The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Separation
2.2. Analysis
2.2.1. Petrographic and Mineralogical Analysis
2.2.2. Particle-Size Analysis and Heavy-Liquid Separation
2.2.3. Chemical Analysis
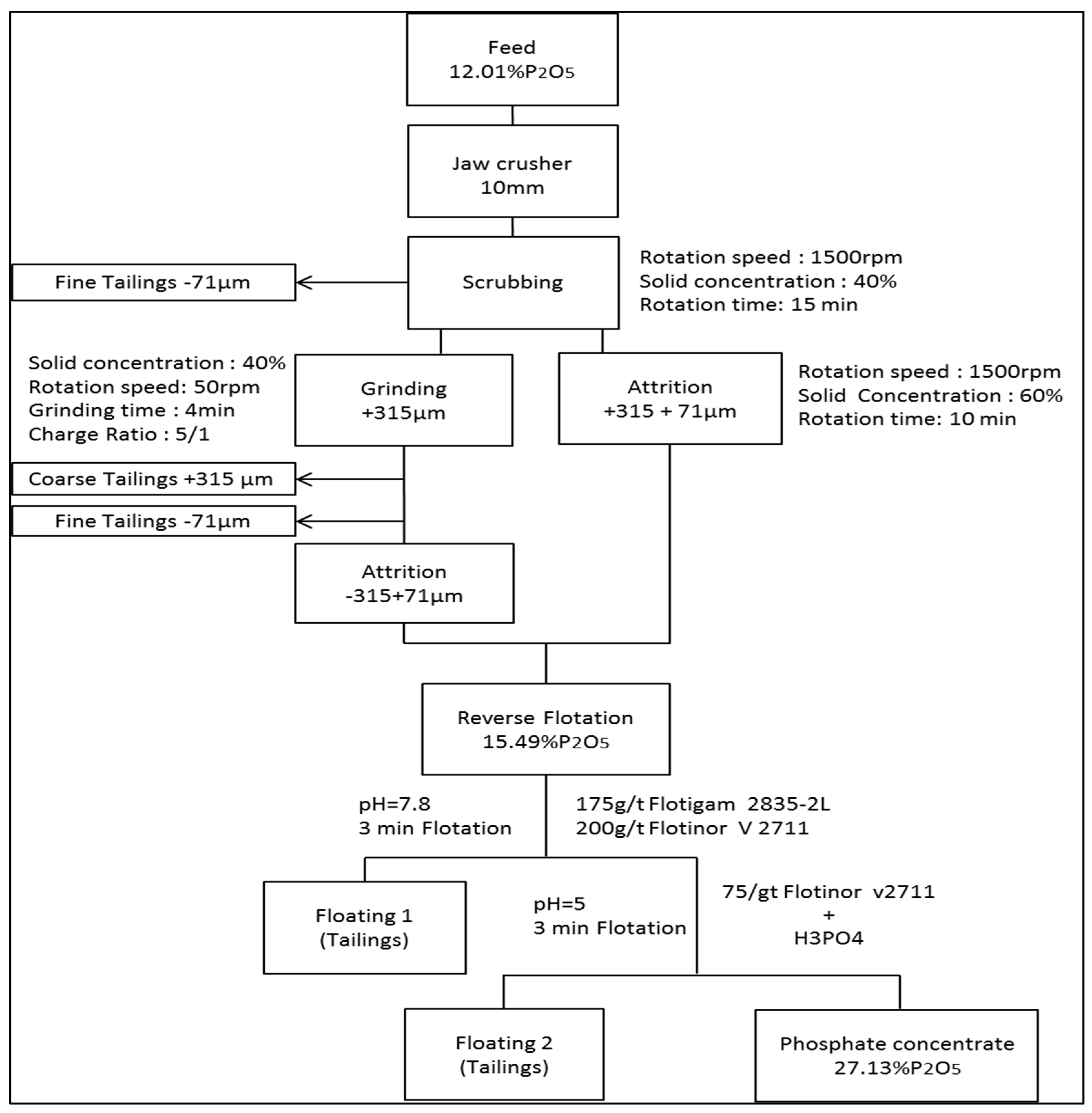
2.3. Beneficiation Processes
2.3.1. Sizing, Scrubbing (Washing) and Attrition
2.3.2. Grinding
2.3.3. Flotation
3. Results and Discussion
3.1. Petrographic Analysis
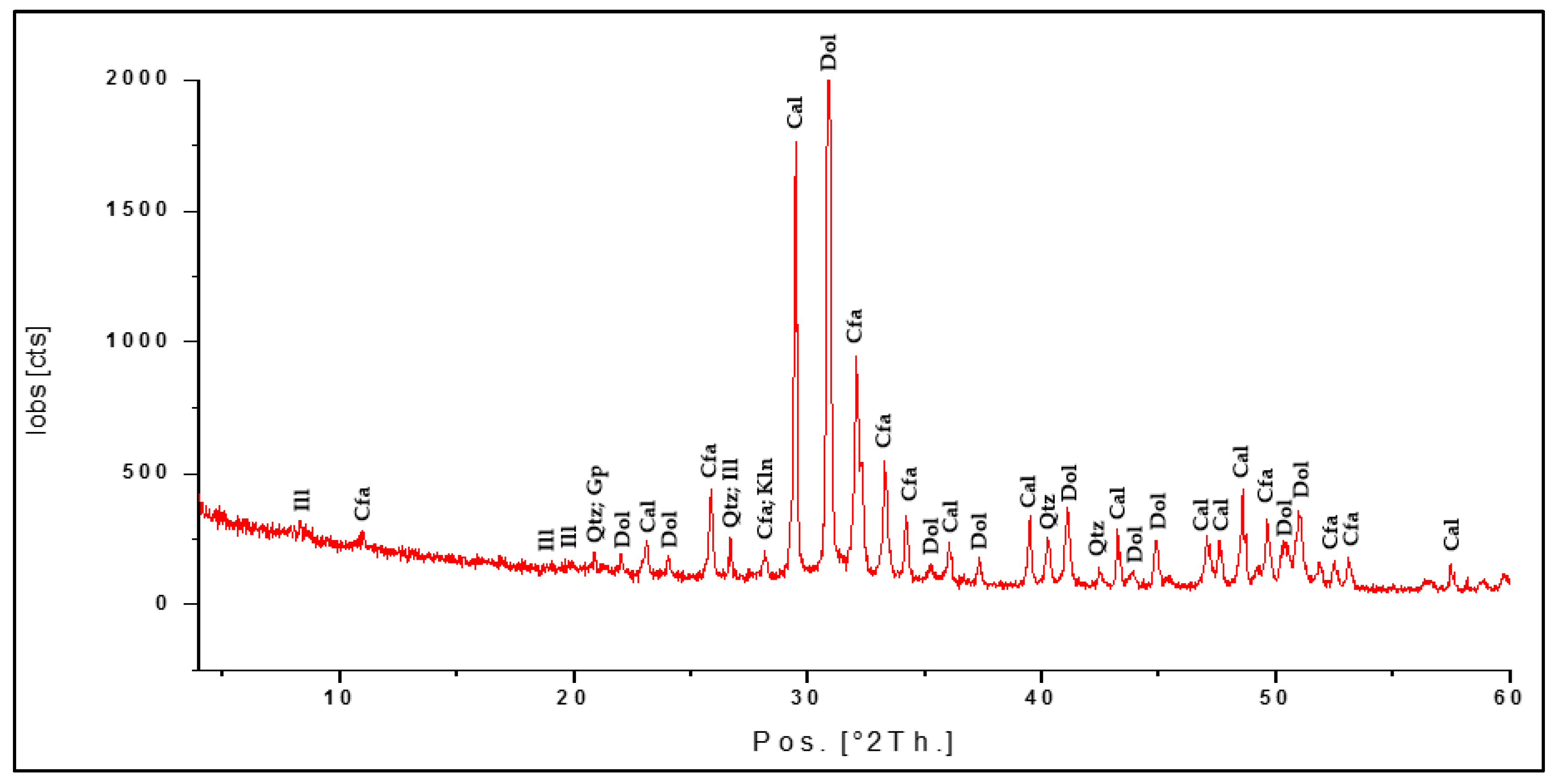
3.2. Mineralogical Analysis
3.3. Chemical Analysis
3.3.1. Raw Phosphate Analysis
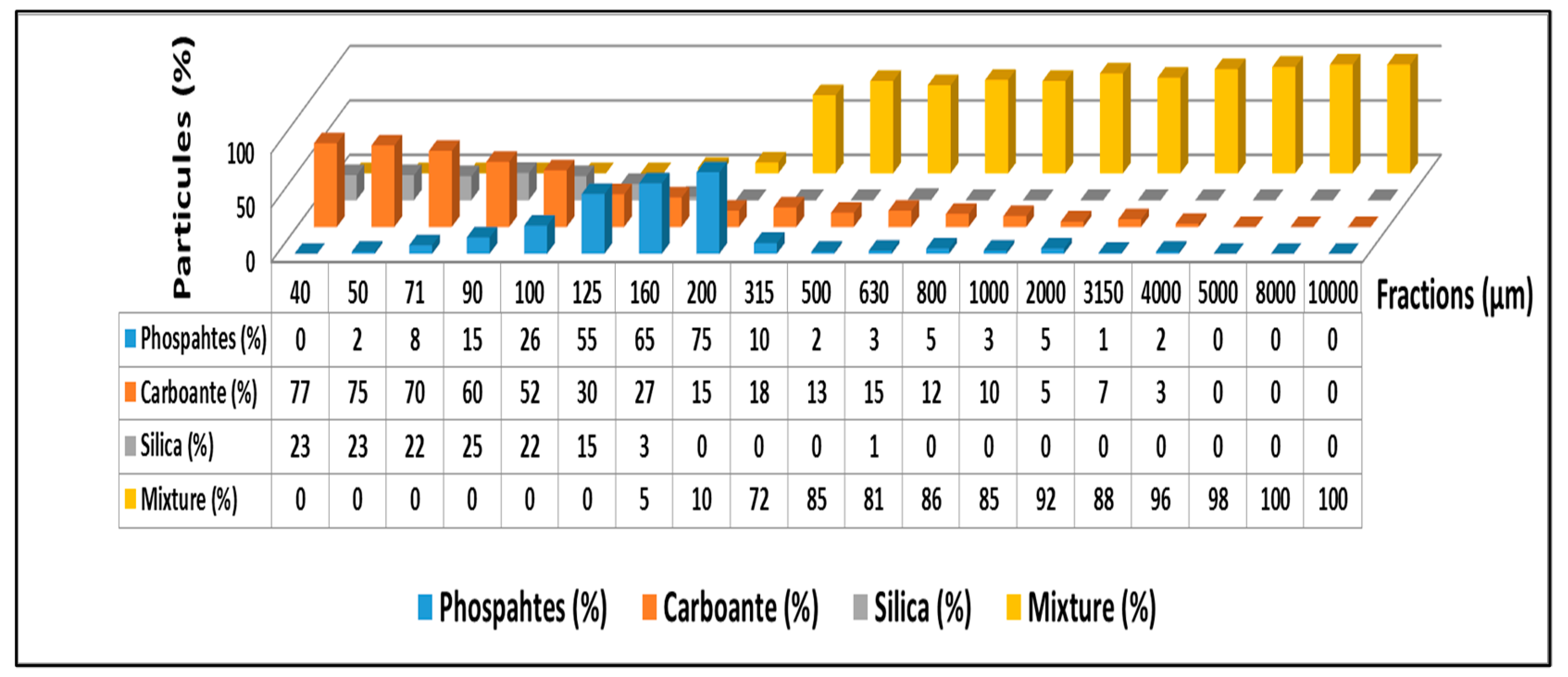
3.3.2. Granulo-Chemical Analysis
3.4. Enrichment Methods
3.4.1. Scrubbing and Attrition
3.4.2. Grinding
Effect of the Grinding Time on the Grade and Recovery of P2O5
Effect of the Charge Ratio on the Grade and Recovery of P2O5
Effect of Solid Concentration on the Grade and Recovery of P2O5
Optimum Grinding Parameters
3.4.3. Flotation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Galfati, I. Etude de l’impact des rejets de l’industrie Phosphatière sur l’environnement dans le bassin de Gafsa Métlaoui. Ph.D. Thesis, University of Tunis El Manar, Tunis, Tunisia, 2010. [Google Scholar]
- IFDC. Available online: https://ifdc.org/wp-content/uploads/2015/03/100922_kauwenbergh_presentation_0.pdf (accessed on 14 September 2018).
- Burollet, P.F. Contribution à l’étude stratigraphique de la Tunisie centrale. Ann. Mines Geol. 1956, 18, 1–350. [Google Scholar]
- Sassi, S. La sédimentation phosphatée au Paléocène dans le Sud et le Centre oust de la Tunisie. Ph.D. Thesis, Université d’Orsay, Paris-Sud, France, 1974. [Google Scholar]
- Chaabani, F. Dynamique de la partie orientale du bassin de Gafsa au crétacé et au paléogène. Etude minéralogique et géochimique de la série phosphatée éocène (Tunisie méridionale). Ph.D. Thesis, University of Tunis El Manar, Tunis, Tunisia, 1995. [Google Scholar]
- Zaier, A. Evolution tecto-sédimentaire du bassin phosphate du Centre-Ouest de la Tunisie, minéralogie, pétrographie, géochimie et genèse des phosphorites. Ph.D. Thesis, University of Tunis El Manar, Tunis, Tunisia, 1999. [Google Scholar]
- Bel Haj Khalifa, M. Etude géostatistique du gisement de phosphates multicouches de Nefta-Tozeur (Tunisie). Ph.D. Thesis, University of Tunis El Manar, Tunis, Tunisia, 1996. [Google Scholar]
