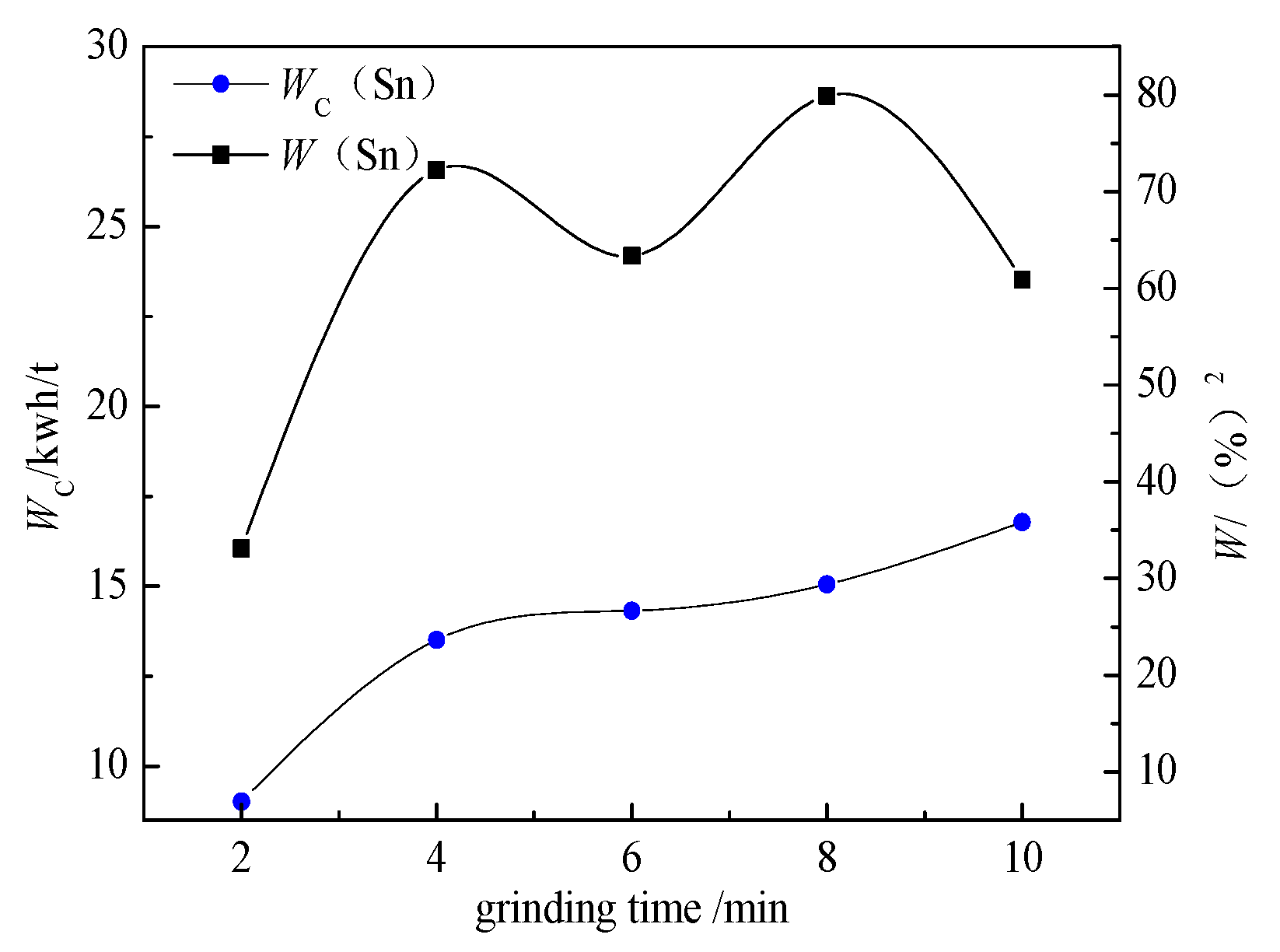
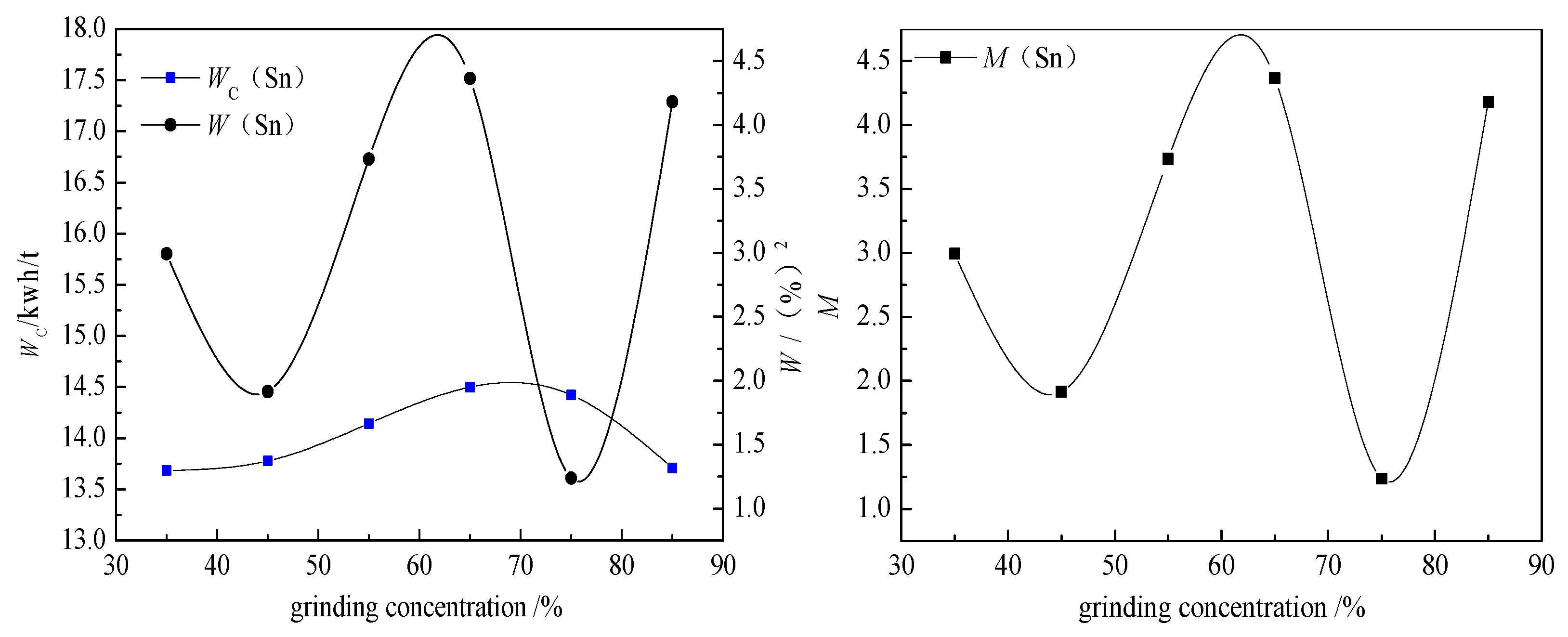
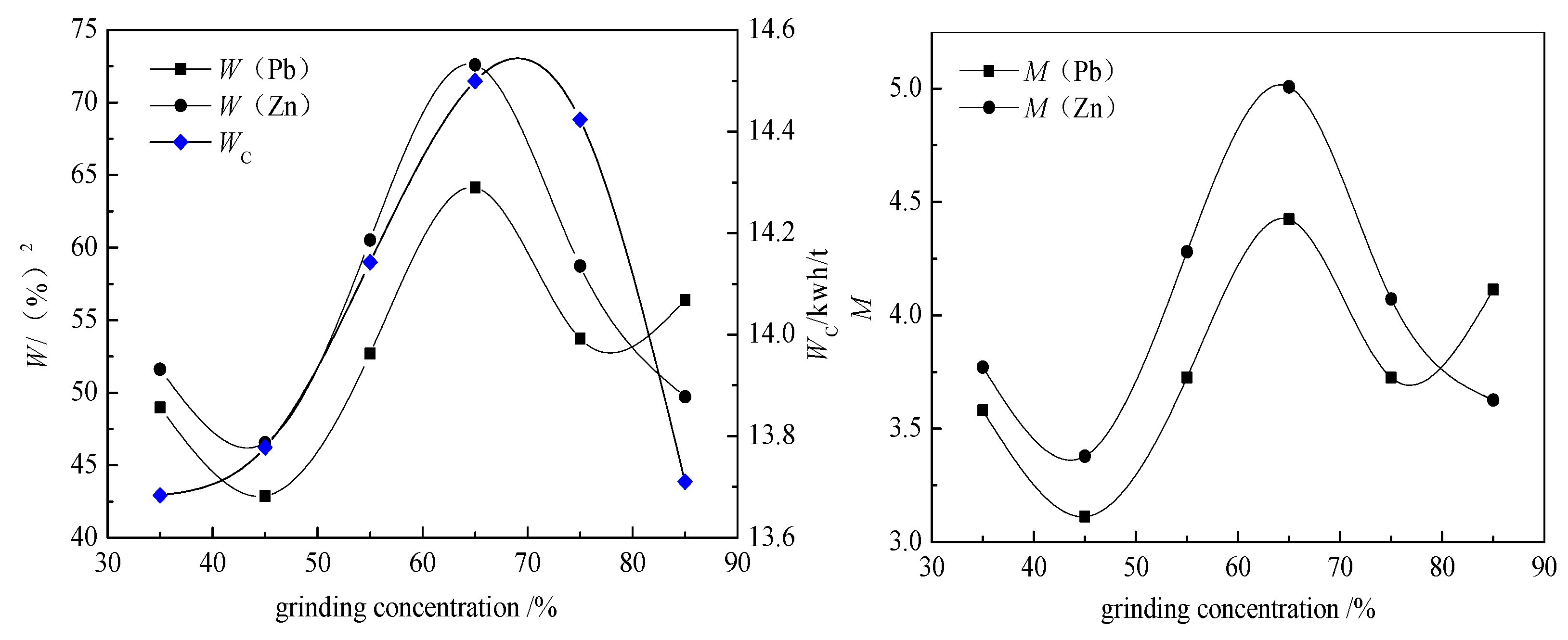
The prediction and verification of optimization conditions are based on a response surface method. The principle of this method is regression design, which involves finding the quantitative relationship between variables and independent variables. By analyzing the regression equation, the optimal process parameters were found, and the multivariable statistical method was solved by experimental analysis. According to the optimum grinding conditions, the prediction and verification experiments were carried out. The response surface method can be used to determine the optimum conditions for grinding time, grinding concentration, and filling ratio of the medium.
