A Study of Bubble Mineralization by Modified Glass Microspheres Based on a High-Speed Dynamic Microscopic Test System
Abstract
:1. Introduction
2. Materials and Methods
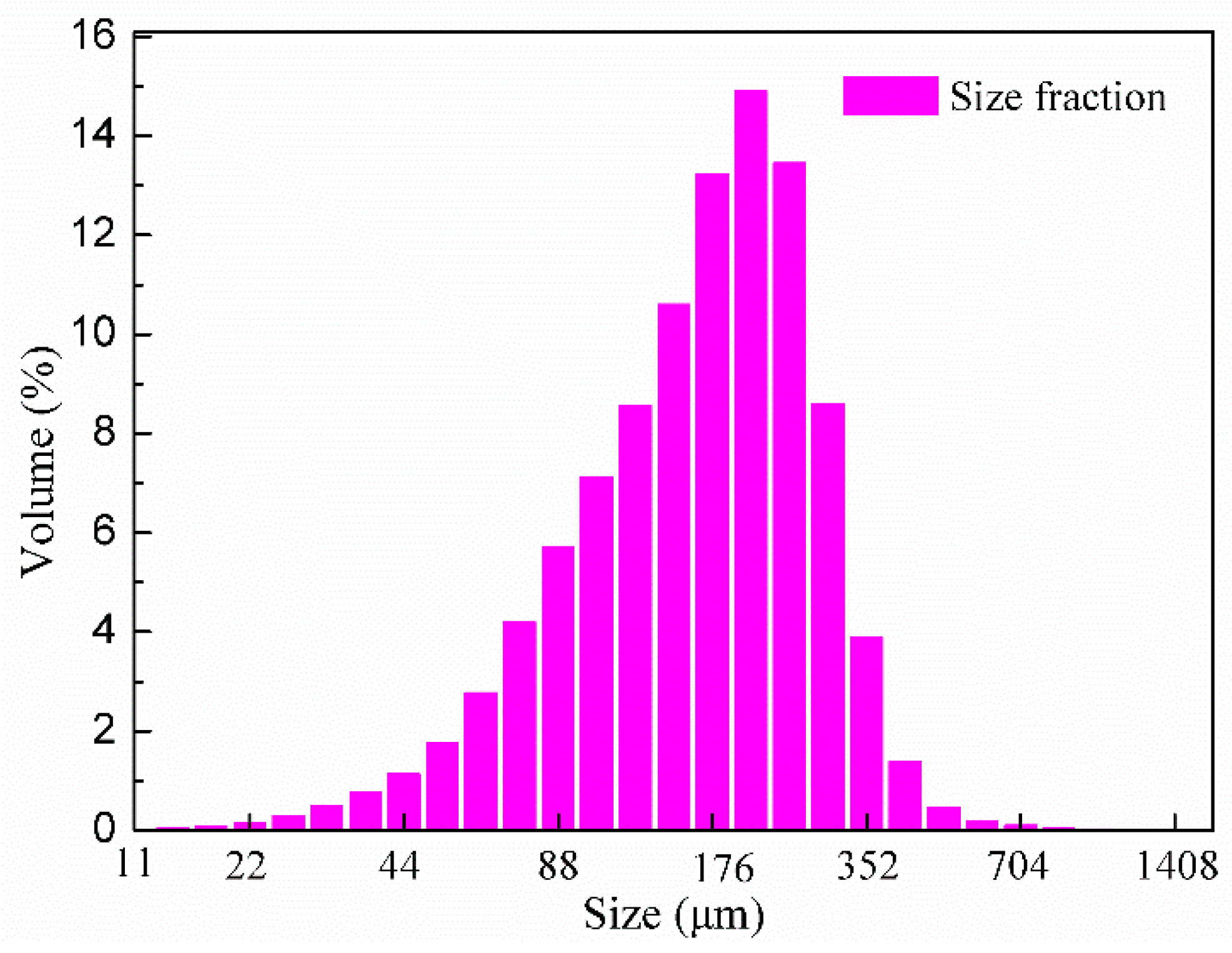
2.1. Experimental Samples
2.2. Experimental Agents
2.3. Experimental Methods
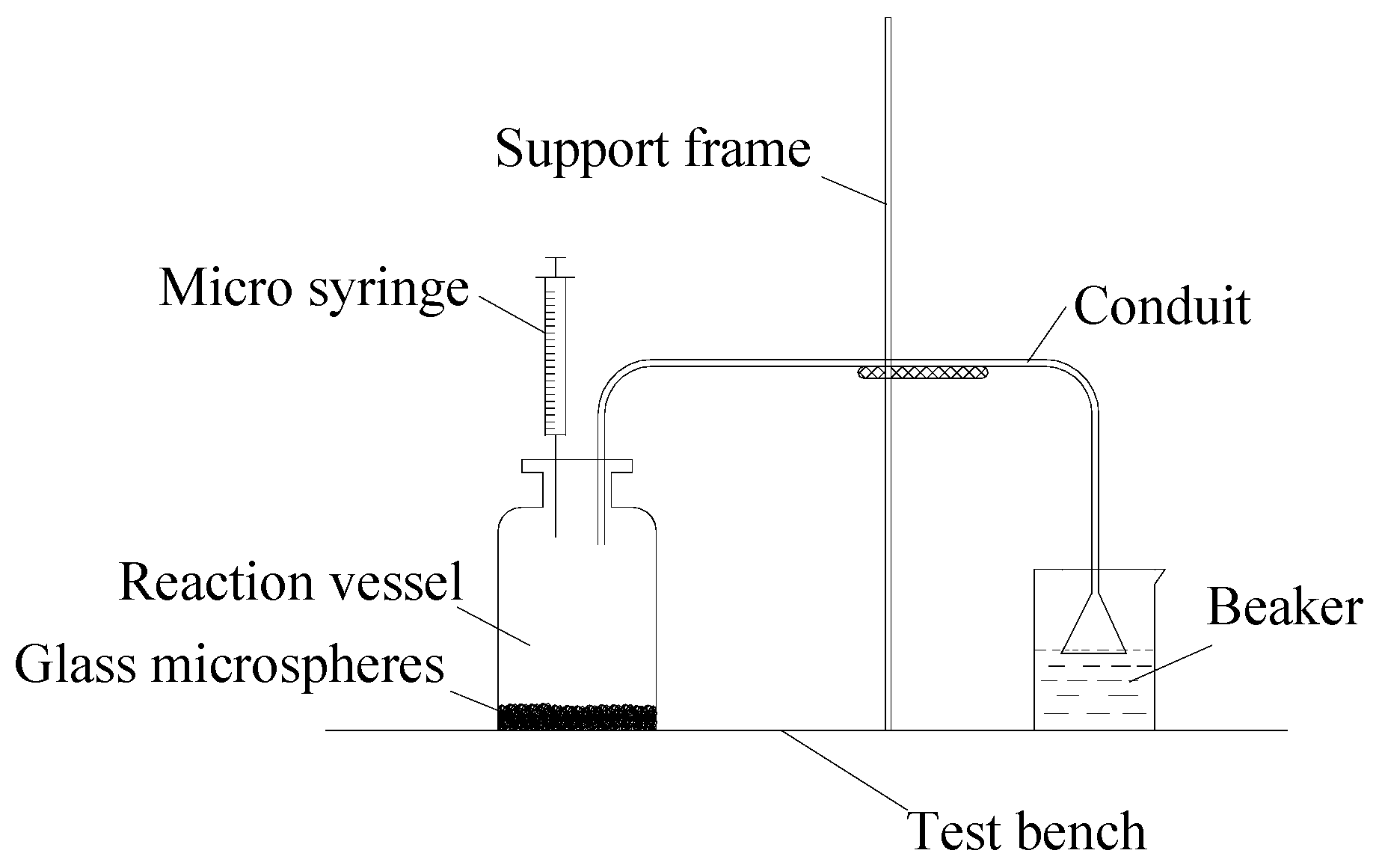
2.3.1. Surface Modification
2.3.2. Verification of Modification
Functional Group Analysis
Flotation Test
Contact Angle Measurement
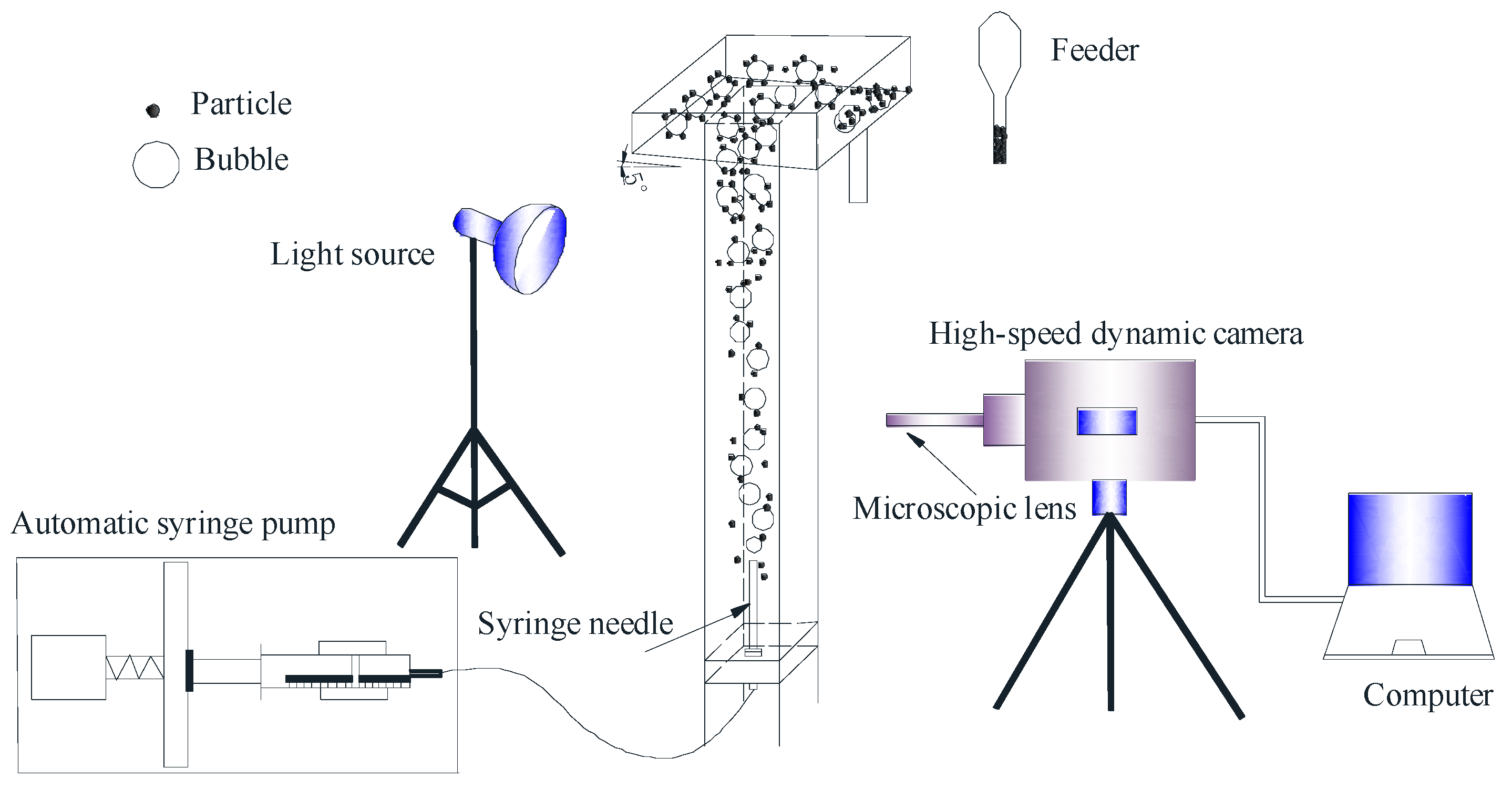
2.3.3. Bubble Mineralization
3. Results and Discussion
3.1. FTIR Spectra Analysis and Flotation Results of the Modified Glass Microspheres
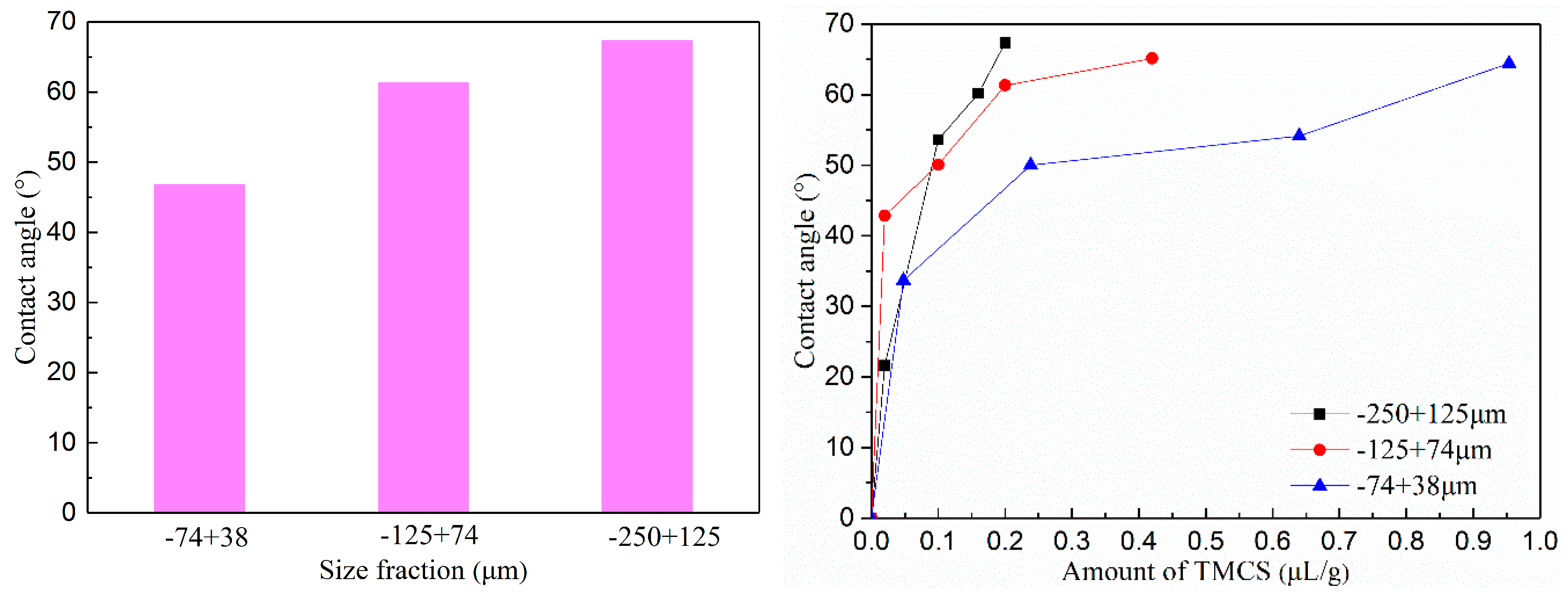
3.2. Change in Contact Angle after Surface Modification
3.3. Bubble Mineralization
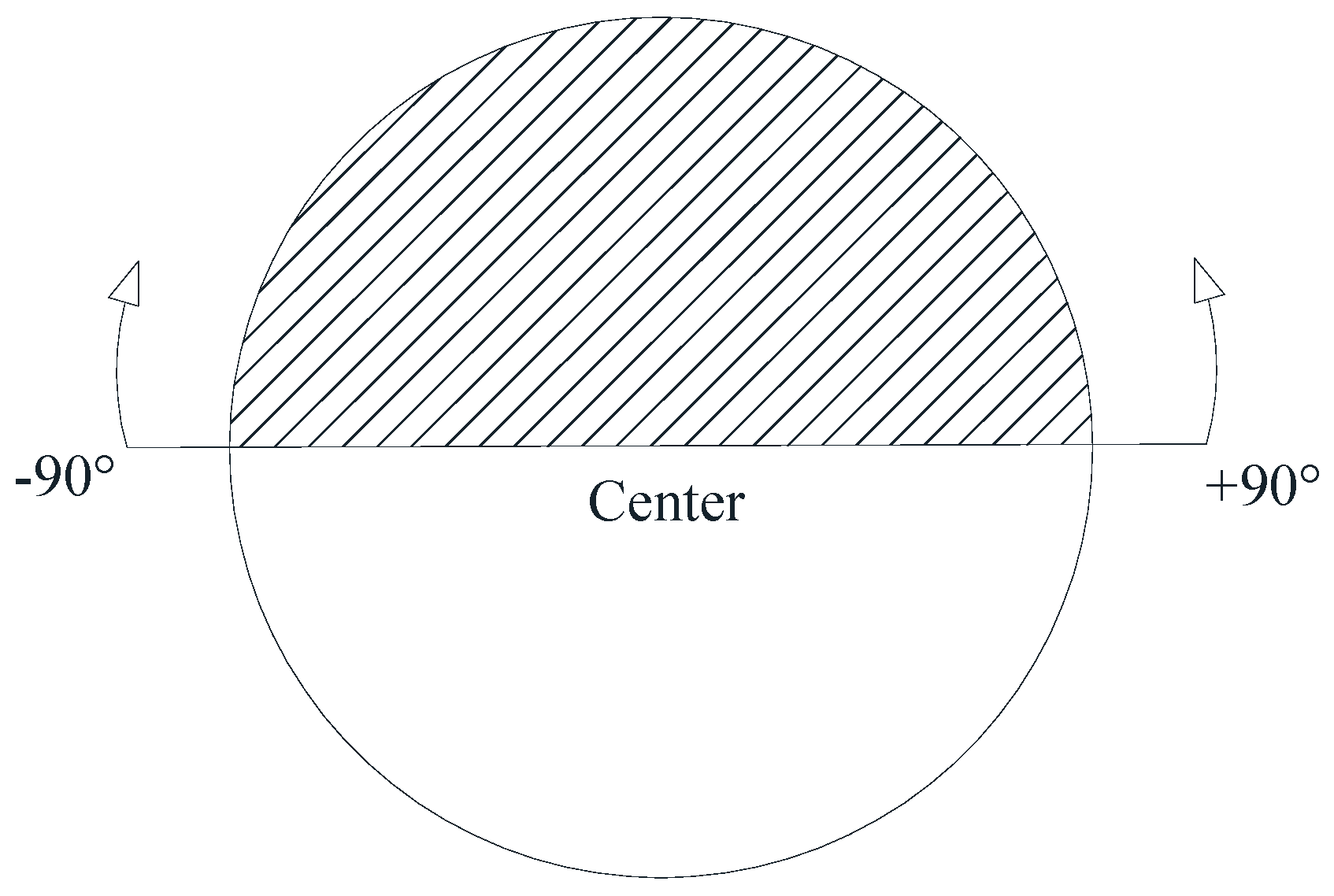
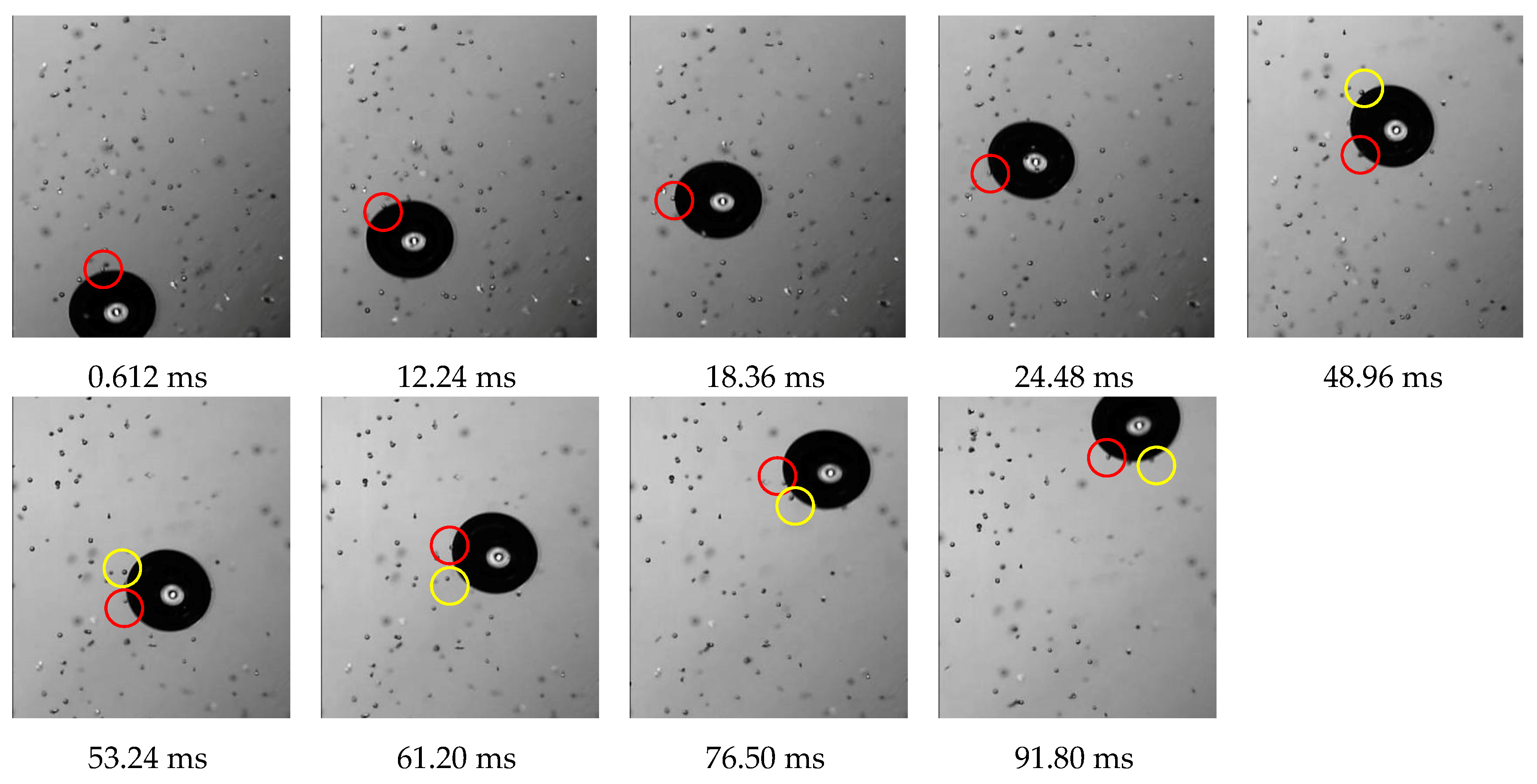
3.3.1. Collision between Bubble and Glass Microspheres
3.3.2. Adhesion between Bubble and Glass Microspheres
3.3.3. Detachment of the Glass Microspheres from Bubbles
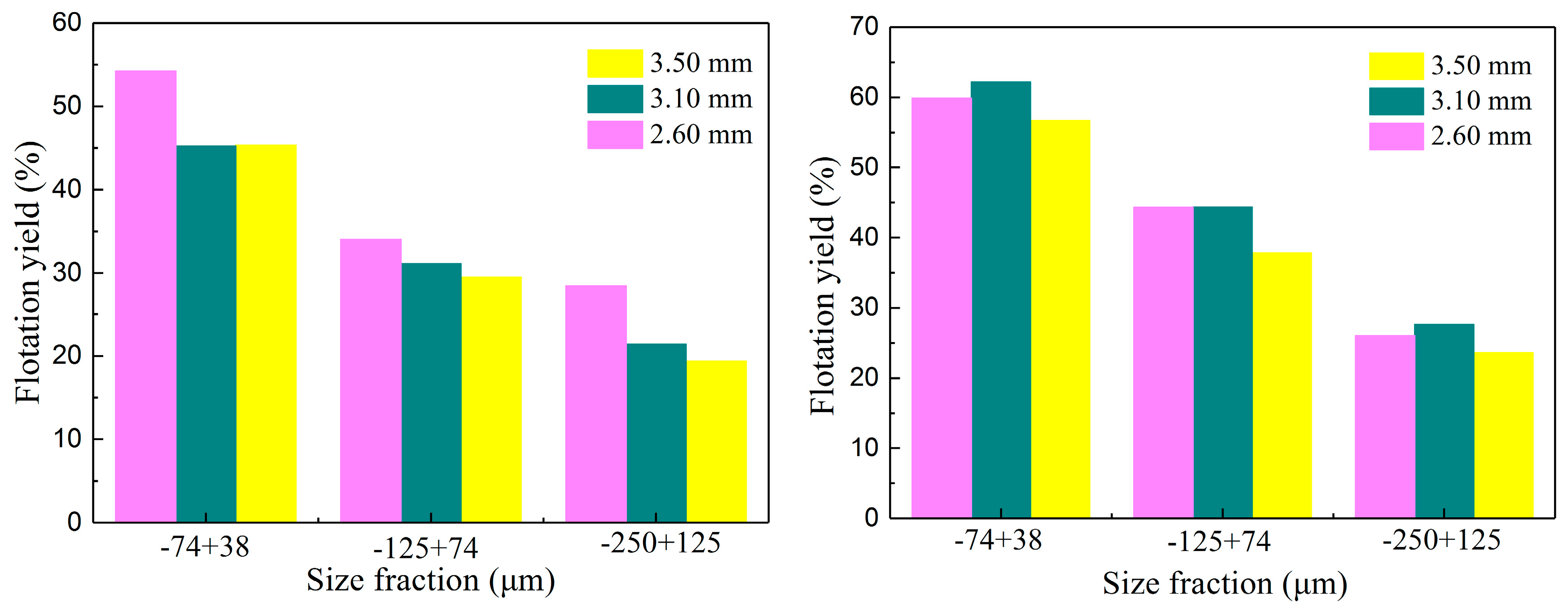
3.3.4. The Effects of Bubble and Glass Microsphere Sizes on Flotation Recovery
