Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis
Abstract
:1. Introduction
1.1. Background
1.2. Particle Size Distribution Using Microscopy and Image Processing and Analysis
2. Methodology
2.1. Reagents
2.2. Generation of Precipitation Aggregates
2.3. Size Distribution Analysis Techniques
2.3.1. Laser Diffraction Analysis
2.3.2. Microscopy and Semiautomated Image Processing and Analysis
2.4. Measurement of Copper Content in the Sulfide Precipitate
2.5. Copper Sulfide Conversion
2.6. PSD Estimation and Statistical Analysis
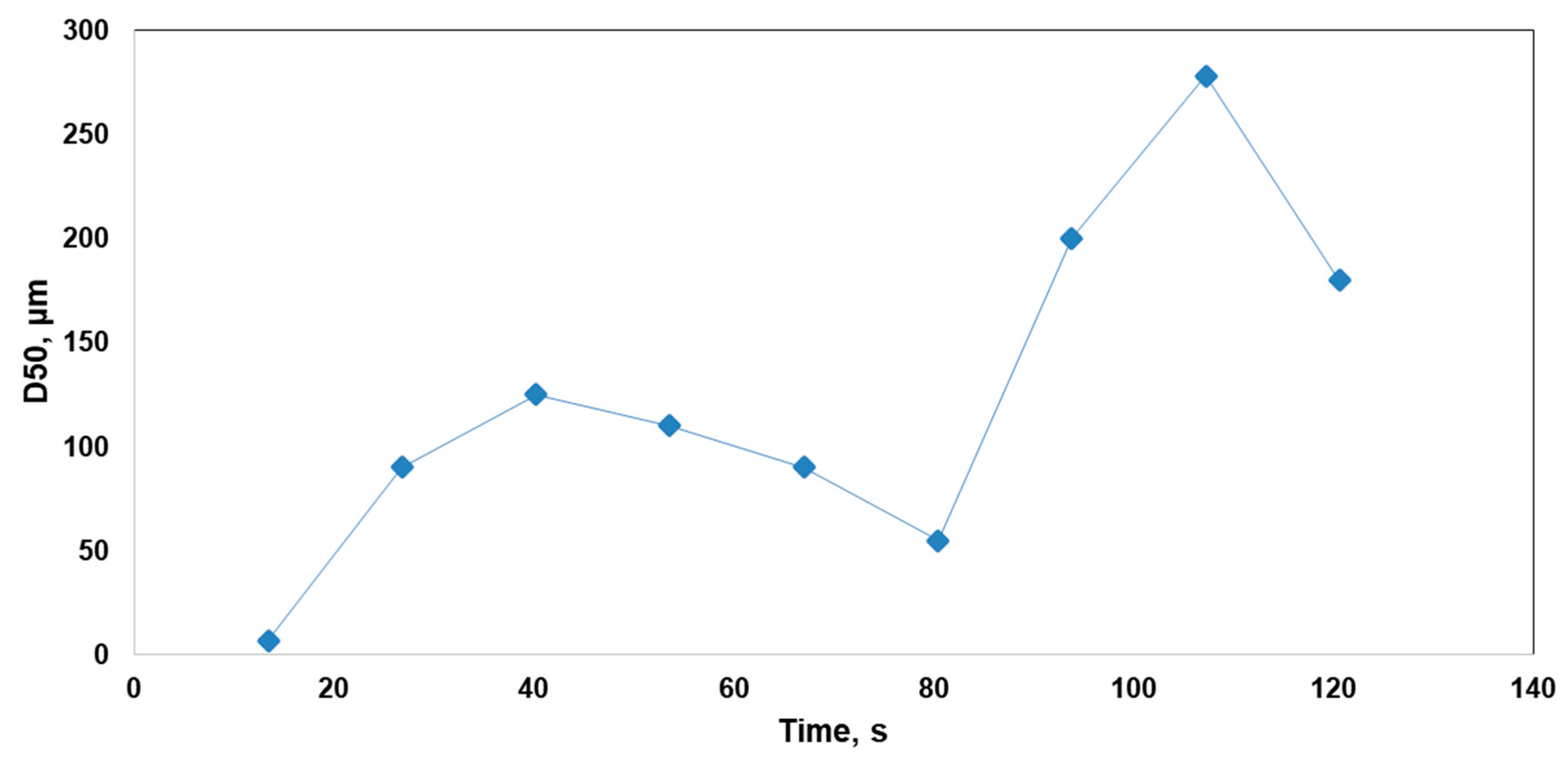
2.7. Validation of Method Using an Unbiased Sample
3. Results and Discussion
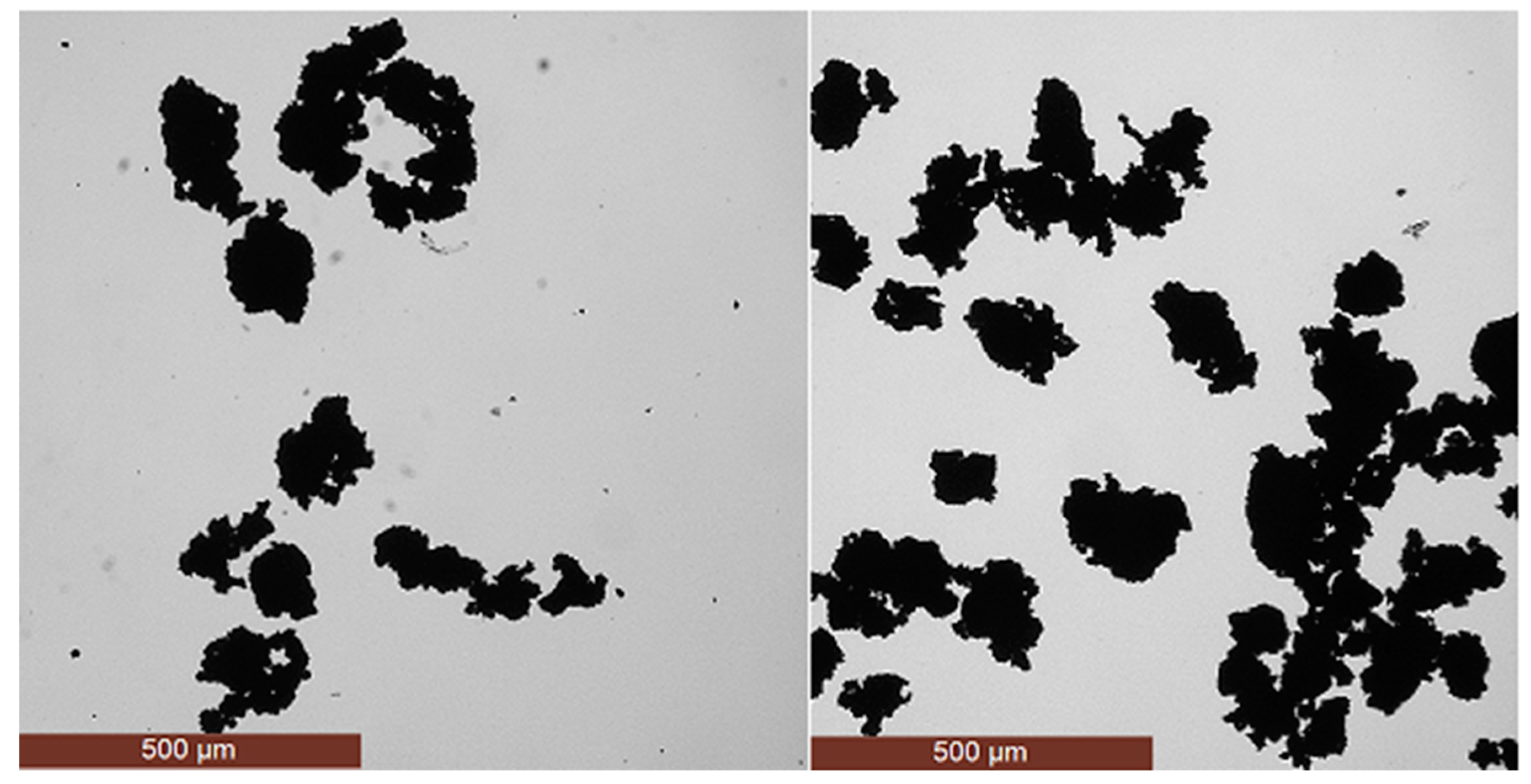
3.1. Generation of Precipitation Aggregates
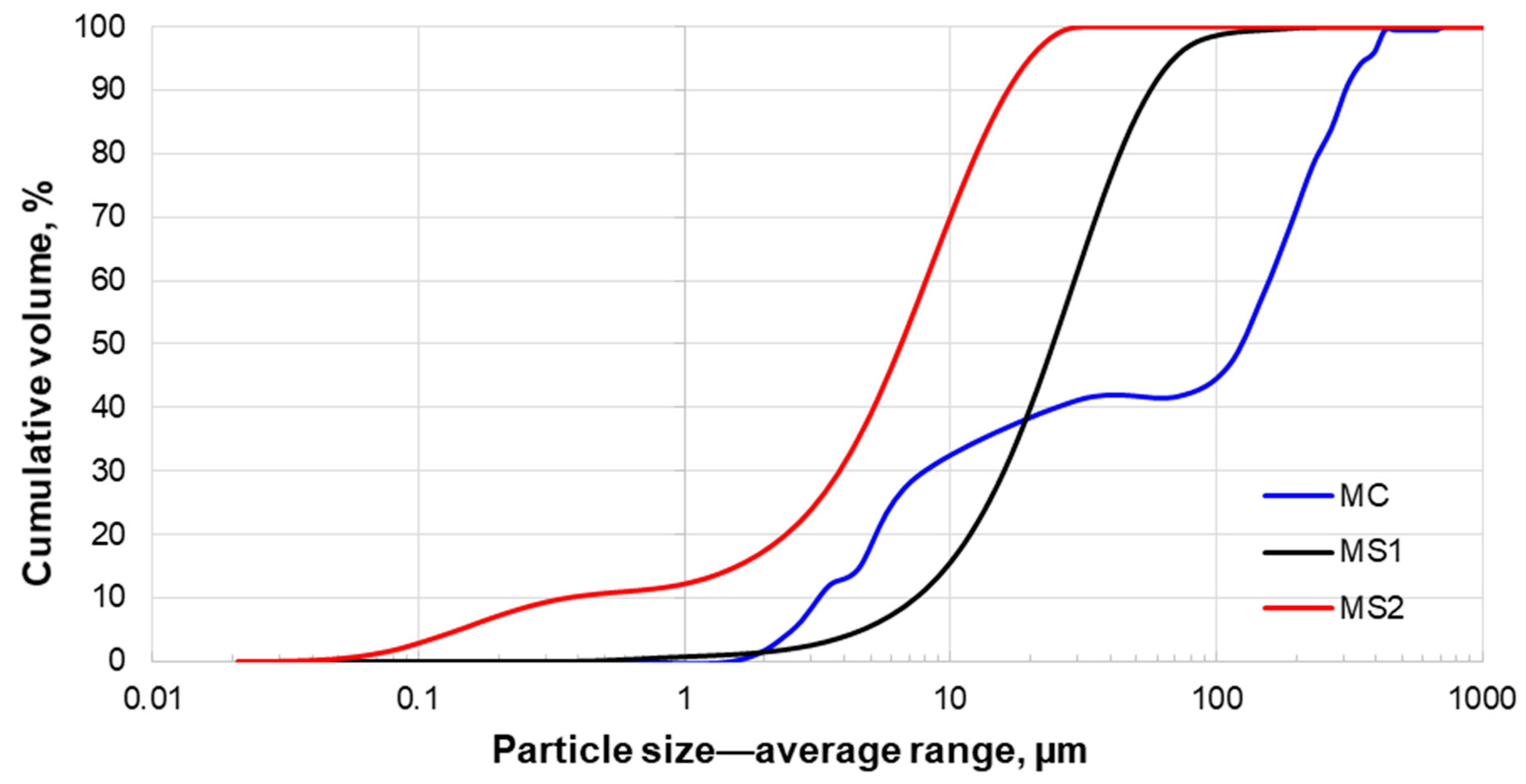
3.2. Size Distribution Analysis
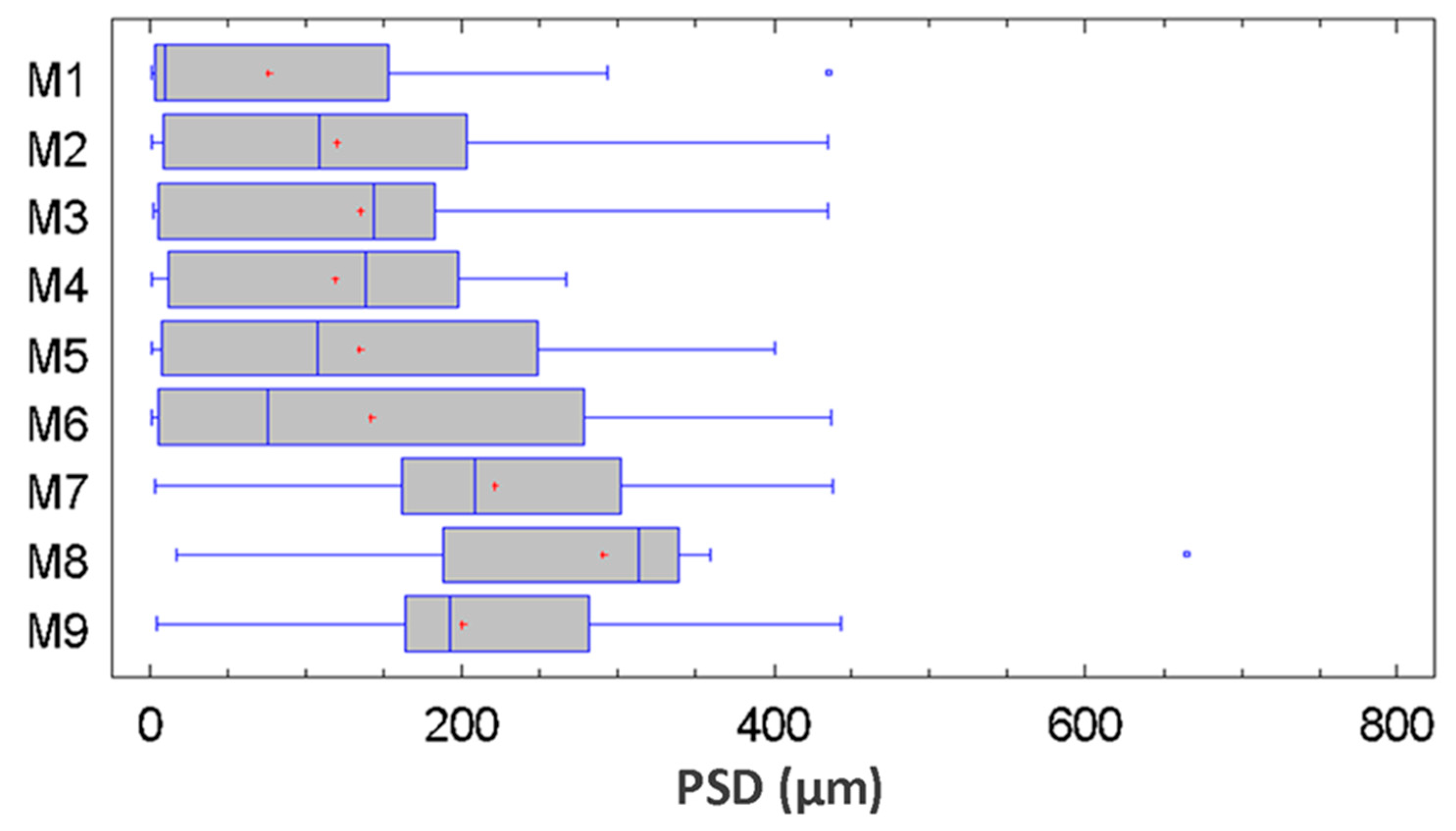
3.3. Statistical Analysis of the Particle Size Distribution for Copper Aggregates
3.4. Validation of Method Using an Unbiased Sample
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Habashi, F. Handbook of Extractive Metallurgy. J. Light Met. 1997, 19, 21–22. [Google Scholar]
- Walton, R. Zinc cementation. In Gold Ore Process, 2nd ed.; Adams, M.D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 553–560. [Google Scholar] [CrossRef]
