Ore Genesis of the Chuduoqu Pb-Zn-Cu Deposit in the Tuotuohe Area, Central Tibet: Evidence from Fluid Inclusions and C–H–O–S–Pb Isotopes Systematics
Abstract
:1. Introduction
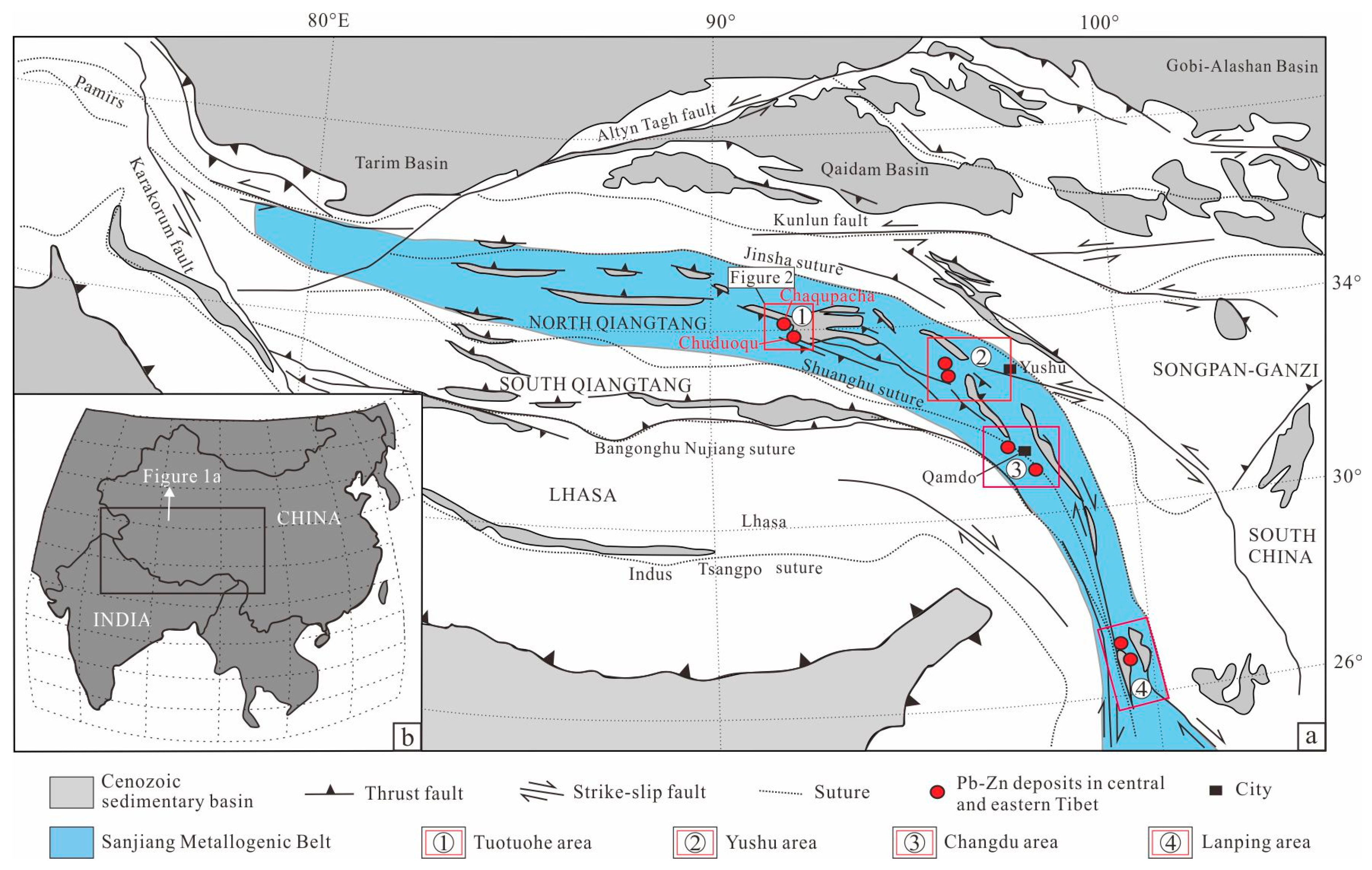
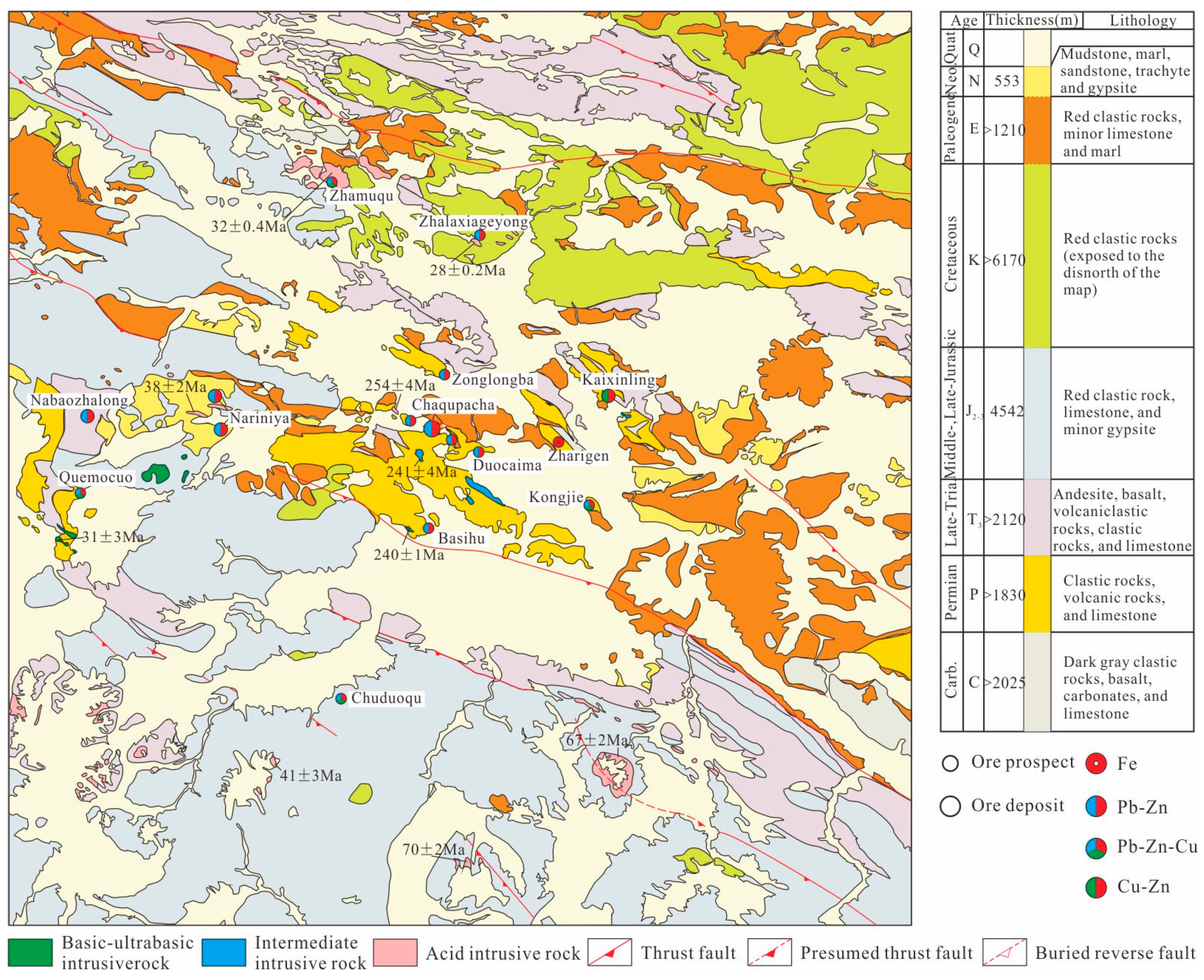
2. Geological Background
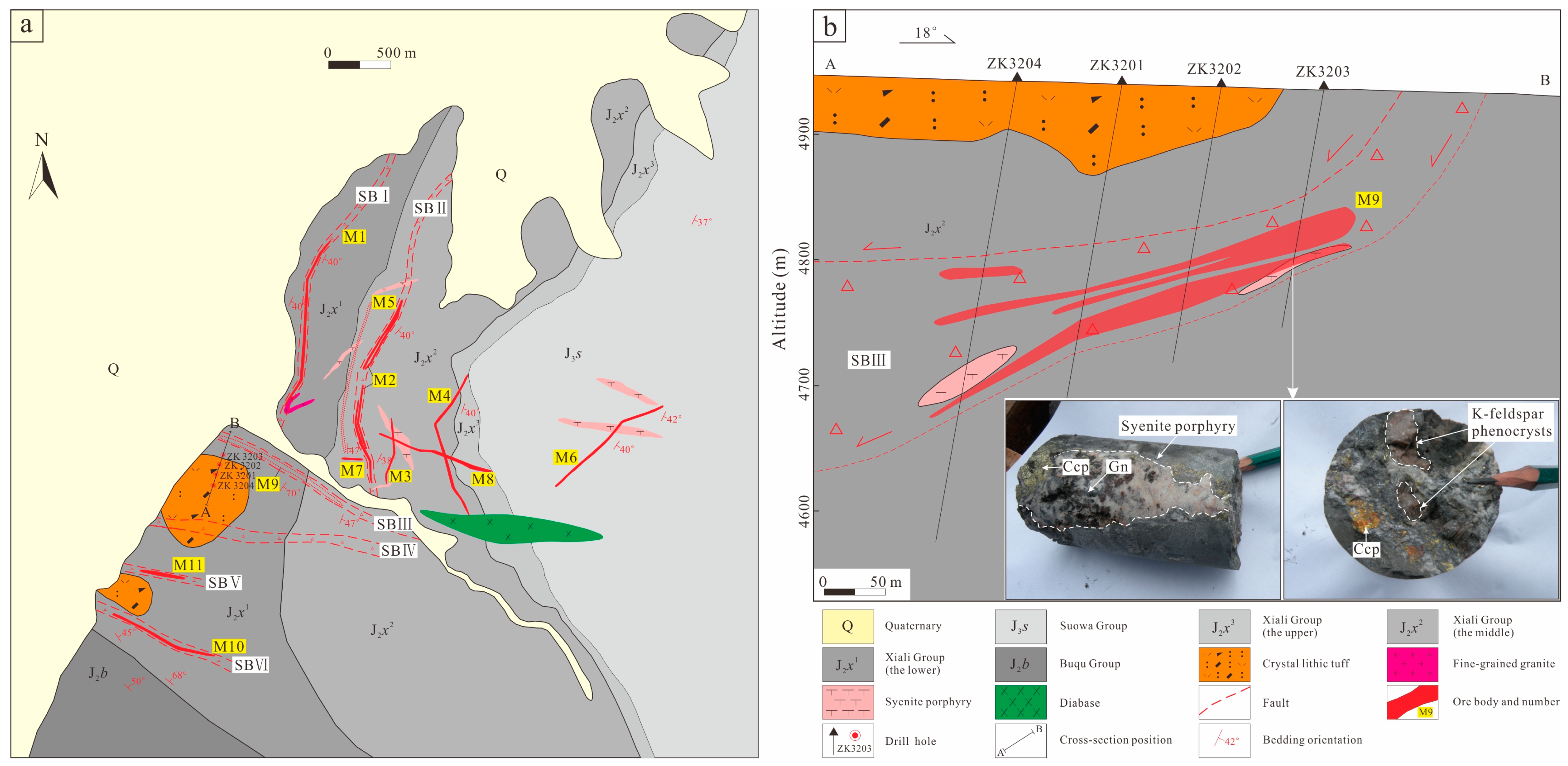
3. Ore Deposit Geology
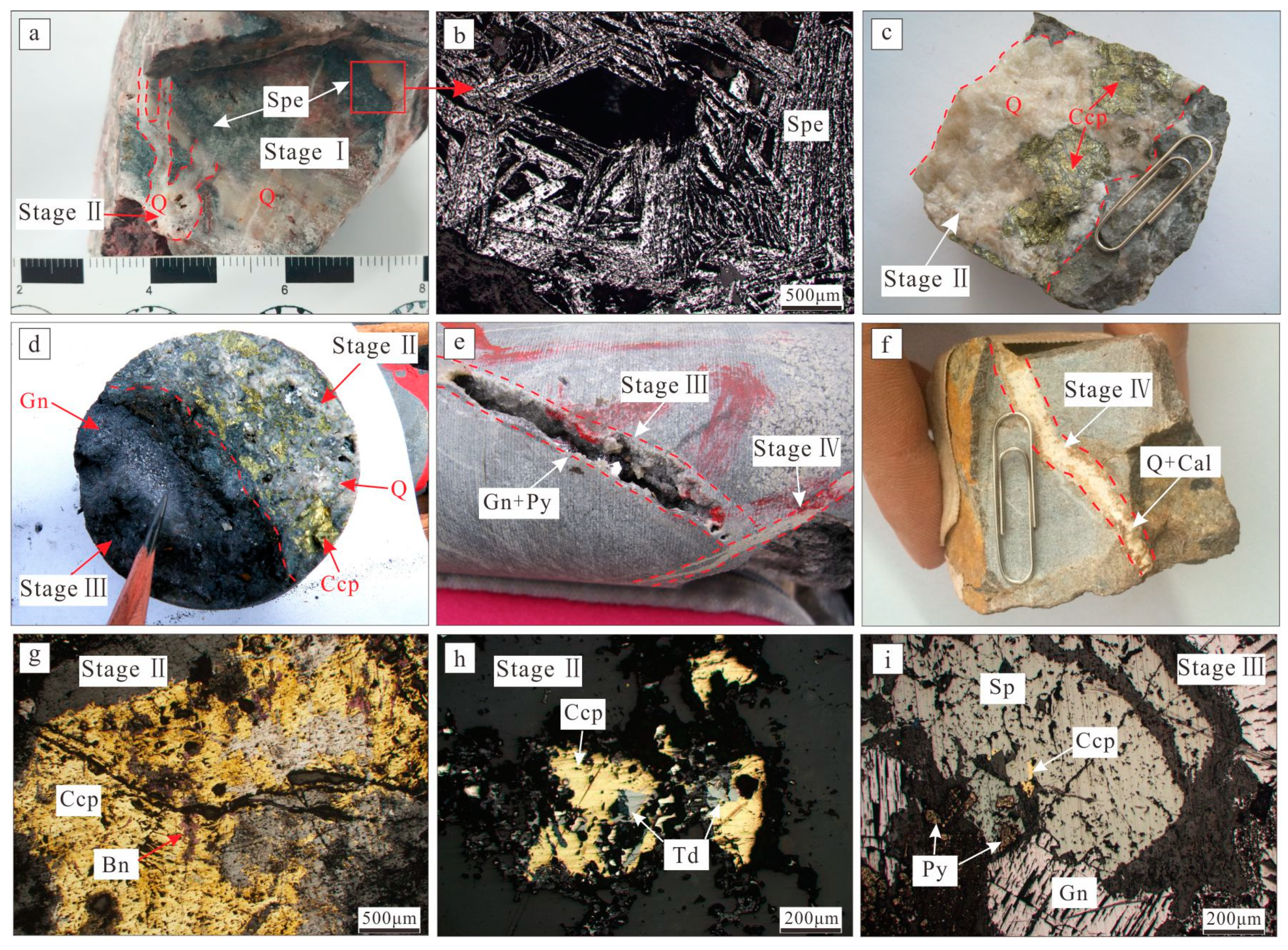
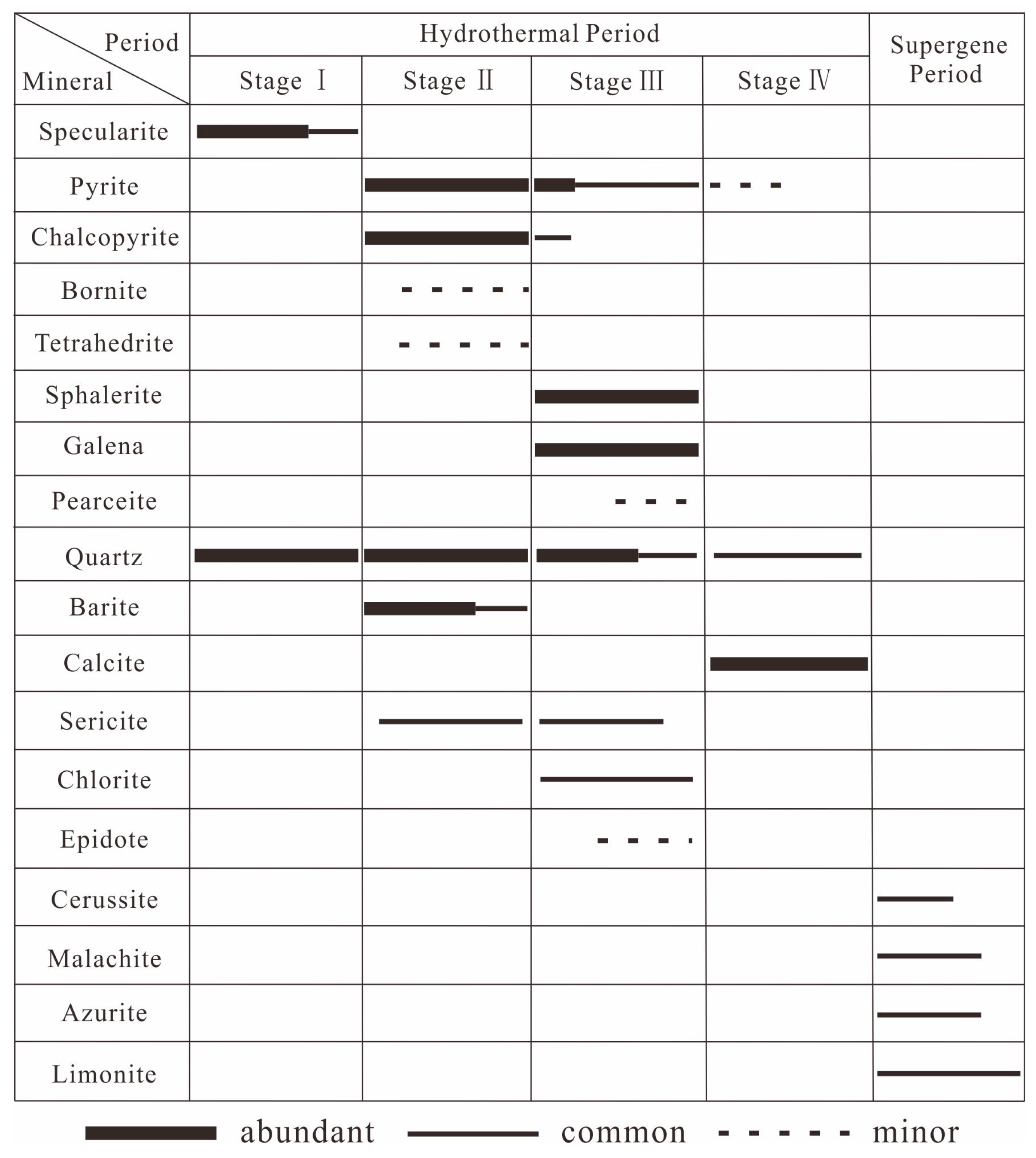
3.1. Quartz–Specularite Ore (Stage I)
3.2. Quartz–Barite–Chalcopyrite (Stage II)
3.3. Quartz–Polymetallic Sulfide Stage (Stage III)
3.4. Quartz–Carbonate Stage (Stage IV)
4. Samples and Analytical Methods
4.1. Fluid Inclusion Measurements
4.2. Ion Chromatography Analysis
4.3. Hydrogen and Oxygen Isotope Analyses
4.4. Carbon and Oxygen Isotope Analyses
4.5. Sulfur and Lead Isotope Analyses
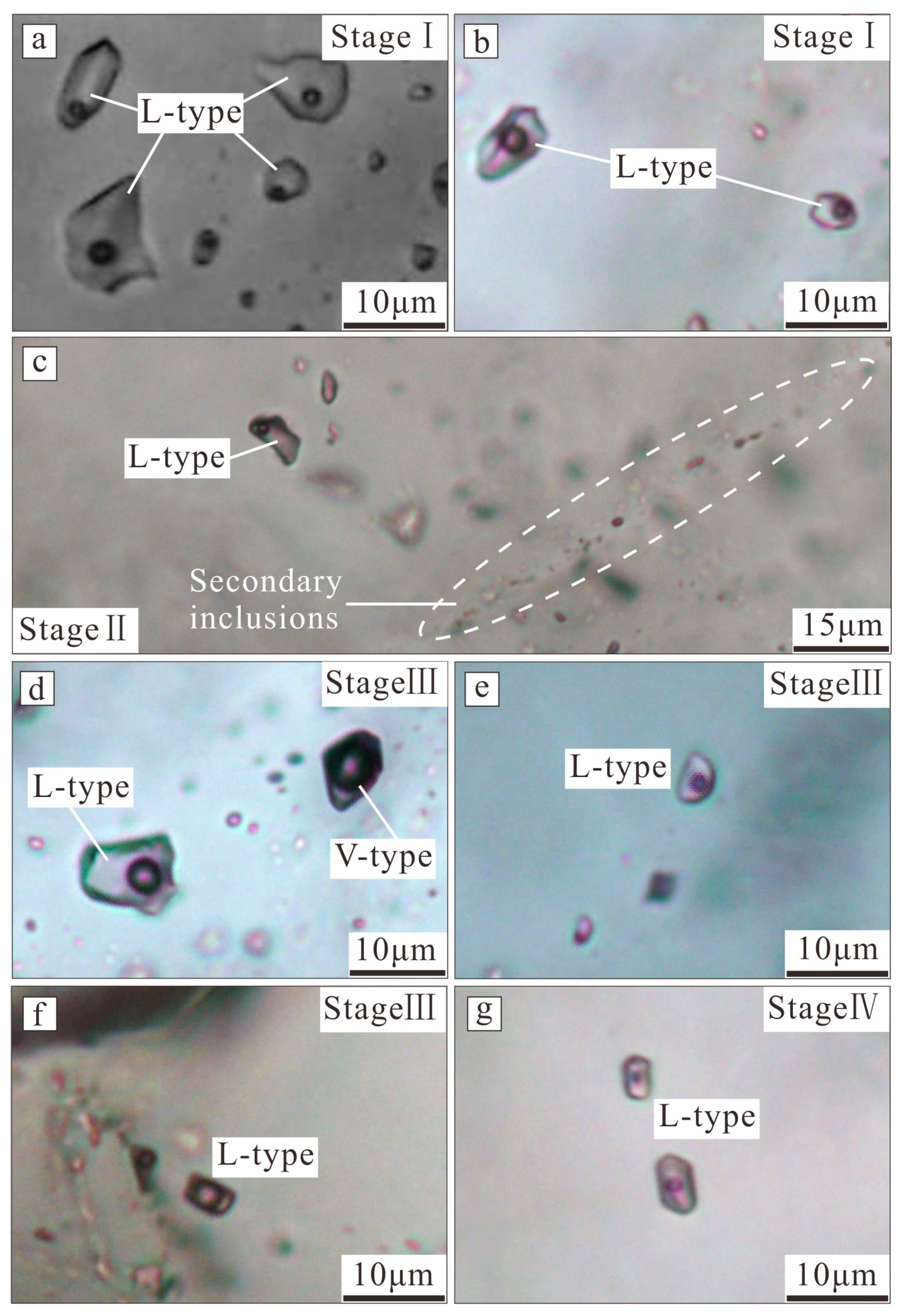
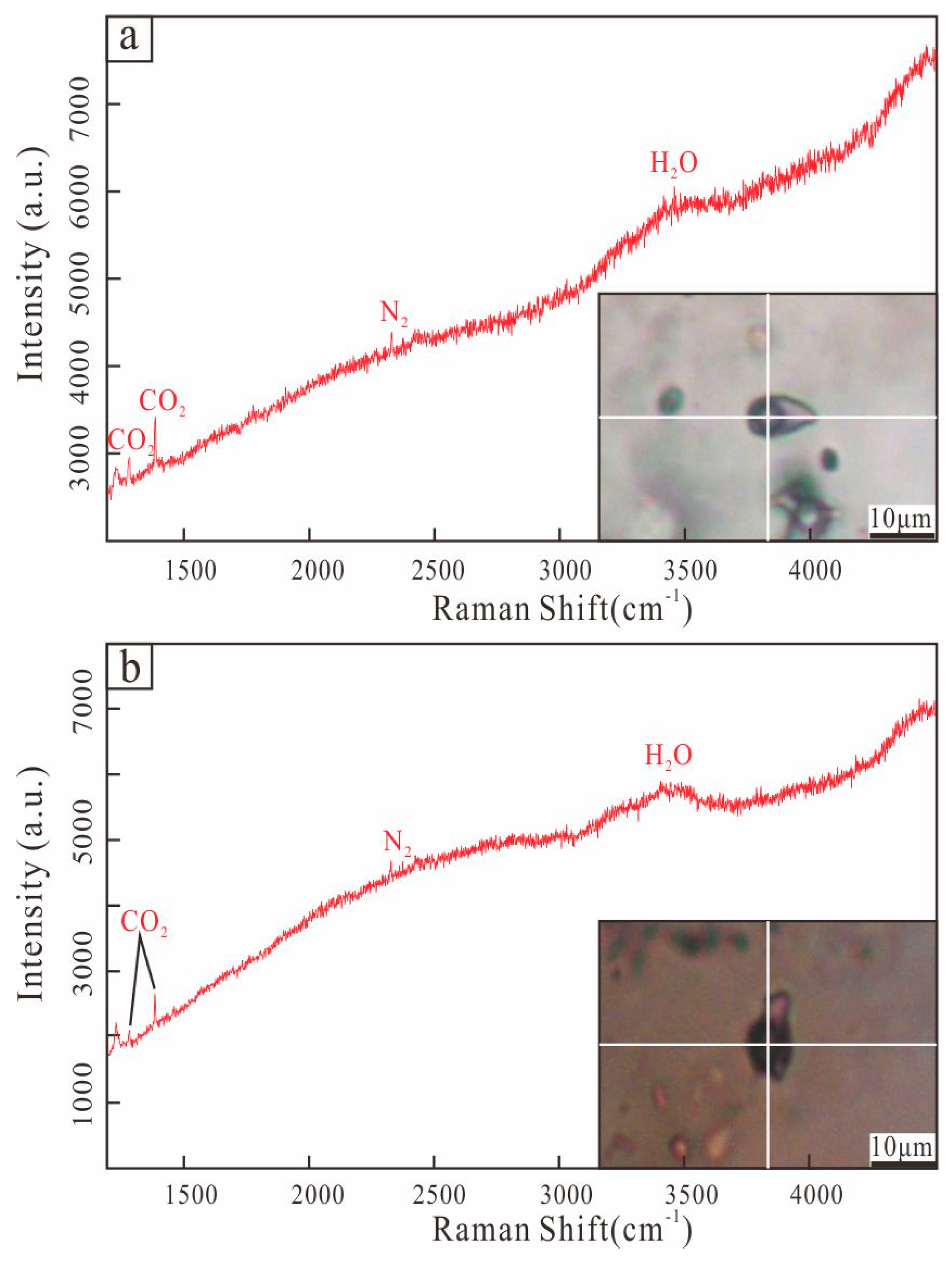
5. Fluid Inclusion Results
5.1. Petrographic Characteristics
5.2. Microthermometry
5.3. Laser Raman Spectroscopy
5.4. Ion Chromatography
6. Isotopes Systematics
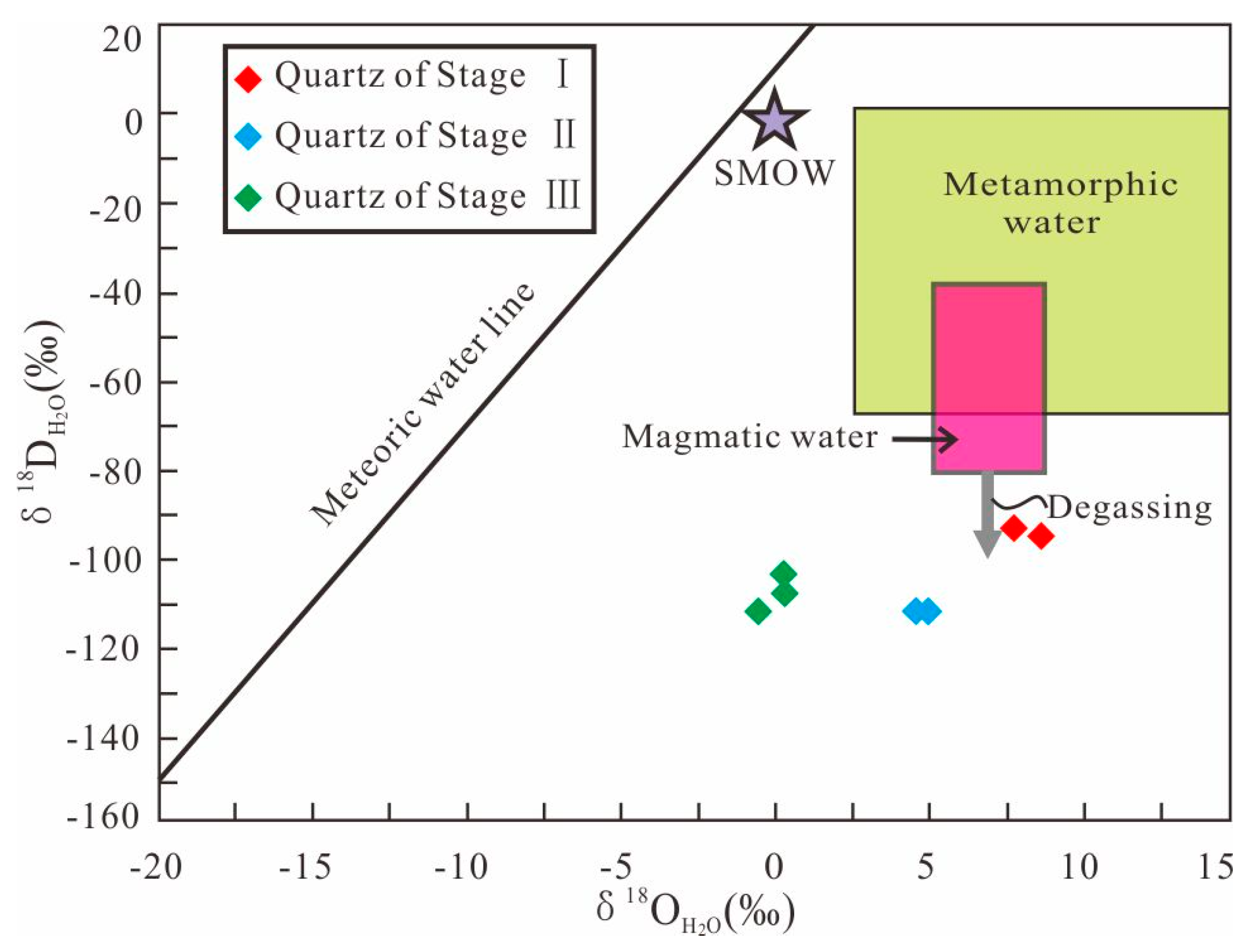
6.1. Oxygen and Hydrogen Isotopes
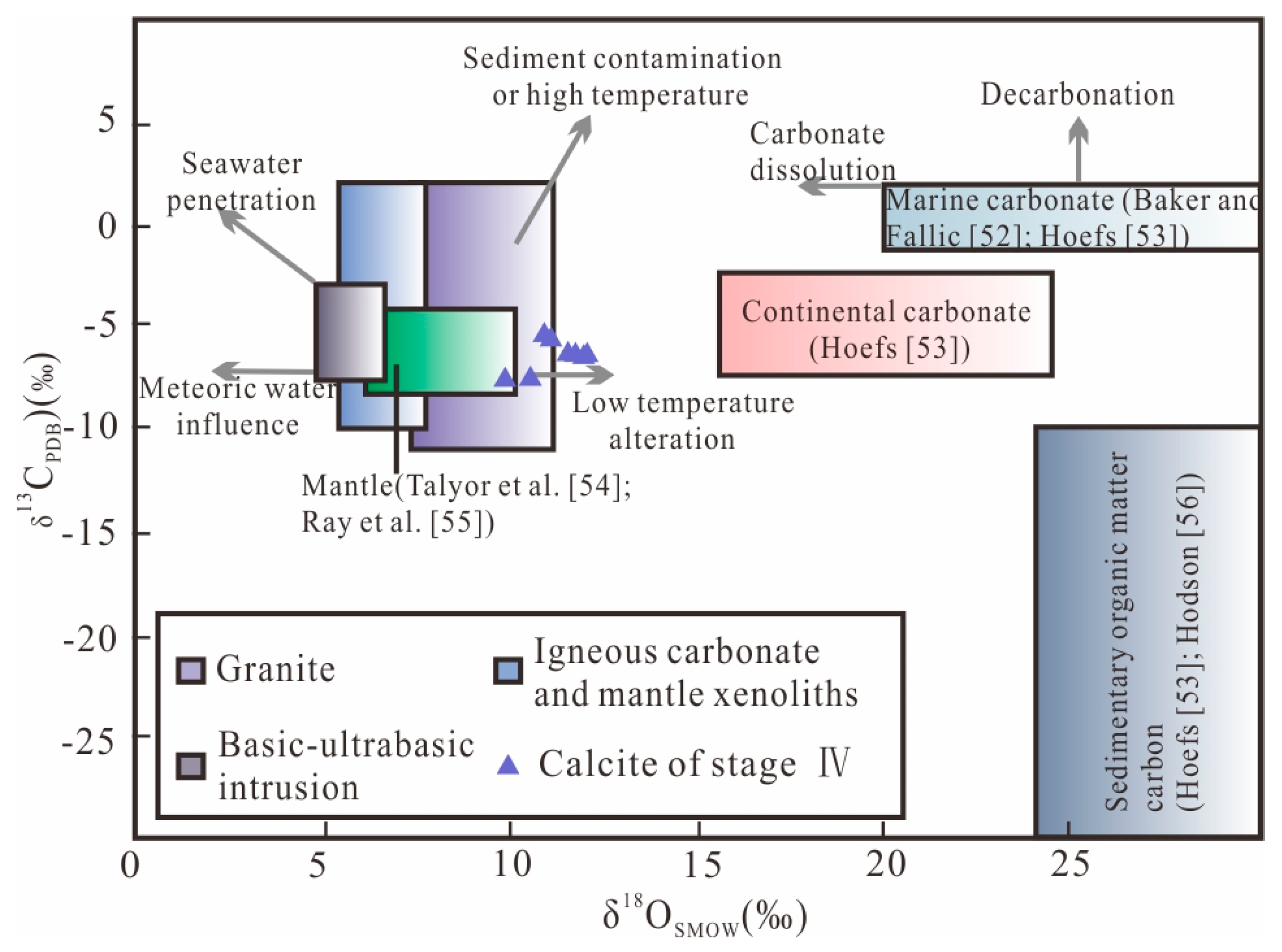
6.2. Carbon and Oxygen Isotopes
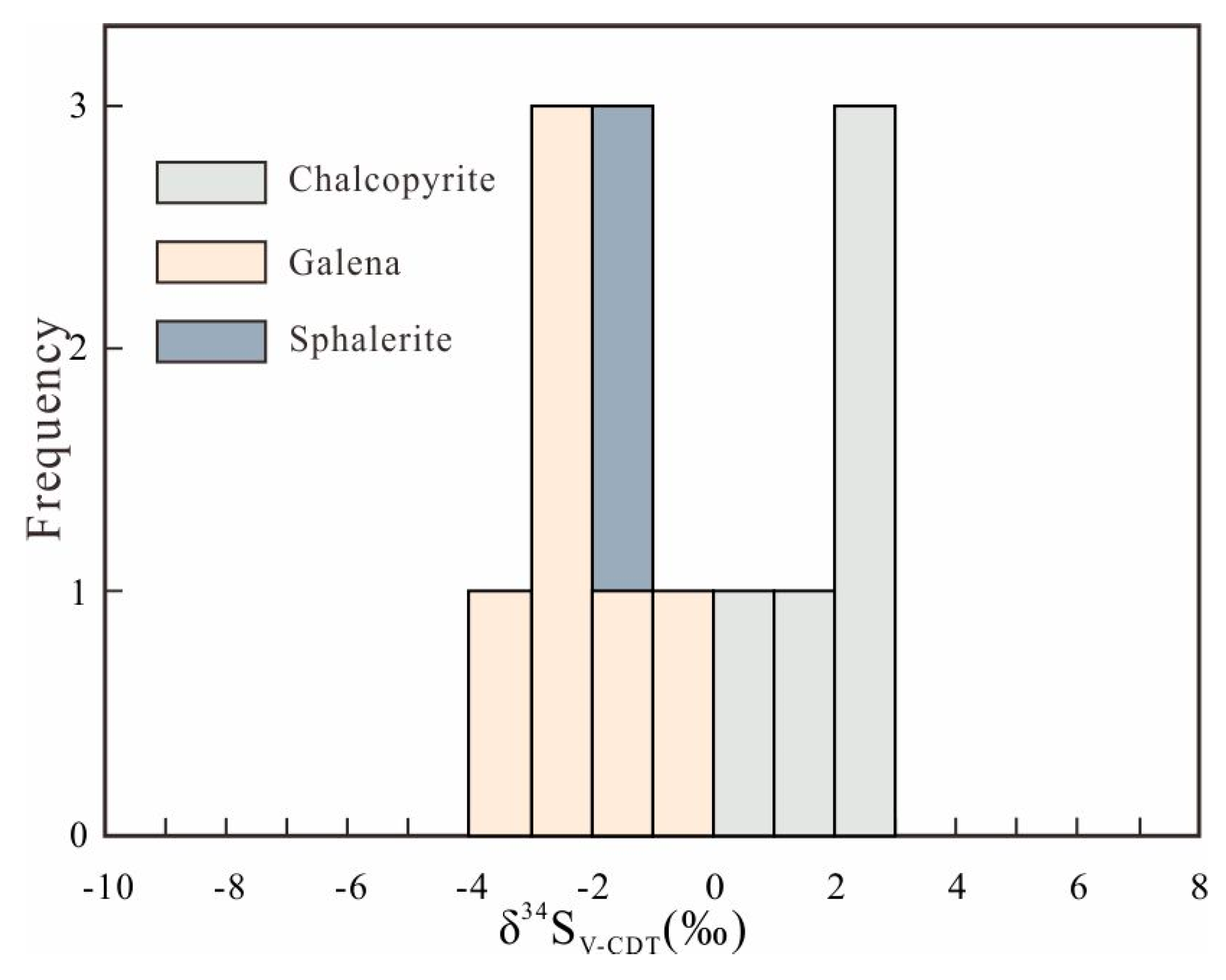
6.3. Sulfur Isotopes
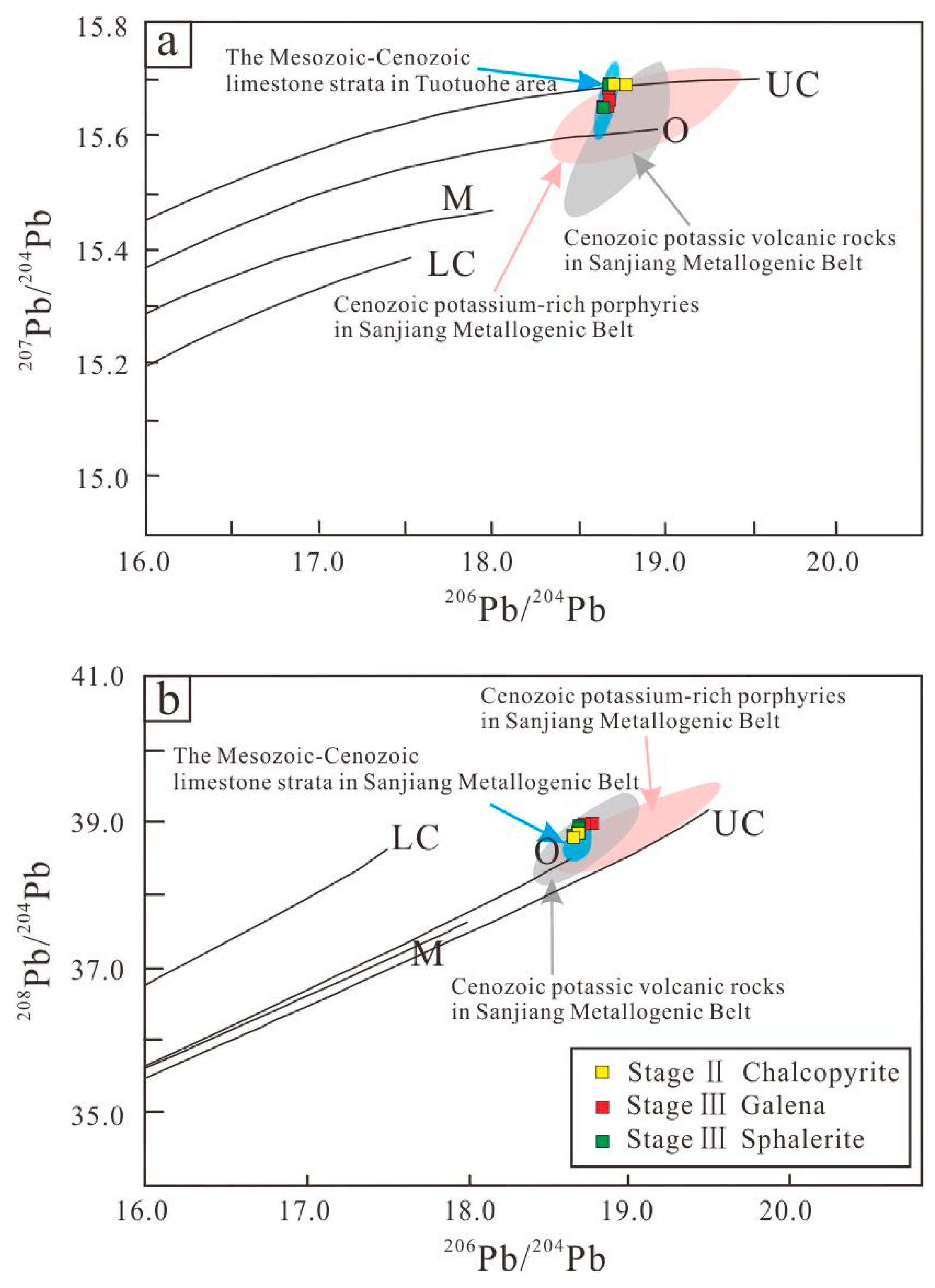
6.4. Lead Isotopes
7. Discussion
7.1. Origin and Evolution of the Ore-Forming Fluids
7.2. Source of the Ore-Forming Materials
7.3. P-T Conditions of Ore Deposition
7.4. Ore Precipitation Mechanism
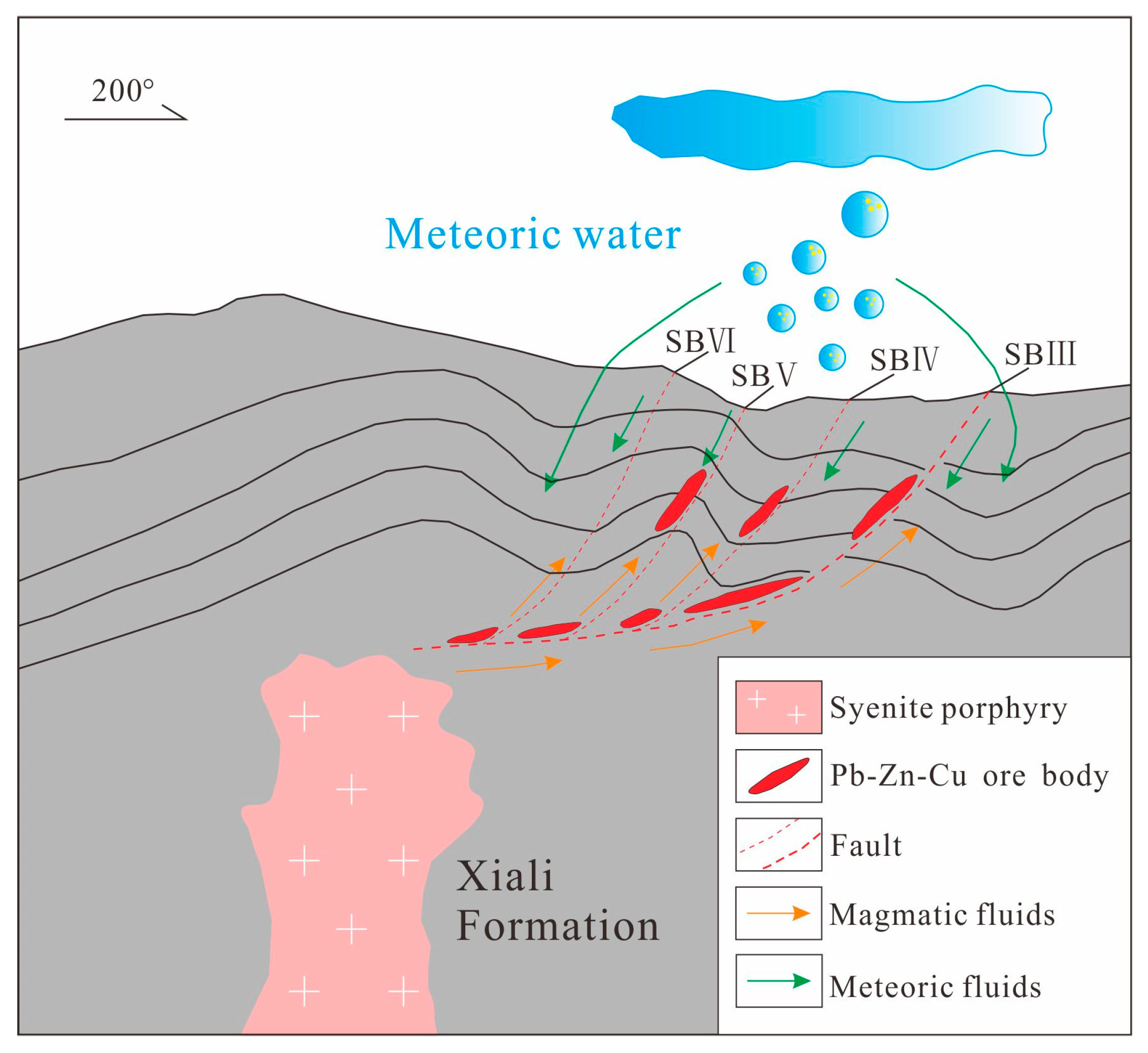
7.5. Metallogenic Model
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, D.C.; Zhao, Z.D.; Niu, Y.L.; Dilek, Y.; Hou, Z.Q.; Mo, X.X. The origin and pre-Cenozoic evolution of the Tibetan Plateau. Gondwana Res. 2013, 23, 1429–1454. [Google Scholar] [CrossRef]
- Hou, Z.Q.; Song, Y.C.; Li, Z.; Wang, Z.L.; Yang, Z.M. Thrust-controlled, sediments-hosted Pb-Zn –Ag–Cu deposits in eastern and northern margins of Tibetan orogenic belt: Geological features and tectonic model. Miner. Depos. 2008, 27, 123–144. (In Chinese) [Google Scholar]
- Hou, Z.Q.; Pan, G.T.; Wang, A.J.; Mo, X.X.; Tian, S.H. Metallogenesis in Tibetan collisional orogenic belt: II. Mineralization in late-collisional transformation setting. Miner. Depos. 2006, 25, 521–543. (In Chinese) [Google Scholar]
- Spurlin, M.S.; Yin, A.; Horton, B.K.; Zhou, J.; Wang, J. Structural evolution of the Yushu-Nangqian region and its relationship to syncollisional igneous activity, east-central Tibet. Geol. Soc. Am. Bull. 2005, 117, 1293–1317. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, Z.D.; Zhu, D.C.; Niu, Y.; DePaolo, D.J.; Harrison, T.M.; Mo, X.; Dong, G.; Zhou, S.; Sun, C.; et al. Postcollisional potassic and ultrapotassic rocks in southern Tibet: Mantle and crustal origins in response to India-Asia collision and convergence. Geochim. Cosmochim. Acta 2014, 143, 207–231. [Google Scholar] [CrossRef]
- Deng, J.; Wang, Q.F. Gold mineralization in China: Metallogenic provinces, deposit types and tectonic framework. Gondwana Res. 2016, 36, 219–274. [Google Scholar] [CrossRef]
- Li, G.J.; Wang, Q.F.; Huang, Y.H.; Gao, L.; Li, Y. Petrogenesis of Middle Ordovician peraluminous granites in the Baoshan block: Implications for the Early Paleozoic tectonic evolution along East Gondwana. Lithosphere 2016, 245, 76–92. [Google Scholar] [CrossRef]
- Xue, C.J.; Wang, R.; Liu, S.W.; Chi, G.X.; Qing, H.R.; Chen, Y.C.; Yang, J.M.; Wang, D.H. Geologic, fluid inclusion and isotopic characteristics of the Jinding Zn-Pb deposit, western Yunnan, South China: A review. Ore Geol. Rev. 2007, 31, 337–359. [Google Scholar] [CrossRef]
