Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing
Abstract
1. Introduction
1.1. Challenges in Tungsten Ore Processing
1.2. Enhanced Gravity Concentration Using Centrifugal Separators
2. Material and Methods
2.1. Materials
2.2. Chemical Analyzes
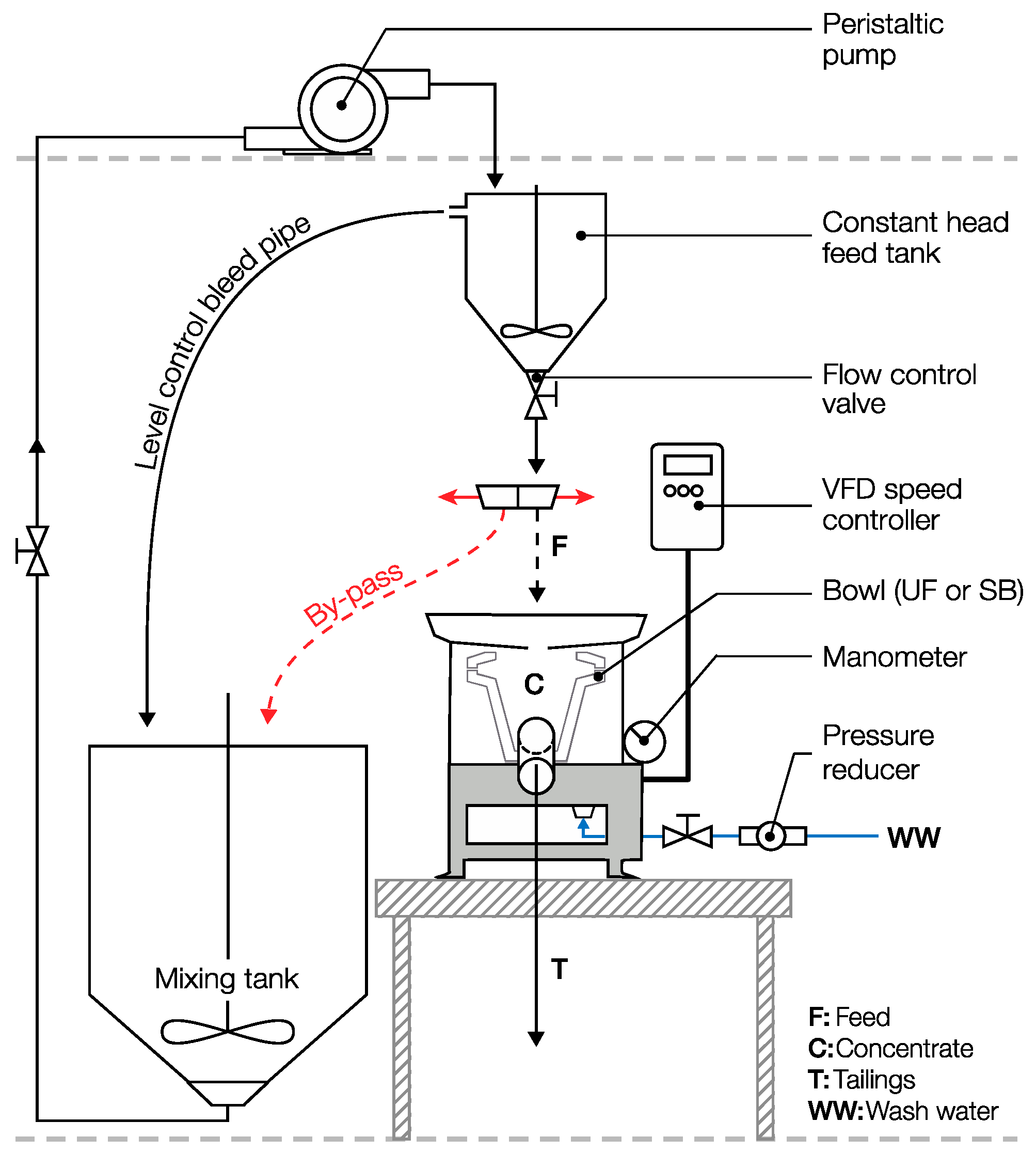
2.3. Falcon Tests
2.4. Hydrocyclone Test
2.5. Design of Experiments Method
3. Results and Discussion
3.1. Falcon UF
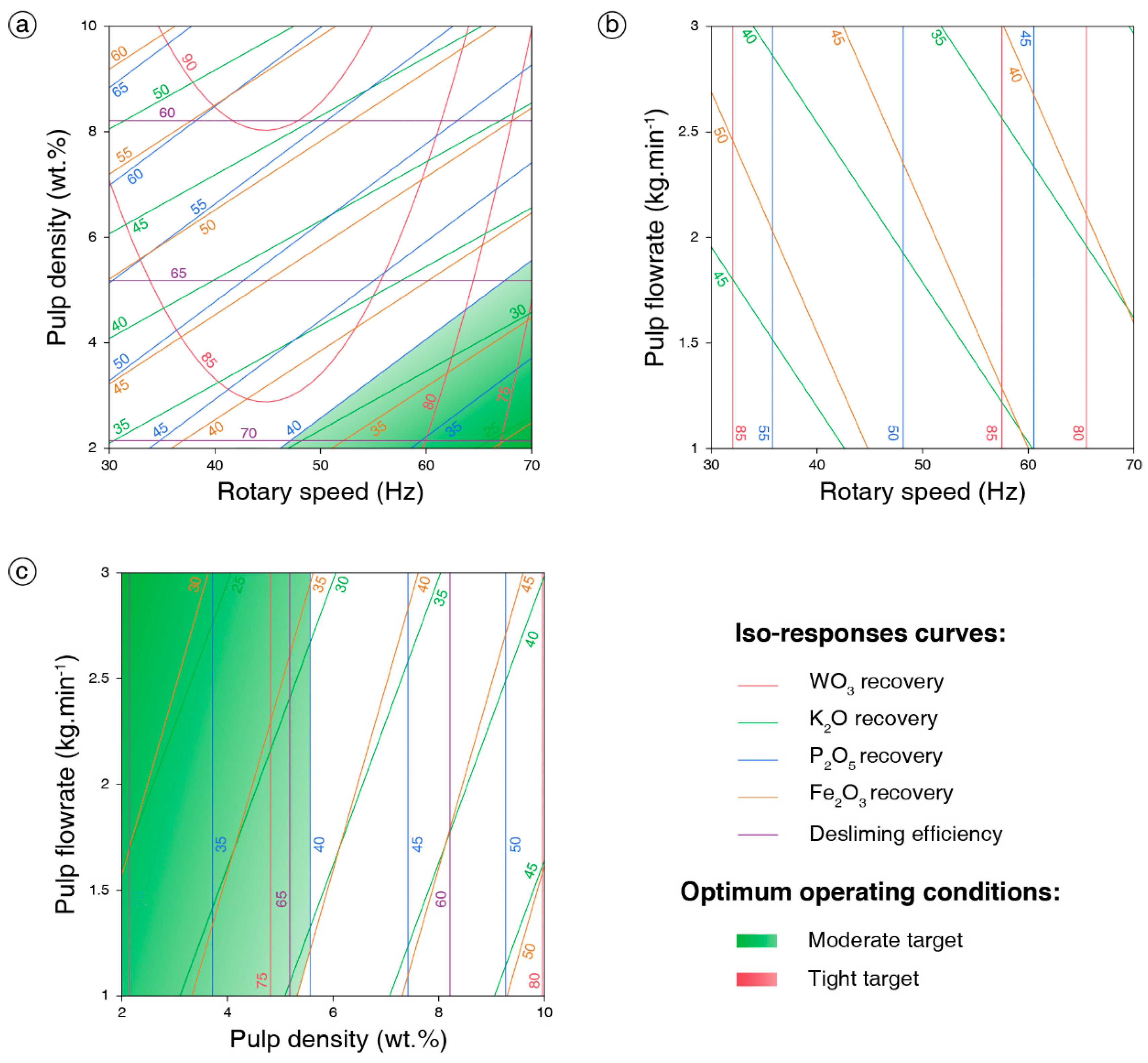
3.1.1. Design of Experiments Results
3.1.2. Interpretation of the Models
3.1.3. Optimization and Validation
3.2. Falcon SB
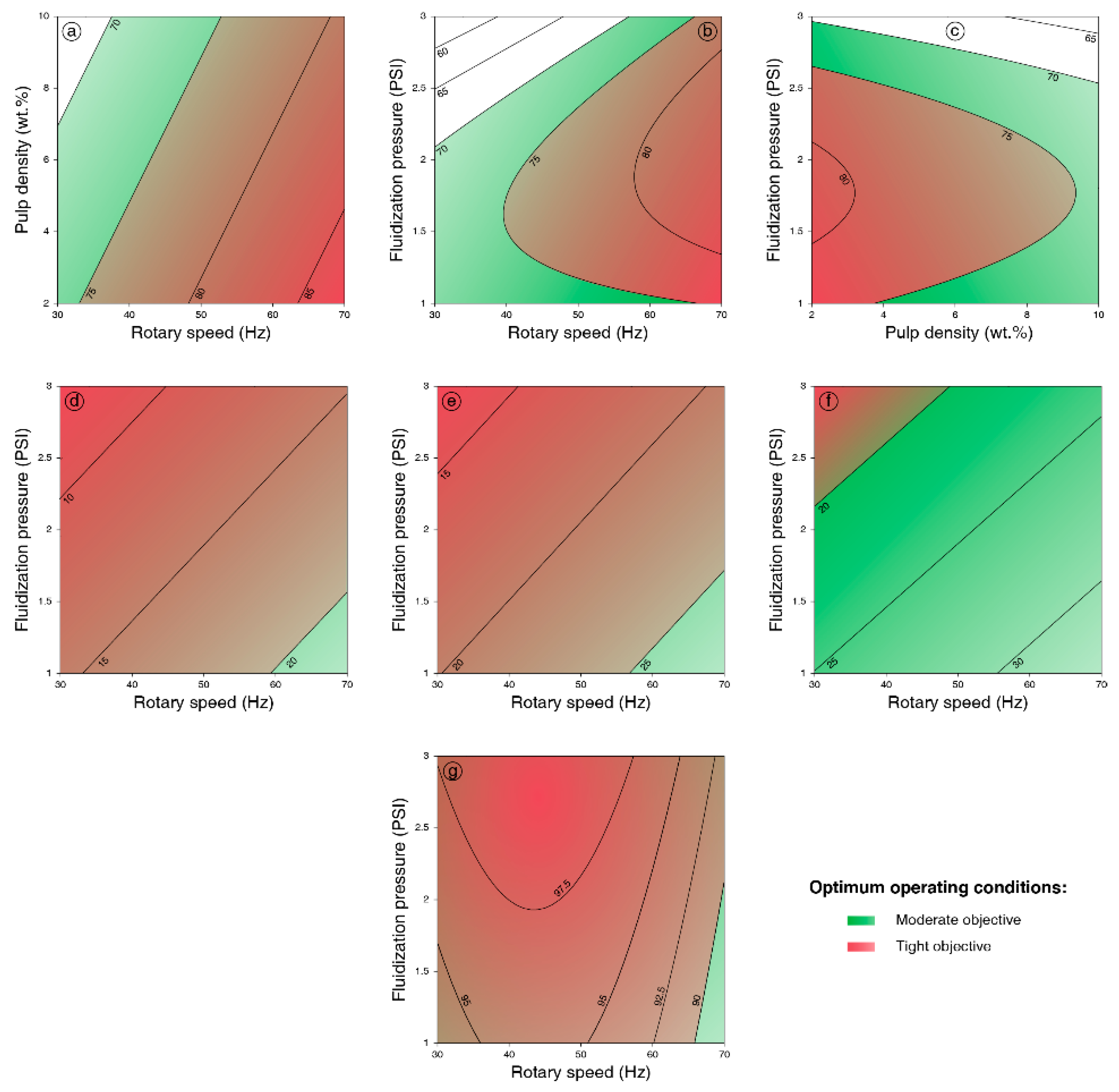
3.2.1. Design of Experiments Results
3.2.2. Interpretation of the Models
3.2.3. Optimization and Validation
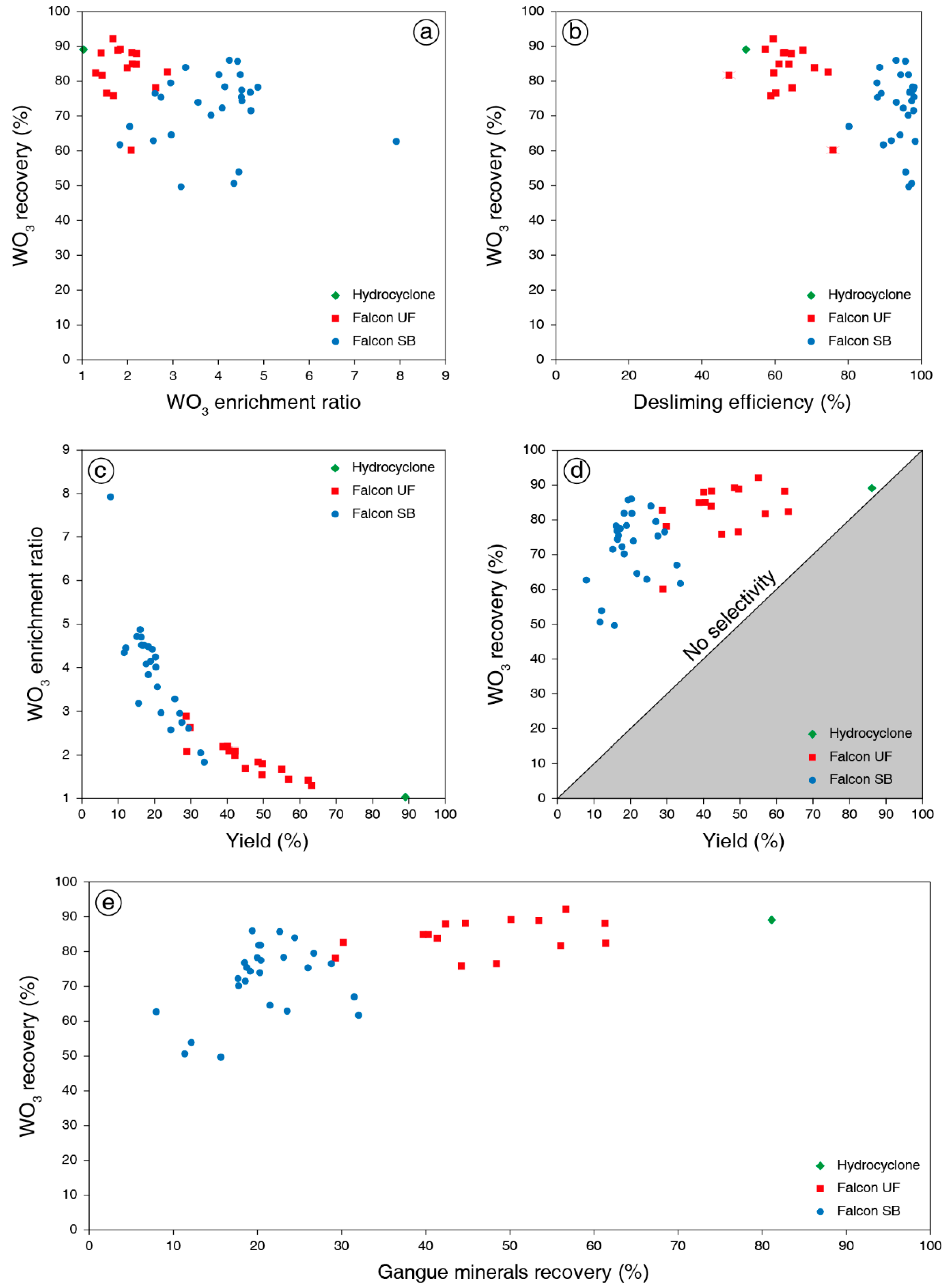
3.3. Discussion on Experimental Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Audion, A.S.; Labbé, J.F. Panorama 2011 du Marché du Tungstène; BRGM: Orléans, France, 2012. [Google Scholar]
- Pitfield, P.; Brown, T.; Gunn, G.; Rayner, D. British Geological Survey. Tungsten Profile. Available online: https://www.bgs.ac.uk/downloads/start.cfm?id=1981 (accessed on 19 July 2019).
- European Commission. Critical Raw Materials for the EU: Report of the Ad-hoc Working Group on Defining Critical Raw Materials; European Commision: Brussels, Belgium, 2010. [Google Scholar]
- Lassner, E.; Schubert, W.-D. Tungsten: Properties, Chemistry, Technology of the Element, Alloys, and Chemical Compounds; Springer: Boston, MA, USA, 1999. [Google Scholar]
