Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles
Abstract
:1. Introduction
2. Materials and methods
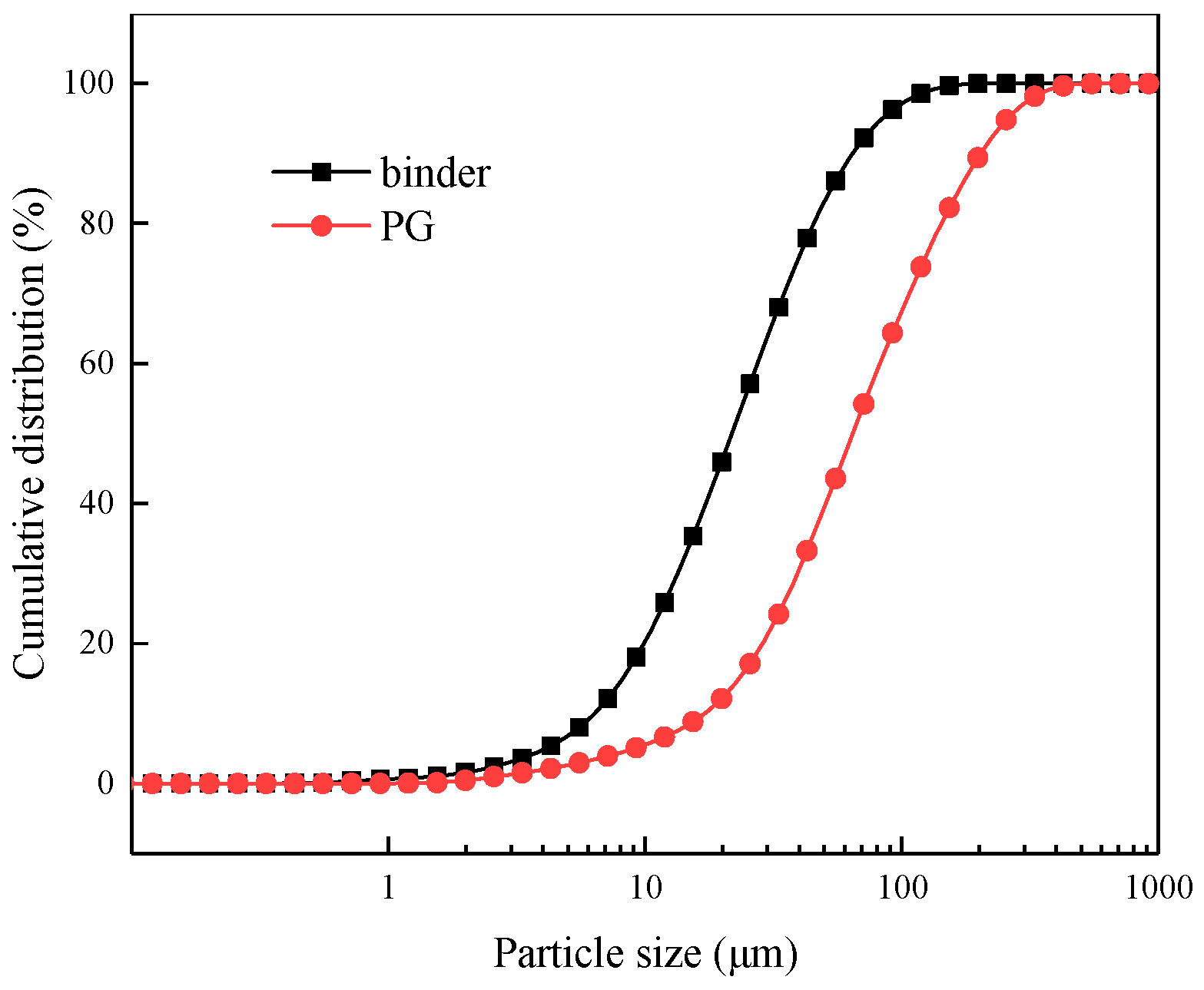
2.1. Raw Material and the Preparation of Backfill Specimens
2.2. Drying-Wetting Test
2.3. UCS Tests
2.4. Chemical Analysis
2.5. Porosity Measurement
2.6. Microstructural Studies
3. Results and Discussion
3.1. Properties of Backfill Exposed to Chemical Solutions
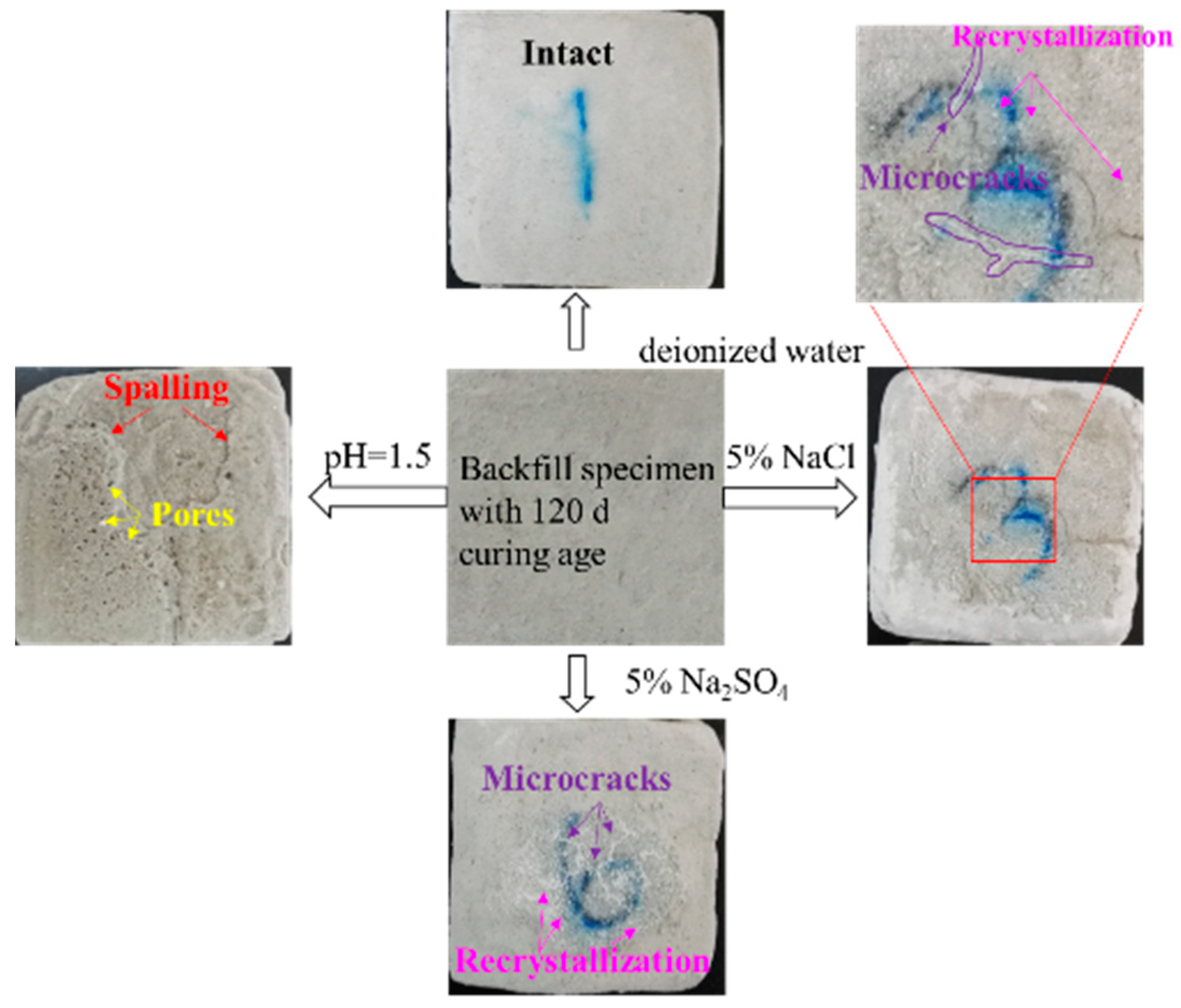
3.1.1. Visual Assessment
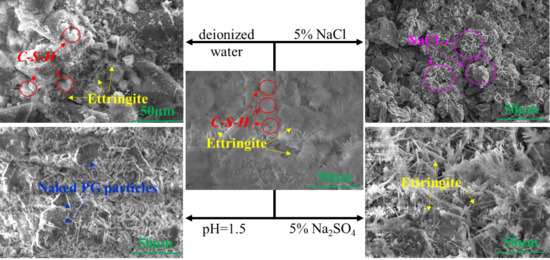
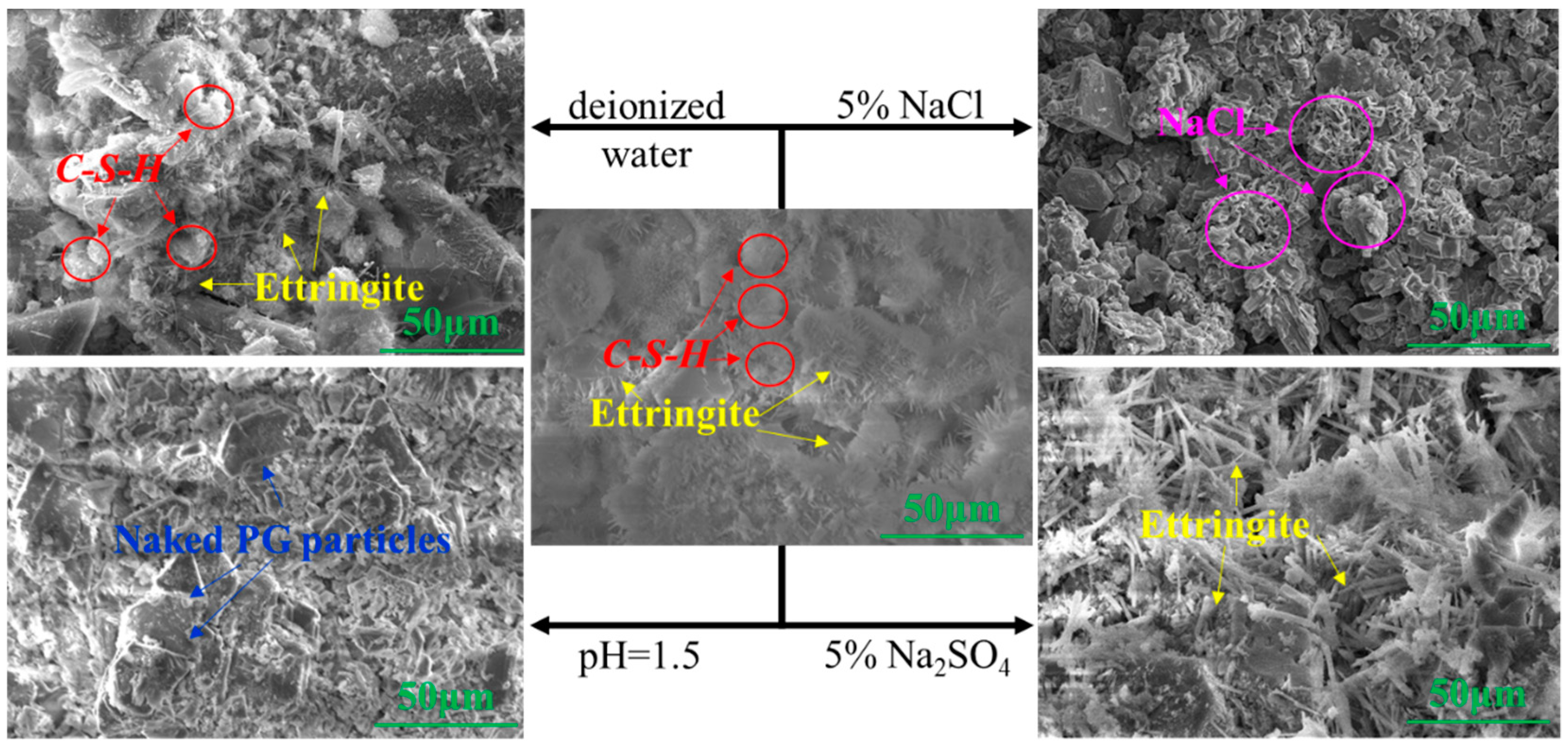
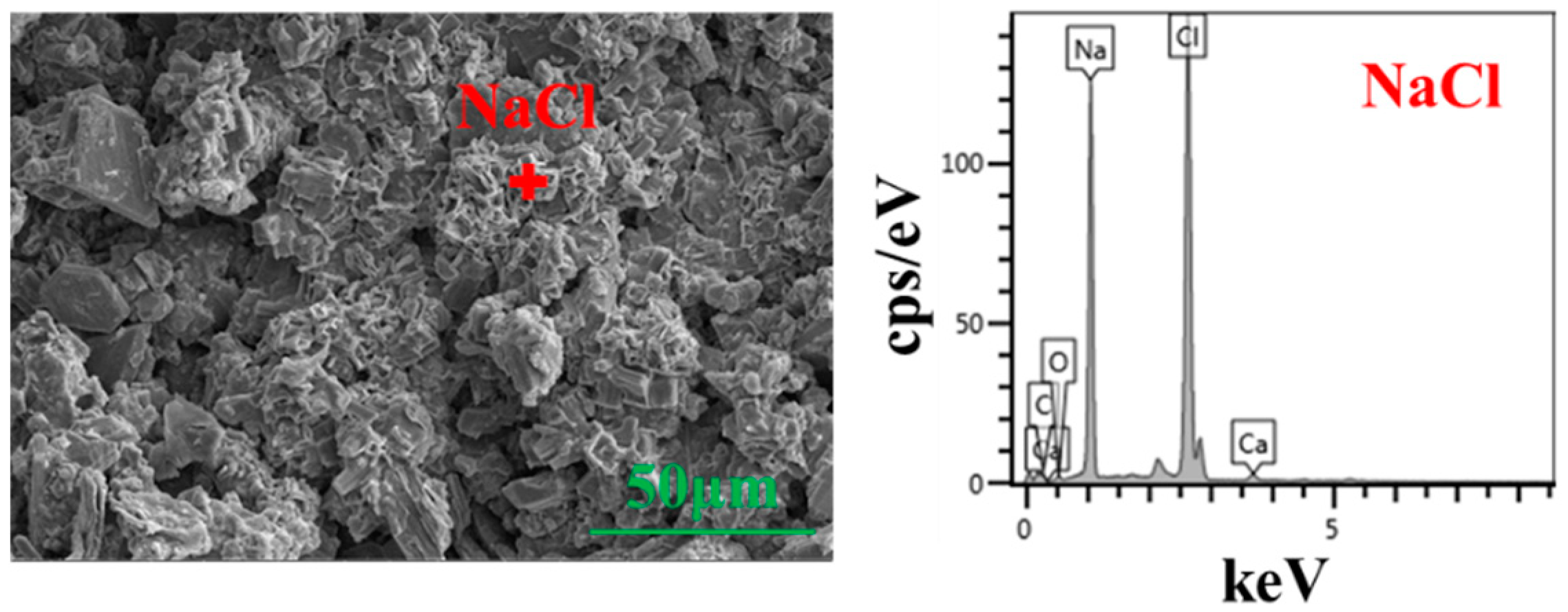
3.1.2. Microstructure
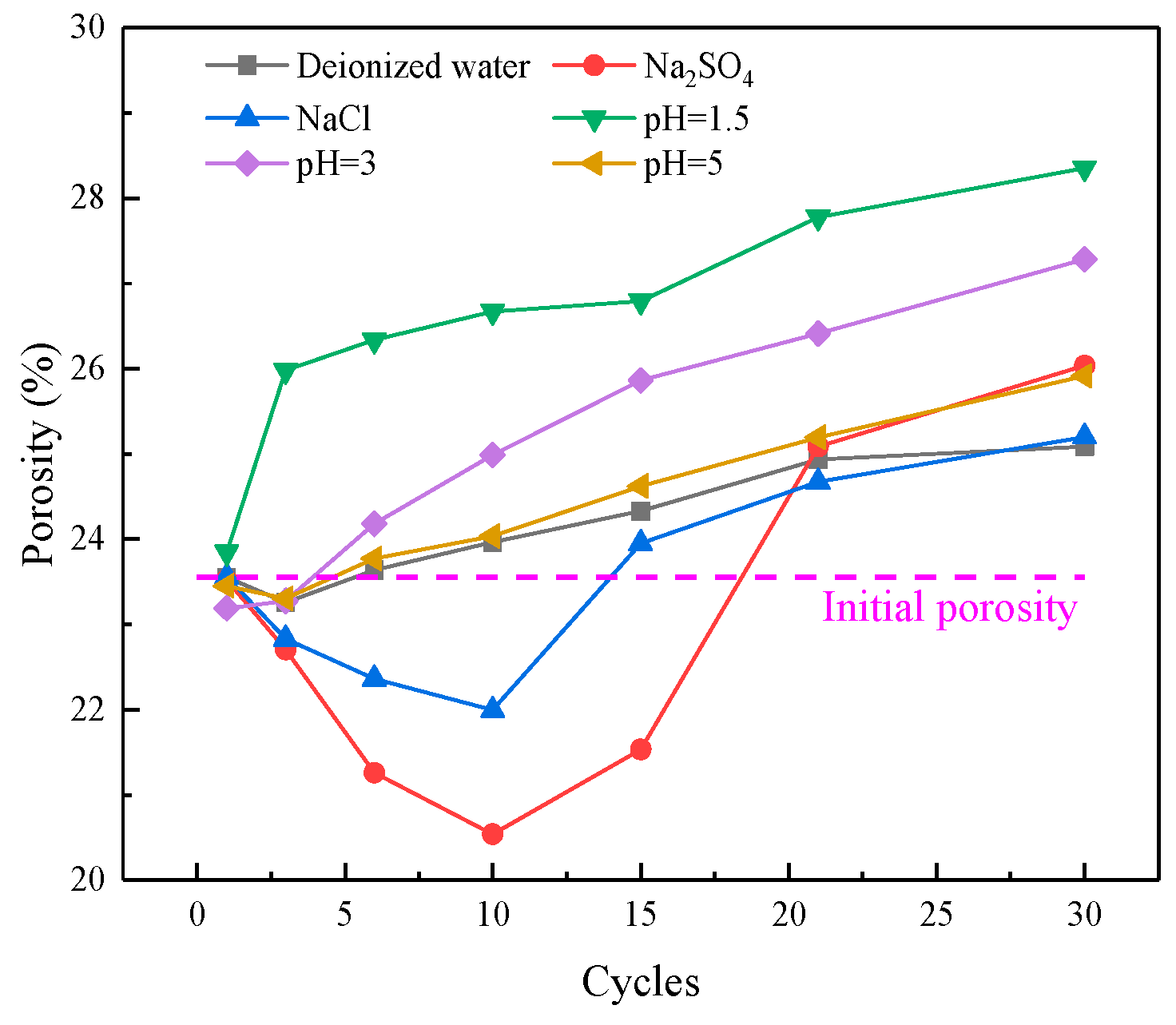
3.1.3. Pore Structure
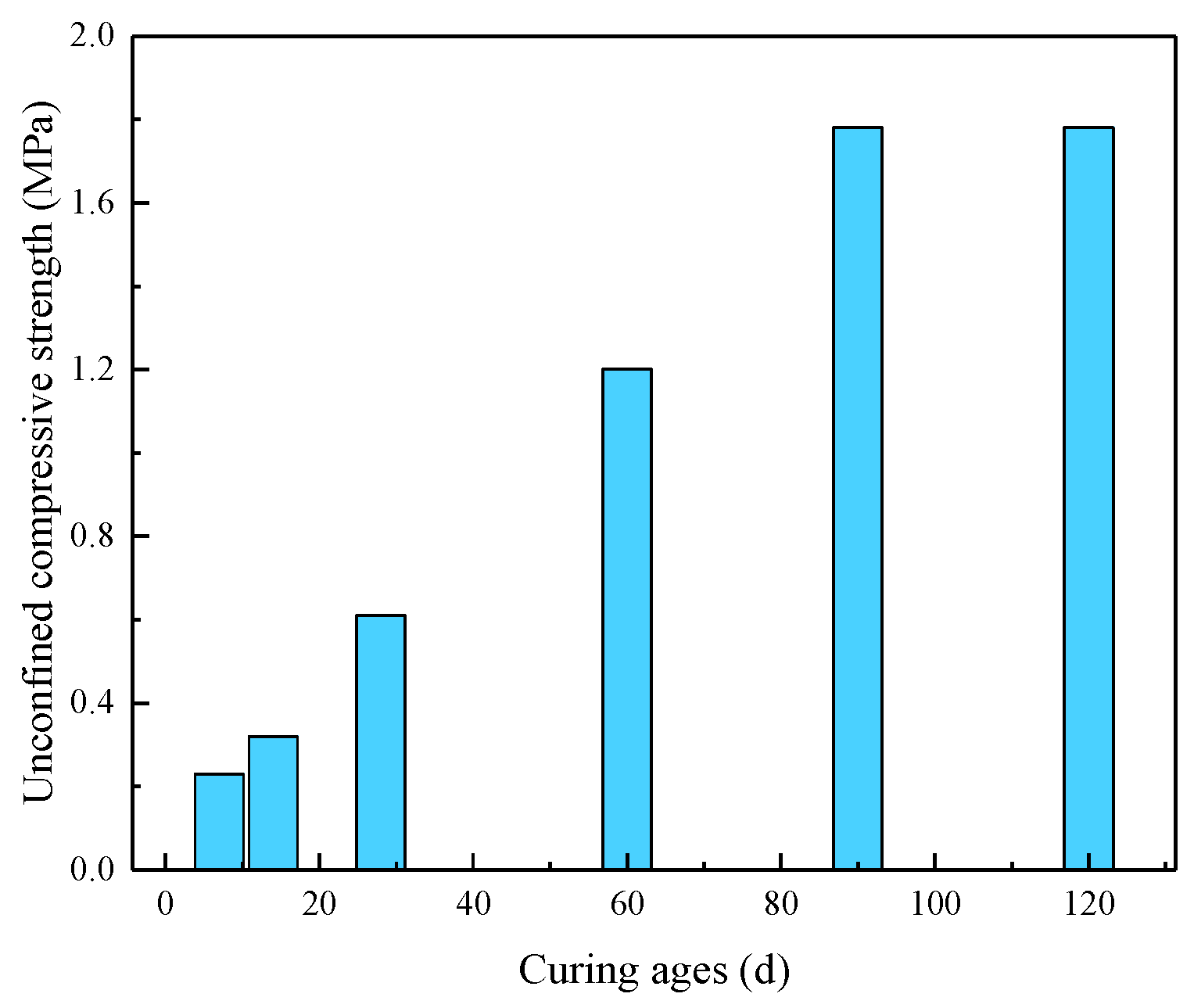
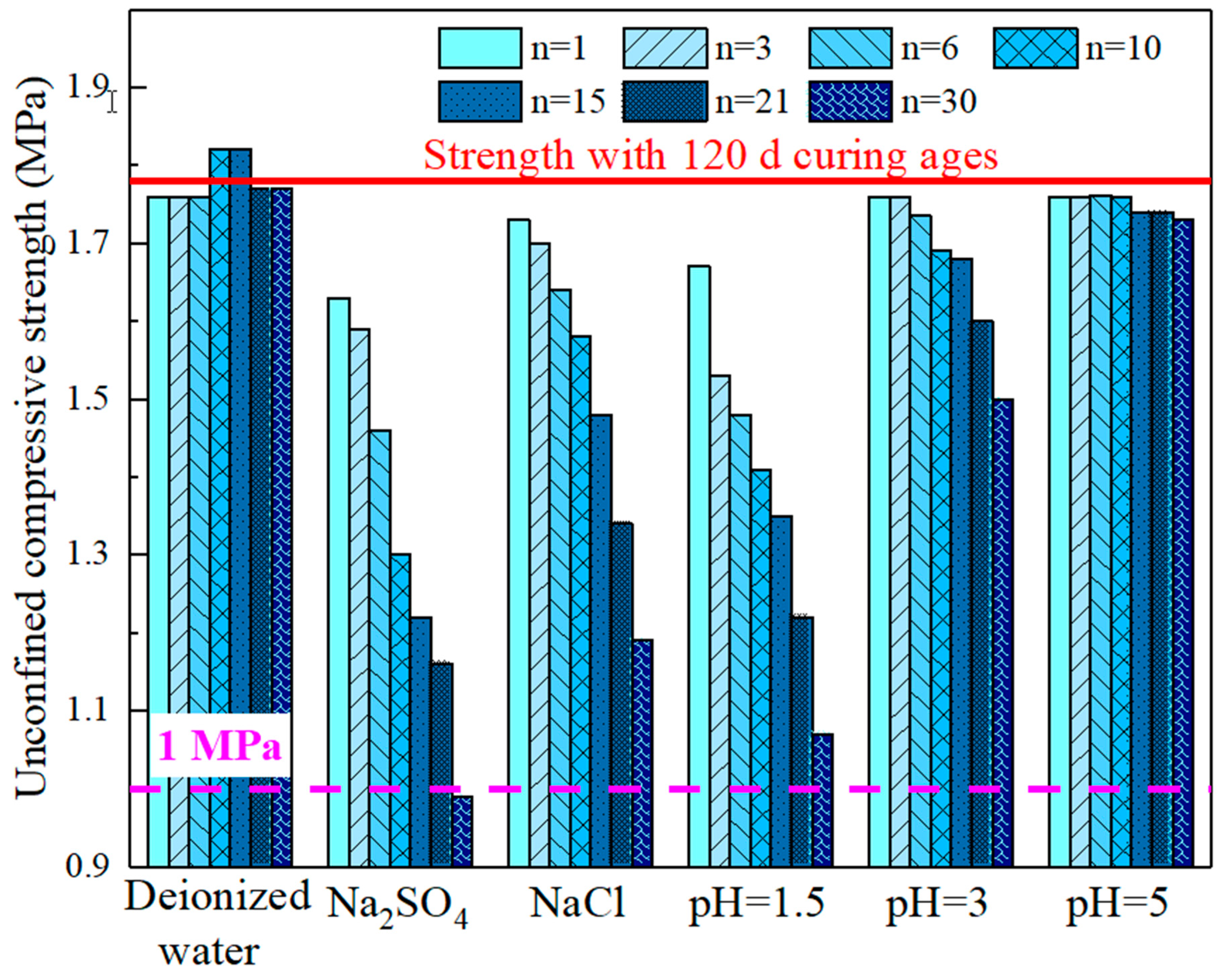
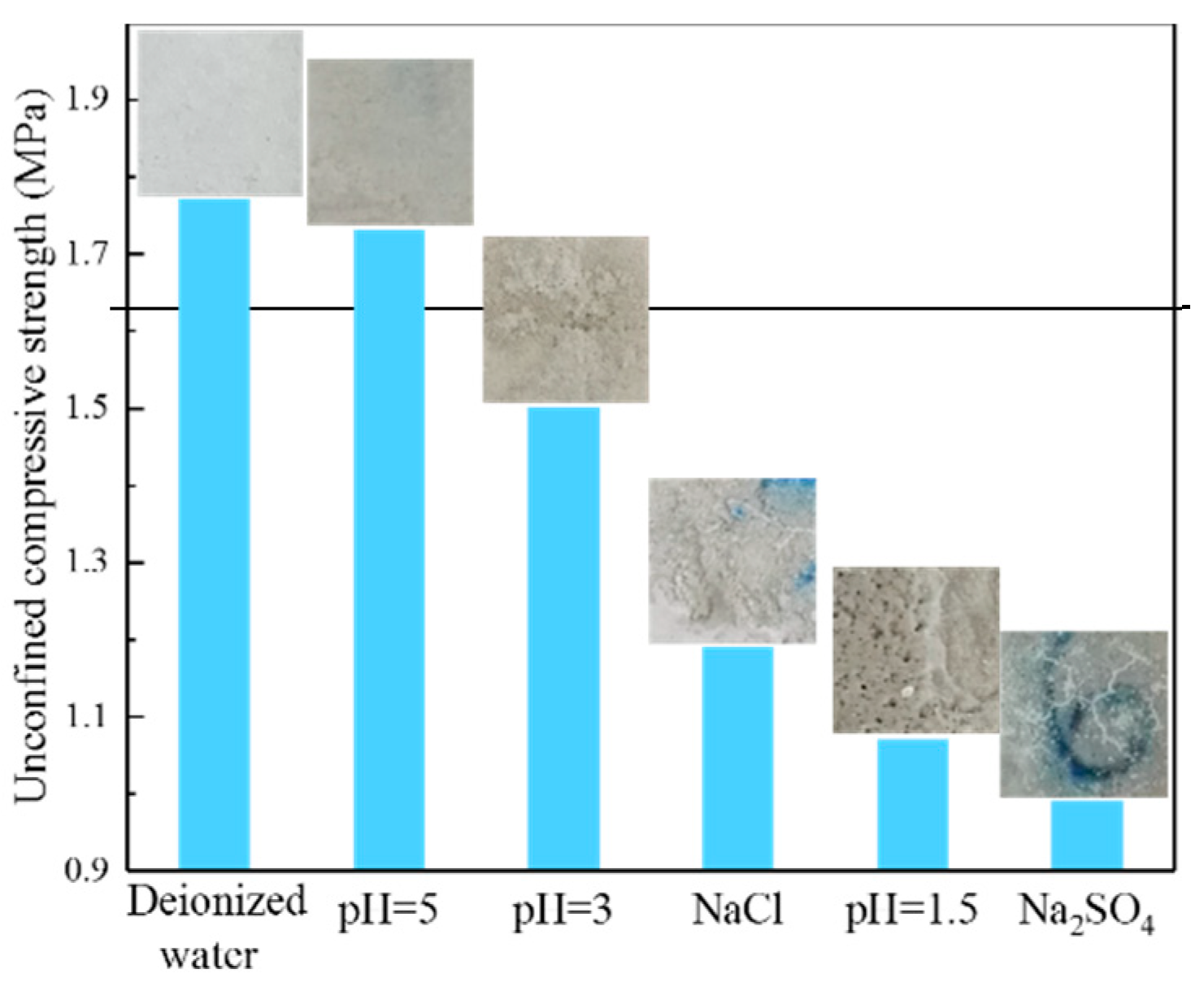
3.1.4. Strength Evolution
3.2. Impurity Dynamics Through Drying-Wetting Cycles
3.2.1. Fluctuation of pH Values
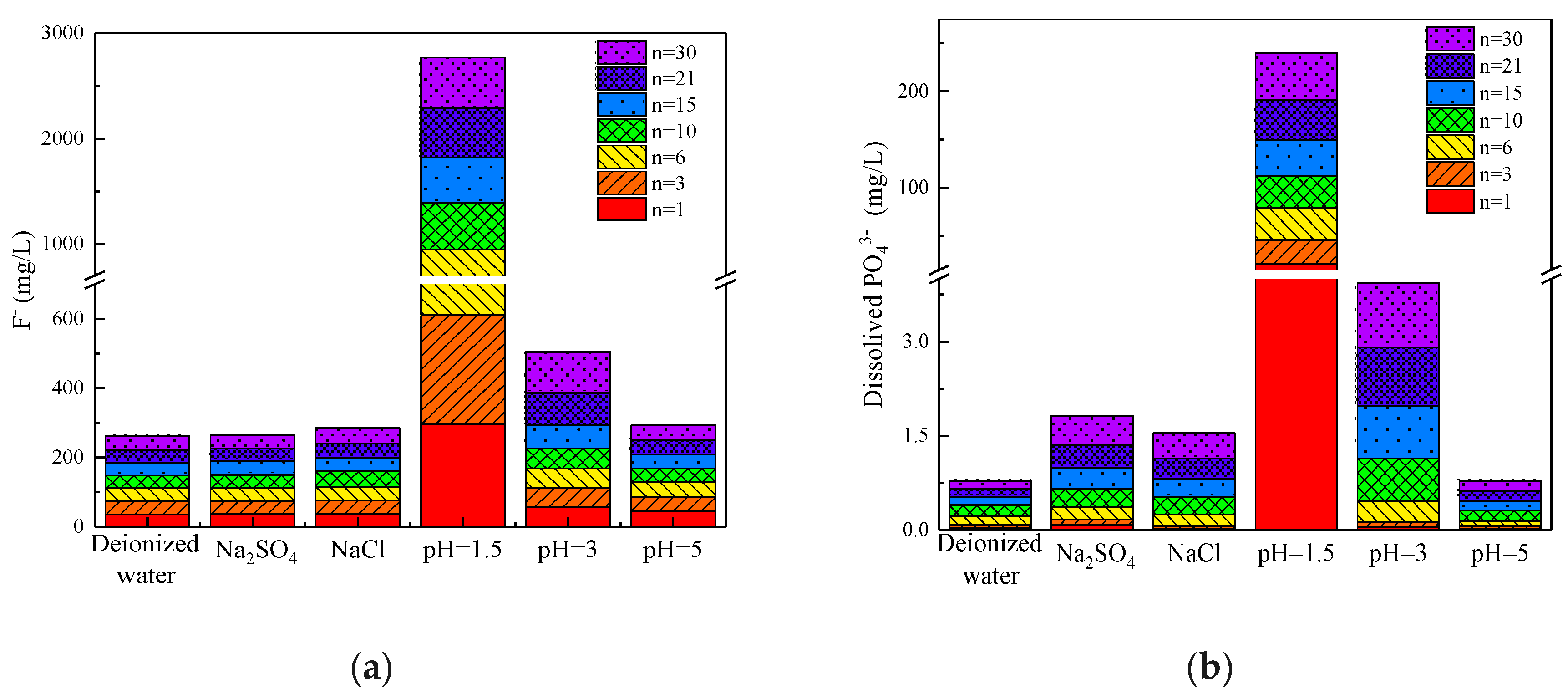
3.2.2. Dynamics of Phosphate and Fluoride
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Hou, Y.B.; Deng, T.F.; Chen, Y.Z.; Zhao, X.L. Thermal, hydraulic and mechanical performances of cemented coal gangue-fly ash backfill. Int. J. Miner. Process. 2017, 162, 12–18. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Fall, M.; Liang, C. Yield stress of cemented paste backfill in sub-zero environments: experimental results. Miner. Eng. 2016, 92, 141–150. [Google Scholar]
- Kesimal, A.; Yilmaz, E.; Ercikdi, B.; Alp, I.; Deveci, H. Effect of properties of tailings and binder on the short-and long-term strength and stability of cemented paste backfill. Mater. Lett. 2005, 59, 3703–3709. [Google Scholar] [CrossRef]
