Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range
Abstract
1. Introduction
2. Methods
3. EoS of Pb, NaCl and LiF
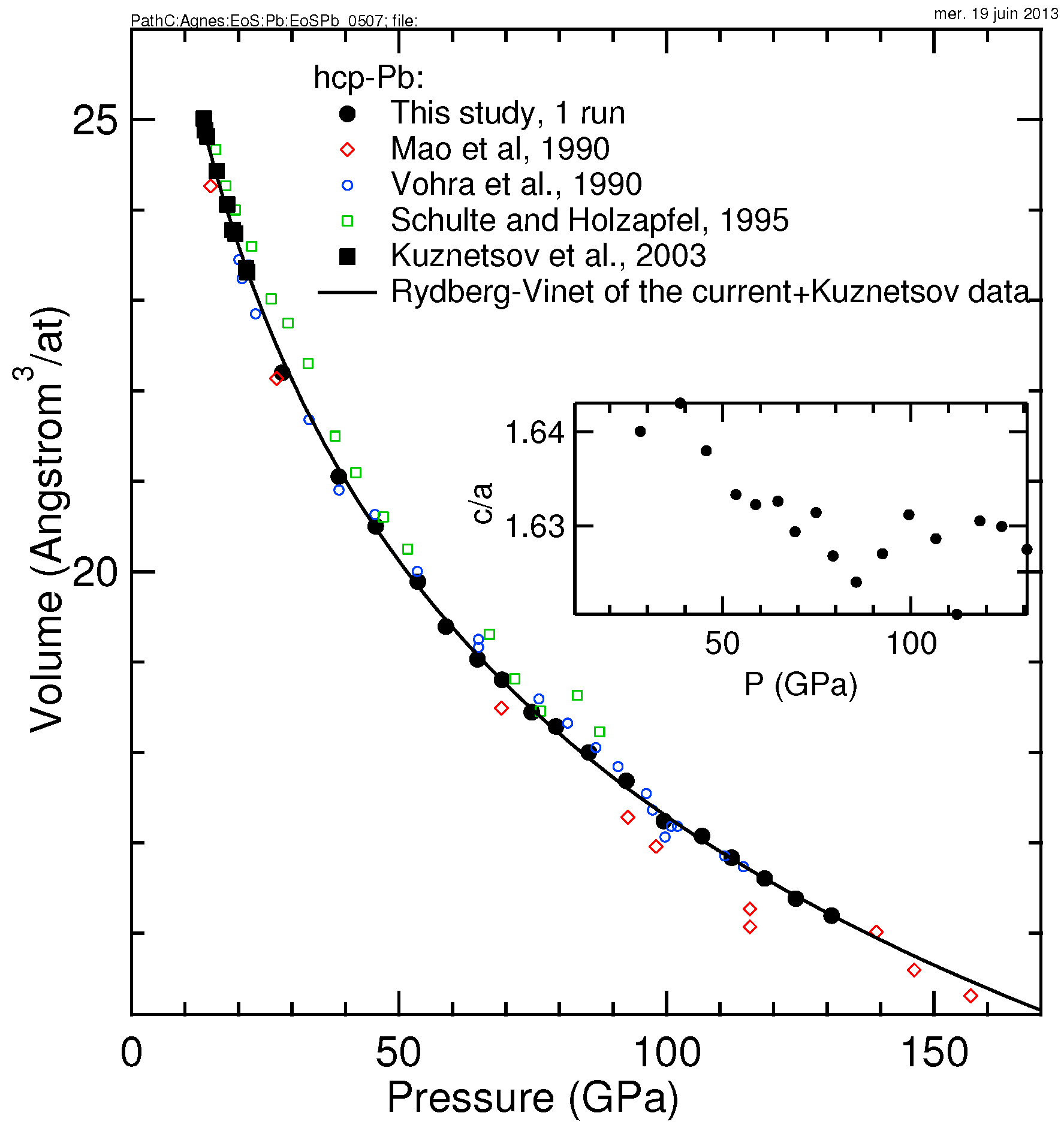
3.1. Pb
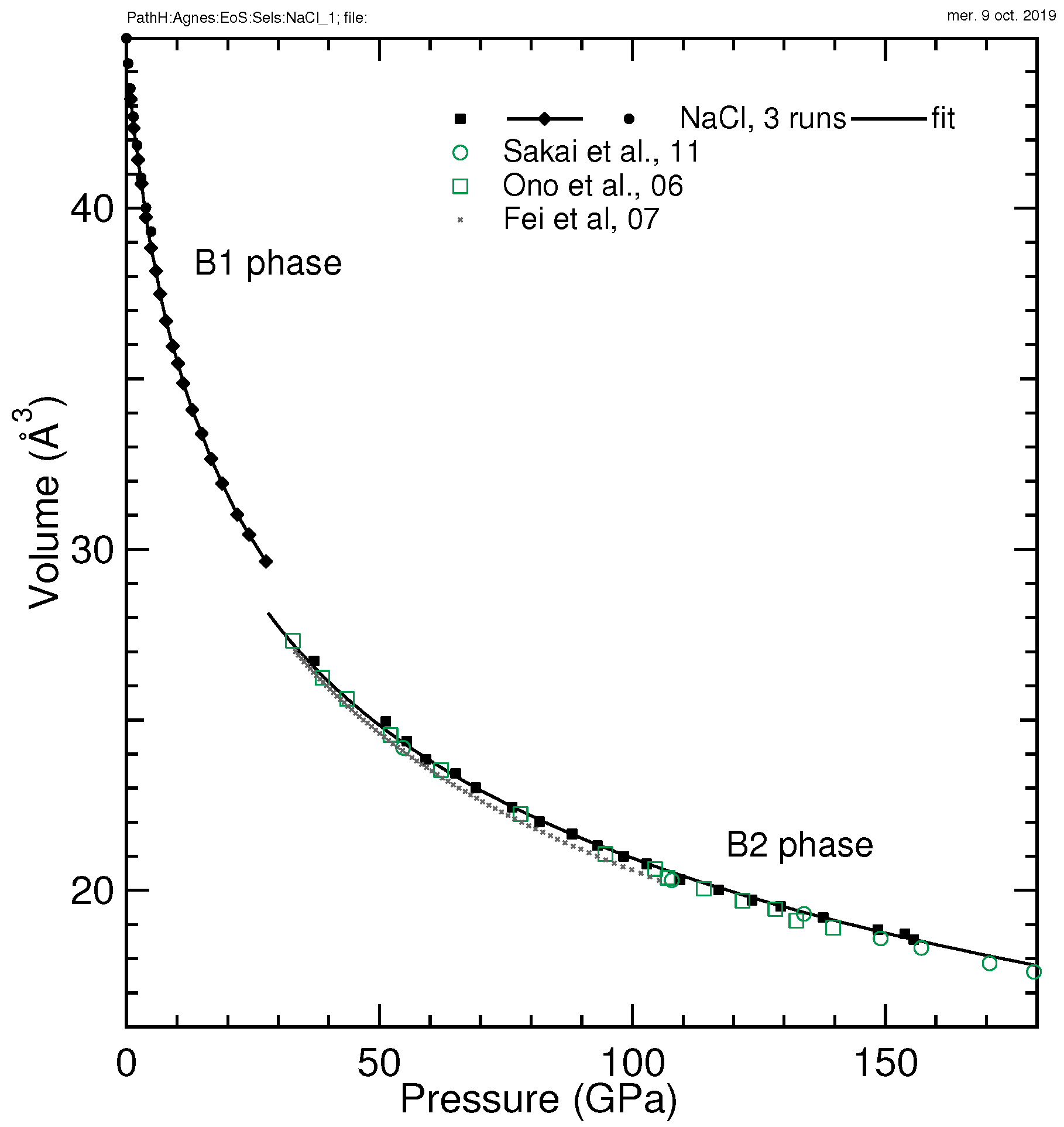
3.2. NaCl
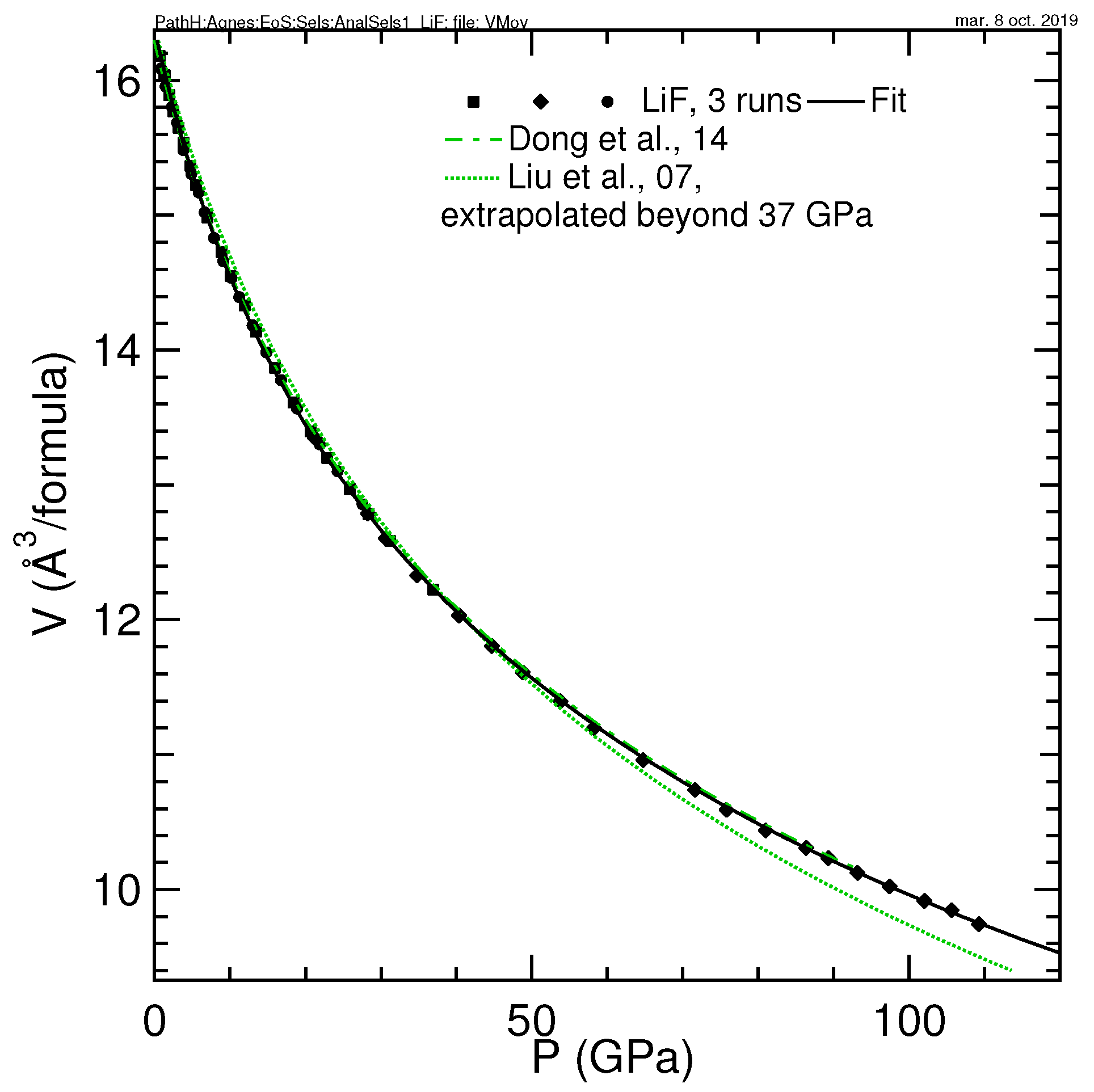
3.3. LiF
4. Equation of State Parameters
Funding
Acknowledgments
Conflicts of Interest
References
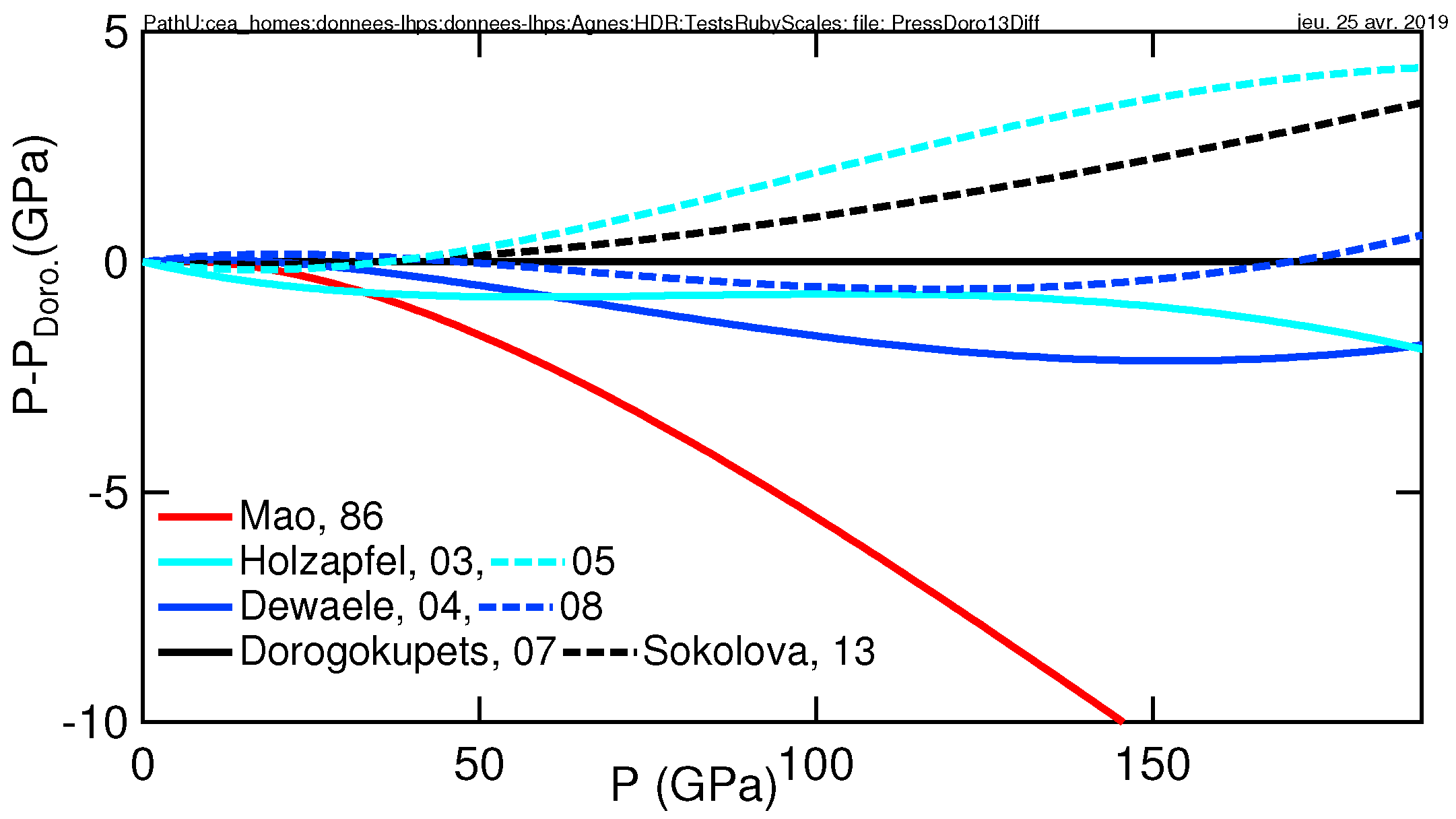
- Holzapfel, W. Refinement of the ruby luminescence pressure scale. J. Appl. Phys. 2003, 93, 1813–1818. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Mezouar, M. Equations of state of six metals above 94 GPa. Phys. Rev. B 2004, 70, 094112–094119. [Google Scholar] [CrossRef]
- Holzapfel, W.B. Progress in the realization of a practical pressure scale for the range 1–300 GPa. High Press. Res. 2005, 25, 187–196. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Oganov, A.R. Ruby, metals, and MgO as alternative pressure scales: A semiempirical description of shock-wave, ultrasonic, X-ray, and thermochemical data at high temperatures and pressures. Phys. Rev. B 2007, 75, 024115. [Google Scholar] [CrossRef]
- Dewaele, A.; Torrent, M.; Loubeyre, P.; Mezouar, M. Compression curves of transition metals in the mbar range: Experiments and projector augmented-wave calculations. Phys. Rev. B 2008, 78, 104102–104114. [Google Scholar] [CrossRef]
