Further Characterization of the BB Zircon via SIMS and MC-ICP-MS for Li, O, and Hf Isotopic Compositions
Abstract
1. Introduction
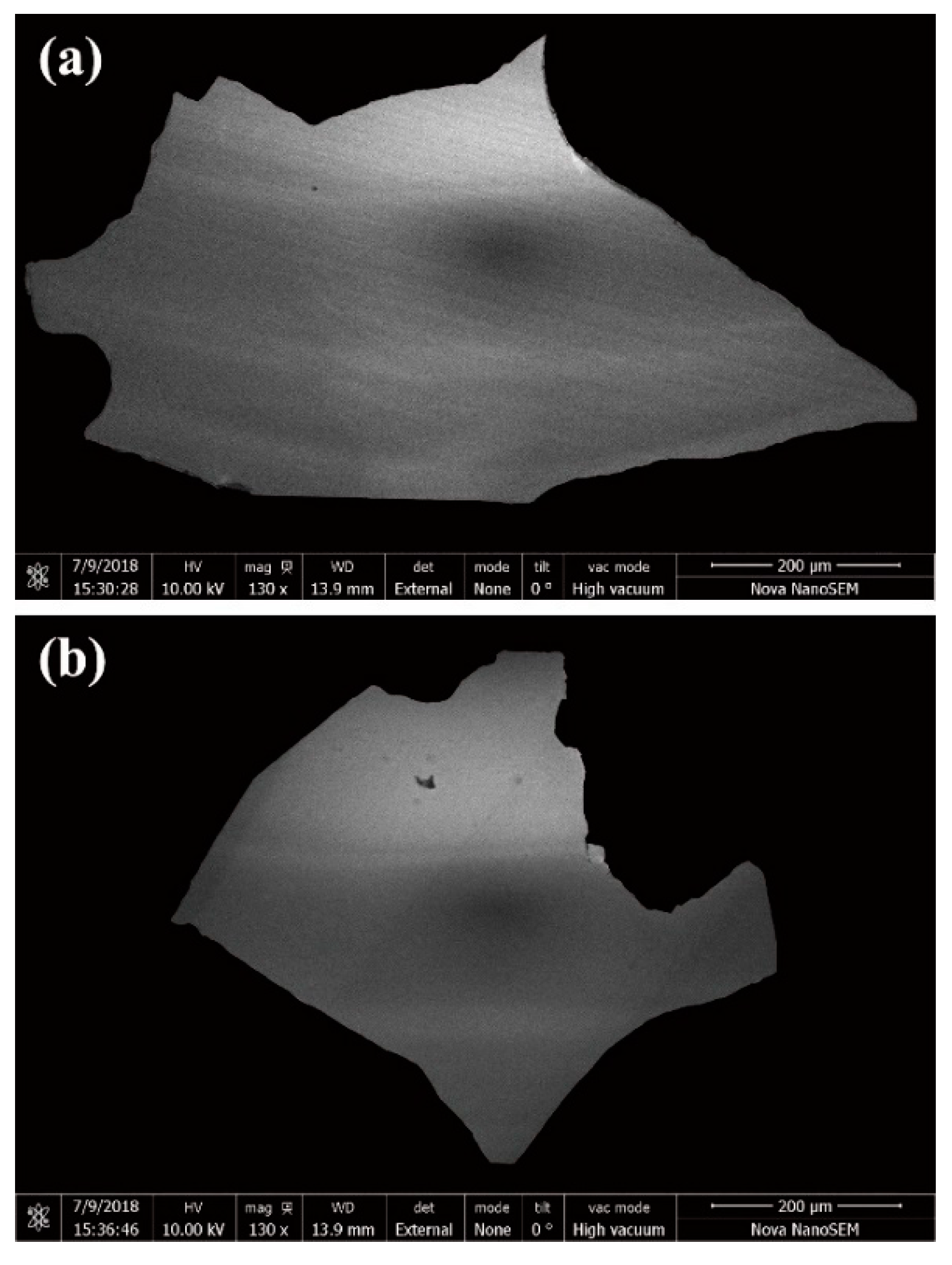
2. Sample Descriptions
3. Analytical Methods
3.1. SIMS Li Isotope Analysis
3.2. SIMS O Isotope Analysis
3.3. LA-MC-ICP-MS Hf Isotope Analysis
3.4. Solution MC-ICP-MS Hf Isotope Analysis
4. Results and Discussion
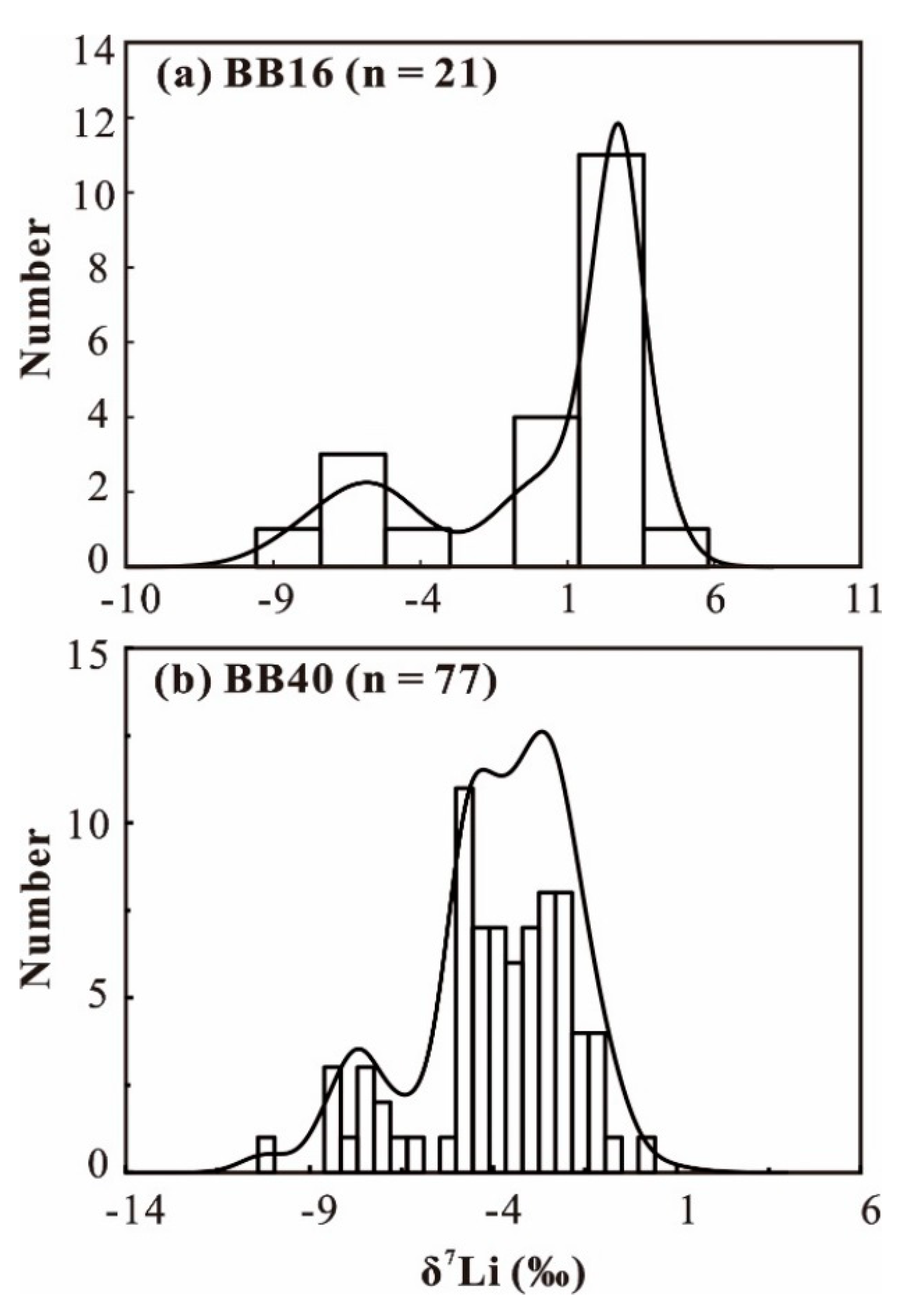
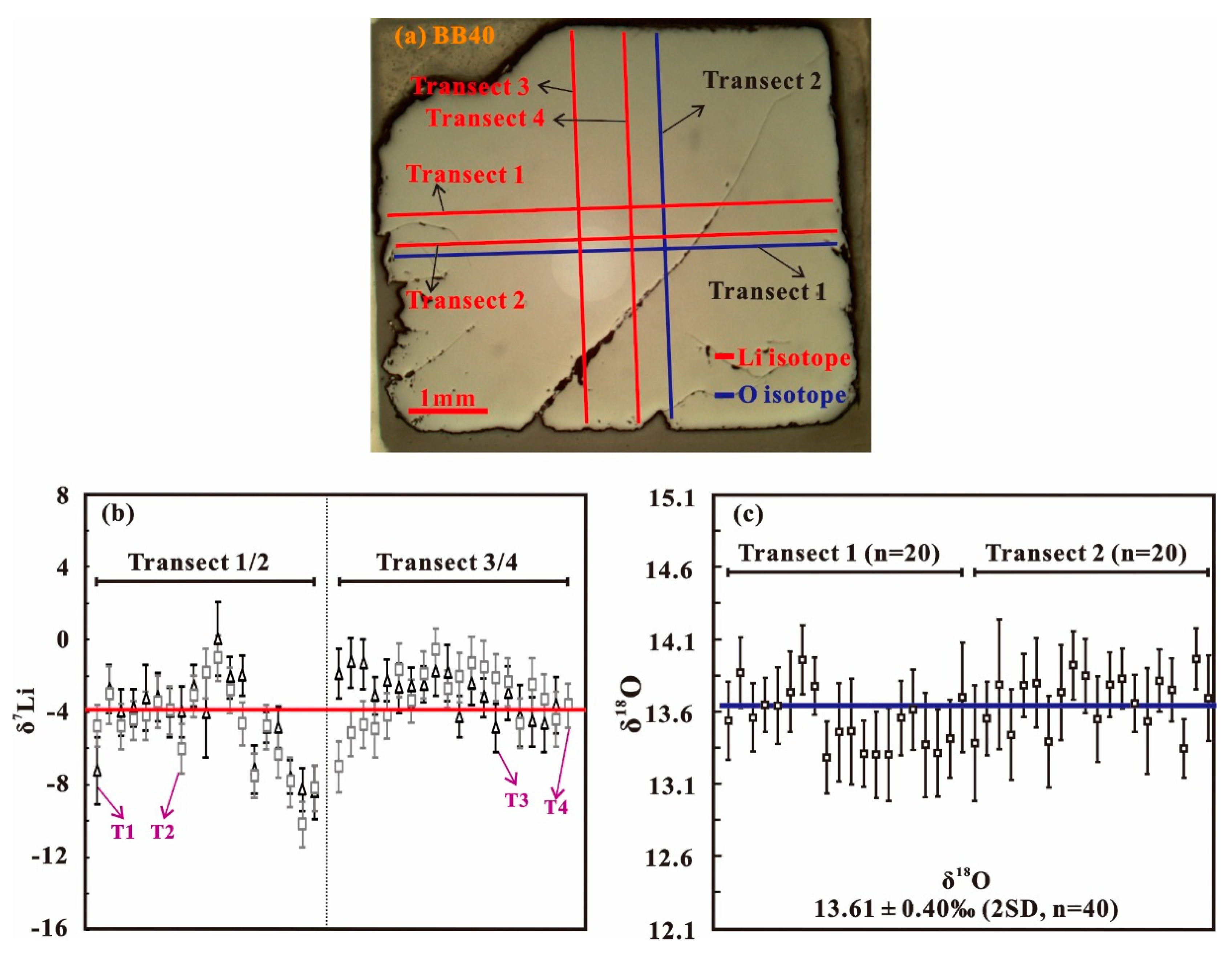
4.1. SIMS Li Isotope Composition
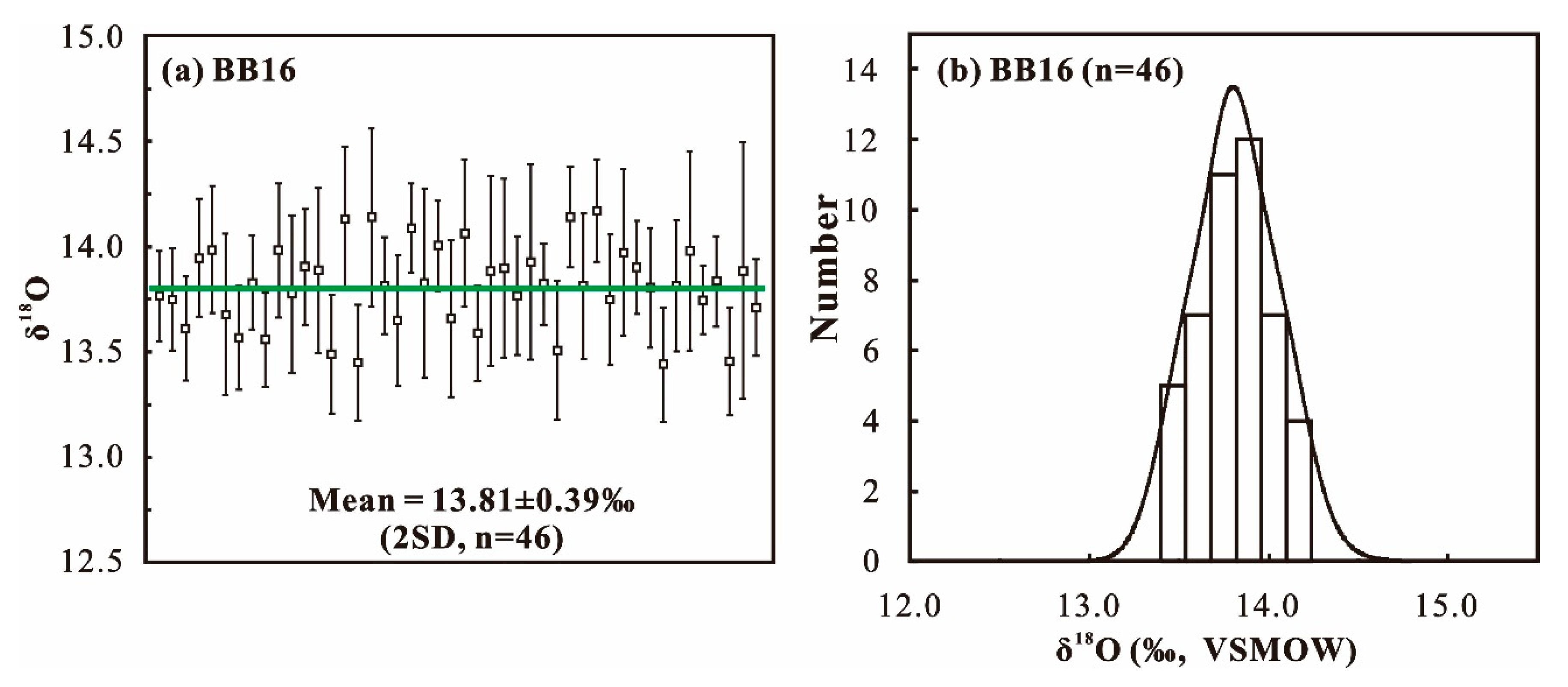
4.2. SIMS O Isotope Composition
4.3. Solution and LA-MC-ICP-MS Hf Isotope Data
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Yang, J.H.; Kroner, A.; Zhu, Y.S.; Li, R. Non-subduction origin for 3.2 Ga high-pressure metamorphic rocks in the Barberton granitoid-greenstone terrane, South Africa. Terra Nova 2019, 31, 373–380. [Google Scholar] [CrossRef]
- Kosler, J.; Sylvester, P.J. Present trends and the future of zircon in geochronology: Laser ablation ICPMS. Rev. Mineral. Geochem. 2003, 53, 243–275. [Google Scholar] [CrossRef]
- Ireland, T.R.; Williams, I.S. Considerations in zircon geochronology by SIMS. Rev. Mineral. Geochem. 2003, 53, 215–241. [Google Scholar] [CrossRef]
- Zhu, Y.S.; Yang, J.H.; Sun, J.F.; Wang, H. Zircon Hf–O isotope evidence for recycled oceanic and continental crust in the sources of alkaline rocks. Geology 2017, 45, 407–410. [Google Scholar] [CrossRef]
- Yang, J.H.; Wu, F.Y.; Wilde, S.A.; Xie, L.W.; Yang, Y.H.; Liu, X.M. Tracing magma mixing in granite genesis: In situ U–Pb dating and Hf-isotope analysis of zircons. Contrib. Mineral. Petr. 2007, 153, 177–190. [Google Scholar] [CrossRef]
- Wang, H.; Wu, Y.B.; Yang, J.H.; Qin, Z.W.; Duan, R.C.; Zhou, L.; Yang, S.H. Crustal basement controls granitoid magmatism, and implications for generation of continental crust in subduction zones: A Sr–Nd–Hf–O isotopic study from the Paleozoic Tongbai orogen, central China. Lithos 2017, 282, 298–315. [Google Scholar] [CrossRef]
- Ushikubo, T.; Kita, N.T.; Cavosie, A.J.; Wilde, S.A.; Rudnick, R.L.; Valley, J.W. Lithium in Jack Hills zircons: Evidence for extensive weathering of Earth’s earliest crust. Earth Planet. Sci. Lett. 2008, 272, 666–676. [Google Scholar] [CrossRef]