- Wang, S.F.; Li, X.B.; Wang, S.Y. Three-dimensional mineral grade distribution modelling and longwall mining of an underground bauxite seam. Int. J. Rock. Mech. Min. 2018, 103, 123–136. [Google Scholar] [CrossRef]
- Guo, G.L.; Zhu, X.J.; Zha, J.F.; Wang, Q. Subsidence prediction method based on equivalent mining height theory for solid backfilling mining. T. Nonferr. Metal. Soc. 2014, 24, 3302–3308. [Google Scholar] [CrossRef]
- Ma, D.; Duan, H.Y.; Liu, J.F.; Li, X.B.; Zhou, Z.L. The role of gangue on the mitigation of mining-induced hazards and environmental pollution: An experimental investigation. Sci. Total Environ. 2019, 664, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Li, X.B.; Du, J.; Gao, L.; He, S.Y.; Gan, L.; Sun, C.; Shi, Y. Immobilization of phosphogypsum for cemented paste backfill and its environmental effect. J. Clean. Prod. 2017, 156, 137–146. [Google Scholar] [CrossRef]
- Chen, Q.S.; Zhang, Q.L.; Qi, C.C.; Fourie, A.; Xiao, C.C. Recycling phosphogypsum and construction demolition waste for cemented paste backfill and its environmental impact. J. Clean. Prod. 2018, 186, 418–429. [Google Scholar] [CrossRef]
- Ercikdi, B.; Cihangir, F.; Kesimal, A.; Deveci, H.; İbrahim, A. Utilization of water-reducing admixtures in cemented paste backfill of sulphide-rich mill tailings. J. Hazard Mater. 2010, 179, 940–946. [Google Scholar] [CrossRef]
- Jiang, H.Q.; Fall, M. Yield stress and strength of saline cemented tailings in sub-zero environments: portland cement paste backfill. Int. J. Miner. Process. 2017, 160, 68–75. [Google Scholar] [CrossRef]
- Helson, O.; Eslami, J.; Beaucour, A.L.; Noumowe, A.; Gotteland, P. Durability of soil mix material subjected to wetting/drying cycles and external sulfate attacks. Constr. Build. Mater. 2018, 192, 416–428. [Google Scholar] [CrossRef]
