Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas Smyrnensis WMS-3
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strain and Culture Medium
2.2. Identification of H. smyrnensis WMS-3 Bacteria
2.3. Characterization of H. smyrnensis WMS-3 Bacteria
2.3.1. Cell Morphology, Gram Staining, and Ammonia Test
2.3.2. Bacterial Growth Curve and pH Changes
2.3.3. NH4+ Concentrations and pH Values Based on NH4+ Concentrations
2.3.4. CA Activity, CO32− and HCO3− Concentrations, and pH Values Based on CO32− and HCO3− Concentrations
2.4. Biomineralization Experiments
2.5. Concentrations of Ca2+ and Mg2+ Ions
2.6. Characterization of Precipitates
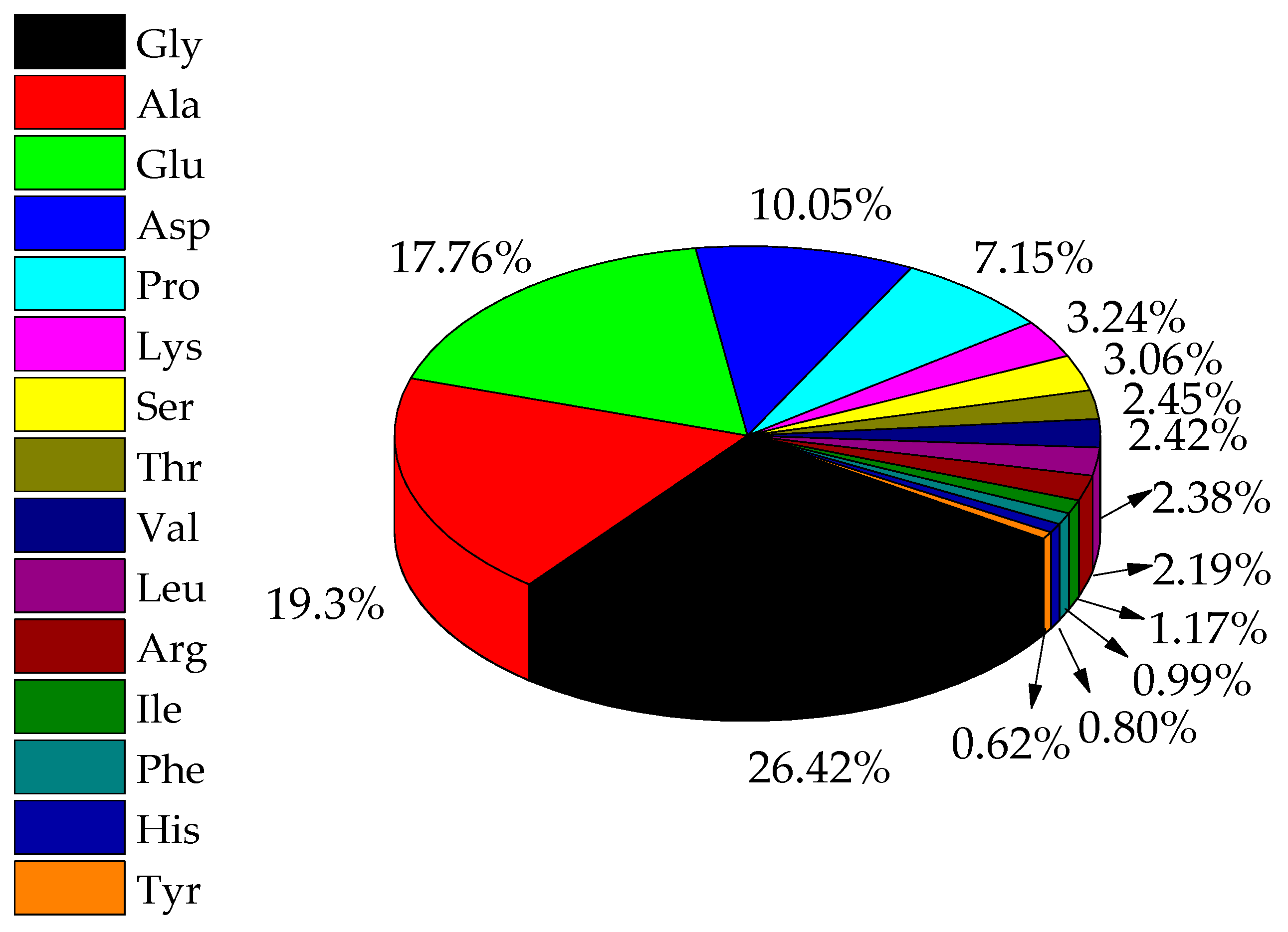
2.7. Amino Acid Composition of EPS
2.8. Analyses of Ultrathin Slices of H. smyrnensis WMS-3 Bacteria
2.9. Fluorescence Intensity of Intracellular Ca2+ Ions
3. Results and Discussions
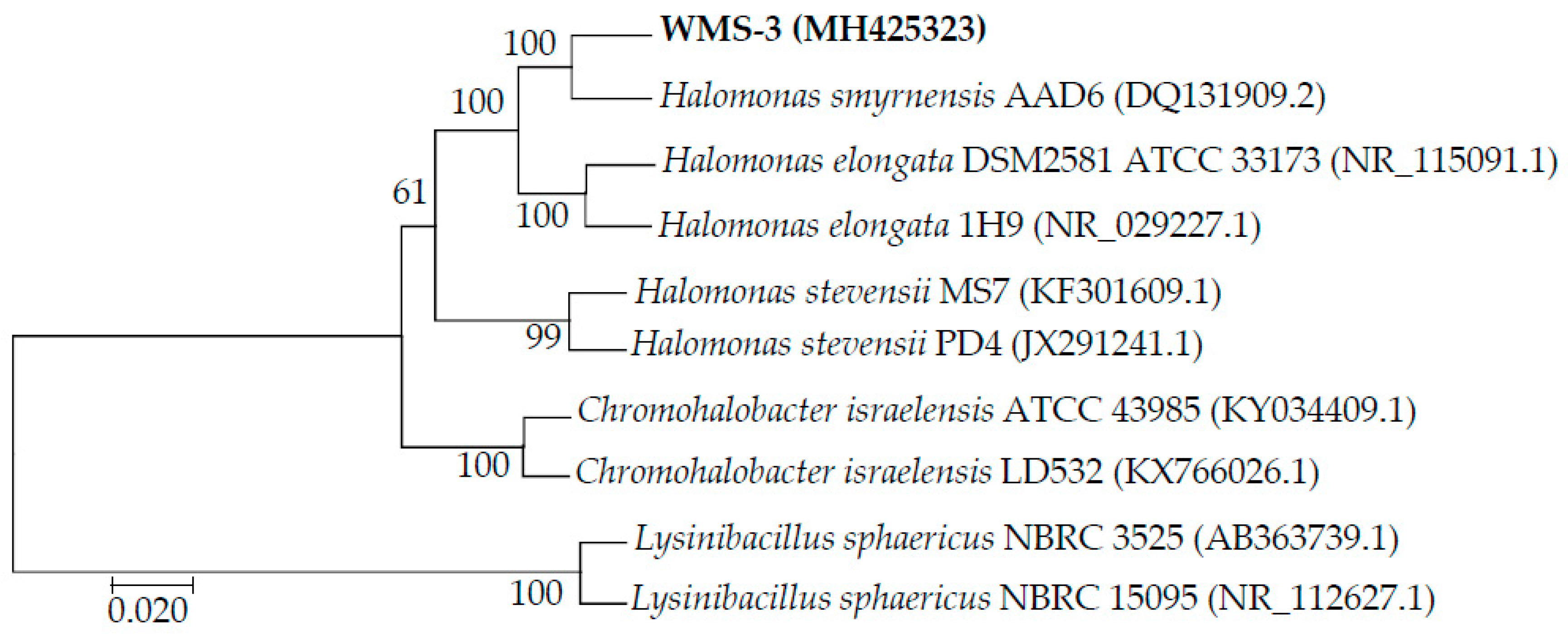
3.1. 16S rDNA Identification of WMS-3 Bacteria
3.2. Characterization of H. smyrnensis WMS-3 Bacteria
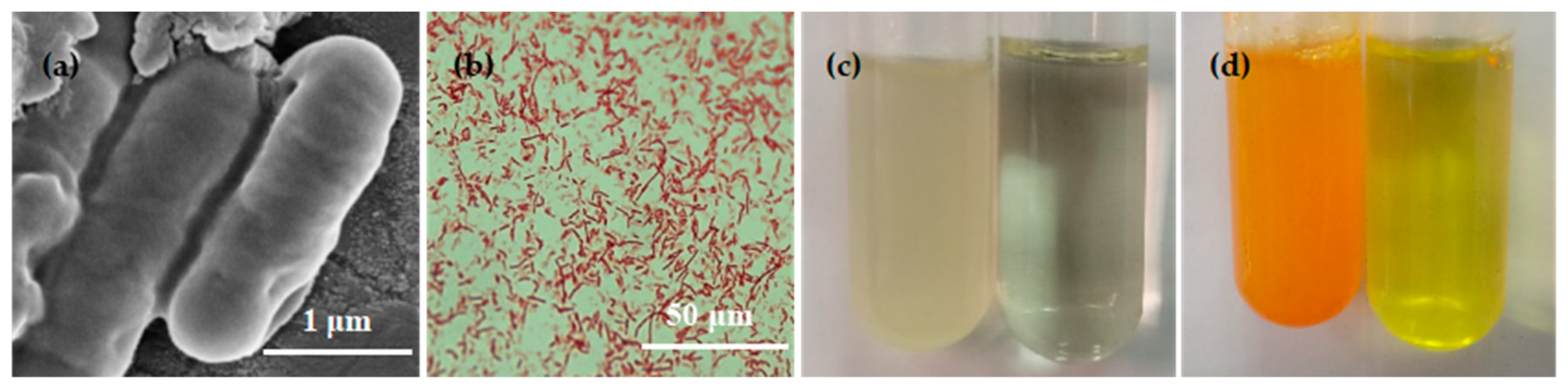
3.2.1. Cell Morphology, Gram Staining, and Ammonia Test
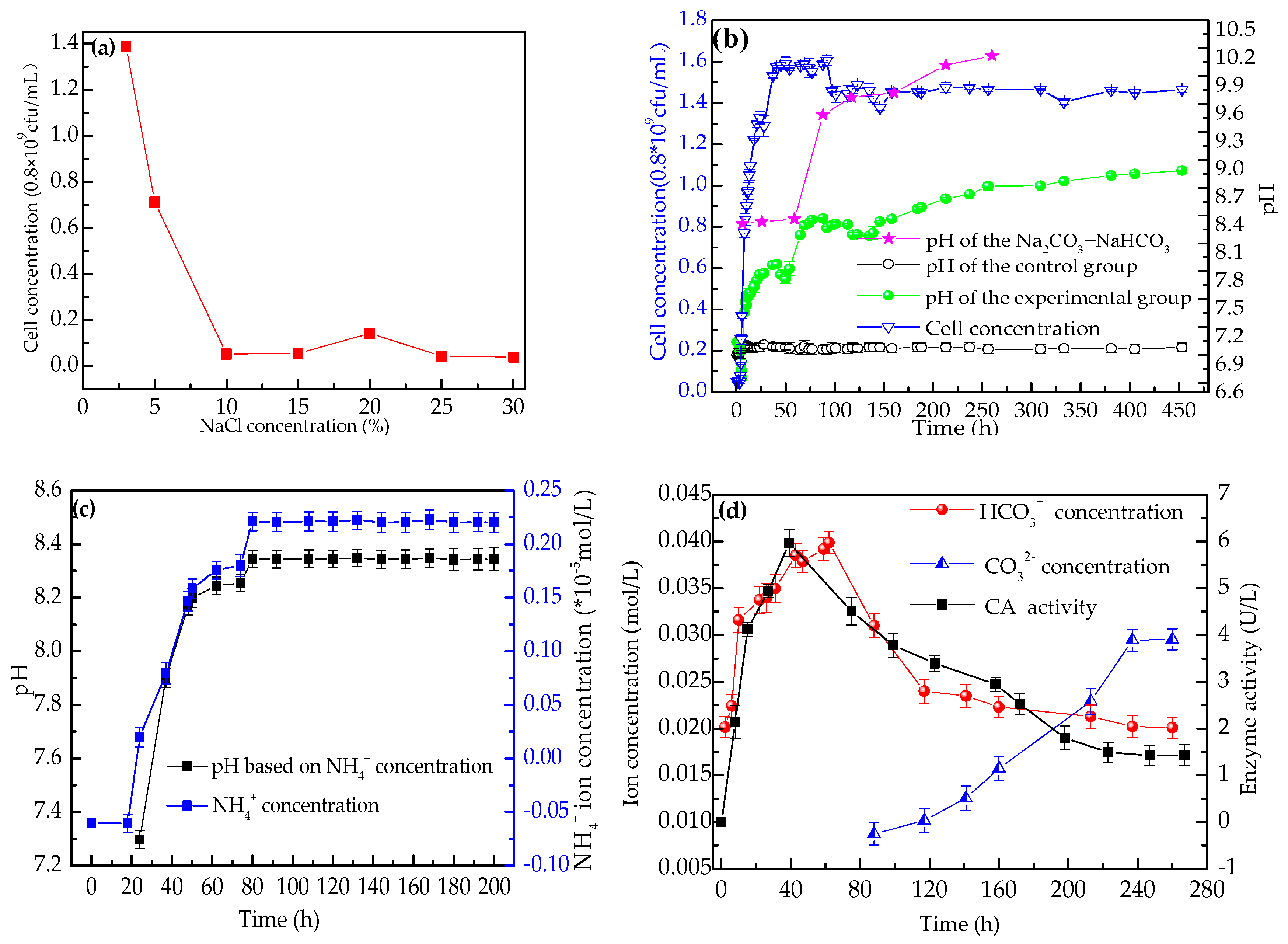
3.2.2. The Growth Curve of H. smyrnensis WMS-3 and pH Variation
3.2.3. Ammonium Concentration and pH Value Based on the Concentration of Ammonium
3.2.4. CA Activity and the Concentration of CO32− and HCO3− Ions
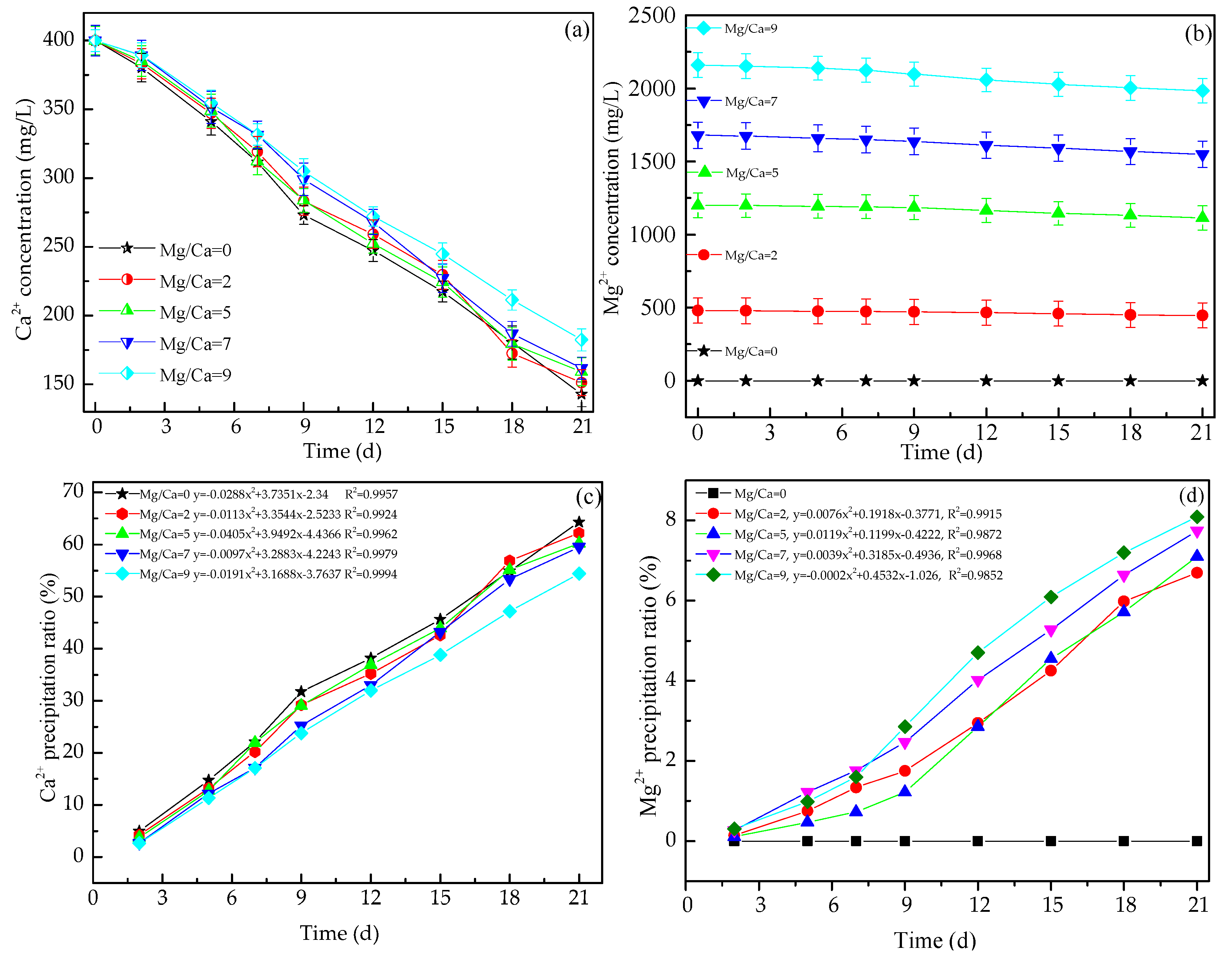
3.3. Changes in the Concentration of Ca2+ and Mg2+ Ions
3.4. Characterization of the Biominerals
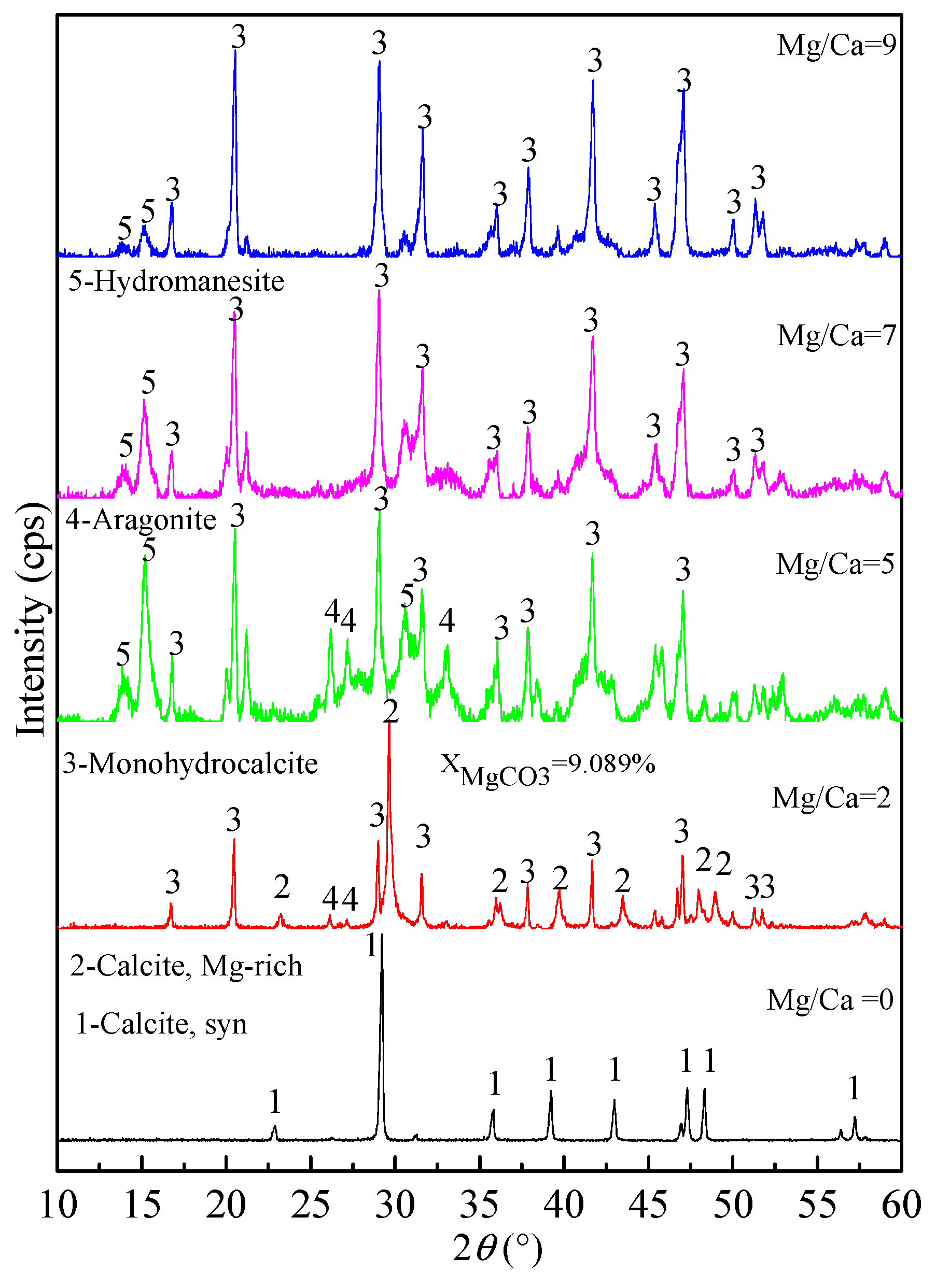
3.4.1. XRD Analyses
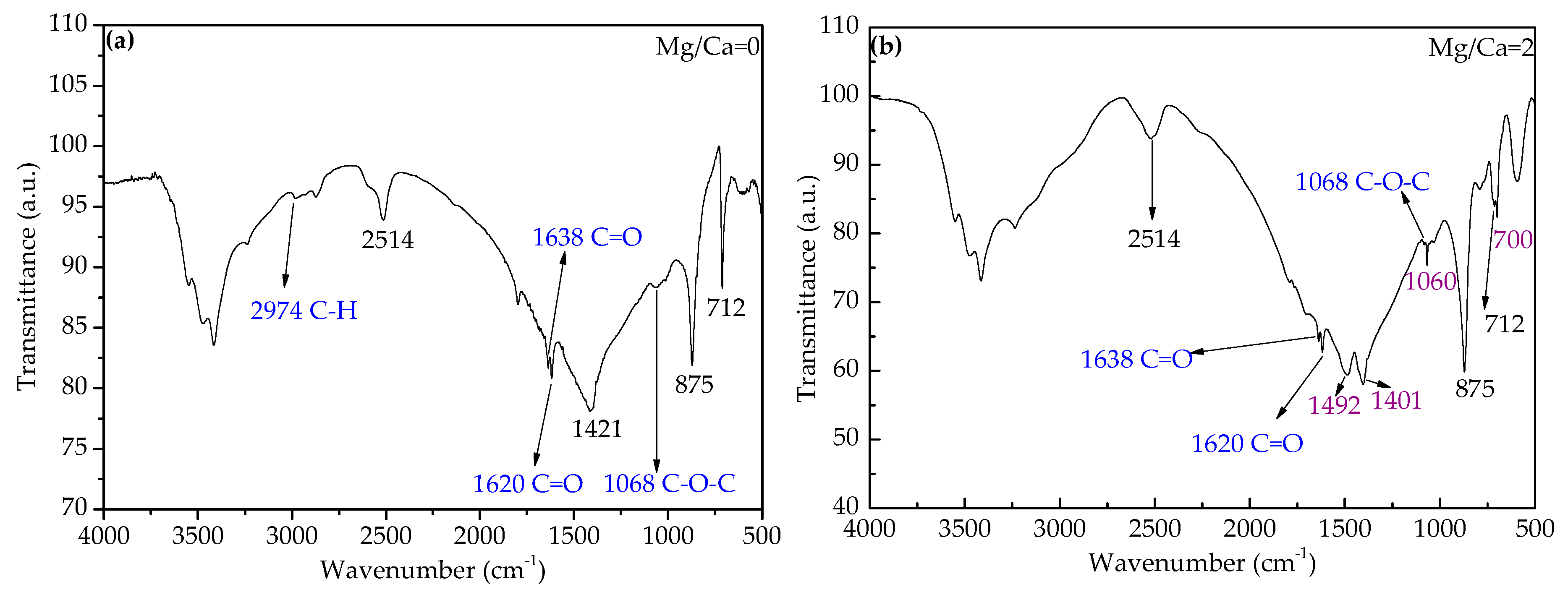
3.4.2. FTIR Analyses
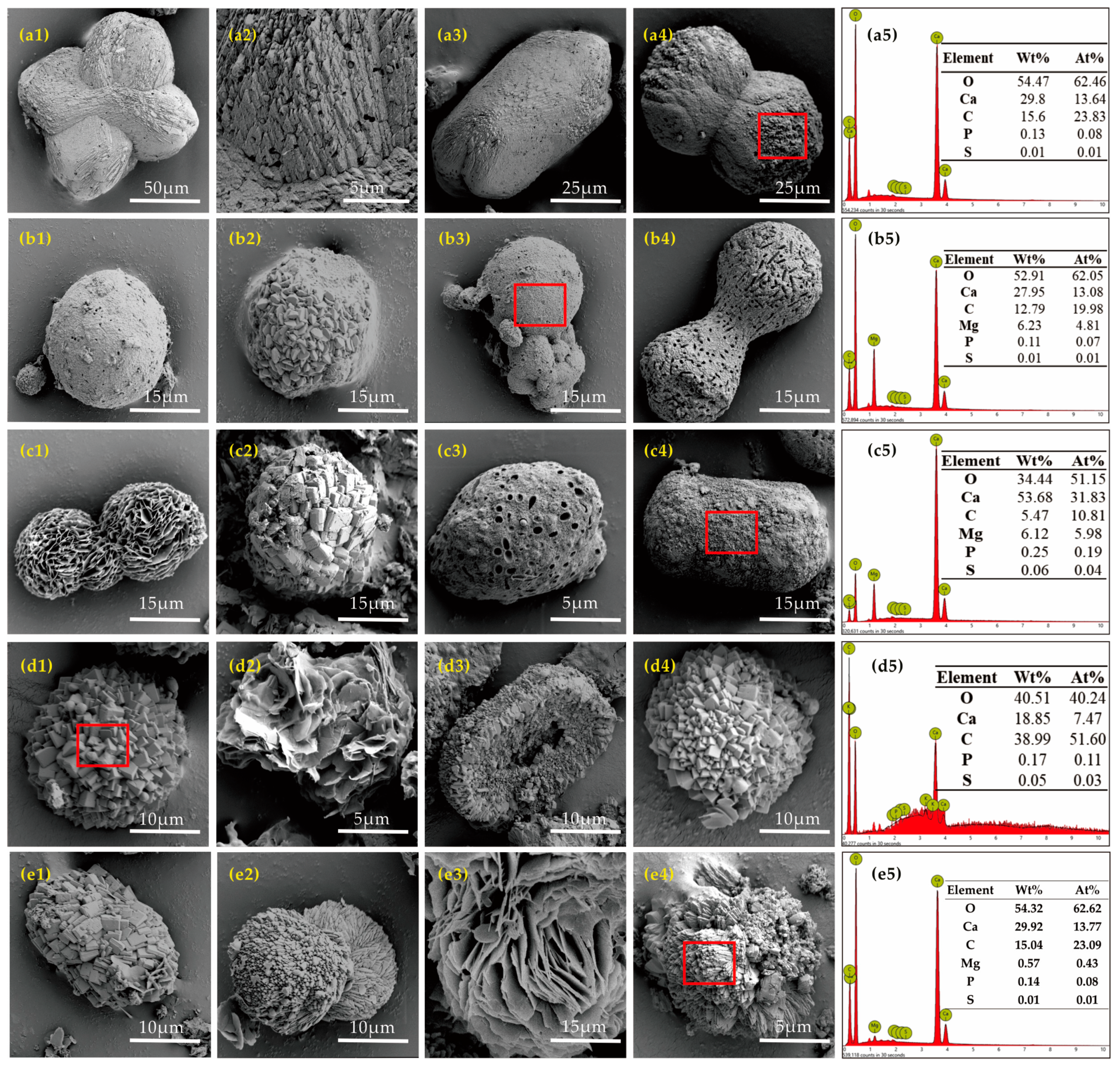
3.4.3. Morphology and Elemental Composition of the Biominerals
3.4.4. Stable Carbon Isotope Composition of the Biominerals
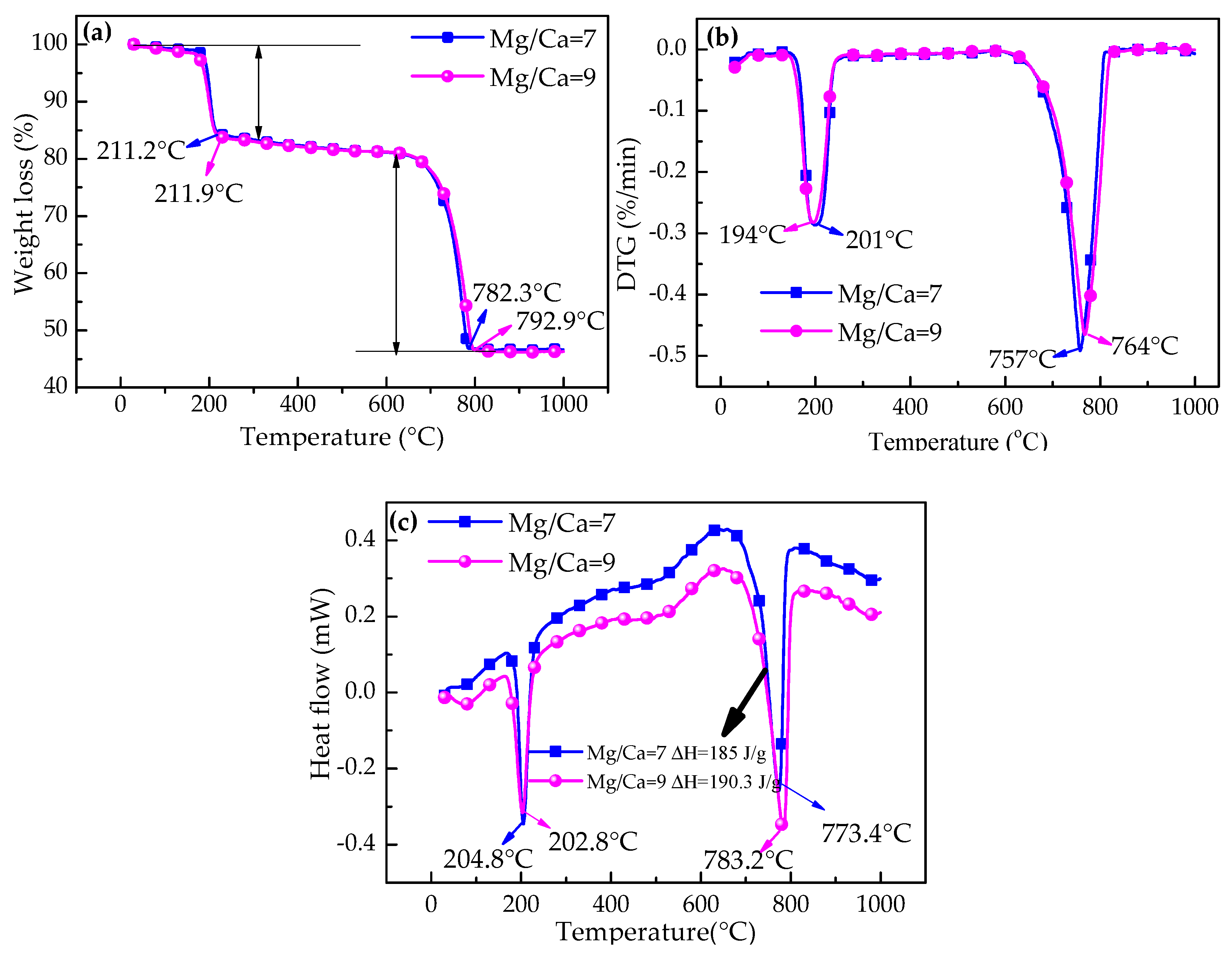
3.4.5. Thermogravimetry-Derivative Thermogravimetry (TG-DTG) and Differential Scanning Calorimetry (DSC) Analyses of Monohydrocalcite at Mg/Ca Molar Ratios 7 and 9
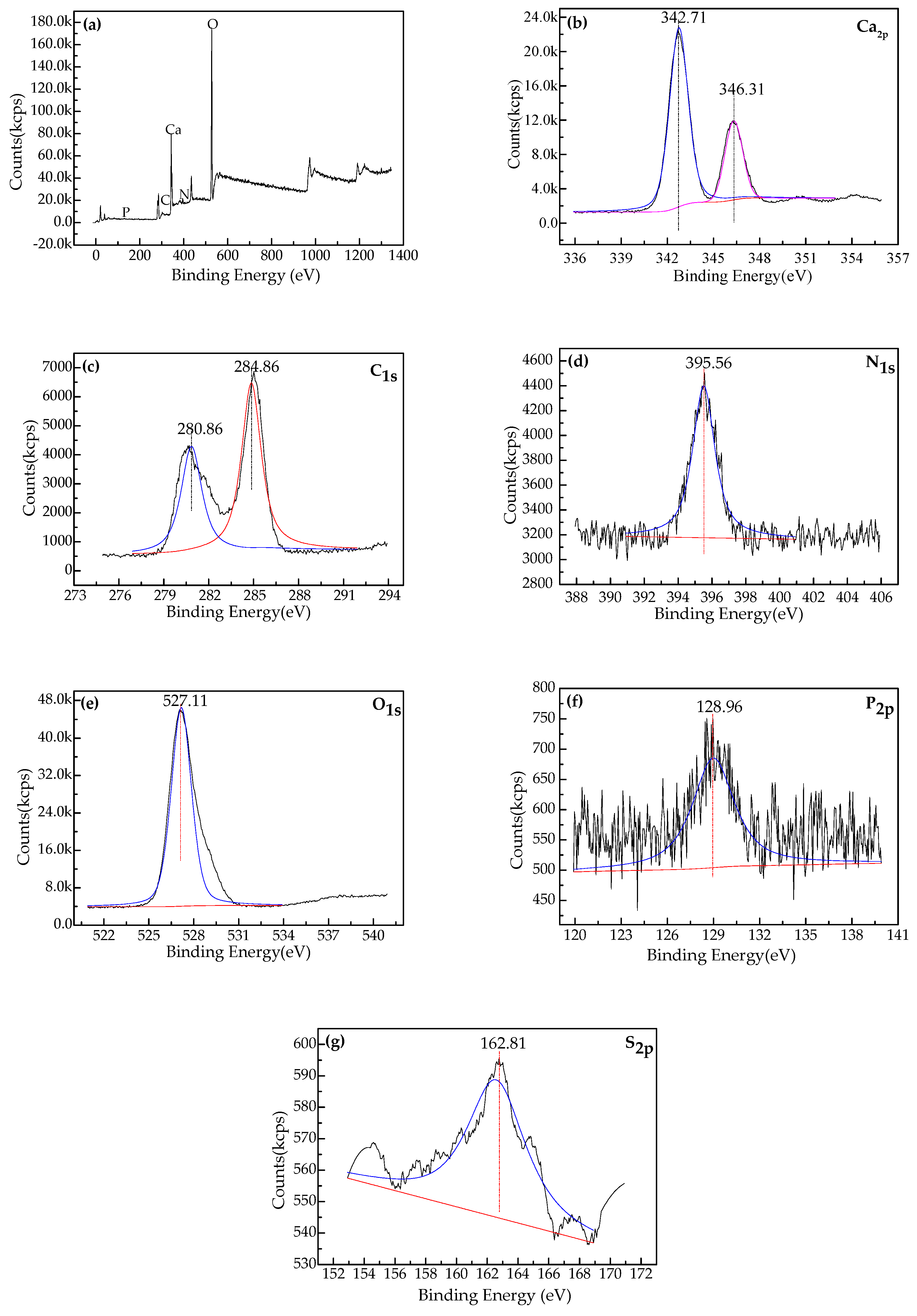
3.4.6. Characterization of Surface Chemistry with XPS
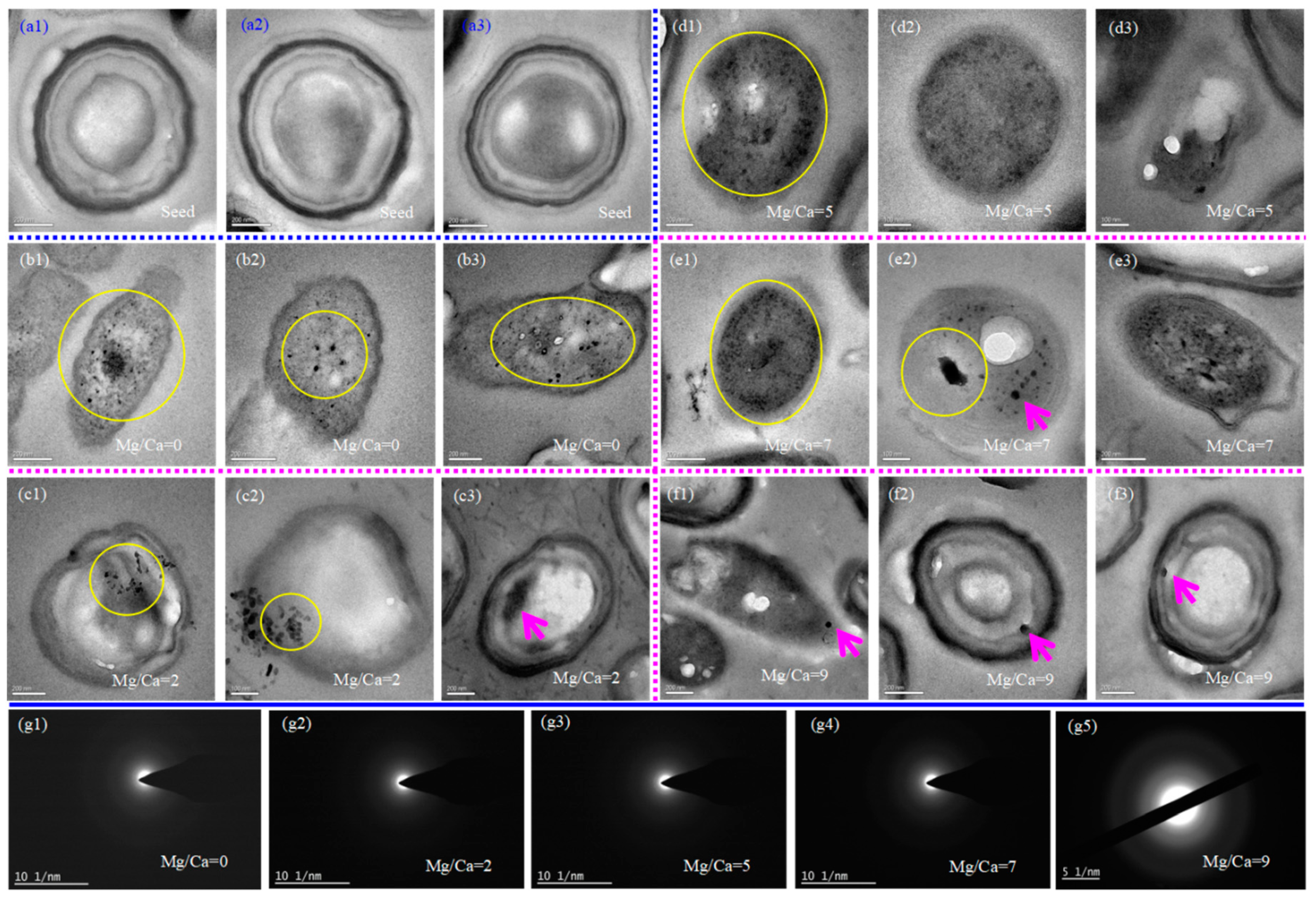
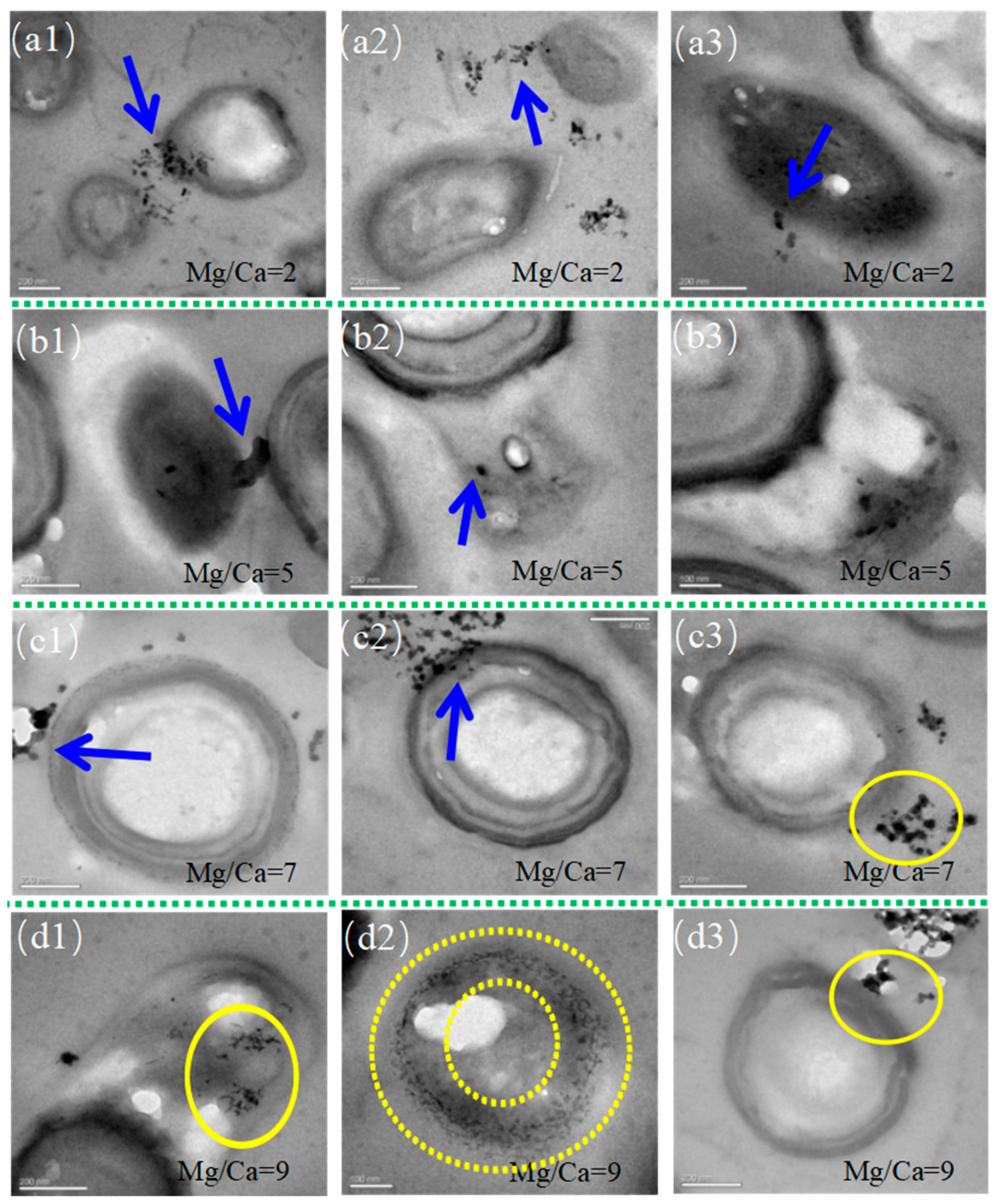
3.5. Intracellular Biomineralization of H. smyrnensis WMS-3 Bacteria
3.6. EPS Acting as the Nucleation Sites
3.7. Changes in the Intracellular Ca2+ Concentration
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lv, D.; Li, Z.; Chen, J.; Liu, H.; Guo, J.; Shang, L. Characteristics of the Permian coal-formed gas sandstone reservoirs in Bohai Bay Basin and the adjacent areas, North China. J. Pet. Sci. Eng. 2011, 78, 516–528. [Google Scholar] [CrossRef]
- Yu, H.; Yuan, J.; Guo, W.; Cheng, J.; Hu, Q. A preliminary laboratory experiment on coalbed methane displacement with carbon dioxide injection. Int. J. Coal. Geol. 2008, 73, 156–166. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, G.; Fan, W.; Ye, J. Predicted CO2 enhanced coalbed methane recovery and CO2 sequestration in China. Int. J. Coal Geol. 2007, 71, 345–357. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, L.; Guo, W.; Cheng, J.; Hu, Q. Predictions of the adsorption equilibrium of methane/carbon dioxide binary gas on coals using Langmuir and ideal adsorbed solution theory under feed gas conditions. Int. J. Coal Geol. 2008, 73, 115–129. [Google Scholar] [CrossRef]