- Kemp, A.I.S.; Hawkesworth, C.J.; Foster, G.L.; Paterson, B.A.; Woodhead, J.D.; Hergt, J.M.; Gray, C.M.; Whitehouse, M.J. Magmatic and crustal differentiation history of granitic rocks from Hf–O isotopes in zircon. Science 2007, 315, 980–983. [Google Scholar] [CrossRef]
- Su, B.X.; Chen, C.; Pang, K.N.; Sakyi, P.A.; Uysal, I.; Avci, E.; Liu, X.; Zhang, P.F. Melt Penetration in Oceanic Lithosphere: Li Isotope Records from the Pozanti–Karsanti Ophiolite in Southern Turkey. J. Petrol. 2018, 59, 191–205. [Google Scholar] [CrossRef]
- Valley, J.W. Oxygen isotopes in zircon. Rev. Mineral. Geochem. 2003, 53, 343–385. [Google Scholar] [CrossRef]
- Bizzarro, M.; Simonetti, A.; Stevenson, R.K.; David, J. Hf isotope evidence for a hidden mantle reservoir. Geology 2002, 30, 771–774. [Google Scholar] [CrossRef]
- Kinny, P.D.; Maas, R. Lu–Hf and Sm–Nd isotope systems in zircon. Rev. Mineral. Geochem. 2003, 53, 327–341. [Google Scholar] [CrossRef]
- Harley, S.L.; Kelly, N.M. Zircon–Tiny but timely. Elements 2007, 3, 13–18. [Google Scholar] [CrossRef]
- Gao, Y.Y.; Li, X.H.; Griffin, W.L.; Tang, Y.J.; Pearson, N.J.; Liu, Y.; Chu, M.F.; Li, Q.L.; Tang, G.Q.; O’Reilly, S.Y. Extreme lithium isotopic fractionation in three zircon standards (Plesovice, Qinghu and Temora). Sci Rep. 2015, 5, 16878. [Google Scholar] [CrossRef] [PubMed]
- Kinny, P.D.; Compston, W.; Williams, I.S. A Reconnaissance Ion-Probe Study of Hafnium Isotopes in Zircons. Geochim. Cosmochim. Acta 1991, 55, 849–859. [Google Scholar] [CrossRef]
- Pidgeon, R.T. Calibration of zircon standards for the Curtin SHRIMP II. In Abstracts of the Eighth International Conference on Geochronology, Cosmochronology, and Isotope Geology; Lanphere, M.A., Dalrymple, G.B., Turrin, B.D., Eds.; United States Geological Survey: Reston, VA, USA, 1994; 251p. [Google Scholar]
- Claoué-Long, J.C.; Compston, W.; Roberts, J.; Fanning, C.M. Two carboniferous ages: A comparison of SHRIMP zircon dating with conventional zircon ages and 40Ar/39Ar analysis. In Geochronology Time Scales and Global Stratigraphic Correlation; Berggren, W.A., Kent, D.V., Aubrey, M.P., Hardenbol, J., Eds.; SEPM Society for Sedimentary Geology Special Publication: Tulsa, OK, USA, 1995; Volume 54, pp. 3–21. [Google Scholar] [CrossRef]
