Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules
Abstract
:1. Introduction
2. Materials and Methods
3. Results
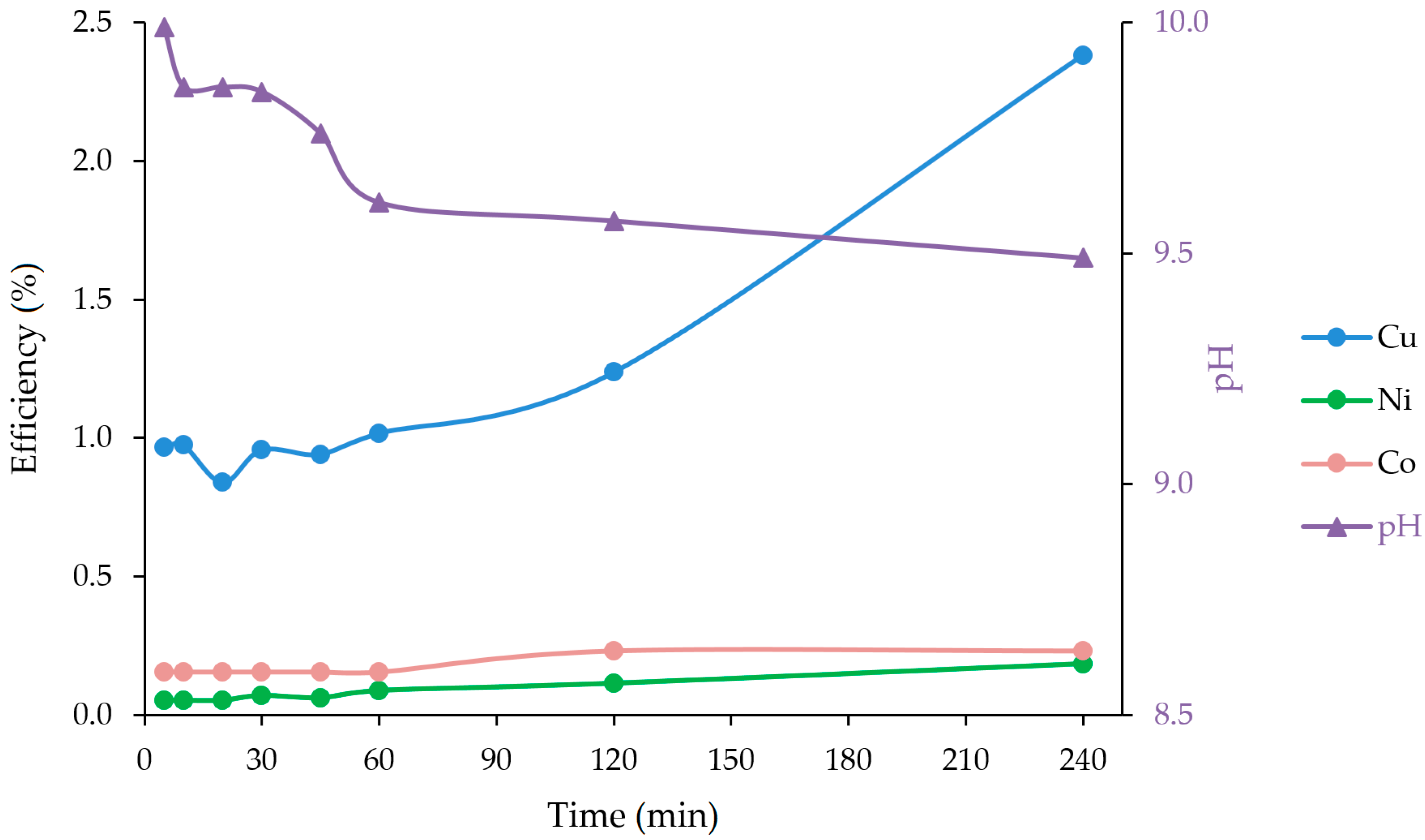
3.1. Ammoniacal Leaching
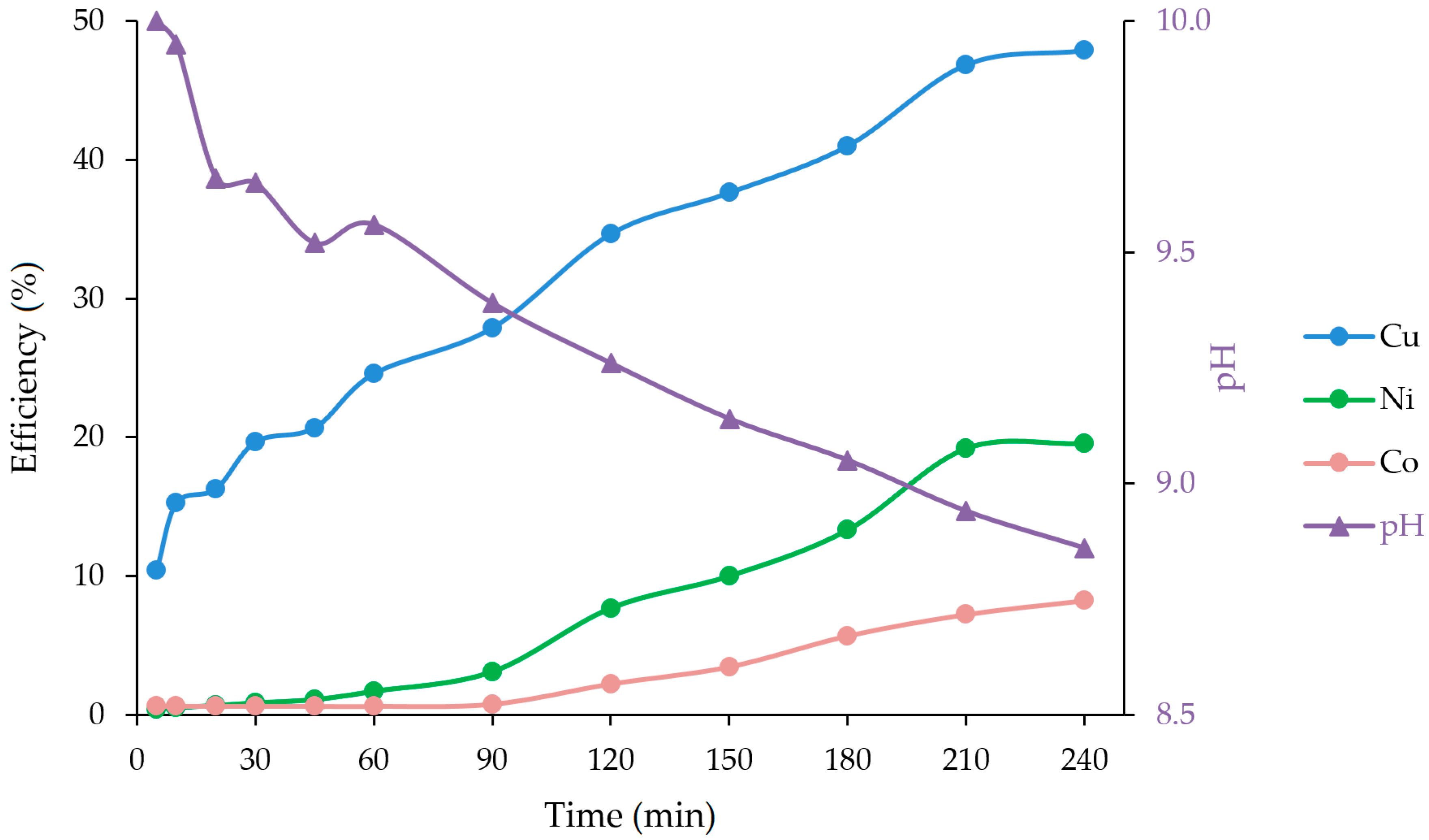
3.2. Ammoniacal Leaching with Ultrasound
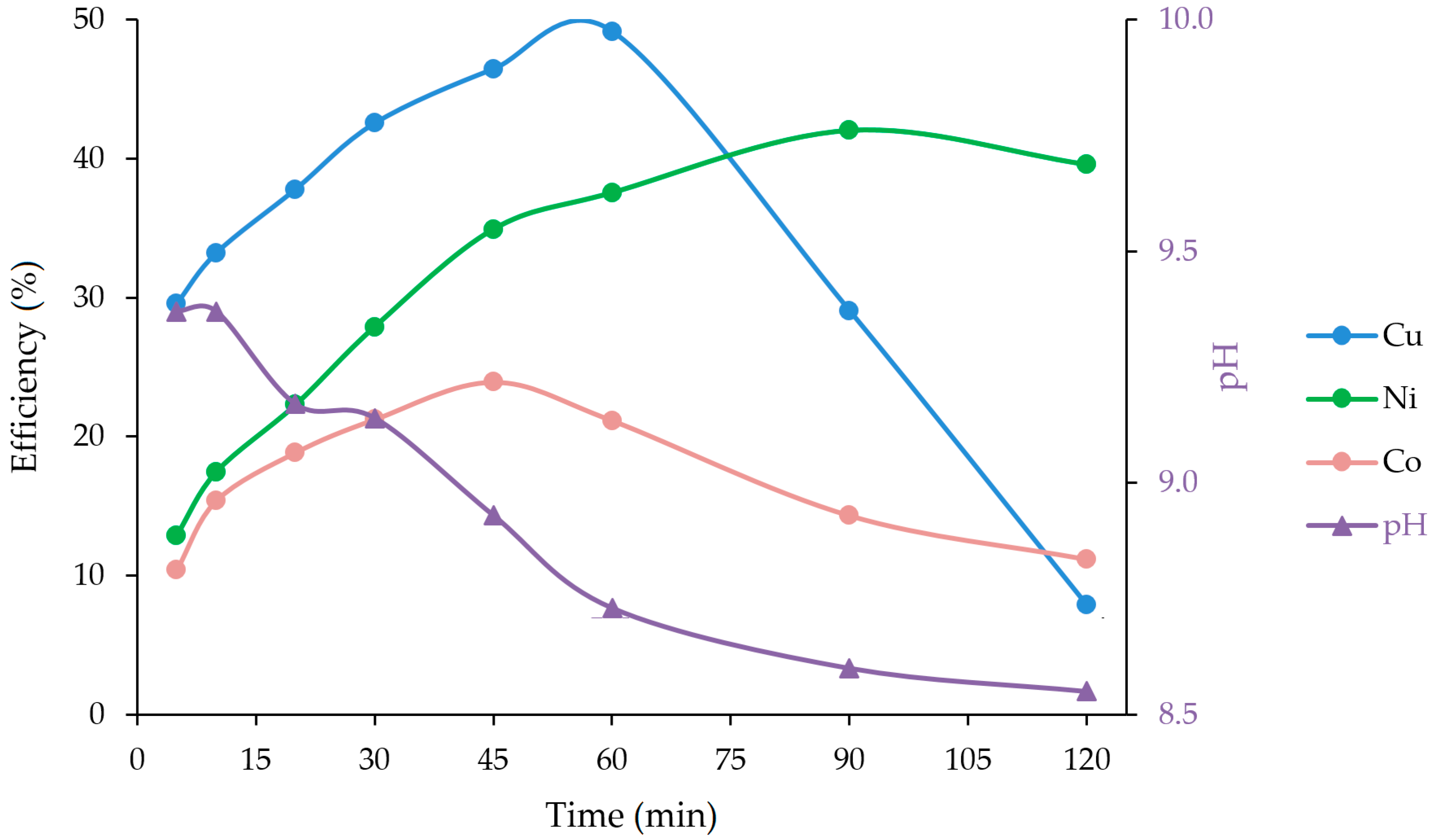
3.3. Ammoniacal Leaching with Microwave
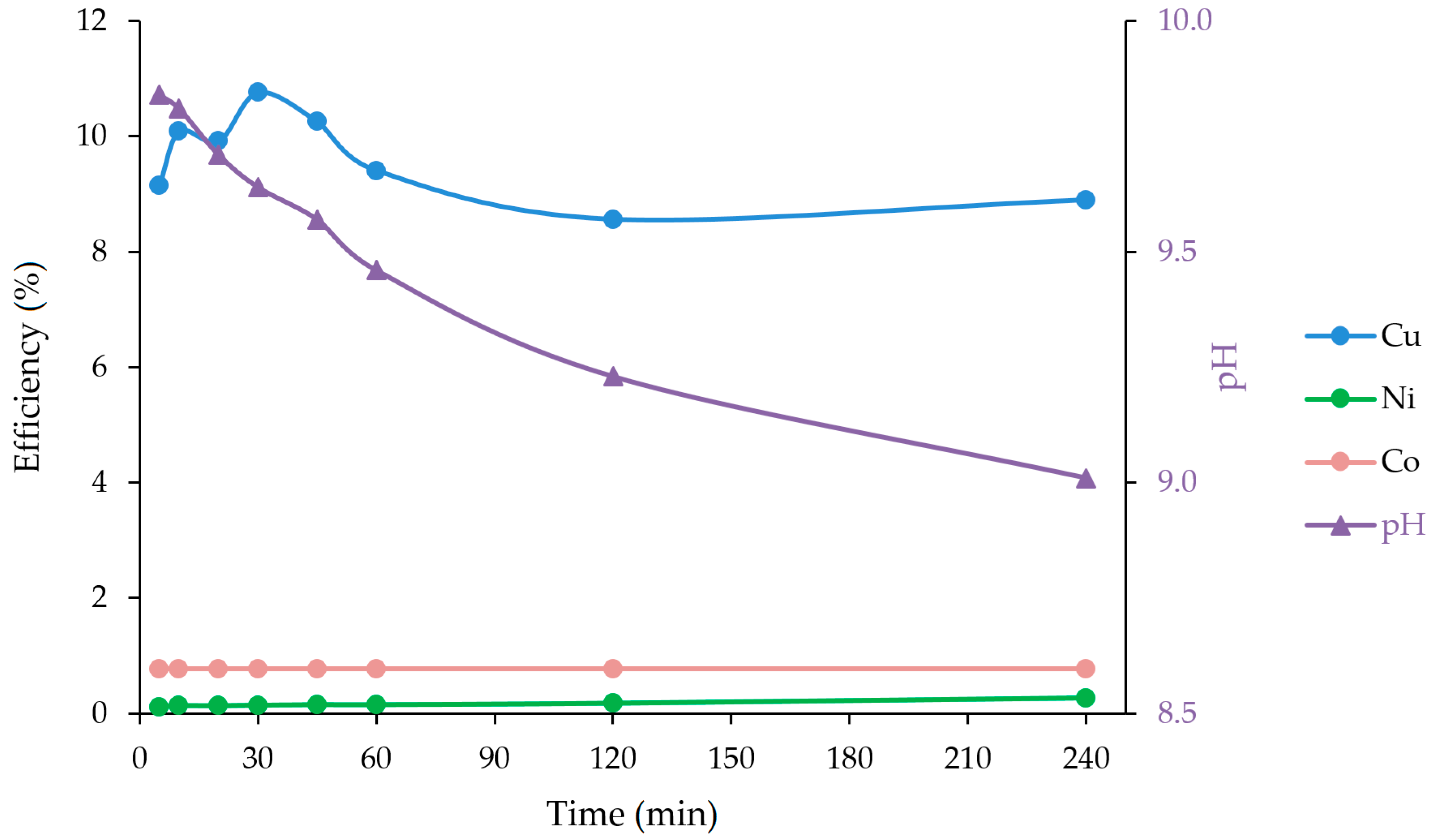
3.4. Ammoniacal Leaching with Ultrasound and Microwave
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Von Stackelberg, U.; Beiersdorf, H. The formation of manganese nodules between the clarion and clipperton fracture zones southeast of Hawaii. Mar. Geol. 1991, 98, 411–423. [Google Scholar] [CrossRef]
- Kužvart, M.; Pešek, J.; René, M. Geologie Ložisek Nerostných Surovin; Státní pedagogické nakladatelství Praha: Praha, Czech Republic, 1986. (In Czech) [Google Scholar]
- Pařízek, A. Metodika průzkumu hlubokomořských surovinových zdrojů a účast čr v těchto aktivitách (aktuální stav a perspektivy do budoucnosti). Sborník vědeckých prací Vysoké školy báňské-Technické univerzity Ostrava 2004, L, 23–40. (In Czech) [Google Scholar]
- Kukal, Z. Zázračné Kuličky Z Mořského Dna. 2006. Available online: https://21stoleti.cz/2006/09/23/zazracne-kulicky-z-morskeho-dna/ (accessed on 17 July 2018).