- Mao, H.-K.; Xu, J.; Bell, P. Calibration of the ruby pressure gauge to 800 kbar under quasi-hydrostatic conditions. J. Geophys. Res. 1986, 91, 4673–4676. [Google Scholar] [CrossRef]
- Sokolova, T.S.; Dorogokupets, P.I.; Litasov, K.D. Self-consistent pressure scales based on the equations of state for ruby, diamond, MgO, B2-NaCl, as well as au, pt, and other metals to 4 mbar and 3000 k. Russ. Geol. Geophys. 2013, 54, 181–199. [Google Scholar] [CrossRef]
- Vinet, P.; Ferrante, J.; Smith, J.R.; Rose, J.H. A universal equation of state for solids. J. Phys. Condens. Matter 1986, 19, L467. [Google Scholar] [CrossRef]
- Takemura, K.; Dewaele, A. Isothermal equation of state for gold with a He-pressure medium. Phys. Rev. B 2008, 78, 104119–104131. [Google Scholar] [CrossRef]
- Lazicki, A.; Dewaele, A.; Loubeyre, P.; Mezouar, M. High pressure-high temperature phase diagram and the equation of state of beryllium. Phys. Rev. B 2012, 86, 174118–174128. [Google Scholar] [CrossRef]
- Anzellini, S.; Dewaele, A.; Occelli, F.; Loubeyre, P.; Mezouar, M. Equation of state of rhenium and application for ultra high pressure calibration. J. Appl. Phys. 2014, 115, 043511. [Google Scholar] [CrossRef]
- Occelli, F.; Loubeyre, P.; Letoullec, R. Properties of diamond under hydrostatic pressures up to 140 GPa. Nat. Mater. 2003, 2, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Dewaele, A.; Datchi, F.; Loubeyre, P.; Mezouar, M. High pressure high temperature equations of state of neon and diamond. Phys. Rev. B 2008, 77, 094106–094114. [Google Scholar] [CrossRef]
