Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Characterization
2.2. Thermodynamic Analysis
2.3. Statistical Design, Mathematical Modeling and Experiments
2.3.1. Screening Experiments
2.3.2. Mathematical Modeling
2.4. Experimental
3. Results
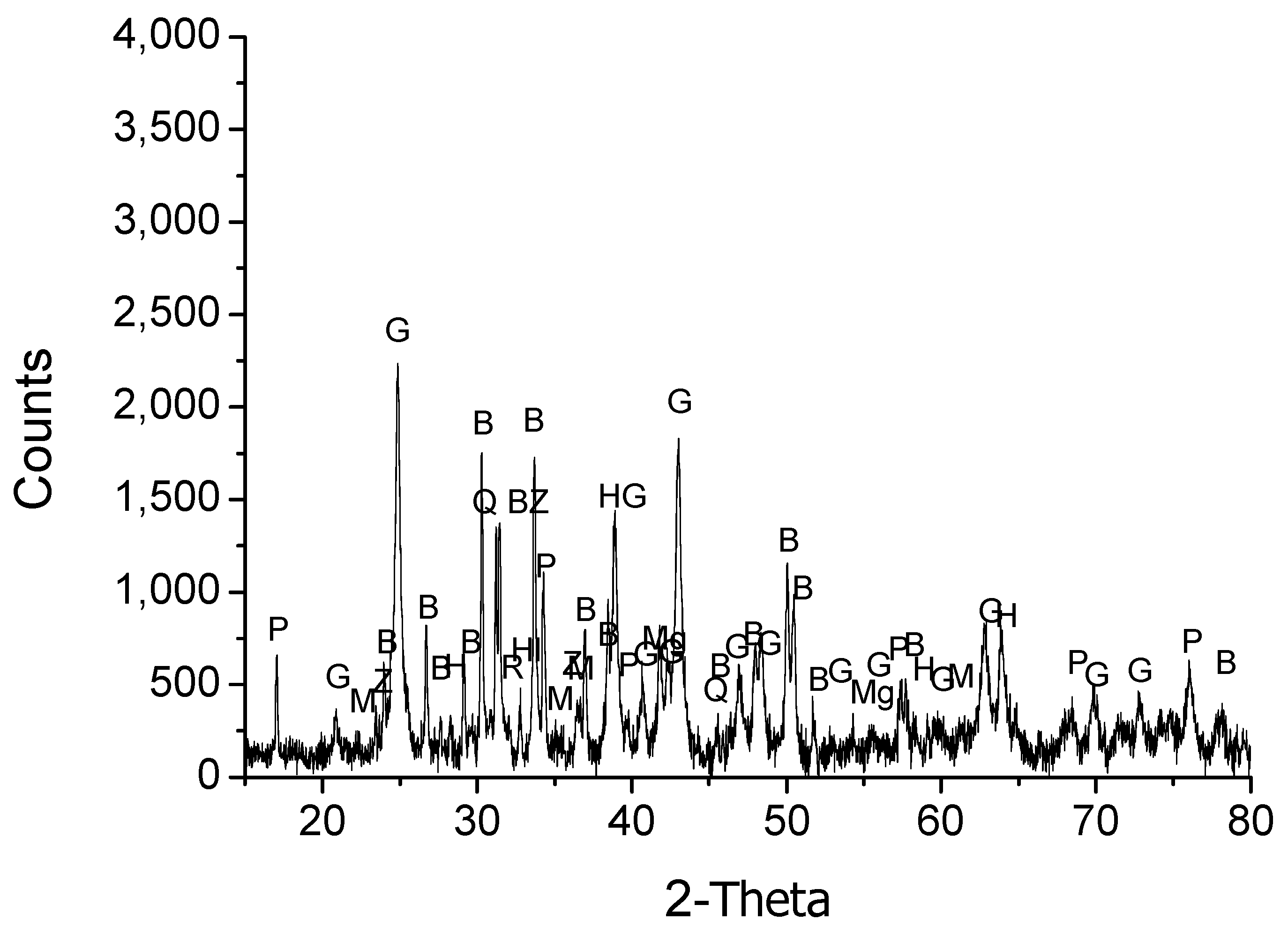