- Wojciech, P. Analysis of carbonate and sulphate attack on concrete structures. Eng. Fail. Anal. 2017, 79, 606–614. [Google Scholar]
- Kunther, W.; Lothenbach, B.; Scrivener, K.L. On the relevance of volume increase for the length changes of mortar bars in sulfate solutions. Cement Concrete Res. 2013, 46, 23–29. [Google Scholar] [CrossRef]
- Fall, M.; Pokharel, M. Coupled effects of sulphate and temperature on the strength development of cemented tailings backfills: Portland cement-paste backfill. Cement Concrete Res. 2010, 32, 819–828. [Google Scholar] [CrossRef]
- Cihangir, F.; Ercikdi, B.; Kesimal, A.; Ocak, S.; Akyol, Y. Effect of sodium-silicate activated slag at different silicate modulus on the strength and microstructural properties of full and coarse sulphidic tailings paste backfill. Constr. Build. Mater. 2018, 185, 555–566. [Google Scholar] [CrossRef]
- Zhang, J.R.; Sun, M.; Hou, D.S.; Li, Z.J. External sulfate attack to reinforced concrete under drying-wetting cycles and loading condition: Numerical simulation and experimental validation by ultrasonic array method. Constr. Build. Mater. 2017, 139, 365–373. [Google Scholar] [CrossRef]
- Cihangir, F.; Akyol, Y. Mechanical, hydrological and microstructural assessment of the durability of cemented paste backfill containing alkali-activated slag. Int. J. Min. Reclam. Env. 2016, 185, 1–21. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, P.Y.; Yang, J.W.; Zhang, B. Influence of steel slag on mechanical properties and durability of concrete. Constr. Build. Mater. 2013, 47, 1414–1420. [Google Scholar] [CrossRef]
