The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia
Abstract
1. Introduction
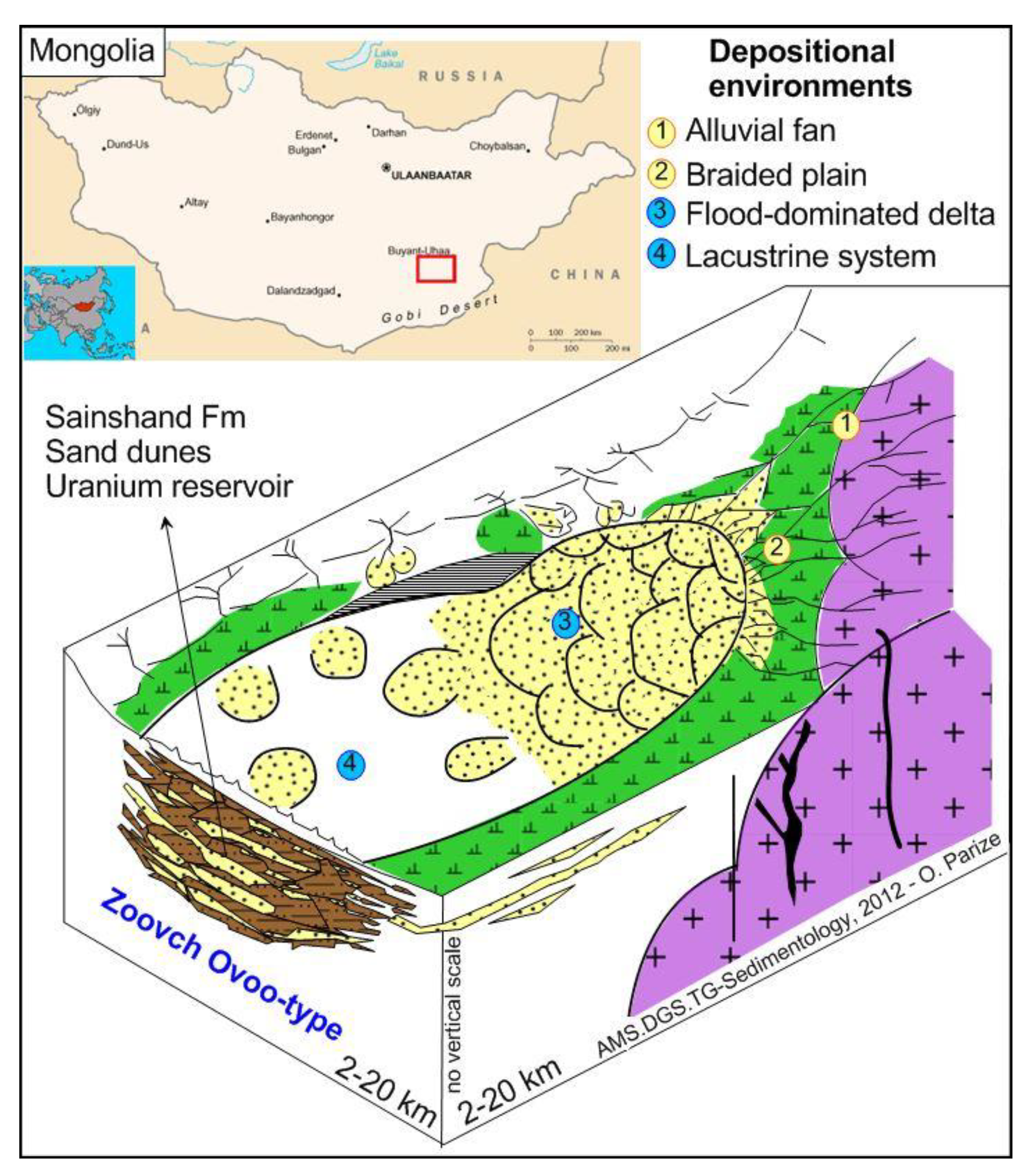
2. Geological Setting
3. Materials and Methods
3.1. Petrography
3.1.1. Optical Microscopy
3.1.2. Scanning Electron Microscopy
3.1.3. Organic Petrography
3.1.4. In Situ Analysis
3.1.5. Micro X-Ray Fluorescence Mapping
4. Results