- He, L.Q.; Song, Y.C.; Chen, K.X.; Hou, Z.Q.; Yu, F.M.; Yang, Z.S.; Wei, J.Q.; Li, Z.; Liu, Y.C. Thrust-controlled, sediment-hosted, Himalayan Zn-Pb-Cu-Ag deposits in the Lanping foreland fold belt, eastern margin of Tibetan Plateau. Ore Geol. Rev. 2009, 36, 106–132. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hou, Z.Q.; Yang, Z.S.; Tian, S.; Song, Y.; Yu, Y.; Ma, W. Geology and chronology of the Zhaofayong carbonate-hosted Pb-Zn ore cluster: Implication for regional Pb-Zn metallogenesis in the Sanjiang belt, Tibet. Gondwana Res. 2016, 35, 15–26. [Google Scholar] [CrossRef]
- Liu, Y.C.; Hou, Z.Q.; Yang, Z.S.; Tian, S.H.; Yang, T.N.; Song, Y.C.; Zhang, H.R.; Carranza, E.J.M. Formation of the Dongmozhazhua Pb-Zn deposit in the thrust-fold setting of the Tibetan plateau, China: Evidence from fluid inclusion and stable isotope data. Resour. Geol. 2011, 61, 384–406. [Google Scholar] [CrossRef]
- Liu, Y.C.; Yang, Z.S.; Tian, S.H.; Song, Y.C.; Zhang, H.R. Fluid origin of fluorite-rich carbonate-hosted Pb-Zn mineralization of the Himalayan–Zagros collisional orogenic system: A case study of the Mohailaheng deposit, Tibetan Plateau, China. Ore Geol. Rev. 2015, 70, 546–561. [Google Scholar] [CrossRef]
- Qian, Y. Metallogenic Setting and Metallogenesis Lead and Zinc in Tuotuohe Region, Qinghai-Tibet Plateau. Ph.D. Thesis, Jilin University, Changchun, China, 2014. (In Chinese). [Google Scholar]
- Song, Y.C.; Hou, Z.Q.; Wang, G.R.; Liu, Y.C.; Tian, S.H.; Yang, Z.S.; Liu, Y.X.; Zhang, H.R.; Yang, T.N.; LI, L.S. Metallogenic regularity and ore exploration targeting in Tuotuohe area, northern Sanjiang orogenic belt. Miner. Depos. 2015, 1, 1–20. (In Chinese) [Google Scholar]
- Sun, Y.G. Study on Ore-Controlling Structure Characteristics and Ore Genesis of Chuduoqu Lead-Zinc Mine in Tuotuohe Region, Qinghai Province. Master’s Thesis, Jilin University, Changchun, China, 2016. (In Chinese). [Google Scholar]
- Song, Y.C.; Yang, T.N.; Zhang, H.R.; Liu, Y.C.; Hao, H.D.; Li, Z. The Chaqupacha Mississippi Valley-type Pb-Zn deposit, central Tibet: Ore formation in a fold and thrust belt of the India-Asia continental collision zone. Ore Geol. Rev. 2015, 70, 533–545. [Google Scholar] [CrossRef]
- Yin, A.; Harrison, T.M. Geologic Evolution of the Himalayan-Tibetan Orogen. Annu. Rev. Earth Planet. Sci. 2000, 28, 211–280. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, C.S.; Zhao, X.X.; Yin, A.; Ma, C. Cenozoic thrust system, basin evolution, and uplift of the Tanggula Range in the Tuotuohe region, central Tibet. Gondwana Res. 2012, 22, 482–492. [Google Scholar] [CrossRef]
- Yang, T.N.; Zhang, H.R.; Liu, Y.X.; Wang, Z.L.; Song, Y.C.; Yang, Z.S.; Tian, S.H.; Xie, H.Q.; Hou, K.J. Permo-Triassic arc magmatism in central Tibet: Evidence from zircon U–Pb geochronology, Hf isotopes, rare earth elements, and bulk geochemistry. Chem. Geol. 2011, 284, 270–282. [Google Scholar] [CrossRef]
- Yang, T.N.; Hou, Z.Q.; Wang, Y.; Zhang, H.R.; Wang, Z.L. Late Paleozoic to Early Mesozoic tectonic evolution of northeast Tibet: Evidence from the Triassic composite western Jinsha–Garzê–Litang suture. Tectonics 2012, 31, 1–20. [Google Scholar] [CrossRef]
- Li, Y.L.; Wang, C.S.; Ma, C.; Xu, G.Q.; Zhao, X.X. Balanced cross-section and crustal shortening analysis in the Tanggula-Tuotuohe area, northern Tibet. J. Earth. Sci. 2011, 22, 1–10. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Zhang, K.J.; Li, Y.; Li, B.; Xie, Y. Characteristics and sedimentary facies of the Middle-Upper Jurassic clastic rocks in Qiangtang Basin, Tibet. Geo Meta. 2007, 31, 51–62. (In Chinese) [Google Scholar]