- Agarwal, J.C.; Beecher, N.; Davies, D.S.; Hubred, G.L.; Kakaria, V.K.; Kust, R.N. Processing of ocean nodules: A technical and economic review. JOM 1976, 28, 24–31. [Google Scholar] [CrossRef]
- Brooks, P.T.; Martin, D.A. Processing Manganiferous Sea Nodules; U.S. Department of Interior, Bureau of Mines: Washington, DC, USA, 1971; p. 19.
- Kane, W.S.; Mccutchen, H.L.; Cardwell, P.H. Recovery of Metal Values from Ocean Floor Nodule Ores by Halidation in Molten Salt Bath. U.S. Patent 3,894,924, 27 November 1975. [Google Scholar]
- Kane, W.S.; Cardwell, P.H. Reduction Method for Separating Metal Values from Ocean Floor Nodule Ore. U.S. Patent 3,869,360, 4 March 1975. [Google Scholar]
- Kanungo, S.B.; Jena, P.K. Reduction leaching of manganese nodules of Indian Ocean origin in dilute hydrochloric acid. Hydrometallurgy 1988, 21, 41–58. [Google Scholar] [CrossRef]
- Han, K.N.; Fuerstenau, D.W. Acid leaching of ocean manganese nodules at elevated temperatures. Int. J. Miner. Process. 1975, 2, 163–171. [Google Scholar] [CrossRef]
- Jana, R.K.; Singh, D.D.N.; Roy, S.K. Alcohol-modified hydrochloric acid leaching of sea nodules. Hydrometallurgy 1995, 38, 289–298. [Google Scholar] [CrossRef]
- Szabo, L.J. Recovery of Metal Values from Manganese Deep Sea Nodules Using Ammoniacal Cuprous Leach Solutions. U.S. Patent 3,983,017, 28 September 1976. [Google Scholar]
- Das, R.P.; Anand, S.; Das, S.C.; Jena, P.K. Leaching of manganese nodules in ammoniacal medium using glucose as reductant. Hydrometallurgy 1986, 16, 335–344. [Google Scholar] [CrossRef]
- Acharya, S. Reductive ammonia leaching of manganese nodules by thiosulfate. MTB 1991, 22, 259–261. [Google Scholar] [CrossRef]
- Gomez-Ariza, J.L.; Morales, E.; Beltran, R.; Giraldez, I.; Ruiz-Benitez, M. Ultrasonic treatment of molluscan tissue for organotin speciation. Analyst 1995, 120, 1171–1174. [Google Scholar] [CrossRef]
- Amoedo, L.; Luis Capelo, J.; Lavilla, I.; Bendicho, C. Ultrasound-assisted extraction of lead from solid samples: A new perspective on the slurry-based sample preparation methods for electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 1999, 14, 1221–1226. [Google Scholar] [CrossRef]
- Wibetoe, G.; Takuwa, D.T.; Lund, W.; Sawula, G. Coulter particle analysis used for studying the effect of sample treatment in slurry sampling electrothermal atomic absorption spectrometry. Fresenius J. Anal. Chem. 1999, 363, 46–54. [Google Scholar] [CrossRef]
- Wu, C.H.; Kuo, C.Y.; Lo, S.L. Recovery of heavy metals from industrial sludge using various acid extraction approaches. Water Sci. Technol. 2009, 59, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Vinodgopal, K.; Peller, J.; Makogon, O.; Kamat, P.V. Ultrasonic mineralization of a reactive textile azo dye, remazol black B. Water Res. 1998, 32, 3646–3650. [Google Scholar] [CrossRef]
- Pack, B.W.; Ray, S.J.; Potyrailo, R.A.; Hiettje, G.M. Evaluation of ultrasonic nebulization for the analysis of transient samples: A theoretical model and practical considerations. Appl. Spectrosc. 1998, 52, 1515–1521. [Google Scholar] [CrossRef]
- Luigi Buldini, P.; Mevoli, A.; Lal Sharma, J. LA-ICO-MS, IC and DPASV-DPCSV determination of metallic impurities in solar-grade silicon. Talanta 1998, 47, 203–212. [Google Scholar] [CrossRef]
- Rauret, G.; Rubio, R.; Lopez-sanchez, J.F.; Casassas, E. Specific procedure for metal solid speciation in heavily polluted river sediments. Int. J. Environ. Anal. Chem. 1989, 35, 89–100. [Google Scholar] [CrossRef]
- De Castro, M.D.L.; Priego-Capote, F. Ultrasound-assisted crystallization (sonocrystallization). Ultrason. Sonochem. 2007, 14, 717–724. [Google Scholar] [CrossRef]
- Pinto, I.S.S.; Soares, H.M.V.M. Selective leaching of molybdenum from spent hydrodesulphurisation catalysts using ultrasound and microwave methods. Hydrometallurgy 2012, 129–130, 19–25. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Kingman, S.W. Microwave-assisted leaching—A review. Hydrometallurgy 2004, 73, 189–203. [Google Scholar] [CrossRef]
- Xia, D.K.; Picklesi, C.A. Microwave caustic leaching of electric arc furnace dust. Miner. Eng. 2000, 13, 79–94. [Google Scholar] [CrossRef]
- Luque-García, J.L.; Luque de Castro, M.D. Ultrasound: A powerful tool for leaching. TrAC Trends Anal. Chem. 2003, 22, 41–47. [Google Scholar] [CrossRef]
- Filgueiras, A.V.; Capelo, J.L.; Lavilla, I.; Bendicho, C. Comparison of ultrasound-assisted extraction and microwave-assisted digestion for determination of magnesium, manganese and zinc in plant samples by flame atomic absorption spectrometry. Talanta 2000, 53, 433–441. [Google Scholar] [CrossRef]
- Konishi, H. Selective Separation and Recovery of Copper from Iron and Copper Mixed Waste by Ammonia Solution. Available online: http://www.jfe-21st-cf.or.jp/jpn/hokoku_pdf_2009/08.pdf (accessed on 17 July 2018).




