Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.1.1. Iron Ore Tailings
2.1.2. Reducing Coal
2.1.3. Additives
2.1.4. S75, Portland Cement PI42.5, and Standard Sand
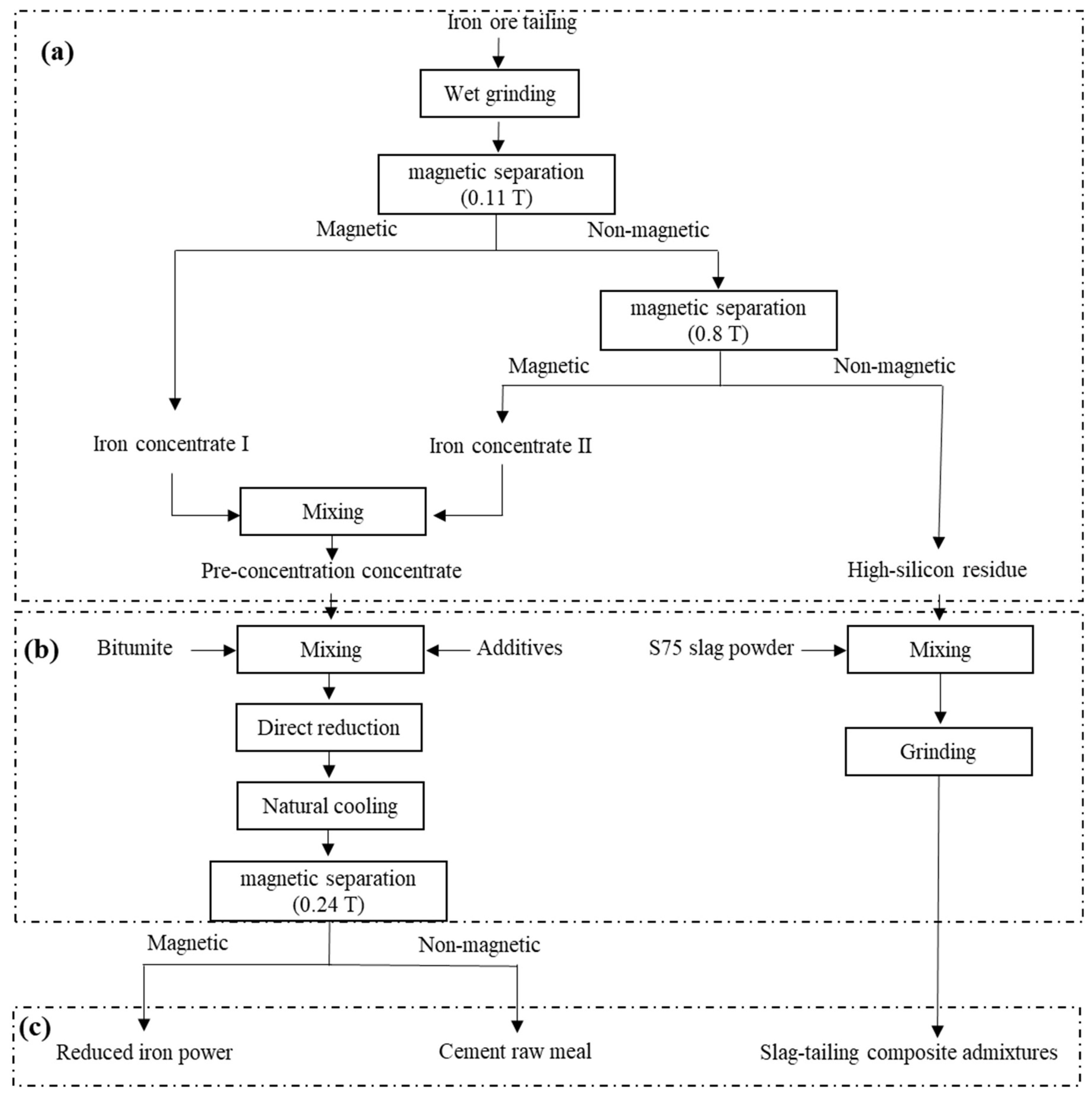
2.2. Experimental Methods
3. Results and Discussion
3.1. Pre-Concentration
3.2. Iron Recovery through Direct Reduction Followed by Magnetic Separation
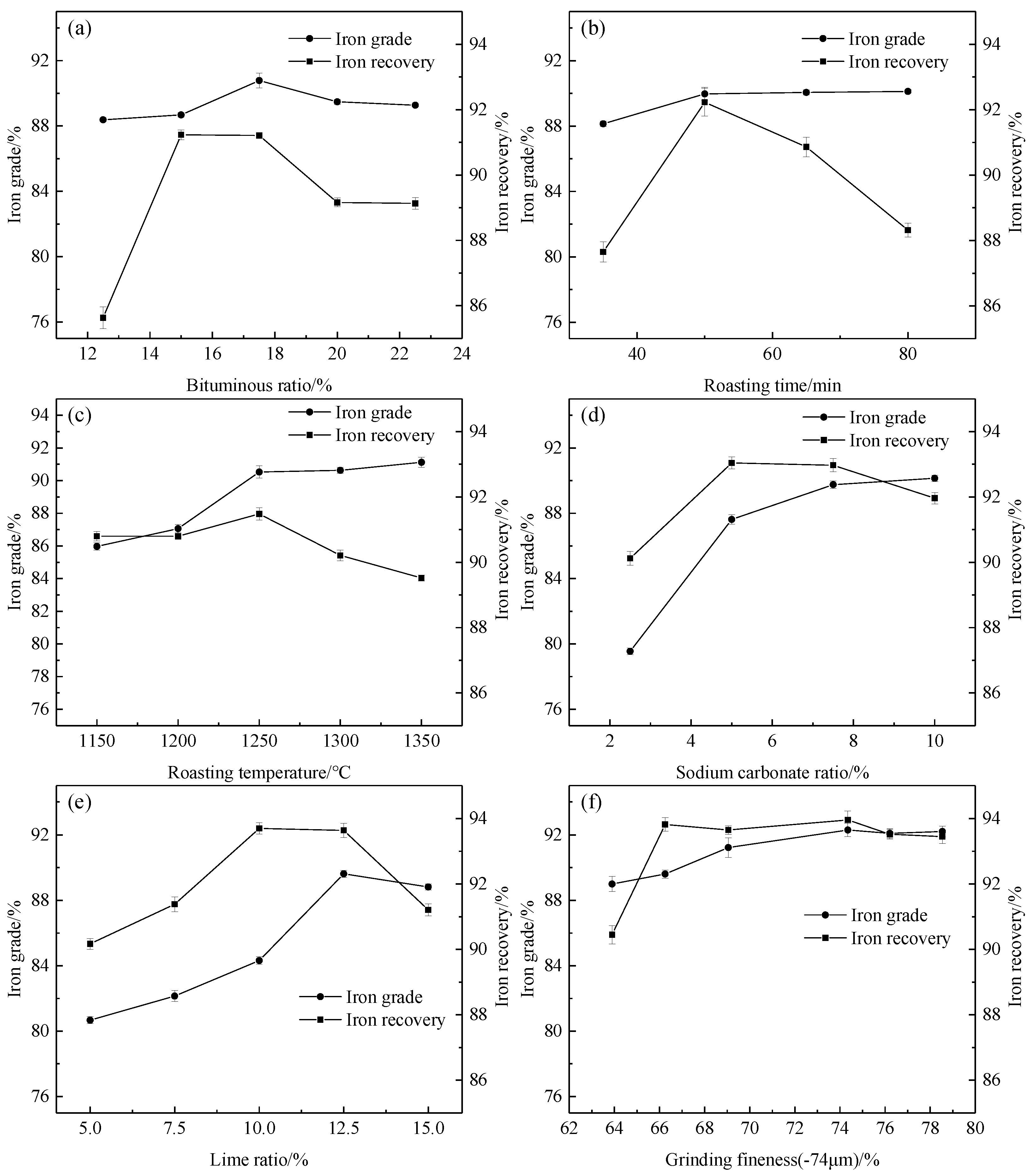
3.2.1. Effect of Bitumite Ratio on Iron Recovery
3.2.2. Effect of Roasting Time on Iron Recovery
3.2.3. Effect of Roasting Temperature on Iron Recovery
3.2.4. Effect of Sodium Carbonate Ratio on Iron Recovery
3.2.5. Effect of Lime Ratio on Iron Recovery
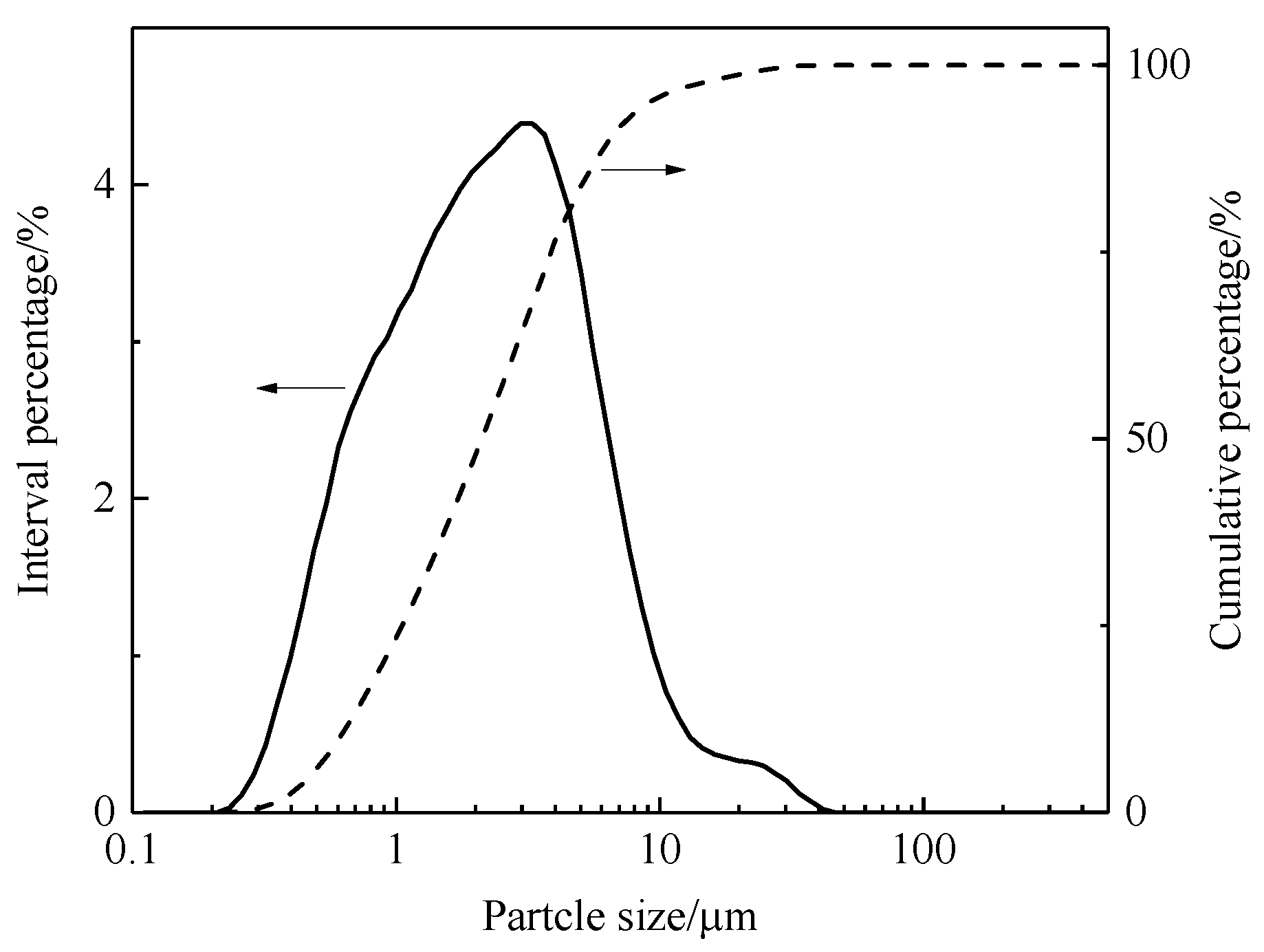
3.2.6. Effect of the Grinding Fineness of the Roasted Product on Iron Recovery
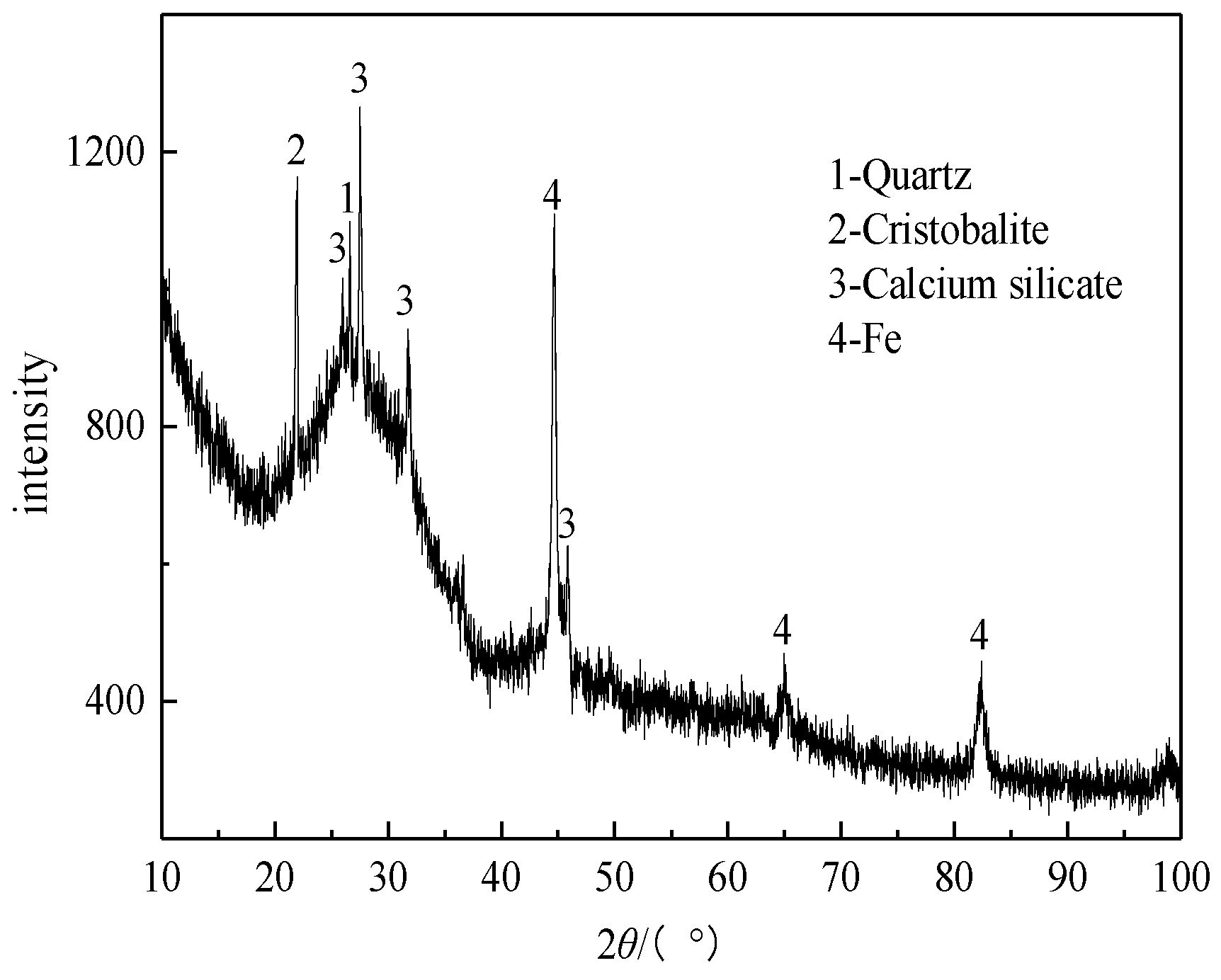
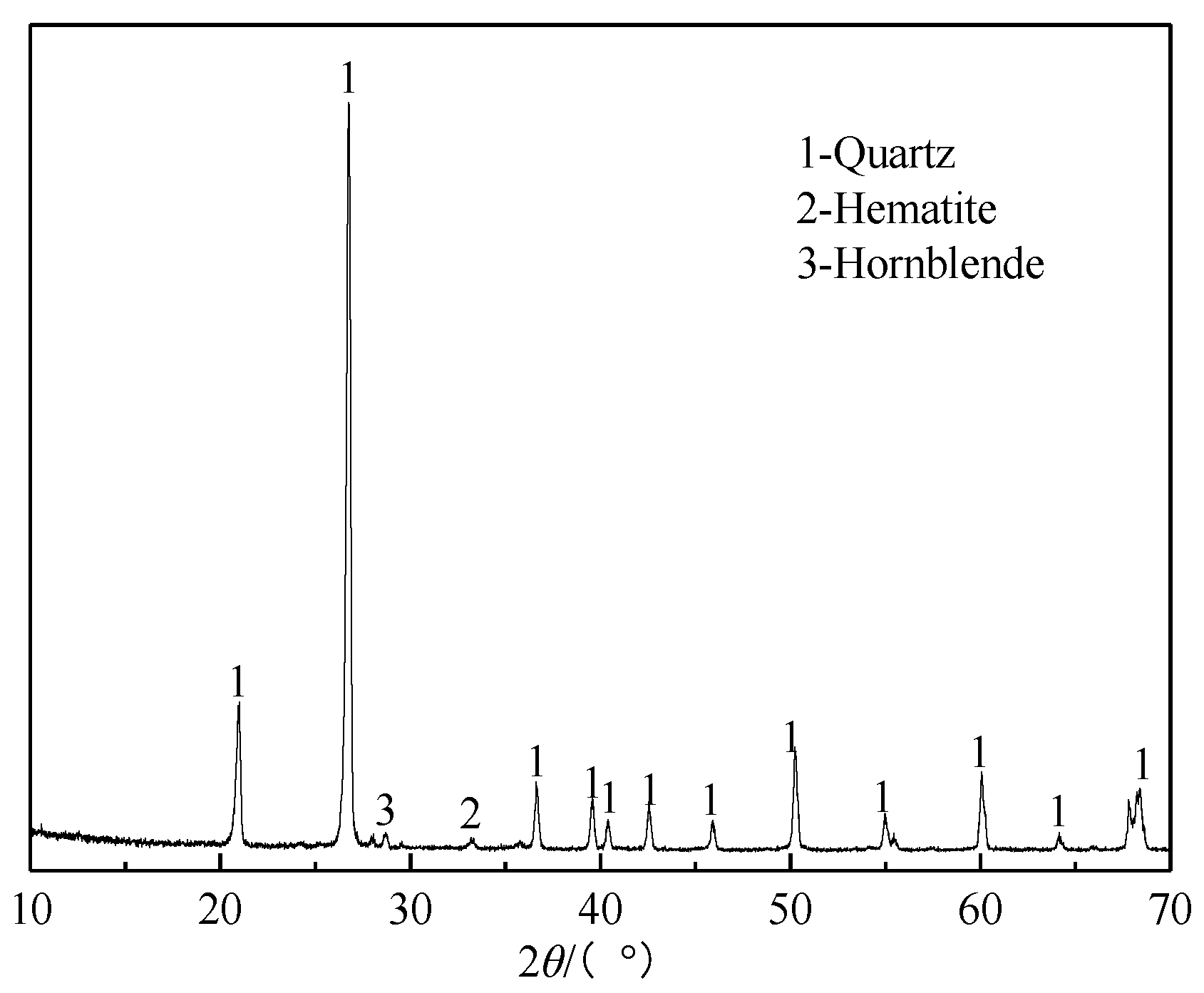
3.2.7. XRD Analysis of the Roasted Product
3.3. Preparation of Slag-Tailing Concrete Composite Admixtures Using the High-Silica Residues
3.3.1. Chemical Composition and Mineral Phases of the High-Silica Residues
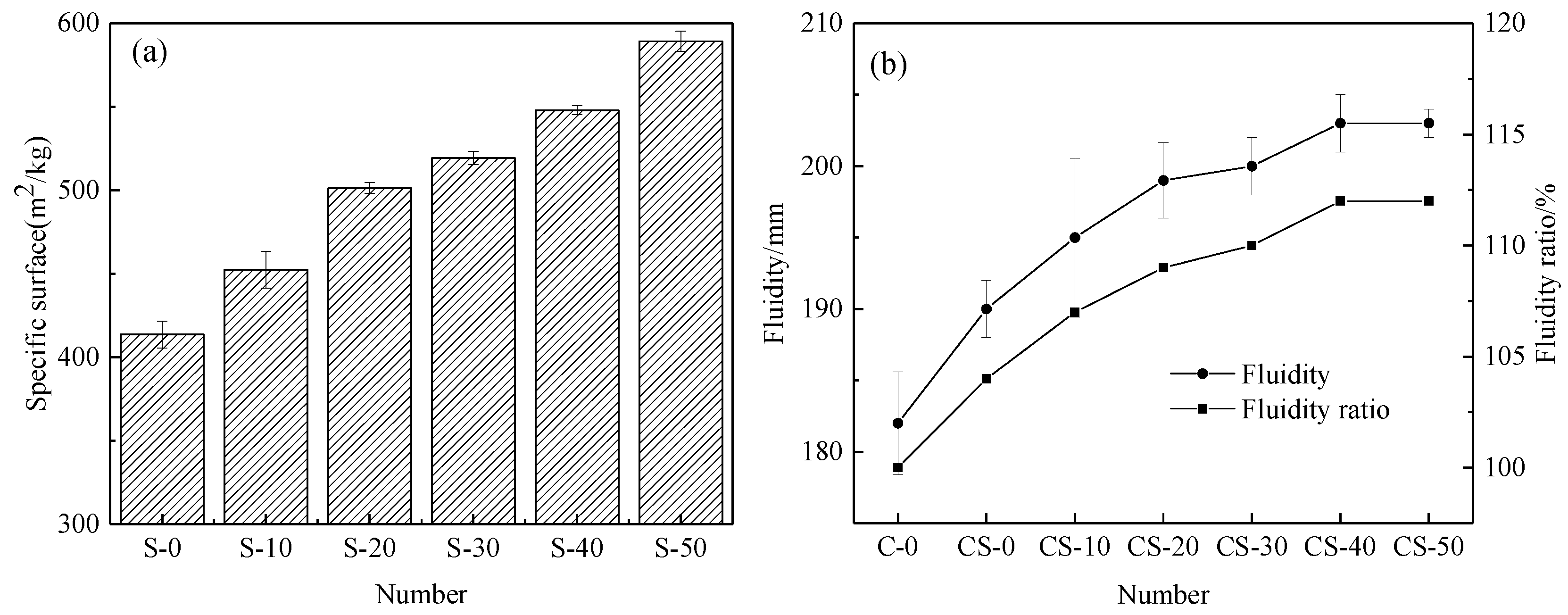
3.3.2. Specific Surface Area of the Slag-Tailing Composite Admixture and the Working Performance of the Mortar
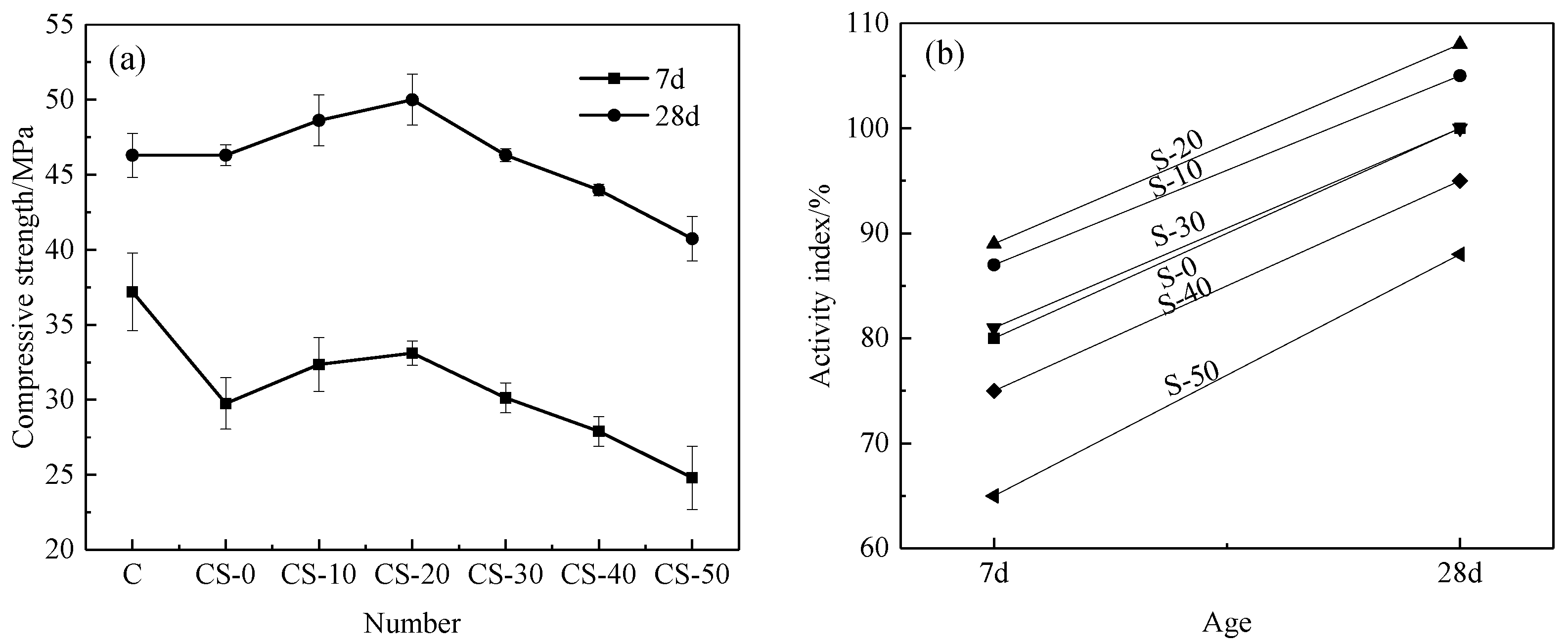
3.3.3. Compressive Strength and Activity Index of Mortar
