Separation of Monazite from Placer Deposit by Magnetic Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
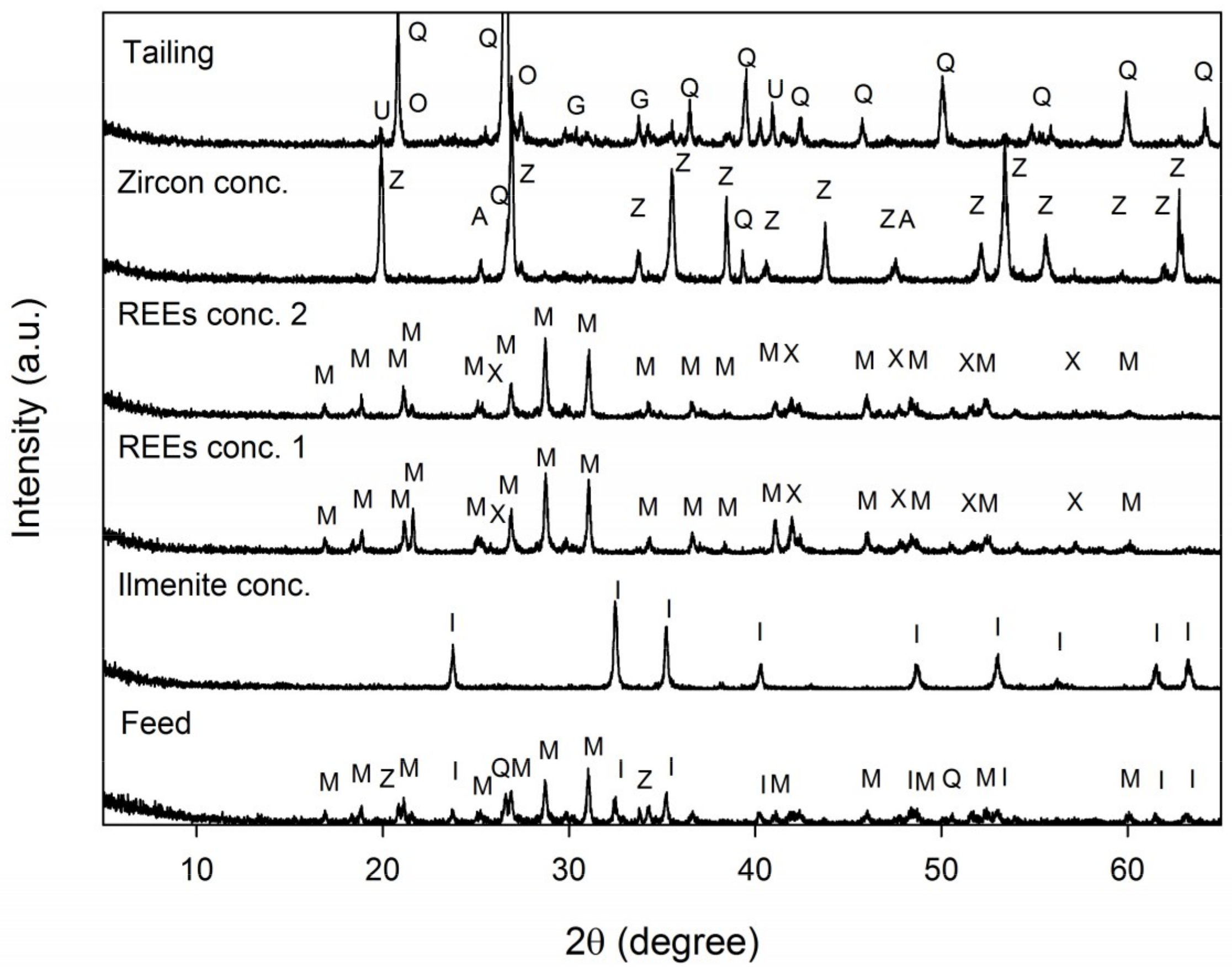
3.1. Feed Sample Analysis
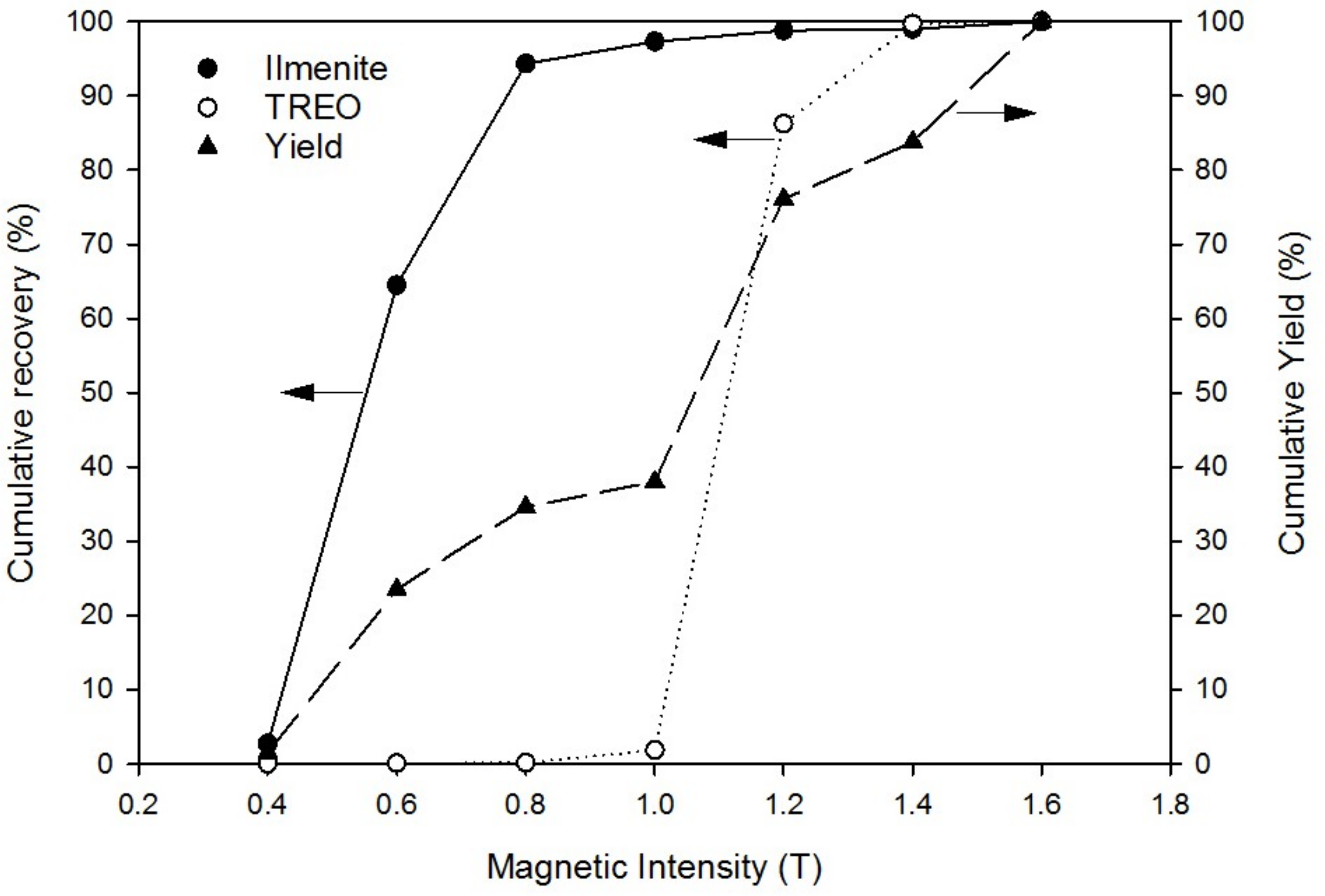
3.2. Dry Magnetic Separation