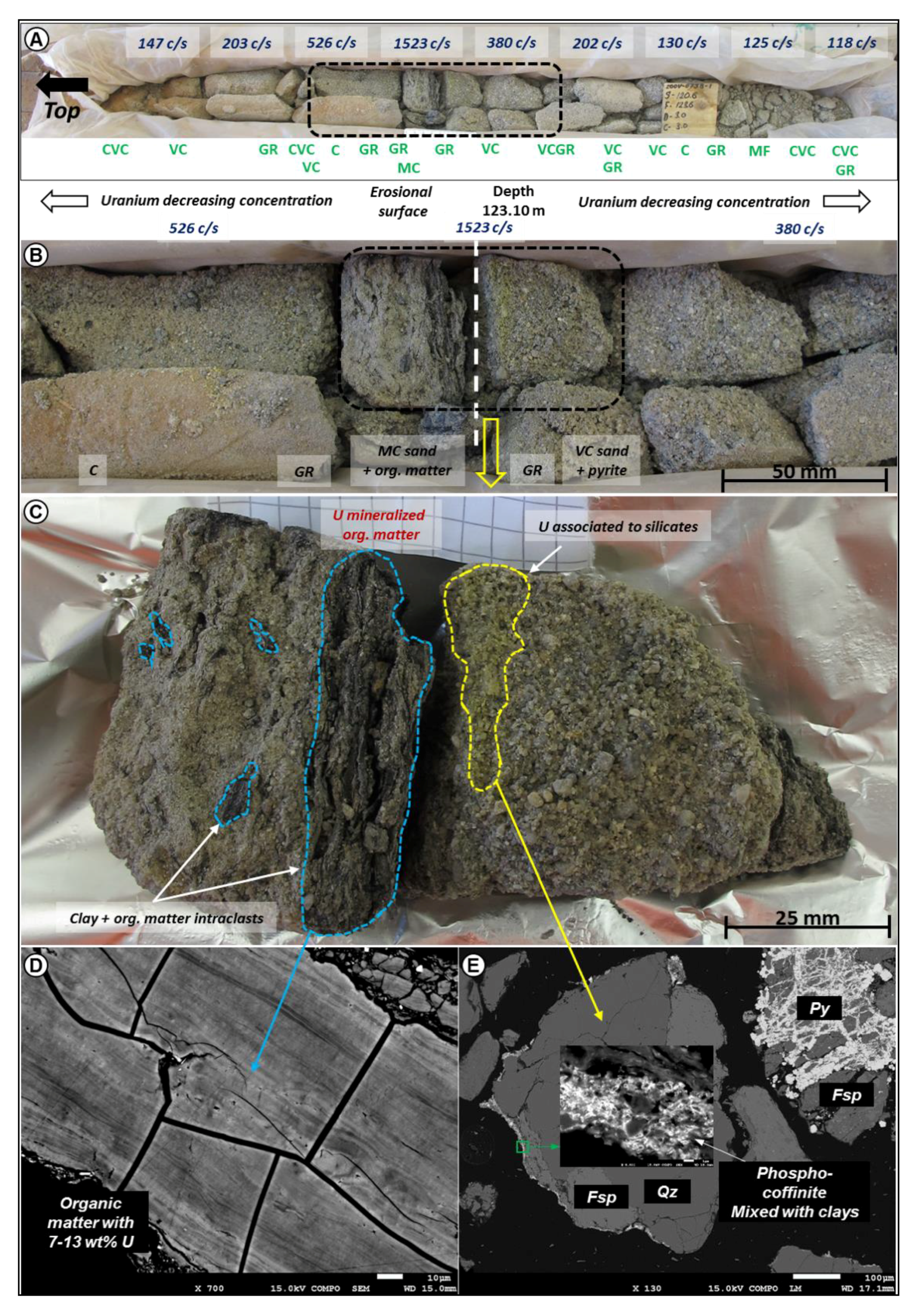
4.1. Organic Matter Sedimentology
4.2. Organic Petrography
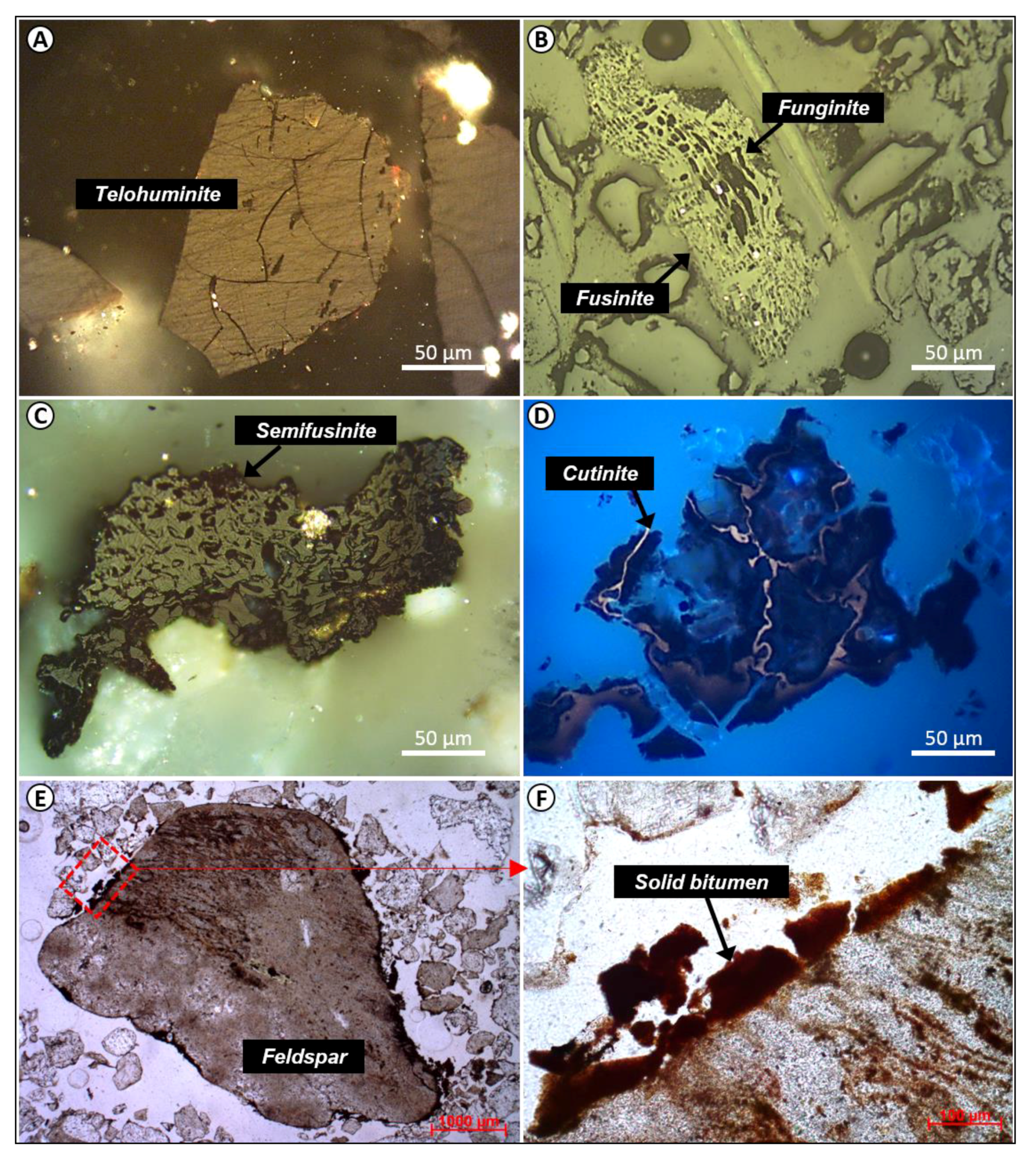
4.2.1. Huminite Macerals
4.2.2. Inertinite Macerals
4.2.3. Liptinite Macerals
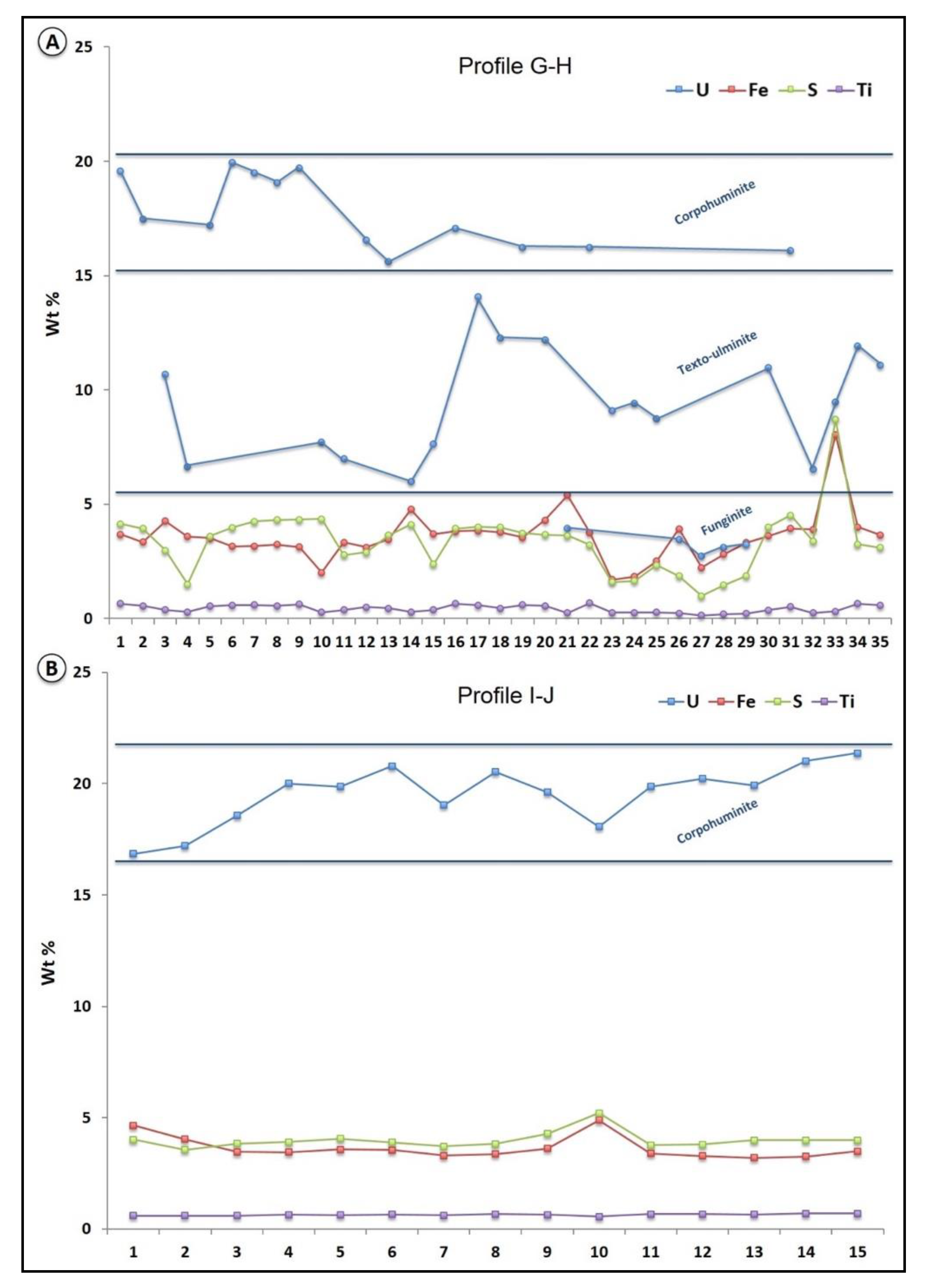
4.3. Petrography of Organic Matter—Uranium Associations and Mineral Geochemistry
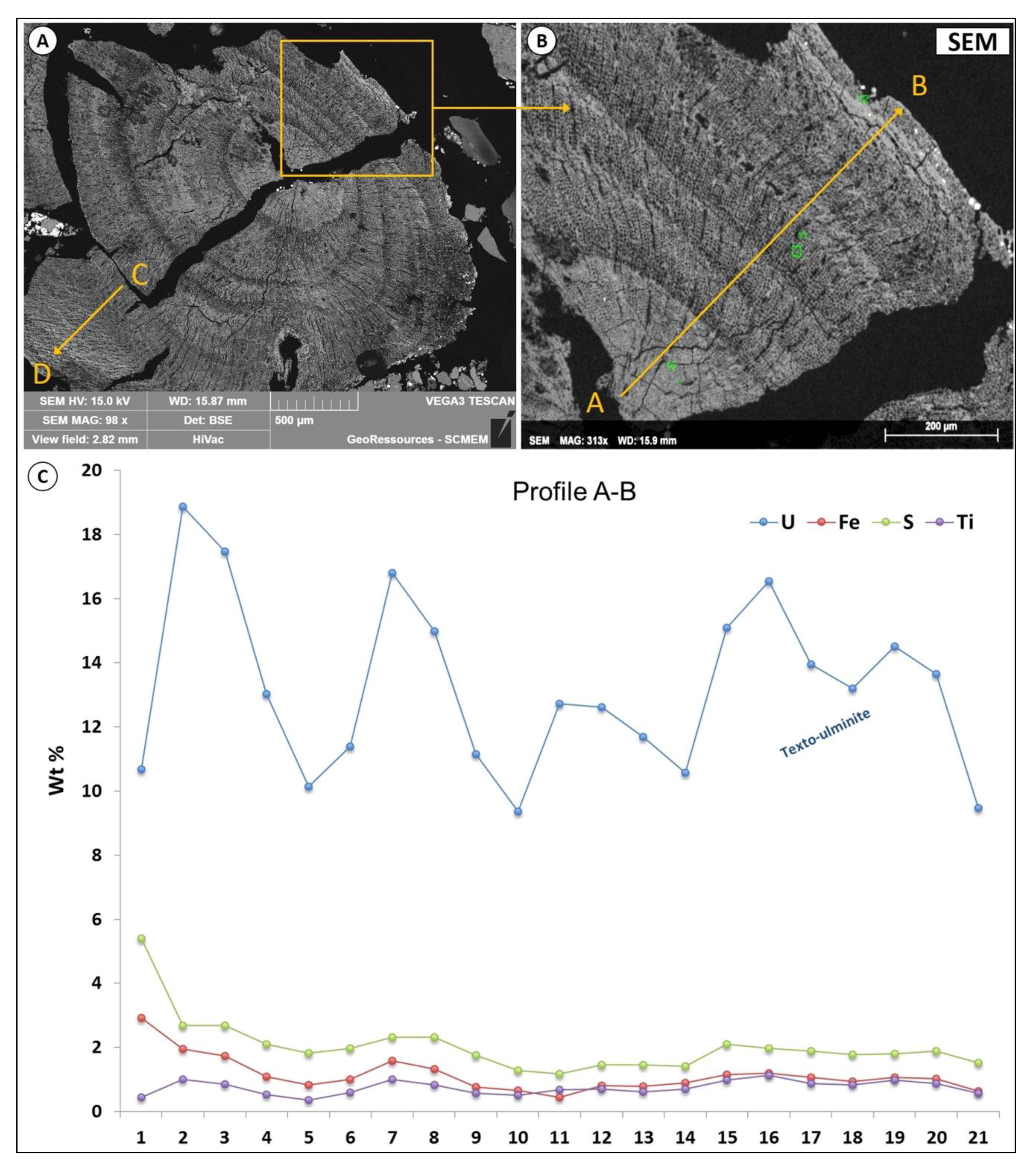