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, J.H.; Ye, G.H.; Hu, S.M. The technology status and research progress of iron tailings re-beneficiation. Min. Metall. 2018, 27, 1–4. [Google Scholar]
- Zhizhen, L.; Xinjian, G.; Resource, S.O. Current Situations of Tailings Utilization and Sustainable Development of Mines. Miner. Eng. Res. 2018, 116, 37–41. [Google Scholar]
- Chen, H.A.; Shen, W.G.; Shan, L.; Xiong, C.B.; Su, Y.Q.; Liu, B.; Rao, J.L. Situation of discharge and comprehensive utilization of iron tailings domestic and abroad. Concrete 2012, 2, 88–91. [Google Scholar]
- Jiabin, C.; Wenlong, J.; Lianghui, Y. Survey and Evaluation of the Iron Tailings Resources in China. Min. Res. Dev. 2010, 3, 60–62. [Google Scholar]
- Ajaka, E.O. Recovering fine iron minerals from Itakpe iron ore process tailing. ARPN J. Eng. Appl. Sci. 2009, 4, 17–28. [Google Scholar]
- Rao, K.H.; Narasimhan, K.S. Selective flocculation applied to Barsuan iron ore tailings. Int. J. Miner. Process. 1985, 14, 67–75. [Google Scholar]
- Han, J.; Zhu, S.; Xun, Z.; Zhao, G. Experimental Research on Mineral Separation of a Magnetic-flotation Tailings Mixture. Min. Res. Dev. 2012, 2, 37–43. [Google Scholar]
- Sakthivel, R.; Vasumathi, N.; Sahu, D.; Mishra, B.K. Synthesis of magnetite powder from iron ore tailings. Powder Technol. 2010, 201, 187–190. [Google Scholar] [CrossRef]