4.1. Prediction Test of Grinding Parameters
In this experiment, three parameters—grinding time, grinding concentration, and filling ratio, expressed by X1, X2 and X3, respectively—were selected to analyze the response surface. The grinding optimization degree of Sn, Pb, and Zn was selected as the evaluation index of the response surface optimization in cassiterite polymetallic sulfide ore. These were called face values, with surface test values and surface predictive values included. Cassiterite mineral was represented by Sn, and the sulfide minerals were represented by Pb and Zn, and M1, M2 and M3 were used to represent the predicted values of Sn, Pb, Zn.
Based on the selected grinding parameters, 17 test combinations of three factors and three levels were designed from the Box–Behnken model. The test sequence was randomly generated by Design Expert 8.0.7 software, and the experimental value ranges of Sn, Pb and Zn were 1.87–5.78, 3.50–4.93, and 3.86–5.77. The analysis shows that the correction coefficient and complex correlation coefficient of three metals in Sn, Pb and Zn are the highest. These two models can be used as the regression equation between the surface test value and the grinding parameter. Through the Design Expert 8.0.7 software analysis and calculation, the fitting equations for Sn, Pb and Zn between the surface test value and the grinding parameter are shown in Equations (6), (7) and (8), with
X1,
X2, and
X3 representing encoded values.
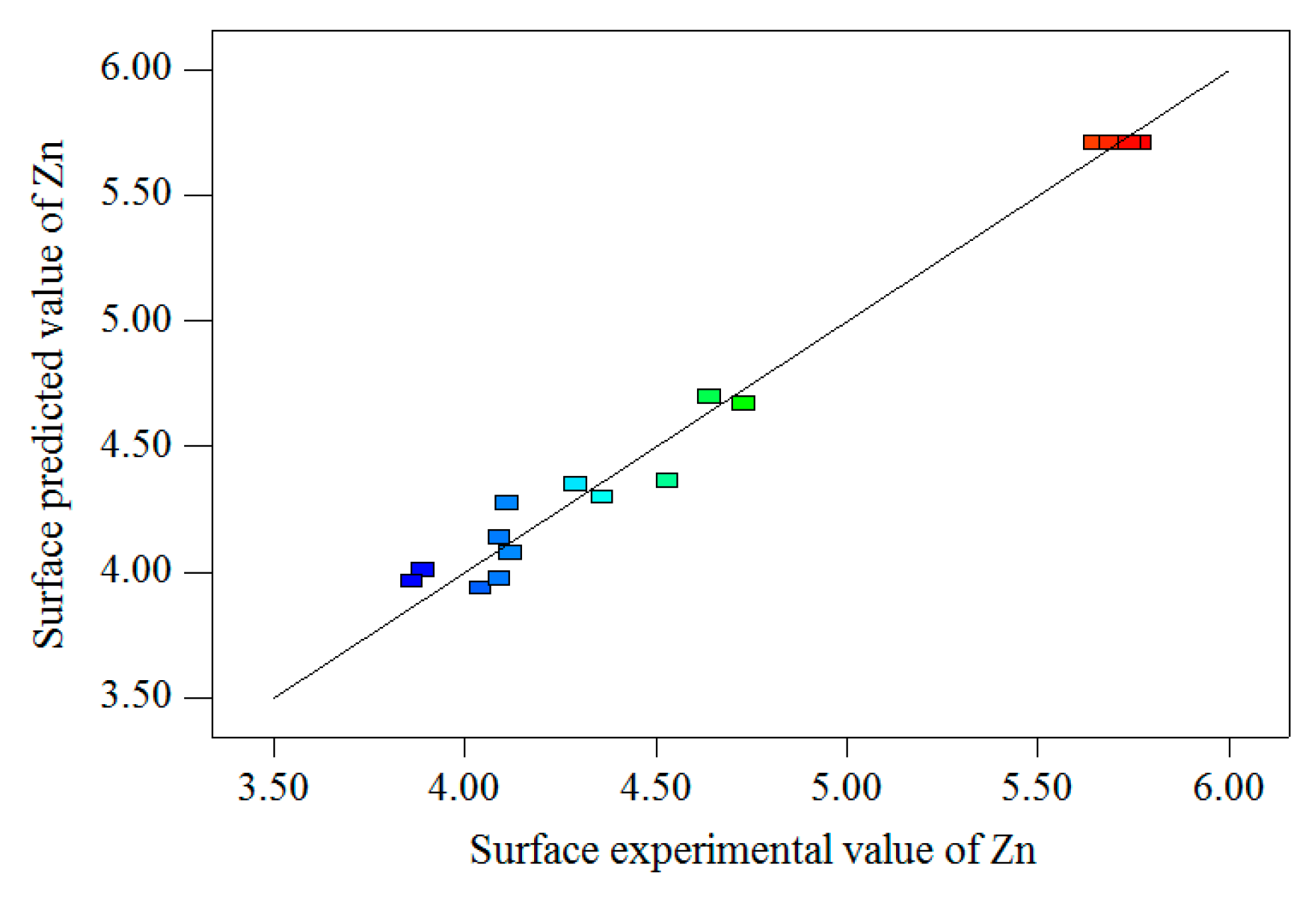
The surface test values, model predictive values and residuals of Sn, Pb and Zn metal elements are shown in
Figure 7 and
Figure 8, respectively. As shown in
Figure 7 and
Figure 8, the experimental values and predicted values have a high fitting degree, indicating that the fitting of Equations (6), (7), and (8) can well describe the relationship between the surface test values and grinding parameters.