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Fan, X.; Liu, K.; Tan, C.; Zhang, J. Research on Mineralization Mechanism of Flotation Bubble. Express Inf. Min. Ind. 2005, 21, 12–14. [Google Scholar]
- Mao, L.; Zhang, H. Progress in the research on foreign flotation theory. Nonferrous Min. Metall. 1989, 10–13. [Google Scholar]
- Mahnke, J.; Schulze, H.J.; Stöckelhuber, K.W.; Radoev, B. Rupture of thin wetting films on hydrophobic surfaces Part I: Methylated glass surfaces. Colloids Surf. 1999, 157, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Nandakumar, K.; Xu, Z.; Masliyah, J.H. Attachment of individual particles to a stationary air bubble in model systems. Int. J. Miner. Process. 2003, 68, 47–69. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Nandakumar, K.; Xu, Z.; Masliyah, J.H. Effect of surface mobility on the particle sliding along a bubble or a solid sphere. J. Colloid Interface Sci. 2003, 259, 81–88. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Evans, G.M. Movement of fine particles on an air bubble surface studied using high-speed video microscopy. J. Colloid Interface Sci. 2004, 273, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.V.; Evans, G.M. Attachment interaction between air bubbles and particles in froth flotation. Exp. Therm. Fluid Sci. 2004, 28, 381–385. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Evans, G.M.; Nalaskowski, J.; Miller, J.D. Hydrodynamic interaction between an air bubble and a particle: Atomic force microscopy measurements. Exp. Therm. Fluid Sci. 2004, 28, 387–394. [Google Scholar] [CrossRef]
- Zachery, I.E. Particle and Bubble Interaction in Flotation Systems. Ph.D. Thesis, Auburn University, Auburn, AL, USA, May 2007. [Google Scholar]
- Verrelli, D.I.; Koh PT, L.; Nguyen, A.V. Particle–bubble interaction and attachment in flotation. Chem. Eng. Sci. 2011, 66, 5910–5921. [Google Scholar] [CrossRef]
- Koh, P.T.; Hao, F.P.; Smith, L.K.; Chau, T.T.; Bruckard, W.J. The effect of particle shape and hydrophobicity in flotation. Int. J. Miner. Process. 2009, 93, 128–134. [Google Scholar] [CrossRef]
- Koh PT, L.; Smith, L.K. The effect of stirring speed and induction time on flotation. Miner. Eng. 2011, 24, 442–448. [Google Scholar]
- Qin, W.Q.; Wang, P.P.; Ren, L.Y.; Wei, Q.; Peng, Z.B.; Gu, Y.L. Effect of matching relationship between particles and bubbles on the flotation of fine cassiterite. J. China Univ. Min. Technol. 2012, 41, 420–424. [Google Scholar]