- Wiedenbeck, M.; Alle, P.; Corfu, F.; Griffin, W.L.; Meier, M.; Oberli, F.; Vonquadt, A.; Roddick, J.C.; Speigel, W. 3 Natural Zircon Standards for U–Th–Pb, Lu–Hf, Trace-Element and Ree Analyses. Geostand. Newsl. 1995, 19, 1–23. [Google Scholar] [CrossRef]
- Black, L.P.; Kamo, S.L.; Allen, C.M.; Aleinikoff, J.N.; Davis, D.W.; Korsch, R.J.; Foudoulis, C. TEMORA 1: A new zircon standard for Phanerozoic U–Pb geochronology. Chem. Geol. 2003, 200, 155–170. [Google Scholar] [CrossRef]
- Stern, R.A. A new isotopic and trace element standard for the ion microprobe: Preliminary TIMS U–Pb and electron microprobe data, current research. In Radiogenic Age and Isotopic Studies: Report 14; Geological Survey of Canada: Ottawa, ON, USA, 2001; 120p. [Google Scholar]
- Jackson, S.E.; Pearson, N.J.; Griffin, W.L.; Belousova, E.A. The application of laser ablation-inductively coupled plasma-mass spectrometry to in situ U–Pb zircon geochronology. Chem. Geol. 2004, 211, 47–69. [Google Scholar] [CrossRef]
- Morel, M.L.A.; Nebel, O.; Nebel-Jacobsen, Y.J.; Miller, J.S.; Vroon, P.Z. Hafnium isotope characterization of the GJ-1 zircon reference material by solution and laser-ablation MC-ICPMS. Chem. Geol. 2008, 255, 231–235. [Google Scholar] [CrossRef]
- Woodhead, J.D.; Hergt, J.M. A preliminary appraisal of seven natural zircon reference materials for in situ Hf isotope determination. Geostand. Geoanal. Res. 2005, 29, 183–195. [Google Scholar] [CrossRef]
- Nasdala, L.; Hofmeister, W.G.; Norberg, N.; Mattinson, J.M.; Corfu, F.; Dorr, W.; Kamo, S.L.; Kennedy, A.K.; Kronz, A.; Reiners, P.W.; et al. Zircon M257—A homogeneous natural reference material for the ion microprobe U–Pb analysis of zircon. Geostand. Geoanal. Res. 2008, 32, 247–265. [Google Scholar] [CrossRef]
- Slama, J.; Kosler, J.; Condon, D.J.; Crowley, J.L.; Gerdes, A.; Hanchar, J.M.; Horstwood, M.S.A.; Morris, G.A.; Nasdala, L.; Norberg, N.; et al. Plesovice zircon—A new natural reference material for U–Pb and Hf isotopic microanalysis. Chem. Geol. 2008, 249, 1–35. [Google Scholar] [CrossRef]
- Gehrels, G.E.; Valencia, V.A.; Ruiz, J. Enhanced precision, accuracy, efficiency, and spatial resolution of U–Pb ages by laser ablation-multicollector-inductively coupled plasma-mass spectrometry. Geochem. Geophy. Geosy. 2008, 9, Q03017. [Google Scholar] [CrossRef]