- Liu, J.J.; Zha, F.S.; Xu, L.; Yang, C.B.; Chu, C.F.; Tan, X.H. Effect of chloride attack on strength and leaching properties of solidified/stabilized heavy metal contaminated soils. Eng. Geol. 2018, 246, 28–35. [Google Scholar] [CrossRef]
- Ragoug, R.; Metalssi, O.O.; Barberon, F.; Torrenti, J.M.; Roussel, N.; Divet, L.; Jean-Baptiste, E.L. Durability of cement pastes exposed to external sulfate attack and leaching: Physical and chemical aspects. Cement Concrete Res. 2019, 116, 134–145. [Google Scholar] [CrossRef]
- Chen, M.C.; Wang, K.; Xie, L. Deterioration mechanism of cementitious materials under acid rain attack. Title of the article. Eng. Fail. Anal. 2013, 27, 272–285. [Google Scholar] [CrossRef]
- Shi, Y.; Gan, L.; Li, X.B.; He, S.Y.; Sun, C.; Gao, L. Dynamics of metals in backfill of a phosphate mine of guiyang, China using a three-step sequential extraction technique. Chemosphere 2018, 192, 354–361. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Tyrer, M.; Hills, C.D.; Yang, X.M.; Carey, P. Immobilisation of heavy metal in cement-based solidification/stabilisation: a review. Waste Manag. 2009, 29, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Wu, H.L. Effect of carbonation on leachability, strength and microstructural characteristics of KMP binder stabilized Zn and Pb contaminated soils. Chemosphere 2015, 144, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.J.; Wei, M.L.; Reddy, K.R.; Liu, Z.P.; Jin, F. Effect of acid rain ph on leaching behavior of cement stabilized lead-contaminated soil. J. Hazard. Mater. 2014, 271, 131–140. [Google Scholar] [CrossRef]
- Qu, Q.L.; Deng, Z.L.; Yang, Y.B.; Li, Z.G.; Yang, B.L. A Manufacturing Method for Producing Composite Phosphorus Slag Powder. China Patent ZL2012102079216, 21 June 2012. (In Chinese). [Google Scholar]
