3.1. Thermodynamic Analysis
To understand the solving behavior of metal elements in the process of alkali leaching-dilute acid washing, ternary potential-pH diagrams were drawn. The results are shown in
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8. The molarity concentration used for the calculation is 1 mol/L.
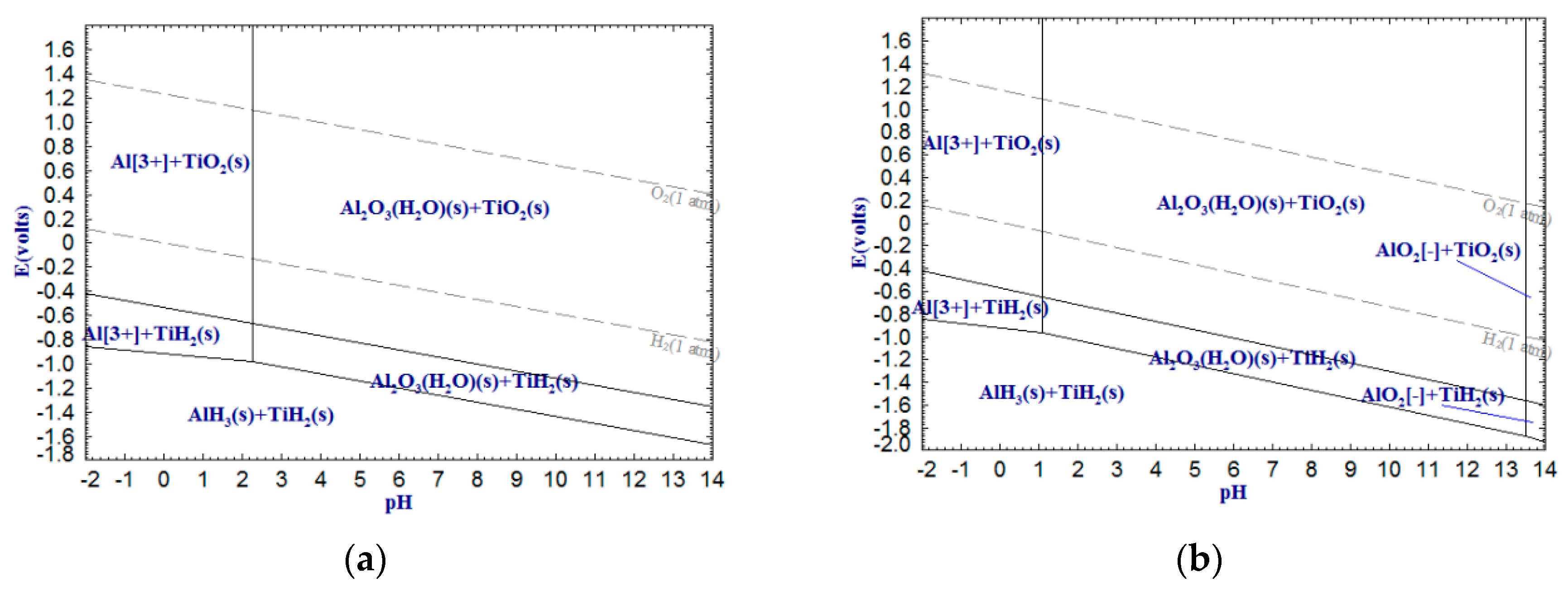
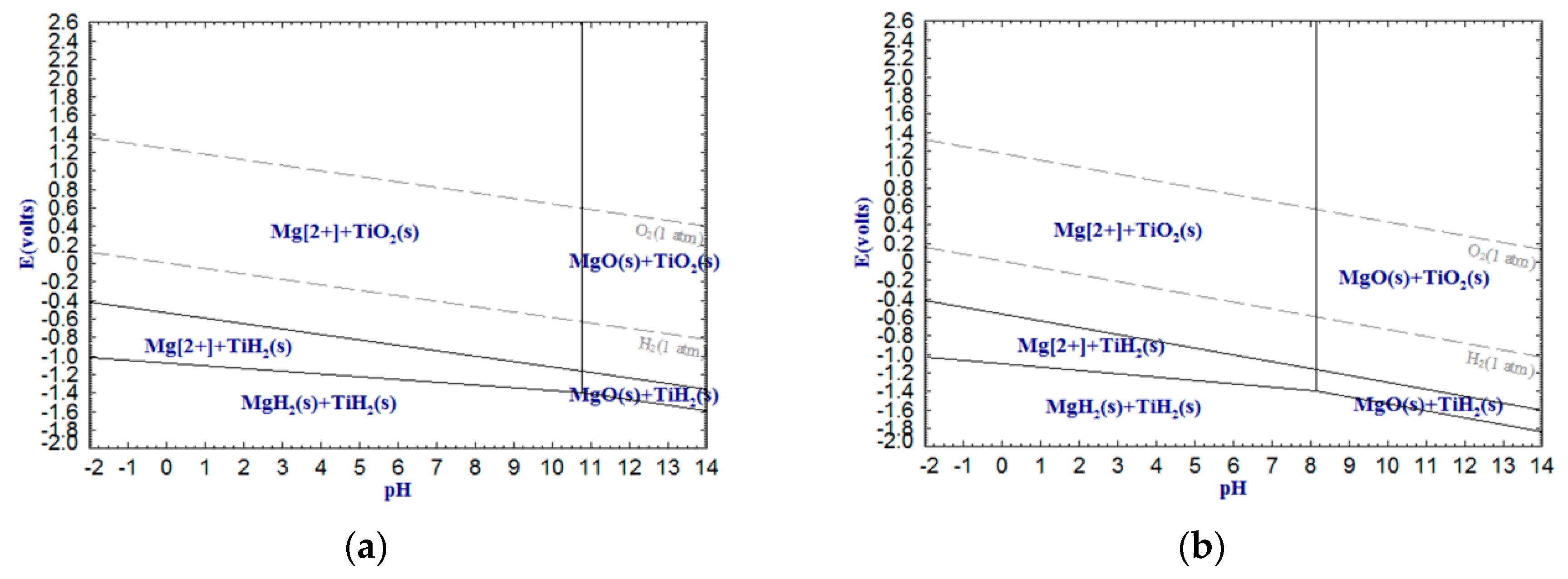
As can be seen from
Figure 3,
Figure 4 and
Figure 5, Al
2O
3, MgO and VO
2 in low-grade titanium slag can be removed by dilute acid as Al
3+, Mg
2+ and VO
2+, respectively, whereas titanium is still present as oxide. When the temperature increases to 100 °C, Al
2O
3 can be leached by alkali with a minimum pH 13.50, indicating that the alkaline leaching of Al
2O
3 can be achieved through increasing the temperature. The V
2O
5 can also be leached by alkali in the form of
, whereas V
2O
3 and VO cannot be leached by acid or alkali. Additionally, as the temperature rises from 25 to 100 °C, the stable areas of Al
3+, Mg
2+ and VO
2+ decrease, whereas those
and
increase, suggesting that increasing temperature not only reduces the required pH values of the dilute acid washing of Al
2O
3, MgO and VO
2 but also decreases the required pH values of the alkali leaching of Al
2O
3 and VO
5.