- Ren, L. Interaction between Fine Cassiterite Particles and Bubbles and Its Effects on Flotation. Ph.D. Thesis, Central South University, Changsha, China, May 2012. [Google Scholar]
- Meng, X. Study on Effect of Surfactants on Interactions between Ultrafine Diaspore Particles and Bubbles. Master’s Thesis, Central South University, Changsha, China, May 2011. [Google Scholar]
- Gui, X.; Xing, Y.; Rong, G.; Cao, Y.; Liu, J. Interaction forces between coal and kaolinite particles measured by atomic force microscopy. Powder Technol. 2016, 301, 349–355. [Google Scholar] [CrossRef]
- Cui, X.; Shi, C.; Zhang, S.; Xie, L.; Liu, J.; Jiang, D.; Zeng, H. Probing effect of salinity and pH on surface interactions between air bubbles and hydrophobic solids: Implications on colloidal assembly at air/water interface. Chemistry 2017, 12, 1568–1577. [Google Scholar] [CrossRef] [PubMed]
- Ali, V.; Jocelyn, B.; Yun, C. Effect of particles on bubble size distribution and gas hold-up in column flotation. Int. J. Miner. Process. 2016, 157, 163–173. [Google Scholar]
- Brozek, M.; Mlynarczykowska, A. Application of the stochastic model for analysis of flotation kinetics with coal as an example. Physicochem. Probl. Miner. Process. 2007, 41, 31–44. [Google Scholar]