- Pang, Y.; Guo, X.; Han, Z.; Zhang, X.; Zhu, X.; Hou, F.; Han, C.; Song, Z.; Xiao, G. Mesozoic–Cenozoic denudation and thermal history in the Central Uplift of the South Yellow Sea basin and the implications for hydrocarbon systems: Constraints from the CSDP-2 borehole. Mar. Petrol. Geol. 2019, 99, 355–369. [Google Scholar] [CrossRef]
- Allwood, A.C.; Walter, M.R.; Kamber, B.S.; Marshall, C.P.; Burch, I.W. Stromatolite reef from the Early Archaean era of Australia. Nature 2006, 441, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Allwood, A.C.; Walter, M.R.; Burch, I.W.; Kamber, B.S. 3.43 billion-year-old stromatolite reef from the Pilbara Craton of western Australia: Ecosystem-scale insights to early life on Earth. Precambrian Res. 2007, 158, 198–227. [Google Scholar] [CrossRef]
- Lindsay, J.F.; Brasier, M.D.; Mcloughlin, N.; Green, O.R.; Fogel, M.; Mcnamara, K.M.; Steele, A.; Mertzman, S.A. Abiotic earth-establishing a baseline for earliest life, data from the Archean of western Australia. In Proceedings of the Lunar & Planetary Science Conference, League, TX, USA, 17–21 March 2003. [Google Scholar]
- Dupraz, C.; Reid, R.P.; Braissant, O.; Decho, A.W.; Norman, R.S.; Visscher, P.T. Processes of carbonate precipitation in modern microbial mats. Earth-Sci. Rev. 2009, 96, 141–162. [Google Scholar] [CrossRef]
- Chough, S.K.; Lee, H.S.; Woo, J.; Chen, J.; Choi, D.K.; Lee, S.-b.; Kang, I.; Park, T.-y.; Han, Z. Cambrian stratigraphy of the North China Platform: Revisiting principal sections in Shandong Province, China. Geosci. J. 2010, 14, 235–268. [Google Scholar] [CrossRef]
- Lee, J.-H.; Chen, J.; Chough, S.K. Paleoenvironmental implications of an extensive maceriate microbialite bed in the Furongian Chaomidian Formation, Shandong Province, China. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 297, 621–632. [Google Scholar] [CrossRef]
- Lv, D.; Chen, J. Depositional environments and sequence stratigraphy of the Late Carboniferous−Early Permian coal-bearing successions (Shandong Province, China): Sequence development in an epicontinental basin. J. Asian Earth Sci. 2014, 79, 16–30. [Google Scholar] [CrossRef]
- Yin, S.; Lv, D.; Jin, L.; Ding, W. Experimental analysis and application of the effect of stress on continental shale reservoir brittleness. J. Geophys. Eng. 2018, 15, 478–494. [Google Scholar] [CrossRef]
- Xu, Y.; Shen, X.; Chen, N.; Yang, C.; Wang, Q. Evaluation of reservoir connectivity using whole-oil gas chromatographic fingerprint technology: A case study from the Es33 reservoir in the Nanpu Sag, China. Pet. Sci. Technol. 2012, 9, 290–294. [Google Scholar] [CrossRef][Green Version]