- Das, S.K.; Kumar, S.; Ramachandrarao, P. Exploitation of iron ore tailing for the development of ceramic tiles. Waste Manag. 2000, 20, 725–729. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, J.; Mandal, A.K.; Singh, N.; Gupta, S. Iron ore tailing: A waste material used in ceramic tile compositions as alternative source of raw materials. Trans. Indian Ceram. Soc. 2012, 71, 21–24. [Google Scholar] [CrossRef]
- Huang, X.; Ranade, R.; Li, V.C. Feasibility study of developing green ECC using iron ore tailings powder as cement replacement. J. Mater. Civ. Eng. 2013, 25, 923–931. [Google Scholar] [CrossRef]
- Adedayo, S.M.; Onitiri, M.A. Mechanical properties of iron ore tailings filled-polypropylene composites. J. Miner. Mater. Charact. Eng. 2012, 11, 671. [Google Scholar] [CrossRef]
- Adedayo, S.M.; Onitiri, M.A. Tensile properties of iron ore tailings filled epoxy composites. West. Indian J. Eng. 2012, 35, 51–59. [Google Scholar]
- Chun, T.J.; Zhu, D.Q.; Pan, J.; He, Z. Preparation of metallic iron powder from red mud by sodium salt roasting and magnetic separation. Can. Metall. Q. 2014, 53, 183–189. [Google Scholar] [CrossRef]
- Yang, H.; Jing, L.; Zhang, B. Recovery of iron from vanadium tailings with coal-based direct reduction followed by magnetic separation. J. Hazard. Mater. 2011, 185, 1405–1411. [Google Scholar] [CrossRef]
