | 0.8 | 0.2 | 11.3 | 0.4 | 0.0 | 0.0 |
| Ye’elimite | 4.5 | 0.0 | 0.1 | 0.7 | 0.4 | 0.1 | 0.5 | 4.6 | 0.0 | 1.9 | 0.0 | 0.0 |
| Barite (Y) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 1.0 | 10.4 | 0.0 | 5.4 | 130.0 | 441.0 |
| Barite (M) | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 145.5 | 0.0 | 4.3 | 0.0 | 1314 |
Table 26.
Partitioning of major and trace elements among opaque minerals identified in Hatrurim larnite CM.
Table 26.
Partitioning of major and trace elements among opaque minerals identified in Hatrurim larnite CM.
| Mineral | Element Incorporation Ratio (KEL) |
|---|
| Ti | Al | Fe | Mg | Ca | Cr | Ni | Zn | Zr | Nb | Sr |
|---|
| Magnesioferrite (Y) | 0.3 | 1.5 | 14.5 | 16.6 | 0.0 | 20.9 | 43.8 | 36.9 | 0.0 | 0.0 | 0.0 |
| Magnesioferrite (M) | 0.3 | 1.1 | 22.7 | 26.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Magnetite | 2.5 | 0.2 | 57.5 | 1.6 | 0.0 | 10.3 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Spinel | 0.4 | 4.3 | 11.8 | 29.7 | 0.0 | 81.3 | 24.2 | 26.3 | 0.0 | 0.0 | 0.0 |
| Periclase | 0.0 | 0.0 | 23.0 | 81.1 | 0.0 | 0.0 | 115 | 47.3 | 0.0 | 0.0 | 0.0 |
| Perovskite | 94.1 | 0.3 | 3.7 | 0.0 | 0.8 | 6.9 | 0.0 | 0.0 | 63.4 | 24.3 | 1.6 |
| Nataliakulikite | 61.8 | 0.2 | 11.9 | 0.0 | 0.9 | 2.6 | 0.0 | 0.0 | 69.1 | 21.6 | 1.6 |
| Sharyginite | 44.7 | 0.6 | 9.5 | 0.1 | 0.8 | n.d. | 0.0 | 0.0 | 80.6 | 0.0 | 0.0 |
| Shulamitite | 46.6 | 0.9 | 8.0 | 0.1 | 0.8 | 8.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Brownmillerite (Y) | 1.6 | 1.0 | 10.6 | 0.4 | 0.8 | 13.4 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Brownmillerite (M) | 7.0 | 1.3 | 12.4 | 0.9 | 0.8 | 18.1 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Srebrodolskite | 0.8 | 0.5 | 12.0 | 0.3 | 0.8 | 0.2 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |