The Characterization and SCR Performance of Mn-Containing α-Fe2O3 Derived from the Decomposition of Siderite
Abstract
1. Introduction
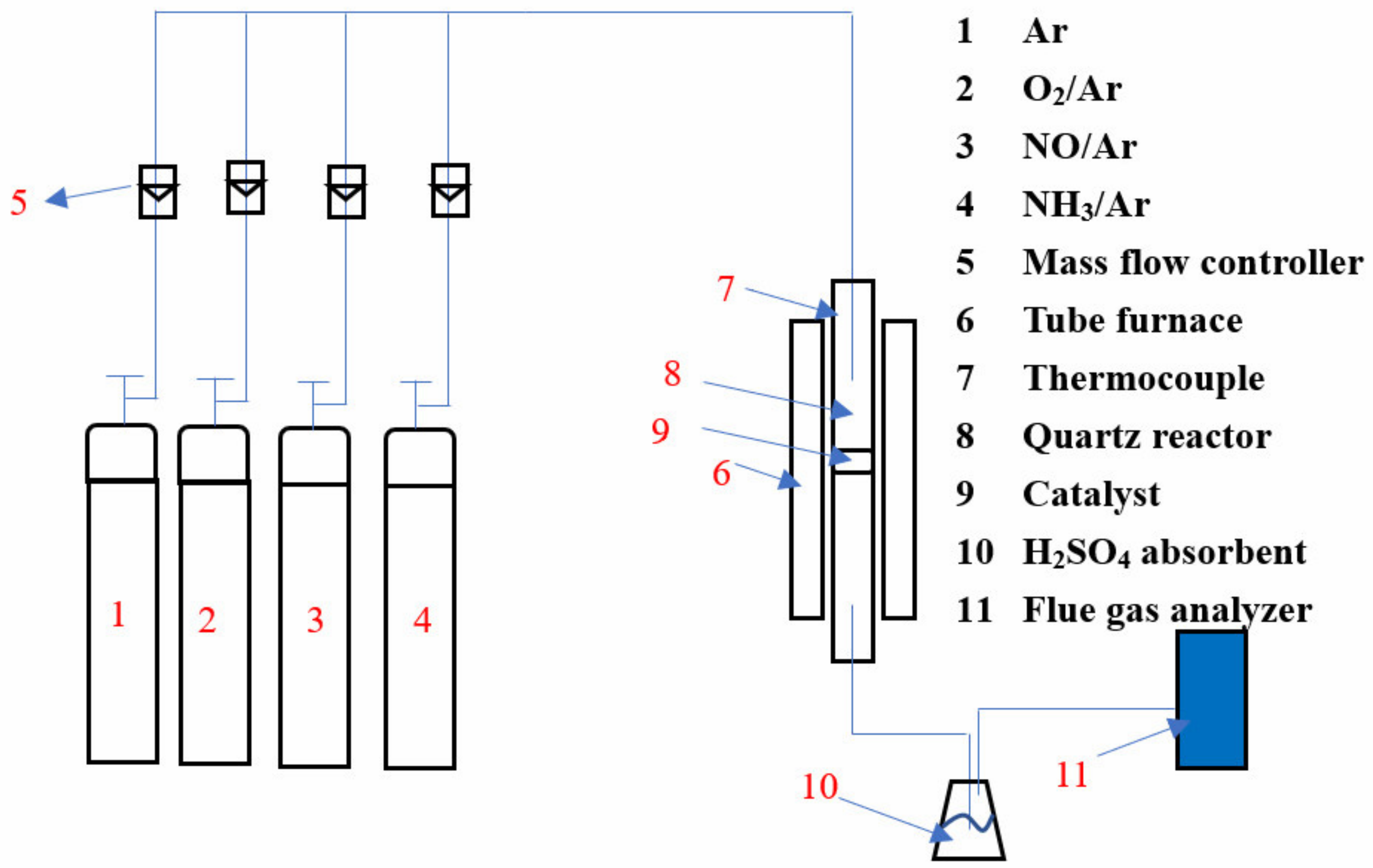
2. Experimental Methods
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Catalytic Activity Testing
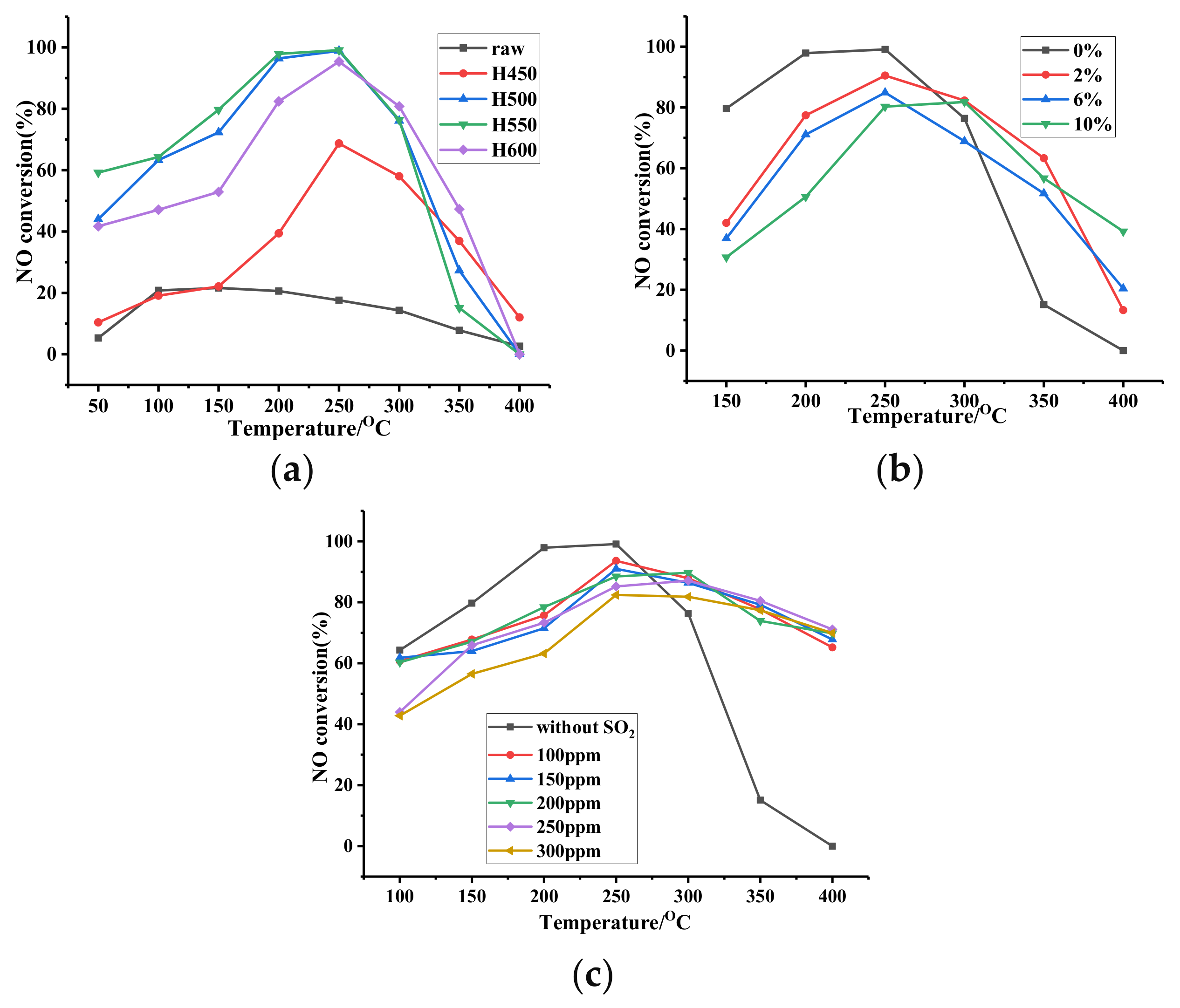
3. Results and Discussion
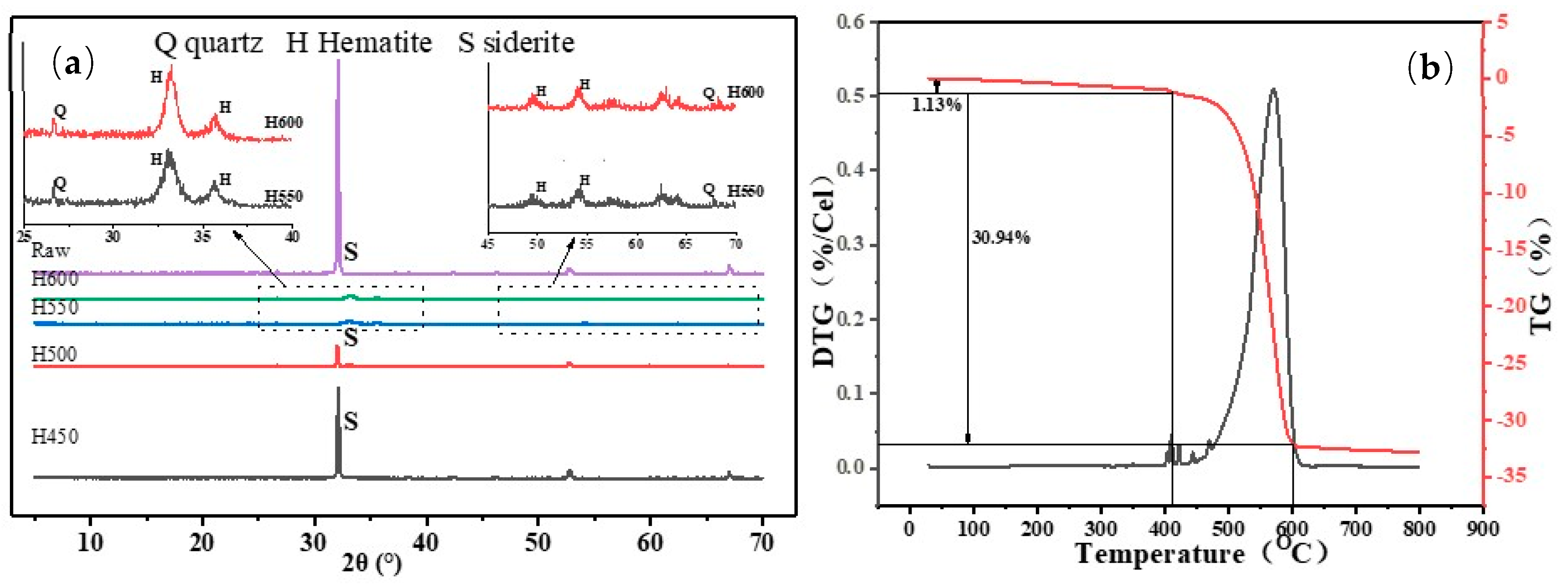
3.1. XRD, XRF, and TG of Siderite before and after Calcination
3.2. XPS
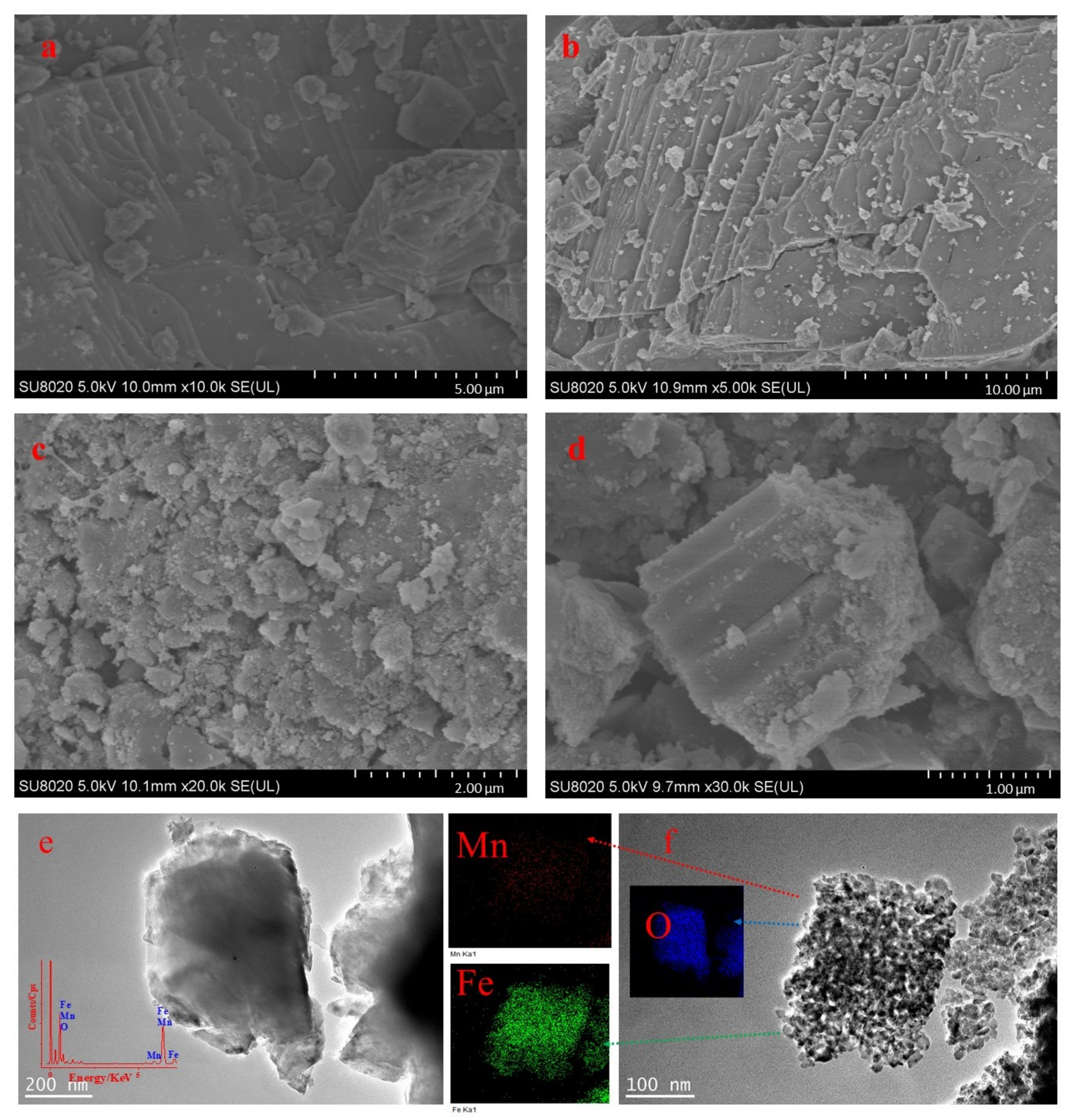
3.3. SEM and TEM
3.4. BET and Raman
3.5. SCR Performance and Resistance
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Mu, J.C.; Li, X.Y.; Sun, W.B.; Fan, S.Y.; Wang, X.Y.; Wang, L.; Qin, M.C.; Gan, G.Q.; Yin, Z.F.; Zhang, D.K. Enhancement of Low-Temperature Catalytic Activity over a Highly Dispersed Fe-Mn/Ti Catalyst for Selective Catalytic Reduction of NOx with NH3. Ind. Eng. Chem. Res. 2018, 57, 10159–10169. [Google Scholar] [CrossRef]
- Liu, C.; Shi, J.W.; Gao, C.; Niu, C. Manganese oxide-based catalysts for low-temperature selective catalytic reduction of NOx with NH3: A review. Appl. Catal. A Gen. 2016, 522, 54–69. [Google Scholar] [CrossRef]
- Cheruiyot, N.K.; Wang, L.-C.; Lin, S.-L.; Yang, H.-H.; Chen, Y.-T. Effects of Selective Catalytic Reduction on the Emissions of Persistent Organic Pollutants from a Heavy-Duty Diesel Engine. Aerosol Air Qual. Res. 2017, 17, 1658–1665. [Google Scholar] [CrossRef]
- Juzsakova, T.; Al-Jammal, N.; Cretescu, I.; Sebestyen, V.; Cuong Le, P.; Domokos, E.; Redey, A.; Stan, C.D. Case Studies for Clean Technology Development in the Chemical Industry Using Zeolite Based Catalysts. Minerals 2018, 8, 462. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, L.; Li, L.; Liu, L.; Cao, Y.; Dong, X.; Gao, F.; Yu, D.; Tang, C.; Chen, Z. Investigation of the structure, acidity, and catalytic performance of CuO/Ti0.95Ce0.05O2 catalyst for the selective catalytic reduction of NO by NH3 at low temperature. Appl. Catal. B Environ. 2014, 150, 315–329. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, D.; Zhang, J.; Cai, S.; Fang, C.; Huang, L.; Li, H.; Gao, R.; Shi, L. Design of meso-TiO2@MnO(x)-CeO(x)/CNTs with a core-shell structure as DeNO(x) catalysts: Promotion of activity, stability and SO2-tolerance. Nanoscale 2013, 5, 9821–9829. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Chen, T.; Liu, H.; Chen, D.; Xu, B.; Qing, C. Low temperature SCR reaction over Nano-Structured Fe-Mn Oxides: Characterization, performance, and kinetic study. Appl. Surf. Sci. 2018, 457, 1116–1125. [Google Scholar] [CrossRef]
- Liu, S.; Ji, P.; Ye, D.; Qu, R.; Zheng, C.; Gao, X. Regeneration of Potassium Poisoned Catalysts for the Selective Catalytic Reduction of NO with NH3. Aerosol Air Qual. Res. 2019, 19, 649–656. [Google Scholar] [CrossRef]
- Schill, L.; Putluru, S.S.R.; Fehrmann, R.; Jensen, A.D. Low-Temperature NH3–SCR of NO on Mesoporous Mn0.6Fe0.4/TiO2 Prepared by a Hydrothermal Method. Catal. Lett. 2014, 144, 395–402. [Google Scholar] [CrossRef]
- Li, Q.; Liu, H.; Chen, T.; Chen, D.; Zhang, C.; Xu, B.; Zhu, C.; Jiang, Y. Characterization and SCR Performance of Nano-Structured Iron-Manganese Oxides: Effect of Annealing Temperature. Aerosol Air Qual. Res. 2017, 17, 2328–2337. [Google Scholar] [CrossRef]
- Yang, S.; Wang, C.; Ma, L.; Peng, Y.; Qu, Z.; Yan, N.; Chen, J.; Chang, H.; Li, J. Substitution of WO3 in V2O5/WO3–TiO2 by Fe2O3 for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2012, 3, 161–168. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, S.; Li, J.; Zhu, J.; Ma, L. Novel V2O5–CeO2/TiO2 catalyst with low vanadium loading for the selective catalytic reduction of NOx by NH3. Appl. Catal. B Environ. 2014, 158, 11–19. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, M.; Du, X.; Ran, J.; Zhang, L.; Tang, D. High Resistance to Na Poisoning of the V2O5-Ce(SO4)(2)/TiO2 Catalyst for the NO SCR Reaction. Aerosol Air Qual. Res. 2018, 18, 2948–2955. [Google Scholar] [CrossRef]
- Liu, F.; He, H. Structure−Activity Relationship of Iron Titanate Catalysts in the Selective Catalytic Reduction of NOx with NH3†. J. Phys. Chem. C 2010, 114, 16929–16936. [Google Scholar] [CrossRef]
- Youn, S.; Song, I.; Kim, D.H. Roles of Promoters in V2O5/TiO2 Catalysts for Selective Catalytic Reduction of NOx with NH3: Effect of Order of Impregnation. J. Nanosci. Nanotechnol. 2016, 16, 4350–4356. [Google Scholar] [CrossRef]
- Ma, L.; Li, J.; Ke, R.; Fu, L. Catalytic Performance, Characterization, and Mechanism Study of Fe2(SO4)3/TiO2 Catalyst for Selective Catalytic Reduction of NOx by Ammonia. J. Phys. Chem. C 2011, 115, 7602–7603. [Google Scholar] [CrossRef]
- Min, K.; Park, E.D.; Ji, M.K.; Yie, J.E. Manganese oxide catalysts for NOx reduction with NH3 at low temperatures. Appl. Catal. A Gen. 2007, 327, 261–269. [Google Scholar]
- Chen, J.P.; Hausladen, M.C.; Yang, R.T. Delaminated Fe2O3-pillared clay: Its preparation, characterization, and activities for selective catalytic reduction of NO by NH3. J. Catal. 1995, 151, 135–146. [Google Scholar] [CrossRef]
- Putluru, S.S.R.; Schill, L.; Jensen, A.D.; Siret, B.; Tabaries, F.; Fehrmann, R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation—Promising for selective catalytic reduction of NO with NH3 at low temperatures. Appl. Catal. B Environ. 2014, 165, 628–635. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, Z.; Li, Q.; Chen, T.; Zhang, C.; Chen, D.; Zhu, C.; Jiang, Y. Novel Method for Preparing Controllable Nanoporous α-Fe2O3 and its Reactivity to SCR De-NOx. Aerosol Air Qual. Res. 2017, 17, 1898–1908. [Google Scholar] [CrossRef]
- Bell, M.S.; Schwandt, C.S.; Zolensky, M.E.; Hörz, F. Experimental Shock Decomposition of Siderite. Meteorit. Planet. Sci. 2002, 37, 1–6. [Google Scholar]
- Zhang, D.; Zhang, L.; Shi, L.; Fang, C.; Li, H.; Gao, R.; Huang, L.; Zhang, J. In situ supported MnO(x)-CeO(x) on carbon nanotubes for the low-temperature selective catalytic reduction of NO with NH3. Nanoscale 2013, 5, 1127–1136. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Kong, T.; Yu, S.; Li, L.; Yang, F.; Dong, L. Influence of different supports on the physicochemical properties and denitration performance of the supported Mn-based catalysts for NH3-SCR at low temperature. Appl. Surf. Sci. 2017, 402, 208–217. [Google Scholar] [CrossRef]
- Qi, G.; Yang, R.T. Low-temperature selective catalytic reduction of NO with NH over iron and manganese oxides supported on titania. Appl. Catal. B Environ. 2003, 44, 217–225. [Google Scholar] [CrossRef]
- Liu, D.; Zhou, A.; Zeng, F.; Zhao, F.; Zou, Y. The Petrography, Mineralogy and Geochemistry of Some Cu- and Pb-Enriched Coals from Jungar Coalfield, Northwestern China. Minerals 2018, 8, 5. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, L.; Jiang, Y.; Wei, J.; Chen, Z. Mineralogical and Geochemical Characteristics of the Early Permian Upper No. 3 Coal from Southwestern Shandong, China. Minerals 2016, 6, 58. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, H.; Ping, L.; Chen, T.; Ma, W. Nanostructured α-Fe2O3 derived from siderite as an effective Hg(II) adsorbent: Performance and mechanism. Appl. Geochem. 2018, 96, 92–99. [Google Scholar] [CrossRef]
- Liu, H.; Chen, T.; Zou, X.; Qing, C.; Frost, R.L. Thermal treatment of natural goethite: Thermal transformation and physical properties. Thermochim. Acta 2013, 568, 115–121. [Google Scholar] [CrossRef]
- Li, M.; Sun, Y.; Liu, H.; Chen, T.; Hayat, T.; Alharbi, N.S.; Chen, C. Spectroscopic and modeling investigation of Eu(III) and U(VI) adsorption on nano-magnetite from aqueous solutions. ACS Sustain. Chem. Eng. 2017, 5, 5493–5502. [Google Scholar] [CrossRef]
- Fan, Y.; Ling, W.; Dong, L.; Li, S.; Yu, C.; Huang, B.; Xi, H. In Situ FT-IR and DFT Study of the Synergistic Effects of Cerium Presence in the Framework and the Surface in NH3-SCR. Aerosol Air Qual. Res. 2018, 18, 655–670. [Google Scholar] [CrossRef]
- Luo, Y.H.; Zhu, D.Q.; Pan, J.; Zhou, X.L. Thermal decomposition behaviour and kinetics of Xinjiang siderite ore. Miner. Process. Extr. Metall. 2016, 125, 17–25. [Google Scholar] [CrossRef]
- Zhang, X.; Han, Y.; Li, Y.; Sun, Y. Effect of Heating Rate on Pyrolysis Behavior and Kinetic Characteristics of Siderite. Minerals 2017, 7, 211. [Google Scholar] [CrossRef]
- Zhang, W.; Shi, Y.; Li, C.; Zhao, Q.; Li, X. Synthesis of Bimetallic MOFs MIL-100(Fe-Mn) as an Efficient Catalyst for Selective Catalytic Reduction of NOx with NH3. Catal. Lett. 2016, 146, 1956–1964. [Google Scholar] [CrossRef]
- Zhu, L.; Zhong, Z.; Yang, H.; Wang, C. NH3-SCR Performance of Mn-Fe/TiO2 Catalysts at Low Temperature in the Absence and Presence of Water Vapor. Water Air Soil Pollut. 2016, 227, 476. [Google Scholar] [CrossRef]
- Liu, F.; He, H.; Lian, Z.; Shan, W.; Xie, L.; Asakura, K.; Yang, W.; Deng, H. Highly dispersed iron vanadate catalyst supported on TiO2 for the selective catalytic reduction of NOx with NH3. J. Catal. 2013, 307, 340–351. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Wang, X.; Zou, W.; Cao, Y.; Sun, J.; Tang, C.; Gao, F.; Deng, Y.; Dong, L. Synthesis, characterization and catalytic performance of FeMnTiOx mixed oxides catalyst prepared by a CTAB-assisted process for mid-low temperature NH3-SCR. Appl. Catal. A Gen. 2015, 505, 235–242. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; Mcintyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Heuer, J.K.; Stubbins, J.F. An XPS characterization of FeCO3 films from CO2 corrosion. Corros. Sci. 1999, 41, 1231–1243. [Google Scholar] [CrossRef]
- Devaiah, D.; Smirniotis, P.G. Role of the Ce and Cr Content of Fe-Ce-Cr Ferrite Spinels for the High Temperature Water-Gas Shift Reaction. Ind. Eng. Chem. Res. 2017, 56, 1772–1781. [Google Scholar] [CrossRef]
- Liu, C.; Yang, S.; Ma, L.; Peng, Y.; Hamidreza, A.; Chang, H.; Li, J. Comparison on the Performance of α-Fe2O3 and γ-Fe2O3 for Selective Catalytic Reduction of Nitrogen Oxides with Ammonia. Catal. Lett. 2013, 143, 697–704. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, L.; Huang, L.; Zhang, J.; Gao, R.; Zhang, D. Rational Design of High-Performance DeNOx Catalysts Based on MNxCO3–xO4 Nanocages Derived from Metal–Organic Frameworks. ACS Catal. 2014, 4, 1753–1763. [Google Scholar] [CrossRef]
- Hu, H.; Zha, K.; Li, H.; Shi, L.; Zhang, D. In situ DRIFTs investigation of the reaction mechanism over MnO x-MOy/Ce0.75Zr0.25O2 (M = Fe, Co, Ni, Cu) for the selective catalytic reduction of NOx with NH3. Appl. Surf. Sci. 2016, 387, 921–928. [Google Scholar] [CrossRef]
- Yu, J.; Guo, F.; Wang, Y.; Zhu, J.; Liu, Y.; Su, F.; Gao, S.; Xu, G. Sulfur poisoning resistant mesoporous Mn-based catalyst for low-temperature SCR of NO with NH3. Appl. Catal.B95: 160-168. Appl. Catal. B Environ. 2010, 95, 160–168. [Google Scholar] [CrossRef]
- Yao, X.; Li, L.; Zou, W.; Yu, S.; An, J.; Li, H.; Yang, F.; Dong, L. Preparation, characterization, and catalytic performance of high efficient CeO2-MnOx-Al2O3 catalysts for NO elimination. Chin. J. Catal. 2016, 37, 1369–1380. [Google Scholar] [CrossRef]
- Ilton, E.S.; Post, J.E.; Heaney, P.J.; Ling, F.T.; Kerisit, S.N. XPS determination of Mn oxidation states in Mn (hydr)oxides. Appl. Surf. Sci. 2016, 366, 475–485. [Google Scholar] [CrossRef]
- Ristić, M.; Krehula, S.; Reissner, M.; Musić, S. 57Fe Mössbauer, XRD, FT-IR, FE SEM Analyses of Natural Goethite, Hematite and Siderite. Croat. Chem. Acta 2017, 90, 499–507. [Google Scholar] [CrossRef]
- Wu, X.; Xu, P.; Duan, Y.; Hu, C.; Li, G. Surface magnetization of siderite mineral. Int. J. Min. Sci. Technol. 2012, 22, 825–830. [Google Scholar] [CrossRef]
- Liu, H.; Shu, D.; Sun, F.; Li, Q.; Chen, T.; Xing, B.; Chen, D.; Qing, C. Effect of manganese substitution on the crystal structure and decomposition kinetics of siderite. J. Therm. Anal. Calorim. 2018, 136, 1315–1322. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, D.; Shi, L.; Gao, R.; Li, H.; Ye, L.; Zhang, J. Highly dispersed CeO2 on carbon nanotubes for selective catalytic reduction of NO with NH3. Catal. Sci. Technol. 2013, 3, 803–811. [Google Scholar] [CrossRef]
- Liu, X.L.; Guo, J.X.; Chu, Y.H.; Luo, D.M.; Yin, H.Q.; Sun, M.C.; Yavuz, R. Desulfurization performance of iron supported on activated carbon. Fuel 2014, 123, 93–100. [Google Scholar] [CrossRef]
- Cooney, T.F.; Scott, E.R.D.; Krot, A.N.; Sharma, S.K.; Yamaguchi, A. Vibrational spectroscopic study of minerals in the Martian meteorite ALH84001. Am. Mineral. 1999, 84, 1569–1576. [Google Scholar] [CrossRef]