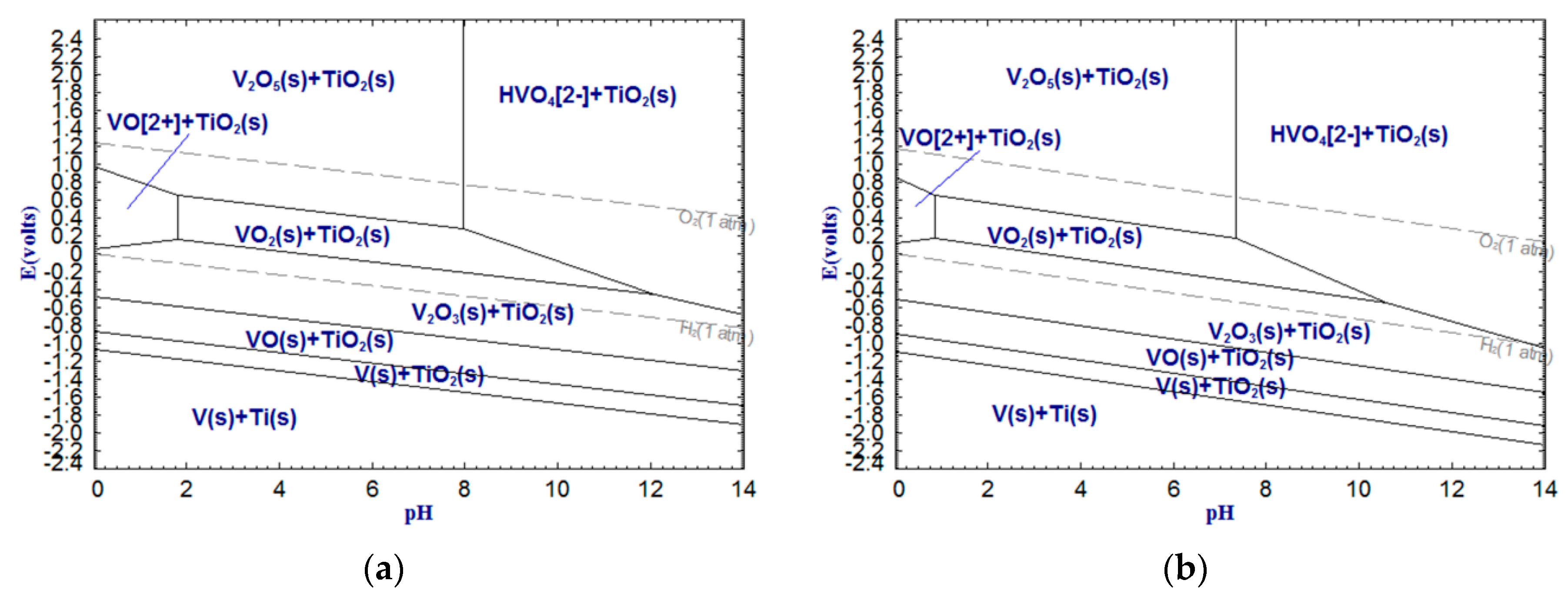
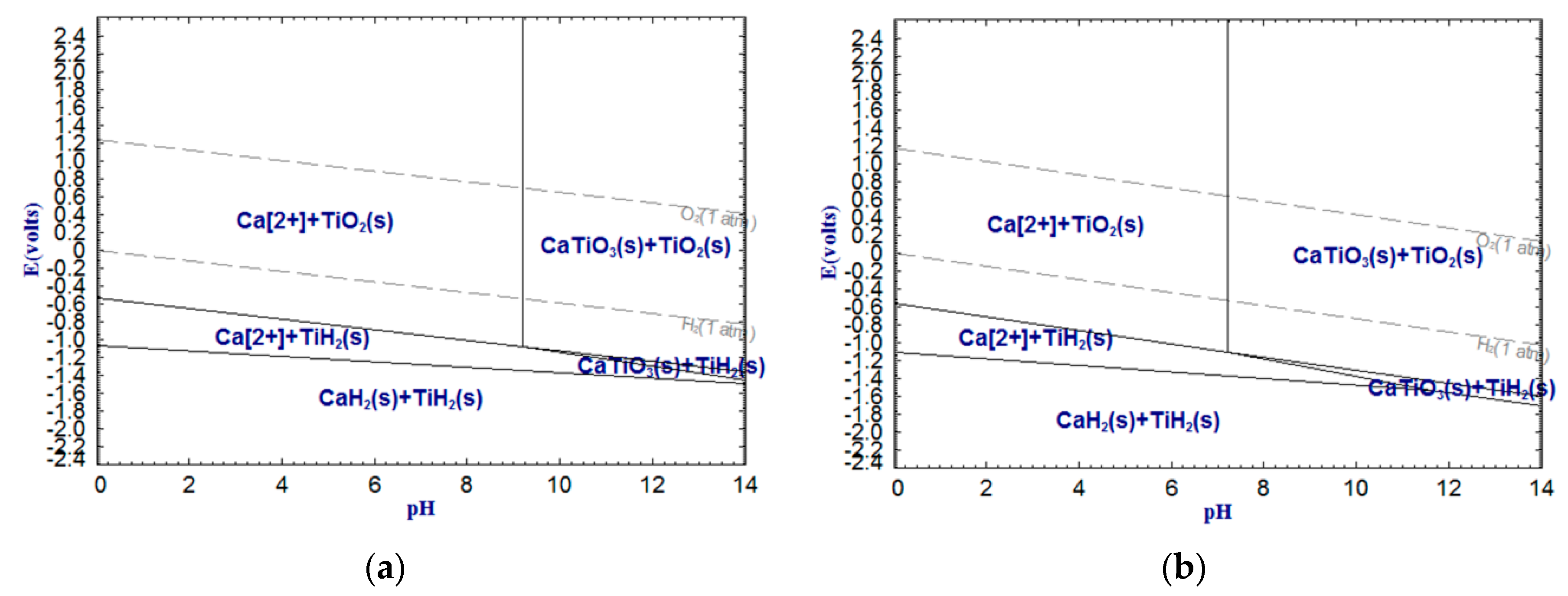
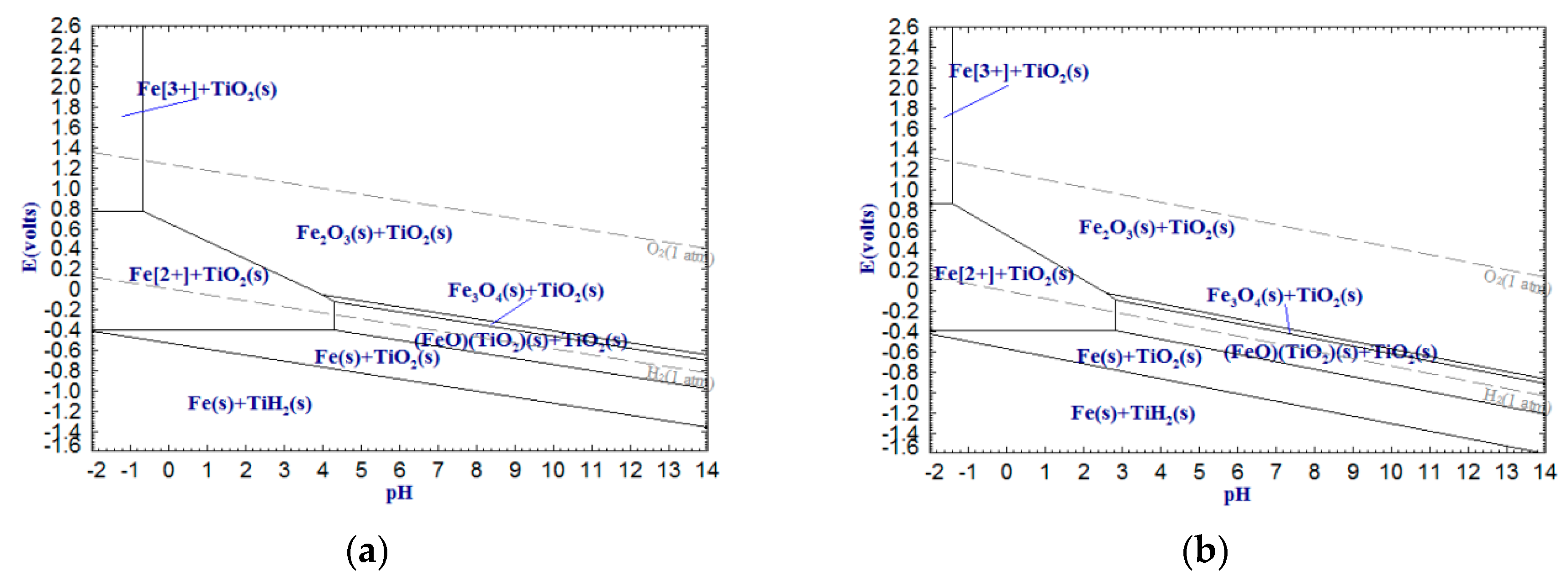
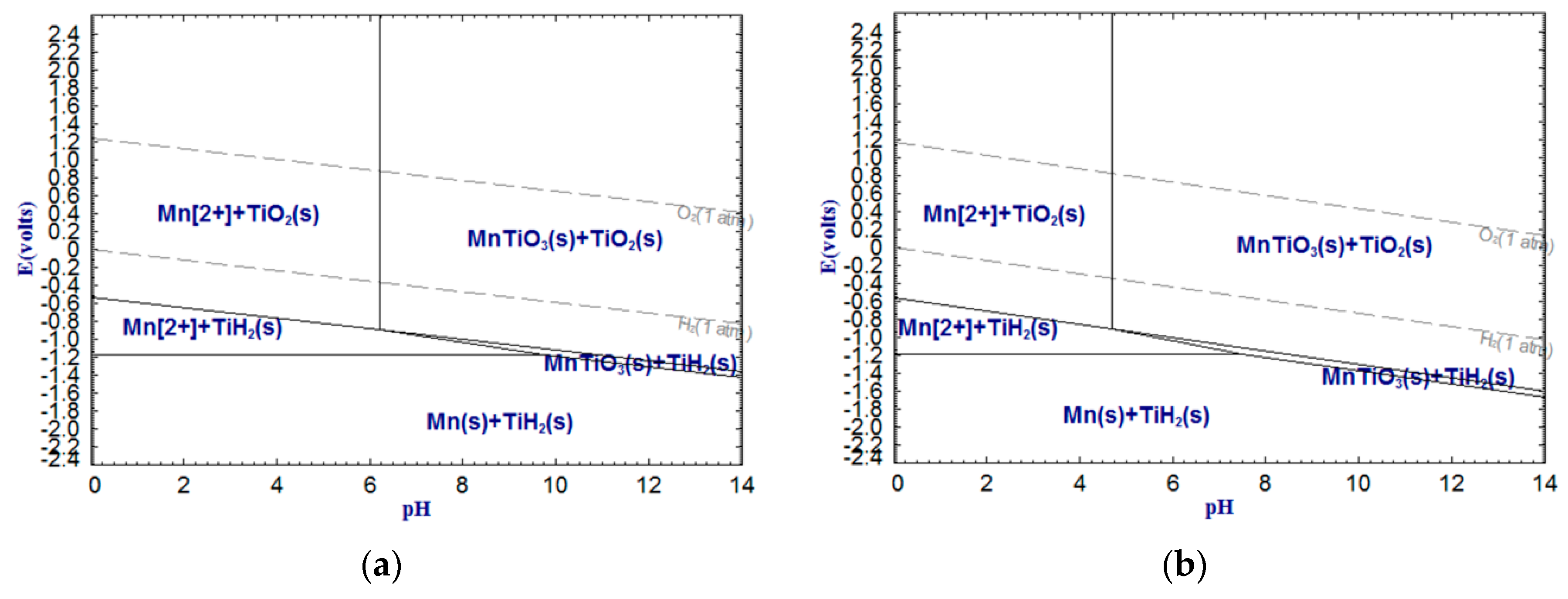
As shown in
Figure 6,
Figure 7 and
Figure 8, CaTiO
3, FeTiO
3 and MnTiO
3 can be removed by dilute acid in the form of Ca
2+, Fe
2+ and Mn
2+, whereas titanium still exists as oxide. It is worth noting that Fe(III) cannot be removed by dilute acid due to the not sufficient low pH of the acid solution. However, Fe(II) can be more easily removed by dilute acid. Therefore, reducing Fe(III) into Fe(II) or metallic iron can significantly enhance the acid solubility of ferrous components in the titanium slag. In addition, with temperature increasing, the stable areas of Ca
2+, Fe
2+ and Mn
2+ decrease sharply, which means that increasing temperature can reduce the required pH values of the dilute acid washing of CaTiO
3, FeTiO
3 and MnTiO
3.
The above analysis indicates that the alkali leaching-dilute acid washing process for upgrading low-grade titanium slag is theoretically feasible. The pH of the alkali leaching should be higher than 13.50 (
Figure 3, Al-Ti-H
2O, 100 °C) and the pH of the dilute acid washing should be lower than 1.00 (
Figure 5, V-Ti-H
2O, 100 °C). A higher temperature is conductive to alkali leaching, but the trend for dilute acid washing is the opposite. Thus, the temperatures for alkali leaching and dilute acid washing can be set at 100 °C and 25 °C, respectively. This can dramatically decrease the NaOH concentration of the alkali leaching and the HCl concentration of the dilute acid washing, which means that a large amount of acid and alkali can be saved.