- Stern, R.A.; Bodorkos, S.; Kamo, S.L.; Hickman, A.H.; Corfu, F. Measurement of SIMS Instrumental Mass Fractionation of Pb Isotopes During Zircon Dating. Geostand. Geoanal. Res. 2009, 33, 145–168. [Google Scholar] [CrossRef]
- Li, X.H.; Long, W.G.; Li, Q.L.; Liu, Y.; Zheng, Y.F.; Yang, Y.H.; Chamberlain, K.R.; Wan, D.F.; Guo, C.H.; Wang, X.C.; et al. Penglai Zircon Megacrysts: A Potential New Working Reference Material for Microbeam Determination of Hf–O Isotopes and U–Pb Age. Geostand. Geoanal. Res. 2010, 34, 117–134. [Google Scholar] [CrossRef]
- Li, X.H.; Tang, G.Q.; Gong, B.; Yang, Y.H.; Hou, K.J.; Hu, Z.C.; Li, Q.L.; Liu, Y.; Li, W.X. Qinghu zircon: A working reference for microbeam analysis of U–Pb age and Hf and O isotopes. Chinese Sci. Bull. 2013, 58, 4647–4654. [Google Scholar] [CrossRef]
- Iwano, H.; Orihashi, Y.; Hirata, T.; Ogasawara, M.; Danhara, T.; Horie, K.; Hasebe, N.; Sueoka, S.; Tamura, A.; Hayasaka, Y.; et al. An inter-laboratory evaluation of OD-3 zircon for use as a secondary U–Pb dating standard. Isl. Arc 2013, 22, 382–394. [Google Scholar] [CrossRef]
- Nasdala, L.; Corfu, F.; Valley, J.W.; Spicuzza, M.J.; Wu, F.Y.; Li, Q.L.; Yang, Y.H.; Fisher, C.; Muenker, C.; Kennedy, A.K.; et al. Zircon M127-A Homogeneous Reference Material for SIMS U–Pb Geochronology Combined with Hafnium, Oxygen and, Potentially, Lithium Isotope Analysis. Geostand. Geoanal. Res. 2016, 40, 457–475. [Google Scholar] [CrossRef]
- Lana, C.; Farina, F.; Gerdes, A.; Alkmim, A.; Goncalves, G.O.; Jardim, A.C. Characterization of zircon reference materials via high precision U–Pb LA-MC-ICP-MS. J. Anal. Atom. Spectrom. 2017, 32, 2011–2023. [Google Scholar] [CrossRef]
- Santos, M.M.; Lana, C.; Scholz, R.; Buick, I.; Schmitz, M.D.; Kamo, S.L.; Gerdes, A.; Corfu, F.; Tapster, S.; Lancaster, P.; et al. A New Appraisal of Sri Lankan BB Zircon as a Reference Materialfor LA-ICP-MS U–Pb Geochronology and Lu–Hf IsotopeTracing. Geostand. Geoanal. Res. 2017, 41, 335–358. [Google Scholar] [CrossRef]
- Tian, Y.T.; Vermeesch, P.; Danisik, M.; Condon, D.J.; Chen, W.; Kohn, B.; Schwanethal, J.; Rittner, M. LGC-1: A zircon reference material for in-situ (U–Th)/He dating. Chem. Geol. 2017, 454, 80–92. [Google Scholar] [CrossRef]
- Nasdala, L.; Corfu, F.; Schoene, B.; Tapster, S.R.; Wall, C.J.; Schmitz, M.D.; Ovtcharova, M.; Schaltegger, U.; Kennedy, A.K.; Kronz, A.; et al. GZ7 and GZ8—Two Zircon Reference Materials for SIMS U–Pb Geochronology. Geostand. Geoanal. Res. 2018, 42, 431–457. [Google Scholar] [CrossRef] [PubMed]