- Yang, X. Beneficiation studies of tungsten ores—A review. Miner. Eng. 2018, 125, 111–119. [Google Scholar] [CrossRef]
- Suárez Sánchez, A.; Krzemień, A.; Riesgo Fernández, P.; Iglesias Rodríguez, F.J.; Sánchez Lasheras, F.; de Cos Juez, F.J. Investment in new tungsten mining projects. Resour. Policy 2015, 46, 177–190. [Google Scholar] [CrossRef]
- Kupka, N.; Rudolph, M. Froth flotation of scheelite—A review. Int. J. Min. Sci. Technol. 2018, 28, 373–384. [Google Scholar] [CrossRef]
- Jébrak, M.; Marcoux, É.; Laithier, M. Geology of Mineral Resources, 2nd ed.; Geological Association of Canada: St. John’s, NL, Canada, 2016. [Google Scholar]
- Foucaud, Y.; Lechenard, B.; Marion, P.; Filippova, I.; Filippov, L. Geology, textural study, ore genesis and processing of the tabuaço tungsten deposit (Northern Portugal). In Contributions to Mineralization; Al-Juboury, A.I., Ed.; InTech: London, UK, 2018. [Google Scholar] [CrossRef]
- Deng, L.; Zhao, G.; Zhong, H.; Wang, S.; Liu, G. Investigation on the selectivity of N-((hydroxyamino)-alkyl) alkylamide surfactants for scheelite/calcite flotation separation. J. Ind. Eng. Chem. 2016, 33, 131–141. [Google Scholar] [CrossRef]
- Liu, C.; Feng, Q.; Zhang, G.; Chen, W.; Chen, Y. Effect of depressants in the selective flotation of scheelite and calcite using oxidized paraffin soap as collector. Int. J. Miner. Process. 2016, 157, 210–215. [Google Scholar] [CrossRef]
- Filippov, L.O.; Foucaud, Y.; Filippova, I.V.; Badawi, M. New reagent formulations for selective flotation of scheelite from a skarn ore with complex calcium minerals gangue. Miner. Eng. 2018, 123, 85–94. [Google Scholar] [CrossRef]
- Agar, G.E. Scheelite Flotation Process. U.S. Patent 4,488,959, 18 December 1984. [Google Scholar]
- Pugh, R.; Stenius, P. Solution chemistry studies and flotation behaviour of apatite, calcite and fluorite minerals with sodium oleate collector. Int. J. Miner. Process. 1985, 15, 193–218. [Google Scholar] [CrossRef]
- Marinakis, K.I.; Shergold, H.L. The mechanism of fatty acid adsorption in the presence of fluorite, calcite and barite. Int. J. Miner. Process. 1985, 14, 161–176. [Google Scholar] [CrossRef]
- Filippova, I.V.; Filippov, L.O.; Duverger, A.; Severov, V.V. Synergetic effect of a mixture of anionic and nonionic reagents: Ca mineral contrast separation by flotation at neutral pH. Miner. Eng. 2014, 66–68, 135–144. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, B.; Wang, R.; Xu, S.; Wang, J.; Xu, Z. Extraction and separation of tungsten from acidic high-phosphorus solution. Hydrometallurgy 2016, 164, 97–102. [Google Scholar] [CrossRef]
- Petrov, N.S. A New Method for Flotation of Poor Scheelite Ores; ONTI, Mekhanobr: Sankt-Peterburg, Russia, 1940. [Google Scholar]
- Feng, B.; Guo, W.; Xu, H.; Peng, J.; Luo, X.; Zhu, X. The combined effect of lead ion and sodium silicate in the flotation separation of scheelite from calcite. Sep. Sci. Technol. 2017, 52, 567–573. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, S.; Zhong, H. Study on the activation of scheelite and wolframite by lead nitrate. Minerals 2015, 5, 247–258. [Google Scholar] [CrossRef]
- Burt, R. The role of gravity concentration in modern processing plants. Miner. Eng. 1999, 12, 1291–1300. [Google Scholar] [CrossRef]
- Falconer, A. Gravity separation: Old technique/new methods. Phys. Sep. Sci. Eng. 2003, 12, 31–48. [Google Scholar] [CrossRef]
- Das, A.; Sarkar, B. Advanced gravity concentration of fine particles: A review. Miner. Process. Extr. Metall. Rev. 2018, 39, 359–394. [Google Scholar] [CrossRef]
- Sepro. Falcon UF Gravity Concentrators Brochure. Sepro Miner. Syst. Website. (n.d.) Available online: http://www.seprosystems.com/images/brochures/sepro-falcon-uf-concentrators.pdf (accessed on 19 November 2015).
- Zhang, Y.; He, Y.; Zhang, T.; Zhu, X.; Feng, Y.; Zhang, G.; Bai, X. Application of Falcon centrifuge in the recycling of electrode materials from spent lithium ion batteries. J. Clean. Prod. 2018, 202, 736–747. [Google Scholar] [CrossRef]
- Duan, C.; Wen, X.; Shi, C.; Zhao, Y.; Wen, B.; He, Y. Recovery of metals from waste printed circuit boards by a mechanical method using a water medium. J. Hazard. Mater. 2009, 166, 478–482. [Google Scholar] [CrossRef]
- Filippov, L.O.; Dehaine, Q.; Filippova, I.V. Rare earths (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-products of kaolin production—Part 3: Processing of fines using gravity and flotation. Miner. Eng. 2016, 95, 96–106. [Google Scholar] [CrossRef]