| Element | Mn | Fe | Cu | Ni | Co | Zn |
| wt % | 30.57 | 4.41 | 1.18 | 1.14 | 0.13 | 0.14 |
| Element | Al | Si | Mg | Ca | Na | Ti |
| wt % | 2.16 | 3.53 | 1.87 | 1.84 | 1.64 | 0.35 |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 11.4 | 0.6 | 0.2 | 0.97 | 0.05 | 0.15 | 9.99 |
| 10 | 11.5 | 0.6 | 0.2 | 0.97 | 0.05 | 0.15 | 9.86 |
| 20 | 9.9 | 0.6 | 0.2 | 0.84 | 0.05 | 0.15 | 9.86 |
| 30 | 11.3 | 0.8 | 0.2 | 0.96 | 0.07 | 0.15 | 9.85 |
| 45 | 11.1 | 0.7 | 0.2 | 0.94 | 0.06 | 0.15 | 9.76 |
| 60 | 12.0 | 1.0 | 0.2 | 1.02 | 0.09 | 0.15 | 9.61 |
| 120 | 14.6 | 1.3 | 0.3 | 1.24 | 0.11 | 0.23 | 9.57 |
| 240 | 28.1 | 2.1 | 0.3 | 2.38 | 0.18 | 0.23 | 9.49 |
| Washing water | 4.7 | 0.9 | 0.3 | 0.40 | 0.08 | 0.23 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 123 | 4.6 | 0.8 | 10.4 | 0.40 | 0.62 | 10.00 |
| 10 | 180 | 5.5 | 0.8 | 15.3 | 0.48 | 0.62 | 9.95 |
| 20 | 192 | 7.5 | 0.8 | 16.3 | 0.66 | 0.62 | 9.66 |
| 30 | 232 | 9.5 | 0.8 | 19.7 | 0.83 | 0.62 | 9.65 |
| 45 | 244 | 12.3 | 0.8 | 20.7 | 1.08 | 0.62 | 9.52 |
| 60 | 290 | 19.1 | 0.8 | 24.6 | 1.68 | 0.62 | 9.56 |
| 90 | 329 | 35.2 | 1.0 | 27.9 | 3.09 | 0.77 | 9.39 |
| 120 | 409 | 87.6 | 2.9 | 34.7 | 7.68 | 2.23 | 9.26 |
| 150 | 444 | 114 | 4.5 | 37.6 | 10.00 | 3.46 | 9.14 |
| 180 | 484 | 152 | 7.4 | 41.0 | 13.33 | 5.69 | 9.05 |
| 210 | 553 | 219 | 9.4 | 46.9 | 19.21 | 7.23 | 8.94 |
| 240 | 565 | 223 | 10.7 | 47.9 | 19.56 | 8.23 | 8.86 |
| Washing water | 55.9 | 26.6 | 0.4 | 4.7 | 2.33 | 0.31 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 349 | 147 | 13.5 | 29.6 | 12.9 | 10.38 | 9.37 |
| 10 | 392 | 199 | 20.0 | 33.2 | 17.5 | 15.38 | 9.37 |
| 20 | 446 | 254 | 24.5 | 37.8 | 22.3 | 18.85 | 9.17 |
| 30 | 502 | 318 | 27.6 | 42.5 | 27.9 | 21.23 | 9.14 |
| 45 | 548 | 398 | 31.1 | 46.4 | 34.9 | 23.92 | 8.93 |
| 60 | 580 | 428 | 27.5 | 49.2 | 37.5 | 21.15 | 8.73 |
| 90 | 343 | 479 | 18.6 | 29.1 | 42.0 | 14.31 | 8.60 |