3.1. Sample Preparation and Characterization
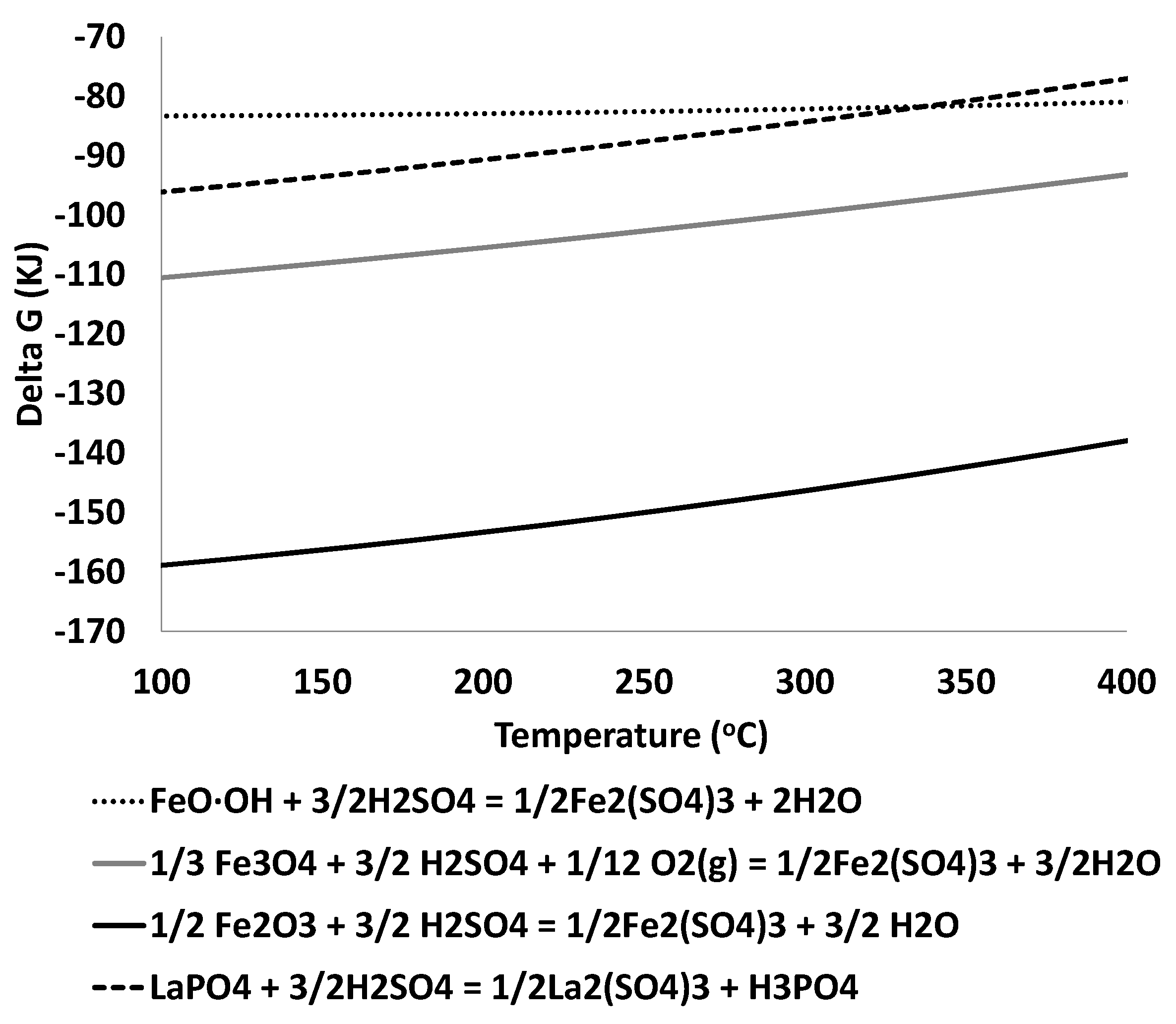
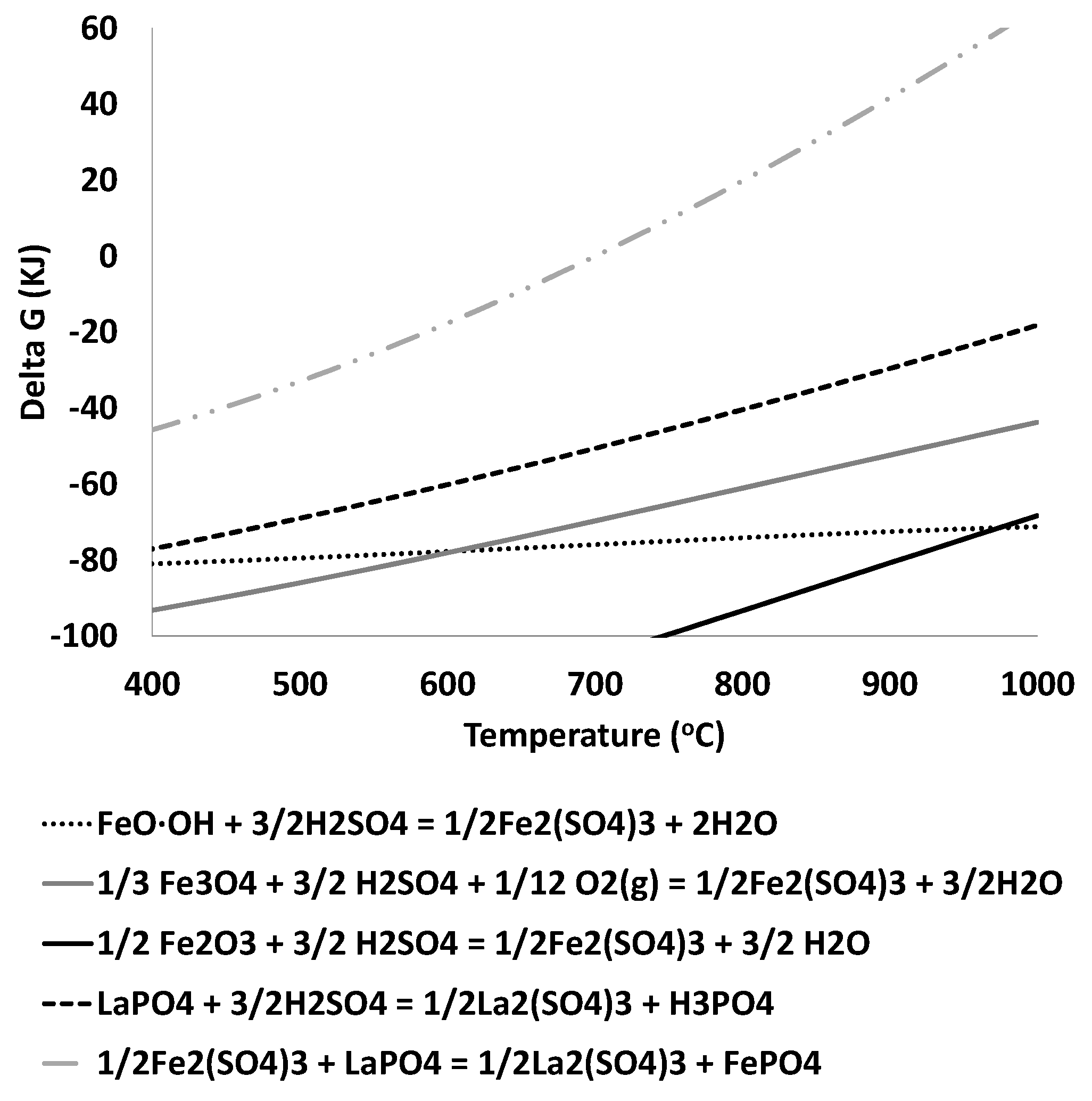
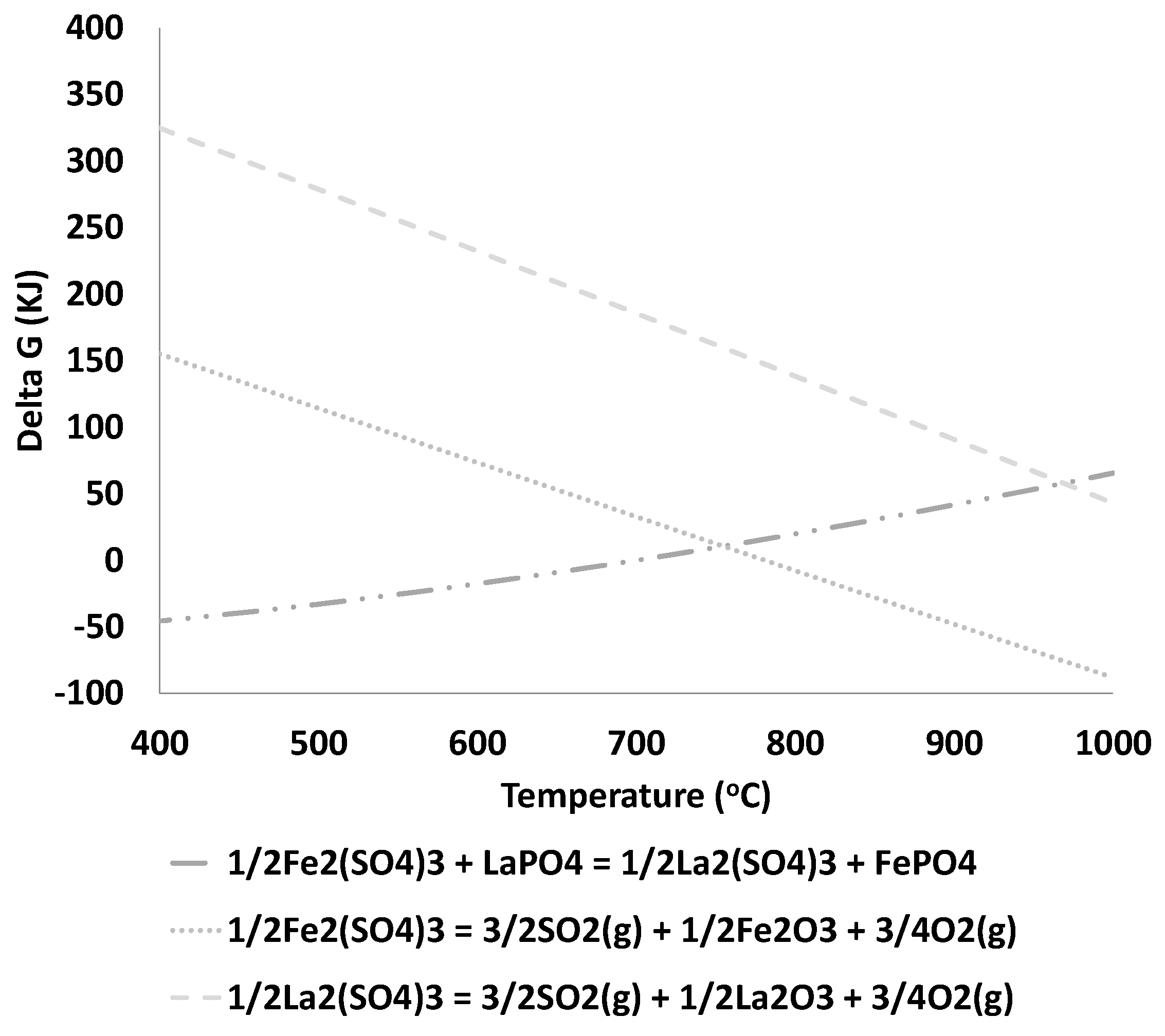
3.2. Thermodynamic Analysis
- Iron sulfate formation is expected for roasting temperature below 700 °C.
- Iron sulfate decomposition is expected for a temperature above 780 °C.
- The amount of acid used in the roasting should be just the necessary to obtain the iron sulfate to react with the monazite of the concentrate.
- The use of an excess of acid in the roasting step leads to iron solubilization in the aqueous leaching.