- Chen, J.; Van Loon, A.J.; Han, Z.; Chough, S.K. Funnel-shaped, breccia-filled clastic dykes in the Late Cambrian Chaomidian Formation (Shandong Province, China). Sediment. Geol. 2009, 221, 1–6. [Google Scholar] [CrossRef]
- Chen, J.; Chough, S.K.; Chun, S.S.; Han, Z. Limestone pseudoconglomerates in the Late Cambrian Gushan and Chaomidian Formations (Shandong Province, China): Soft-sediment deformation induced by storm-wave loading. Sedimentology 2010, 56, 1174–1195. [Google Scholar] [CrossRef]
- Chen, J.; Han, Z.; Zhang, X.; Fan, A.; Yang, R. Early diagenetic deformation structures of the Furongian ribbon rocks in Shandong Province of China—A new perspective of the genesis of limestone conglomerates. Sci. China Earth Sci. 2010, 53, 241–252. [Google Scholar] [CrossRef]
- Chen, J.; Chough, S.K.; Han, Z.; Lee, J.-H. An extensive erosion surface of a strongly deformed limestone bed in the Gushan and Chaomidian formations (late Middle Cambrian to Furongian), Shandong Province, China: Sequence-stratigraphic implications. Sediment. Geol. 2011, 233, 129–149. [Google Scholar] [CrossRef]
- Chen, J.; Chough, S.K.; Lee, J.-H.; Han, Z. Sequence-stratigraphic comparison of the upper Cambrian Series 3 to Furongian succession between the Shandong region, China and the Taebaek area, Korea: High variability of bounding surfaces in an epeiric platform. Geosci. J. 2012, 16, 357–379. [Google Scholar] [CrossRef]
- Lee, J.; Chen, J.; Choh, S.; Lee, D.; Han, Z.; Chough, S.K. Furongian (Late Cambrian) sponge–microbial maze-like reefs in the north china platform. Palaios 2014, 29, 27–37. [Google Scholar] [CrossRef]
- Van Loon, A.J.T.; Han, Z.; Han, Y. Origin of the vertically orientated clasts in brecciated shallow-marine limestones of the Chaomidian Formation (Furongian, Shandong Province, China). Sedimentology 2013, 60, 1059–1070. [Google Scholar] [CrossRef]
- Woo, J.; Chough, S.K.; Han, Z. Chambers of Epiphyton thalli in microbial buildups, Zhangxia Formation (Middle Cambrian), Shandong Province, China. Palaios 2008, 23, 55–64. [Google Scholar] [CrossRef]
- Park, T.Y.; Sang, J.M.; Han, Z.; Choi, D.K. Ontogeny of the middle cambrian trilobite shantungia spinifera walcott, 1905 from north china and its taxonomic significance. J. Paleontol. 2008, 82, 851–855. [Google Scholar] [CrossRef]
- Liu, Y.; Jiao, X.; Li, H.; Yuan, M.; Yang, W.; Zhou, X.; Liang, H.; Zhou, D.; Zheng, C.; Sun, Q.; et al. Primary dolostone formation related to mantle-originated exhalative hydrothermal activities, Permian Yuejingou section, Santanghu area, Xinjiang, NW China. Sci. China Earth Sci. 2012, 55, 183–192. [Google Scholar] [CrossRef]
- Han, Z.; Zhang, X.; Chi, N.; Han, M.; Woo, J.; Lee, H.S.; Chen, J. Cambrian oncoids and other microbial-related grains on the North China Platform. Carbonates Evaporites 2015, 30, 373–386. [Google Scholar] [CrossRef]
- Fan, A.; Yang, R.; van Loon, A.J.; Yin, W.; Han, Z.; Zavala, C. Classification of gravity-flow deposits and their significance for unconventional petroleum exploration, with a case study from the Triassic Yanchang Formation (southern Ordos Basin, China). J. Asian Earth Sci. 2018, 161, 57–73. [Google Scholar] [CrossRef]
