Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Reagents
2.2. Materials Synthesis
2.3. Characterization
3. Results and Discussion
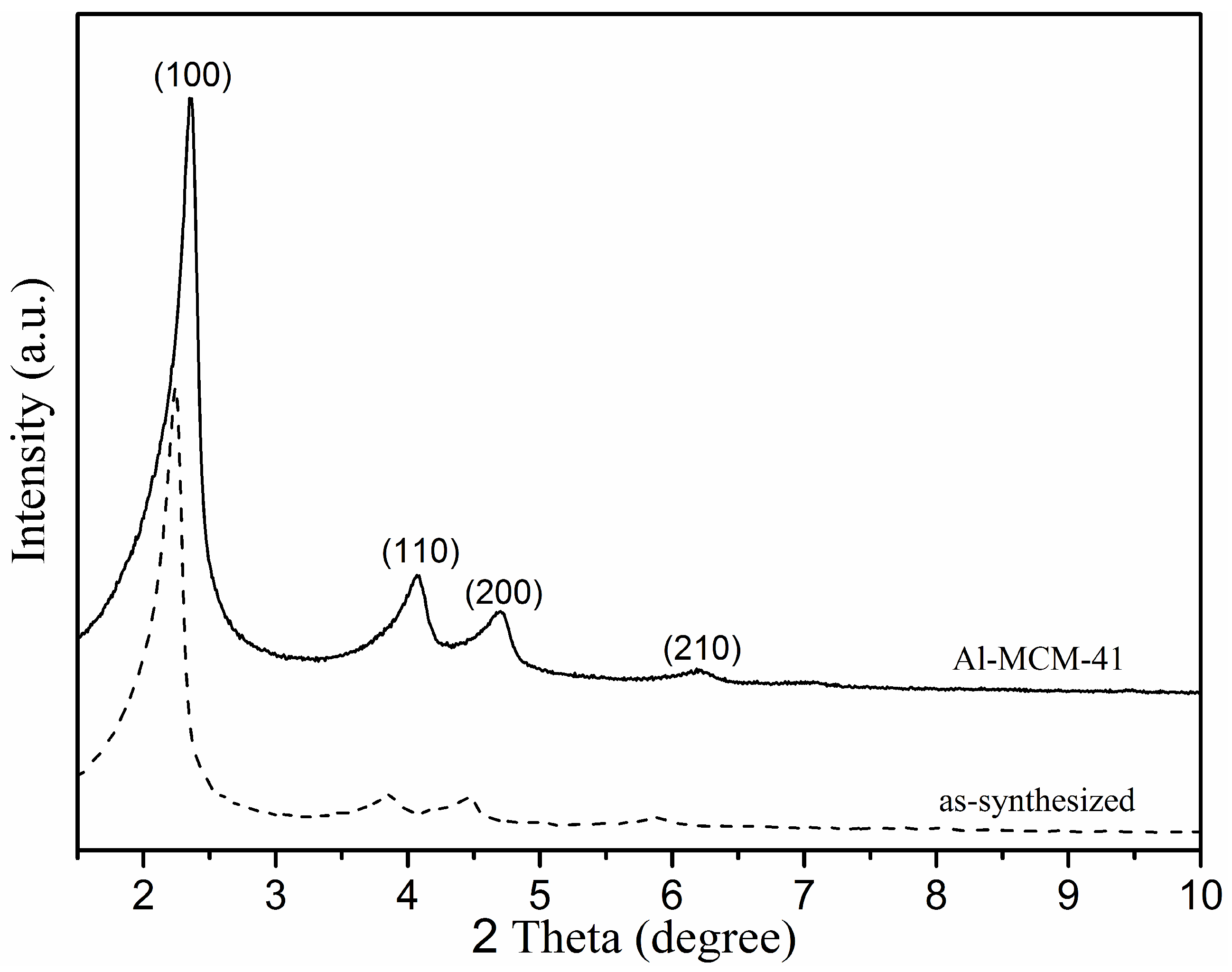
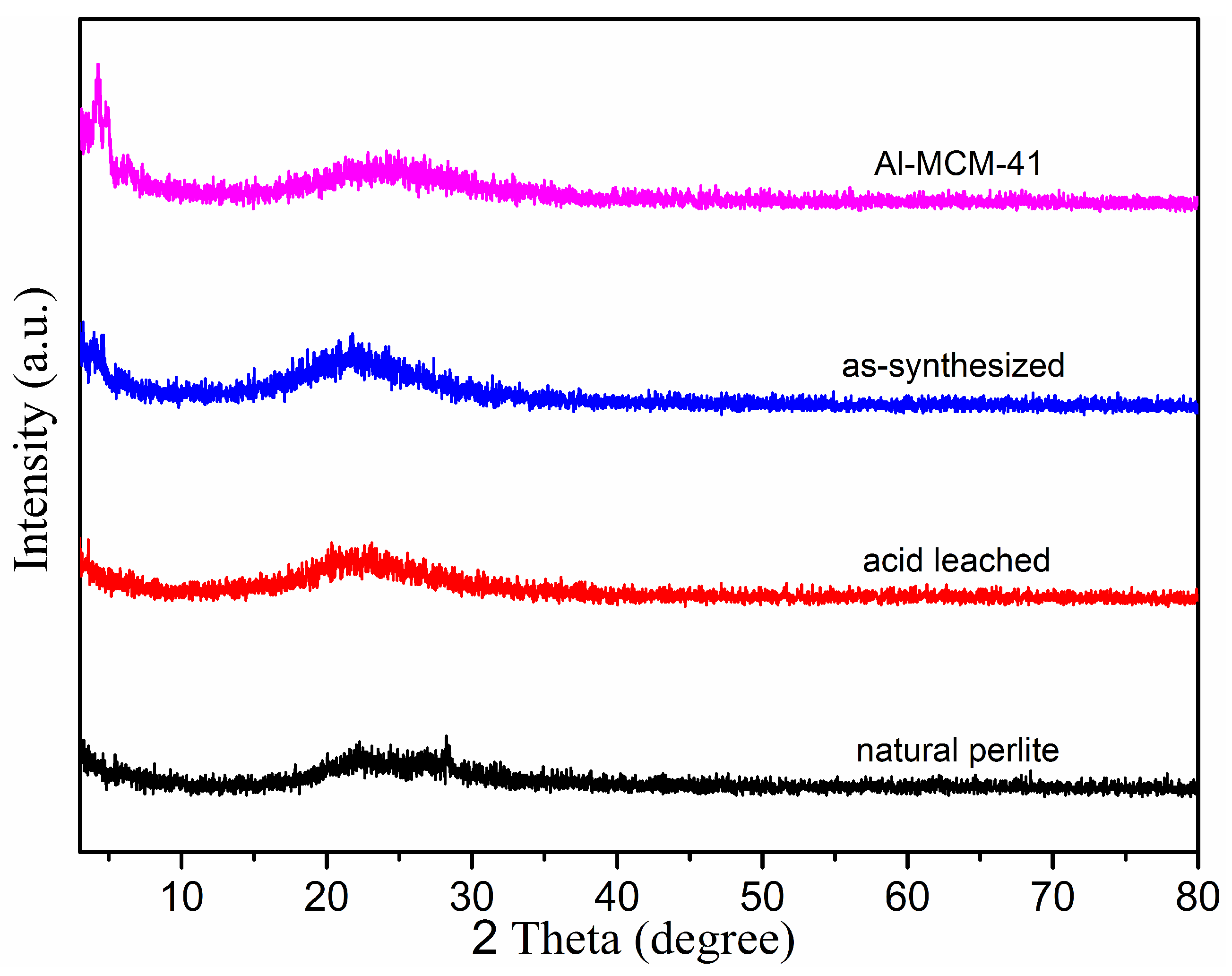
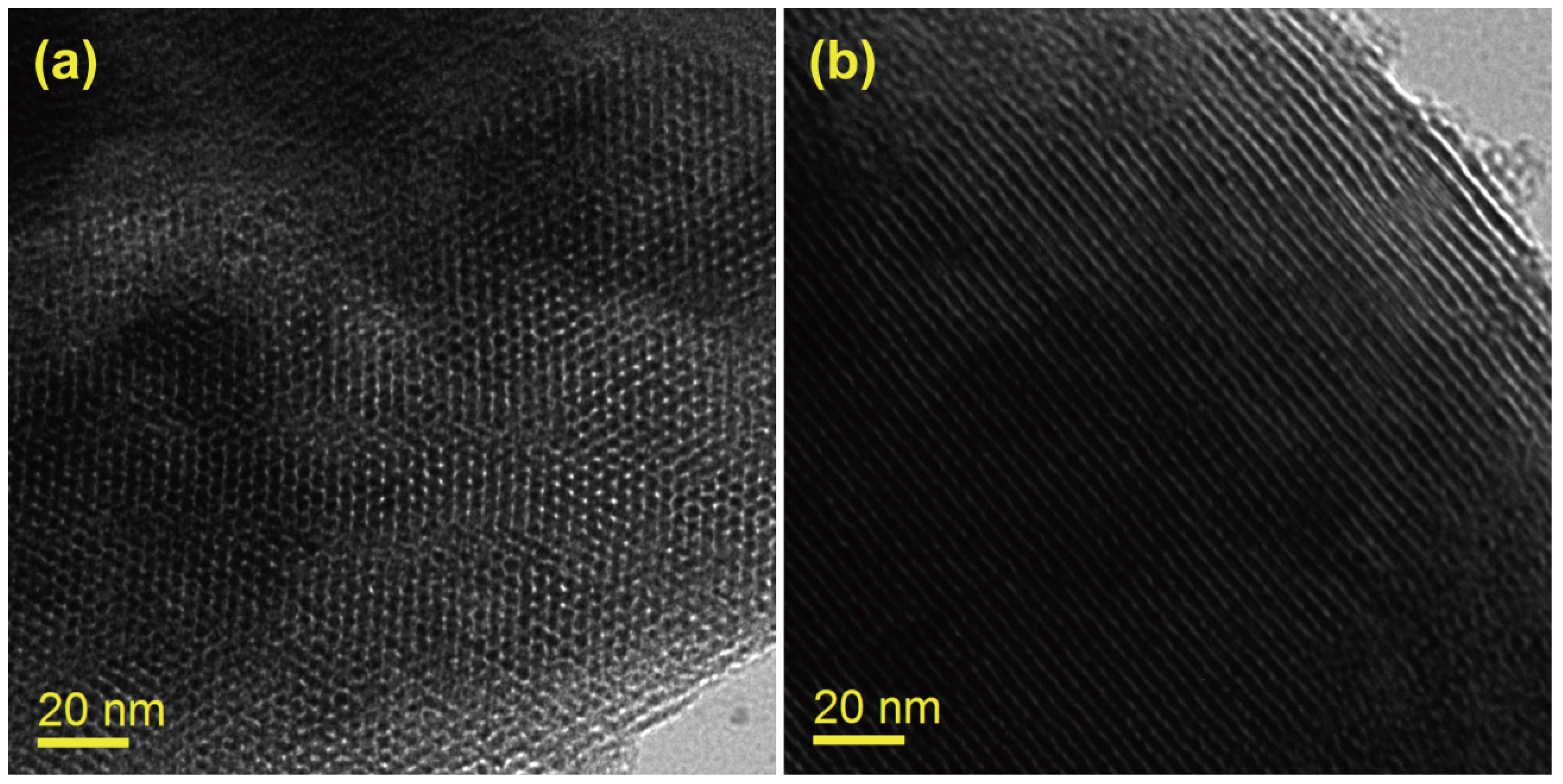
3.1. Phase and Morphology Analysis
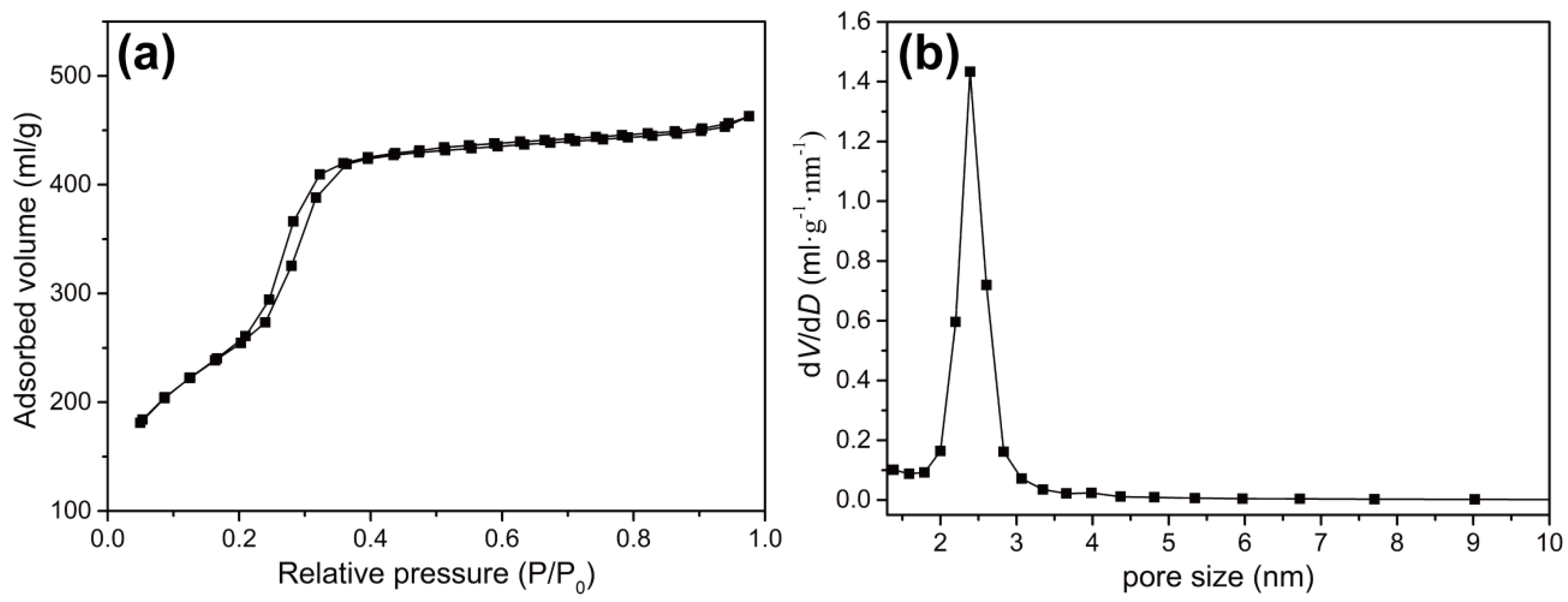
3.2. Porosity Measurements
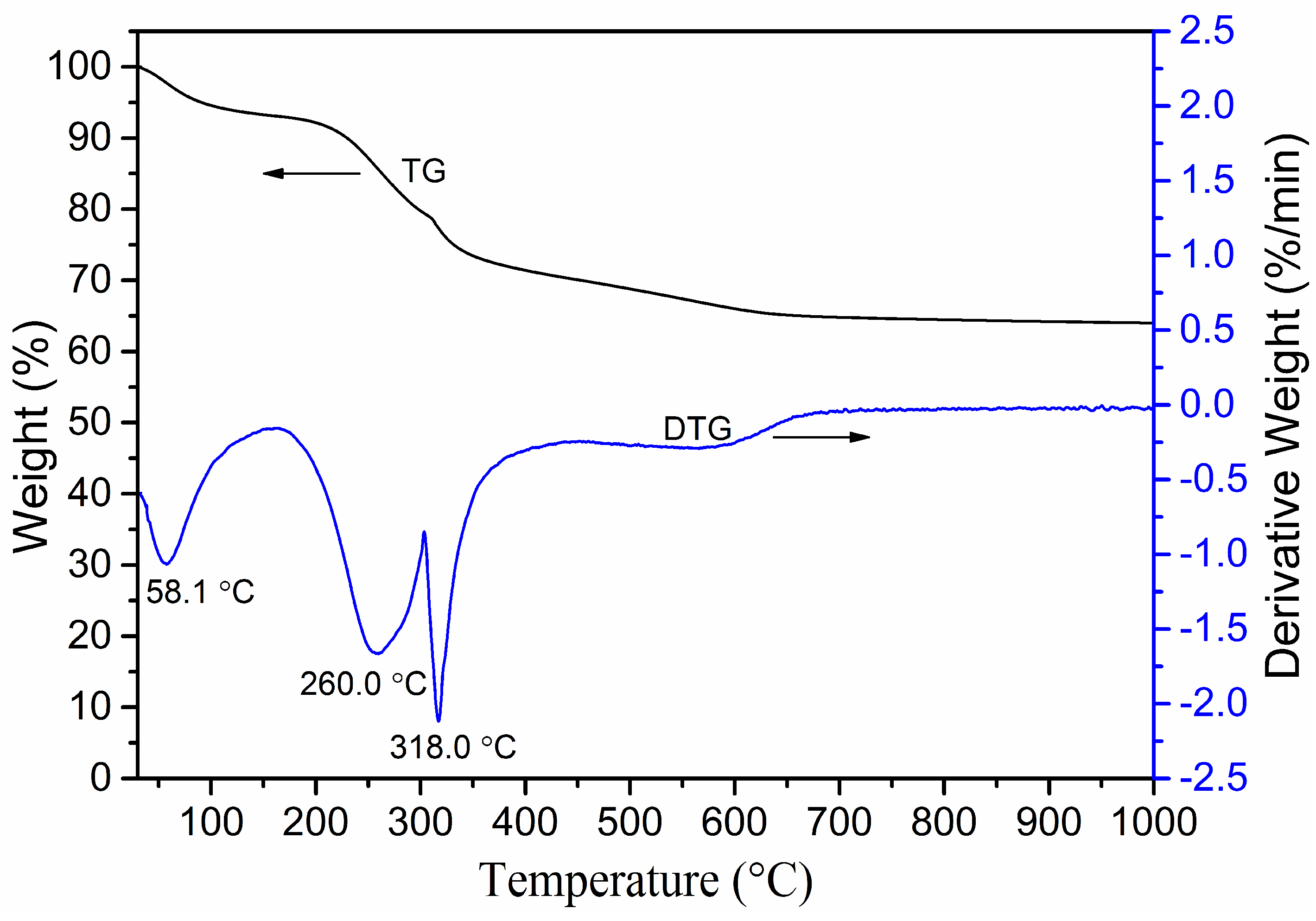
3.3. TG–DTG Analysis
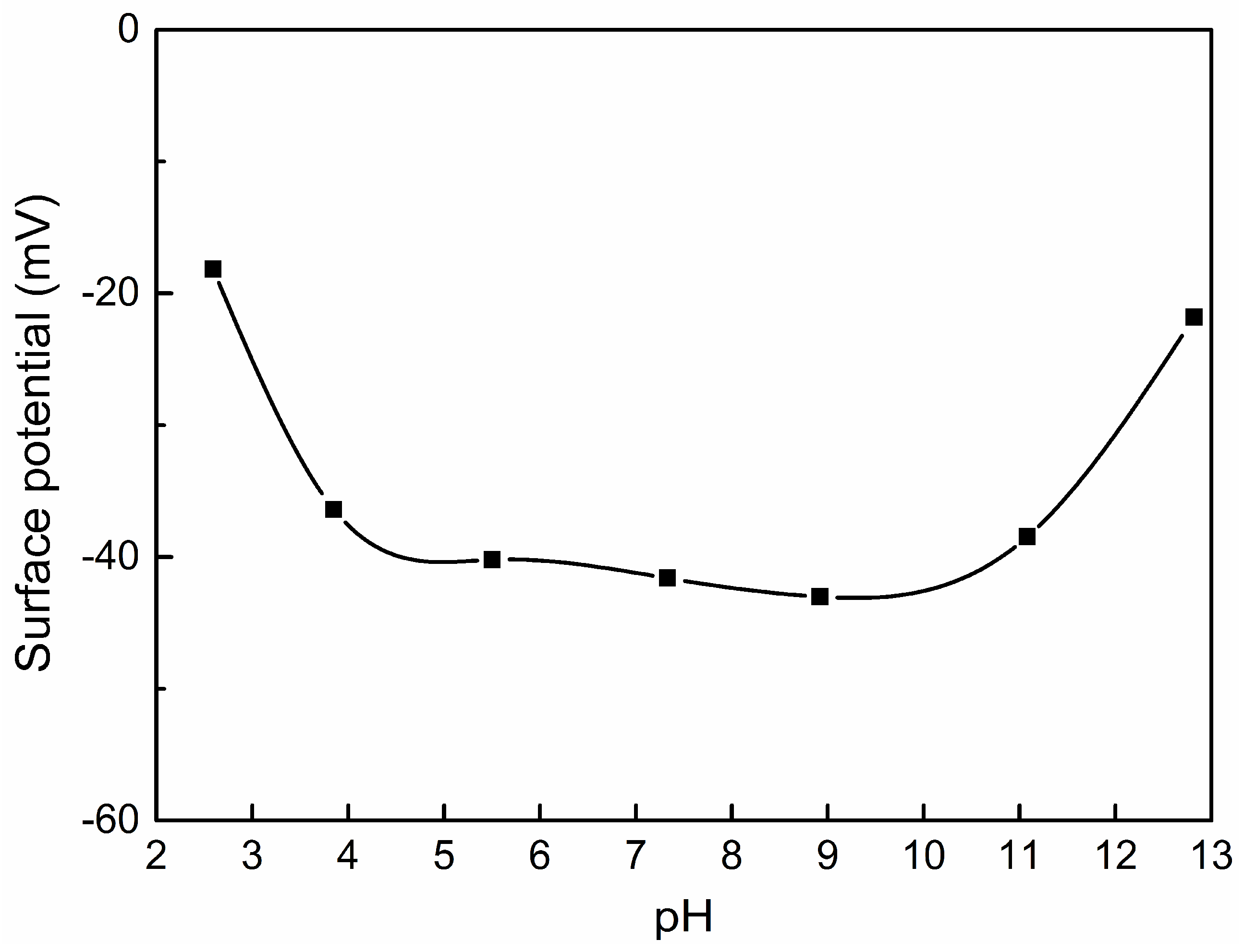
3.4. Surface Charge
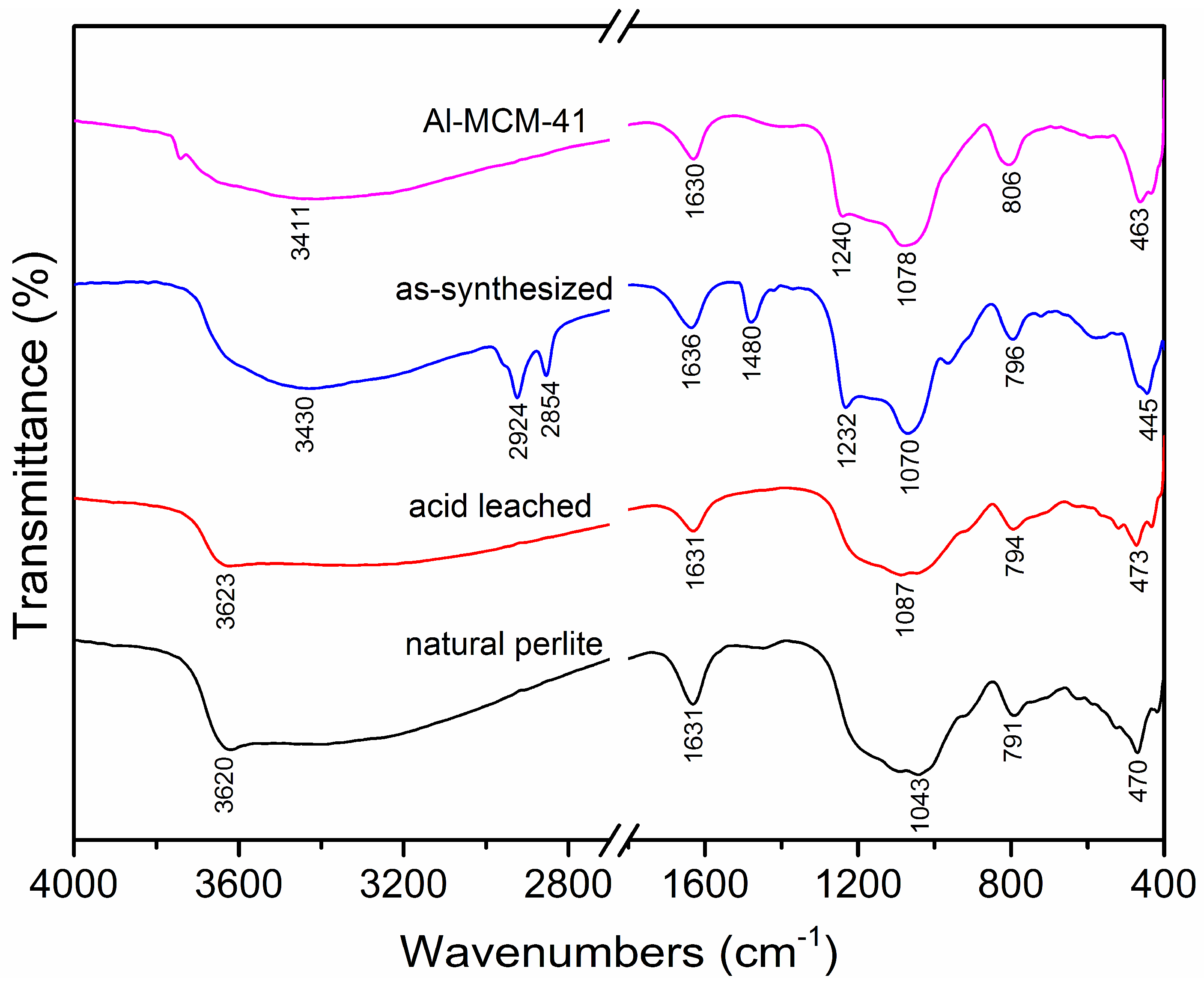
3.5. FTIR Spectra
3.6. Proposed Mechanism from Natural Perlite to Al–MCM–41
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.-W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A new family of mesoporous molecular sieves prepared with liquid crystal templates. J. Am. Chem. Sot. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Zhang, H.; Sun, H.; Zhang, D.; Zhang, W.; Chen, S.; Li, M.; Liang, P. Nanoconfinement of Ag nanoparticles inside mesoporous channels of MCM-41 molecule sieve as a regenerable and H2O resistance sorbent for Hg0 removal in natural gas. Chem. Eng. J. 2019, 361, 139–147. [Google Scholar] [CrossRef]
- Reiser, S.; Türk, M. Influence of temperature and high-pressure on the adsorption behavior of scCO2 on MCM-41 and SBA-15. J. Supercrit. Fluids 2019, 144, 122–133. [Google Scholar] [CrossRef]