- Qian, Y.; Sun, F.Y.; Li, B.L.; Li, S.J.; Zhao, J.W. Early Permian-Late Triassic Magmatism in the Tuotuohe Region of the Qinghai-Tibet Plateau: Constraints on the Tectonic evolution of the Western Segment of the Jinshajiang Suture. Acta Geol. Sin. 2014, 88, 498–516. (In Chinese) [Google Scholar] [CrossRef]
- Hao, H.D.; Song, Y.C.; Li, L.S.; Jia, Z.Y.; Wang, Y.K.; Liu, Q. Characteristics of Breccias and C-O-Sr-S Isotope Geochemistry of the Duocaima Pb-Zn Deposit in Tuotuohe, Qinghai Province: Implications for the Ore-forming Process. Acta Geol. Sin. 2015, 89, 1568–1587. (In Chinese) [Google Scholar]
- Li, Y.L.; Wang, C.S.; Yi, H.S.; Liu, Z.F.; Li, Y. Cenozoic Thrust System and Uplifting of the Tanggula Mountain, Northern Tibet. Acta Geol. Sin. 2006, 80, 1118–1130. (In Chinese) [Google Scholar]
- Wang, J.H.; Yin, A.; Harrison, T.M.; Grove, M.; Zhang, Y.Q.; Xie, G.H. A tectonic model for Cenozoic igneous activities in the eastern Indo-Asian collision zone. Earth Planet Sci. Lett. 2001, 188, 123–133. [Google Scholar] [CrossRef]
- Ding, L.; Zhou, Y.; Zhang, J.J.; Deng, W.M. Geologic relationships and geochronology of the Cenozoic volcanoes and interbedded weathered mantles of Yulinshan in Qiangtang, North Tibet. Chin. Sci. Bull. 2000, 45, 2214–2220. (In Chinese) [Google Scholar] [CrossRef]
- Lai, S.C.; Liu, C.Y.; O'Reilly, S.Y. Petrogenesis and its significance to continental dynamics of the Neogene high-potassium calc-alkaline volcanic rock association from north Qiangtang, Tibetan Plateau. Sci. Chin. Ser. D 2001, 44, 44–45. (In Chinese) [Google Scholar] [CrossRef]
- Xia, L.Q.; Li, X.M.; Ma, Z.P.; Xu, X.Y.; Xia, Z.C. Cenozoic volcanism and tectonic evolution of the Tibetan plateau. Gondwana Res. 2010, 19, 850–866. [Google Scholar] [CrossRef]
- Li, Z. The ore Forming Genesis Research of Chaqupacha Lead–Zinc Deposits in Qinghai Province Tuotuohe Area. Master’s Thesis, Beijing University of Science and Technology, Beijing, China, 2008. (In Chinese). [Google Scholar]
- Sun, J.; Li, B.L.; Zhao, G.Q.; Liu, Y.F.; Zhang, J.Y. Zircon U-Pb chronology and geochemistry of Basihu diorite in Tuotuohe area,Qinghai-Tibetan Plateau. J. Guilin Univ. Technol. 2018, 38, 25–33. (In Chinese) [Google Scholar]
- Duan, Z.M.; Li, Y.; Zhang, Y.; Li, Y.L.; Wang, M. Zircon U–Pb age, continent dynamics significance and geochemical characteristics of the Mesozoic and Cenozoic granites from the Tanggula Range in the Qinghai-Tibet plateau. Acta Geol. Sin. 2005, 79, 88–97. (In Chinese) [Google Scholar]
- Li, L.; Bai, Y.S.; Niu, Z.J.; Yao, H.Z.; Duan, Q.F. The ultrabasic and basic rocks and their tectonic settings in the Qoimaco region, Qiangtang on the Qinghai-Xizang Plateau. Se. Geo. Teth. Geo. 2004, 24, 27–34. (In Chinese) [Google Scholar]
- Wang, G.Z. Study on Geological Features and Genetic of Zhalaxiageyong Cu-Pb-Zn Deposit in the Tuotuohe Area, Qinghai. Master’s Thesis, Jilin University, Changchun, China, 2017. (In Chinese). [Google Scholar]
- Roger, F.; Tapponnier, P.; Arnaud, N.; Schärer, U.; Brunel, M.; Zhiqin, X.; Jingsui, Y. An Eocene magmatic belt across central Tibet: Mantle subduction triggered by the Indian collision. Terra Nova. 2000, 12, 102–108. [Google Scholar] [CrossRef]
- Roedder, E. Fluid inclusions. Rev. Miner. 1984, 12, 1–644. [Google Scholar]
- Clayton, W.M.; Mayeda, T.K. The use of bromine pent a fluoride in the extraction of oxygen from oxides and silicates for isotopic analysis. Geochim. Cosmochim. Acta 1963, 27, 43–52. [Google Scholar] [CrossRef]
- Friedman, I. Deuterium content of natural waters and other substances. Geochim. Cosmochim. Acta 1953, 4, 89–103. [Google Scholar] [CrossRef]
- McCrea, J.M. On the isotopic chemistry of carbonates and a paleotemperature scale. J. Chem. Phys. 1950, 18, 849–857. [Google Scholar] [CrossRef]