- Chang, X.; Wang, T.G.; Li, Q.; Cheng, B.; Tao, X. Geochemistry and possible origin of petroleum in Palaeozoic reservoirs from Halahatang Depression. J. Asian Earth Sci. 2013, 74, 129–141. [Google Scholar] [CrossRef]
- Dahl, K.; Buchardt, B. Monohydrocalcite in the Arctic Ikka Fjord, SW Greenland: First reported marine occurrence. J. Sediment. Res. 2006, 76, 460–471. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Biomineralization of carbonates by Halomonas eurihalina in solid and liquid media with different salinities: Crystal formation sequence. Res. Microbiol. 1998, 149, 277–287. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Ramos-Cormenzana, A.; Delgado, R. Precipitation of carbonates by Deleya halophila in liquid media: Pedological implications in saline soils. Arid Soil Res. Rehab. 1997, 11, 35–47. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, R.; Delgado, G.; Moral, A.D.; Ferrer, M.R.; Ramos-Cormenzana, A. Precipitation of carbonates by Bacillus sp. isolated from saline soils. Geomicrobiol. J. 1993, 11, 175–184. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Párraga, J.; Delgado, R.; Ramos-Cormenzana, A.; Delgado, G. Biomineralization of carbonates by Halobacillus trueperi in solid and liquid media with different salinities. FEMS Microbiol. Ecol. 2004, 48, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Arias, D.; Cisternas, L.A.; Rivas, M. Biomineralization of calcium and magnesium crystals from seawater by halotolerant bacteria isolated from Atacama Salar (Chile)(Article). Desalination 2017, 405, 1–9. [Google Scholar] [CrossRef]
- Han, Z.; Li, D.; Zhao, H.; Yan, H.; Li, P. Precipitation of carbonate minerals induced by the halophilic Chromohalobacter israelensis under high salt concentrations: Implications for natural environments. Minerals 2017, 7, 95. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Martín-Algarra, A.; Sánchez-Navas, A.; Martín-Ramos, D. Carbonate and phosphate precipitation by Chromohalobacter marismortui. Geomicrobiol. J. 2006, 23, 89–101. [Google Scholar] [CrossRef]
- Han, Z.; Wang, J.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Wu, G.; Zhou, J.; Yin, J.; Zhang, H.; Zhang, X.; et al. Mechanism of biomineralization induced by Bacillus subtilis J2 and characteristics of the biominerals. Minerals 2019, 9, 218. [Google Scholar] [CrossRef]
- Zhuang, D.; Yan, H.; Tucker, M.E.; Zhao, H.; Han, Z.; Zhao, Y.; Sun, B.; Li, D.; Pan, J.; Zhao, Y.; et al. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: Further insights into biotic and abiotic calcite. Chem. Geol. 2018, 500, 64–87. [Google Scholar] [CrossRef]
- Han, Z.; Meng, R.; Yan, H.; Zhao, H.; Han, M.; Zhao, Y.; Sun, B.; Sun, Y.; Wang, J.; Zhuang, D. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonates Evaporites 2017. [Google Scholar] [CrossRef]