- Rizzi, V.; Prasetyanto, E.A.; Chen, P.; Gubitosa, J.; Fini, P.; Agostiano, A.; Cola, D.L.; Cosma, P. Amino grafted MCM-41 as highly efficient and reversible ecofriendly adsorbent material for the direct blue removal from wastewater. J. Mol. Liq. 2019, 273, 435–446. [Google Scholar] [CrossRef]
- Chen, X.; Ching, W.K.; Lam, K.F.; Wei, W.; Yeung, K.L. An investigation of the selective adsorptions of metals on mesoporous NH2-MCM-41. J. Phys. Chem. C 2016, 120, 18365–18376. [Google Scholar] [CrossRef]
- Ahmed, S.; Ramli, A.; Yusup, S. Development of polyethylenimine-functionalized mesoporous Si-MCM-41 for CO2 adsorption. Fuel Process. Technol. 2017, 167, 622–630. [Google Scholar] [CrossRef]
- Nkinahamira, F.; Su, T.; Xie, Y.; Ma, G.; Wang, H.; Li, J. High pressure adsorption of CO2 on MCM-41 grafted with quaternary ammonium ionic liquids. Chem. Eng. J. 2017, 326, 831–838. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Zhou, H. Preparation and characterization of DUB-loaded MCM-41 for adsorption of CO2. Energy 2018, 149, 414–423. [Google Scholar] [CrossRef]
- Martínez-Edo, G.; Balmori, A.; Pontón, I.; Martí del Rio, A.; Sánchez-García, D. Functionalized ordered mesoporous silicas (MCM-41): Synthesis and applications in catalysis. Catalysts 2018, 8, 617. [Google Scholar] [CrossRef]