- Akcil, A.; Koldas, S. Acid Mine Drainage (AMD): Causes, treatment and case studies. J. Clean. Prod. 2006, 14, 1139–1145. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Estay, H. Designing the SART process—A Review. Hydrometallurgy 2018, 176, 147–165. [Google Scholar] [CrossRef]
- Mokone, T.P.; van Hille, R.P.; Lewis, A.E. Effect of solution chemistry on particle characteristics during metal sulfide precipitation. J. Colloid Interface Sci. 2010, 351, 10–18. [Google Scholar] [CrossRef]
- Mokone, T.P.; Lewis, A.E.; van Hille, R.P. Effect of post-precipitate conditions on surface properties of colloidal metal sulphide precipitates. Hydrometallurgy 2012, 119–120, 55–66. [Google Scholar] [CrossRef]
- Martin, A.; Fawcett, S.; Kulczycki, E.; Loomer, D.; Al, T.; Rollo, A. Application of High-Resolution Microscopy Methods to the Analysis of Fine-Grained and Amorphous Treatment Sludges. In Proceedings of the Proceedings Tailings and Mine Waste, Vancouver, BC, Canada, 6–9 November 2011. [Google Scholar]
- Fleming, C.A.; Melashvili, M. The SART process: Killing the sacred cows. In Proceedings of the XXVIII International Mineral Processing Congress (IMPC 2016), Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Gim-Krumm, M.; Quilaqueo, M.; Rojas, V.; Seriche, G.; Ruby-Figueroa, R.; Cortés-Arriagada, D.; Romero, J.; Troncoso, E.; Estay, H. Impact of precipitate characteristics and precipitation conditions on the settling performance of a sulfide precipitation process: An exhaustive characterization of the aggregation behavior. Hydrometallurgy 2019, 189, 105150. [Google Scholar] [CrossRef]