3.3. Experimental
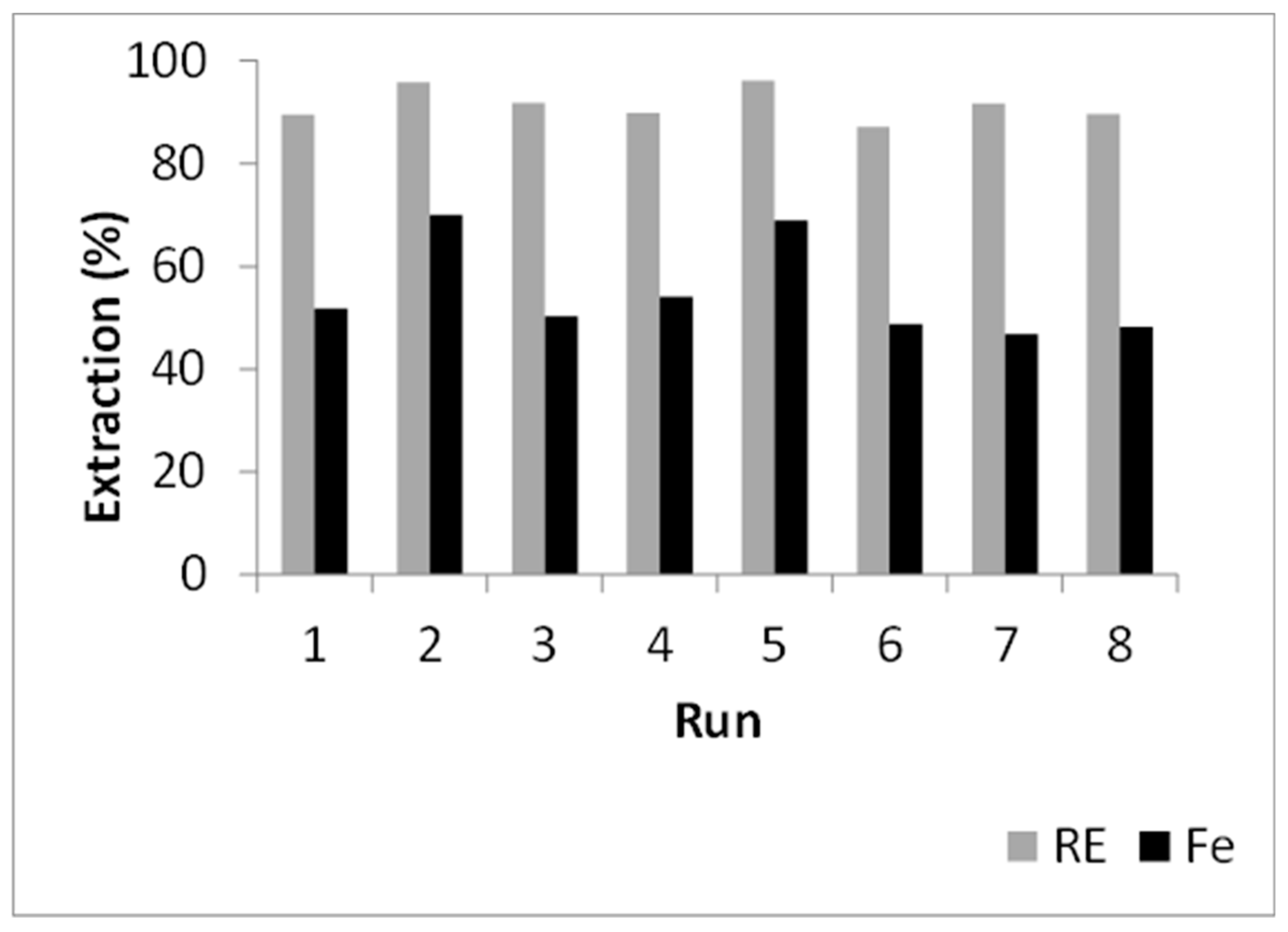
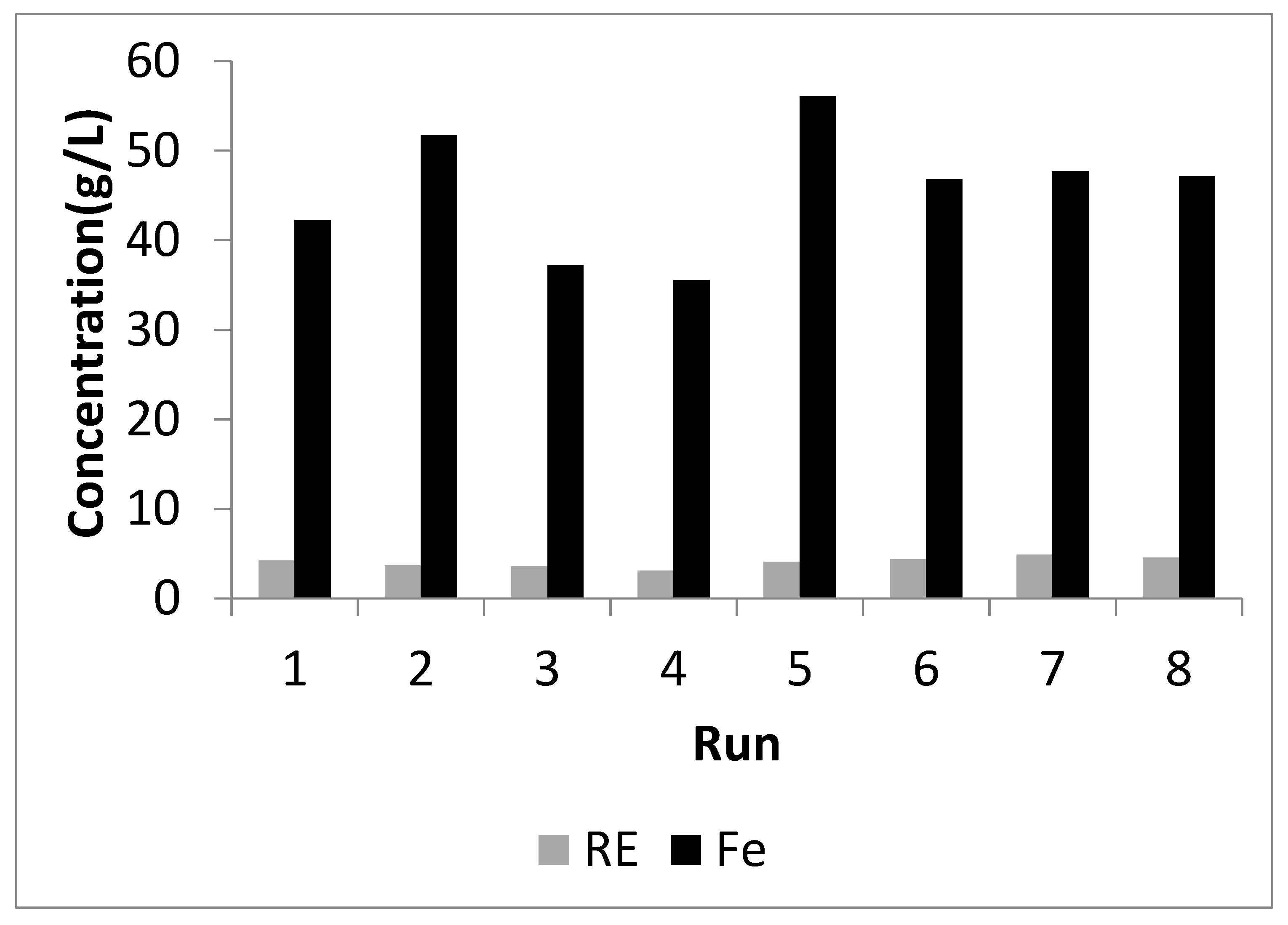
3.3.1. Screening Experiments
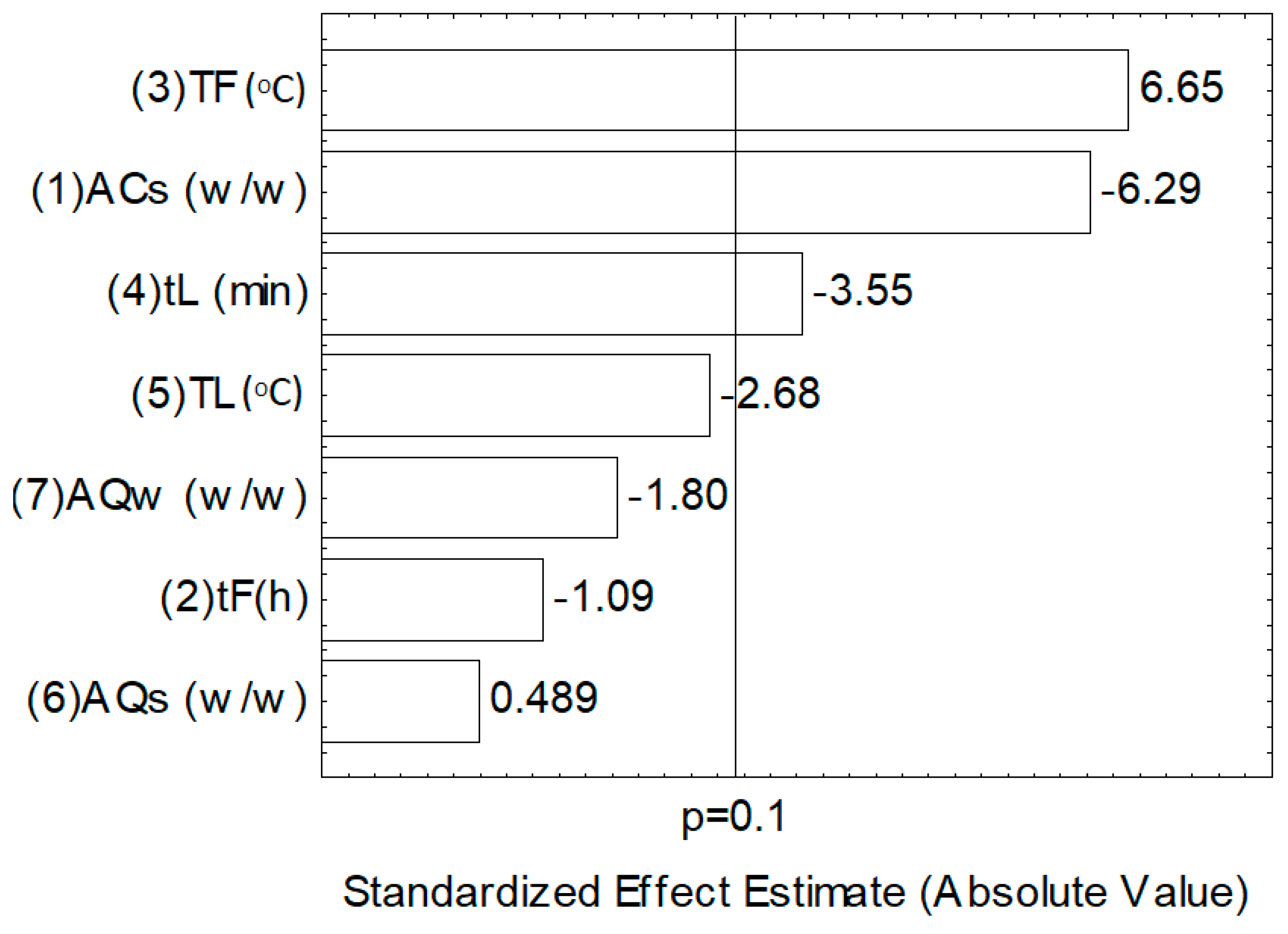