- Li, J.S.; Xue, Q.; Wang, P.; Li, Z.Z.; Liu, L. Effect of drying-wetting cycles on leaching behavior of cement solidified lead-contaminated soil. Chemosphere 2014, 117, 10–13. [Google Scholar] [CrossRef]
- Li, K.G.; Zheng, D.P.; Huang, W.H. Mechanical behavior of sandstone and its neural network simulation of constitutive model considering cyclic drying-wetting effect. Rock. Soil. Mech. 2013, 34, 168–173. [Google Scholar]
- Abdelhadi, K.; Latifa, O.; Khadija, B.; Lahcen, B. Valorization of mining waste and tailings through paste backfilling solution, Imiter operation, Morocco. Int. J. Min. Sci. Tech. 2016, 26, 511–516. [Google Scholar]
- Zhou, K.P.; Liu, T.Y.; Hu, Z.X. Exploration of damage evolution in marble due to lateral unloading using nuclear magnetic resonance. Eng. Geol. 2018, 244, 75–85. [Google Scholar] [CrossRef]
- Nehdi, M.L.; Suleiman, A.R.; Soliman, A.M. Investigation of concrete exposed to dual sulfate attack. Cement Concrete Res. 2014, 64, 42–53. [Google Scholar] [CrossRef]
- Collepardi, M. A state-of-the-art review on delayed ettringite attack on concrete. Cement Concrete Res. 2003, 25, 401–407. [Google Scholar] [CrossRef]
- Tixier, R.; Mobasher, B. Modeling of damage in cement-based materials subjected to external sulfate attack. I: Formulation. J. Mater. Civil Eng. 2003, 15, 305–313. [Google Scholar] [CrossRef]
- Andrés, E.I.; Carlos, M.L.; Ignacio, C. Chemo-mechanical analysis of concrete cracking and degradation due to external sulfate attack: A meso-scale model. Cement Concrete Comp. 2011, 33, 411–423. [Google Scholar]