- Jeirani, Z.; Soltan, J. Improved formulation of Fe-MCM-41 for catalytic ozonation of aqueous oxalic acid. Chem. Eng. J. 2017, 307, 756–765. [Google Scholar] [CrossRef]
- Lin, X.; Zhong, A.; Sun, Y.; Zhang, X.; Song, W.; Lu, R.; Cao, A.; Wan, L. In situ encapsulation of Pd inside the MCM-41 channel. Chem. Commun. 2015, 51, 7482–7485. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- Brezoiu, A.-M.; Deaconu, M.; Nicu, I.; Vasile, E.; Mitran, R.-A.; Matei, C.; Berger, D. Heteroatom modified MCM-41-silica carriers for lomefloxacin delivery systems. Microporous Mesoporous Mater. 2019, 275, 214–222. [Google Scholar] [CrossRef]
- Varache, M.; Bezverkhyy, I.; Saviot, L.; Bouyer, F.; Baras, F.; Bouyer, F. Optimization of MCM-41 type silica nanoparticles for biological applications: Control of size and absence of aggregation and cell cytotoxicity. J. Non-Cryst. Solids 2015, 408, 87–97. [Google Scholar] [CrossRef]
- Zhao, Q.; Fu, L.; Jiang, D.; Xi, Y.; Yang, H. A nanoclay-induced defective g-C3N4 photocatalyst for highly efficient catalytic reactions. Chem. Commun. 2018, 54, 8249–8252. [Google Scholar] [CrossRef]
- Fu, L.; Yan, Z.; Zhao, Q.; Yang, H. Novel 2D nanosheets with potential applications in heavy metal purification: A review. Adv. Mater. Interfaces 2018, 5, 1801094. [Google Scholar] [CrossRef]