- Friedman, I.; O’Neil, J.R. Complication of Stable Isotope Fractionation Factors of Geochemical Interest. In Data of Geochemistry—Sixth Edition; Fleischer, M., Ed.; US Government Printing Office: Washington, DC, USA, 1977. [Google Scholar]
- Hall, D.L.; Sterner, S.M.; Bodnar, R.J. Freezing point depression of NaCl-KCl-H2O solutions. Econ. Geol. 1988, 83, 197–202. [Google Scholar] [CrossRef]
- Crawford, M.L. Phase equilibria in aqueous fluid inclusions. In Fluid Inclusions: Application to Petrology; Hollister, L.S., Crawfor, M.L., Eds.; Mineralogical Association of Canada: Quebec City, QC, Canada, 1981; Volume 83, pp. 197–202. [Google Scholar]
- Bodnar, R.J. Revised equation and table for determining the freezing point depression of H2O–NaCl solutions. Geochim. Cosmochim. Acta 1993, 57, 683–684. [Google Scholar] [CrossRef]
- Clayton, R.N.; O’Neil, J.R.; Mayeda, T.K. Oxygen Isotope Exchange between Quartz and Water. J. Geophys. Res. 1972, 77, 3057–3067. [Google Scholar] [CrossRef]
- Taylor, H.P. The Application of Oxygen and Hydrogen Isotope Studies to Problems of Hydrothermal Alteration and Ore Deposition. Econ. Geol. 1974, 69, 843–883. [Google Scholar] [CrossRef]
- Barnes, H.L. Solubilities of ore minerals. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1979; pp. 404–460. [Google Scholar]
- Rye, R.O.; Ohmoto, H. Sulfur and carbon isotopes and ore genesis: A review. Econ. Geol. 1974, 69, 826–842. [Google Scholar] [CrossRef]
- Hedenquist, J.W.; Arribas, A.; Reynolds, T.J. Evolution of an intrusion- centered hydrothermal system: Far southeast-lepanto porphyry and epithermal Cu-Au deposits, Philippines. Econ. Geol. 1998, 93, 373–404. [Google Scholar] [CrossRef]
- Shepherd, T.J.; Rankin, A.H.; Alderton, D.H.M. A Practical Guide to Fluid Inclusion Studies; Blackie and Son Ltd.: London, UK, 1985. [Google Scholar]
- Taylor, B.E. Magmatic volatiles: Isotope variation of C, H and S. Rev. Miner. Geochem. 1986, 16, 185–225. [Google Scholar]
- Demény, A.; Ahijado, A.; Casillas, R.; Vennemann, T.W. Crustal contamination and fluid/rock interaction in the carbonatites of Fuerteventura (Canary Islands, Spain): A C, O, H isotope study. Lithosphere 1998, 44, 101–115. [Google Scholar] [CrossRef]
- Baker, A.J.; Fallick, A.E. Evidence from Lewisian limestones for isotopically heavy carbon in two-thousand-million-year-old sea water. Nature 1989, 337, 352–354. [Google Scholar] [CrossRef]
- Hoefs, J. Stable Isotope Geochemistry (4); Springer: Berlin, Germany, 1997; pp. 199–201. [Google Scholar]
- Taylor, H.P.; Frechen, J.; Degens, E.T. Oxygen and carbon isotope studies of carbonatites from the Laacher See District, West Germany and the Alnö District, Sweden. Geochim. Cosmochim. Acta 1967, 31, 407–430. [Google Scholar] [CrossRef]
- Ray, J.S.; Ramesh, R.; Pande, K. Carbon isotopes in Kerguelen plume-derived carbonatites: Evidence for recycled inorganic carbon. Earth Planet. Sci. Lett. 1999, 170, 205–214. [Google Scholar] [CrossRef]
- Hudson, J.D. Stable isotopes and limestone lithification. J. Geol. Soc. 1977, 133, 637–660. [Google Scholar] [CrossRef]
- Deines, P. Stable isotope variations in carbonatites. In Carbonatites: Genesis and Evolution; Bell, K., Ed.; Unwin Hyman: London, UK, 1989; pp. 301–359. [Google Scholar]
- Demeny, A.; Harangi, S.Z. Stable isotope studies on carbonate formations in alkaline basalt and lamprophyre series: Evolution of magmatic fluids and magma-sediment interactions. Lithosphere 1996, 37, 335–349. [Google Scholar]
- Hoernle, K.; Tilton, G.; Le Bas, M.J.; Duggen, S.; Garbe-Schönberg, D. Geochemistry of oceanic carbonatites compared with continental carbonatites: Mantle recycling of oceanic crustal carbonate. Contrib. Miner. Petrol. 2002, 142, 520–542. [Google Scholar] [CrossRef]