3.2. Alkali Leaching
According to the relevant literature [
24] and
Figure 3, it can be seen that SiO
2 and Al
2O
3 in the slag can be theoretically leached by alkali. Thus, the TiO
2 content in the slag, SiO
2 extraction ratio and Al
2O
3 extraction ratio were determined by chemical analysis. The results are shown in
Figure 9. The experimental program of the alkali leaching is provided in
Table 2. The SiO
2 (
G) and Al
2O
3 (
H) extraction ratios were calculated using the following Equations (1) and (2):
where
m0 is the mass of low-grade titanium slag (20 g),
m1 is the mass of the cake after alkali leaching (g),
wG0 and
wH0 are the SiO
2 and Al
2O
3 content, respectively, of low-grade titanium slag (wt %), and
wG1 and
wH1 are the SiO
2 and Al
2O
3 content, respectively, of the cake after alkali leaching (wt %).
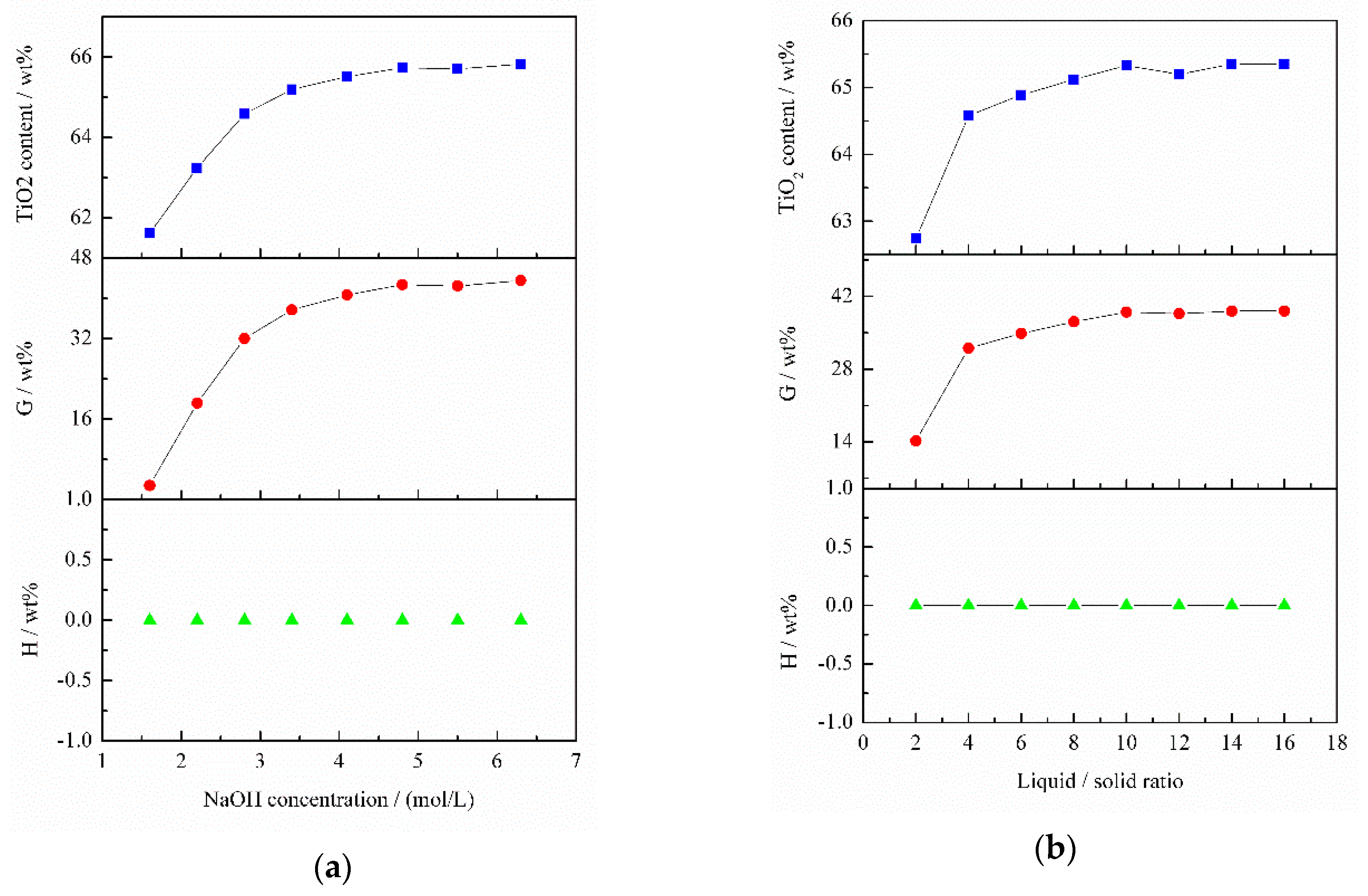
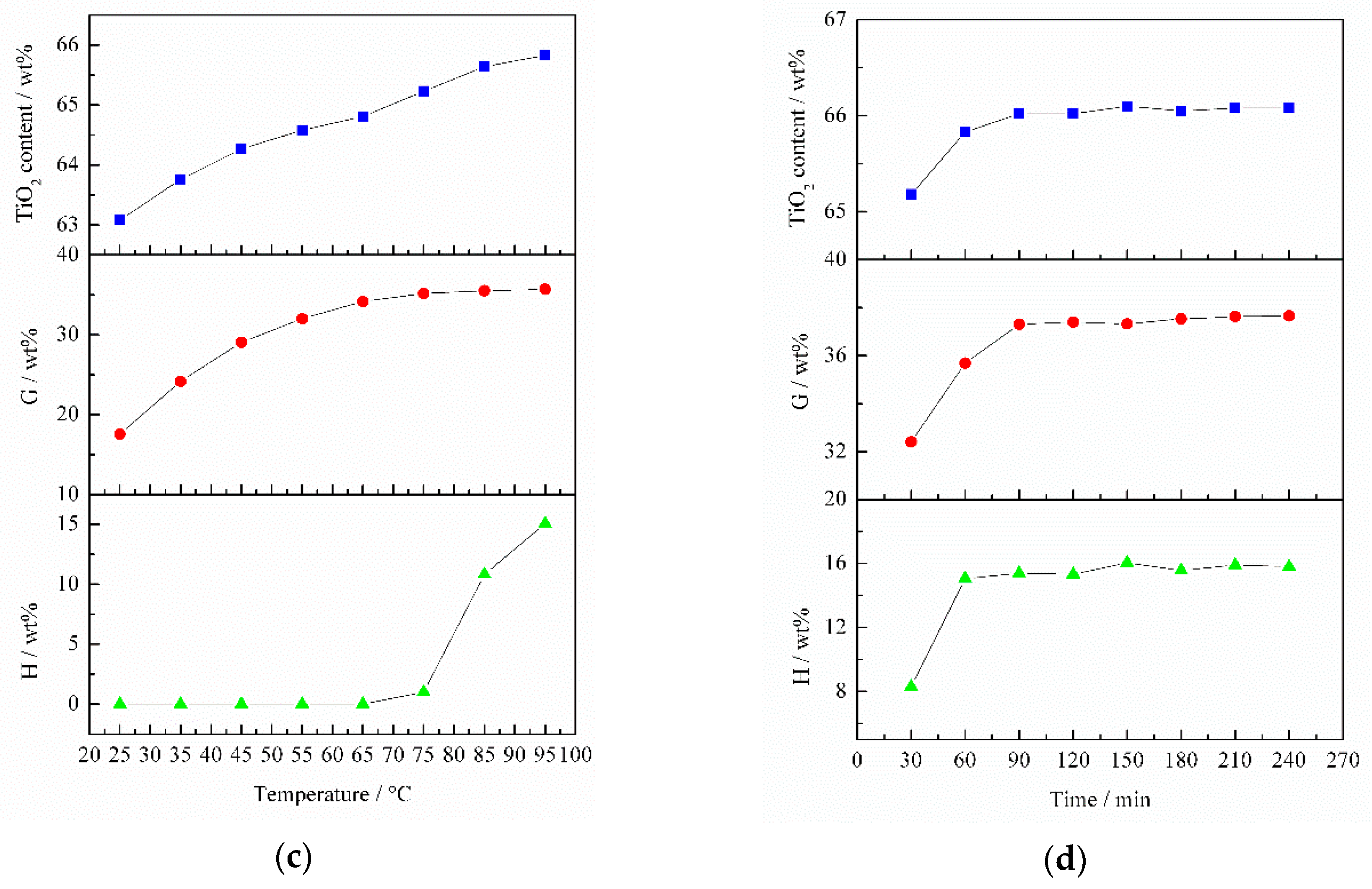
The effect of NaOH concentration on TiO
2 content is delineated in
Figure 9a. The TiO
2 content increased rapidly from 61.62 to 64.58 wt % as NaOH concentration increased from 1.6 to 2.8 mol/L. Within a NaOH concentration range of 2.8–4.8 mol/L, TiO
2 content increased slowly. When NaOH concentration was higher than 4.8 mol/L, the TiO
2 content remained unchanged. Thus, a NaOH concentration of 2.8 mol/L was used in the following experiments. It can be seen that SiO
2 in the slag can be leached by alkali and Al
2O