- Dewaele, A.; Belonoshko, A.B.; Garbarino, G.; Occelli, F.; Bouvier, P.; Hanfland, M.; Mezouar, M. High pressure-high temperature equation of state of KCl and KBr. Phys. Rev. B 2012, 85, 214105–214112. [Google Scholar] [CrossRef]
- Klotz, S.; Chervin, J.-C.; Munsch, P.; Le Marchand, G. Hydrostatic limits of 11 pressure transmitting media. J. Phys. D Appl. Phys. 2009, 42, 075413. [Google Scholar] [CrossRef]
- Holzapfel, W. Equations of State for Ideal and Real Solids Under Strong Compression. Europhys. Lett. 1991, 16, 67. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P.; Occelli, F.; Marie, O.; Mezouar, M. Toroidal diamond anvil cell for detailed measurements under extreme static pressures. Nat. Commun. 2018, 9, 2913. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, A.; Dmitriev, V.; Dubrovinsky, L.; Prakapenka, V.; Weber, H.P. Fcc-hcp phase boundary in lead. Solid State Commun. 2002, 122, 125–127. [Google Scholar] [CrossRef]
- Mao, H.K.; Wu, Y.; Shu, J.F.; Hu, J.Z.; Hemley, R.J.; Cox, D.E. High pressure phase transition and equation of state of lead to 238 GPa. Solid State Commun. 1990, 74, 1027–1029. [Google Scholar] [CrossRef]
- Dewaele, A.; Mezouar, M.; Guignot, N.; Loubeyre, P. Melting of lead under high pressure using second-scale times resolved X-ray diffraction. Phys. Rev. B 2007, 76, 144106. [Google Scholar] [CrossRef]