- Yan, Z.; Yang, H.; Ouyang, J.; Tang, A. In situ loading of highly-dispersed CuO nanoparticles on hydroxyl group-rich SiO2-AlOOH composite nanosheets for CO catalytic oxidation. Chem. Eng. J. 2017, 316, 1035–1046. [Google Scholar] [CrossRef]
- Zhang, Y.; Tang, A.; Yang, H.; Ouyang, J. Applications and interfaces of halloysite nanocomposites. Appl. Clay Sci. 2016, 119, 8–17. [Google Scholar] [CrossRef]
- Ali-dahmane, T.; Adjdir, M.; Hamacha, R.; Villieras, F.; Bengueddach, A.; Weidler, P.G. The synthesis of MCM-41 nanomaterial from algerian bentonite: The effect of the mineral phase contents of clay on the structure properties of MCM-41. C. R. Chim. 2014, 17, 1–6. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Liu, X.; Sun, J.; Sha, G.; Yang, J.; Meng, C. A novel pathway for the synthesis of ordered mesoporous silica from diatomite. Mater. Lett. 2014, 119, 150–153. [Google Scholar] [CrossRef]
- Jin, S.; Cui, K.; Guan, H.; Yang, M.; Liu, L.; Lan, C. Preparation of mesoporous MCM-41 from natural sepiolite and its catalytic activity of cracking waste polystyrene plastics. Appl. Clay Sci. 2012, 56, 1–6. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, T.; Gao, Q.; Alshameri, A.; Zhu, P.; Wang, H.; Qiu, X.; Ma, Y.; Yan, C. Synthesis and characterization of ordered mesoporous aluminosilicate molecular sieve from natural halloysite. J. Taiwan Inst. Chem. Eng. 2014, 45, 1073–1079. [Google Scholar] [CrossRef]