- Ben Hassen, A. Données nouvelles sur la matière organique associée aux séries du bassin phosphaté du sud-tunisien (Gisement de Ras-Draâ) et sur la phosphatogenèse. Ph.D. Thesis, Université d’ Orléans, Orléans, France, 2007. [Google Scholar]
- Ben Hassen, A.; Trichet, J.; Disnar, J.R.; Belayouni, H. Données nouvelles sur le contenu organique des dépôts phosphatés du gisement de Ras-Draâ (Tunisie). C. R. Geosci. 2009, 341, 319–326. [Google Scholar] [CrossRef]
- Ben Hassen, A.; Trichet, J.; Disnar, J.R.; Belayouni, H. Pétrographie et géochimie comparées des pellets phosphatés et de leur gangue dans le gisement phosphaté de Ras-Draâ (Tunisie). Implications sur la genèse des pellets phosphatés. Swiss J. Geosci. 2010, 103, 457–473. [Google Scholar] [CrossRef]
- Gallala, W.; Saïdi, M.; El Haji, S.; Zayani, K.; Gaied, M.E.; Montacer, M. Characterization and Valorization of Tozeur-Nefta Phosphate Ore Deposit (Southwestern Tunisia). Procedia Eng. 2016, 138, 8–18. [Google Scholar] [CrossRef]
- El-Jallad, I.S.; Abouzeid, A.Z.; El-Sinbawy, H.A. Calcination of phosphates: Reactivity of calcined phosphate. Powder Technol. 1980, 26, 187–197. [Google Scholar] [CrossRef]
- Zafar, Z.I.; Anwar, M.M.; Pritchard, D.W. Optimization of thermal beneficiation of a low-grade dolomitic phosphate rock. Int. J. Miner. Process. 1995, 43, 123–131. [Google Scholar] [CrossRef]
- Mohammad Khani, M.; Noaparast, M.; Shafaei, S.Z.; Amini, A.; Amini, E.; Abdollahi, H. Double reverse flotation of a very low-grade sedimentary phosphate rock, rich in carbonate and silicate. Int. J. Miner. Process. 2011, 100, 157–165. [Google Scholar] [CrossRef]
- Good, P.C. Beneficiation of Unweathered Indian Calcareous Phosphate Rock by Calcination and Hydration; US Bureau of Mines: Washington, DC, USA, 1976. [Google Scholar]
- Hollick, C.T.; Wright, R. Recent trends in phosphate mineral beneficiation. Trans. Inst. Min. Metall. Sect. A 1986, 95, 150–154. [Google Scholar]
- Rao, T.C.; Rao, L.S.; Rao, G.M. Beneficiation of Indian Low-Grade Phosphate Deposits-Problems and Prospects. Trans. Indian Inst. Met. 1992, 45, 195–205. [Google Scholar]
- Van Straaten, P. Rocks for Crops: Agrominerals of Sub-Saharan Africa; ICRAF: Nairobi, Kenya, 2002; ISBN 0-88955-512-5. [Google Scholar]
- Woodrooffe, H.M. Phosphate in the Kola Peninsula, USSR. Miner. Eng. 1972, 24, 54–56. [Google Scholar]