- Zhang, G.F.; Yang, Q.R.; Yang, Y.D.; Wu, P.; McLean, A. Recovery of iron from waste slag of pyrite processing using reduction roasting magnetic separation method. Can. Metall. Q. 2013, 52, 153–159. [Google Scholar] [CrossRef]
- Maweja, K.; Mukongo, T.; Mutombo, I. Cleaning of a copper matte smelting slag from a water-jacket furnace by direct reduction of heavy metals. J. Hazard. Mater. 2009, 164, 856–862. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.; Yu, X. Recovery of iron from cyanide tailings with reduction roasting—Water leaching followed by magnetic separation. Adv. Mater. Res. 2012, 396, 486–489. [Google Scholar] [CrossRef]
- Li, C.; Sun, H.; Bai, J.; Li, L. Innovative methodology for comprehensive utilization of iron ore tailings: Part 1. The recovery of iron from iron ore tailings using magnetic separation after magnetizing roasting. J. Hazard. Mater. 2010, 174, 71–77. [Google Scholar] [CrossRef]
- Ni, W.; Fu, C.H.; Fan, D.C.; Li, J.; Qiu, X.J.; Li, Y. A Method of Extracting Iron Ore Tailings by Deep Reduction after Using Strong Magnetism to Pre-concentrate the Tailings. Chinese Patent No. 201210362689.3, 11 September 2013. [Google Scholar]
- Li, J.; Ni, W.; Fan, D.C.; Li, Y.; Fu, C.H. Process Mineralogy Research on Iron Tailings from Qidashan. Metal Mine 2014, 1, 158–162. [Google Scholar]