- Du, C.; Yang, H. Simple synthesis and characterization of nanoporous materials from talc. Clays Clay Min. 2009, 57, 290–301. [Google Scholar] [CrossRef]
- Yang, H.; Tang, A.; Ouyang, J.; Li, M.; Mann, S. From natural attapulgite to mesoporous materials: Methodology, characterization and structural evolution. J. Phys. Chem. B 2010, 114, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Deng, Y.; Du, C.; Jin, S. Novel synthesis of ordered mesoporous materials Al–MCM–41 from bentonite. Appl. Clay Sci. 2010, 47, 351–355. [Google Scholar] [CrossRef]
- Jin, J.; Ouyang, J.; Yang, H. One-step synthesis of highly ordered Pt/MCM-41 from natural diatomite and the superior capacity in hydrogen storage. Appl. Clay Sci. 2014, 99, 246–253. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, Y.; Ouyang, J.; Yang, H. Mesoporous material Al–MCM–41 from natural halloysite. Phys. Chem. Miner. 2014, 41, 497–503. [Google Scholar] [CrossRef]
- Du, C.; Yang, H. Investigation of the physicochemical aspects from natural kaolin to Al–MCM–41 mesoporous materials. J. Colloid Interface Sci. 2012, 369, 216–222. [Google Scholar] [CrossRef]
- Chen, H.; Yang, H.; Xi, Y. Highly ordered and hexagonal mesoporous silica materials with large specific surface from natural rectorite mineral. Microporous Mesoporous Mater. 2019, 279, 53–60. [Google Scholar] [CrossRef]