As shown in
Figure 9,
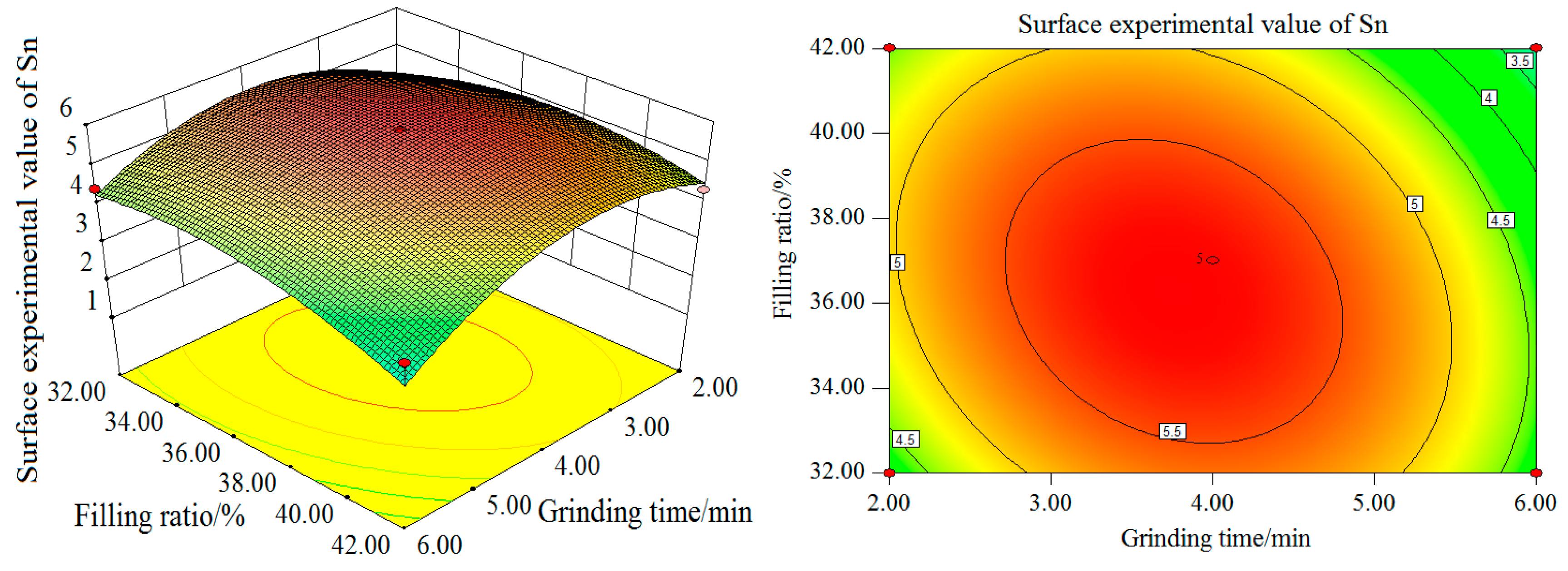
Figure 10 and
Figure 11, with the increase of grinding time and grinding concentration, the Sn surface test value firstly increases and then decreases, with the value reaching its maximum at grinding time of 4 min and grinding concentration of 65%. In terms of the single factor, the grinding time and grinding concentration have a significant influence on the experimental value of the Sn curved surface. As can be seen from the contour map, the interaction between grinding time and grinding concentration is more significant. The influence curve between the filling ratio and grinding time on the experimental value of the Sn curved surface also shows a trend of increasing first and then decreasing. When the grinding time is 4 min and the filling ratio is about 37%, the maximum Sn surface test value can be obtained. In terms of the single factor, the influence of grinding time on the experimental value of the Sn surface is greater than that of the filling ratio.