- Vohra, Y.K.; Ruoff, A.L. Static compression of metals mo, pb and pt to 272 GPa: Comparison with shock data. Phys. Rev. B 1990, 42, 8651–8653. [Google Scholar] [CrossRef] [PubMed]
- Schulte, O.; Holzapfel, W.B. Equation-of-state behavior for different phases of lead under strong compression. Phys. Rev. B 1995, 52, 12636–12639. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M. The NaCl pressure standard. J. Appl. Phys. 1999, 86, 5801–5809. [Google Scholar] [CrossRef]
- Dewaele, A.; Loubeyre, P. Pressurizing conditions in helium-pressure-transmitting medium. High Press. Res. 2007, 27, 419–429. [Google Scholar] [CrossRef]
- Boehler, R.; Bargen, N.V.; Chopelas, A. Melting, thermal expansion, and phase transitions of iron at high pressures. J. Geophys. Res. 1990, 95, 21731–21736. [Google Scholar] [CrossRef]
- Ono, S.; Kikegawa, T.; Ohishi, Y. Structural property of CsCl-type sodium chloride under pressure. Solid State Commun. 2006, 137, 517–521. [Google Scholar] [CrossRef]
- Sakai, T.; Ohtani, E.; Hirao, N.; Ohishi, Y. Equation of state of the NaCl-b2 phase up to 304 GPa. J. Appl. Phys. 2011, 109, 084912. [Google Scholar] [CrossRef]
- Fei, Y.; Ricolleau, A.; Frank, M.; Mibe, K.; Shen, G.; Prakapenka, V. Toward an internally consistent pressure scale. Proc. Natl. Acad. Sci. USA 2007, 104, 9182–9186. [Google Scholar] [CrossRef]
- Dong, H.; Dorfman, S.M.; Holl, C.M.; Meng, Y.; Prakapenka, V.B.; He, D.; Duffy, T.S. Compression of lithium fluoride to 92 GPa. High Press. Res. 2014, 34, 39–48. [Google Scholar] [CrossRef]