- Dehaine, Q.; Foucaud, Y.; Kroll-Rabotin, J.-S.; Filippov, L.O. Experimental investigation into the kinetics of Falcon UF concentration: Implications for fluid dynamic-based modelling. Sep. Purif. Technol. 2019, 215, 590–601. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, D.M.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- McAlister, S.; Armstrong, K. Development of the Falcon concentrators. In Proceedings of the Society for Mining, Metallurgy & Exploration, Preprint 98-172, Orlando, FL, USA, 9–11 March 1998. [Google Scholar]
- Buonvino, M. A Study of the Falcon Concentrator. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1993. [Google Scholar]
- Laplante, A.R.; Buonvino, M.; Veltmeyer, A.; Robitaille, J.; Naud, G. A Study of the Falcon Concentrator. Can. Metall. Q. 1994, 33, 279–288. [Google Scholar] [CrossRef]
- Kroll-Rabotin, J.-S.; Bourgeois, F.; Climent, É. Fluid dynamics based modelling of the Falcon concentrator for ultrafine particle beneficiation. Miner. Eng. 2010, 23, 313–320. [Google Scholar] [CrossRef][Green Version]
- Kroll-Rabotin, J.-S.; Bourgeois, F.; Climent, É. Physical analysis and modeling of the Falcon concentrator for beneficiation of ultrafine particles. Int. J. Miner. Process. 2013, 121, 39–50. [Google Scholar] [CrossRef]
- Sepro; Falcon Concentrators Inc. FALCON L40 Operations and Maintenance Manual. Available online: https://www.adss.net/documents/manuals/F40_OP_MAN.pdf. (accessed on 19 July 2019).