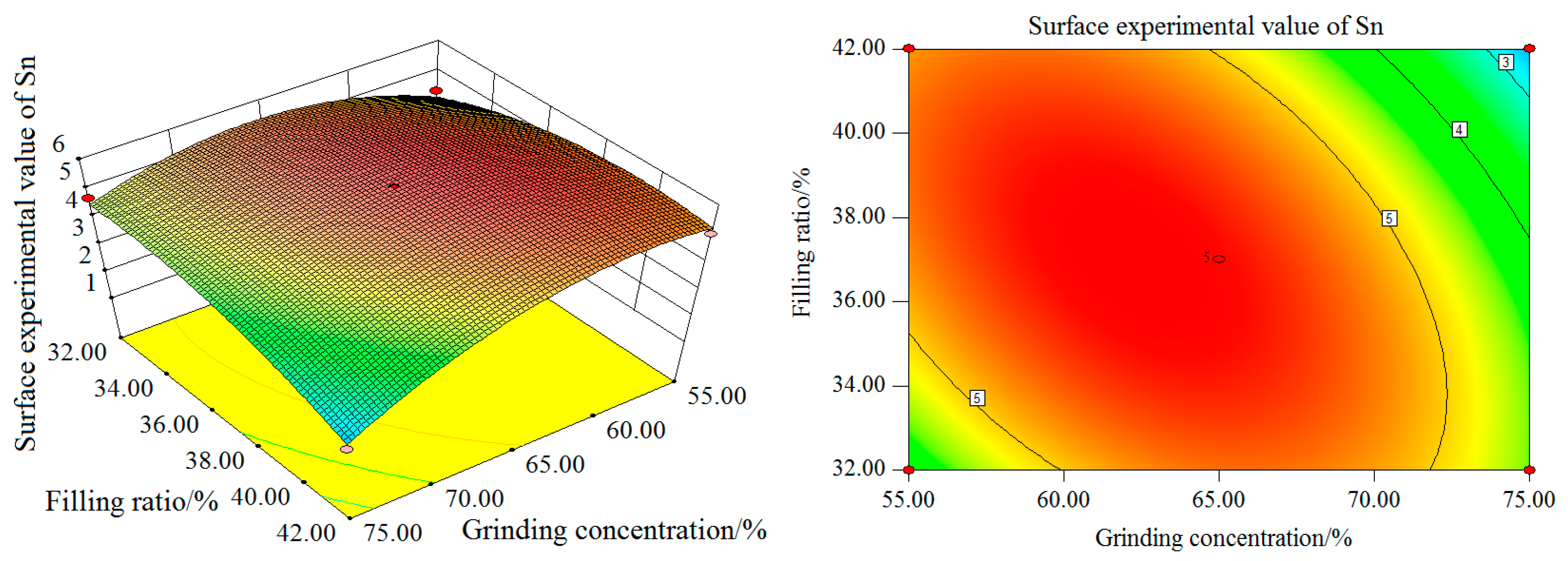
As can be seen from the contour map, the interaction between grinding time and the filling ratio is more significant. With the increase of the grinding concentration and filling ratio, the Sn surface test value increases first and then decreases. The maximum value is reached when the grinding concentration is 65% and the filling ratio about 37%. In terms of the single factor, the influence of the grinding concentration on the experimental value of the Sn surface is greater than that of the filling ratio.
The contour map shows that the interaction between the grinding concentration and filling ratio is not significant. In summary, the order of influence degree on Sn surface test value from high to low is as follows: grinding time, grinding concentration, and filling ratio. The interaction between the grinding concentration and filling ratio is weak, while the interaction between the grinding time and grinding concentration and between the grinding time and filling ratio, is stronger.
As shown in
Figure 12,
Figure 13 and
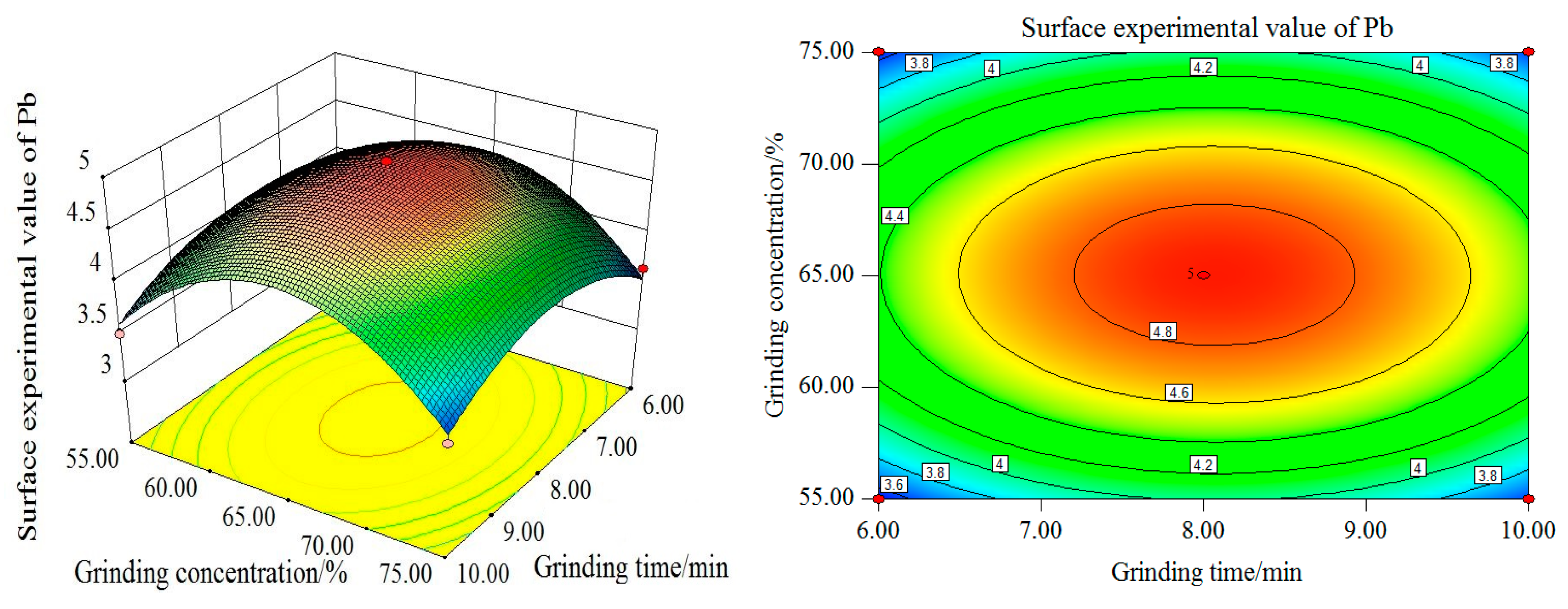