- Blazy, P.; Jdid, E.A. Calcination du phosphate sédimentaire à gangue carbonatée d’Akashat (Irak) en four rotatif et en four éclair (Flash). C. R. Acad. Sci 1997, 325, 761–764. [Google Scholar] [CrossRef]
- Bangar, K.C.; Yadav, K.S.; Mishra, M.M. Transformation of rock phosphate during composting and the effect of humic acid. Plant Soil 1985, 25, 259–266. [Google Scholar] [CrossRef]
- Sadeddin, W.; Abu-Eishah, S.I. Minimization of free calcium carbonate in hard and medium-hard phosphate rocks using dilute acetic acid solution. Int. J. Miner. Process. 1990, 30, 113–125. [Google Scholar] [CrossRef]
- Abu-Eishah, S.I.; Muthaker, M.; Touqan, N. A new technique for the beneficiation of low-grade carbonate-rich phosphate rocks by digestion with dilute acetic acid solutions: Pilot plant testing results. Miner. Eng. 1991, 4, 573–586. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; El Kammar, A.M.; Guda, A. Characterization and separation of pyrite from Abu Tartur black shale. Int. J. Min. Sci. Technol. 2015, 25, 565–571. [Google Scholar] [CrossRef]
- Mâamri, A.J.; Abbassi, L.; Batis, H.N. Characterization of the Oum El Khacheb phosphorites (South Tunisia) and enrichment of big rejections by grinding. Int. J. Min. Sci. Technol. 2016, 26, 833–842. [Google Scholar] [CrossRef]
- Zidi, R.; Babbou-Abdelmalek, C.; Chaabani, F.; Abbassi, L. Enrichment of low-grade phosphate coarse particles by froth-flotation process, at the Kef-Eddour washing plant, Tunisia. Arab. J. Geosci. 2016, 9, 462. [Google Scholar] [CrossRef]
- Lawver, J.E.; Weigel, R.L.; Snow, R.E.; Hwang, C.L. Phosphate reserves enhanced by beneficiation. Min. Congr. 1982, 68, 27–31. [Google Scholar]
- Elgillani, D.A.; Abouzeid, A.Z. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores-an overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- El-Midany, A.A. Separating dolomite from phosphate rock by reactive flotation: Fundamentals and application. Ph.D. Thesis, University of Florida, Gainesville, FL, USA, 2004. [Google Scholar]
- Henchiri, A.; Cecile, J.L.; Baudet, G.; Barbery, G.; Bloise, R.U.S. Process of the treatment of phosphate ores with silico-carbonate gangue. U.S. Patent 4,324,653, 13 April 1982. [Google Scholar]