- Greet, C.J.; Smart, R.S.C. The effect of separation by cyclosizing and sedimentation/decantation on mineral surfaces. Miner. Eng. 1997, 10, 995–1011. [Google Scholar] [CrossRef]
- Arasan, S.; Akbulut, S.; Hasiloglu, A.S. Effect of particle size and shape on the grain-size distribution using Image analysis. Int. J. Civ. Struct. Eng. 2011, 1, 968–985. [Google Scholar] [CrossRef]
- Coster, M.; Chermant, J.L. Image analysis and mathematical morphology for civil engineering materials. Cem. Concr. Compos. 2001, 23, 133–151. [Google Scholar] [CrossRef]
- Ghasemy, A.; Rahimi, E.; Malekzadeh, A. Introduction of a new method for determining the particle-size distribution of fine-grained soils. Meas. J. Int. Meas. Confed. 2019, 132, 79–86. [Google Scholar] [CrossRef]
- Ramírez, C.; Troncoso, E.; Muñoz, J.; Aguilera, J.M. Microstructure analysis on pre-treated apple slices and its effect on water release during air drying. J. Food Eng. 2011, 106, 253–261. [Google Scholar] [CrossRef]
- Orrego, M.; Troncoso, E.; Zúñiga, R.N. Aerated whey protein gels as new food matrices: Effect of thermal treatment over microstructure and textural properties. J. Food Eng. 2015, 163, 37–44. [Google Scholar] [CrossRef]