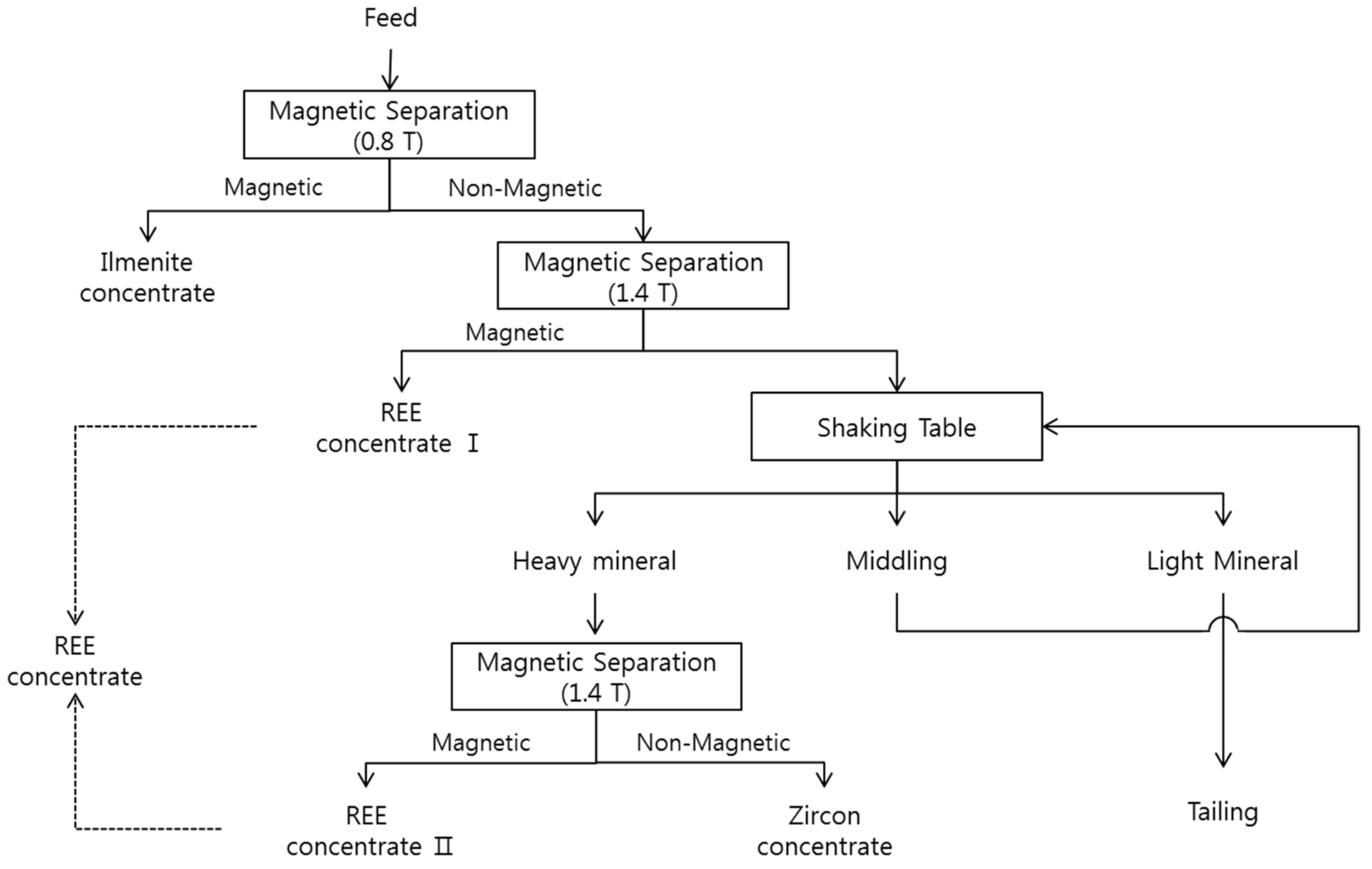
3.3. Batch Test for Beneficiation Process
4. Conclusions
- 1.
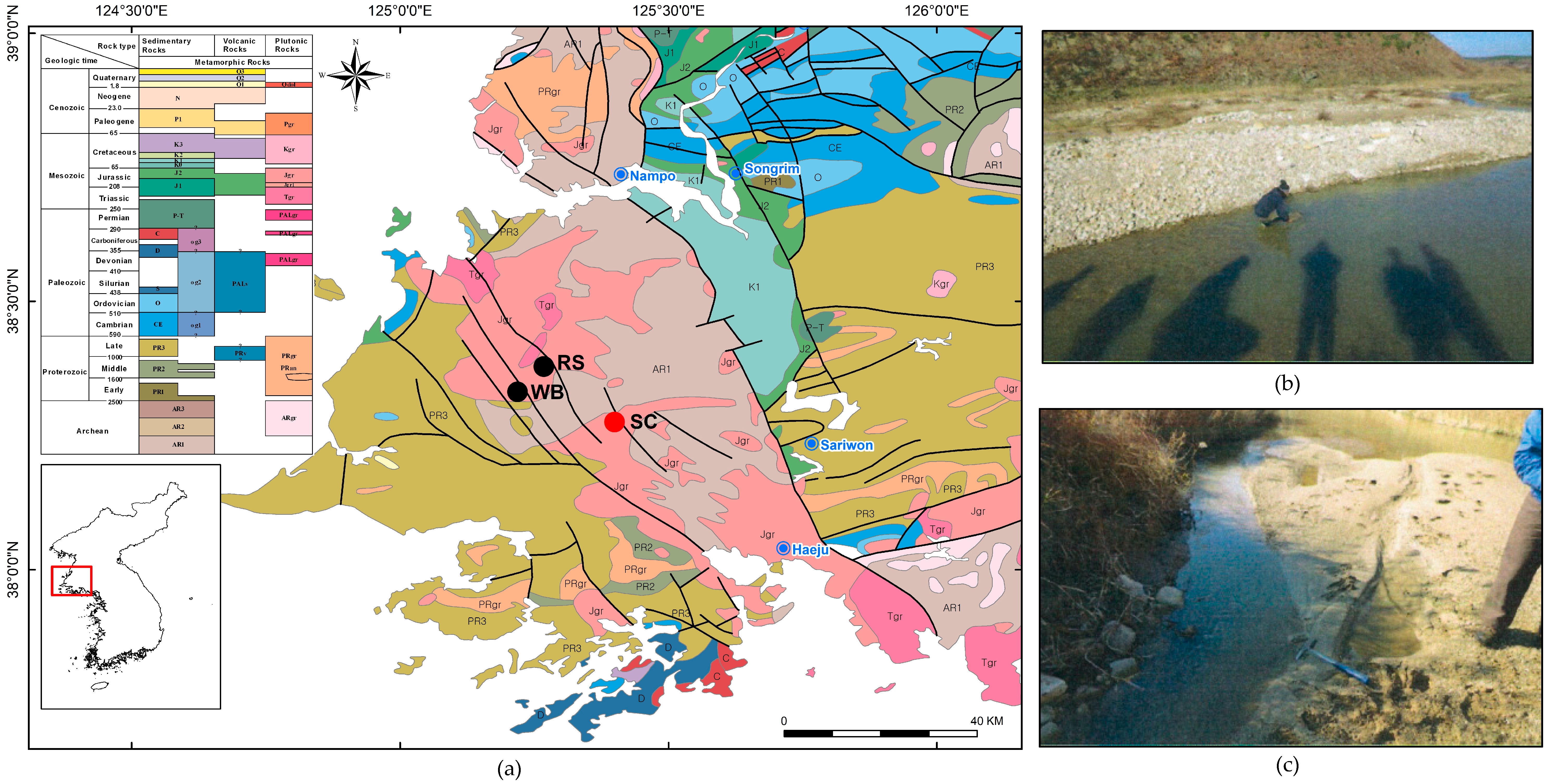
- The feed sample was obtained from the bottom of the active river channel and sand bar of the Sam-Cheon