- Liu, J.; Dubrovinsky, L.; Ballaran, T.B.; Crichton, W. Equation of state and thermal expansivity of LiF and NaF. High Press. Res. 2007, 27, 483–489. [Google Scholar] [CrossRef]
- Simmons, G.; Wang, H. Single Crystal Elastic Constants and Calculated Aggregate Properties: A Handbook; The MIT Press: Cambridge, UK, 1971. [Google Scholar]
- Dewaele, A.; Bouchet, J.; Occelli, F.; Hanfland, M.; Garbarino, G. Refinement of the equation of state of α-uranium. Phys. Rev. B 2013, 88, 134202. [Google Scholar] [CrossRef]
| Element | [6] | [4] | P | PTM | P | Ref. |
|---|---|---|---|---|---|---|
| or Compound | , , | , , | Domain | Gauge | ||
| Au | 16.983(20), 166.4(2.0), 5.47(6) | 16.986, 163.4, 6.04 | 0–131 | He | ruby | [9] |
| Pt | 15.099(25), 273.4(2.5), 4.83(8) | 15.098, 270.8, 5.50 | 0–95 | He | ruby | [2] |
| Cu | 11.810(15), 135.3(1.5), 4.91(6) | 11.81, 133.1, 5.38 | 0–155 | He | ruby | [2] |
| Ta | 18.020(18), 197.9(3.7), 3.17(10) | 18.019, 196.1, 3.64 | 0–90 | He | ruby | [2] |
| Al | 16.573(19), 76.32(1.5), 4.16(6) | 16.584, 74.2, 4.52 | 0–155 | He | ruby | [2] |
| W | 15.862(16), 298.3(4.1), 3.82(11) | 15.858, 298.6, 4.37 | 0–155 | He | ruby | [2] |
| Co | 11.077(12), 197.0(3.2), 3.85(20) | 11.077, 194.85, 4.36 | 0–66 | He | ruby | [5] |
| Ag | 17.070(16), 100.2(1.6), 5.70(9) | 17.088, 96.6, 6.22 | 0–124 | He | ruby | [5] |