- Zujovic, Z.; Wheelwright, W.V.K.; Kilmartin, P.A.; Hanna, J.V.; Cooney, R.P. Structural investigations of perlite and expanded perlite using 1H, 27Al and 29Si solid-state NMR. Ceram. Int. 2018, 44, 2952–2958. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Ouyang, J.; Yang, H. Emerging parallel dual 2D composites: Natural clay mineral hybridizing MoS2 and interfacial structure. Adv. Funct. Mater. 2016, 26, 2666–2675. [Google Scholar] [CrossRef]
- Yan, Z.; Fu, L.; Yang, H.; Ouyang, J. Amino-functionalized hierarchical porous SiO2-AlOOH composite nanosheets with enhanced adsorption performance. J. Hazard. Mater. 2018, 344, 1090–1100. [Google Scholar] [CrossRef]
- Peng, K.; Yang, H. Carbon hybridized montmorillonite nanosheets: Preparation, structural evolution and enhanced adsorption performance. Chem. Commun. 2017, 53, 6085–6088. [Google Scholar] [CrossRef]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Majchrzak-Kucęba, I.; Nowak, W. Characterization of MCM-41 mesoporous materials derived from polish fly ashes. Int. J. Miner. Process. 2011, 101, 100–111. [Google Scholar] [CrossRef]
- Kumar, P.; Mal, N.; Oumi, Y.; Yamana, K.; Sano, T. Mesoporous materials prepared using coal fly ash as the silicon and aluminium source. J. Mater. Chem. 2001, 11, 3285–3290. [Google Scholar] [CrossRef]