- Benzaazoua, M.; Belem, T.; Bussière, B. Chemical factors that influence the performance of mine sulphidic paste backfill. Cement Concrete Res. 2002, 32, 1133–1144. [Google Scholar] [CrossRef]
- Cefis, N.; Comi, C. Chemo-mechanical modelling of the external sulfate attack in concrete. Cement Concrete Res. 2017, 93, 57–70. [Google Scholar] [CrossRef]
- Hadigheh, S.A.; Gravina, R.J.; Smith, S.T. Effect of acid attack on FRP-to-concrete bonded interfaces. Constr. Build. Mater. 2017, 152, 285–303. [Google Scholar] [CrossRef]
- Andre, B.; Moien, R.; Tilo, P.; Cyrill, G.; Florian, S.; Marlene, S.; Claudia, B.; Isabel, G.; Florian, E.; Florian, M. Effect of very high limestone content and quality on the sulfate resistance of blended cements. Constr. Build. Mater. 2018, 188, 1065–1076. [Google Scholar]
- Wang, Y.; Yuan, Q.; Deng, D.; Ye, T.; Fang, L. Measuring the pore structure of cement asphalt mortar by nuclear magnetic resonance. Constr. Build. Mater. 2017, 137, 450–458. [Google Scholar] [CrossRef]
- Zhang, J.; Deng, H.W.; Taheri, A.; Deng, J.R.; Ke, B. Effects of Superplasticizer on the Hydration, Consistency, and Strength Development of Cemented Paste Backfill. Minerals 2018, 8, 381. [Google Scholar] [CrossRef]
- Min, C.D.; Li, X.B.; He, S.Y.; Zhou, S.T.; Zhou, Y.N.; Yang, S.; Shi, Y. Effect of mixing time on the properties of phosphogypsum-based cemented backfill. Constr. Build. Mater. 2019, 210, 564–573. [Google Scholar] [CrossRef]
- Fall, M.; Benzaazoua, M. Modeling the effect of sulphate on strength development of paste backfill and binder mixture optimization. Cement Concrete Res. 2005, 35, 301–314. [Google Scholar] [CrossRef]
- Wu, J.Y.; Feng, M.M.; Chen, Z.Q.; Mao, X.B.; Han, G.S.; Wang, Y.M. Particle Size Distribution Effects on the Strength Characteristic of Cemented Paste Backfill. Minerals 2018, 8, 322. [Google Scholar] [CrossRef]