area in North Korea. The Sam-Cheon area is located on the Rimjingang belt in the middle Korean Peninsula, and there are two REE mines, Wolbong mine and Ryeonsan mine, in the vicinity. The sample was preliminary concentrated in the mining site by gravity separation, and mineralogical analysis and beneficiation tests were conducted using preliminary concentrated heavy minerals sample.
- 2.
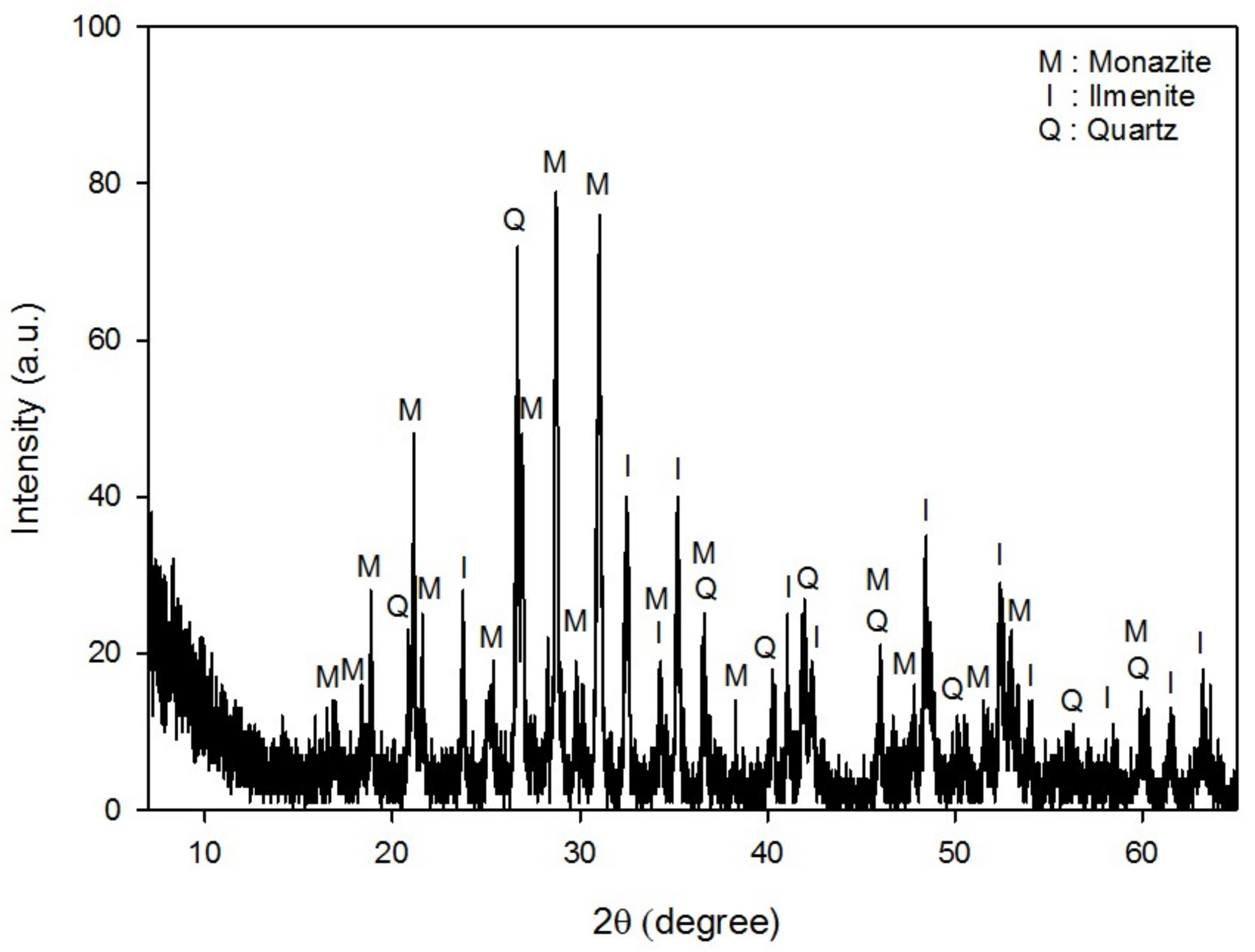
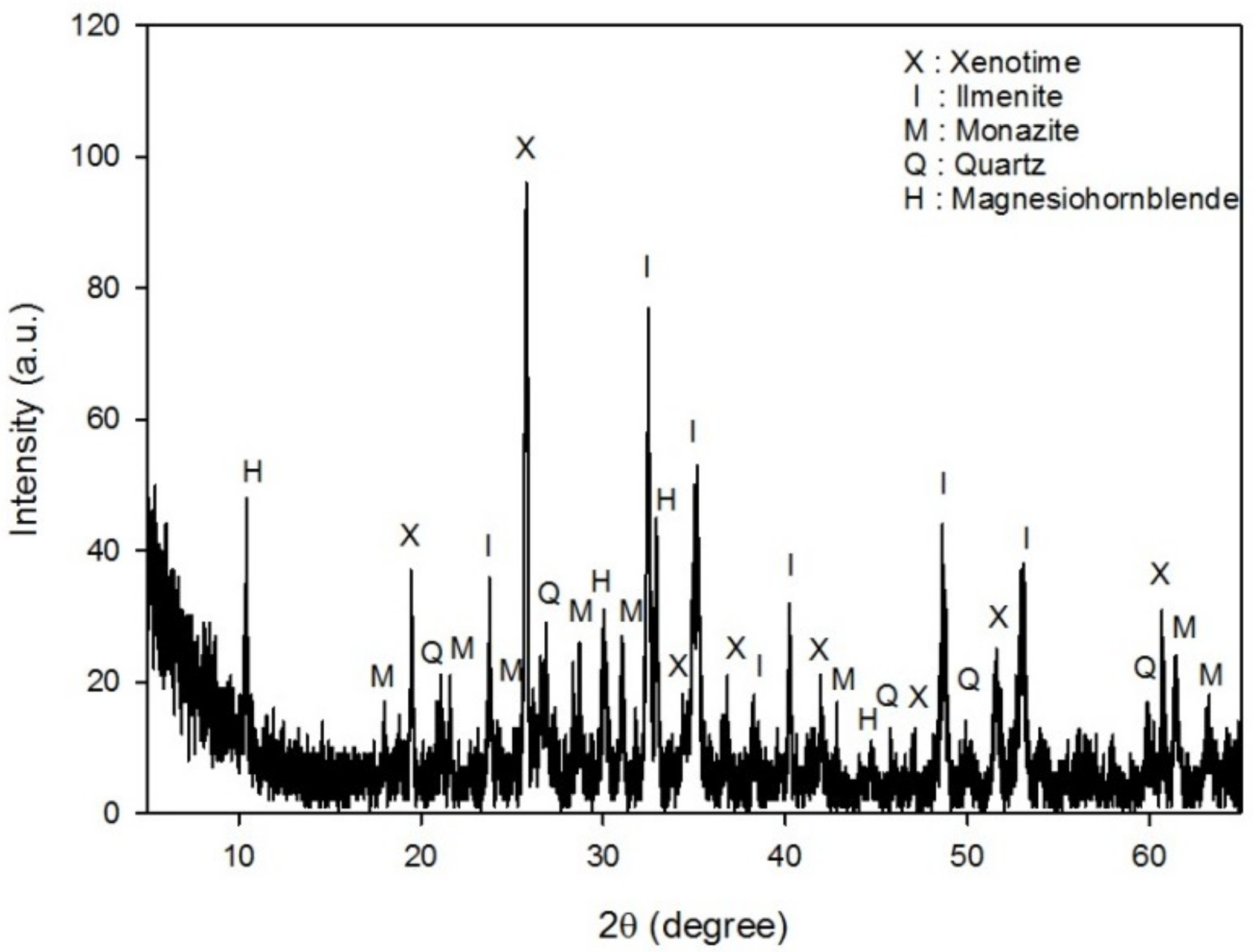
- The mineralogical analyses by XRD, XRF, ICP, and MLA indicated that the REE-bearing minerals in this sample were mostly monazite, and to a lesser extent, xenotime. Besides these REE-bearing minerals, ilmenite and zircon were valuable minerals to be concentrated.