- Graham, D.; Rice, S.P.; Reid, I. A transferable method for the automated grain sizing of rivergravels. Water Resour. Res. 2005, 41, 1–12. [Google Scholar] [CrossRef]
- Alvarez-Iglesias, J.C.; Gomes, O.; Paciornik, S. Automatic recognition of hematite grains under polarized reflected light microscopythrough image analysis. Min. Eng. 2011, 24, 1264–1270. [Google Scholar] [CrossRef]
- Presles, B.; Debayle, J.; Févotte, G.; Pinoli, J.C. Novel image analysis method for in situ monitoring theparticle size distribution of batch crystallization processes. J. Electron. Imaging 2010, 19, 031207. [Google Scholar] [CrossRef]
- Singh, M.R.; Chakraborty, J.; Nere, N.; Tung, H.H.; Bordawekar, S.; Ramkrishna, D. Image-Analysis-Based Method for 3D Crystal Morphology Measurement and Polymorph Identification Using ConfocalMicroscopy. Crist. Growth Des. 2012, 12, 3735–3748. [Google Scholar] [CrossRef]
- Maerz, N.H. Aggregate sizing and shape determination using digital image processing. In Proceedings of the Center for Aggregates Research (ICAR) Sixth Annual Symposium Proceedings, St. Louis, MO, USA, 19–20 April 1998; pp. 195–203. [Google Scholar]
- Ramírez, C.; Aguilera, J.M. Determination of a representative area element (RAE) based on nonparametric statistics in bread. J. Food Eng. 2011, 102, 197–201. [Google Scholar] [CrossRef]