- Daisuke, S. Chemical alteration of calcium silicate hydrate (C–S–H) in sodium chloride solution. Cement Concrete Res. 2008, 38, 1270–1275. [Google Scholar]
- Zhou, X.S.; Lin, X.; Huo, M.J.; Zhang, Y. The hydration of saline oil-well cement. Cement Concrete Res. 1996, 26, 1753–1759. [Google Scholar] [CrossRef]
- Komljenović, M.M.; Baščarević, Z.; Marjanović, N.; Nikolić, V. Decalcification resistance of alkali-activated slag. J. Hazard Mater. 2012, 233–234, 112–121. [Google Scholar] [CrossRef]
- Najjar, M.F.; Nehdi, M.L.; Soliman, A.M.; Azabi, T.M. Damage mechanisms of two-stage concrete exposed to chemical and physical sulfate attack. Constr. Build. Mater. 2017, 137, 141–152. [Google Scholar] [CrossRef]
- Tang, Z.; Li, W.G.; Ke, G.J.; Zhou, J.L.; Tam, V.W.Y. Sulfate attack resistance of sustainable concrete incorporating various industrial solid wastes. J. Clean. Prod. 2019, 218, 810–822. [Google Scholar] [CrossRef]
- Fan, Y.F.; Hu, Z.Q.; Zhang, Y.Z.; Liu, J.L. Deterioration of compressive property of concrete under simulated acid rain environment. Constr. Build. Mater. 2010, 24, 1975–1983. [Google Scholar] [CrossRef]
- Brackebusch, F.W. Basics of paste backfill systems. Miner. Eng. 1994, 46, 1175–1178. [Google Scholar]
- Zhao, Y.; Soltani, A.; Taheri, A.; Karakus, M.; Deng, A. Application of Slag–Cement and Fly Ash for Strength Development in Cemented Paste Backfills. Minerals 2019, 9, 22. [Google Scholar] [CrossRef]
- Geng, J.; Easterbrook, D.; Li, L.Y.; Mo, L.W. The stability of bound chlorides in cement paste with sulfate attack. Cement Concrete Res. 2015, 68, 211–222. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Amin, Y.; Ibrahim, S.; Al-Bich, F. Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 2004, 19, 747–753. [Google Scholar] [CrossRef]