- Lorrain, A.; Savoye, N.; Chauvaud, L.; Paulet, Y.M.; Naulet, N. Decarbonation and preservation method for the analysis of organic C and N contents and stable isotope ratios of low-carbonated suspended particulate material. Anal. Chim. Acta 2003, 491, 125–133. [Google Scholar] [CrossRef]
- Chen, F.C.; Deng, J.; Wang, Q.F.; Li, G.J.; Shu, Q.H.; Yang, C.H.; Liu, J.Y.; Xu, R. The source and evolution of ore fluids in the Heiniuwa gold deposit, Baoshan block, Sanjiang region: Constraints from sulfide trace element, fluid inclusion and stable isotope studies. Ore. Geol. Rev. 2018, 95, 725–745. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Reynolds, T.J.; Kuehn, C.A. Fluid inclusion systematics in epithermal systems. Rev. Econ. Geol. 1985, 2, 25. [Google Scholar]
- Heinrich, C.A. Fluid-fluid interactions in magmatic-hydrothermal ore formation. Rev. Mineral. Geochem. 2007, 65, 363–387. [Google Scholar] [CrossRef]
- Ohmoto, H. Stable Isotope Geochemistry of Ore Deposits. Rev. Miner. 1986, 16, 491–559. [Google Scholar]
- Ripley, E.M. Origin and concentration mechanisms of copper and nickel in Duluth Complex sulfide zones, a dilemma. Econ. Geol. 1986, 81, 974–978. [Google Scholar] [CrossRef]
- Zhang, C.; Song, Y.C.; Hou, Z.Q. Geological and geochemical studies of the Nariniya lead-zinc depositin Tuotuohe area, Qinghai Province. Acta Petrol. Miner. 2013, 32, 291–304. (In Chinese) [Google Scholar]
- Li, Y.Z.; Kong, H.L.; Nanka, E.W. Analysis of Matter Source and Metallogenic Setting of Metallogenic Rock of Narigongma Porphyry Copper Molybdenum Deposit in Qinhai Province. Geol. Sci. Technol. Inf. 2015, 34, 1–7. [Google Scholar]
- Zartman, R.E.; Doe, B.R. Plumbotectonics—The model. Tectonophysics 1981, 75, 135–162. [Google Scholar] [CrossRef]
- Zhu, B.Q.; Zhang, Y.Q.; Xie, Y.W. Nd, Sr and Pb isotopic characterstics of cenozoic ultra-potassic volcanic rocks from eastern Erhai, Yunnan and their implications for subcontinent–mantle evolution in South China. Geochim 1992, 3, 201–212. [Google Scholar]
- Zhang, Y.Q.; Xie, Y.W.; Li, X.H.; Qiu, H.N.; Zhao, Z.H.; Liang, H.Y.; Zhong, S.L. Isotopic characteristics of potassium basalt series magmatic rocks in eastern Qinghai-Tibet Plateau: Petrogenesis and its tectonic significance. Sci. Chin. D 2000, 30, 493–498. (In Chinese) [Google Scholar]
- Deng, W.M.; Zhang, H.J.; Zhang, Y.Q. Petrogenesis of cenozoic potassic volcanic rocks in Nangqen basin. Chin. J. Geol. 2001, 36, 304–318. (In Chinese) [Google Scholar]
- Zhao, X.; Yu, X.H.; Mo, X.X.; Zhang, J.; Lv, B.X. Petrological and geochemical characteristics of Cenozoic alkali -rich porphyries and xenoliths hosted in Western Yunnan Province. Geoscience 2004, 18, 217–228. (In Chinese) [Google Scholar]
- Zhang, C. Study on the metalgenesis of the Nariniya lead-zinc deposits in the Tuotuohe area, Qinghai province. Master’s Thesis, East China Institute of Technology, Nanchang, China, 2013. (In Chinese). [Google Scholar]
- Hao, J.H.; Chen, J.P.; Dong, Q.J.; Li, Y.L.; Wang, T. Element and Sr-Nd-Pb isotope geochemistry of ore-bearing porphyries in northern Sanjiang Mo(Cu) ore belt, Qinghai Province: Implications for tectonic setting and petrogenesis. Acta Petrol. Miner. 1998, 30, 427–437. (In Chinese) [Google Scholar]
- Yang, Z.M.; Hou, Z.Q.; Yang, Z.S.; Wang, S.X.; Wang, G.R.; Tian, S.H.; Wen, D.Y.; Wang, Z.L.; Liu, Y.C. Genesis of porphyries and tectonic controls on the Narigongma porphyry Mo (-Cu) deposit, southern Qinghai. Acta Petrol. Sin. 2008, 24, 489–502. (In Chinese) [Google Scholar]
- Tian, S.H.; Yang, Z.S.; Hou, Z.Q.; Liu, Y.C.; Song, Y.C.; Wang, F.C.; Xue, W.W. Sulfur, lead, strontium and neodymium isotope compositions of the Dongmozhazhua lead-zinc ore deposit in the Yushu area, southern Qinghai: Implications for the sources of ore-forming material in the deposit. Acta Petrol. Sin. 2011, 27, 2173–2183. (In Chinese) [Google Scholar]