- Fan, D. Research on Pre-Concentration and Deep Reduction of Qidashan Iron Ore Tailings and the Comprehensive Utilization of Tailings; University of Science and Technology Beijing: Beijing, China, 2018. [Google Scholar]
- Zhu, D.Q.; Chun, T.J.; Pan, J.; He, Z. Recovery of Iron from High-Iron Red Mud by Reduction Roasting with Adding Sodium Salt. J. Iron Steel Res. Int. 2012, 19, 1–5. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Y.; Liu, Z.; Kou, J.; Xu, C. Effects of particle sizes of iron ore and coal on the strength and reduction of high phosphorus oolitic hematite-coal composite briquettes. ISIJ Int. 2014, 54, 56–62. [Google Scholar] [CrossRef]
- Yu, W.; Sun, T.; Kou, J.; Wei, Y.; Xu, C.; Liu, Z. The function of Ca(OH)2 and Na2CO3 as additive on the reduction of high-phosphorus oolitic hematite-coal mixed pellets. ISIJ Int. 2013, 53, 427–433. [Google Scholar] [CrossRef]
- Zhu, D.; Chun, T.; Pan, J.; Lu, L.; He, Z. Upgrading and dephosphorization of Western Australian iron ore using reduction roasting by adding sodium carbonate. Int. J. Miner. Metall. Mater. 2013, 20, 505–513. [Google Scholar] [CrossRef]
- Fan, D.; Ni, W.; Wang, J.; Wang, K. Effects of CaO and Na2CO3 on the reduction of high silicon iron ores. J. Wuhan Univ. Technol. -Mater. Sci. Ed. 2017, 32, 508–516. [Google Scholar] [CrossRef]