4.3.1. U-Rich Organic Matter without any Distinguishable U-Phase
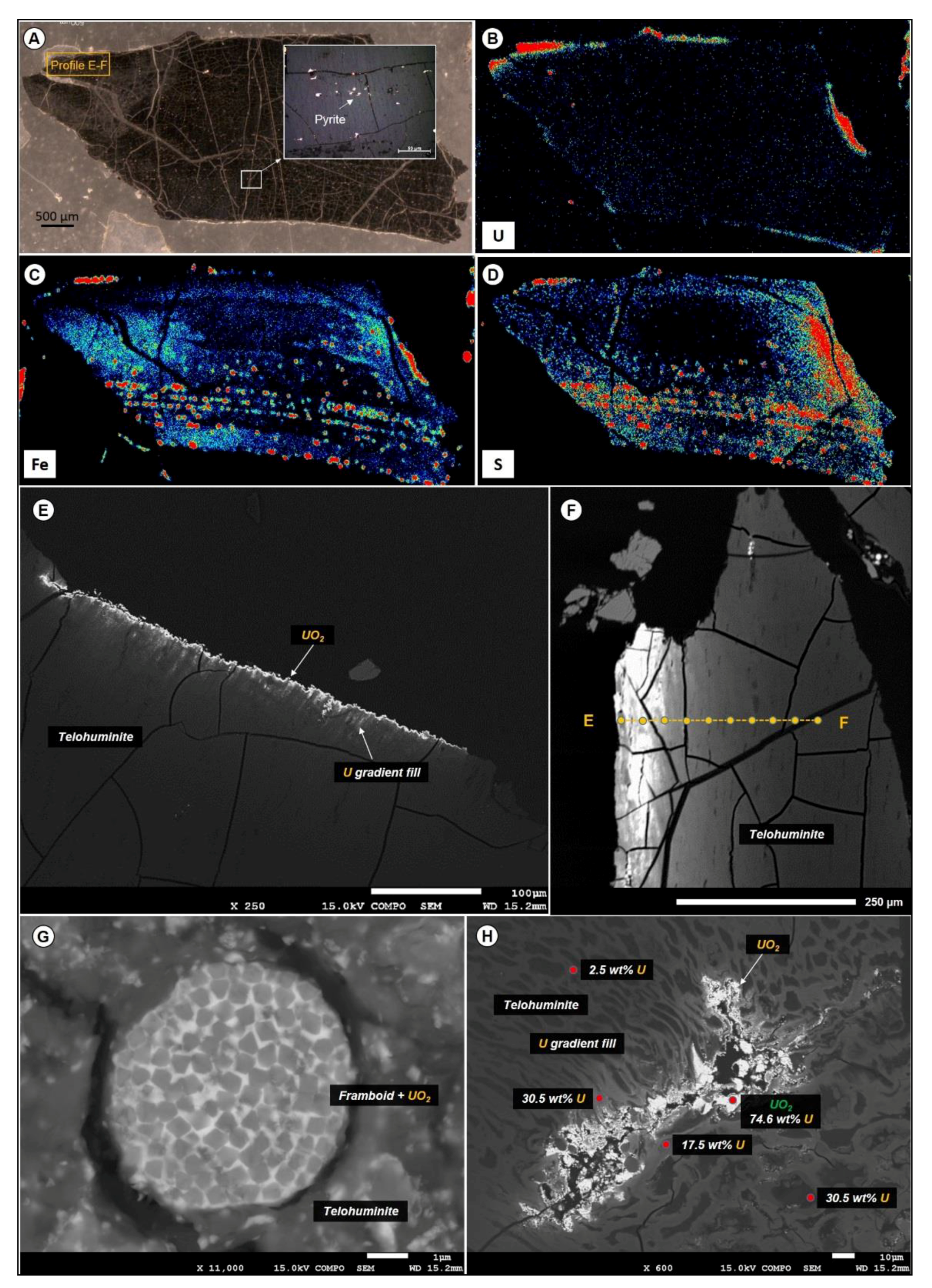
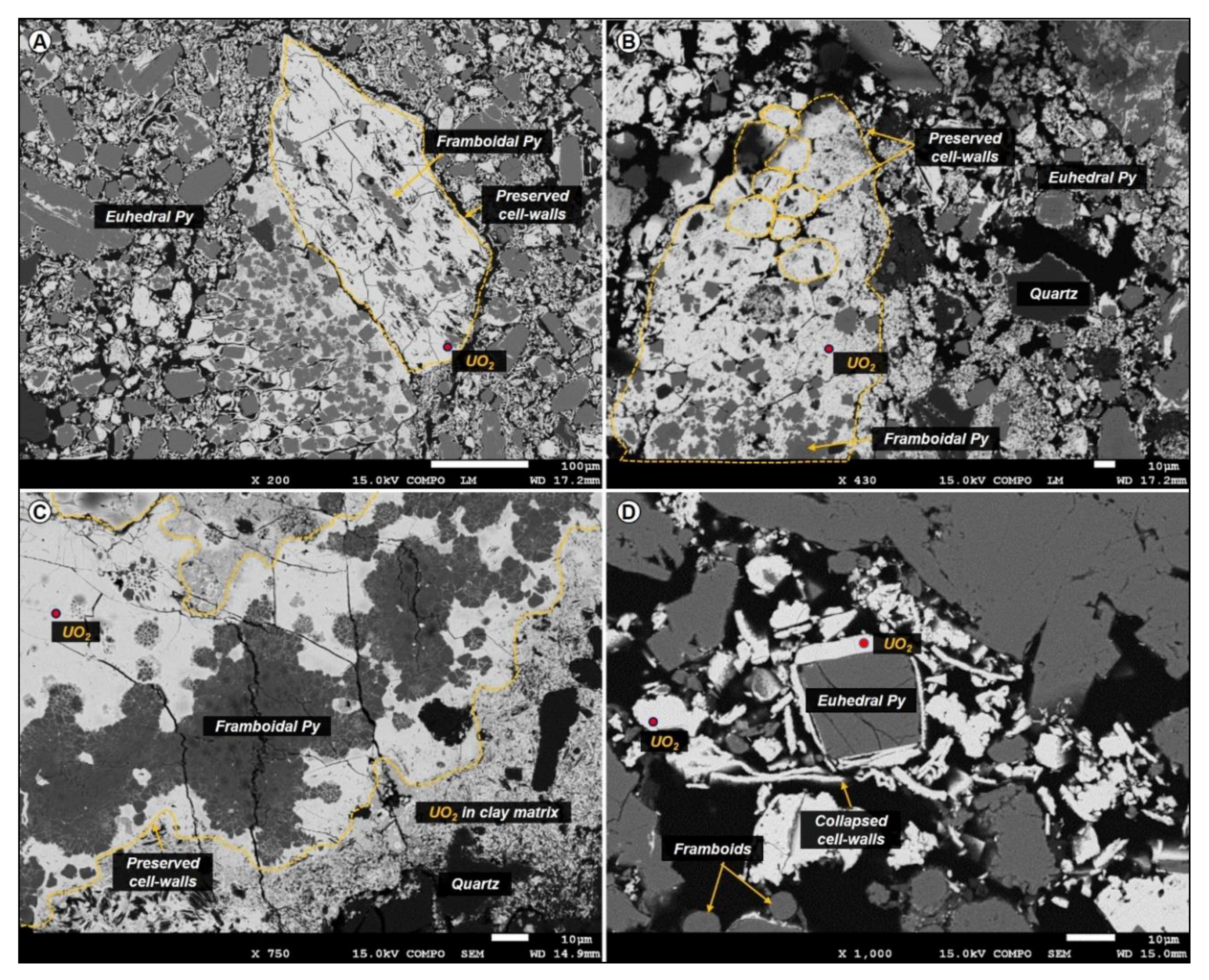
4.3.2. Uranium Oxides Precipitated at the Contact of the Organic Particle
4.3.3. Uranium Phases Replacing Entirely Organic Matter
5. Discussion
5.1. Detrital Organic Matter and U Distribution in Sediments