- Li, X.B.; Zhou, Y.N.; Zhu, Q.Q.; Zhou, S.T.; Min, C.D.; Shi, Y. Slurry Preparation Effects on the Cemented Phosphogypsum Backfill through an Orthogonal Experiment. Minerals 2019, 9, 31. [Google Scholar] [CrossRef]

| Chemical Composition | PG | Binder |
|---|---|---|
| % | % | |
| SiO2 | 1.7 | 23.4 |
| Fe2O3 | 0.3 | 2.6 |
| CaO | 35.9 | 54.4 |
| MgO | 0.1 | 1.7 |
| SO3 | 50.8 | 5.5 |
| P2O5 | 2.6 | 1.7 |
| Na2O | 0.1 | 1.3 |
| K2O | 0.1 | 0.9 |
| TiO2 | 0.1 | 0.5 |
| Physical Characteristic | ||
| D10 (µm) | 17.51 | 6.31 |
| D30 (µm) | 42.79 | 13.56 |
| D60 (µm) | 92.05 | 29.18 |
| Cu = D60/D10 | 5.26 | |
| Cc = D302/(D60 × D10) | 1.14 | |
| Specific gravity | 2.35 | 3.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhou, S.; Zhou, Y.; Min, C.; Cao, Z.; Du, J.; Luo, L.; Shi, Y. Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles. Minerals 2019, 9, 321. https://doi.org/10.3390/min9050321
Li X, Zhou S, Zhou Y, Min C, Cao Z, Du J, Luo L, Shi Y. Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles. Minerals. 2019; 9(5):321. https://doi.org/10.3390/min9050321
Chicago/Turabian StyleLi, Xibing, Shitong Zhou, Yanan Zhou, Chendi Min, Zhiwei Cao, Jing Du, Lin Luo, and Ying Shi. 2019. "Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles" Minerals 9, no. 5: 321. https://doi.org/10.3390/min9050321
APA StyleLi, X., Zhou, S., Zhou, Y., Min, C., Cao, Z., Du, J., Luo, L., & Shi, Y. (2019). Durability Evaluation of Phosphogypsum-Based Cemented Backfill Through Drying-Wetting Cycles. Minerals, 9(5), 321. https://doi.org/10.3390/min9050321