- Chung, S.L.; Lo, C.H.; Lee, T.Y.; Zhang, Y.; Xie, Y.; Li, X.; Wang, K.L.; Wang, P.L. Diachronous uplift of the Tibetan plateau starting 40 Myr ago. Nature 1998, 394, 769–773. [Google Scholar] [CrossRef]
- Zhao, Z.D.; Mo, X.X.; Dong, G.C. Pb isotopic geochemistry of Tibetan Plateau and its implications. Geoscience 2007, 2, 265–274. (In Chinese) [Google Scholar]
- Roedder, E.; Bodnar, R.J. Geologic pressure determinations from fluid inclusion studies. Annu. Rev. Earth Planet. Sci. 1980, 8, 263–301. [Google Scholar] [CrossRef]
- Roedder, E.; Bodnar, R.J. Fluid inclusion studies of hydrothermal ore deposits. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley: New York, NY, USA, 1997; pp. 657–697. [Google Scholar]
- Driesner, T.; Heinrich, C.A. The system H2O-NaCl. Part I: Correlation formulae for phase relations in temperature-pressure-composition space from 0 to 1000 °C, 0 to 5000 bar, and 0 to 1 XNaCl. Geochim. Cosmochim. Acta 2007, 71, 4880–4901. [Google Scholar] [CrossRef]
- Sibson, R.H.; Robert, F.; Poulsen, K.H. High angle reverse faults, fluid-pressure cycling, and mesothermal gold-quartz deposits. Geology 1988, 16, 551–555. [Google Scholar] [CrossRef]
- Zhong, R.C.; Brugger, J.; Chen, Y.J.; Li, W.B. Contrasting regimes of Cu, Zn and Pb transport in ore-forming hydrothermal fluids. Chem. Geol. 2015, 154–164, 154–164. [Google Scholar] [CrossRef]
- Seward, T.; Barnes, H. Metal transport by hydrothermal ore fluids. In Geochemistry of Hydrothermal Ore Deposits, 3rd ed.; Barnes, H., Ed.; Wiley: New York, NY, USA, 1997; pp. 435–486. [Google Scholar]
- Mao, G.Z.; Hua, R.M.; Long, G.M.; Lu, H.J. Rb-Sr Dating of Pyrite and Quartz Fluid Inclusions and Origin of Ore-forming Materials of the Jinshan Gold Deposit, Northeast Jiangxi Province, South China. Acta Geol. Sin. Engl. Ed. 2013, 87, 1658–1667. [Google Scholar] [CrossRef]
- Calagari, A.A. Fluid inclusion studies in quartz veinlets in the porphyry copper deposit at Sungun, East-Azarbaidjan, Iran. J. Asian Earth Sci. 2004, 23, 179–189. [Google Scholar] [CrossRef]
- Wang, L.L.; Sun, F.Y.; Xu, J.H.; Wang, K.Y.; Wang, L.; Xu, Q.L. Ore-forming fluid and evolution of Luming molybdenum deposit, Heilongjiang Province. Acta Petrol. Sin 2015, 31, 979–990. (In Chinese) [Google Scholar]
- O’Neil, J.R.; Silberman, M.L. Stable isotope relations in epithermal Au-Ag deposits. Econ. Geol. 1974, 69, 902–909. [Google Scholar] [CrossRef]
- Landtwing, M.; Pettke, T.; Halter, W.; Heinrich, C.; Redmond, P.; Einaudi, M.; Kunze, K. Copper deposition during quartz dissolution by cooling magmatichydrothermal magmatic-hydrothermal fluids: The Bingham porphyry. Earth Planet. Sci. Lett. 2005, 235, 229–243. [Google Scholar] [CrossRef]
- Beane, R.; Titley, S. Porphyry copper deposits; Part II: Hydrothermal alteration and mineralization. Econ. Geol. 1981, 75, 235–269. [Google Scholar]
- Partida, E.G.; Chavez, A.A.; Levresse, G.; Tritlla, J.; Camprubi, A. Genetic implications of fluid inclusions in skarn chimney ore, Las Animas Zn–Pb–Ag(–F) deposit, Zimapan, Mexico. Ore. Geol. Rev. 2003, 23, 91–96. [Google Scholar]
- Hezarkhani, A.; Williams-Jones, A.E.; Gammons, C.H. Factors controlling copper solubility and chalcopyrite deposition in the Sungun porphyry copper deposit, Iran. Miner. Depos. 1999, 34, 770–783. [Google Scholar] [CrossRef]
- Fournier, R.O. Hydrothermal processes related to movement of fluid from plastic into brittle rock in the magmatic-epithermal environment. Econ. Geol. 1999, 94, 1193–1211. [Google Scholar] [CrossRef]