- Sommer, A.J.; Bogdan, C.E.; Simpson, D.R.; Herman, R.G. Discrimination Among Carbonate Minerals by Raman Spectroscopy Using the Laser Microprobe. Appl. Spectrosc. 1987, 41, 437–440. [Google Scholar]
- Zoppi, A.; Lofrumento, C.; Castellucci, E.M.; Migliorini, M.G. The Raman spectrum of hematite: Possible indicator for a compositional or firing distinction among Terra Sigillata wares. Ann. Di Chim. 2010, 95, 239–246. [Google Scholar] [CrossRef]
- León, C.P.; Kador, L.; Zhang, M.; Müller, A.H.E. In situ laser-induced formation of α-Fe2O3 from Fe3+ ions in a cylindrical core-shell polymer brush. J. Raman Spectrosc. 2004, 35, 165–169. [Google Scholar] [CrossRef]
- Chen, H.; Wang, Y.; Lv, Y.K. Catalytic oxidation of NO over MnO2 with different crystal structures. RSC Adv. 2016, 6, 54032–54040. [Google Scholar] [CrossRef]
- Hong, W.J.; Iwamoto, S.; Hosokawa, S.; Wada, K.; Kanai, H.; Inoue, M. Effect of Mn content on physical properties of CeOx–MnOy support and BaO–CeOx–MnOy catalysts for direct NO decomposition. J. Catal. 2011, 277, 208–216. [Google Scholar] [CrossRef]
- Wu, Z.; Jin, R.; Wang, H.; Liu, Y. Effect of ceria doping on SO2 resistance of Mn/TiO2 for selective catalytic reduction of NO with NH3 at low temperature. Catal. Commun. 2009, 10, 935–939. [Google Scholar] [CrossRef]
- Liu, F.; He, H. Selective catalytic reduction of NO with NH3 over manganese substituted iron titanate catalyst: Reaction mechanism and H2O/SO2 inhibition mechanism study. Catal. Today 2010, 153, 70–76. [Google Scholar] [CrossRef]
- Lian, Z.; Liu, F.; He, H.; Shi, X.; Mo, J.; Wu, Z. Manganese–niobium mixed oxide catalyst for the selective catalytic reduction of NOx with NH3 at low temperatures. Chem. Eng. J. 2014, 250, 390–398. [Google Scholar] [CrossRef]
- Long, R.Q.; Yang, R.T.; Chang, R. Low temperature selective catalytic reduction (SCR) of NO with NH3 over Fe-Mn based catalysts. Chem. Commun. 2002, 5, 452–453. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, X.; Wu, W. Effects of H2O and SO2 on the Performance of V2O5/TiO2 Catalysts for Selective Catalytic Reduction of NO in Flue Gas. Proc. CSEE 2013, 33, 28–33. [Google Scholar]