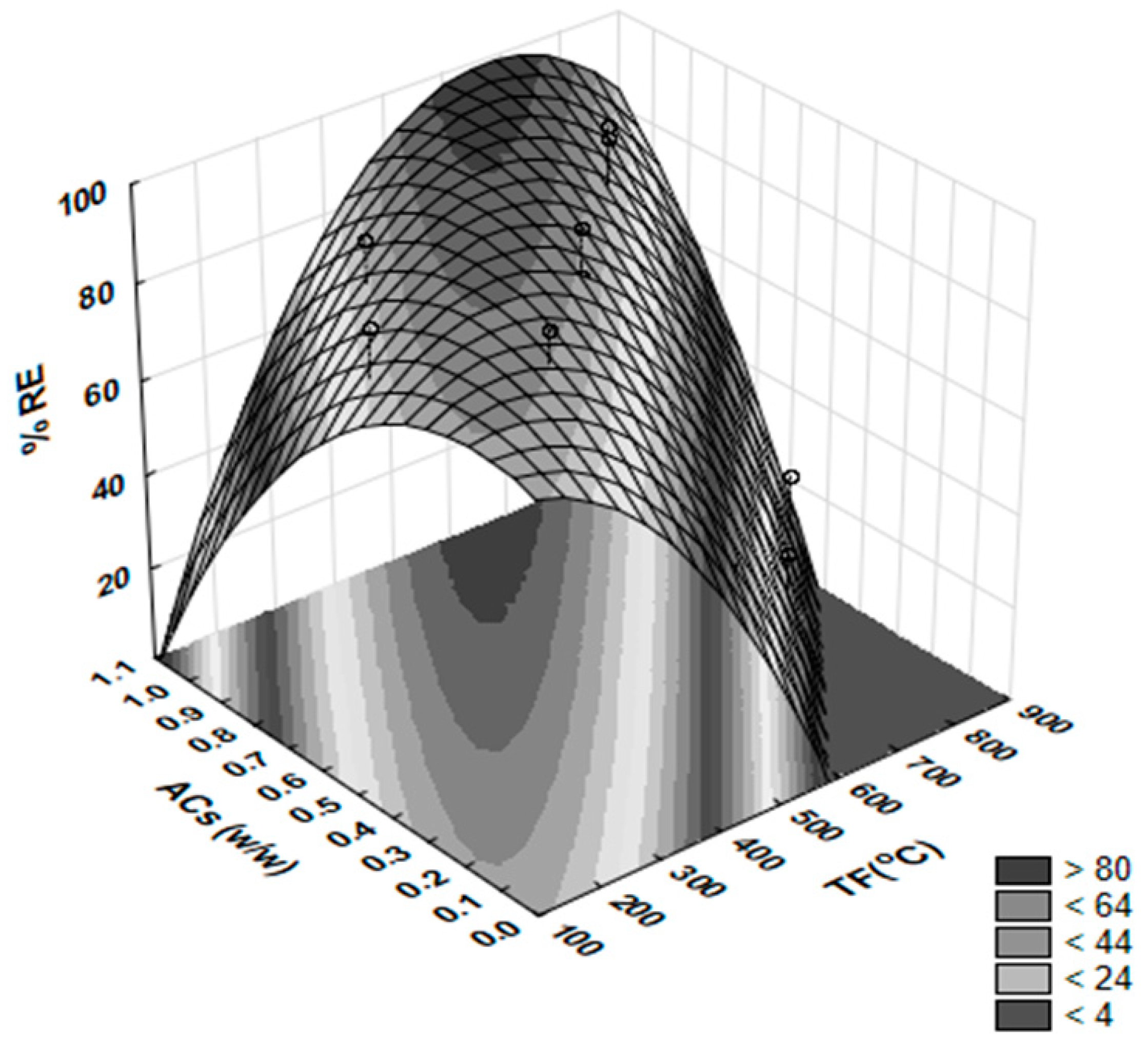
3.3.2. Mathematical Modeling
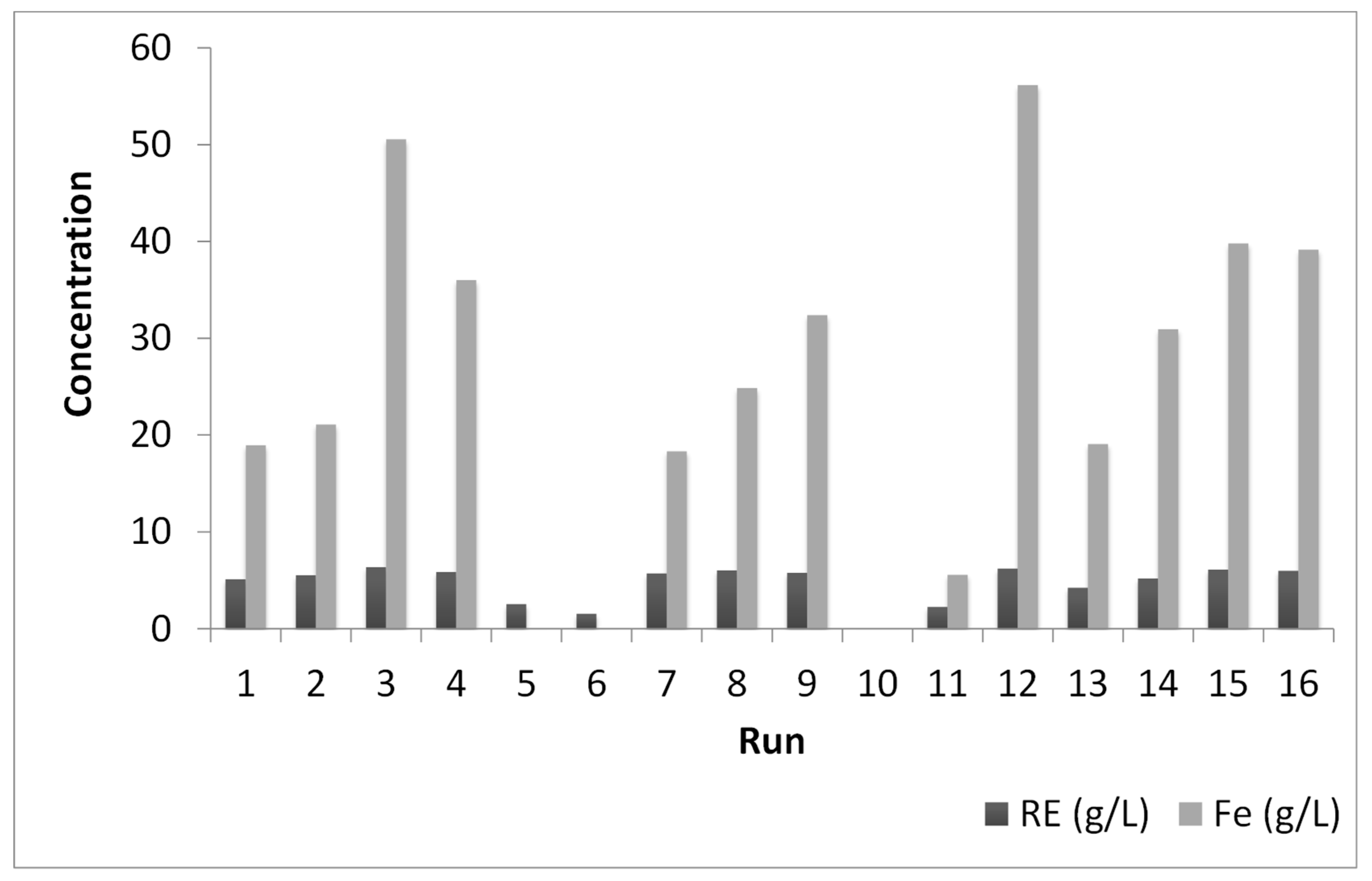
3.3.3. Multi-Objective Optimization
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Geological Survey, Mineral Commodity Summaries, Rare Earths. February 2014. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/rare_earths/mcs-2014-raree.pdf (accessed on 10 May 2018).