| 120 | 93 | 451 | 14.5 | 7.9 | 39.6 | 11.15 | 8.55 |
| Washing water | 96.5 | 139 | 6.0 | 8.2 | 12.2 | 4.62 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 108 | 1.3 | 1 | 9.2 | 0.11 | 0.77 | 9.84 |
| 10 | 119 | 1.5 | 1 | 10.1 | 0.13 | 0.77 | 9.81 |
| 20 | 117 | 1.5 | 1 | 9.9 | 0.13 | 0.77 | 9.71 |
| 30 | 127 | 1.6 | 1 | 10.8 | 0.14 | 0.77 | 9.64 |
| 45 | 121 | 1.7 | 1 | 10.3 | 0.15 | 0.77 | 9.57 |
| 60 | 111 | 1.7 | 1 | 9.4 | 0.15 | 0.77 | 9.46 |
| 120 | 101 | 2.0 | 1 | 8.6 | 0.18 | 0.77 | 9.23 |
| 240 | 105 | 3.0 | 1 | 8.9 | 0.26 | 0.77 | 9.01 |
| Washing water | 23.8 | 1.1 | 0.5 | 2.0 | 0.10 | 0.38 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 197 | 3.9 | 0.4 | 16.7 | 0.34 | 0.31 | 9.70 |
| 10 | 213 | 4.7 | 0.4 | 18.1 | 0.41 | 0.31 | 9.67 |
| 20 | 255 | 7.4 | 0.4 | 21.6 | 0.65 | 0.31 | 9.55 |
| 30 | 272 | 9.2 | 0.4 | 23.1 | 0.81 | 0.31 | 9.49 |
| 45 | 312 | 16.6 | 0.5 | 26.4 | 1.46 | 0.38 | 9.31 |
| 60 | 350 | 25.5 | 0.5 | 29.7 | 2.24 | 0.38 | 9.19 |
| 90 | 441 | 53.6 | 0.6 | 37.4 | 4.70 | 0.46 | 8.96 |
| 120 | 490 | 78.3 | 0.6 | 41.5 | 6.87 | 0.46 | 8.95 |
| 150 | 523 | 118 | 0.7 | 44.3 | 10.35 | 0.54 | 8.94 |
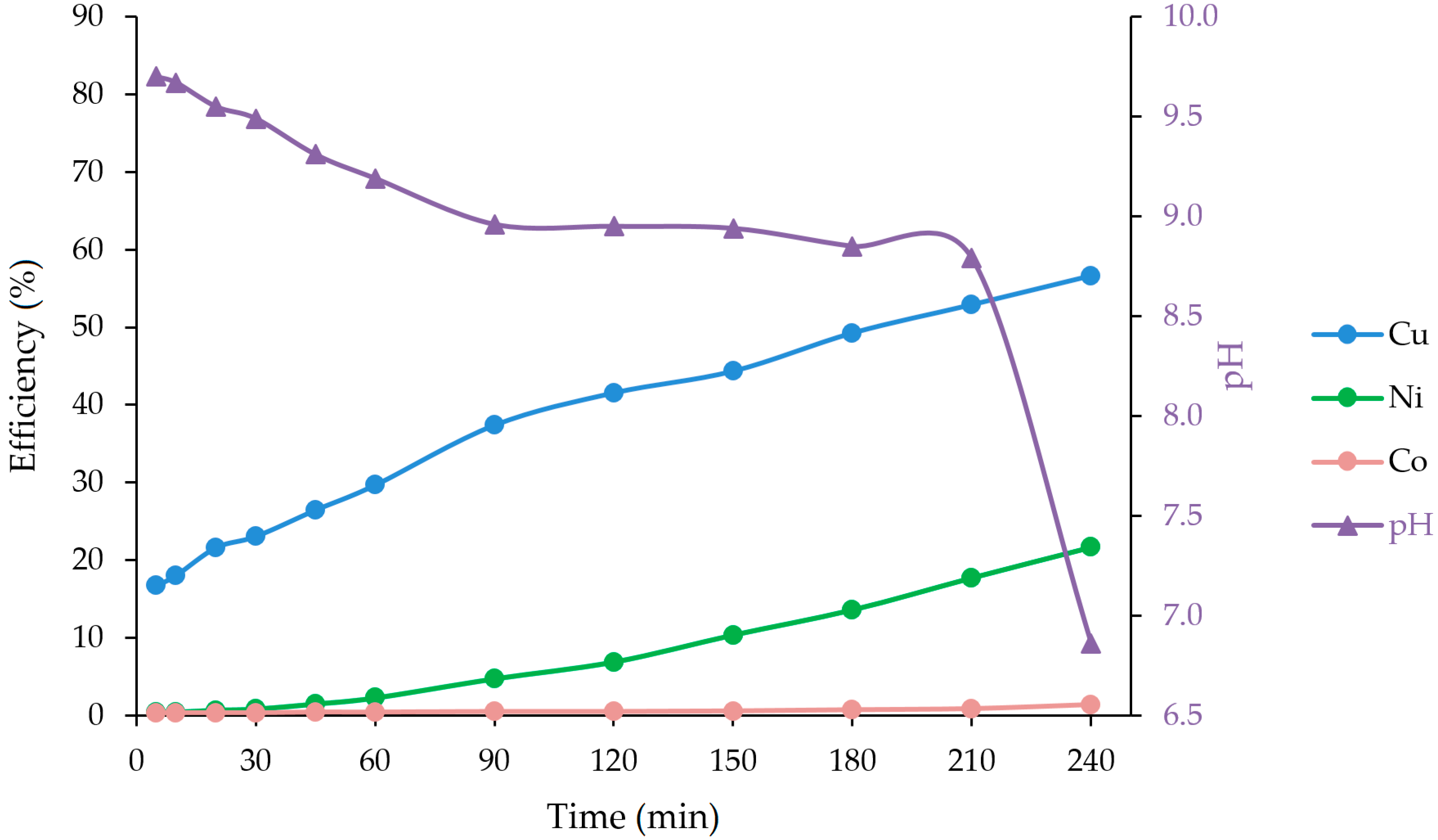
| 180 | 581 | 155 | 0.9 | 49.2 | 13.60 | 0.69 | 8.85 |
| 210 | 624 | 202 | 1.1 | 52.9 | 17.72 | 0.85 | 8.79 |
| 240 | 668 | 247 | 1.8 | 56.6 | 21.67 | 1.38 | 6.86 |
| Washing water | 33.9 | 16.6 | 0.1 | 2.9 | 1.46 | 0.08 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | pH | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 401 | 118 | 2.7 | 34.0 | 10.4 | 2.08 | 9.16 |
| 10 | 512 | 229 | 5.1 | 43.4 | 20.1 | 3.92 | 8.90 |
| 20 | 632 | 364 | 14.3 | 53.6 | 31.9 | 11.00 | 8.69 |
| 30 | 677 | 443 | 25.1 | 57.4 | 38.9 | 19.31 | 8.48 |
| 45 | 717 | 545 | 32.6 | 60.8 | 47.8 | 25.08 | 8.36 |
| 60 | 817 | 619 | 35.1 | 69.2 | 54.3 | 27.00 | 8.41 |
| 90 | 978 | 808 | 42.0 | 82.9 | 70.9 | 32.31 | 7.40 |
| 120 | 551 | 856 | 36.0 | 46.7 | 75.1 | 27.69 | 7.30 |