3 cannot be removed by alkali under the above conditions.
The effect of liquid/solid ratio (L/S) on TiO
2 content is illustrated in
Figure 9b. It was found that TiO
2 content increased markedly when L/S increased from 2 to 4. As L/S increased from 4 to 10, TiO
2 content increased insignificantly. At L/S > 10, TiO
2 content remained unchanged. Thus, a L/S of 4 was used in subsequent experiments. As shown in
Figure 9b, the variation tendency of SiO
2 extraction ratio was consistent with that of TiO
2 content, whereas the Al
2O
3 extraction ratio remained 0 wt %. This means that only part of SiO
2 was leached under the aforementioned conditions.
Figure 9c shows the effect of temperature on TiO
2 content. It is obvious that TiO
2 content monotonically increased with the rise in temperature. Therefore, the optimum temperature was 95 °C. When temperature was more than 75 °C, the SiO
2 extraction rate remained unchanged, but the TiO
2 content continued to increase. This is because Al
2O
3 started to be leached by a 2.8 mol/L NaOH solution (pH 14.44) at 75 °C. This also confirmed the previous conclusion (
Figure 3) that increasing temperature can achieve the alkali leaching of Al
2O
3.
Figure 9d illustrates the effect of time on TiO
2 content. When time increased from 30 min to 60 min, TiO
2 content rapidly increased. As time continued to increase to 90 min, TiO
2 content increased slightly. This is because both SiO
2 and Al
2O
3 can be leached before 30 min, and only a small quantity of SiO
2 was leached after 30 min. Hence, the optimum time was 60 min.