- Houot, R. Beneficiation of phosphatic ores through flotation: Review of industrial applications and potential developments. Int. J. Miner. Process. 1982, 9, 353–384. [Google Scholar] [CrossRef]
- Abdel-Khalek, N.A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonates gangues. Miner. Eng. 2000, 13, 789–793. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.; Negm, A.T.; Elgillani, D.A. Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- Lisiansky, L.; Baker, M.; Larmour-Ship, K.; Elyash, O. A Tailor Made Approach for the Beneficiation of Phosphate Rock. In Beneficiation of Phosphates; Zhang, P., Miller, J.B., Wingate, E., Filho, L.L., Eds.; SME CO.: Englewood, CO, USA, 2016; pp. 55–61. ISBN 978-0-87335-427-1. [Google Scholar]
- Liu, X.; Zhang, Y.; Liu, T.; Cai, Z.; Sun, K. Characterization and Separation Studies of a Fine Sedimentary Phosphate Ore Slime. Minerals 2017, 7, 94. [Google Scholar] [CrossRef]
- Somasundaran, P.; Lin, I.J. Effect of the Nature of Environment on Comminution Processes. Ind. Eng. Chem. Process. Des. Dev. 1972, 11, 321–331. [Google Scholar] [CrossRef]
- Anglaret, P.; Filippi, J.; Kazmierczak, S. Technologie Génie Chimique, Tome I; CRDM: Amiens, France, 1998; pp. 17–384. ISBN 2-86615-223-9. [Google Scholar]
- Houot, R.; Joussemet, R.; Tracez, J.; Brouard, R. Selective flotation of phosphatic ores having a siliceous and/or a carbonated gangue. Int. J. Miner. Process. 1985, 14, 245–264. [Google Scholar] [CrossRef]
- Henchiri, A. A contribution to carbonates–phosphate separation by flotation technique. In Beneficiation of Phosphate: Theory and Practice; El-Shall, H., Moudgil, R., Wiegel, R., Eds.; SME: Littleton, CO, USA, 1993; pp. 225–243. [Google Scholar]
- Baudet, G.; Save, M. Phosphoric esters as carbonate collectors in the flotation of sedimentary phosphate ores. In Beneficiation of Phosphates: Advances in Research and Practice; Zhang, P., El-Shall, H., Wiegel, R., Eds.; SME: Littleton, CO, USA, 1999; pp. 163–185. [Google Scholar]
- Blazy, P. La valorisation des Minerais: Manuels de Minéralurgie; Presses Universitaires de France: Vendôme, France, 1970; pp. 5–415. [Google Scholar]
- Sengul, H.; Ozer, A.K.; Gulaboglu, M.S. Beneficiation of Mardin-Mazıdaği (Turkey) calcareous phosphate rock using dilute acetic acid solutions. Chem. Eng. J. 2006, 122, 135–140. [Google Scholar] [CrossRef]