- Watanabe, M.; Kowalczuk, P.B.; Drzymala, J. Analytical solution of equation relating maximum size of floating particle and its hydrophobicity. Physicochem. Probl. Min. Process. 2011, 46, 13–20. [Google Scholar]
- Kowalczuk, P.B.; Drzymala, J. Surface flotation of particles on liquids. Principles and applications. Colloids Surf. A Physicochem. Eng. Asp. 2012, 393, 81–85. [Google Scholar] [CrossRef]
- Shen, D.; Xiao, J.; Chen, X. Comparison of Height Method and Pressure Method for Measurement of Powder Contact Angle. Sulphur Phosphorus Bulk Mater. Handl. Relat. Eng. 2007, 1, 9–12. [Google Scholar]
- Zou, W.; Cao, Y.; Liu, J. Surface thermodynamic characterization of fine coal by Washburn dynamic method. J. China Coal Soc. 2013, 7, 1271–1276. [Google Scholar]
- Siebold, A.; Walliser, A.; Nardin, M.; Oppliger, M.; Schultz, J. Capillary rise for thermodynamic characterization of solid particle surface. J. Colloid Interface Sci. 1997, 186, 60–70. [Google Scholar] [CrossRef]
- Washburn, E.W. The dynamics of capillary flow. Phys. Rev. 1921, 17, 273–283. [Google Scholar] [CrossRef]
- Tristan, P.; Ljiljana, P. FTIR spectroscopy of nanodiamonds: Methods and interpretation. Diam. Relat. Mater. 2018, 89, 52–66. [Google Scholar]