- Ohmoto, H. Systematics of sulfur and carbon isotopes in hydrothermal ore deposits. Econ. Geol. 1972, 67, 551–578. [Google Scholar] [CrossRef]
- Sawkins, F.J. Sulfide ore deposits in relation to plate tectonics. J. Geol. 1972, 80, 377–397. [Google Scholar] [CrossRef]
- Benedezú, R.; Fonteboté, L. Cordilleran Epithermal Cu-Zn-Pb-(Au-Ag) Mineralization in the Colquijirca District, Central Peru: Deposit-Scale Mineralogical Patterns. Econ. Geol. 2009, 104, 905–944. [Google Scholar] [CrossRef]
- Kissin, S.A.; Mango, H. Silver vein deposits. In Treatise on Geochemistry, 2nd ed.; Elsevier: Oxford, UK, 2014; pp. 425–432. [Google Scholar]
- Aitchison, J.C.; Xia, X.; Baxter, A.T.; Ali, J.R. Detrital zircon U-Pb ages along the Yarlung-Tsangpo suture zone, Tibet: Implications for oblique convergence and collision between India and Asia. Gondwana Res. 2011, 20, 691–709. [Google Scholar] [CrossRef]
- Zhang, J.J.; Santosh, M.; Wang, X.X.; Guo, L.; Yang, X.; Zhang, B. Tectonics of the northern Himalaya since the India-Asia collision. Gondwana Res. 2011, 20, 939–960. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Yang, J.S.; Li, H.B.; Zhang, J.X.; Wu, C.L. Orogenic Plateau-Terrane Amalgamation, Collisional Orogeny and Uplifting of the Qinghai-Tibet Plateau; Geological Publishing House: Beijing, China, 2007. (In Chinese) [Google Scholar]
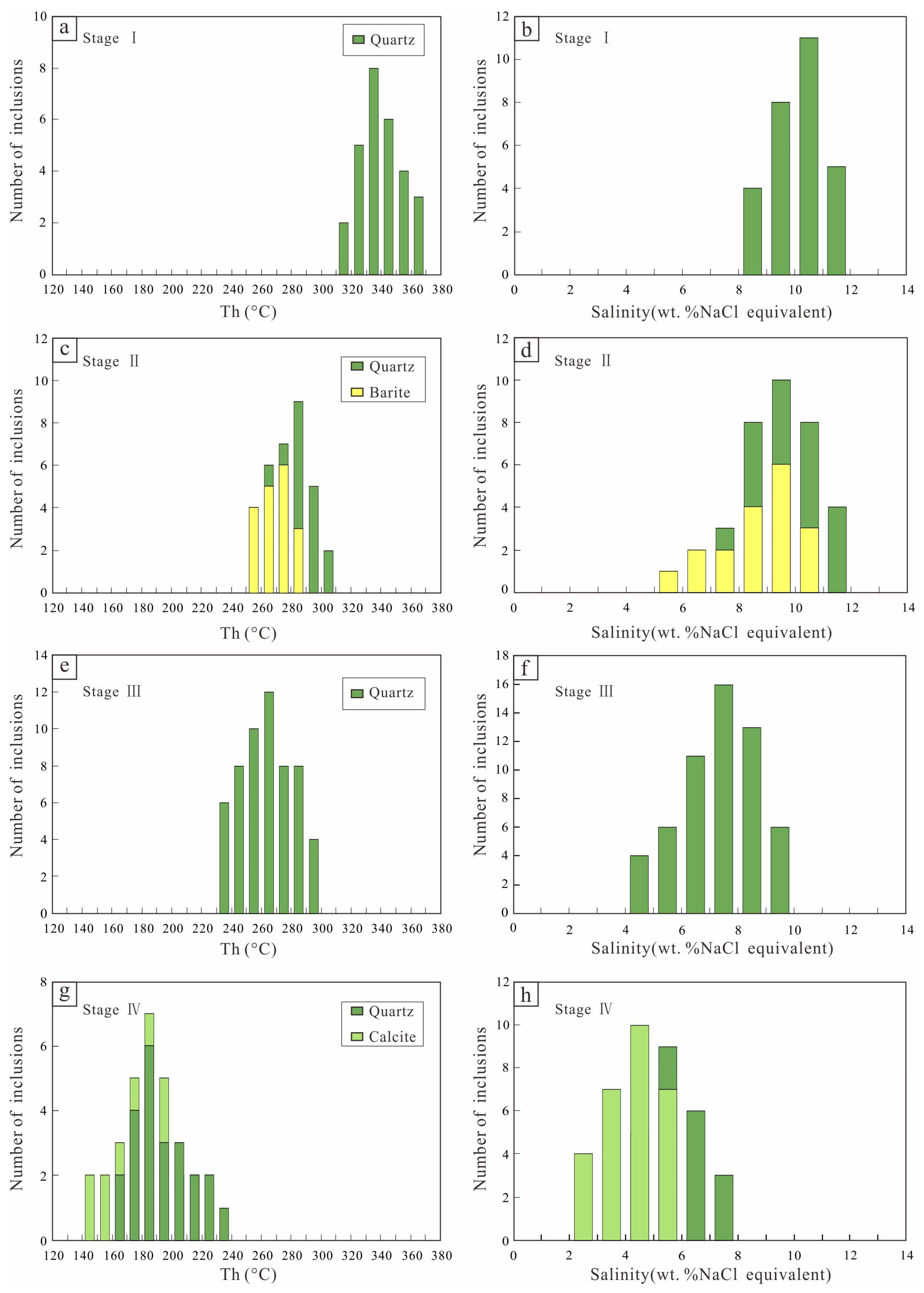

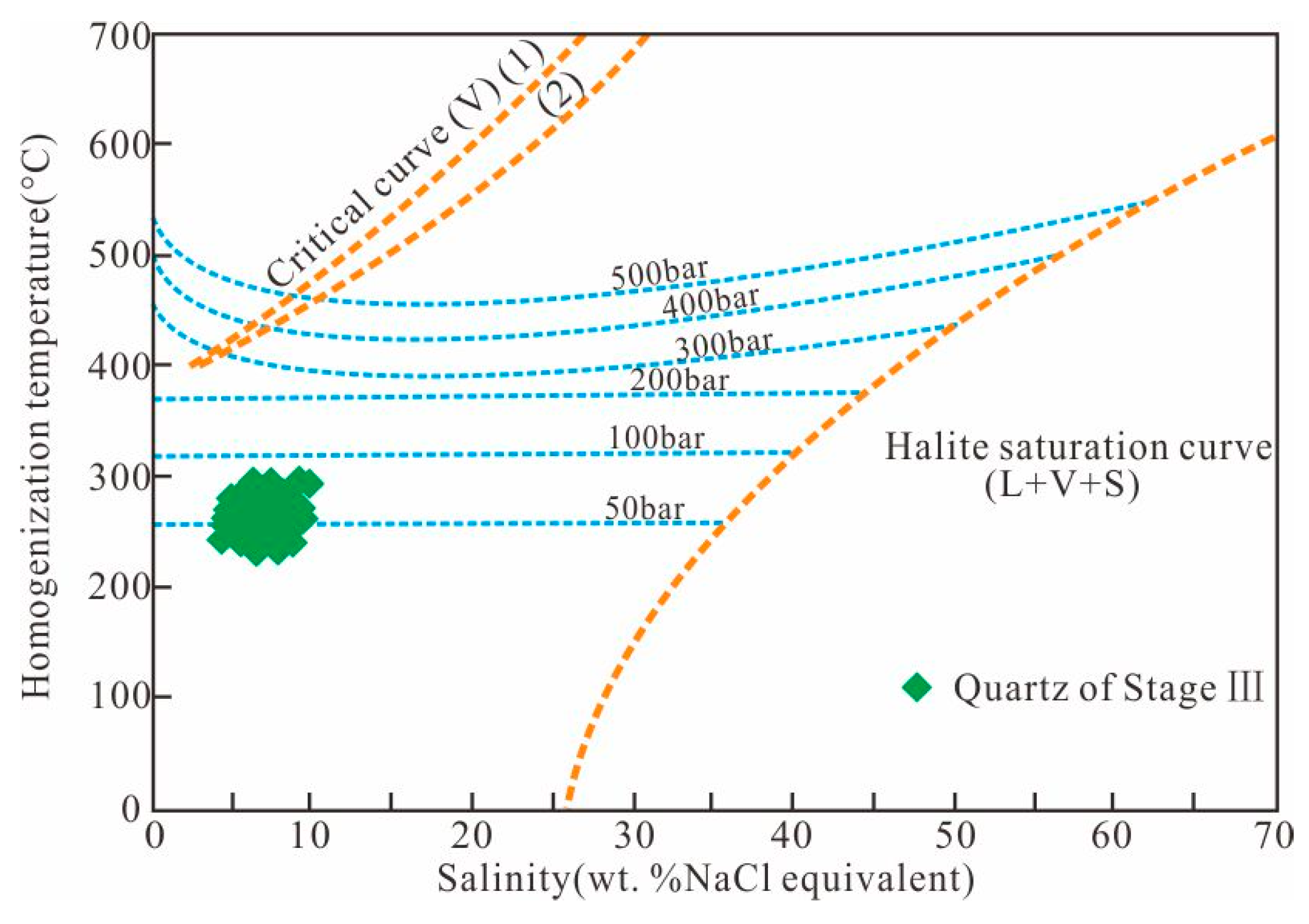
| Mineralized Stages | Host Minerals | Inclusion Type | No. | Tm-eu (°C) | Tm-ice (°C) | Th (°C) | Salinity (NaCl wt. %) |
|---|---|---|---|---|---|---|---|
| I | Quartz | L-type | 28 | from −8.1 to −5.6 | 318–370 | 8.68–11.81 | |
| II | Quartz | L-type | 33 | −29.5 ± 0.5 | from −7.8 to −4.8 | 250–308 | 7.59–11.46 |
| II | Barite | L-type | 18 | −31.2 ± 0.5 | from −7.2 to −3.3 | 250–287 | 5.41–10.73 |
| III | Quartz | L-type | 45 | −31.5 ± 0.5 | from −6.5 to −2.9 | 231–294 | 4.80–9.86 |
| III | Quartz | V-type | 11 | from −5.7 to −2.7 | 230–259 | 4.49–8.81 | |
| IV | Quartz | L-type | 23 | from −4.4 to −2.6 | 162–233 | 4.34–7.02 | |
| IV | Calcite | L-type | 28 | from −3.1 to −1.2 | 144–219 | 2.07–5.10 |
| Sample No. | CDQ-TC72-B5 | CDQ-TC72-B6 | CDQ-TC72-B7 | CDQ-P1-26 | CDQ-LX1-25 |
|---|---|---|---|---|---|
| Mineral | Quartz | Quartz | Quartz | Quartz | Quartz |
| Sample Location | M8 Orebody | M8 Orebody | M8 Orebody | M9 Orebody | M9 Orebody |
| Mineralization Stages | II | II | II | II | II |
| Major Ion Components (μg/g) | |||||
| Na+ | 1.4 | 2.92 | 3.53 | 2.7 | 3.07 |
| K+ | 3.49 | 1.61 | 2.61 | 1.57 | 3.69 |
| Mg2+ | 1.46 | 1.15 | 4.15 | 2.85 | 8.09 |
| Ca2+ | 8.2 | 4.96 | 17.89 | 16.93 | 48.92 |
| F− | 0.22 | 0.04 | 0.12 | 0.05 | 0.33 |
| Cl− | 5.53 | 8.49 | 10.64 | 2.73 | 4.23 |
| NO2− | 0.36 | 0.59 | 0.6 | 0.73 | 0.77 |
| NO3− | 0.51 | 0.66 | 1.17 | 0.22 | 0.79 |
| SO42− | 13.41 | 9.91 | 55.12 | 5.53 | 20.32 |
| Sample No. | Mineral | Sample Location | Temperature (°C) | Mineralization Stages | δ18OV-SMOW (‰) | δ18OH2O (‰) | δDV-SMOW (‰) |
|---|---|---|---|---|---|---|---|
| CDQ-ZK006-B3 | Quartz | M5 orebody | 319.2 | I | 15.3 | 9.1 | −96.2 |
| CDQ-ZK006-B4 | Quartz | M5 orebody | 318.1 | I | 14.2 | 7.9 | −93.8 |
| CDQ-TC72-B5 | Quartz | M8 orebody | 272.6 | II | 12.7 | 4.8 | −112.6 |
| CDQ-TC72-B6 | Quartz | M8 orebody | 261.9 | II | 13.6 | 5.2 | −112.7 |
| CDQ-TC72-B8 | Quartz | M8 orebody | 230.1 | III | 9.5 | −0.4 | −113.2 |
| CDQ-PM1-26 | Quartz | M9 orebody | 235.3 | III | 10.1 | 0.4 | −108.6 |
| CDQ-LX1-25 | Quartz | M9 orebody | 231.1 | III | 10.3 | 0.4 | −105.8 |
| Sample No. | Mineral | Sample Location | Mineralization Stages | δ13CPDB (‰) | δ18OPDB (‰) | δ18OSMOW (‰) | Reference |
|---|---|---|---|---|---|---|---|
| CDQ-PM1-B | Calcite | M11 orebody | IV | −5.4 | −19.3 | 11.0 | This study |