- Han, Z.; Yan, H.; Zhao, H.; Zhou, S.; Han, M.; Meng, X.; Zhang, Y.; Zhao, Y.; Sun, B.; Yao, C.; et al. Bio-precipitation of calcite with preferential orientation induced by Synechocystis sp. PCC6803. Geomicrobiol. J. 2014, 31, 884–899. [Google Scholar] [CrossRef]
- Arvidson, R.S.; Mackenzie, F.T. The dolomite problem: Control of precipitation kinetics by temperature and saturation state. Am. J. Sci. 1999, 299, 257–288. [Google Scholar] [CrossRef]
- Liu, F.; Csetenyi, L.; Gadd, G.M. Amino acid secretion influences the size and composition of copper carbonate nanoparticles synthesized by ureolytic fungi. Appl. Microbiol. Biot. 2019, 103, 7217–7230. [Google Scholar] [CrossRef]
- Morgan, J.W.; Forster, C.F.; Evison, L. A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water. Res. 1990, 24, 743–750. [Google Scholar] [CrossRef]
- Su, T.; Shao, Q.; Qin, Z.; Guo, Z.; Wu, Z. Role of interfaces in two-dimensional photocatalyst for water splitting. Acs. Catal. 2018, 8, 2253–2276. [Google Scholar] [CrossRef]
- Ding, Y.; Yang, B.; Hao, L.; Liu, Z.; Xiao, Z.; Zheng, X.; Liu, Q. FePt-Au ternary metallic nanoparticles with the enhanced peroxidase-like activity for ultrafast colorimetric detection of H2O2. Sensor Actuat. B-chem 2017, 259, 775–783. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, Y.; Sun, Y.; Ding, P.; Liu, Q.; Lin, T.; Guo, J. Hybrid of Fe4 [Fe(CN)6]3 nanocubes and MoS2 nanosheets on nitrogen-doped graphene realizing improved electrochemical hydrogen production. Electrochim. Acta. 2018, 263, 140–146. [Google Scholar] [CrossRef]
- Wei, C.; Liu, Q.; Zhu, X.; Min, F. One-step in situ growth of magnesium ferrite nanorods on graphene and their microwave-absorbing properties. Appl. Organomet. Chem. 2018, 32, 4011–4019. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Liu, Q.; Guo, J.; Xiao, Z. FePt nanoalloys on N-doped graphene paper as integrated electrode towards efficient formic acid electrooxidation. J. Appl. Electrochem. 2018, 48, 95–103. [Google Scholar] [CrossRef]
- Lin, C.; Hu, L.; Cheng, C.; Sun, K.; Guo, X.; Shao, Q.; Li, J.; Wang, N.; Guo, Z. Nano-TiNb2O7/carbon nanotubes composite anode for enhanced lithium-ion storage. Electrochim. Acta 2018, 260, 65–72. [Google Scholar] [CrossRef]
- Pan, D.; Ge, S.; Zhang, X.; Mai, X.; Li, S.; Guo, Z. Synthesis and photoelectrocatalytic activity of In2O3 hollow microspheres via a bio-template route using yeast templates. Dalton Trans. 2018, 47, 708–715. [Google Scholar] [CrossRef]
- Han, Z.; Gao, X.; Zhao, H.; Tucker, M.E.; Zhao, Y.; Bi, Z.; Pan, J.; Wu, G.; Yan, H. Extracellular and intracellular biomineralization induced by Bacillus Licheniformis DB1-9 at different Mg/Ca molar ratios. Minerals 2018, 8, 585. [Google Scholar] [CrossRef]
- Han, Z.; Sun, B.; Zhao, H.; Yan, H.; Han, M.; Zhao, Y.; Meng, R.; Zhuang, D.; Li, D.; Ma, Y.; et al. Isolation of Leclercia adcarboxglata strain JLS1 from dolostone sample and characterization of its induced struvite. Geomicrobiol. J. 2017, 34, 500–510. [Google Scholar] [CrossRef]