- Cheong, A.C.S.; Jeong, Y.J.; Lee, S.; Yi, K.; Jo, H.J.; Lee, H.S.; Park, C.; Kim, N.K.; Li, X.H.; Kamo, S.L. LKZ-1: A New Zircon Working Standard for the In Situ Determination of U–Pb Age, O–Hf Isotopes, and Trace Element Composition. Minerals 2019, 9, 325. [Google Scholar] [CrossRef]
- Eddy, M.P.; Ibanez-Mejia, M.; Burgess, S.D.; Coble, M.A.; Cordani, U.G.; DesOrmeau, J.; Gehrels, G.E.; Li, X.H.; MacLennan, S.; Pecha, M.; et al. GHR1 Zircon—A New Eocene Natural Reference Material for Microbeam U–Pb Geochronology and Hf Isotopic Analysis of Zircon. Geostand. Geoanal. Res. 2019, 43, 113–132. [Google Scholar] [CrossRef]
- Huang, C.; Wang, H.; Yang, J.H.; Ramezani, J.; Yang, C.; Zhang, S.B.; Yang, Y.H.; Xia, X.P.; Feng, L.J.; Lin, J.; et al. SA01—A Proposed Zircon Reference Material for Micro-beam U–Pb Age and Hf–O Isotopic Analysis. Geostand. Geoanal. Res. 2020, in press. [Google Scholar] [CrossRef]
- Li, X.H.; Li, Q.L.; Liu, Y.; Tang, G.Q. Further characterization of M257 zircon standard: A working reference for SIMS analysis of Li isotopes. J. Anal. Atom. Spectrom. 2011, 26, 352–358. [Google Scholar] [CrossRef]
- Tang, G.Q.; Li, X.H.; Li, Q.L.; Liu, Y.; Ling, X.X.; Yin, Q.Z. Deciphering the physical mechanism of the topography effect for oxygen isotope measurements using a Cameca IMS-1280 SIMS. J. Anal. Atom. Spectrom 2015, 30, 950–956. [Google Scholar] [CrossRef]
- Baertschi, P. Absolute O-18 Content of Standard Mean Ocean Water. Earth Planet. Sci. Lett. 1976, 31, 341–344. [Google Scholar] [CrossRef]
- Wu, F.Y.; Yang, Y.H.; Xie, L.W.; Yang, J.H.; Xu, P. Hf isotopic compositions of the standard zircons and baddeleyites used in U–Pb geochronology. Chem. Geol. 2006, 234, 105–126. [Google Scholar] [CrossRef]
- Huang, C.; Yang, Y.H.; Yang, J.H.; Xie, L.W. In situ simultaneous measurement of Rb–Sr/Sm–Nd or Sm–Nd/Lu–Hf isotopes in natural minerals using laser ablation multi-collector ICP-MS. J. Anal. Atom. Spectrom. 2015, 30, 994–1000. [Google Scholar] [CrossRef]
- Yang, Y.H.; Zhang, H.F.; Chu, Z.Y.; Xie, L.W.; Wu, F.Y. Combined chemical separation of Lu, Hf, Rb, Sr, Sm and Nd from a single rock digest and precise and accurate isotope determinations of Lu–Hf, Rb–Sr and Sm–Nd isotope systems using Multi-Collector ICP-MS and TIMS. Int. J. Mass. Spectrom. 2010, 290, 120–126. [Google Scholar] [CrossRef]