- Weissberger, S.; Zimmels, Y.; Lin, I.J. Mechanism of growth of metallic phase in direct reduction of iron bearing oolites. Metall. Trans. B 1986, 17, 433–442. [Google Scholar] [CrossRef]
- Ni, W.; Yan, J.; Cheng, Y. Beneficiation of unwieldy oolitic hematite by deep reduction and magnetic separation process. Chin. J. Eng. 2010, 32, 287–291. [Google Scholar]
- Hao, Z.; Wu, S.; Wang, Y.; Luo, G.; Wu, H.; Duan, X. Acting mechanism of F, K, and Na in the solid phase sintering reaction of the Baiyunebo iron ore. Int. J. Miner. Metall. Mater. 2010, 17, 137–142. [Google Scholar] [CrossRef]
- Nadiv, S.; WeissbergerI, S.; Lin, J.; Zimmels, Y. Diffusion mechanisms and reactions during reduction of oolitic iron-oxide mineral. J. Mater. Sci. 1988, 23, 1050–1055. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Rao, M.; Zhang, Y.; Jiang, T. Effects of sodium salts on reduction roasting and Fe–P separation of high-phosphorus oolitic hematite ore. Int. J. Miner. Process. 2013, 124, 26–34. [Google Scholar] [CrossRef]
- Huang, X.Y.; Ni, W.; Zhu, L.-P.; Wang, Z.-J. Grinding characteristic of Qidashan iron tailings. J. Univ. Sci. Technol. Beijing 2010, 32, 1253–1257. [Google Scholar]
- Kronlöf, A. Effect of very fine aggregate on concrete strength. Mater. Struct. 1994, 27, 15–25. [Google Scholar] [CrossRef]