- Han, Z.; Yu, W.; Zhao, H.; Zhao, Y.; Tucker, M.E.; Yan, H. The significant roles of Mg/Ca ratio, Cl− and SO42− in carbonate mineral precipitation by the Halophile Staphylococcus epidermis Y2. Minerals 2018, 8, 594. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating mineral carbonation using carbonic anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef]
- Lee, S.W.; Park, S.B.; Jeong, S.K.; Lim, K.S.; Lee, S.H.; Trachtenberg, M.C. On carbon dioxide storage based on biomineralization strategies. Micron 2010, 41, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Southam, G. Carbon sequestration via carbonic anhydrase facilitated magnesium carbonate precipitation. Int. J. Greenh. Gas Control 2013, 16, 145–155. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.; Yao, Y.; Duan, Y. High salinity facilitates dolomite precipitation mediated by Haloferax volcanii DS52. Earth. Planet. Sci. Lett. 2017, 472, 197–205. [Google Scholar] [CrossRef]
- Katz, A.K.; Glusker, J.P.; Beebe, S.A.; Bock, C.W. Calcium ion coordination: A comparison with that of beryllium, magnesium, and zinc. J. Am. Chem. Soc. 1996, 118, 5752–5763. [Google Scholar] [CrossRef]
- Xu, J.; Yan, C.; Zhang, F.; Konishi, H.; Xu, H.; Teng, H.H. Testing the cation-hydration effect on the crystallization of Ca-Mg-CO3 systems. Proc. Natl. Acad. Sci. USA 2013, 110, 17750–17755. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallization of monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef]
- Stoffers, P.; Fischbeck, R. Monohydrocalcite in the sediments of Lake Kivu (East Africa). Sedimentology 2010, 21, 163–170. [Google Scholar] [CrossRef]
- Hull, H.; Turnbull, A.G. A thermochemical study of monohydrocalcite. Geochim. Cosmochim. Acta 1973. [Google Scholar] [CrossRef]
- Munemoto, T.; Fukushi, K. Transformation kinetics of monohydrocalcite to aragonite in aqueous solutions. J. Miner. Petrol. Sci. 2008, 103, 345–349. [Google Scholar] [CrossRef]
- Kalmar, L.; Homola, D.; Varga, G.; Tompa, P. Structural disorder in proteins brings order to crystal growth in biomineralization. Bone 2012, 51, 528–534. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, M.; Persson, D.; Thierry, D. Corrosion product formation during NaCl induced atmospheric corrosion of magnesium alloy AZ91D. Corros. Sci. 2007, 49, 1540–1558. [Google Scholar] [CrossRef]
- Bizani, D.; Motta, A.S.; Morrissy, J.A.; Terra, R.M.; Souto, A.A.; Brandelli, A. Antibacterial activity of cerein 8A, a bacteriocin-like peptide produced by Bacillus cereus. Int. Microbiol. 2005, 8, 125–131. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, Q.; Zhou, G.; Fu, S. Formation of elongated calcite mesocrystals and implication for biomineralization. Chem. Geol. 2013, 360, 126–133. [Google Scholar] [CrossRef]
- Zhou, G.; Guan, Y.; Yao, Q.; Fu, S. Biomimetic mineralization of prismatic calcite mesocrystals: Relevance to biomineralization. Chem. Geol. 2010, 279, 63–72. [Google Scholar] [CrossRef]