- Prestidge, C.A.; Ralston, J. Contact angle studies of particulate sulphide minerals. Miner. Eng. 1996, 9, 85–102. [Google Scholar] [CrossRef]
- Fronzi, M.; Assadi, M.; Hanaor, D. Theoretical insights into the hydrophobicity of low index CeO2 surfaces. Appl. Surf. Sci. 2019, 478, 68–74. [Google Scholar] [CrossRef]
- Zhang, F.; Xu, Y.; Yan, X.; Cao, Y.; Liu, G. Research of bubbles mineralization based on high-speed dynamic microscopic Test. J. China Univ. Min. Technol. 2016, 45, 1–7. [Google Scholar]
- Dai, Z.; Fornasiero, D.; Ralston, J. Particle-bubble collision models—A review. Adv. Colloid Interface Sci. 2000, 85, 231–256. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Pan, L.; Pinchasik, B.E.; Cao, Y.; Liu, J.; Kappl, M.; Butt, H.J. Recent experimental advances for understanding bubble-particle attachment in flotation. Adv. Colloid Interface Sci. 2017, 246, 105–132. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Ralston, J.; Schulze, H.J. On modelling of bubble–particle attachment probability in flotation. Int. J. Miner. Process. 1998, 53, 225–249. [Google Scholar] [CrossRef]
- Goel, S.; Jameson, G.J. Detachment of particles from bubbles in an agitated vessel. Miner. Eng. 2012, 36–38, 324–330. [Google Scholar] [CrossRef]