| CDQ-ZK3205-B10 | Calcite | M9 orebody | IV | −7.5 | −20.3 | 9.9 | This study |
| CDQ-SZK802-B2 | Calcite | M10 orebody | IV | −6.3 | −18.5 | 11.8 | This study |
| CDQ-LX3-W8 | Calcite | M1 orebody | IV | −6.5 | −18.3 | 12.0 | This study |
| CDQ-ZK006-C20 | Calcite | M5 orebody | IV | −5.6 | −19.2 | 11.1 | [13] |
| CDQ-ZK006-W14 | Calcite | M5 orebody | IV | −6.2 | −18.2 | 12.1 | [13] |
| CDQ-ZK006-W25 | Calcite | M5 orebody | IV | −6.3 | −18.7 | 11.6 | [13] |
| CDQ-ZK006-W26 | Calcite | M5 orebody | IV | −7.5 | −19.7 | 10.6 | [13] |
| Sample No. | Mineral | Sample Location | Mineralization Stages | δ34SCDT (‰) |
|---|---|---|---|---|
| CDQ-P1-B1 | Chalcopyrite | M11 orebody | II | 1.2 |
| CDQ-SZK802-B2 | Chalcopyrite | M10 orebody | II | 0.4 |
| CDQ-ZK3205-B5 | Chalcopyrite | M9 orebody | II | 2.9 |
| CDQ-ZK3205-B6 | Chalcopyrite | M9 orebody | II | 2.8 |
| CDQ-ZK3205-B7 | Chalcopyrite | M9 orebody | II | 2.5 |
| CDQ-ZK3205-B8 | Galena | M9 orebody | III | −0.3 |
| CDQ-ZK3205-B9 | Galena | M9 orebody | III | −1.7 |
| CDQ-ZK3205-B10 | Galena | M9 orebody | III | −2.6 |
| CDQ-TC29-B13 | Galena | M9 orebody | III | −3.8 |
| CDQ-ZK3205-B11 | Galena | M9 orebody | III | −2.3 |
| CDQ-ZK3205-B12 | Galena | M9 orebody | III | −2.1 |
| CDQ-ZK3205-B13 | Sphalerite | M9 orebody | III | −1.6 |
| CDQ-ZK3205-B14 | Sphalerite | M9 orebody | III | −1.4 |
| CDQ-ZK3205-B15 | Sphalerite | M9 orebody | III | −1.2 |
| Sample No. | Mineral | Sample Location | Mineralization Stages | 206Pb/204Pb | 207Pb/204Pb | 208Pb/204Pb | Reference |
|---|---|---|---|---|---|---|---|
| CDQ-ZK3205-B6 | Chalcopyrite | M9 orebody | II | 18.773 | 15.691 | 39.014 | This study |
| CDQ-ZK3205-B7 | Chalcopyrite | M9 orebody | II | 18.724 | 15.694 | 38.992 | This study |
| CDQ-ZK3205-B8 | Galena | M9 orebody | III | 18.652 | 15.653 | 38.803 | This study |
| CDQ-ZK3205-B9 | Galena | M9 orebody | III | 18.682 | 15.664 | 38.854 | This study |
| CDQ-TC29-B13 | Galena | M9 orebody | III | 18.689 | 15.664 | 38.858 | [13] |
| CDQ-ZK3205-B11 | Galena | M9 orebody | III | 18.687 | 15.682 | 38.904 | [13] |
| CDQ-ZK3205-B12 | Galena | M9 orebody | III | 18.695 | 15.689 | 38.932 | [13] |
| CDQ-ZK3205-B13 | Sphalerite | M9 orebody | III | 18.655 | 15.651 | 38.810 | [13] |
| CDQ-ZK3205-B14 | Sphalerite | M9 orebody | III | 18.692 | 15.692 | 38.937 | [13] |
| CDQ-ZK3205-B15 | Sphalerite | M9 orebody | III | 18.694 | 15.694 | 38.943 | [13] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-G.; Li, B.-L.; Sun, F.-Y.; Qian, Y.; Yu, R.-T.; Zhao, T.-F.; Dong, J.-L. Ore Genesis of the Chuduoqu Pb-Zn-Cu Deposit in the Tuotuohe Area, Central Tibet: Evidence from Fluid Inclusions and C–H–O–S–Pb Isotopes Systematics. Minerals 2019, 9, 285. https://doi.org/10.3390/min9050285
Sun Y-G, Li B-L, Sun F-Y, Qian Y, Yu R-T, Zhao T-F, Dong J-L. Ore Genesis of the Chuduoqu Pb-Zn-Cu Deposit in the Tuotuohe Area, Central Tibet: Evidence from Fluid Inclusions and C–H–O–S–Pb Isotopes Systematics. Minerals. 2019; 9(5):285. https://doi.org/10.3390/min9050285
Chicago/Turabian StyleSun, Yong-Gang, Bi-Le Li, Feng-Yue Sun, Ye Qian, Run-Tao Yu, Tuo-Fei Zhao, and Jun-Lin Dong. 2019. "Ore Genesis of the Chuduoqu Pb-Zn-Cu Deposit in the Tuotuohe Area, Central Tibet: Evidence from Fluid Inclusions and C–H–O–S–Pb Isotopes Systematics" Minerals 9, no. 5: 285. https://doi.org/10.3390/min9050285
APA StyleSun, Y.-G., Li, B.-L., Sun, F.-Y., Qian, Y., Yu, R.-T., Zhao, T.-F., & Dong, J.-L. (2019). Ore Genesis of the Chuduoqu Pb-Zn-Cu Deposit in the Tuotuohe Area, Central Tibet: Evidence from Fluid Inclusions and C–H–O–S–Pb Isotopes Systematics. Minerals, 9(5), 285. https://doi.org/10.3390/min9050285