- Becker, P. Phosphates and Phosphoric Acid: Raw Materials, Technology, and Economics of the Wet Process; Marcel Dekker: New York, NY, USA, 1989; pp. 1–579. ISBN 0-8247-1712-0. [Google Scholar]
- Khelifi, L. Contribution à l’étude géochimique des phosphates du bassin de Gafsa-Metlaoui. Exemple du gisement d’Oum Lakhcheb. Master’s Thesis, University of Tunis El Manar, Tunis, Tunisia, 2012. [Google Scholar]
- Galfati, I.; Bilal, E.; Abdallah, H.; Beji-Sassi, A. Geochemistry of solid effluents and phosphate ore washed from Metlaoui-Gafsa basin, Tunisia. Rom. J. Miner. Depos. 2014, 87, 83–86. [Google Scholar]
- Pease, J.D.; Curry, D.C.; Young, M.F. Designing flotation circuits for high fines recovery. Miner. Eng. 2016, 19, 831–840. [Google Scholar] [CrossRef]
- Al-Fariss, T.F.; El-Aleem, F.A.A.; El-Nagdy, K.A. Beneficiation of Saudi phosphate ores by column flotation technology. J. King Saud Univ. Sci 2013, 25, 113–117. [Google Scholar] [CrossRef]
- Clerc, L. Broyage ultrafin de carbonates naturels: Paramétrisation, modélisation et conséquences physico-chimiques. Ph.D. Thesis, Ecole Nationale Supérieure des Mines, Saint-Etienne, France, 1983. [Google Scholar]
- Bafghi, M.S.; Emami, A.H.; Zakeri, A.; Khak, J.V. Development and verification of a mathematical model for variations of the specific surface area of mineral powders during intensive milling. Powder Technol. 2010, 197, 87–90. [Google Scholar] [CrossRef]
- Jian, T.; Longhua, X.; Wei, D.; Hao, J.; Zhiyong, G.; Yuehua, H. Adsorption mechanism of new mixed anionic-cationic collectors in a spodumene-Feldspar flotation system. Chem. Eng. Sci. 2017, 164, 99–107. [Google Scholar] [CrossRef]
- Houqin, W.; Jia, T.; Longhua, X.; Shuai, F.; Zhenye, Z.; Ruan, C. Flotation and adsorption of new mixed anionic/cationic collector in the spodumene-feldspar system. Miner. Eng. 2018, 127, 42–47. [Google Scholar] [CrossRef]
- Amankonah, J.O.; Somasundaran, P. Effects of dissolved mineral species on the electrokinetic behavior of calcite and apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Hsieh, S.S.; Lehr, J.R. Beneficiation of dolomitic Idaho phosphate rock by the TVA diphosphoric acid depressant process. Min. Metall. Explor. 1985, 12, 10–13. [Google Scholar] [CrossRef]

| Grinding Ball | Diameter (mm) | Ball Ratio (%) | Weight (kg) |
|---|---|---|---|
| B1 | 40 | 7.06 | 0.29 |
| B2 | 30 | 55.5 | 0.12 |