- Long, M.; Zhang, Y.; Huang, P.; Chang, S.; Hu, Y.; Yang, Q.; Mao, L.; Yang, H. Emerging nanoclay composite for effective hemostasis. Adv. Funct. Mater. 2018, 28, 1704452. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, M.; Huang, P.; Yang, H.; Chang, S.; Hu, Y.; Tang, A.; Mao, L. Intercalated 2D nanoclay for emerging drug delivery in cancer therapy. Nano Res. 2017, 10, 2633–2643. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Li, X.; Ouyang, J.; Yang, H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Majouli, A.; Younssi, S.A.; Tahiri, S.; Albizane, A.; Loukili, H.; Belhaj, M. Characterization of flat membrane support elaborated from local moroccan perlite. Desalination 2011, 277, 61–66. [Google Scholar] [CrossRef]
- Amama, P.B.; Lim, S.; Ciuparu, D.; Pfefferle, L.; Haller, G.L. Hydrothermal synthesis of MCM-41 using different ratios of colloidal and soluble silica. Microporous Mesoporous Mater. 2005, 81, 191–200. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, A.; Yang, H. Synthesis of nanoporous materials Al–MCM–41 from natural halloysite. Nano 2015, 10, 1550005. [Google Scholar] [CrossRef]
| Minerals | Synthesized MCM–41 | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) | References |