| Mo | 15.569(21), 270.3(3.9), 3.34(12) | 15.567, 269.3, 3.87 | 0–124 | He | ruby | [5] |
| Ni | 10.954(18), 177.5(2.4), 4.83(9) | 10.952, 176, 5.322 | 0–157 | He | ruby | [5] |
| Zn | 15.147(19), 64.3(1.2), 5.30(10) | 15.155, 62.2, 5.705 | 0–157 | He | ruby | [5] |
| Be | 8.133(5), 115.2(1.1), 2.94(5) | 8.134, 113.4, 3.29 | 0–95 | He | ruby | [10] |
| Pb-hcp | 28.063(55), 71.8(2.1), 4.40(8) | 28.058, 70.0, 4.77 | 13–131 | He | ruby | this work |
| Re | 14.737(20), 350.5(8.0), 3.98(17) | 14.734, 350.5, 4.62 | 0–144 | He | W | [11] |
| -Fe | 11.209(50), 164.5(7.9), 4.96(16) | 11.177, 168.4, 5.33 | 17–204 | He, Ne | W | [5] |
| C | 5.673(8), 446.8(5.0), 3.01(60) | 5.672, 448.9, 3.66 | 0–151 | He, Ne | ruby | [12,13] |
| KCl-B2 | 55.98(76), 15.4(2.5), 5.75(15) | 56.86, 13.1, 6.21 | 0–165 | He | ruby | [14] |
| KBr-B2 | 64.37(81), 14.5(2.5), 5.58(20) | 65.29, 12.44, 6.01 | 0–165 | He | ruby | [14] |
| NaCl-B1 | 44.90(12), 24.0(8), 5.09(6) | 44.93, 23.4, 5.29 | 0–35 | He | ruby | this work |
| NaCl-B2 | 42.3, 24.0(1.2), 5.37(20) | 42.3, 22.664, 5.735 | 37–155 | He | ruby | this work |
| LiF | 16.371(30), 64.6(1.4), 4.62(60) | 16.391, 62.3, 5.01 | 0–109 | He | ruby | this work |
| P (GPa) | V (Å/at) |
|---|---|
| 28.16 | 22.2010 |
| 38.74 | 21.0521 |
| 45.62 | 20.5023 |
| 53.51 | 19.8897 |
| 58.77 | 19.3907 |
| 64.69 | 19.0305 |
| 69.24 | 18.8032 |
| 74.79 | 18.4456 |
| 79.34 | 18.2875 |
| 85.48 | 17.9984 |
| 92.47 | 17.6854 |
| 99.5 | 17.2410 |
| 106.6 | 17.0756 |