| B3 | 25 | 8.91 | 0.07 |
| B4 | 20 | 28.5 | 0.04 |
| Element | Concentration |
|---|---|
| P2O5 (%) | 12.0 |
| CaO (%) | 40.7 |
| MgO (%) | 4.95 |
| SiO2 (%) | 20.5 |
| SO3 (%) | 1.27 |
| Fe2O3 (%) | 0.65 |
| Al2O3 (%) | 1.75 |
| Na2O (%) | 0.39 |
| K2O (%) | 0.45 |
| Corg (%) | 0.28 |
| F (%) | 1.27 |
| CO2 | 20.2 |
| LOI | 20.7 |
| Cd (mg/kg) | 65 |
| Cu (mg/kg) | 31 |
| Zn (mg/kg) | 215 |
| Mn (mg/kg) | 296 |
| Ni (mg/kg) | 80 |
| Fraction (µm) | Weight (%) | P2O5 (%) | CaO (%) | CaO/P2O5 | MgO (%) | SiO2 (%) | SO3 (%) | Fe2O3 (%) | Al2O3 (%) | Na2O (%) | K2O (%) | Corg (%) | CO2 (%) | F (%) | LOI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10,000 | 4.64 | 4.25 | 47.3 | 11.1 | 3.93 | 17.0 | 0.57 | 0.43 | 1.10 | 0.18 | 0.10 | 0.10 | 23.4 | 1.74 | 25.0 |
| 8000 | 2.11 | 4.63 | 45.1 | 9.74 | 4.55 | 15.6 | 0.54 | 0.49 | 1.24 | 0.17 | 0.11 | 0.12 | 25.8 | 1.74 | 27.5 |
| 5000 | 2.50 | 5.16 | 44.2 | 8.56 | 3.99 | 18.5 | 0.61 | 0.41 | 1.20 | 0.19 | 0.11 | 0.34 | 23.3 | 2.24 | 25.4 |
| 4000 | 0.78 | 5.53 | 46.2 | 8.36 | 3.67 | 16.6 | 0.67 | 0.47 | 1.20 | 0.19 | 0.10 | 0.04 | 23.7 | 2.04 | 25.2 |
| 3150 | 0.89 | 6.89 | 44.2 | 6.42 | 3.34 | 18.0 | 0.75 | 0.36 | 1.14 | 0.21 | 0.10 | 0.24 | 22.7 | 2.60 | 24.59 |
| 2000 | 1.25 | 7.95 | 45.4 | 5.71 | 3.17 | 18.2 | 0.86 | 0.38 | 1.10 | 0.23 | 0.10 | 0.21 | 20.2 | 1.20 | 22.1 |
| 1000 | 1.92 | 8.83 | 45.7 | 5.17 | 3.15 | 17.9 | 1.23 | 0.28 | 1.09 | 0.23 | 0.10 | 0.24 | 19.1 | 2.40 | 21.0 |
| 800 | 1.06 | 8.42 | 46.4 | 5.51 | 2.49 | 17.7 | 1.09 | 0.29 | 1.11 | 0.22 | 0.10 | 0.10 | 20.2 | 3.36 | 21.9 |
| 630 | 1.25 | 8.27 | 46.4 | 5.61 | 1.35 | 17.9 | 1.10 | 0.34 | 1.10 | 0.24 | 0.12 | 0.38 | 20.9 | 3.44 | 23. 9 |
| 500 | 2.02 | 9.56 | 46.8 | 4.89 | 3.13 | 17.1 | 1.14 | 0.35 | 1.09 | 0.39 | 0.11 | 0.37 | 17.4 | 3.12 | 19.5 |
| 315 | 6.05 | 16.8 | 49.6 | 2.94 | 2.21 | 16.3 | 1.67 | 0.41 | 1.03 | 0.57 | 0.10 | 0.42 | 9.09 | 2.72 | 11.3 |
| 200 | 9.99 | 21.5 | 50.7 | 2.36 | 1.53 | 11.3 | 2.03 | 0.29 | 0.95 | 0.73 | 0.09 | 0.49 | 8.56 | 2.44 | 10.9 |
| 160 | 2.31 | 18.9 | 49.8 | 2.64 | 2.06 | 16.5 | 1.8 | 0.19 | 0.94 | 0.59 | 0.09 | 0.16 | 7.38 | 2.00 | 9.16 |
| 125 | 3.94 | 11.3 | 45.5 | 4.04 | 3.66 | 13.8 | 1.13 | 0.26 | 0.91 | 0.43 | 0.11 | 0.24 | 20.9 | 3.32 | 22.8 |
| 100 | 3.75 | 6.86 | 39.9 | 5.81 | 5.25 | 16.3 | 0.79 | 0.24 | 0.91 | 0.33 | 0.11 | 0.12 | 26.6 | 2.52 | 28.3 |