- Vervoort, J.D.; Patchett, P.J.; Soderlund, U.; Baker, M. Isotopic composition of Yb and the determination of Lu concentrations and Lu/Hf ratios by isotope dilution using MC-ICPMS. Geochem Geophy Geosy 2004, 5. [Google Scholar] [CrossRef]
- Sliwinski, J.T.; Kueter, N.; Marxer, F.; Ulmer, P.; Guillong, M.; Bachmann, O. Controls on lithium concentration and diffusion in zircon. Chem. Geol. 2018, 501, 1–11. [Google Scholar] [CrossRef]
- Fisher, C.M.; Vervoort, J.D.; Hanchar, J.M. Guidelines for reporting zircon Hf isotopic data by LA-MC-ICPMS and potential pitfalls in the interpretation of these data. Chem. Geol. 2014, 363, 125–133. [Google Scholar] [CrossRef]
| MC-ICP-MS Cup Configuration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cup | L4 | L3 | L2 | L1 | C | H1 | H2 | H3 | H4 |
| Solution | 173Yb | 175Lu | 176(Lu + Hf + Yb) | 177Hf | 178Hf | 179Hf | 180(Hf + Ta + W) | 181Ta | 183W |
| Laser | 172Yb | 173Yb | 175Lu | 176Hf | 177Hf | 178Hf | 179Hf | 180Hf | 182W |
| Instrumentation | |||||||||
| Mass spectrometry | Thermo Fisher Neptune Plus (MC-ICP-MS) | ||||||||
| RF forward power | ~1200 W for Laser; ~1100 W for Solution | ||||||||
| Interface cones | Nickel Standard Sampler cones and “H” Skimmer cones | ||||||||
| Sampling mode | 1 block of 200 cycles for Laser; 9 blocks of 10 cycles for Solution | ||||||||
| Integration times | 0.131 s for Laser; 4.191 s for Solution | ||||||||
| Background/baseline | No baseline was collected | ||||||||
| Carrier gas (L/min) | ~0.8 for Laser; ~1 for Solution | ||||||||
| Laser ablation system | Geolas Pro | ||||||||
| Fluence | ~4.5 J/cm2 | ||||||||
| Spot size | 60 µm for BB16 and SA01; 44 µm for Mud Tank | ||||||||
| Ablation duration | 26 s | ||||||||
| Sampling mode/Repetition rate | Static spot ablation/6 Hz | ||||||||
| Sample preparation | Conventional mineral separation, 1 inch resin mount | ||||||||
| Imaging | Transmissive and reflected light imaging | ||||||||
| Data processing | |||||||||
| Reference material information | Mud Tank and SA01 used as the quality control standard | ||||||||