| 112.2 | 16.8330 |
| 118.3 | 16.6038 |
| 124.2 | 16.3813 |
| 130.8 | 16.1940 |
| P (GPa) | V (Å/fu) (B1) | P (GPa) | V (Å/fu) (B1) | P (GPa) | V (Å/fu) (B2) |
|---|---|---|---|---|---|
| 0.868 | 43.2022 | 0.319 | 44.2471 | 37.06 | 26.7309 |
| 1.46 | 42.3505 | 0.768 | 43.5143 | 51.22 | 24.9611 |
| 2.29 | 41.4177 | 1.37 | 42.6836 | 55.45 | 24.3764 |
| 2.96 | 40.7222 | 2.11 | 41.8422 | 59.18 | 23.8456 |
| 3.85 | 39.7304 | 2.91 | 40.8914 | 65.03 | 23.4403 |
| 4.88 | 38.8363 | 0.0171 | 44.9814 | 69.06 | 23.0081 |
| 5.87 | 38.1616 | 76.24 | 22.4425 | ||
| 6.68 | 37.4870 | 81.68 | 22.0179 | ||
| 7.87 | 36.6937 | 88.07 | 21.6523 | ||
| 9.12 | 35.9584 | 93.12 | 21.3184 | ||
| 10.2 | 35.4483 | 98.24 | 21.0017 | ||
| 11.3 | 34.8669 | 102.8 | 20.7833 | ||
| 13.0 | 34.0953 | 109.5 | 20.3174 | ||
| 14.8 | 33.3907 | 117.1 | 20.0107 | ||
| 16.7 | 32.6566 | 123.7 | 19.7202 | ||
| 18.9 | 31.9352 | 129.3 | 19.5347 | ||
| 21.9 | 31.0243 | 137.7 | 19.2101 | ||
| 24.3 | 30.4328 | 148.5 | 18.8551 | ||
| 27.6 | 29.6501 | 153.9 | 18.7279 | ||
| 155.6 | 18.5570 |
| P (GPa) | V (Å/fu) | P (GPa) | V (Å/fu) | P (GPa) | V (Å/fu) |
|---|---|---|---|---|---|
| 0.722 | 16.181 | 21.3 | 13.3466 | 0.868 | 16.0879 |
| 1.32 | 16.040 | 28.3 | 12.7874 | 1.46 | 15.9574 |
| 1.95 | 15.8910 | 30.6 | 12.6060 | 2.29 | 15.8032 |
| 2.50 | 15.7684 | 34.8 | 12.3274 | 2.96 | 15.6871 |
| 3.14 | 15.6498 | 40.4 | 12.0328 | 3.85 | 15.4849 |
| 3.81 | 15.5367 | 44.7 | 11.8066 | 4.88 | 15.3078 |