Figure 14, with the increase of grinding time and grinding concentration, the Pb surface test value firstly increases and then decreases, reaching its maximum when the grinding time is at 8 min and grinding concentration at 65%. From the perspective of single factor, the influence of grinding time and grinding concentration on the experimental value of the Pb curved surface is remarkable. As can be seen from the contour map, the interaction between the grinding time and grinding concentration is more significant. The influence curve between the filling ratio and grinding time on the experimental value of Pb curved surface also increases first and then decreases. When the grinding time is 8 min and the filling ratio is about 37%, the maximum Pb surface test value can be obtained.
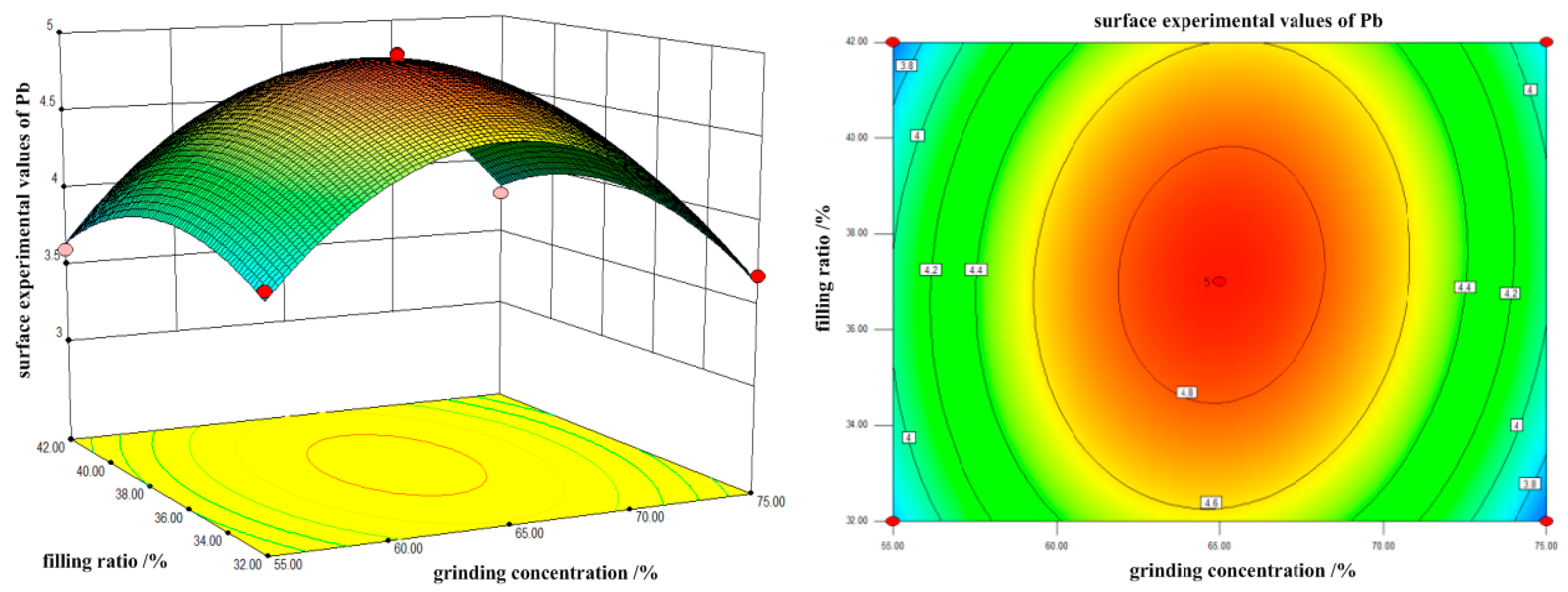
In terms of the single factor, the experimental value of Pb curved surface is significantly affected by grinding time and filling ratio. As can be seen from the contour map, the interaction between the grinding time and filling ratio is more significant. With the increase in grinding concentration and filling ratio, the Pb surface test value increases first and then decreases. The maximum value is reached when the grinding concentration is 65% and the filling ratio is about 37%. From the single factor, the filling ratio and grinding concentration have a significant influence on the experimental value of the Pb curved surface.