| 150 | 52 | 836 | 21.9 | 4.4 | 73.3 | 16.85 | 7.23 |
| 180 | 7 | 827 | 11.5 | 0.6 | 72.5 | 8.85 | 7.33 |
| 210 | 32 | 855 | 6.5 | 2.7 | 75.0 | 5.00 | 7.00 |
| Washing water | 23.2 | 64.6 | 1.0 | 2.0 | 5.7 | 0.77 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 76.5 | 1.3 | 0.2 | 6.5 | 0.11 | 0.15 | 39 |
| 10 | 86.6 | 1.4 | 0.2 | 7.3 | 0.12 | 0.15 | 45 |
| 20 | 88.5 | 2.4 | 0.4 | 7.5 | 0.21 | 0.31 | 58 |
| 30 | 95.2 | 6.0 | 0.8 | 8.1 | 0.53 | 0.62 | 65 |
| 45 | 130 | 15.4 | 1.2 | 11.0 | 1.35 | 0.92 | 63 |
| 60 | 164 | 24.8 | 1.2 | 13.9 | 2.18 | 0.92 | 63 |
| 90 | 200 | 39.1 | 1.5 | 16.9 | 3.43 | 1.15 | 64 |
| 120 | 254 | 59.8 | 2.2 | 21.5 | 5.25 | 1.69 | 63 |
| 150 | 315 | 90.0 | 3.4 | 26.7 | 7.89 | 2.62 | 60 |
| 180 | 372 | 117 | 4.4 | 31.5 | 10.26 | 3.38 | 62 |
| 210 | 416 | 153 | 6.5 | 35.3 | 13.42 | 5.00 | 62 |
| 240 | 499 | 200 | 9.2 | 42.3 | 17.54 | 7.08 | 60 |
| Washing water | 79.7 | 30.5 | 1.1 | 6.8 | 2.68 | 0.85 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 95.7 | 1.2 | 0.3 | 8.1 | 0.11 | 0.23 | 32 |
| 10 | 117 | 1.5 | 0.2 | 9.9 | 0.13 | 0.15 | 39 |
| 20 | 151 | 2.2 | 0.3 | 12.8 | 0.19 | 0.23 | 49 |
| 30 | 187 | 4.4 | 0.3 | 15.8 | 0.39 | 0.23 | 58 |
| 45 | 249 | 19.5 | 0.5 | 21.1 | 1.71 | 0.38 | 62 |
| 60 | 350 | 81.9 | 1.8 | 29.7 | 7.18 | 1.38 | 66 |
| 90 | 608 | 255 | 10.4 | 51.5 | 22.37 | 8.00 | 71 |
| 120 | 700 | 357 | 17.3 | 59.3 | 31.32 | 13.31 | 73 |
| 150 | 774 | 436 | 19.1 | 65.6 | 38.25 | 14.69 | 76 |
| 180 | 772 | 544 | 14.6 | 65.4 | 47.72 | 11.23 | 75 |
| 210 | 787 | 550 | 10.9 | 66.7 | 48.25 | 8.38 | 81 |
| 240 | 663 | 430 | 7.6 | 56.2 | 37.72 | 5.85 | 81 |
| Washing water | 219 | 171 | 2.3 | 18.6 | 15.00 | 1.77 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 175 | 1.8 | 0.4 | 14.8 | 0.16 | 0.31 | 23 |
| 10 | 182 | 2.0 | 0.4 | 15.4 | 0.18 | 0.31 | 26 |
| 20 | 197 | 2.1 | 0.4 | 16.7 | 0.18 | 0.31 | 29 |