| 4.75 | 15.3677 | 48.8 | 11.6103 | 5.87 | 15.1685 |
| 5.54 | 15.2234 | 53.8 | 11.3973 | 6.68 | 15.0231 |
| 7.03 | 14.9857 | 58.2 | 11.1980 | 7.87 | 14.8317 |
| 8.85 | 14.7288 | 64.8 | 10.9605 | 9.12 | 14.6591 |
| 10.1 | 14.5479 | 71.7 | 10.7381 | 10.2 | 14.5370 |
| 11.9 | 14.3310 | 75.8 | 10.5914 | 11.3 | 14.3930 |
| 13.5 | 14.1368 | 81.0 | 10.4369 | 13.0 | 14.1842 |
| 15.9 | 13.8710 | 86.4 | 10.3086 | 14.8 | 13.9848 |
| 18.4 | 13.6116 | 89.3 | 10.2328 | 16.7 | 13.7792 |
| 20.7 | 13.3988 | 93.2 | 10.1232 | 18.9 | 13.5672 |
| 22.9 | 13.2003 | 97.4 | 10.0220 | 21.9 | 13.2994 |
| 25.9 | 12.9683 | 102.1 | 9.91426 | 24.3 | 13.1015 |
| 28.4 | 12.7853 | 105.7 | 9.84496 | 27.6 | 12.8561 |
| 31.2 | 12.5880 | 109.3 | 9.74253 | ||
| 37 | 12.2217 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewaele, A. Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range. Minerals 2019, 9, 684. https://doi.org/10.3390/min9110684
Dewaele A. Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range. Minerals. 2019; 9(11):684. https://doi.org/10.3390/min9110684
Chicago/Turabian StyleDewaele, Agnès. 2019. "Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range" Minerals 9, no. 11: 684. https://doi.org/10.3390/min9110684
APA StyleDewaele, A. (2019). Equations of State of Simple Solids (Including Pb, NaCl and LiF) Compressed in Helium or Neon in the Mbar Range. Minerals, 9(11), 684. https://doi.org/10.3390/min9110684