- Zhang, C.; Lv, J.; Li, F.; Li, X. Nucleation and growth of mg-calcite spherulites induced by the bacterium Curvibacter Lanceolatus strain HJ-1. Microsc. Microanal. 2017, 23, 1–8. [Google Scholar] [CrossRef]
- Buczynski, C.; Chafetz, H. Habit of bacterially induced precipitates of calcium carbonate and the influences of medium viscosity on mineralogy. J. Sediment. Res. 1991, 61, 226–233. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; McKenzie, J.A.; Wagener, A.D.L.R.; Rivadeneyra, M.A.; Vasconcelos, C. Presence of sulfate does not inhibit low-temperature dolomite precipitation. Earth. Planet. Sci. Lett. 2009, 285, 131–139. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; McKenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef]
- Jones, M.; Donnelly, A. Carbon sequestration in temperate grassland ecosystems and the influence of management, climate and elevated CO2: Tansley review. New Phytol. 2004, 164, 423–439. [Google Scholar] [CrossRef]
- Haurie, L.; Fernandez, A.I.; Velasco, J.I.; Chimenos, J.M.; Lopez-Cuesta, J.M.; Espiell, F. Effects of milling on the thermal stability of synthetic hydromagnesite. Mater. Res. Bull. 2007, 42, 1010–1018. [Google Scholar] [CrossRef]
- Kimura, T.; Koga, N. Monohydrocalcite in comparison with hydrated amorphous calcium carbonate: Precipitation condition and thermal behavior. Crysl. Growth Des. 2011, 11, 3877–3884. [Google Scholar] [CrossRef]
- Ma, K.; Cui, L.; Dong, Y.; Wang, T.; Da, C.; Hirasaki, G.J.; Biswal, S.L. Adsorption of cationic and anionic surfactants on natural and synthetic carbonate materials. J. Colloid Interf. Sci 2013, 408, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bianchi, C.L.; Fadoni, M.; Vercelli, B. Magnesium salts and oxide: An XPS overview. Appl. Surf. Sci. 1997, 119, 253–259. [Google Scholar] [CrossRef]
- Paulo, C.; Kenney, J.P.L.; Persson, P.; Dittrich, M. Effects of phosphorus in growth media on biomineralization and cell surface properties of marine cyanobacteria synechococcus. Geosciences 2018, 8, 471. [Google Scholar] [CrossRef]
- Huang, Y.; Yao, Q.; Li, H.; Wang, F.; Zhou, G.; Fu, S. Aerobically incubated bacterial biomass-promoted formation of disordered dolomite and implication for dolomite formation. Chem. Geol. 2019, 523, 19–30. [Google Scholar] [CrossRef]
- Hsu, M.; Chuang, H.; Cheng, F.; Huang, Y.; Han, C.; Pao, K.; Chou, S.; Shieh, F.K.; Tsai, F.Y.; Lin, C.; et al. Simple and highly efficient direct thiolation of the surface of carbon nanotubes. RSC Adv. 2014, 4, 14777–14780. [Google Scholar] [CrossRef]
- Fein, J.B.; Martin, A.M.; Wightman, P.G. Metal adsorption onto bacterial surfaces: Development of a predictive approach. Geochim. Cosmochim. Acta 2001, 65, 4267–4273. [Google Scholar] [CrossRef]
- Couradeau, E.; Benzerara, K.; Gérard, E.; Moreira, D.; Bernard, S.; Brown, G.; Lopez-Garcia, P. An early-branching microbialite cyanobacterium forms intracellular carbonates. Science 2012, 336, 459–462. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouri-Panet, F.; Li, J.; Ferard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N.; et al. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933. [Google Scholar] [CrossRef] [PubMed]