- Taran, E.; Nguyen, A.V. Surface deformation during bubble-particle colloidal interaction quantified by atomic force microscopy and numerical modeling. In Proceedings of the International Symposium on Separation Technologies for Minerals, Coal, and Earth Resources, Part of the 2011 SME Annual Meeting, Denver, CO, USA, 27 February–2 March 2012; pp. 19–26. [Google Scholar]
- Wang, G.; Evans, G.M.; Jameson, G.J. Bubble–particle detachment in a turbulent vortex I: Experimental. Miner. Eng. 2016, 92, 196–207. [Google Scholar] [CrossRef]
- Wang, G.; Evans, G.M.; Jameson, G.J. Experiments on the detachment of particles from bubbles in a turbulent vortex. Powder Technol. 2016, 302, 196–206. [Google Scholar] [CrossRef]
- Xing, Y.; Gui, X.; Cao, Y. Effect of bubble size on bubble-particle attachment and film drainage kinetics—A theoretical study. Powder Technol. 2017, 322, 140–146. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, Z.; Nandakumar, K.; Masliyah, J.H.; Xu, Z. An induction time model for the attachment of an air bubble to a hydrophobic sphere in aqueous solutions. Int. J. Miner. Process. 2005, 75, 69–82. [Google Scholar] [CrossRef]