Taking the above results into consideration, the optimal alkali-leaching conditions were as follows: NaOH concentration 2.8 mol/L (pH 14.44), L/S 4, temperature 95 °C and time 60 min. The TiO
2 content of the titanium slag, SiO
2 extraction ratio and Al
2O
3 extraction ratio were 65.83 wt %, 35.69 wt % and 15.06 wt %, respectively, which indicates single alkali leaching cannot effectively increase TiO
2 content in the slag. This may be because CaAl
2O
4 (calcium aluminum spinel) produced in the process of alkali leaching covers the surface of unreacted CaAl
2(SiO
4)
2 (anorthite) and prevents the reaction between NaOH and CaAl
2(SiO
4)
2. The reliability of potential-pH diagrams for Al-Ti-H
2O systems was confirmed by measuring the Al
2O
3 extraction ratio in the slag. Moreover, it was found that the extraction ratio of Al
2O
3 is much lower than that of SiO
2. As shown in
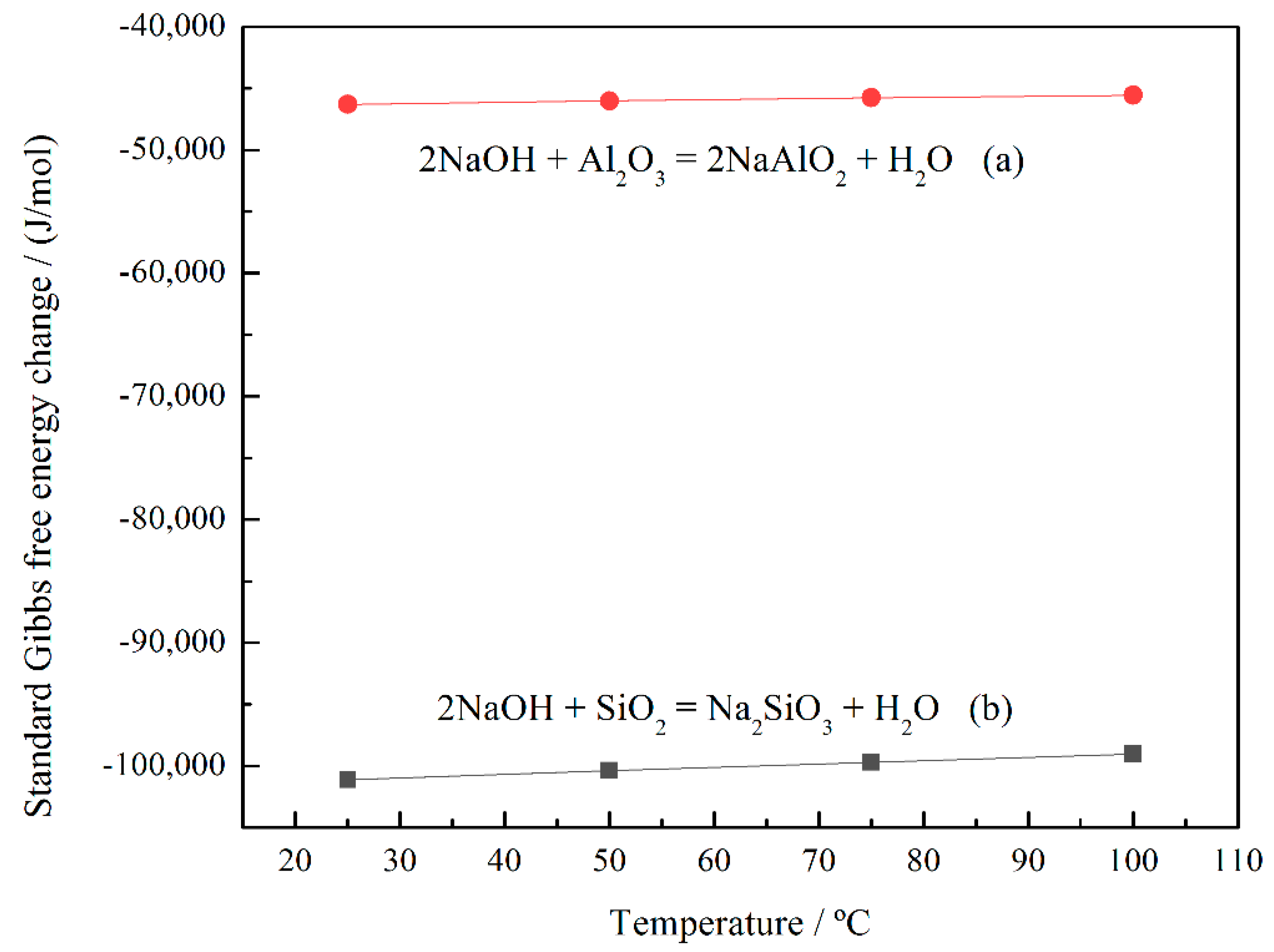
Figure 10, it can be explained by the fact that the standard Gibbs free energy change of chemical reactions (b) is much smaller than that of reaction (a), i.e., NaOH prefers to react with SiO
2 rather than Al
2O
3. On the other hand, a small amount of insoluble NaAl(OH)
4 was formed during the alkali-leaching process, resulting in a lower extraction rate of Al
2O
3. The NaAl(OH)
4 content can be determined by the sodium content of the cake after alkali leaching. As shown in Table 4, the Na
2O content of the cake increased after alkali leaching. Thus, the main reaction that occurred in the alkali-leaching process is as follows:
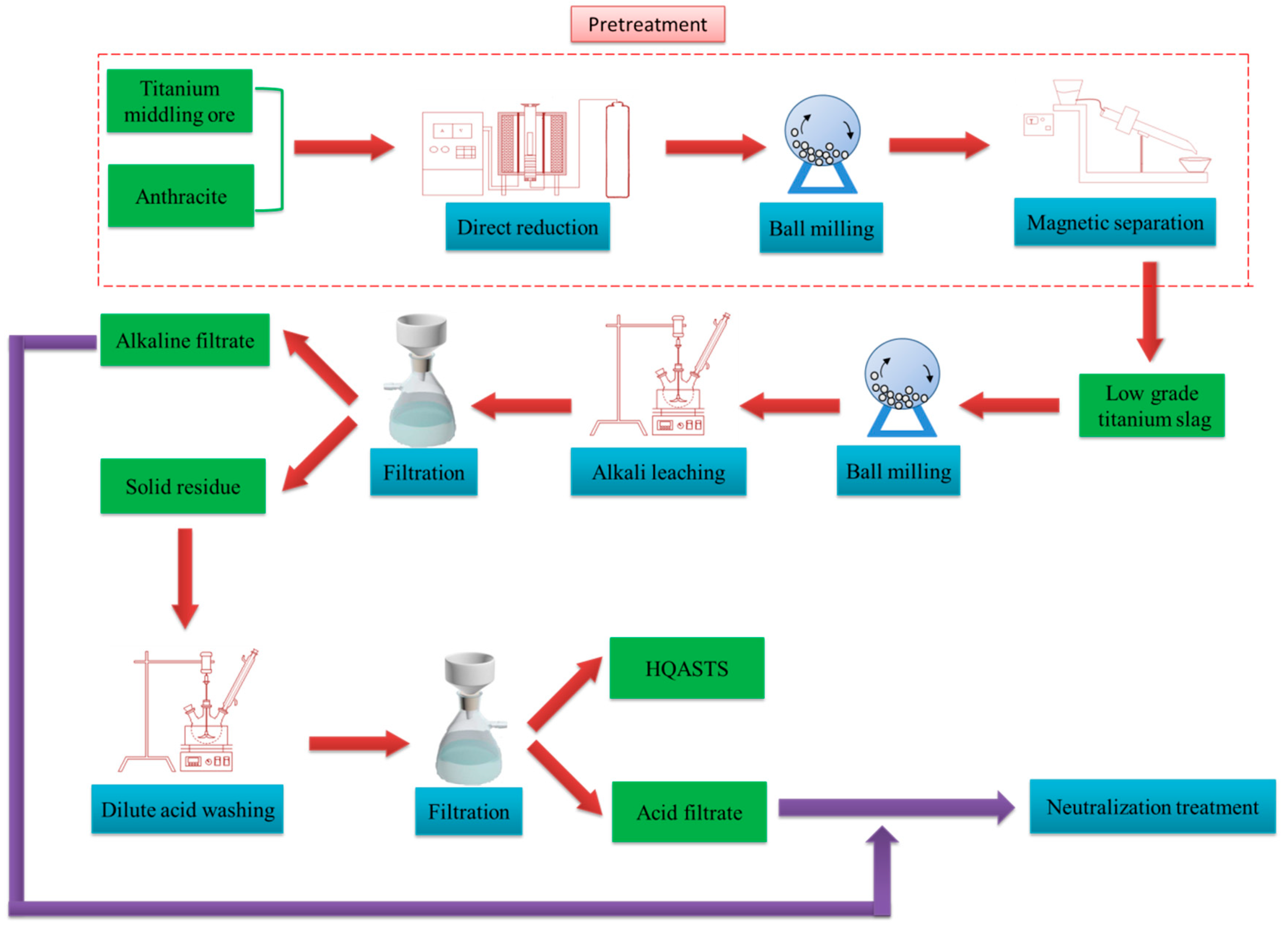
3.3. Alkali Leaching-Dilute Acid Washing
In this work, the low-grade titanium slag was first leached by alkali under the above-mentioned optimal conditions before dilute acid washing. The experimental program of dilute acid washing is shown in
Table 3. Based on
Figure 3,
Figure 4,
Figure 5,
Figure 6,
Figure 7 and
Figure 8, it was observed that Al
2O
3, MgO, VO
2, CaO, FeO, Fe and MnO in the slag can be theoretically leached by dilute acid. The MgO and MnO extraction ratios were not measured because MgO mainly exists in the form of the anosovite phase, which is insoluble in acid, and the MnO content is relatively low. Therefore, the TiO
2 content in the slag, CaO extraction ratio, Al
2O
3 extraction ratio, Fe
2O
3 extraction ratio (the total extraction ratio of various valence iron oxides) and V
2O
5 extraction ratio (the total extraction ratio of various valence vanadium oxides) were determined by chemical analysis during the dilute acid washing process. The results are presented in
Figure 11. The calculation equations of CaO extraction ratio (
B), Al
2O
3 extraction ratio (
C), Fe
2O
3 extraction ratio (
D) and V
2O
5 extraction ratio (
E) were given in Equations (4), (5), (6) and (7), respectively:
where
m0 is the mass of low-grade titanium slag (20 g),
m2 is the mass of the cake after alkali leaching-dilute acid washing (g),
wB0,
wC0,
wD0 and
wE0 are the CaO, Al
2O
3, Fe
2O
3 and V
2O
5 content, respectively, of low-grade titanium slag (wt %) and
wB2,
wC2,
wD2 and
wE2 are the CaO, Al
2O
3, Fe
2O
3 and V
2O
5 content, respectively, of the cake after alkali leaching-dilute acid washing (wt %).