| 90 | 1.92 | 6.26 | 36.6 | 5.85 | 4.56 | 19.6 | 0.73 | 0.19 | 1.10 | 0.32 | 0.12 | 0.19 | 28.3 | 0.73 | 30.1 |
| 71 | 7.11 | 6.11 | 35.8 | 5.86 | 6.82 | 21.3 | 0.66 | 0.15 | 0.96 | 0.22 | 0.13 | 0.30 | 25.4 | 0.66 | 27.4 |
| 50 | 5.77 | 5.26 | 37.8 | 7.19 | 7.21 | 16.9 | 0.63 | 0.20 | 0.82 | 0.25 | 0.08 | 0.48 | 28.5 | 0.63 | 30.9 |
| 40 | 1.92 | 5.43 | 38.5 | 7.09 | 7.19 | 15.8 | 0.68 | 0.24 | 0.86 | 0.20 | 0.08 | 0.12 | 29.2 | 0.68 | 30.9 |
| −40 | 38.8 | 13.2 | 35.5 | 2.68 | 5.62 | 22.5 | 1.33 | 0.85 | 3.35 | 0.42 | 1.07 | 0.28 | 15.1 | 1.33 | 17.1 |
| Fraction (µm) | Cd | Cu | Zn | Mn | Ni |
|---|---|---|---|---|---|
| 10,000 | 95 | 31 | 148 | 574 | 77 |
| 8000 | 129 | 27 | 157 | 589 | 134 |
| 5000 | 102 | 26 | 138 | 486 | 97 |
| 4000 | 108 | 30 | 151 | 492 | 127 |
| 3150 | 82 | 24 | 146 | 544 | 106 |
| 2000 | 93 | 22 | 151 | 530 | 143 |
| 1000 | 98 | 19 | 113 | 522 | 88 |
| 800 | 104 | 22 | 112 | 575 | 144 |
| 630 | 110 | 21 | 112 | 613 | 138 |
| 500 | 103 | 27 | 120 | 621 | 142 |
| 315 | 106 | 27 | 110 | 439 | 133 |
| 200 | 80 | 22 | 85 | 340 | 93 |
| 160 | 67 | 27 | 84 | 378 | 21 |
| 125 | 76 | 23 | 95 | 418 | 58 |
| 100 | 57 | 19 | 91 | 296 | 116 |
| 90 | 47 | 20 | 84 | 253 | 66 |
| 71 | 40 | 15 | 80 | 220 | 70 |
| 50 | 40 | 15 | 79 | 211 | 68 |
| 40 | 45 | 17 | 83 | 236 | 63 |
| −40 | 49 | 49 | 345 | 173 | 87 |
| Fraction (µm) | Weight (%) | P2O5 (%) | CaO (%) | CaO/P2O5 | MgO (%) | SiO2 (%) | Cd (mg/kg) | |
|---|---|---|---|---|---|---|---|---|
| Grade | Recovery | |||||||
| +315 | 23.1 | 10.1 | 19.7 | 46.8 | 4.66 | 3.19 | 18.8 | 97 |
| −315+71 | 30.0 | 13.5 | 34.3 | 43.8 | 3.26 | 3.80 | 16.5 | 55 |
| −71 | 46.9 | 11.5 | 46.0 | 35.9 | 3.11 | 5.95 | 22.9 | 56 |
| Reconstituted | 100 | 11.8 | 100 | 40.78 | 3.46 | 4.67 | 20.0 | 65.2 |
| Time (min) | Fraction (µm) | Weight (%) | P2O5 (%) | |
|---|---|---|---|---|
| Grade | Recovery | |||
| 5 | −315+71 | 88.8 | 13.7 | 90.6 |
| 10 | −315+71 | 84.2 | 15.5 | 97.2 |
| 15 | −315+71 | 86.6 | 14.4 | 92.4 |
| Element | P2O5 (%) | CaO (%) | CaO/P2O5 | MgO (%) | SiO2 (%) | Cd (mg/kg) |
|---|---|---|---|---|---|---|
| Concentration | 30.9 | 49.5 | 1.60 | 1.09 | 2.22 | 27 |
| Parameter to be Varied | Test | Time (min) | Cr | Sc (%) | Rotation Speed (Rpm) | Weight (%) | P2O5 (%) | |
|---|---|---|---|---|---|---|---|---|