|---|---|---|---|---|---|
| Bentonite | MCM–41 | 494 | 0.72 | 3.8 | [20] |
| Diatomite | MCM–41 | 1124 | 1.65 | 4.9 | [21] |
| Sepiolite | MCM–41 | 1030 | 1.06 | 3.0 | [22] |
| Talc | MCM–41 | 974 | 1.00 | 2.8 | [24] |
| Attapulgite | Al–MCM–41 | 1030 | 0.96 | 3.7 | [25] |
| Bentonite | Al–MCM–41 | 1018 | 0.72 | 3.0 | [26] |
| Halloysite | Al–MCM–41 | 509 | 0.48 | 3.8 | [28] |
| Kaolin | Al–MCM–41 | 1041 | 0.97 | 3.7 | [29] |
| Rectorite | Al–MCM–41 | 1032 | 0.97 | 2.6 | [30] |
| Perlite | Al–MCM–41 | 1027 | 0.72 | 2.8 | This work |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, H.; Fu, S.; Fu, L.; Yang, H.; Chen, D. Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite. Minerals 2019, 9, 264. https://doi.org/10.3390/min9050264
Chen H, Fu S, Fu L, Yang H, Chen D. Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite. Minerals. 2019; 9(5):264. https://doi.org/10.3390/min9050264
Chicago/Turabian StyleChen, Hongyun, Siyao Fu, Liangjie Fu, Huaming Yang, and Deliang Chen. 2019. "Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite" Minerals 9, no. 5: 264. https://doi.org/10.3390/min9050264
APA StyleChen, H., Fu, S., Fu, L., Yang, H., & Chen, D. (2019). Simple Synthesis and Characterization of Hexagonal and Ordered Al–MCM–41 from Natural Perlite. Minerals, 9(5), 264. https://doi.org/10.3390/min9050264