5.1.1. Origin of Organic Matter
5.1.2. Uranium Uptake from Interstitial Waters
5.1.3. Uranium Trapping Processes Involving Organic Matter at Zoovch Ovoo
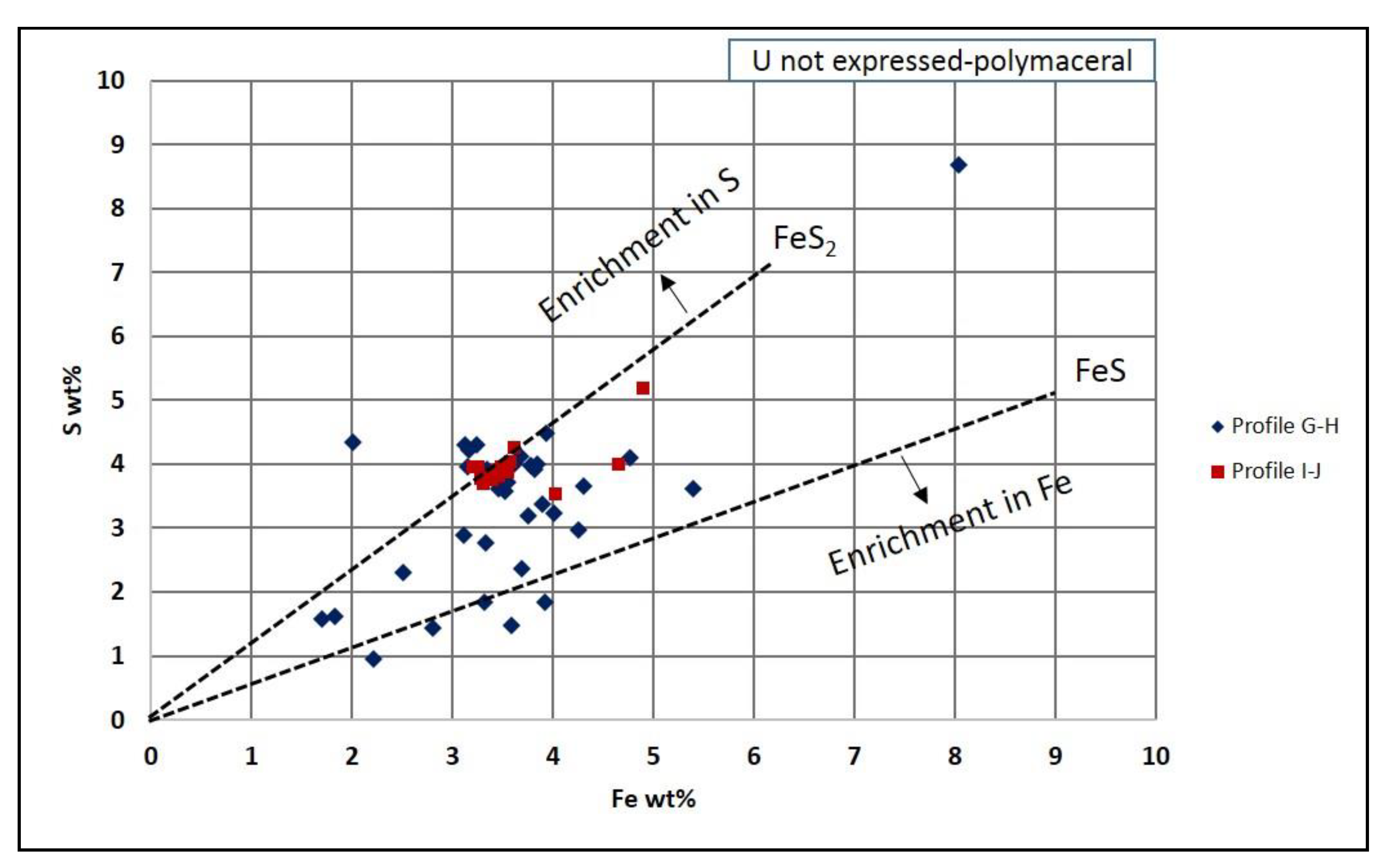
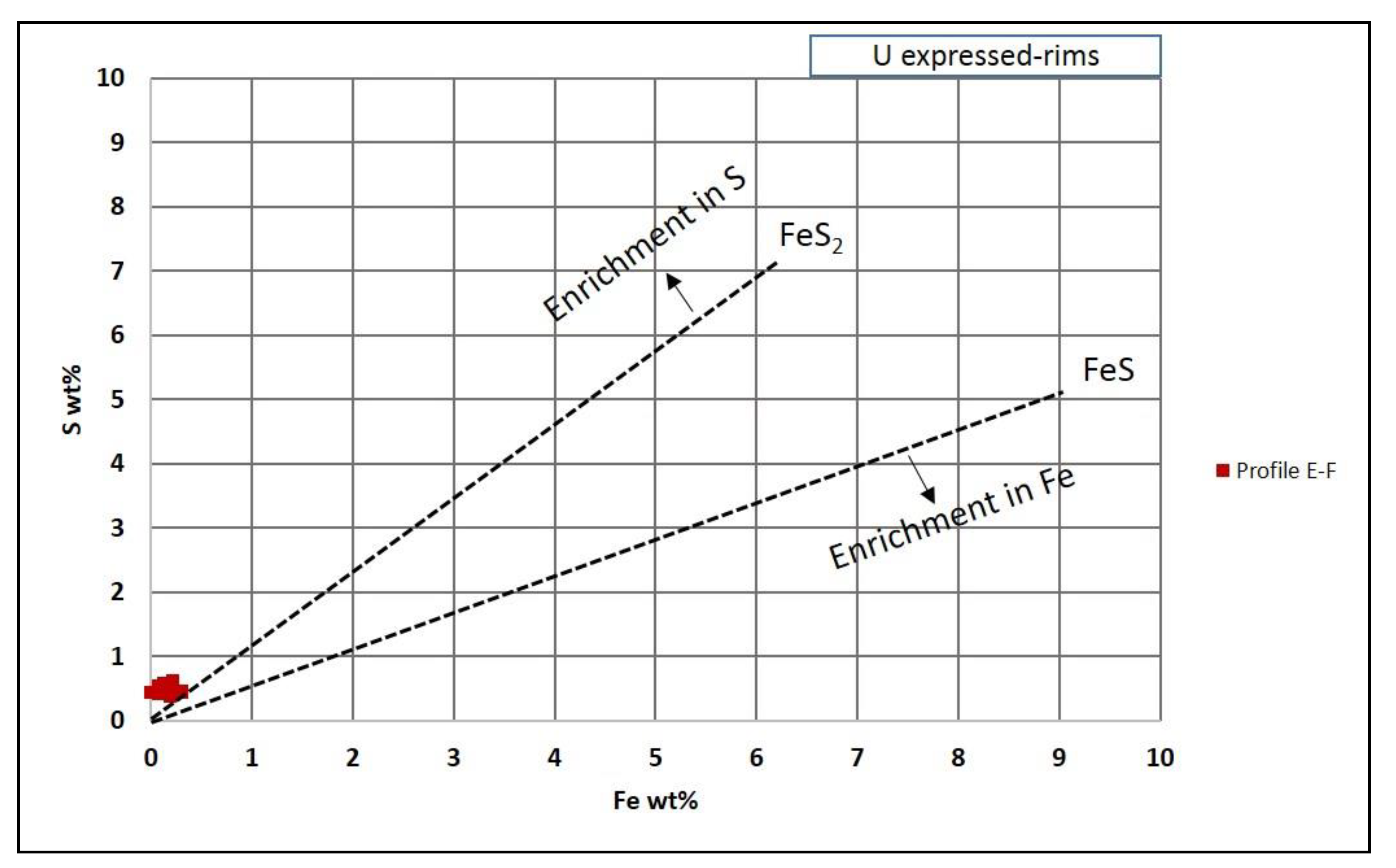
5.1.4. Iron and Sulfur in Organic Matter Particles
5.1.5. Sedimentological Control of Organic Matter and Uranium Distribution
6. Conclusions
- Organic matter present at Zoovch Ovoo is land plant derived, and occurs as detrital particles concentrated into clay layers, clay intraclasts or sandy laminae. Biological features of particles are more or less preserved, depending on their transportation (proximal or distal origin). Its thermal maturity is very low (peat to lignite stage; %Rr = 0.3).