The contour map illustrates that the interaction between the grinding concentration and the filling ratio is not significant. In conclusion, the order of the influence degree on the Sn surface test value from high to low is as follows: grinding concentration, grinding time and filling ratio. The interaction between grinding concentration and filling ratio is weak, while the interaction between the grinding time and grinding concentration and between the grinding time and filling ratio, is stronger.
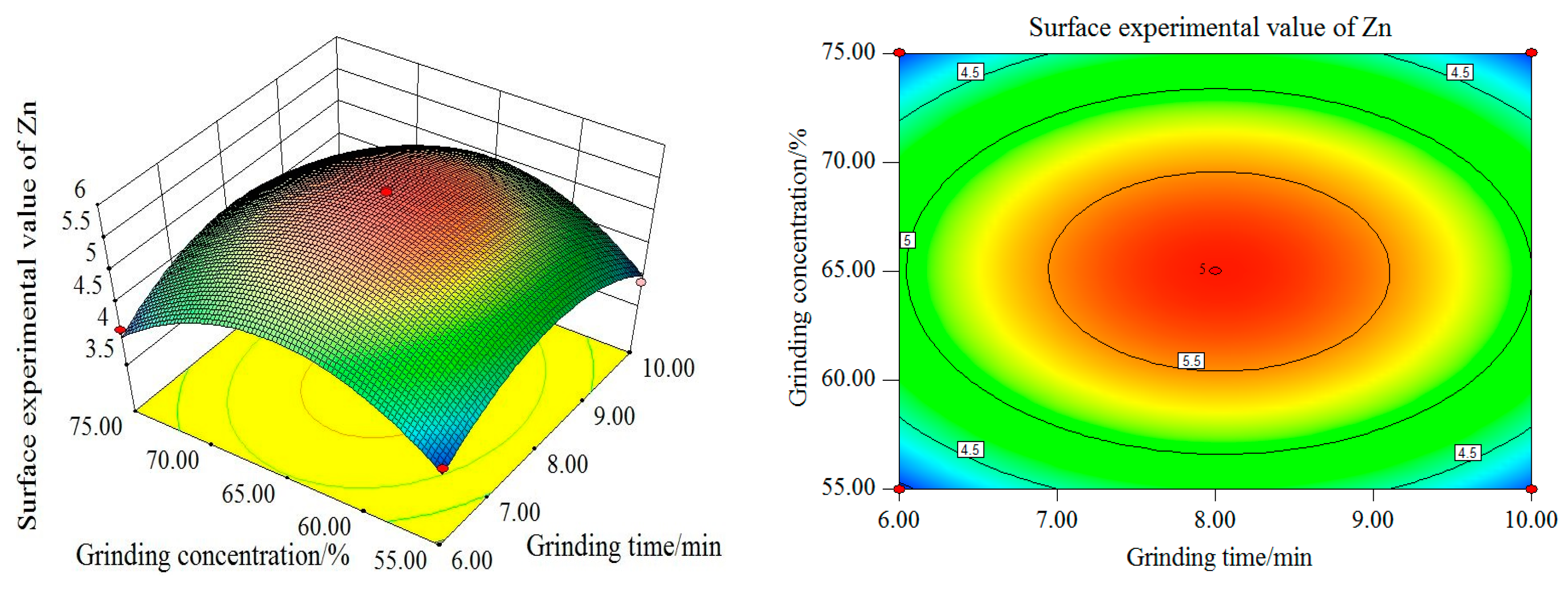
As shown in
Figure 15,
Figure 16 and
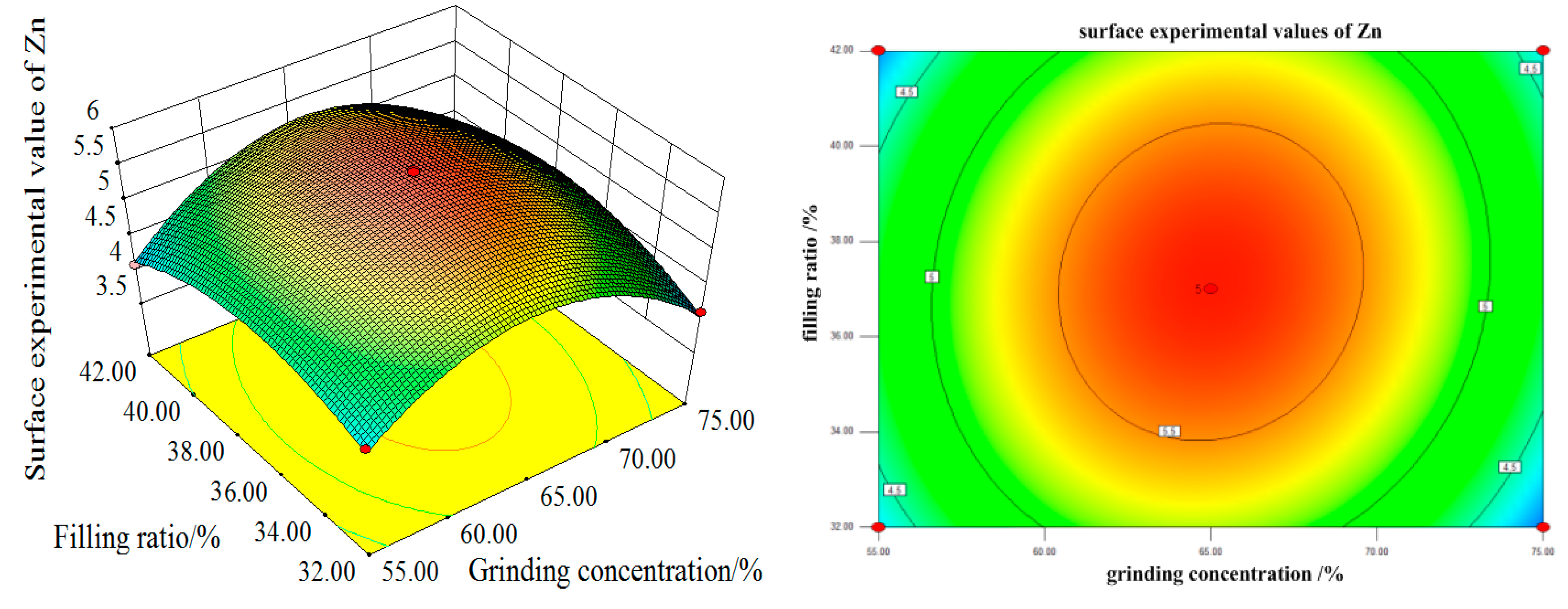
Figure 17, with the increase of grinding time and grinding concentration, the Zn surface test value firstly increases and then decreases, reaching its maximum at 8 min of grinding time and 65% of grinding concentration. From the single factor, the influence of grinding time and grinding concentration on the experimental value of the Zn curved surface is remarkable. As can be seen from the contour map, the interaction between the grinding time and the grinding concentration is more significant. The influence curve between the filling ratio and grinding time on the experimental value of the Zn curved surface also increases first and then decreases. When the grinding time is 8 min and the filling ratio is about 37%, the maximum Zn surface test value can be obtained. From the single factor, the influence of grinding concentration on the experimental value of the Zn surface is more significant than that of the filling ratio.
It can be seen from the contour map that the interaction between the grinding time and filling ratio is not significant. With the increase in the grinding concentration and filling ratio, the Zn surface test value increases first and then decreases. The maximum value is reached when the grinding concentration is 65% and the filling ratio is about 37%. From the single factor, the influence of grinding concentration on the experimental value of the Zn surface is more significant than that of the filling ratio.