| Grade | Recovery | |||||||
| Grinding time (Gt) | 1 | 2 | 5/1 | 40 | 50 | 28.1 | 14.2 | 39.7 |
| 2 | 3 | 5/1 | 40 | 50 | 32.9 | 14.4 | 47.0 | |
| 3 | 4 | 5/1 | 40 | 50 | 40 | 14.5 | 57.6 | |
| Charge ratio (Cr) | 1 | 4 | 3/1 | 40 | 50 | 29.8 | 14.4 | 42.6 |
| 2 | 4 | 4/1 | 40 | 50 | 30 | 14.4 | 43.1 | |
| 3 | 4 | 5/1 | 40 | 50 | 40.2 | 14.5 | 58.1 | |
| Solid concentration (Sc) | 1 | 4 | 5/1 | 30 | 50 | 28.6 | 14.4 | 40.9 |
| 2 | 4 | 5/1 | 40 | 50 | 40.2 | 14.5 | 58.0 | |
| 3 | 4 | 5/1 | 50 | 50 | 31.2 | 14.4 | 44.7 | |
| Experiment | Fraction (µm) | Weight (%) | P2O5 (%) | CaO (%) | |
|---|---|---|---|---|---|
| Grade | Recovery | ||||
| Grinding | >315 | 33.7 | 6.71 | 22.5 | 47.8 |
| 71–315 | 40.2 | 14.5 | 58.0 | 46.5 | |
| <71 | 26.1 | 5.45 | 14.1 | 44.8 | |
| Attrition | 71–315 | 87.2 | 15.2 | 91.4 | 46.8 |
| Product | Weight (%) | P2O5 (%) | CaO (%) | CaO/P2O5 | MgO (%) | SiO2 (%) | |
|---|---|---|---|---|---|---|---|
| Grade | Recovery | ||||||
| Flotation feed | - | 15.5 | - | 44.6 | 2.9 | 3.39 | 13.2 |
| Floating 1 (Tailings) | 21.3 | 3.23 | 4.40 | 36.6 | 11.3 | 5.96 | 26.0 |
| Floating 2 (Tailings) | 25.3 | 2.01 | 3.20 | 47.2 | 23.5 | 4.89 | 23.9 |
| Non-floating (Phosphate-concentrate) | 53.4 | 27.1 | 92.4 | 46.5 | 1.71 | 1.03 | 2.52 |
| Reconstituted | 100 | 15.7 | 100 | 44.6 | 2.80 | 3.05 | 12.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boujlel, H.; Daldoul, G.; Tlil, H.; Souissi, R.; Chebbi, N.; Fattah, N.; Souissi, F. The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia). Minerals 2019, 9, 2. https://doi.org/10.3390/min9010002
Boujlel H, Daldoul G, Tlil H, Souissi R, Chebbi N, Fattah N, Souissi F. The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia). Minerals. 2019; 9(1):2. https://doi.org/10.3390/min9010002
Chicago/Turabian StyleBoujlel, Haïfa, Ghassen Daldoul, Haïfa Tlil, Radhia Souissi, Noureddine Chebbi, Nabil Fattah, and Fouad Souissi. 2019. "The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia)" Minerals 9, no. 1: 2. https://doi.org/10.3390/min9010002
APA StyleBoujlel, H., Daldoul, G., Tlil, H., Souissi, R., Chebbi, N., Fattah, N., & Souissi, F. (2019). The Beneficiation Processes of Low-Grade Sedimentary Phosphates of Tozeur-Nefta Deposit (Gafsa-Metlaoui Basin: South of Tunisia). Minerals, 9(1), 2. https://doi.org/10.3390/min9010002