| Data processing package used | For Hf isotope, an in-house Microsoft Excel macro written in VBA (Visual Basic for Applications) was used for mass fraction correction, interference correction, and uncertainty propagation | ||||||||
| Sample | Analysis Numbers | δ7Li (‰) a | 7Li+ Count Rate (cps/nA) | Li (μg g−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | 2SD | Max | Min | Mean | Max | Min | Mean | Max | Min | |||
| BB16 | Small Shard1 | 5 | 2.6 | 0.6 | 3.0 | 2.2 | 4705 | 4874 | 4577 | 0.80 | 0.83 | 0.78 |
| Small Shard2 | 4 | 1.6 | 2.7 | 3.0 | −0.1 | 930 | 1213 | 703 | 0.16 | 0.21 | 0.12 | |
| Small Shard3 | 3 | 2.8 | 0.6 | 3.1 | 2.4 | 2785 | 3430 | 2179 | 0.47 | 0.58 | 0.37 | |
| Small Shard4 | 3 | 2.3 | 2.2 | 3.7 | 1.1 | 3585 | 4369 | 2438 | 0.61 | 0.74 | 0.41 | |
| Large Shard | 6 | −5.2 | 4.4 | −0.6 | −7.5 | 755 | 977 | 579 | 0.13 | 0.17 | 0.10 | |
| BB40 | Traverse 1 | 19 | −4.4 | 4.6 | 0 | −8.5 | 5658 | 7311 | 3423 | 0.96 | 1.2 | 0.58 |
| Traverse 2 | 19 | −4.9 | 4.5 | −1 | −10.2 | 5810 | 8068 | 3357 | 1.03 | 1.4 | 0.59 | |
| Traverse 3 | 19 | −3 | 2.2 | −1.3 | −4.9 | 5396 | 6460 | 4584 | 0.92 | 1.1 | 0.78 | |
| Traverse 4 | 20 | −3.2 | 3.2 | −0.6 | −7.1 | 5718 | 7115 | 5227 | 1.01 | 1.3 | 0.92 | |
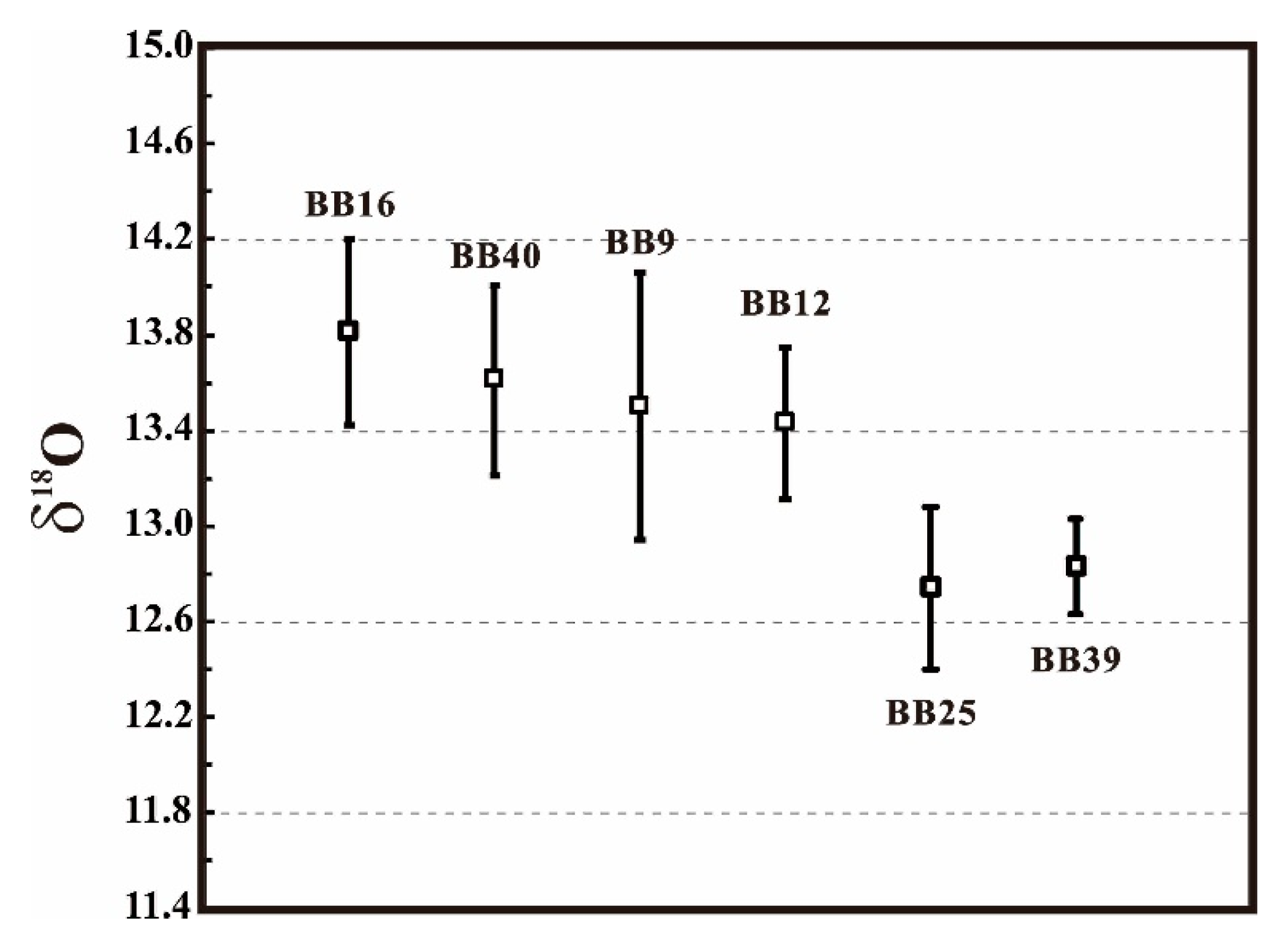
| Reference Material | Number of Analysis | δ18O (‰) | References |
|---|---|---|---|
| BB16 | 46 | 13.81 ± 0.39‰ (2SD) | This study |
| BB40 | 40 | 13.61 ± 0.40‰ (2SD) | This study |
| BB9 | 31 | 13.50 ± 0.56‰ (2SD) | Santos et al. [33] |
| BB12 | 29 | 13.43 ± 0.32‰ (2SD) | Santos et al. [33] |
| BB25 | 19 | 12.74 ± 0.34‰ (2SD) | Santos et al. [33] |
| BB39 | 30 | 12.83 ± 0.20‰ (2SD) | Santos et al. [33] |
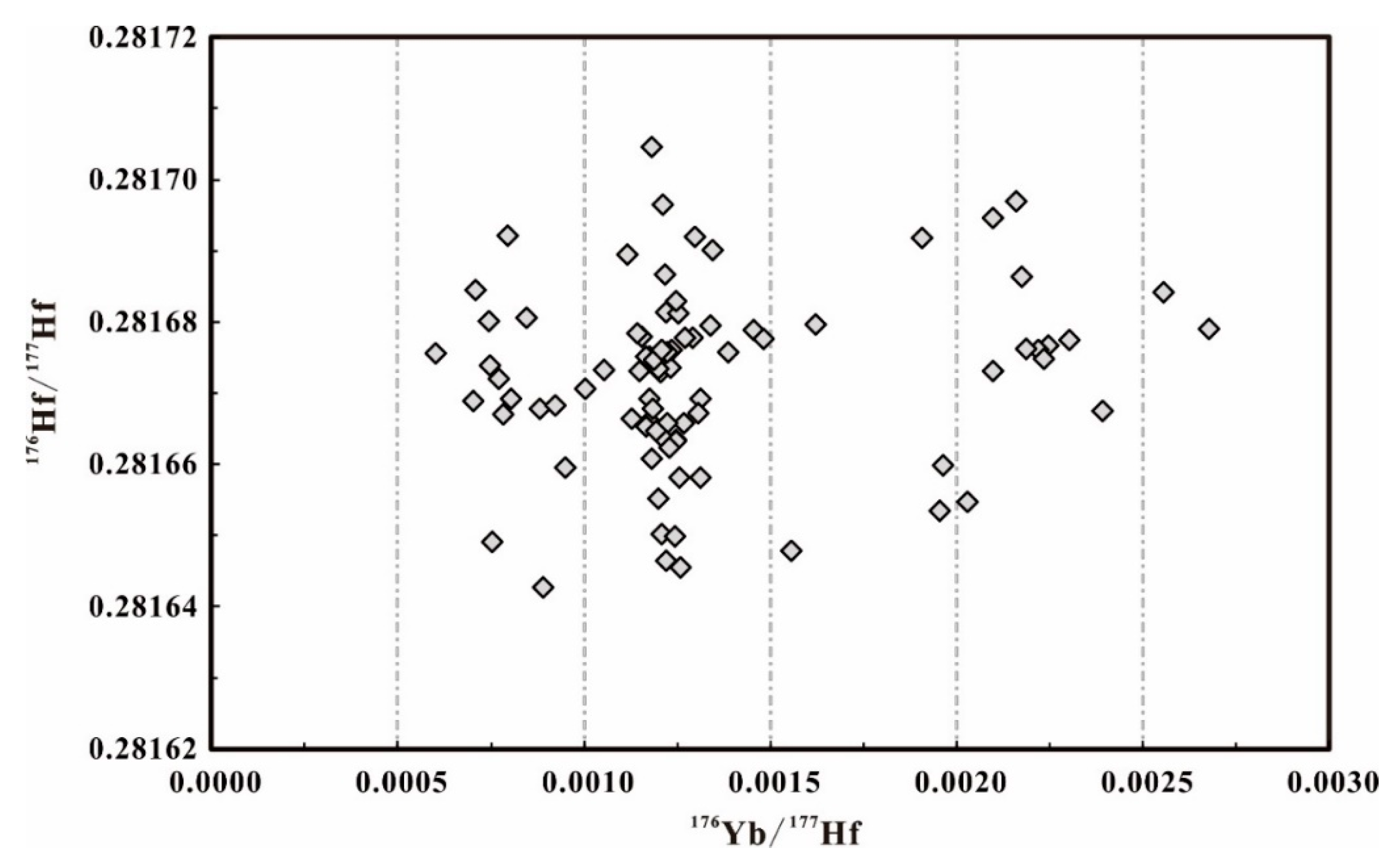
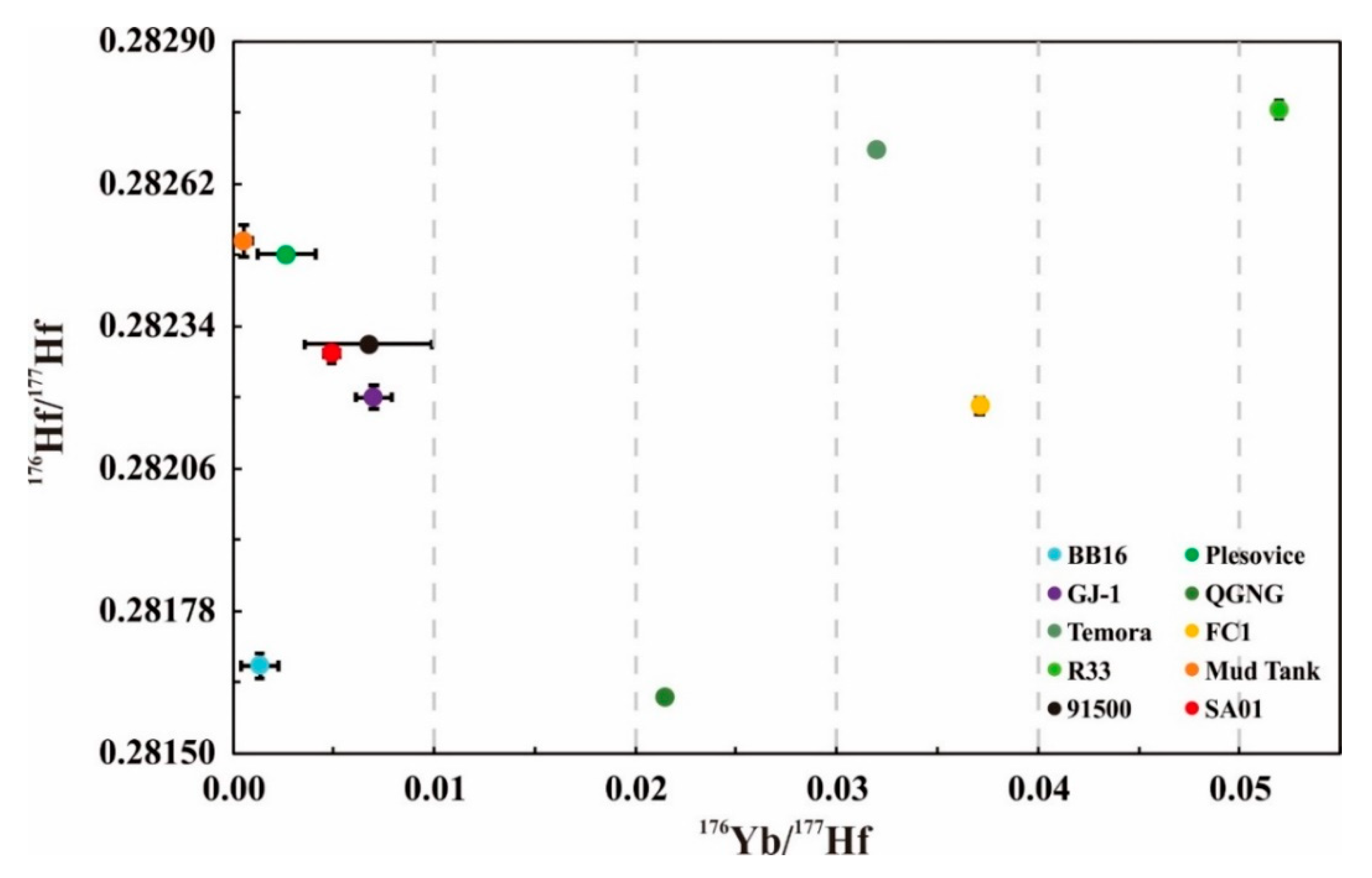
| Sample No. | Method | Analysis Numbers | 176Yb/177Hf | 2SD | 176Lu/177Hf | 2SD | 176Hf/177Hf | 2SD |
|---|---|---|---|---|---|---|---|---|
| This Work | ||||||||
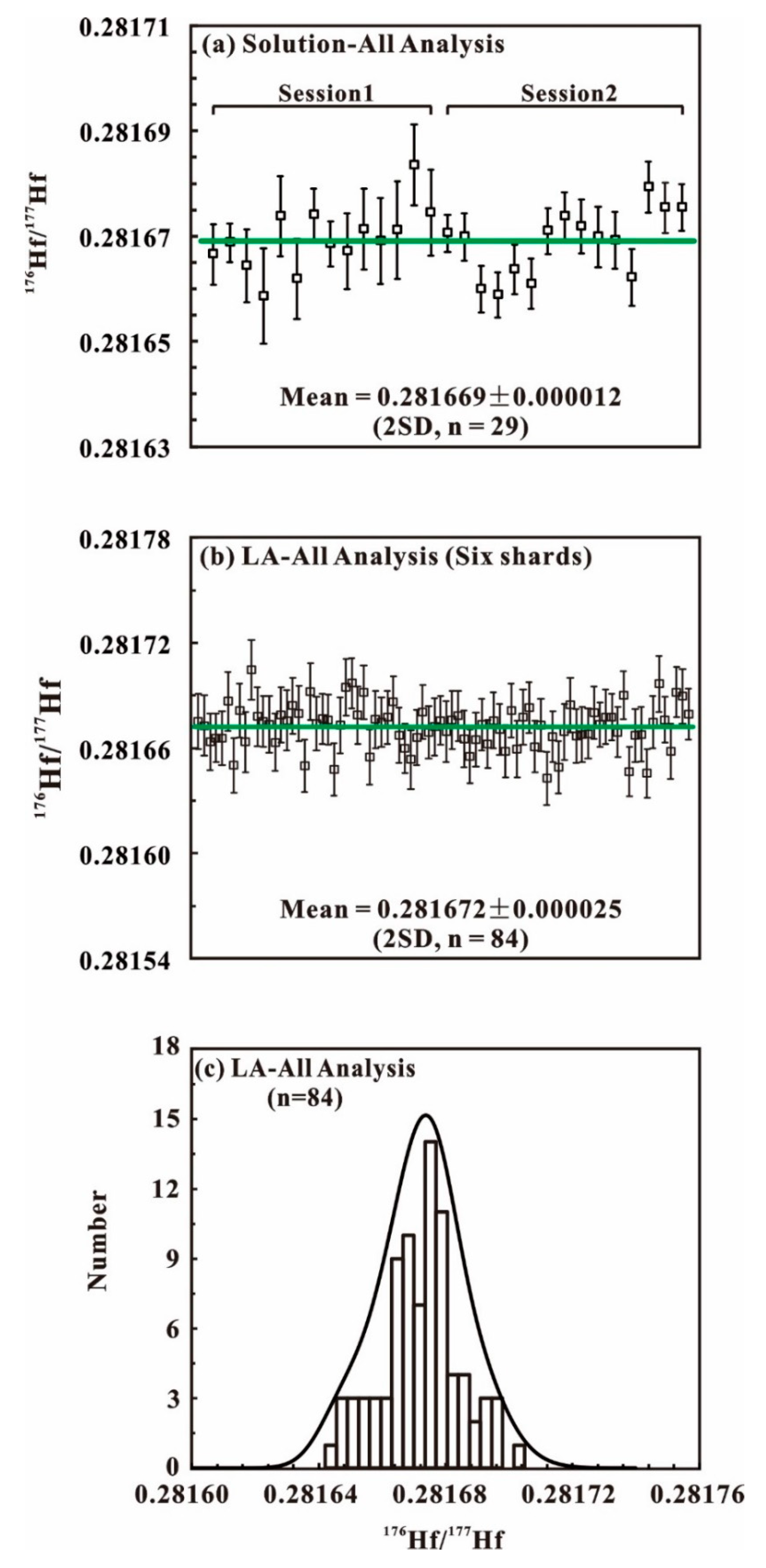
| BB16@session1 | Solution | 14 | - | - | - | - | 0.281670 | 0.000012 |
| BB16@session2 | Solution | 15 | 0.281669 | 0.000012 | ||||
| BB16@shard1 | LA | 14 | 0.00121 | 0.00006 | 0.000044 | 0.000001 | 0.281673 | 0.000025 |
| BB16@shard2 | LA | 14 | 0.00178 | 0.00117 | 0.000066 | 0.000043 | 0.281677 | 0.000027 |
| BB16@shard3 | LA | 14 | 0.00166 | 0.00130 | 0.000060 | 0.000046 | 0.281672 | 0.000021 |