- MacPhail, P.K.; Fleming, C.; Sarbutt, K. Cyanide Recovery by the SART Process for the Lobo-Marte Project. In Proceedings of the Chile, Randol Gold and Silver Forum, Denver, CO, USA, 26–29 April 1998. [Google Scholar]
- Botz, M.; Acar, S. Copper Precipitation and Cyanide Recovery Pilot Testing for the Newmont Yanacocha Project. In Proceedings of the Society for Mining, Metallurgy & Exploration (SME) Annual Meeting, Denver, CO, USA, 25–28 February 2007. [Google Scholar]
- Botz, M.; Kaczmarek, A.; Orser, S. Managing copper in leach solution at the Copler gold mine: Laboratory testing and process design. Min. Metall. Proc. 2011, 28, 133–138. [Google Scholar] [CrossRef]
- Estay, H.; Carvajal, P.; Hedjazi, F.; Van Zeller, T. The SART process experience in the Gedabek plant. In Proceedings of the HydroProcess, 4th International Workshop on Process Hydrometallurgy, Santiago, Chile, 12–13 July 2012. [Google Scholar]
- Marsden, J.O.; House, C.I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy and Exploration SME: Englewood, CO, USA, 2006. [Google Scholar]
- Puigdomenech, I. Hydra-Medusa Software (Hydrochemical Equilibrium Constant, Database and Make Equilibrium Diagrams Using Sophisticated Algorithms), 1st ed.; Royal Institute of Technology: Stockholm, Sweden, 2004. [Google Scholar]
- Malvern Instruments Ltd. Mastersizer 2000 User Manual UK. 2007. Available online: https://www.labmakelaar.com/fjc_documents/mastersizer-2000-2000e-manual-eng1.pdf (accessed on 23 November 2018).