- 3.
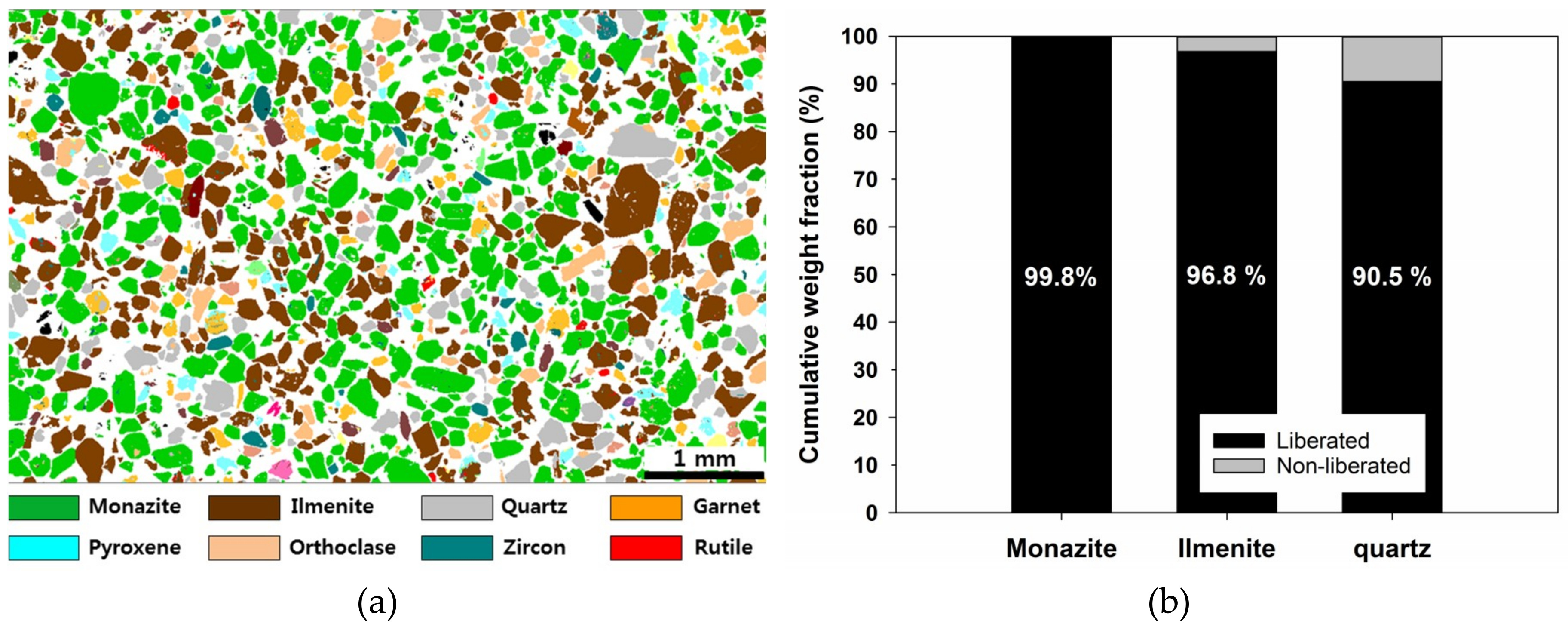
- Because this sample came from a placer deposit, the degree of liberation was sufficiently high. The “liberated” minerals, which have a mineral composition (wt %) of more than 80%, exceeded 90% for main minerals (monazite, ilmenite, and quartz).
- 4.
- Valuable minerals could be recovered via magnetic separation through various magnetic intensities. Ilmenite was recovered first between the magnetic intensities of 0.6 T and 0.8 T, and the REE-bearing minerals, xenotime and monazite were then recovered between 1.0 T and 1.4 T.
- 5.
- The 10 kg batch test was conducted to confirm the feasibility of the process using the unit separation result. As a result, the grade of ilmenite increased from 32.5 to 90.9%, and TREO(%) was enhanced from 20.5% to 45.0%. Additionally, zircon, another useful mineral, could be concentrated to 42.8% of the grade in heavy minerals of non-magnetic products. Consequently, it is confirmed that it was possible to separate valuable minerals selectively by simple magnetic separation and additional gravity separation.
Author Contributions
Funding
Conflicts of Interest
References
- Gupta, C.; Krishnamurthy, N. Extractive metallurgy of rare earths. Int. Mater. Rev. 1992, 37, 197–248. [Google Scholar] [CrossRef]
- Gupta, C.; Krishnamurthy, N. Extractive Metallurgy of Rare Earths; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- U.S. Geological Survey. Available online: https://pubs.er.usgs.gov/publication/70170140 (accessed on 25 February 2019).