| Content | SiO2 | Na2O | CaO | MgO | Al2O3 | K2O | Fe2O3 | SO3 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| % | 71.86 | 13.76 | 8.43 | 3.91 | 1.18 | 0.48 | 0.22 | 0.1 | 0.03 |
| Size Fraction, μm | Specific Surface Area, m2/g |
|---|---|
| −250 + 125 | 0.0699 |
| −125 + 74 | 0.1093 |
| −74 + 38 | 0.1890 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, F.; Cao, Y.; Yan, X.; Wang, L.; Xu, Y. A Study of Bubble Mineralization by Modified Glass Microspheres Based on a High-Speed Dynamic Microscopic Test System. Minerals 2019, 9, 350. https://doi.org/10.3390/min9060350
Zhang F, Cao Y, Yan X, Wang L, Xu Y. A Study of Bubble Mineralization by Modified Glass Microspheres Based on a High-Speed Dynamic Microscopic Test System. Minerals. 2019; 9(6):350. https://doi.org/10.3390/min9060350
Chicago/Turabian StyleZhang, Fanfan, Yijun Cao, Xiaokang Yan, Lijun Wang, and Yajun Xu. 2019. "A Study of Bubble Mineralization by Modified Glass Microspheres Based on a High-Speed Dynamic Microscopic Test System" Minerals 9, no. 6: 350. https://doi.org/10.3390/min9060350
APA StyleZhang, F., Cao, Y., Yan, X., Wang, L., & Xu, Y. (2019). A Study of Bubble Mineralization by Modified Glass Microspheres Based on a High-Speed Dynamic Microscopic Test System. Minerals, 9(6), 350. https://doi.org/10.3390/min9060350