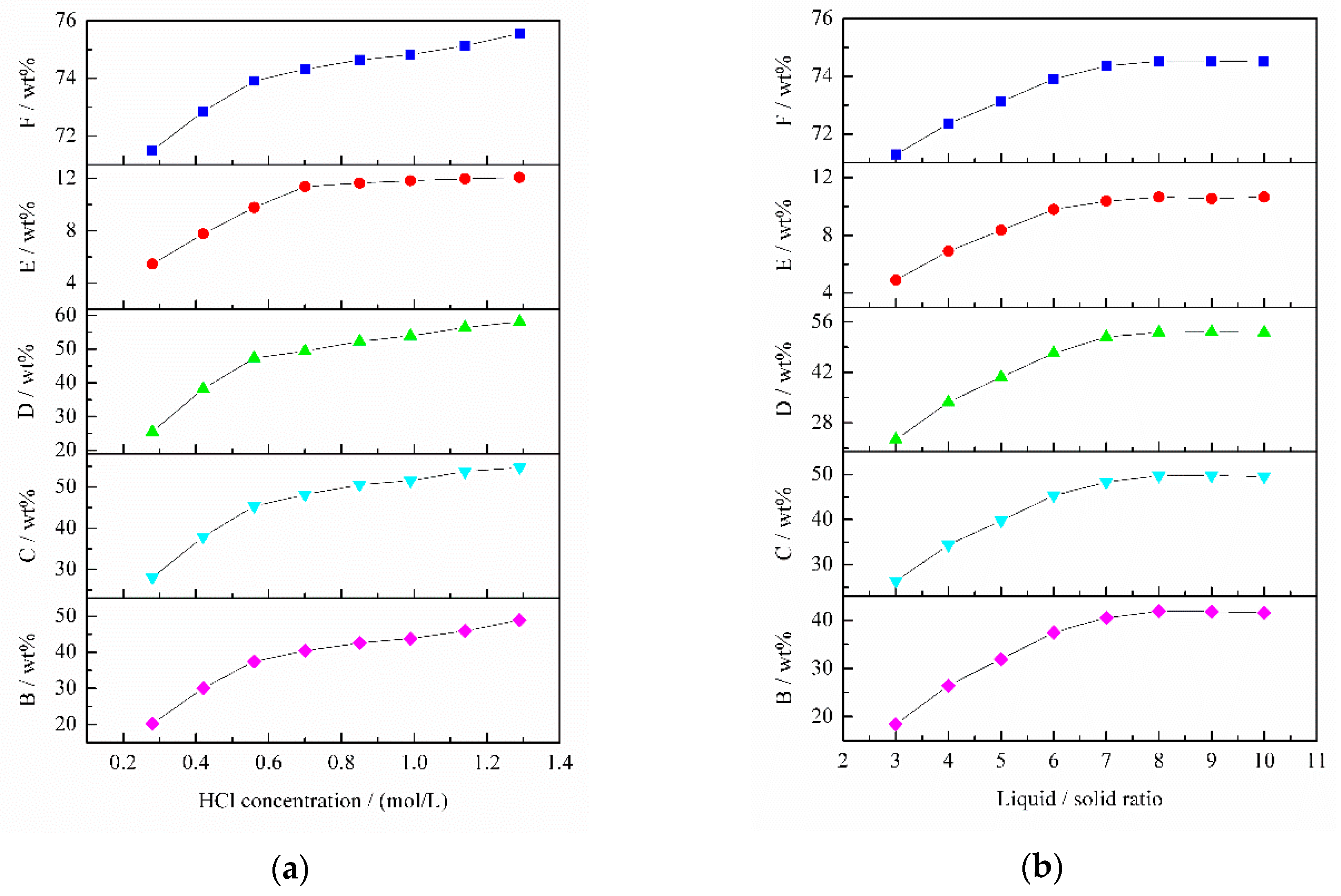
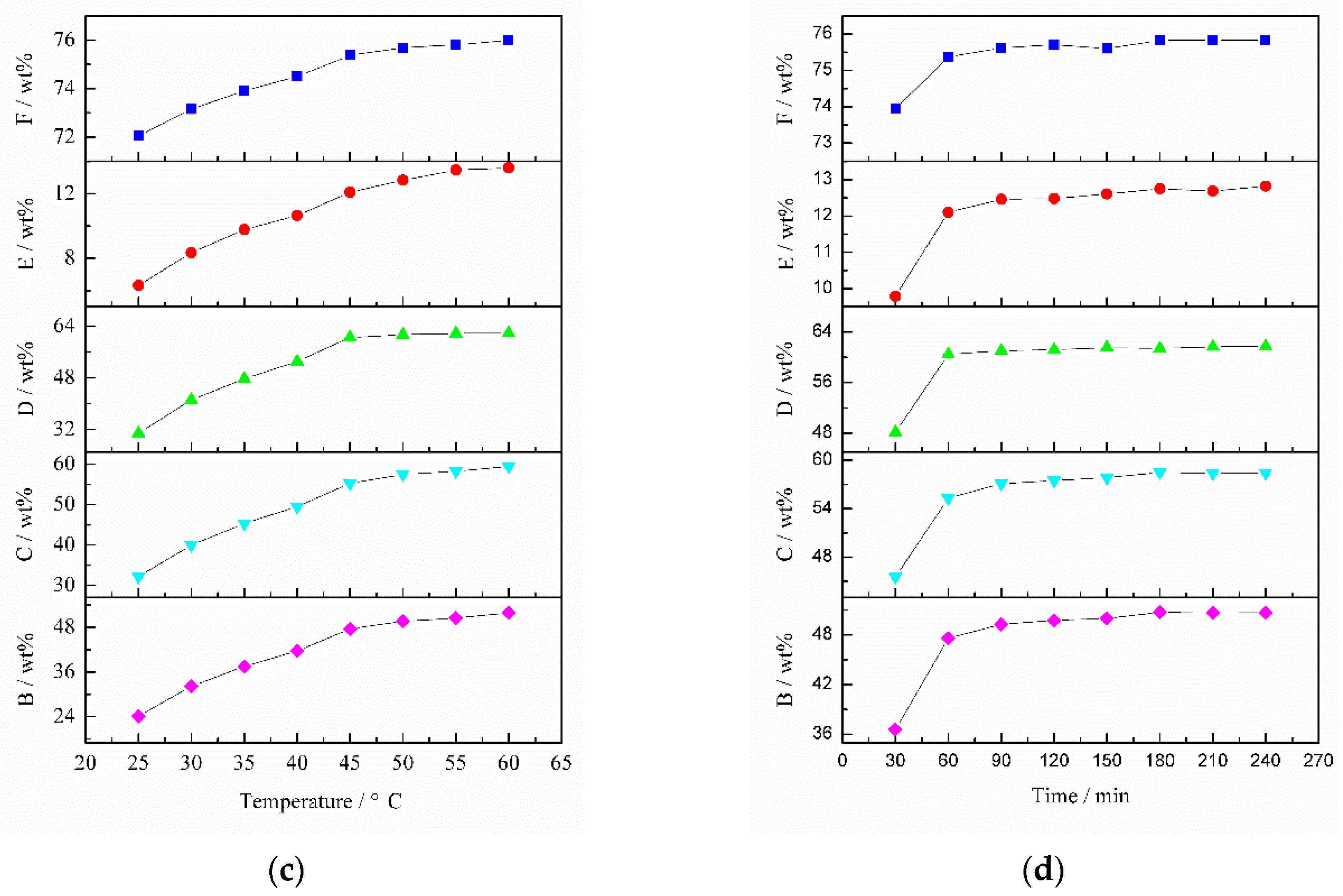
The effect of HCl concentration on TiO
2 content is shown in
Figure 11a. The TiO
2 content sharply increased with HCl concentration increasing from 0.28 to 0.56 mol/L. Thereafter, in the HCl concentration range of 0.56–1.29 mol/L, the TiO
2 content continued to increase at a slow rate. This can be attributed to the fact that CaO, Al
2O
3, Fe
2O
3 and V
2O
5 extraction ratios increased significantly as HCl concentration increased from 0.28 to 0.56 mol/L, and then these extraction ratios increased slowly when the HCl concentration was more than 0.56 mol/L. Taking into account cleaner production and reducing cost, 0.56 mol/L HCl was chosen as the optimum concentration.
The effect of L/S on TiO
2 content is illustrated in
Figure 11b. The TiO
2 content rapidly increased from 71.29 wt % with L/S 3 to 73.91 wt % with L/S 6. When L/S increases from 6 to 8, the TiO
2 content increased slowly. As for the case of L/S > 8, the TiO
2 content in the slag was almost constant. It can be explained by the fact that the variation tendencies of Al
2O
3, CaO, Fe
2O
3 and V
2O
5 extraction ratios were consistent with that of TiO
2 content. Thus, the optimum L/S was 6.
Figure 11c shows the effect of temperature on TiO
2 content. The TiO
2 content dramatically increased when temperature increased from 25 to 45 °C. Nevertheless, the TiO
2 content slightly increased in the temperature range of 45–60 °C. This is because CaO, Al
2O
3, Fe
2O
3 and V
2O
5 extraction ratios rapidly increased at 25–45 °C. When temperature was more than 45 °C, the extraction ratio of Fe
2O
3 remained unchanged and the extraction ratios of CaO and Al
2O
3 increased at a low ratio. Thus, the optimum temperature was 45 °C.
Figure 11d illustrates the effect of time on TiO
2 content. The TiO
2 content sharply increased as time increased from 30 to 60 min, and then slightly increased with more than 60 min leaching. This is because CaO, Al
2O
3, Fe
2O
3 and V
2O
5 can be removed at a high rate as the time increased from 30 to 60 min. When the time was longer than 60 min, the CaO, Al
2O
3 and V
2O
5 extraction ratios increased slightly and Fe
2O
3 extraction rate was almost constant. Hence, the optimum time was 60 min.