- Lawrence, P.; Cyr, M.; Ringot, E. Mineral admixtures in mortars: Effect of inert materials on short-term hydration. Cem. Concr. Res. 2003, 33, 1939–1947. [Google Scholar] [CrossRef]
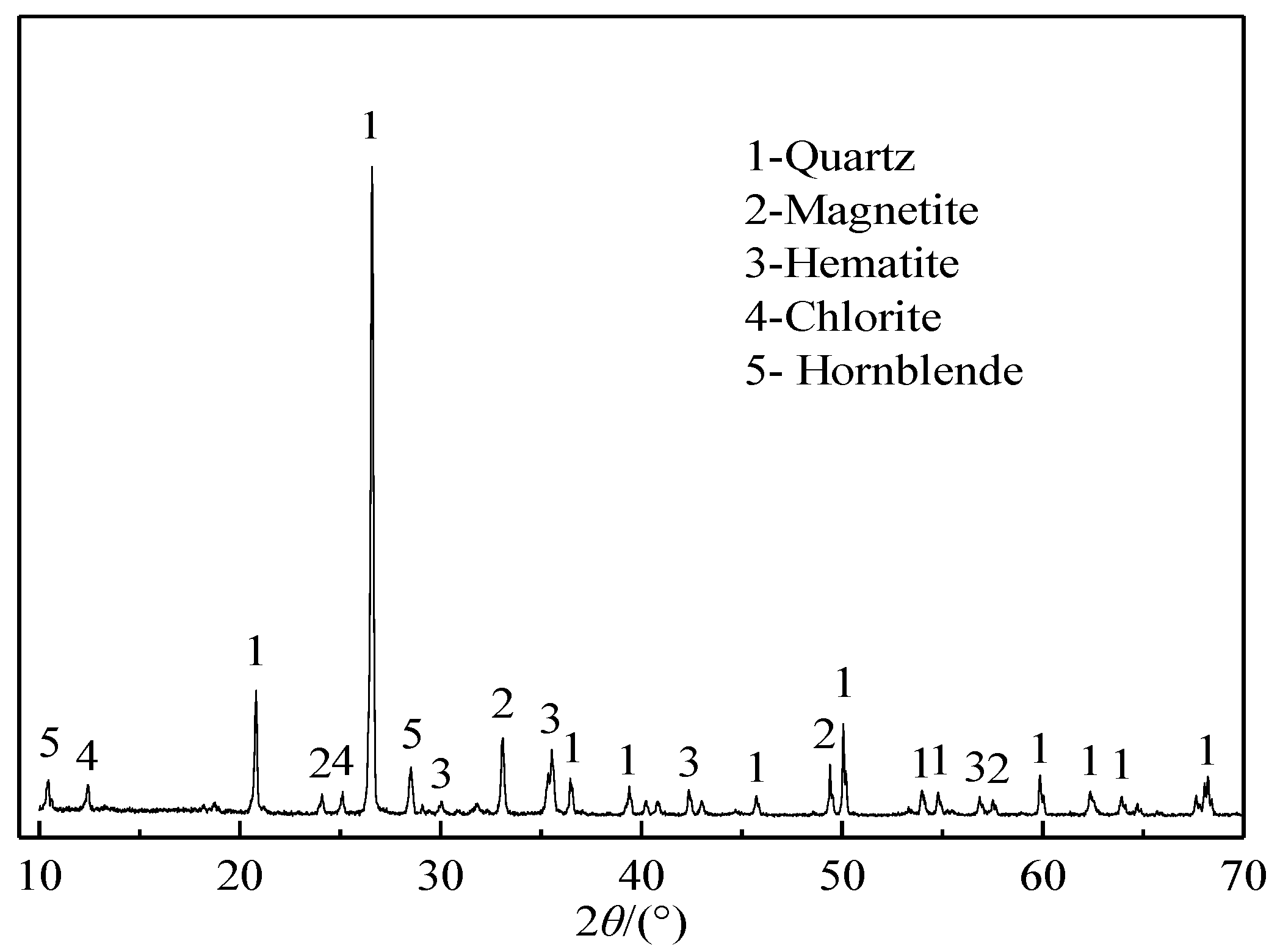
| Components | TFe | SiO2 | CaO | MgO | Al2O3 | Na2O | K2O | MnO | TiO2 | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Content/wt % | 12.61 | 75.46 | 1.70 | 1.75 | 1.65 | 0.32 | 0.34 | 0.13 | 0.06 | 0.10 | 0.03 |
| Phases | Magnetic Iron | Hematite and Limonite | Iron Carbonate | Iron Silicate | Iron Sulfide | TFe |
|---|---|---|---|---|---|---|
| Iron content/wt % | 2.29 | 5.16 | 0.39 | 4.72 | 0.08 | 12.64 |
| Fraction/wt % | 18.12 | 40.82 | 3.09 | 37.34 | 0.63 | 100.00 |
| Ingredients | Moisture | Ash | Volatiles | Fixed Carbon |
|---|---|---|---|---|
| Content/% | 9.88 | 16.20 | 28.76 | 45.16 |
| Components | SiO2 | Fe2O3 | FeO | Al2O3 | CaO | MgO |
| Content | 59.57 | 1.36 | 1.93 | 3.10 | 24.81 | 2.65 |
| Components | Na2O | K2O | MnO | S | P | Loss |
| Content | 0.97 | 0.52 | 0.10 | 0.10 | 0.02 | 4.27 |
| Products | Productivity Rate | Iron Grade | Iron Recovery Rate |
|---|---|---|---|
| PC | 28.82 | 36.58 | 83.86 |
| High-silicon residue | 71.18 | 2.85 | 16.14 |
| IOTs | 100.00 | 12.57 | 100.00 |
| Products | SiO2 | Fe2O3 | FeO | CaO | MgO | Al2O3 | Na2O | K2O | MnO | TiO2 | S | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PC * | 41.71 | 42.83 | 8.18 | 1.82 | 1.69 | 1.82 | 0.41 | 0.27 | 0.14 | 0.072 | 0.1 | 0.02 |