- Jordens, A.; Cheng, Y.P.; Waters, K.E. A review of the beneficiation of rare earth element bearing minerals. Miner. Eng. 2013, 41, 97–114. [Google Scholar] [CrossRef]
- Cui, H.; Anderson, C.G. Alternative flowsheet for rare earth beneficiation of Bear Lodge ore. Miner. Eng. 2017, 110, 166–178. [Google Scholar] [CrossRef]
- Jordens, A.; Sheridan, R.S.; Rowson, N.A.; Waters, K.E. Processing a rare earth mineral deposit using gravity and magnetic separation. Miner. Eng. 2014, 62, 9–18. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Langlois, R.; Grammatikopoulos, T.; Rowson, N.A.; Waters, K.E. Beneficiation of the Nechalacho rare earth deposit. Part 1: Gravity and magnetic separation. Miner. Eng. 2016, 99, 111–122. [Google Scholar] [CrossRef]
- Jordens, A.; Marion, C.; Langlois, R.; Grammatikopoulos, T.; Sheridan, R.S.; Teng, C.; Demers, H.; Gauvin, R.; Rowson, N.A.; Waters, K.E. Beneficiation of the Nechalacho rare earth deposit. Part 2: Characterisation of products from gravity and magnetic separation. Miner. Eng. 2016, 99, 96–110. [Google Scholar] [CrossRef]
- Li, L.Z.; Yang, X. China’s rare earth ore deposits and beneficiation techniques. In Proceedings of the European Rare Earth Resource Conference, Milos Island, Greece, 4–7 September 2014. [Google Scholar]
- Panda, R.; Kumari, A.; Jha, M.K.; Hait, J.; Kumar, V.; Kumar, J.R.; Lee, J.Y. Leaching of rare earth metals (REMs) from Korean monazite concentrate. J. Ind. Eng. Chem. 2014, 20, 2035–2042. [Google Scholar] [CrossRef]
- Yang, X.; Satur, J.V.; Sanematsu, K.; Laukkanen, J.; Saastamoinen, T. Beneficiation studies of a complex REE ore. Miner. Eng. 2015, 71, 55–64. [Google Scholar] [CrossRef]
- Moustafa, M.I.; Abdelfattah, N.A. Physical and chemical beneficiation of the Egyptian beach monazite. Resour. Geol. 2010, 60, 288–299. [Google Scholar] [CrossRef]
- Rajan, G.R.; Sundararajan, M. Combined magnetic, electrostatic, and gravity separation techniques for recovering strategic heavy minerals from beach sands. Marine Georesour. Geotechnol. 2018, 36, 959–965. [Google Scholar] [CrossRef]
- Xiong, W.L.; Deng, J.; Chen, B.Y.; Deng, S.Z.; Wei, D.Z. Flotation-magnetic separation for the beneficiation of rare earth ores. Miner. Eng. 2018, 119, 49–56. [Google Scholar] [CrossRef]
- Kang, M.S.; Choi, B.S.; Ryu, D.M.; Huh, D.H.; Lim, D.H.; Seo, E.R. Chosun Geology Series 7; Gong-Up Press: Pyongyang, Korea, 2011. [Google Scholar]
- Kang, P.C.; Chwae, U.C.; Kim, K.B.; Hong, S.H.; Lee, B.J.; Hwang, J.H.; Park, K.H.; Hwang, S.K.; Choi, P.Y.; Song, K.Y. Geological Map of Korea, Scale 1:1,000,000; Korea Institute of Geology, Mining and Materials: Daejeon, Korea, 1995. [Google Scholar]
- Kumari, A.; Panda, R.; Jha, M.K.; Kumar, J.R.; Lee, J.Y. Process development to recover rare earth metals from monazite mineral: A review. Miner. Eng. 2015, 79, 102–115. [Google Scholar] [CrossRef]
| Mineral | wt % | Mineral | wt % |
|---|---|---|---|
| Monazite | 42.6 | Rutile | 0.8 |
| Ilmenite | 33.8 | Xenotime | 0.6 |
| Quartz | 6.7 | Tourmaline | 0.4 |
| 1 Garnet Group | 6.3 | Titanite | 0.4 |
| Orthoclase | 2.9 | Muscovite | 0.4 |
| Pyroxene | 1.8 | Magnetite | 0.4 |