| 30 | 202 | 2.2 | 0.4 | 17.1 | 0.19 | 0.31 | 33 |
| 45 | 230 | 2.6 | 0.4 | 19.5 | 0.23 | 0.31 | 36 |
| 60 | 250 | 2.9 | 0.4 | 21.2 | 0.25 | 0.31 | 42 |
| 90 | 302 | 5.2 | 0.5 | 25.6 | 0.46 | 0.38 | 50 |
| 120 | 354 | 10.5 | 0.5 | 30.0 | 0.92 | 0.38 | 54 |
| 150 | 455 | 49.5 | 0.7 | 38.6 | 4.34 | 0.54 | 62 |
| 180 | 528 | 141 | 1.3 | 44.7 | 12.37 | 1.00 | 67 |
| 210 | 611 | 274 | 3.0 | 51.8 | 24.04 | 2.31 | 70 |
| 240 | 656 | 336 | 4.5 | 55.6 | 29.47 | 3.46 | 70 |
| Washing water | 263 | 154 | 1.5 | 22.3 | 13.51 | 1.15 | - |
| Time (min) | Concentration (mg/L) | Efficiency (%) | Temperature (°C) | ||||
|---|---|---|---|---|---|---|---|
| Cu | Ni | Co | Cu | Ni | Co | ||
| 5 | 110 | 1.5 | 0.2 | 9.3 | 0.13 | 0.15 | 33 |
| 10 | 118 | 1.7 | 0.2 | 10.0 | 0.15 | 0.15 | 44 |
| 20 | 173 | 5.6 | 0.3 | 14.7 | 0.49 | 0.23 | 62 |
| 30 | 248 | 19.4 | 0.5 | 21.0 | 1.70 | 0.38 | 65 |
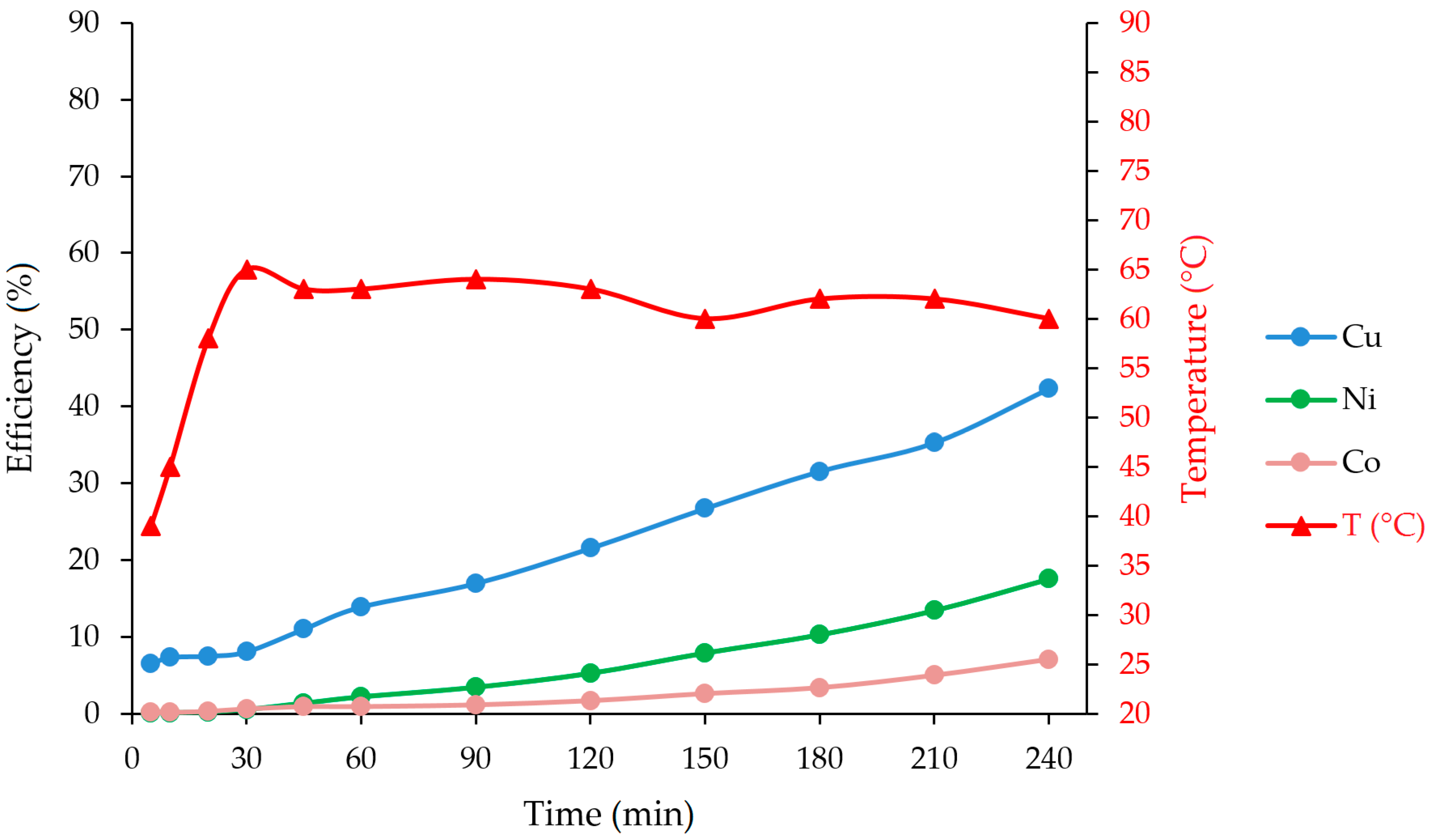
| 45 | 369 | 115 | 2.0 | 31.3 | 10.09 | 1.54 | 74 |
| 60 | 658 | 392 | 9.0 | 55.8 | 34.39 | 6.92 | 82 |
| 120 | 721 | 489 | 20.0 | 61.1 | 42.89 | 15.38 | 89 |
| 240 | 744 | 545 | 22.4 | 63.1 | 47.81 | 17.23 | 89 |
| Washing water | 204 | 144 | 7.0 | 17.3 | 12.63 | 5.38 | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Knaislová, A.; Vu, H.N.; Dvořák, P. Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules. Minerals 2018, 8, 351. https://doi.org/10.3390/min8080351
Knaislová A, Vu HN, Dvořák P. Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules. Minerals. 2018; 8(8):351. https://doi.org/10.3390/min8080351
Chicago/Turabian StyleKnaislová, Anna, Hong Ng. Vu, and Petr Dvořák. 2018. "Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules" Minerals 8, no. 8: 351. https://doi.org/10.3390/min8080351
APA StyleKnaislová, A., Vu, H. N., & Dvořák, P. (2018). Microwave and Ultrasound Effect on Ammoniacal Leaching of Deep-Sea Nodules. Minerals, 8(8), 351. https://doi.org/10.3390/min8080351