| BB16@shard4 | LA | 14 | 0.00119 | 0.00023 | 0.000044 | 0.000008 | 0.281669 | 0.000016 |
| BB16@shard5 | LA | 14 | 0.00097 | 0.00039 | 0.000036 | 0.000016 | 0.281669 | 0.000023 |
| BB16@shard6 | LA | 14 | 0.00126 | 0.00013 | 0.000048 | 0.000005 | 0.281674 | 0.000031 |
| Santos et al. | ||||||||
| BB1 | LA | 7 | 0.000002 | 0.00003 | 0.281670 | 0.000027 | ||
| BB2 | LA | 13 | 0.000009 | 0.00015 | 0.281670 | 0.000033 | ||
| BB3 | LA | 20 | 0.000004 | 0.00006 | 0.281669 | 0.000023 | ||
| BB4 | LA | 5 | 0.000003 | 0.00005 | 0.281684 | 0.000016 | ||
| BB5 | LA | 16 | 0.000004 | 0.00006 | 0.281669 | 0.000018 | ||
| BB6 | LA | 5 | 0.000002 | 0.00004 | 0.281668 | 0.000029 | ||
| BB7 | LA | 7 | 0.000002 | 0.00004 | 0.281678 | 0.000023 | ||
| BB9 | LA | 20 | 0.000004 | 0.00007 | 0.281671 | 0.000012 | ||
| BB10 | LA | 13 | 0.000010 | 0.00016 | 0.281677 | 0.000014 | ||
| BB11 | LA | 12 | 0.000006 | 0.00010 | 0.281676 | 0.000008 | ||
| BB12 | LA | 15 | 0.000009 | 0.00015 | 0.281677 | 0.000011 | ||
| BB13 | LA | 12 | 0.000003 | 0.00004 | 0.281675 | 0.000009 | ||
| BB14 | LA | 9 | 0.000003 | 0.00005 | 0.281678 | 0.000010 | ||
| BB16 | LA | 16 | 0.000003 | 0.00005 | 0.281676 | 0.000009 | ||
| BB17 | LA | 11 | 0.000003 | 0.00005 | 0.281677 | 0.000006 | ||
| BB18 | LA | 16 | 0.000007 | 0.00012 | 0.281675 | 0.000010 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Wang, H.; Yang, J.-H.; Xie, L.-W.; Yang, Y.-H.; Wu, S.-T. Further Characterization of the BB Zircon via SIMS and MC-ICP-MS for Li, O, and Hf Isotopic Compositions. Minerals 2019, 9, 774. https://doi.org/10.3390/min9120774
Huang C, Wang H, Yang J-H, Xie L-W, Yang Y-H, Wu S-T. Further Characterization of the BB Zircon via SIMS and MC-ICP-MS for Li, O, and Hf Isotopic Compositions. Minerals. 2019; 9(12):774. https://doi.org/10.3390/min9120774
Chicago/Turabian StyleHuang, Chao, Hao Wang, Jin-Hui Yang, Lie-Wen Xie, Yue-Heng Yang, and Shi-Tou Wu. 2019. "Further Characterization of the BB Zircon via SIMS and MC-ICP-MS for Li, O, and Hf Isotopic Compositions" Minerals 9, no. 12: 774. https://doi.org/10.3390/min9120774
APA StyleHuang, C., Wang, H., Yang, J.-H., Xie, L.-W., Yang, Y.-H., & Wu, S.-T. (2019). Further Characterization of the BB Zircon via SIMS and MC-ICP-MS for Li, O, and Hf Isotopic Compositions. Minerals, 9(12), 774. https://doi.org/10.3390/min9120774