The contour map illustrates that the interaction between the grinding concentration and the filling ratio is not significant. In summary, the order of the influence degree on the Zn surface test value from high to low is as follows: the grinding concentration, grinding time, and filling ratio. The interaction between the grinding concentration and grinding time is strong, the interaction between the grinding concentration and filling ratio is weak, and the interaction between the grinding time and filling ratio is weak.
4.2. Grinding Verification Test
The Sn surface design model can be obtained by accurately solving the model. The optimal theoretical parameters were a grinding time of 3.81 min, grinding concentration of 62.42%, and filling ratio of 37.07%. Therefore, the predicted value of the Sn surface was 5.847. The optimal theoretical parameters of Pb and Zn surface design models were a grinding time of 8.03 min, grinding concentration of 65.03%, and filling ratio of 37.13%. Accordingly, the prediction values of the Pb and Zn surface tests were 4.887 and 5.712, respectively. To verify the reliability and validity of the three equation models, A and B tests were carried out respectively on the prediction conditions and results. The results are shown in
Table 13 and
Table 14.
Through two verification tests, the conclusion can be reached that the predicted results were close to the experimental results, and the errors were within the range of ±2%. This finding shows that the model has strong reliability and validity. Therefore, on the premise of meeting the minimum energy consumption and the highest qualified particle size index, the grinding optimization condition of the cassiterite polymetallic sulfide ore can be obtained. Thus, Sn, as the representative of cassiterite, achieves optimum grinding conditions with a grinding time of 3.8 min, a grinding concentration of 63%, and a filling ratio of 37%. Polymetallic sulphide ore, represented by Pb and Sn, achieves the optimum grinding conditions with a grinding time of 8 min, a grinding concentration of 65% and a filling ratio of 37%.