- Dehaine, Q.; Filippov, L.O. Rare earth (La, Ce, Nd) and rare metals (Sn, Nb, W) as by-product of kaolin production, Cornwall: Part1: Selection and characterisation of the valuable stream. Miner. Eng. 2015, 76, 141–153. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Goupy, J.; Creighton, W.L. Introduction to Design of Experiments with JMP Examples, 3rd ed.; SAS Institute: Cary, NC, USA, 2007. [Google Scholar]
- Kroll-Rabotin, J.-S. Analyse Physique et Modélisation de la Séparation Centrifuge de Particules Ultrafines en Film Fluant: Application au Séparateur Industriel Falcon. Ph.D. Thesis, Université de Toulouse, Toulouse, France, 2010. [Google Scholar]
- Kroll-Rabotin, J.-S.; Climent, E.; Bourgeois, F. Beneficiation of concentrated ultrafine suspensions with a Falcon UF concentrator. Can. Inst. Min. J. 2011, 2, 189–198. [Google Scholar]
- Burt, R.O.; Mills, C. Gravity Concentration Technology; Elsevier: Amsterdam, The Netherlands, 1984. [Google Scholar]
- Burt, R.O. Gravity concentration methods. In Mineral Processing Design; Yarar, B., Dogan, Z.M., Eds.; Springer Netherlands: Dordrecht, The Netherlands, 1987; pp. 106–137. [Google Scholar] [CrossRef]
- Kademli, M.; Gulsoy, O. The role of particle size and solid contents of feed on mica-feldspar separation in gravity concentration. Physicochem. Probl. Miner. Process. 2012, 48, 645–654. [Google Scholar] [CrossRef]
- Laplante, A.; Nickoletopoulos, N. Validation of a falcon model with a synthetic ore. Can. Metall. Q. 1997, 36, 7–13. [Google Scholar] [CrossRef]
- Majumder, A.K.; Barnwal, J.P. Modeling of enhanced gravity concentrators–Present status. Miner. Process. Extr. Metall. Rev. 2006, 27, 61–86. [Google Scholar] [CrossRef]
- Ancia, P.; Frenay, J.; Dandois, P. Comparison of Knelson and Falcon centrifugal separators. In Innovation in Physical Separation Technologies; Mozeley, R., Ed.; Richard Mozely Symposium Volume IMM.: Falmouth, UK, 1997; pp. 53–62. [Google Scholar]
- Laplante, A.R.; Shu, Y.; Marois, J. Experimental characterization of a laboratory centrifugal separator. Can. Metall. Q. 1996, 35, 23–29. [Google Scholar] [CrossRef]
- Coulter, T.; Subasinghe, G.K.N. A mechanistic approach to modelling Knelson concentrators. Miner. Eng. 2005, 18, 9–17. [Google Scholar] [CrossRef]
- Ghaffari, A.; Farzanegan, A. An investigation on laboratory Knelson Concentrator separation performance: Part 2: Two-component feed separation modelling. Miner. Eng. 2017, 112, 114–124. [Google Scholar] [CrossRef]
- Ghaffari, A.; Farzanegan, A. An investigation on laboratory Knelson Concentrator separation performance: Part 3: Multi-component feed separation modelling. Miner. Eng. 2018, 122, 185–194. [Google Scholar] [CrossRef]