| Zircon | 1.7 | Others | 1.2 |
| Size Fraction | Mass Fraction (wt %) | Concentration (%) | ||
|---|---|---|---|---|
| Fe | Ti | TREO | ||
| >500 μm | 2.5 | 22.2 | 17.9 | 2.9 |
| 300–500 μm | 22.6 | 15.1 | 13.2 | 13.2 |
| 212–300 μm | 27.9 | 12.8 | 11.2 | 22.1 |
| 150–212 μm | 31.6 | 10.9 | 9.6 | 24.6 |
| 106–150 μm | 11.2 | 10.0 | 9.1 | 25.1 |
| 74–106 μm | 2.4 | 10.8 | 9.7 | 19.5 |
| <74 μm | 1.9 | 10.2 | 8.0 | 12.7 |
| Total | 100.0 | 12.5 | 11.0 | 20.5 |
| Yield (wt %) | Chemical Composition | ||||||
|---|---|---|---|---|---|---|---|
| Fe | Ti | 1 TREO | |||||
| 2 C (%) | 3 R (%) | C (%) | R (%) | C (%) | R (%) | ||
| Sink fraction (Heavy mineral) | 86.2 | 14.4 | 97.2 | 12.7 | 99.5 | 23.7 | 99.7 |
| Float fraction (Light mineral) | 13.8 | 1.0 | 2.8 | 0.2 | 0.5 | 0.17 | 0.3 |
| Magnetic Intensity (T) | Yield (wt %) | Ilmenite | REE | SiO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe (%) | Ti (%) | 1 C (%) | 2 R (%) | 3 LREO (%) | 4 HREO (%) | TREO (%) | 2 R (%) | 1 C (%) | 2 R (%) | ||
| Non-Magnetic | 19.1 | 0.7 | 1.2 | 2.0 | 1.2 | 0.3 | 0.04 | 0.34 | 0.3 | 62.2 | 75.9 |
| 0.4 | 1.5 | 42.3 | 18.2 | 57.7 | 2.7 | 0.3 | 0.02 | 0.32 | 0.02 | 4.9 | 0.5 |
| 0.6 | 22.0 | 33.6 | 30.7 | 91.3 | 61.8 | 0.04 | 0.0 | 0.04 | 0.04 | 5.0 | 7.0 |
| 0.8 | 11.1 | 32.1 | 29.4 | 87.2 | 29.8 | 0.09 | 0.03 | 0.12 | 0.06 | 4.7 | 3.3 |
| 1.0 | 3.4 | 15.9 | 9.2 | 29.2 | 3.1 | 3.0 | 9.1 | 12.1 | 2.0 | 4.2 | 0.9 |
| 1.2 | 38.1 | 0.5 | 0.4 | 1.3 | 1.5 | 46.2 | 1.8 | 48.0 | 89.2 | 4.6 | 11.2 |
| 1.4 | 4.7 | 0.6 | 0.3 | 1.0 | 0.1 | 34.8 | 1.1 | 35.9 | 8.2 | 4.1 | 1.2 |
| Total | 100.0 | 12.5 | 11.0 | 32.5 | 100.0 | 19.4 | 1.1 | 20.5 | 100.0 | 15.8 | 100.0 |
| Product | Yield (wt %) | Ilmenite | REE | Zircon | SiO2 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fe (%) | Ti (%) | 1 C (%) | 2 R (%) | TREO (%) | 2 R (%) | Zr (%) | 2 R (%) | 1 C (%) | 2 R (%) | |||
| REEs | Conc.I | 44.5 | 1.4 | 0.3 | 0.9 | 1.3 | 45.0 | 97.6 | 0.1 | 2.8 | 4.4 | 12.4 |
| Conc.II | 0.9 | 0.4 | 0.2 | 0.6 | <0.05 | 45.0 | 2.0 | 0.1 | 0.1 | 4.3 | 0.3 | |
| Sub-total | 45.4 | 1.4 | 0.3 | 0.9 | 1.3 | 45.0 | 99.6 | 0.1 | 2.9 | 4.4 | 12.7 | |
| Ilmenite | 35.2 | 33.5 | 30.5 | 90.9 | 98.5 | 0.2 | 0.3 | 0.1 | 1.6 | 5.0 | 11.2 | |
| Zircon | 2.1 | 1.5 | 4.3 | 0.9 | <0.05 | 0.8 | 0.1 | 42.8 | 77.2 | 30.7 | 4.0 | |
| Gangue mineral | 17.3 | 0.1 | 0.2 | 0.3 | 0.2 | 0.1 | 0.1 | 1.2 | 18.3 | 66.0 | 72.1 | |
| Total | 100.0 | 12.5 | 11.0 | 32.5 | 100 | 20.5 | 100 | 1.1 | 100 | 15.8 | 100 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.; Jeong, S. Separation of Monazite from Placer Deposit by Magnetic Separation. Minerals 2019, 9, 149. https://doi.org/10.3390/min9030149
Kim K, Jeong S. Separation of Monazite from Placer Deposit by Magnetic Separation. Minerals. 2019; 9(3):149. https://doi.org/10.3390/min9030149
Chicago/Turabian StyleKim, Kwanho, and Soobok Jeong. 2019. "Separation of Monazite from Placer Deposit by Magnetic Separation" Minerals 9, no. 3: 149. https://doi.org/10.3390/min9030149
APA StyleKim, K., & Jeong, S. (2019). Separation of Monazite from Placer Deposit by Magnetic Separation. Minerals, 9(3), 149. https://doi.org/10.3390/min9030149