In summary, the optimal dilute acid washing conditions were 0.56 mol/L HCl concentration (pH 0.26), L/S 6, temperature 45 °C and time 60 min. The TiO
2 content of the HQASTS, CaO extraction ratio, Al
2O
3 extraction ratio, Fe
2O
3 extraction ratio and V
2O
5 extraction ratio were 75.37 wt %, 47.57 wt %, 55.33 wt %, 60.49 wt % and 12.10 wt %, respectively; indicating that the process of alkaline leaching-dilute acid washing can significantly improve TiO
2 content in titanium-bearing materials. In this present study, vanadium oxides in the low-grade titanium slag mainly consist of V
2O
3 and a small amount of VO
2. It can be seen from
Figure 5 that V
2O
3 cannot be removed by acid but VO
2 can. Therefore, only a small quantity of VO
2 was dissolved in acid solution. This is the reason why the V
2O
5 extraction rate is only 12.10 wt %. On the other hand, the reliability of potential-pH diagrams (Ca-Ti-H
2O, Al-Ti-H
2O, Fe-Ti-H
2O and V-Ti-H
2O) was confirmed by measuring the CaO, Al
2O
3, Fe
2O
3 and V
2O
5 extraction ratios in the slag. In addition, as shown in
Figure 11, the variation trends of the Al
2O
3 and CaO extraction ratios under the same conditions were very similar. It can be inferred that Al
2O
3 and CaO were leached in equal proportions. Thus, the main reaction that occurred in the dilute acid washing process is as follows:
3.4. Leaching Mechanism
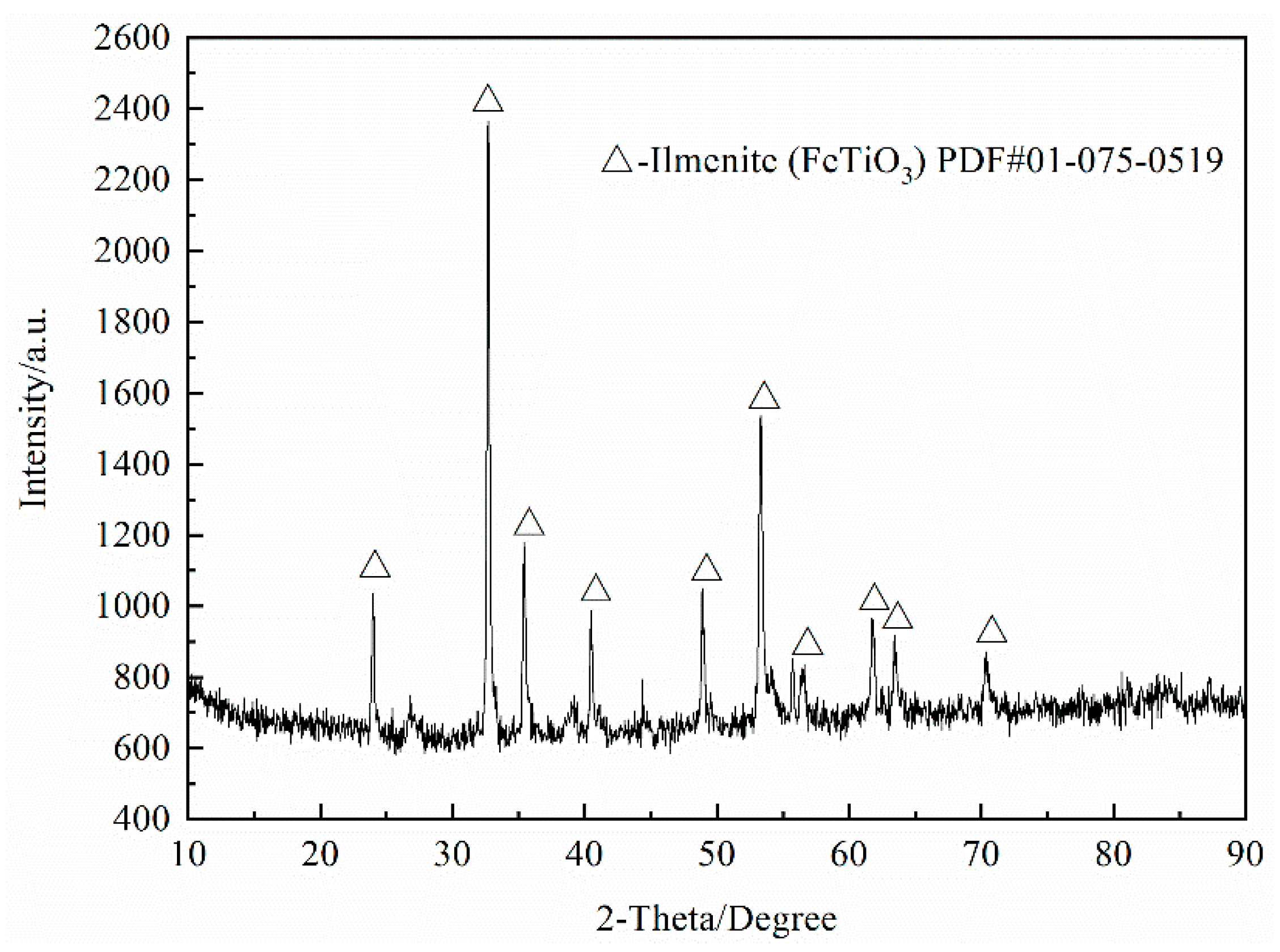
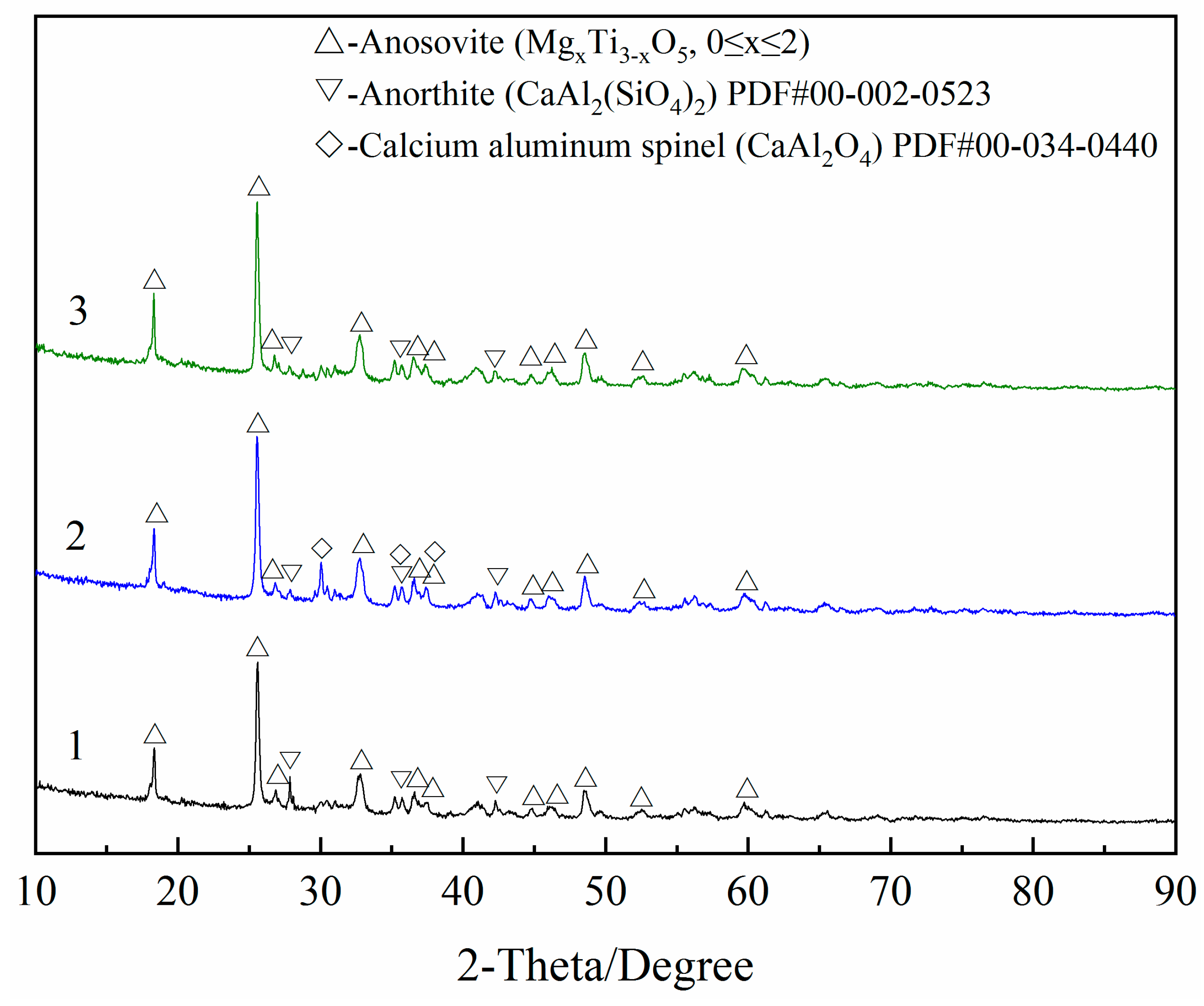
In order to gain insight into the leaching mechanism of process, the chemical analysis of resulting solids and solutions as well as XRD test were performed. The results are shown in
Table 4 and
Table 5 and
Figure 12.
As seen from
Table 4 and
Table 5, more than one-third of SiO
2 and a small amount of Al
2O
3 were dissolved in an alkaline solution. In the process of dilute acid washing, most of the Fe
2O
3, Al
2O
3, CaO and MnO and a small quantity of V
2O
5 were dissolved in acid solution. The results indicated that the main reactions that occurred in the alkali leaching and dilute acid washing were (3) and (8), respectively. Despite anosovite (Mg
xTi
3−xO