- Wrighton-Araneda, K.; Ruby-Figueroa, R.; Estay, H.; Cortés-Arriagada, D. Interaction of H2O with (CuS)n, (Cu2S)n, and (ZnS)n small clusters (n = 1–4, 6): Relation to the aggregation characteristics of metal sulfides at aqueous solutions. J. Mol. Model. 2019, 25, 291. [Google Scholar] [CrossRef]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for Non-statiticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, D.C. Design and analysis of experiments-second edition. Qual. Reliab. Eng. Int. 1987, 3, 212. [Google Scholar] [CrossRef]
- Peng, X.; Xia, Z.; Kong, L.; Hu, X.; Wang, X. UV light irradiation improves the aggregation and settling performance of metal sulfide particles in strongly acidic wastewater. Water Res. 2019, 163, 114860. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Oraby, E.A.; Eksteen, J.J. Sulfide precipitation of copper from alkaline glycine-cyanide solutions: Precipitate characterization. Min. Eng. 2020, 145, 106102. [Google Scholar] [CrossRef]
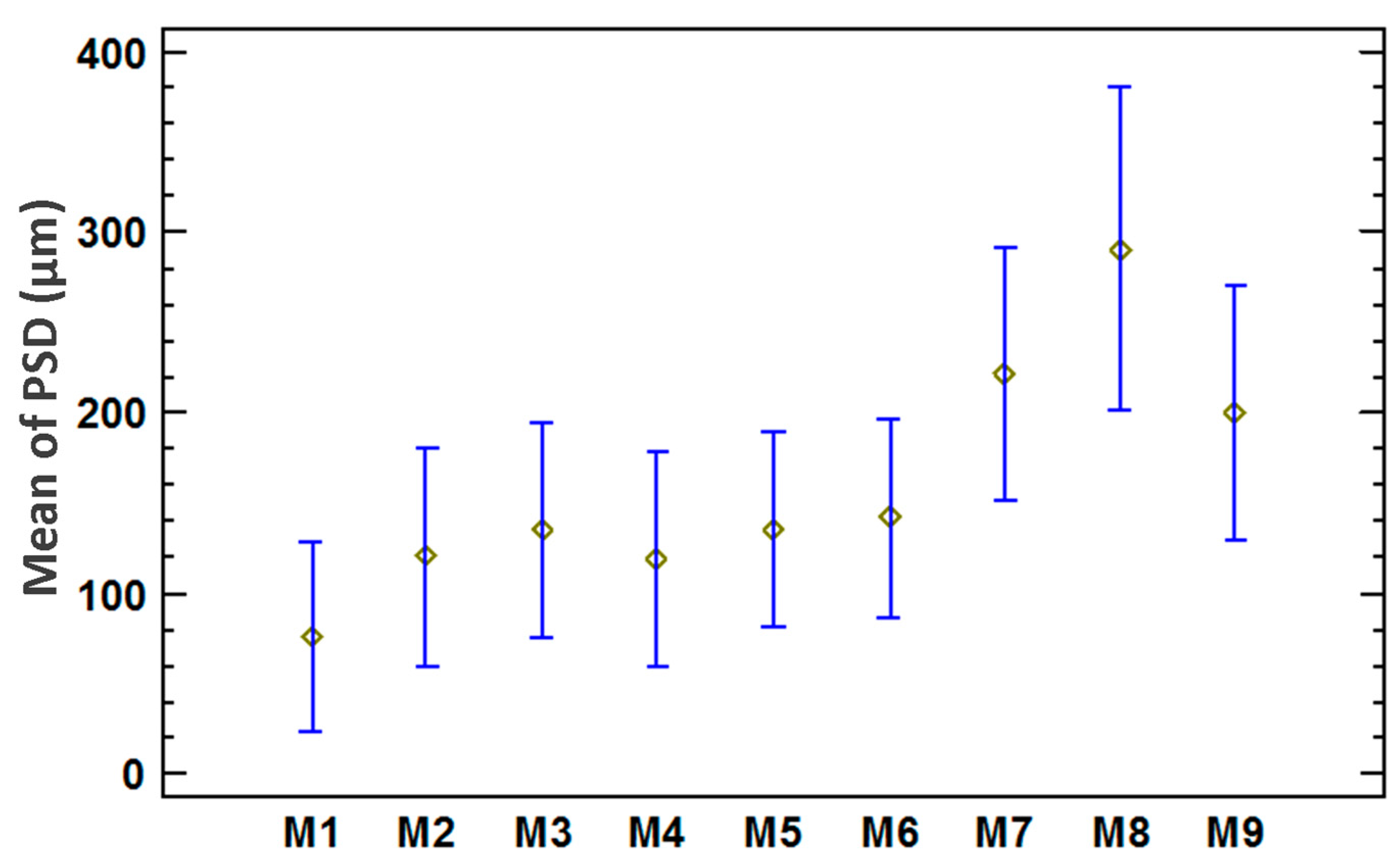

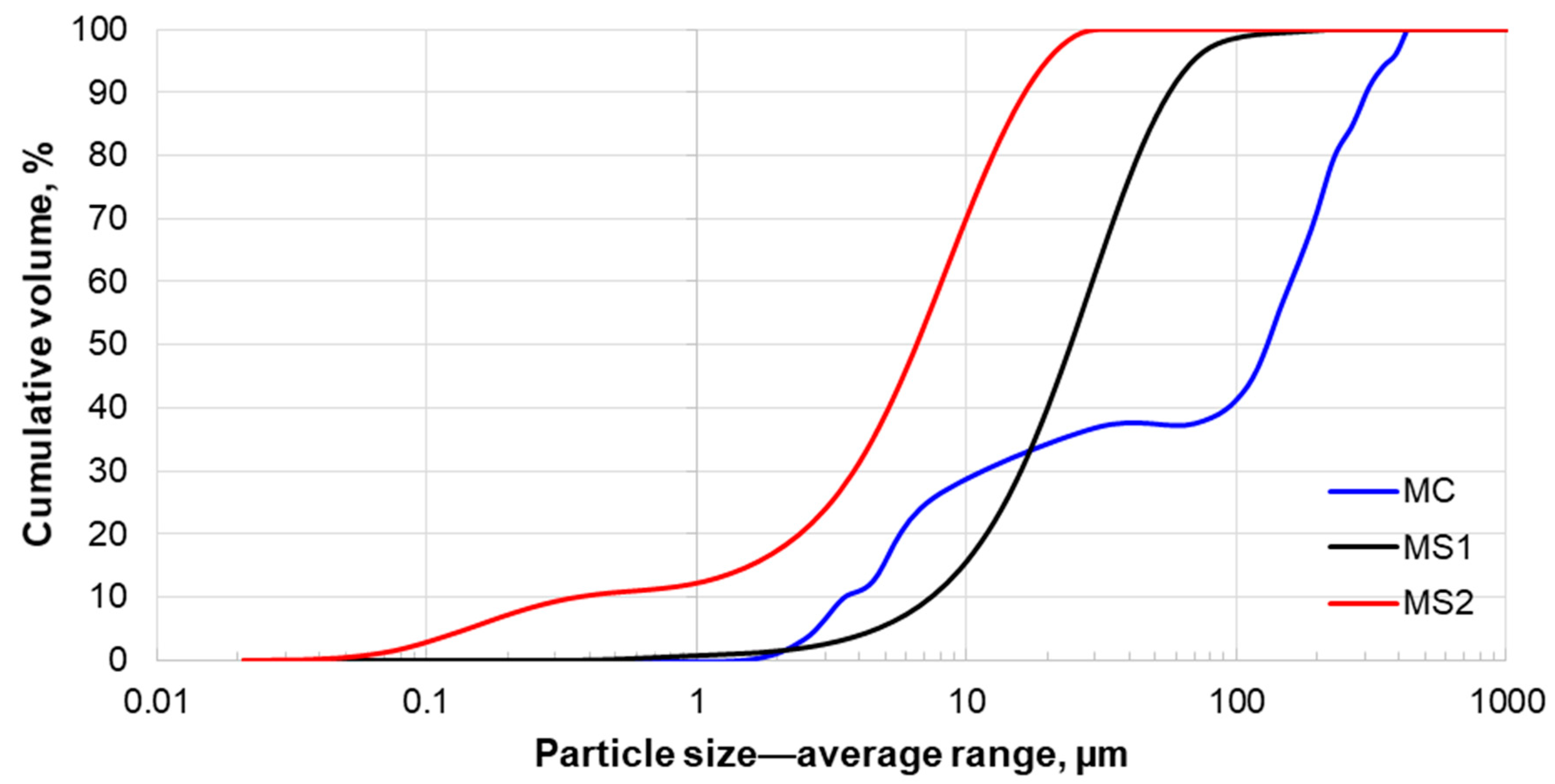
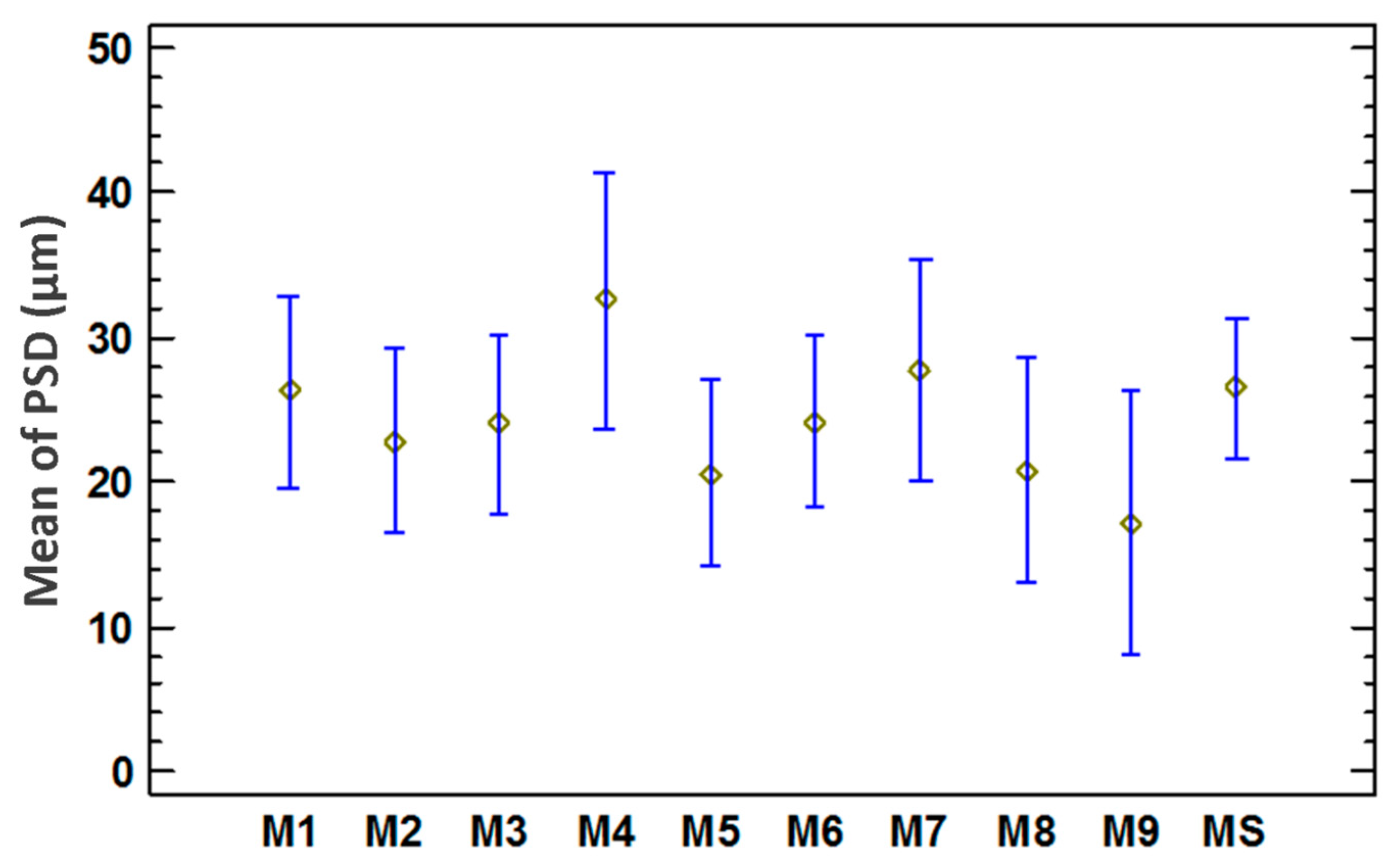
| Description | Value |
|---|---|
| Copper content, % w/w | 67.69 |
| Copper sulfide conversion, % | 99.97 |
| Total suspended solids, mg/L | 2658 |
| Particle Size, µm | MC | MS1 | MS2 |
|---|---|---|---|
| P50 | 119.0 | 25.7 | 6.9 |
| P90 | 307.0 | 59.6 | 17.4 |
| Statistics | MC | MS1 | MS2 | |||
|---|---|---|---|---|---|---|
| F-Ratio | p-Value | F-Ratio | p-Value | F-Ratio | p-Value | |
| Shapiro-Wilk | 0.87288 | <0.05 | 0.60841 | <0.05 | 0.77999 | <0.05 |
| Modified Kolmogorov-Smirnov | 2.73516 | <0.05 | 5.82743 | <0.05 | 5.03322 | <0.05 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quilaqueo, M.; Gim-Krumm, M.; Ruby-Figueroa, R.; Troncoso, E.; Estay, H. Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis. Minerals 2019, 9, 724. https://doi.org/10.3390/min9120724
Quilaqueo M, Gim-Krumm M, Ruby-Figueroa R, Troncoso E, Estay H. Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis. Minerals. 2019; 9(12):724. https://doi.org/10.3390/min9120724
Chicago/Turabian StyleQuilaqueo, Michelle, Minghai Gim-Krumm, René Ruby-Figueroa, Elizabeth Troncoso, and Humberto Estay. 2019. "Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis" Minerals 9, no. 12: 724. https://doi.org/10.3390/min9120724
APA StyleQuilaqueo, M., Gim-Krumm, M., Ruby-Figueroa, R., Troncoso, E., & Estay, H. (2019). Determination of Size Distribution of Precipitation Aggregates Using Non-Invasive Microscopy and Semiautomated Image Processing and Analysis. Minerals, 9(12), 724. https://doi.org/10.3390/min9120724