- Sumário Mineral. 2016. Available online: http://www.anm.gov.br/dnpm/publicacoes/ serie-estatisticas-e-economia-mineral/sumario-mineral/sumario-mineral-brasileiro-2016 (accessed on 8 August 2018).
- Neumann, R.; Medeiros, E.B. Comprehensive mineralogical and technological characterization of the Araxá (SE Brazil) complex REE (Nb-P) ore, and the fate of its processing. Int. J. Min. Proc. 2015, 144, 1–10. [Google Scholar] [CrossRef]
- Kim, R.; Cho, H.; Han, K.N.; Kim, K.; Min, M. Optimization of acid leaching of rare-earth elements from Mongolian apatite-based ore. Minerals 2016, 6, 63. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, H.; Xue, X.; Yuan, S. Separation and Recovery of Iron and Rare Earth from Bayan Obo Tailings by Magnetizing Roasting and (NH4)2SO4 Activation Roasting. Metals 2017, 7, 195. [Google Scholar] [CrossRef]
- Li, L.Z.; Yang, X. China’s rare earth ore deposits and beneficiation techniques. In Proceedings of the 1st European Rare Earth Resources Conference, Milos Island, Greece, 4–7 September 2014; pp. 26–36. [Google Scholar]
- Lapidus, G.T.; Doyle, F.M. Selective thorium and uranium extraction from monazite: I. Single-stage oxalate leaching. Hydrometallurgy 2015, 154, 102–110. [Google Scholar] [CrossRef]
- Sadri, F.; Nazari, A.M.; Ghahreman, A. A review on the cracking, baking and leaching process of rare earth element concentrates. J. Rare Earths 2017, 35, 739–752. [Google Scholar] [CrossRef]
- Gupta, C.K.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Habashi, F. Handbook of Extractive Metallurgy; Wiley-VCH: Weinheim, Germany, 1997. [Google Scholar]
- Habashi, F. Extractive metallurgy of rare earths. Can. Met. Q. 2013, 52, 224–233. [Google Scholar] [CrossRef]
- Ponou, J.; Garrouste, M.; Dodbiba, G.; Fujita, T.; Ahn, J. Sulfation–Roasting–Leaching–Precipitation Processes for Selective Recovery of Erbium from Bottom Ash. Sustainability 2019, 11, 3461. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, B. Separation Hydrometallurgy of Rare Earth Elements; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Ritcey, G.M.; Ashbrook, A.W. Solvent Extraction–Principles and Applications to Process Metallurgy; Elsevier Science Publishing Company Inc.: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Ritcey, G.M. Solvent Extraction-Principles and Applications to Process Metallurgy; Ritcey, G.M., Ed.; Associates Inc.: Ottawa, ON, Canada, 2006. [Google Scholar]
- Beltrami, D.; Deblonde, G.J.P.; Bélair, S.; Weigel, V. Recovery of yttrium and lanthanides from sulfate solutions with high concentration of iron and low rare earth content. Hydrometallurgy 2015, 157, 356–362. [Google Scholar] [CrossRef]
- Karshigina, Z.; Abisheva, Z.; Bochevskaya, Y.; Akcil, A.; Salgelova, E.; Sukorov, B.; Silachyov, I. Recovery of rare earth metals (REMs) from primary raw material: Sulphatization-leaching-precipitation-extraction. Min. Proc. Ext. Met. Rev. 2018, 39, 319–338. [Google Scholar] [CrossRef]
- Kim, E.; Osseo-Asare, K. Aqueous stability of thorium and rare earth metals in monazite hydrometallurgy: Eh–pH diagrams for the systems Th–, Ce–, La–, Nd– (PO4)–(SO4)–H2O at 25 °C. Hydrometallurgy 2012, 113–114, 67–78. [Google Scholar] [CrossRef]
- Ma, Y.; Stopic, S.; Gronen, L.; Milivojevic, M.; Obradovic, S.; Friedrich, B. Neural network modeling for the extraction of rare earth elements from eudialyte concentrate by dry digestion and leaching. Metals 2018, 8, 267. [Google Scholar] [CrossRef]
- Georgiou, T.; Tsakanika, P.; Hatzilyberis, L.; Ochsenkuehn-Petropoulou, K.M. Optimizing conditions for scandium extraction from bauxite residue using taguchi methodology. Minerals 2019, 9, 236. [Google Scholar]
- Neumann, R. (Rio de Janeiro, RJ, Brazil). Centro de Tecnologia Mineral–CETEM/MCTIC. Personal communication, 2018. [Google Scholar]
- Lapido-Loureiro, F.E.D.V. Terras-Raras no Brasil: Depósitos Recursos Identificados Reservas; Centro de Tecnologia Mineral–CETEM: Rio de Janeiro, Brazil, 1994.
- Silva, M.C.; Matiolo, E. Estudo da concentração de monazita em uma amostra de carbonatito friável brasileiro. In Proceedings of the Anais da XXXIV Jornada de Iniciação Científica CETEM, CETEM/MCTIC, Rio de Janeiro, Brazil, 29 July 2016; pp. 76–80. [Google Scholar]
- Outotec. Outotec HSC 7.15 Chemistry Software. Available online: https://www.outotec.com/products/digitalsolutions/hsc-chemistry/ (accessed on 15 May 2018).