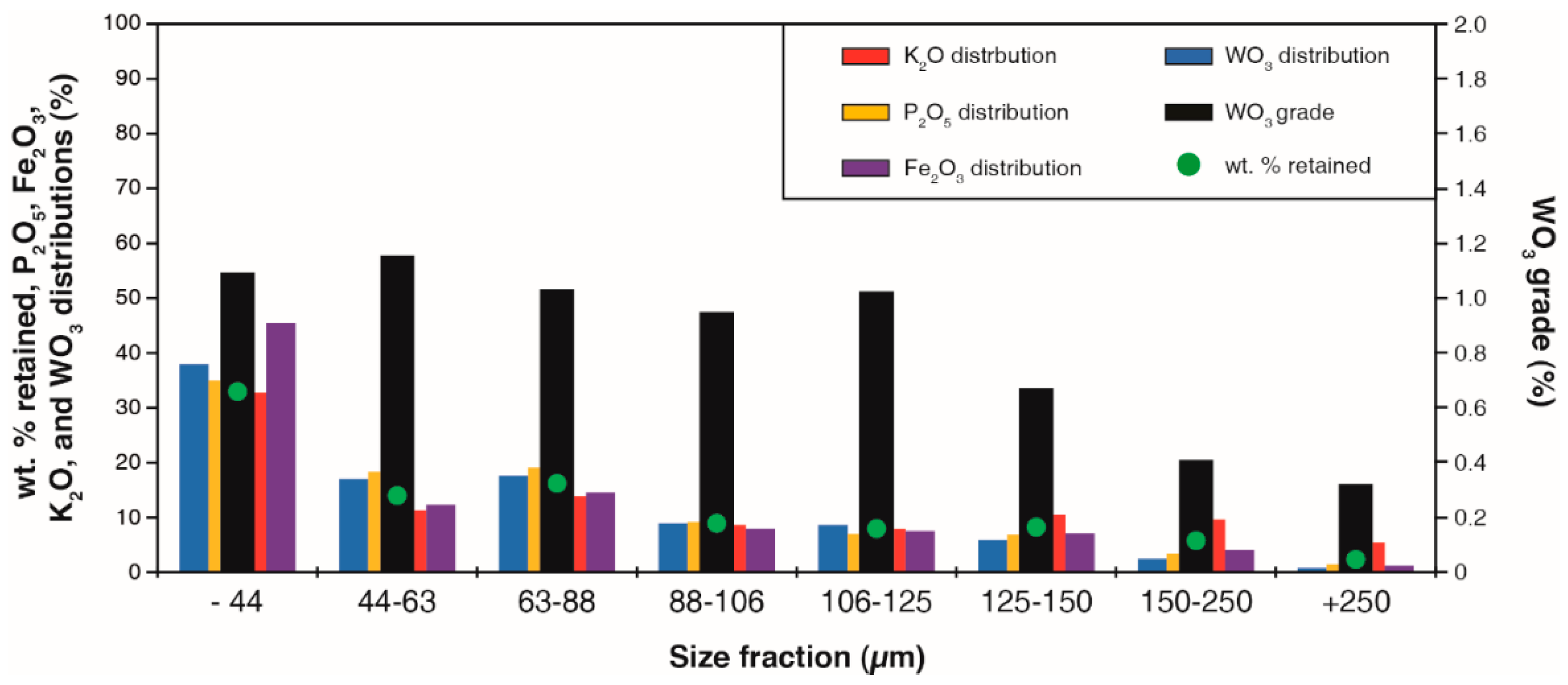


| Mineral | Formula | Abundance (wt %) | Density (g∙cm−3) | Group |
|---|---|---|---|---|
| Vesuvianite | Ca10(Mg,Fe)2Al4(SiO4)5(Si2O7)2(OH)4 | 45 | 3.30 | Dense silicates |
| Epidote (zoisite) | Ca2(Al,Fe)3(SiO4)3(OH) | 15 | 3.20 | Dense silicates |
| Fluorite | CaF2 | 10 | 3.18 | Calcium salts |
| Feldspars | (K,Na)AlSi3O8 | 15 | 2.65 | Light silicates |
| Phyllosilicates | - | 1-5 | 2.65 | Light silicates |
| Garnet (grossular) | Ca3(Al,Fe)2(SiO4)3 | 1-5 | 3.70 | Dense silicates |
| Fluorapatite | Ca5(PO4)3F | 3 | 3.18 | Calcium salts |
| Pyroxene (diopside) | Ca(Fe,Mg)Si2O6 | 1 | 3.20 | Dense silicates |
| Quartz | SiO2 | 1 | 2.65 | Light silicates |
| Scheelite | CaWO4 | 1 | 6.10 | Target mineral |
| Factors | Symbol | Levels | Coded Variables | ||
|---|---|---|---|---|---|
| Low (−1) | Center (0) | High (+1) | |||
| Rotary speed (Hz) | ω | 30 | 50 | 70 | x1 = (ω − 50)/20 |
| Pulp density (wt % solid) | %s | 2 | 6 | 10 | x2 = (%s − 6)/4 |
| Pulp flowrate (kg∙min−1) | Q | 1 | 2 | 3 | x3 = (Q − 2)/1 |
| Fluidization pressure (PSI) * | f | 1 | 2 | 3 | x4 = (f − 2)/1 |
| Response | Criterion | |
|---|---|---|
| Moderate | Tight | |
| WO3 recovery | 70% | 75% |
| P2O5 recovery | 40% | 25% |
| K2O recovery | 40% | 20% |
| Fe2O3 recovery | 40% | 20% |
| Desliming efficiency | 60% | 90% |
| Model Statistics | RW | RK | RP | RFe | Deff |
|---|---|---|---|---|---|
| R2 | 0.5802 | 0.9420 | 0.8810 | 0.9121 | 0.5492 |
| RMSE | 5.5592 | 2.7470 | 4.3503 | 3.5020 | 5.0488 |
| F | 5.5300 | 64.9100 | 48.1000 | 41.5300 | 17.0600 |
| P | 0.0128 | <0.0001 | <0.0001 | <0.0001 | 0.0010 |
| Term | Estimate | STD Error | T | P |
|---|---|---|---|---|
| RW | ||||
| Constant | 87.52 | 2.27 | 38.56 | <0.0001 |
| x1 (ω) | −3.90 | 1.76 | −2.22 | 0.0464 |
| x2 (%s) | 3.89 | 1.76 | 2.21 | 0.0472 |
| x1 (ω × ω) | −7.47 | 2.87 | −2.60 | 0.0232 |
| RK | ||||
| Constant | 39.21 | 0.69 | 57.09 | <0.0001 |
| x1 (ω) | −5.62 | 0.87 | −6.47 | <0.0001 |
| x2 (%s) | 10.08 | 0.87 | 11.60 | <0.0001 |
| x3 (Q) | −3.71 | 0.87 | −4.28 | 0.0011 |
| Rp | ||||
| Constant | 49.26 | 1.09 | 45.29 | <0.0001 |
| x1 (ω) | −8.08 | 1.38 | −5.87 | <0.0001 |
| x2 (%s) | 10.81 | 1.38 | 7.85 | <0.0001 |
| RFe | ||||
| Constant | 45.41 | 0.88 | 51.87 | <0.0001 |
| x1 (ω) | −6.58 | 1.11 | −5.94 | <0.0001 |
| x2 (%s) | 10.06 | 1.11 | 9.08 | <0.0001 |
| x3 (Q) | −2.89 | 1.11 | −2.61 | 0.0230 |
| Deff | ||||
| Constant | 63.65 | 1.26 | 50.42 | <0.0001 |
| x2 (%s) | −6.59 | 1.60 | −4.13 | 0.0010 |
| Validation Data | RW | RK | RP | RFe | Deff |
|---|---|---|---|---|---|
| Mean | 63.73 | 24.19 | 25.45 | 22.62 | 72.51 |