| High-silicon residue ** | 91.29 | 2.96 | 0.72 | 1.3 | 1.25 | 1.19 | 0.42 | 0.17 | 0.074 | 0.024 | 0.066 | 0.023 |
| Grinding Time/min | 10 | 15 | 20 | 25 | 30 | 35 |
|---|---|---|---|---|---|---|
| Grinding fineness (−74 μm)/wt % | 63.9 | 66.25 | 69.05 | 74.35 | 76.21 | 78.54 |
| Number | S 75 | Tailing |
|---|---|---|
| S-0 | 100 | 0 |
| S-10 | 90 | 10 |
| S-20 | 80 | 20 |
| S-30 | 70 | 30 |
| S-40 | 60 | 40 |
| S-50 | 50 | 50 |
| Number | Cement | Composite Admixture | ISO Sand | Water |
|---|---|---|---|---|
| C-0 | 450 | 0 | 1350 | 225 |
| CS-0~CS50* | 225 | 225 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, C.; Li, K.; Ni, W.; Fan, D. Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures. Minerals 2019, 9, 232. https://doi.org/10.3390/min9040232
Tang C, Li K, Ni W, Fan D. Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures. Minerals. 2019; 9(4):232. https://doi.org/10.3390/min9040232
Chicago/Turabian StyleTang, Chang, Keqing Li, Wen Ni, and Duncheng Fan. 2019. "Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures" Minerals 9, no. 4: 232. https://doi.org/10.3390/min9040232
APA StyleTang, C., Li, K., Ni, W., & Fan, D. (2019). Recovering Iron from Iron Ore Tailings and Preparing Concrete Composite Admixtures. Minerals, 9(4), 232. https://doi.org/10.3390/min9040232