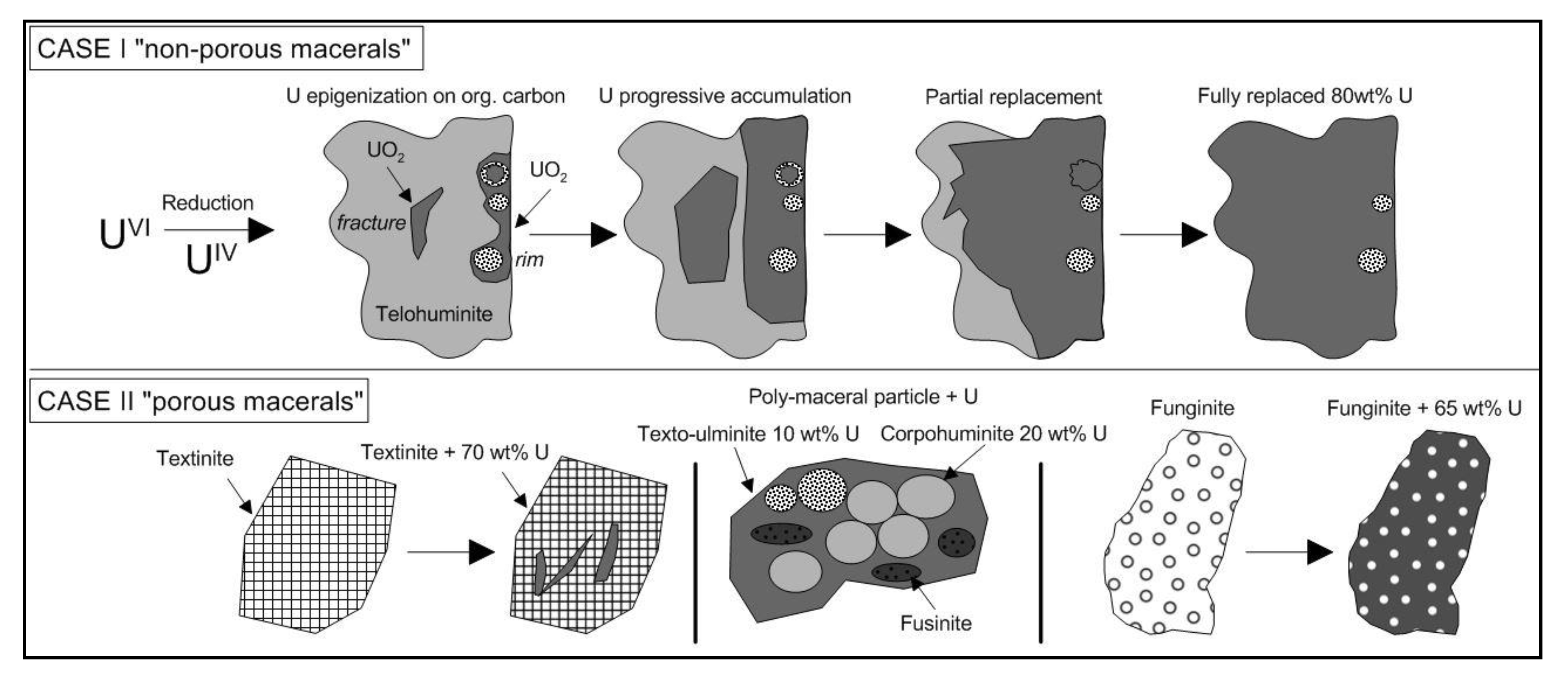
- Maceral particles show high concentrations (up to 20 wt %) of U not expressed as oxide (detectable under SEM). It is very likely that U(VI) is adsorbed as uranyl–carboxyl groups. The organic matter particles have therefore captured U from circulating fluids at a low temperature (T < 40 °C). It is not yet clear if the trapping occurred during sedimentation (pre-concentration stage), during the roll-front events or both.
- The land plant particles concentrated microbiological activity, which triggered biodegradation as well as iron and bacterial sulfate reduction. The absence of detectable pyrite crystals in the organic matter, despite the presence of Fe and S in often non-FeS2 stoichiometric proportions, suggests the presence of other forms of sulfur, such as amorphous FeS phase (e.g., mackinawite) and/or elemental sulfur.
- The distribution of UO2 in the organic particles seems to be linked to porosity/permeability of the organic structure, which may be a control of fluid accessibility to the macerals. Organic matter particles can be fully replaced by UO2, with partial preservation of organic structure (suggesting an epigenesis).
- As burial diagenesis is too low to consider reduction of U(VI) by carbonaceous moieties, it is suggested that microorganisms are mainly responsible for the reduction of U(VI), either directly through their physiological activity, or by providing reduced sulfur, which is an efficient reducing agent for U(VI). Furthermore, Fe(II) carboxylates may also be considered as a possible reducing agent [58]. The U(IV) speciation can be better understood by using EXAFS. Thus, it is necessary to identify the potential presence of biogenic non-crystalline U(IV) compounds within the macerals.
- At the scale of the sedimentary particles, organic matter plays a capital role in uranium deposition, as it acts as a uranium trap through complexation and sustains the U(VI) reduction mechanism into UO2 through biological activity.
- At the scale of the sedimentary deposit, organic matter distribution as well as the permeability network that allows the circulation of uranium-bearing fluids are controlled by the sedimentary architecture.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nash, J.T.; Granger, H.C.; Adams, S.S. Geology and Concepts of Genesis of Important Types of Uranium Deposits; Economic Geology Publishing Company: New Haven, CT, USA, 1981; pp. 63–116. [Google Scholar] [CrossRef]
- Meunier, J.D.; Landais, P.; Monthioux, M.; Pagel, M. Oxidation reduction processes in the genesis of the uranium-vanadium tabular deposits of the Cottonwood Wash mining area (Utah, USA): Evidence from petrological study and organic matter analysis. Bull. Minéral. 1987, 110, 145–156. [Google Scholar] [CrossRef]
- Landais, P.; Connan, J.; Dereppe, J.M.; George, E.; Meunier, J.D.; Monthioux, M.; Pagel, M.; Pironon, J.; Poty, B. Alterations of the organic matter, a clue for uranium ore genesis. Uranium 1987, 3, 307–342. [Google Scholar]
- Landais, P. Organic geochemistry of sedimentary uranium ore deposits. Ore Geol. Rev. 1996, 11, 33–51. [Google Scholar] [CrossRef]
- Spirakis, C.S. The roles of organic matter in the formation of uranium deposits in sedimentary rocks. Ore Geol. Rev. 1996, 11, 53–69. [Google Scholar] [CrossRef]
- Cuney, M. Ore deposits, 2007. In Encyclopedia of Geochemistry; Springer International Publishing AG: Berlin, Germany, 2016; 13p. [Google Scholar] [CrossRef]
- Nakashima, S. Complexation and reduction of uranium by lignite. Sci. Total Environ. 1992, 117–118, 425–437. [Google Scholar] [CrossRef]
- Cumberland, S.A.; Etschmann, B.; Brugger, J.; Douglas, G.; Evans, K.; Fisher, L.; Kappen, P.; Moreau, J.W. Characterization of uranium redox state in organic-rich Eocene sediments. Chemosphere 2018, 194, 602–613. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.F. Redox behavior of uranium in an anoxic marine basin. Uranium 1987, 3, 145–164. [Google Scholar]
- Goldhaber, M.B.; Hemingway, B.S.; Mohagheghi, A.; Reynolds, R.L.; Northrop, H.R. Origin of coffinite in sedimentary rocks by a sequential adsorption-reduction mechanism. Bulletin de la Société de l’industrie Minérale 1987, 110, 131–144. [Google Scholar] [CrossRef]
- Nakashima, S.; Disnar, J.R.; Perruchot, A.; Trichet, J. Experimental study of mechanisms of fixation and reduction of uranium by sedimentary organic matter under diagenetic or hydrothermal conditions. Geochim. Cosmochim. Acta 1984, 48, 2321–2329. [Google Scholar] [CrossRef]