- Montgomery, D.C.; Peck, E.A.; Vining, G.G. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1997. [Google Scholar]
- Calado, V.; Montgomery, D.C. Planejamento de Experimentos Usando o Statistica; e-Papers: Rio de Janeiro, Brasil, 2003. (In Portuguese) [Google Scholar]
- Montgomery, D.C.; Runger, G.C.; Hubele, N.F. Design and Analysis of Experiments; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Costa, N.R.; Lourenço, J.; Pereira, Z.L. Desirability function approach: a review and performance evaluation in adverse conditions, Chemom. Int. Lab. Syst. 2011, 107, 234–244. [Google Scholar] [CrossRef]
- Berni, T.V.; Pereira, A.C.; Mendes, F.D.; Tude, J.A.L. System and Method for Rare Earths Extraction. U.S. Patent US2013/0336856 A1, 19 December 2013. [Google Scholar]






| Species | ΔG°f, 298 (kJ/mol) |
|---|---|
| Fe2O3 | −744.246 |
| Fe3O4 | −1012.34 |
| FeOOH | −490.52 |
| Fe2(SO4)3 | −2264.37 |
| FePO4 | −1182.22 |
| LaPO4 | −1781.49 |
| La2O3 | −1706.49 |
| La2(SO4)3 | −3597.5 |
| H2SO4 | −689.916 |
| SO2(g) | −285.468 |
| H2O | −237.141 |
| Run | ACs (w/w) | tF (h) | TF (°C) | tL (min) | TL (°C) | AQs (w/w) | AQw (w/w) |
|---|---|---|---|---|---|---|---|
| 1 | 1.2 | 3 | 350 | 30 | 80 | 2 | 2 |
| 2 | 1.2 | 3 | 250 | 120 | 25 | 2 | 3 |
| 3 | 1.2 | 1 | 350 | 30 | 25 | 3 | 3 |
| 4 | 0.8 | 3 | 250 | 30 | 80 | 3 | 3 |
| 5 | 1.2 | 1 | 250 | 120 | 80 | 3 | 2 |
| 6 | 0.8 | 1 | 350 | 120 | 80 | 2 | 3 |
| 7 | 0.8 | 3 | 350 | 120 | 25 | 3 | 2 |
| 8 | 0.8 | 1 | 250 | 30 | 25 | 2 | 2 |
| Run | TF (°C) | ACs (w/w) | tL (min) |
|---|---|---|---|
| 1 | 300 | 0.30 | 20 |
| 2 | 300 | 0.30 | 60 |
| 3 | 300 | 0.80 | 20 |
| 4 | 300 | 0.80 | 60 |
| 5 | 700 | 0.30 | 20 |
| 6 | 700 | 0.30 | 60 |
| 7 | 700 | 0.80 | 20 |
| 8 | 700 | 0.80 | 60 |
| 9 | 163.64 | 0.55 | 40 |
| 10 | 836.36 | 0.55 | 40 |
| 11 | 500 | 0.13 | 40 |
| 12 | 500 | 0.97 | 40 |
| 13 | 500 | 0.55 | 6.36 |
| 14 | 500 | 0.55 | 73.63 |
| 15 (C) | 500 | 0.55 | 40 |
| 16 (C) | 500 | 0.55 | 40 |
| Species | %(w/w) | Species | %(w/w) |
|---|---|---|---|
| Na2O | 0.22 | MnO | 3.3 |
| MgO | 0.67 | ZnO | 0.31 |
| Al2O3 | 0.56 | SrO | 0.3 |
| SiO2 | 4.5 | Nb2O5 | 3.9 |
| P2O5 | 3.2 | BaO | 6.7 |
| CaO | 0.2 | La2O3 | 0.87 |
| TiO | 4 | CeO2 | 0.76 |
| Fe2O3 | 57 | Pr6O11 | 0.24 |
| ZrO2 | 0.14 | Nd2O3 | 0.69 |
| PbO | 0.34 | Gd2O3 | 0.17 |
| ThO2 | 0.34 | S | 1.9 |
| Run | d%RE | d%Fe | 1-d%Fe | D |
|---|---|---|---|---|
| 1 | 0.23 | 0.39 | 0.61 | 0.38 |
| 2 | 0.81 | 0.45 | 0.55 | 0.66 |
| 3 | 0.67 | 0.92 | 0.08 | 0.24 |
| 4 | 0.00 | 0.52 | 0.48 | 0.00 |
| 5 | 0.00 | 0.00 | 1.00 | 0.00 |
| 6 | 0.00 | 0.00 | 1.00 | 0.00 |
| 7 | 0.85 | 0.38 | 0.62 | 0.72 |
| 8 | 0.98 | 0.51 | 0.49 | 0.69 |
| 9 | 0.72 | 0.65 | 0.35 | 0.50 |
| 10 | 0.00 | 0.00 | 1.00 | 0.00 |
| 11 | 0.00 | 0.12 | 0.88 | 0.00 |
| 12 | 0.47 | 1.00 | 0.00 | 0.00 |
| 13 | 0.00 | 0.40 | 0.60 | 0.00 |
| 14 | 0.42 | 0.65 | 0.35 | 0.38 |
| 15 | 0.49 | 0.72 | 0.28 | 0.37 |
| 16 | 1.00 | 0.81 | 0.19 | 0.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, M.; Lemos, F.; Guimarães, R.; Sousa, C.; Soares, P. Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil). Minerals 2019, 9, 451. https://doi.org/10.3390/min9070451
Nascimento M, Lemos F, Guimarães R, Sousa C, Soares P. Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil). Minerals. 2019; 9(7):451. https://doi.org/10.3390/min9070451
Chicago/Turabian StyleNascimento, Marisa, Flávio Lemos, Rogério Guimarães, Clóvis Sousa, and Paulo Soares. 2019. "Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil)" Minerals 9, no. 7: 451. https://doi.org/10.3390/min9070451
APA StyleNascimento, M., Lemos, F., Guimarães, R., Sousa, C., & Soares, P. (2019). Modeling of REE and Fe Extraction from a Concentrate from Araxá (Brazil). Minerals, 9(7), 451. https://doi.org/10.3390/min9070451