- Keim, C.; Solórzano, I.; Farina, M.; Lins, U. Intracellular inclusions of uncultured magnetotactic bacteria. Int. Microbiol. 2005, 8, 111–117. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.; Slowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2017, 65, 1213–1245. [Google Scholar] [CrossRef]
- Obst, M.; Dynes, J.J.; Lawrence, J.R.; Swerhone, G.D.W.; Benzerara, K.; Karunakaran, C.; Kaznatcheev, K.; Tyliszczak, T.; Hitchcock, A.P. Precipitation of amorphous CaCO3 (aragonite-like) by cyanobacteria: A STXM study of the influence of EPS on the nucleation process. Geochim. Cosmochim. Acta 2009, 73, 4180–4198. [Google Scholar] [CrossRef]
- Deng, S.; Dong, H.; Lv, G.; Jiang, H.; Yu, B.; Bishop, M.E. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; Gonzalez, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef]
- Bontognali, T.R.R.; Vasconcelos, C.; Warthmann, R.J.; Bernasconi, S.M.; Dupraz, C.; Strohmenger, C.J.; Mckenzie, J.A. Dolomite formation within microbial mats in the coastal sabkha of Abu Dhabi (United Arab Emirates). Sedimentology 2010, 57, 824–844. [Google Scholar] [CrossRef]
- Lv, J.; Ma, F.; Li, F.; Zhang, C.; Chen, J. Vaterite induced by Lysinibacillus sp. GW-2 strain and its stability. J. Struct. Biol. 2017, 200, 97–105. [Google Scholar] [CrossRef]
- Mann, S.; Heywood, B.R.; Rajam, S.; Wade, V.J. Molecular recognition in biomineralization. In Mechanisms and Phylogeny of Mineralization in Biological Systems; Suga, S., Nakahara, H., Eds.; Springer: Tokyo, Japan, 1991; pp. 47–55. [Google Scholar]
- Zhao, Y.; Yan, H.; Zhou, J.; Tucker, M.E.; Han, M.; Zhao, H.; Mao, G.; Zhao, Y.; Han, Z. Bio-precipitation of calcium and magnesium ions through extracellular and intracellular process induced by Bacillus Licheniformis SRB2. Minerals 2019, 9, 526. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, J.; Zhao, H.; Tucker, M.E.; Zhou, J.; Jiang, M.; Wang, Y.; Zhao, Y.; Sun, B.; Han, Z.; Yan, H. Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas Smyrnensis WMS-3. Minerals 2019, 9, 632. https://doi.org/10.3390/min9100632
Pan J, Zhao H, Tucker ME, Zhou J, Jiang M, Wang Y, Zhao Y, Sun B, Han Z, Yan H. Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas Smyrnensis WMS-3. Minerals. 2019; 9(10):632. https://doi.org/10.3390/min9100632
Chicago/Turabian StylePan, Juntong, Hui Zhao, Maurice E. Tucker, Jingxuan Zhou, Mengzhen Jiang, Yapeng Wang, Yanyang Zhao, Bin Sun, Zuozhen Han, and Huaxiao Yan. 2019. "Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas Smyrnensis WMS-3" Minerals 9, no. 10: 632. https://doi.org/10.3390/min9100632
APA StylePan, J., Zhao, H., Tucker, M. E., Zhou, J., Jiang, M., Wang, Y., Zhao, Y., Sun, B., Han, Z., & Yan, H. (2019). Biomineralization of Monohydrocalcite Induced by the Halophile Halomonas Smyrnensis WMS-3. Minerals, 9(10), 632. https://doi.org/10.3390/min9100632