- Meunier, J.D.; Landais, P.; Pagel, M. Experimental evidence of uraninite formation from diagenesis of uranium-rich organic matter. Geochim. Cosmochim. Acta 1990, 54, 809–817. [Google Scholar] [CrossRef]
- Van Krevelen, D.W. Coal Typology–Chemistry–Physics–Constitution, 1st ed.; Elsevier: Amsterdam, The Netherlands, 1961; 514p. [Google Scholar]
- Tissot, B.P.; Welte, D.H. Petroleum Formation and Occurrence, 2nd ed.; Springer: Berlin/Heildelberg, Germany; New York, NY, USA, 1984; 699p. [Google Scholar]
- Adreyef, P.F.; Chumachenko, A.P. Reduction of uranium by natural organic substances. Geokhimiya 1964, 1, 12–22. [Google Scholar]
- Forbes, P.; Landais, P.; Bertrand, P.; Brosse, E.; Espitalié, J.; Yahaya, M. Chemical transformations of type-III organic matter associated with the Akouta uranium deposit (Niger): Geological implications. Chem. Geol. 1988, 71, 267–282. [Google Scholar] [CrossRef]
- Granger, H.C.; Warren, C.G. Unstable sulfur compounds and the origin of roll-front type uranium deposits. Econ. Geol. 1969, 64, 160–171. [Google Scholar] [CrossRef]
- Boulègue, J. Simultaneous Determination of Sulfide, Polysulfides and Thiosulfat as an Aid to Ore Exploration. Dev. Econ. Geol. 1981, 15, 21–36. [Google Scholar] [CrossRef]
- Petrov, N.N. Les gisements épigénétiques du Kazakhstan. Géologie du Kazakhstan 1998, 2, 22–39. [Google Scholar]
- Munara, A. Formation des gisements d’uranium de type roll: Approche minéralogique et géochimique du gisement uranifère de Muyunkum (Bassin de Chu-Sarysu, Kazakhstan). Ph.D. Thesis, Henri Poincaré University, Nancy, France, 9 July 2012; 327p. [Google Scholar]
- Lach, P.; Cathelineau, M.; Brouand, M.; Fiet, N. In-situ isotopic and chemical study of pyrite from Chu-Sarysu (Kazakhstan) roll-front uranium deposit. Procedia Earth Planet. Sci. 2015, 13, 207–210. [Google Scholar] [CrossRef]
- Cai, C.; Dong, H.; Li, H.; Xiao, X.; Ou, G.; Zhang, C. Mineralogical and geochemical evidence for coupled bacterial uranium mineralization and hydrocarbon oxidation in the Shashagetai deposit, NW China. Chem. Geol. 2007, 236, 167–179. [Google Scholar] [CrossRef]
- Zhu, M.; Wu, R.; Liu, X.; Nie, F.; Yu, D. Geologic Setting of Interformational Braided Channel Type Sandstone Uranium Deposits in North China, East China Institute of Technology, 2012. Available online: https://www.slideshare.net/monatom/05-xiaodong-liu-geologic-setting-of-interformationalbraidedchannel-type-sandstone-uranium-deposits-in-north-china (accessed on 17 May 2019).
- Yue, S.; Wang, G. Relationship between the hydrogeochemical environment and sandstone-type uranium mineralization in the Ili basin, China. Appl. Geochem. 2011, 26, 133–139. [Google Scholar] [CrossRef]
- Bonnetti, C.; Cuney, M.; Malartre, F.; Michels, R.; Liu, X.; Peng, Y. The Nuheting Deposit, Erlian Basin, China: Synsedimentary vs. Diagenetic Uranium Mineralization. Ore Geol. Rev. 2015, 69, 118–139. [Google Scholar] [CrossRef]
- Le Goux, F.; Banzragch, T.O.; Delaunay, A.; Nyamdorj, B.I.; Jaques, E.; Korshunov, A.; Parize, O.; Brouand, M. The Major Gobi Uranium Deposits, Upper Cretaceous East Gobi Basin, Mongolia: Geodynamical and Mineralogical Key Parameters of Uranium Ore Geology. In Proceedings of the 13th SGA Biennial Meeting, Nancy, France, 24–27 August 2015; Volume 5, pp. 1815–1818. [Google Scholar]
- Dahlkamp, F.J. Uranium Deposits of the World: Asia; Springer: Berlin/Heidelberg, Germany, 2009; 493p. [Google Scholar]
- Graham, S.A.; Hendrix, M.S.; Johnson, C.L.; Badamgarav, D.; Badarch, G.; Amory, J.; Porter, M.; Barsbold, R.; Webb, L.E.; Hacker, B.R. Sedimentary record and tectonic implications of Mesozoic rifting in southeast Mongolia. Geol. Soc. Am. Bull. 2001, 113, 1560–1579. [Google Scholar] [CrossRef]
- Johnson, C.L. Polyphase evolution of the East Gobi basin: Sedimentary and structural records of Mesozoic-Cenozoic intraplate deformation in Mongolia. Basin Res. 2004, 16, 79–99. [Google Scholar] [CrossRef]
- Prost, G.L. Tectonics and hydrocarbon systems of the East Gobi basin, Mongolia. Am. Assoc. Pet. Geol. Bull. 2004, 88, 483–513. [Google Scholar] [CrossRef]
- Pontolillo, J.; Stanton, R.W. Coal Petrographic Laboratory Procedures and Safety Manual II. Open-File Report 94-631. U.S Department of Interior Geological Survey, 1994; 74p. Available online: https://pubs.usgs.gov/of/1994/0631/report.pdf (accessed on 4 September 2017).