- Sheng, Z.Y.; Hu, Y.F.; Xue, J.M.; Wang, X.M.; Liao, W.P. SO2 poisoning and regeneration of Mn-Ce/TiO2 catalyst for low temperature NOx reduction with NH3. J. Rare Earths 2012, 30, 676–682. [Google Scholar] [CrossRef]
- Casapu, M.; Kröcher, O.; Elsener, M. Screening of doped MnOx–CeO2 catalysts for low-temperature NO-SCR. Appl. Catal. B Environ. 2009, 88, 413–419. [Google Scholar] [CrossRef]
- Jiang, B.Q.; Wu, Z.B.; Liu, Y.; Lee, S.C.; Ho, W.K. DRIFT Study of the SO2 Effect on Low-Temperature SCR Reaction over Fe−Mn/TiO2. J. Phys. Chem. C 2010, 114, 4961–4965. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, X.; Xing, Z.; Bao, C.; Liang, G. Preparation and Characterization of CeO2-MoO3/TiO2 Catalysts for Selective Catalytic Reduction of NO with NH3. Aerosol Air Qual. Res. 2017, 17, 2726–2734. [Google Scholar] [CrossRef]

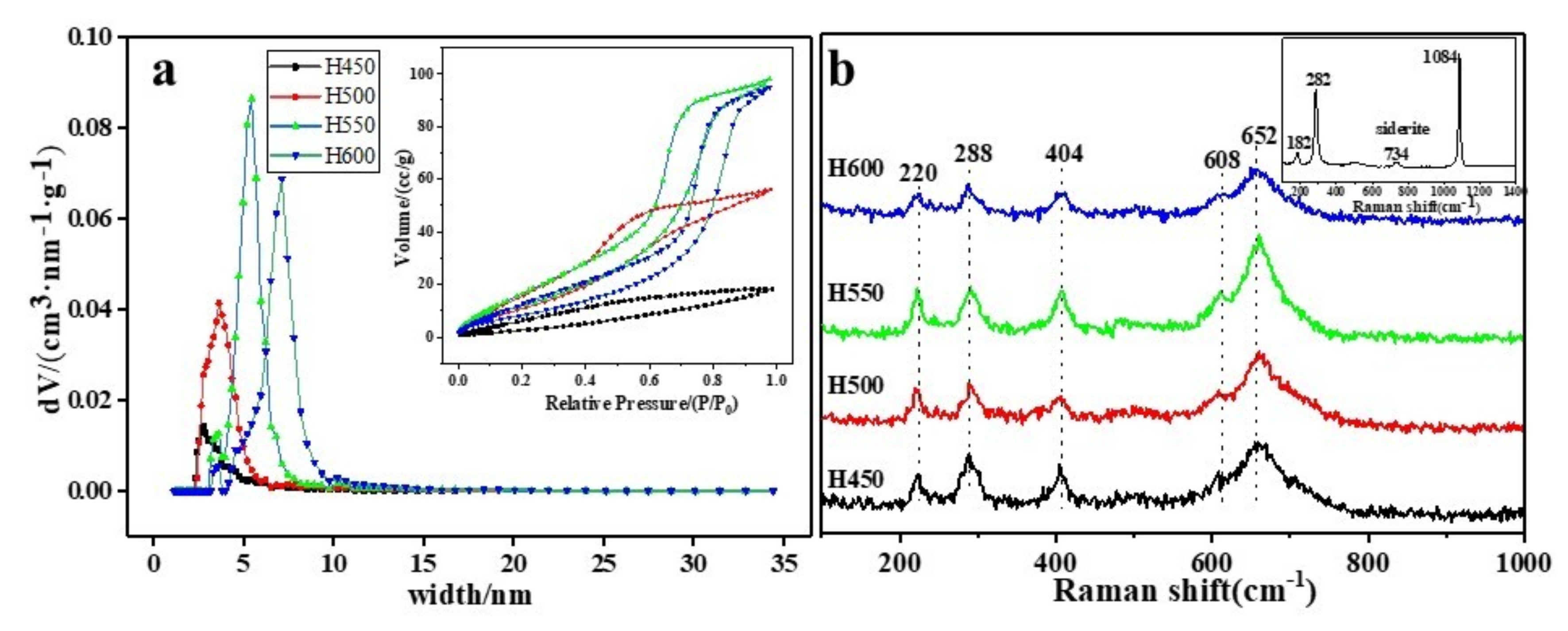
| Sample | BET-SSA (m2/g) | Pore Volume(cc/g) | Average Pore Size(nm) |
|---|---|---|---|
| H450 | 14 | 0.028 | 8.069 |
| H500 | 52 | 0.087 | 6.661 |
| H550 | 54 | 0.152 | 11.033 |
| H600 | 37 | 0.147 | 15.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, F.; Liu, H.; Shu, D.; Chen, T.; Chen, D. The Characterization and SCR Performance of Mn-Containing α-Fe2O3 Derived from the Decomposition of Siderite. Minerals 2019, 9, 393. https://doi.org/10.3390/min9070393
Sun F, Liu H, Shu D, Chen T, Chen D. The Characterization and SCR Performance of Mn-Containing α-Fe2O3 Derived from the Decomposition of Siderite. Minerals. 2019; 9(7):393. https://doi.org/10.3390/min9070393
Chicago/Turabian StyleSun, Fuwei, Haibo Liu, Daobing Shu, Tianhu Chen, and Dong Chen. 2019. "The Characterization and SCR Performance of Mn-Containing α-Fe2O3 Derived from the Decomposition of Siderite" Minerals 9, no. 7: 393. https://doi.org/10.3390/min9070393
APA StyleSun, F., Liu, H., Shu, D., Chen, T., & Chen, D. (2019). The Characterization and SCR Performance of Mn-Containing α-Fe2O3 Derived from the Decomposition of Siderite. Minerals, 9(7), 393. https://doi.org/10.3390/min9070393