5, 0 ≤ x ≤ 2) phase is insoluble in acid, a small quantity of MgO must be dissolved because the solid mass decreased during the dilute acid washing process and the MgO content remained constant. Consequently, the mass of MgO decreased, indicating that some MgO exists in other phases. The experimental results are consistent with the previous analysis of potential-pH diagrams and also confirm their authenticity. Moreover,
Table 5 shows that the Ti content of alkali and acid solutions is quite low, indicating that almost all Ti components in the slag can be recovered. It is remarkable that the Fe
2O
3 content of the HQASTS is only 0.96 wt %. This not only eliminates the processes of the freezing crystallization and separation copperas for sulfate process but also greatly reduces the workload of the subsequent purification of metatitanic acid.
As shown in
Figure 12, CaAl
2(SiO
4)
2 (anorthite) was transformed into CaAl
2O
4 (calcium aluminum spinel) after alkali leaching, and CaAl
2O
4 disappeared after dilute acid washing. It was found from experimental results of
Section 3.2 and
Section 3.3 that the main reactions that occurred in the alkali leaching and dilute acid washing were (3) and (8), respectively. The results were consistent with the XRD analysis. In addition, the elemental content changes in
Table 4 and
Table 5 were also in accordance with the XRD analysis results. Therefore, the leaching mechanism of the process can be represented by
Figure 13. It was observed that CaAl
2O
4 generated in alkali leaching, and it encapsulated the unreacted anorthite, causing the sodium hydroxide to not be able to further react with anorthite. This is the main reason for the low content of TiO
2 in the slag during the alkali leaching process. Thus, it is not an ideal way to deal with the low-grade Ti-slag by only using alkali leaching. However, CaAl
2O
4 can be removed by dilute acid washing. Therefore, the process of alkali leaching-dilute acid washing can avoid the encapsulation of the unreacted anorthite by the calcium aluminum spinel, which is the result of alkali leaching. If an acid leaching-alkali leaching process is employed to upgrade the low-grade titanium slag, silicic acid will be produced during the acid leaching process and encapsulate the unreacted anorthite. Although subsequent alkali leaching can remove the silicic acid, this process wastes a certain amount of the acid and alkali leaching agents. Thus, the alkali leaching-dilute acid washing process is more reasonable.