| Absolute experimental standard deviation | 1.94 | 1.30 | 1.89 | 1.26 | 1.46 |
| Predicted by the model | 72.26 | 19.80 | 30.37 | 25.89 | 70.24 |
| Model absolute error | 8.53 | −4.39 | 4.92 | 3.27 | −2.27 |
| Model Statistics | RW | RK | RP | RFe | Deff |
|---|---|---|---|---|---|
| R2 | 0.6989 | 0.6523 | 0.6904 | 0.7125 | 0.6921 |
| RMSE | 6.2631 | 3.3917 | 3.4524 | 2.9182 | 2.6222 |
| F | 364.1500 | 21.5800 | 25.6500 | 28.5000 | 16.4900 |
| P | 0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Term | Estimate | STD error | T | P |
|---|---|---|---|---|
| RW | ||||
| Constant | 77.33 | 2.21 | 34.92 | <0.0001 |
| x1 (ω) | 6.56 | 1.48 | 4.45 | 0.0002 |
| x2 (%s) | −3.25 | 1.48 | −2.20 | 0.0398 |
| x4 (f) | −3.52 | 1.48 | −2.39 | 0.0270 |
| x1 ∗ x4 (ω ∗ f) | 4.37 | 1.57 | 2.79 | 0.0113 |
| x42 (f ∗ f) | −7.69 | 2.66 | −2.89 | 0.0091 |
| RK | ||||
| Constant | 14.59 | 0.67 | 21.94 | <0.0001 |
| x1 (ω) | 3.82 | 0.80 | 4.77 | <0.0001 |
| x4 (f) | −3.61 | 0.80 | −4.51 | 0.0002 |
| RP | ||||
| Constant | 24.58 | 0.68 | 36.30 | <0.0001 |
| x1 (ω) | 3.87 | 0.81 | 4.75 | <0.0001 |
| x4 (f) | −4.36 | 0.81 | −5.36 | <0.0001 |
| RFe | ||||
| Constant | 20.19 | 0.57 | 35.28 | <0.0001 |
| x1 (ω) | 3.82 | 0.69 | 5.56 | <0.0001 |
| x4 (f) | −3.52 | 0.69 | −5.11 | <0.0001 |
| Deff | ||||
| Constant | 97.16 | 0.93 | 104.81 | <0.0001 |
| x1 (ω) | −2.94 | 0.62 | −4.75 | <0.0001 |
| x4 (f) | 2.02 | 0.62 | 3.26 | 0.0036 |
| x12 (ω ∗ ω) | −4.49 | 1.11 | −4.03 | 0.0006 |
| Validation Data | RW | RK | RP | RFe | Deff |
|---|---|---|---|---|---|
| Mean | 71.61 | 12.63 | 22.62 | 17.15 | 98.78 |
| Absolute experimental standard deviation | 3.52 | 1.29 | 3.16 | 1.24 | 0.06 |
| Predicted by the model | 73.74 | 12.51 | 21.77 | 18.20 | 97.29 |
| Model absolute error | 2.07 | −0.12 | −0.85 | 1.05 | −1.49 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Foucaud, Y.; Dehaine, Q.; Filippov, L.O.; Filippova, I.V. Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing. Minerals 2019, 9, 448. https://doi.org/10.3390/min9070448
Foucaud Y, Dehaine Q, Filippov LO, Filippova IV. Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing. Minerals. 2019; 9(7):448. https://doi.org/10.3390/min9070448
Chicago/Turabian StyleFoucaud, Yann., Quentin Dehaine, Lev. O. Filippov, and Inna V. Filippova. 2019. "Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing" Minerals 9, no. 7: 448. https://doi.org/10.3390/min9070448
APA StyleFoucaud, Y., Dehaine, Q., Filippov, L. O., & Filippova, I. V. (2019). Application of Falcon Centrifuge as a Cleaner Alternative for Complex Tungsten Ore Processing. Minerals, 9(7), 448. https://doi.org/10.3390/min9070448