- (ISO) 7404-2. International Organization for Standardization. Methods for the Petrographic Analysis of Coals—Part 2: Methods of Preparing Coal Samples; International Organization for Standardization: Geneva, Switzerland, 2009; 12p. [Google Scholar]
- (ISO) 7404-5. International Organization for Standardization. Methods for the Petrographic Analysis of Coal—Part 5: Methods of Determining Microscopically the Reflectance of Vitrinite; International Organization for Standardization: Geneva, Switzerland, 2009; 14p. [Google Scholar]
- Sýkorová, I.; Pickel, W.; Christanis, K.; Wolf, M.; Taylor, G.H.; Flores, D. Classification of huminite—ICCP System 1994. Int. J. Coal Geol. 2005, 62, 85–106. [Google Scholar] [CrossRef]
- International Committee for Coal and Organic Petrology (ICCP). ICCP Training Course on Dispersed Organic Matter; International Committee for Coal and Organic Petrology (ICCP): Porto, Portugal, 2011; 322p, ISBN 978-989-8265-67-8. [Google Scholar]
- Rallakis, D.; Michels, R.; Brouand, M.; Parize, O.; Cathelineau, M. Dolomite cements in Cenomanian continental sand deposits: Time evolution and significance (Zoovch Ovoo, U-deposit, East Gobi Basin, Mongolia). J. Sediment. Res. 2019. under review. [Google Scholar]
- Borrego, A.G.; Cook, A. Humic Macerals: Vitrinite/Inertinite, Chapter 9. In 10th ICCP Training Course on Dispersed Organic Matter. Integrating Transmitted and Reflected Light Microscopy; GFZ: Potsdam, Germany, 2017; 276p. [Google Scholar]
- Van Krevelen, D.W. Coal, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 1993. [Google Scholar]
- Langmuir, D. Uranium solution-mineral equilibria at low temperatures with applications to sedimentary ore deposits. Geochim. Cosmochim. Acta 1978, 44, 1753–1766. [Google Scholar] [CrossRef]
- Bone, E.S.; Dynes, J.J.; Cliff, J.; Bargar, J. Uranium (IV) adsorption by natural organic matter in anoxic sediments. Proc. Natl. Acad. Sci. USA 2017, 114, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, S.; Disnar, J.R.; Perruchot, A. Precipitation Kinetics of Uranium by Sedimentary Organic Matter under Diagenetic and Hydrothermal Conditions. Econ. Geol. 1999, 94, 993–1006. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus, Chemistry. Genesis, Composition, Reactions; John Wiley and Sons: New York, NY, USA, 1982; p. 443. [Google Scholar]
- Douglas, G.B.; Butt, C.R.M.; Gray, D.J. Geology, geochemistry and mineralogy of the lignite-hosted Ambassador palaeochannel uranium and multi-element deposit, Gunbarrel Basin, Western Australia. Miner. Depos. 2011, 46, 761–787. [Google Scholar] [CrossRef]
- Bonnetti, C.; Liu, X.; Zhaobin, Y.; Cuney, M.; Michels, R.; Malartre, F.; Mercadier, J.; Cai, J. Coupled uranium mineralization and bacterial sulphate reduction for the genesis of the Baxingtu sandstone-hosted U deposit, SW Songliao Basin, NE China. Ore Geol. Rev. 2017, 82, 108–129. [Google Scholar] [CrossRef]
- Rouzaud, J.N.; Oberlin, A.; Trichet, J. Interaction of uranium and organic matter in uraniferous sediments. In Advances in Organic Geochemistry; Douglas, A.G., Maxwell, J.R., Eds.; Pergamon Press: Oxford, UK, 1979; pp. 505–516. [Google Scholar] [CrossRef]
- Lovley, D.R.; Phillips, E.J.P.; Gorby, Y.A.; Landa, E.R. Microbial reduction of uranium. Nature 1991, 350, 413–416. [Google Scholar] [CrossRef]
- Detmers, J.; Schulte, U.; Strauss, J.; Kuever, J. Sulfate Reduction at a Lignite Seam: Microbial Abundance and Activity. Microb. Ecol. 2001, 42, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Min, M.; Xu, H.; Chen, J.; Fayek, M. Evidence of uranium biomineralization in sandstone-hosted roll-front uranium deposits, northwestern China. Ore Geol. Rev. 2005, 26, 198–206. [Google Scholar] [CrossRef]
- Menor-Salván, C.; Tornos, F.; Fernández-Remolar, D.; Amils, R. Association between catastrophic paleovegetation changes during Devonian-Carboniferous boundary and the formation of giant massive sulfide deposits. Earth Planet. Sci. Lett. 2010, 299, 398–408. [Google Scholar] [CrossRef]
- Gruner, J.W. Syntheses of uranium minerals at room and elevated temperatures. Am. Mineral. 1953, 38, 342. [Google Scholar]
- Kochenov, A.V.; Korolev, K.G.; Dubinchuk, V.T.; Medvelev, Y.L. Experimental data on the conditions of precipitation of uranium from aqueous solutions. Geochem. Int. 1977, 14, 82–87. [Google Scholar]
- Bhattacharyya, A.; Campbell, K.M.; Kelly, S.D.; Roebbert, Y.; Weyer, S.; Bernier-Latmani, R.; Borch, T. Biogenic non-crystalline U(IV) revealed as major component in uranium ore deposits. Nat. Commun. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Davison, W.; Buffle, J.; Devitre, R. Voltammetric characterization of a dissolved iron sulfide species by laboratroy and field studies. Anal. Chim. Acta 1998, 377, 193–203. [Google Scholar] [CrossRef]
- Theberge, S.; Luther, G.W. Determination of the electrochemical properties of a soluble aqueous FeS species present in sulfide solutions. Aquat. Geochem. 1987, 3, 191–211. [Google Scholar] [CrossRef]
- Rickard, D.; Griffith, A.; Oldroyd, A.; Butler, I.B.; Lopez-Caper, E.; Manning, D.A.C.; Apperley, D.C. The composition of nanoparticulate mackinawite, tetragonal iron (II) monosulfide. Chem. Geol. 2006, 235, 286–298. [Google Scholar] [CrossRef]
- Wolthers, M.; Charlet, L.; van Der Linde, P.; Rickard, D.; van Der Weijden, C. Surface chemistry of disordered mackinawite (FeS). Geochim. Cosmochim. Acta 2005, 69, 3469–3481. [Google Scholar] [CrossRef]
- Bura-Nakić, E.; Viollier, E.; Jézéquel, D.; Thiam, A.; Ciglenečki, I. Reduced sulfur and iron species in anoxic water column of meromictic crater Lake Pavin (Massif Central, France). Chem. Geol. 2009, 266, 311–317. [Google Scholar] [CrossRef]
- Boyanov, M.I.; O’Loughlin, E.J.; Roden, E.E.; Fein, J.B.; Kemner, K.M. Adsorption of Fe(II) and U(VI) to carboxyl-functionalized microspheres: The influence of speciation on uranyl reduction studied by titration and XAFS. Geochim. Cosmochim. Acta 2007, 71, 1898–1912. [Google Scholar] [CrossRef]




| Oxides Mass (%) | Uraninite | Coffinite | ||
|---|---|---|---|---|
| UO2 | 76.28 | 76.48 | 61.92 | 56.58 |
| SiO2 | 1.20 | 1.30 | 6.65 | 12.69 |
| P2O5 | 2.06 | 2.18 | 6.23 | 7.40 |
| CaO | 3.58 | 3.39 | 4.00 | 5.67 |
| Total | 83.12 | 83.35 | 78.80 | 82.34 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rallakis, D.; Michels, R.; Brouand, M.; Parize, O.; Cathelineau, M. The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia. Minerals 2019, 9, 310. https://doi.org/10.3390/min9050310
Rallakis D, Michels R, Brouand M, Parize O, Cathelineau M. The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia. Minerals. 2019; 9(5):310. https://doi.org/10.3390/min9050310
Chicago/Turabian StyleRallakis, Dimitrios, Raymond Michels, Marc Brouand, Olivier Parize, and Michel Cathelineau. 2019. "The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia" Minerals 9, no. 5: 310. https://doi.org/10.3390/min9050310
APA StyleRallakis, D., Michels, R., Brouand, M., Parize, O., & Cathelineau, M. (2019). The Role of Organic Matter on Uranium Precipitation in Zoovch Ovoo, Mongolia. Minerals, 9(5), 310. https://doi.org/10.3390/min9050310





