The Compressive Strength and Microstructure of Alkali-Activated Binary Cements Developed by Combining Ceramic Sanitaryware with Fly Ash or Blast Furnace Slag
Abstract
1. Introduction
2. Experimental Process
2.1. Materials
2.2. Experimental Process and Tests Performed
- -
- System 1: 0 to 50 wt % CSW was replaced with BFS, a high-calcium waste material. A 100 wt % BFS sample was also prepared for comparison purposes, and no Ca(OH)2 was added to this system.
- -
- -
- System 3: 0 to 50 wt % CSW was replaced with BFS and 4 wt % Ca(OH)2 was added to these blended samples. The microstructure and mechanical properties of this system were compared with those observed in the alkali-activated CSW/BFS with no added Ca(OH)2 (System 1) and the alkali-activated CSW/FA binders (System 2).
2.3. Mix Proportions and Preparing Pastes and Mortars
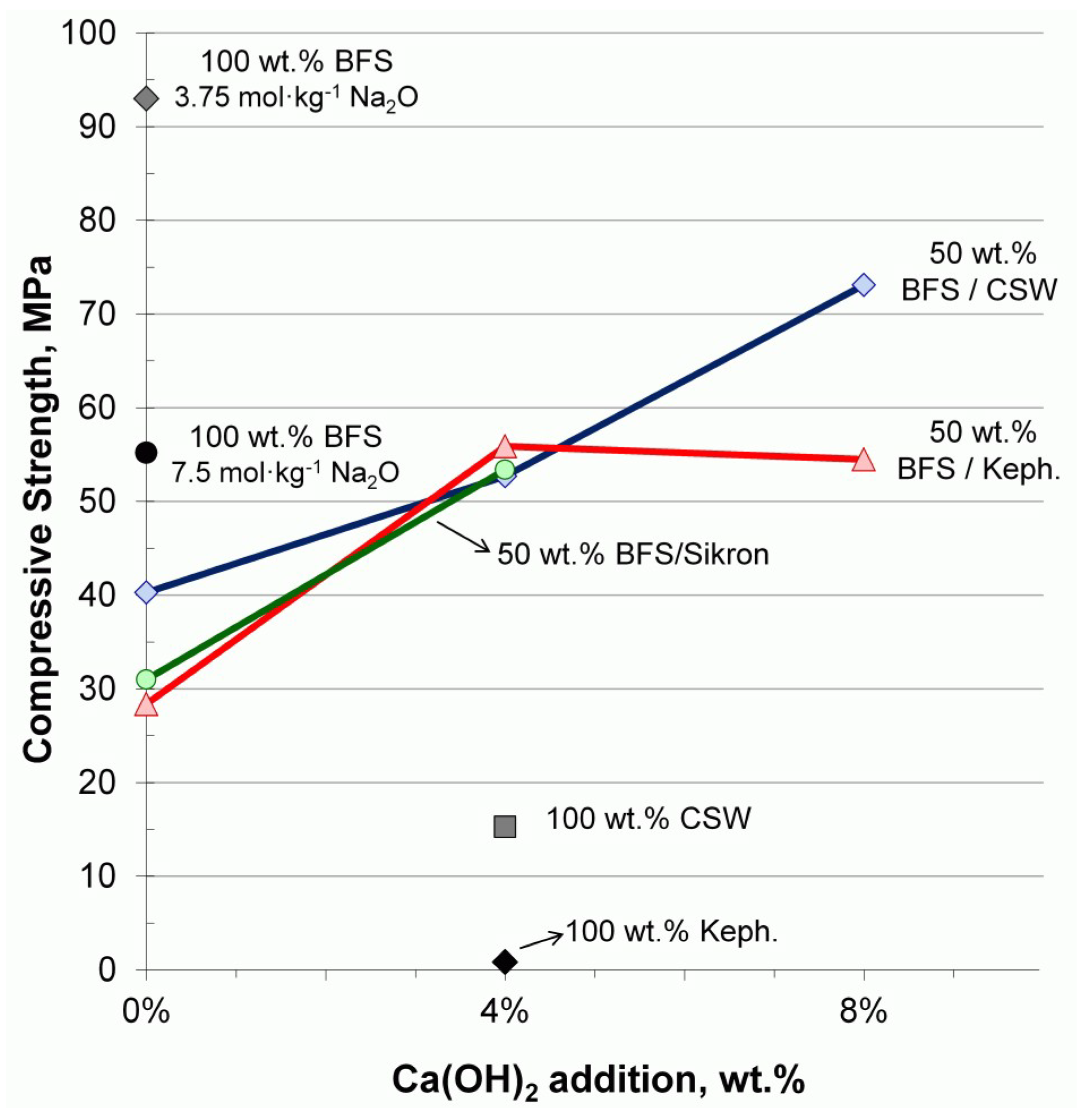
2.4. Further Studies on the Influence of BFS and Ca(OH)2 in the Alkali-Activated CSW Blended Systems
- -
- a 100 wt % BFS mortar with a higher Na2O concentration (7.5 mol·kg−1 instead of 3.75 mol·kg−1);
- -
- 50 wt % BFS mortars blended with two different high-crystallinity materials: kephalite (and alucite, silicoaluminate in nature) and sikron (flour quartz, siliceous in nature). Both were prepared without Ca(OH)2 and with the addition of 4 wt %;
- -
- 50 wt % XXX/BFS mortars (where XXX: CSW or kephalite), with 0, 4 and 8 wt % Ca(OH)2;
- -
- a 100 wt % kephalite with the addition of 4 wt % Ca(OH)2.
3. Results
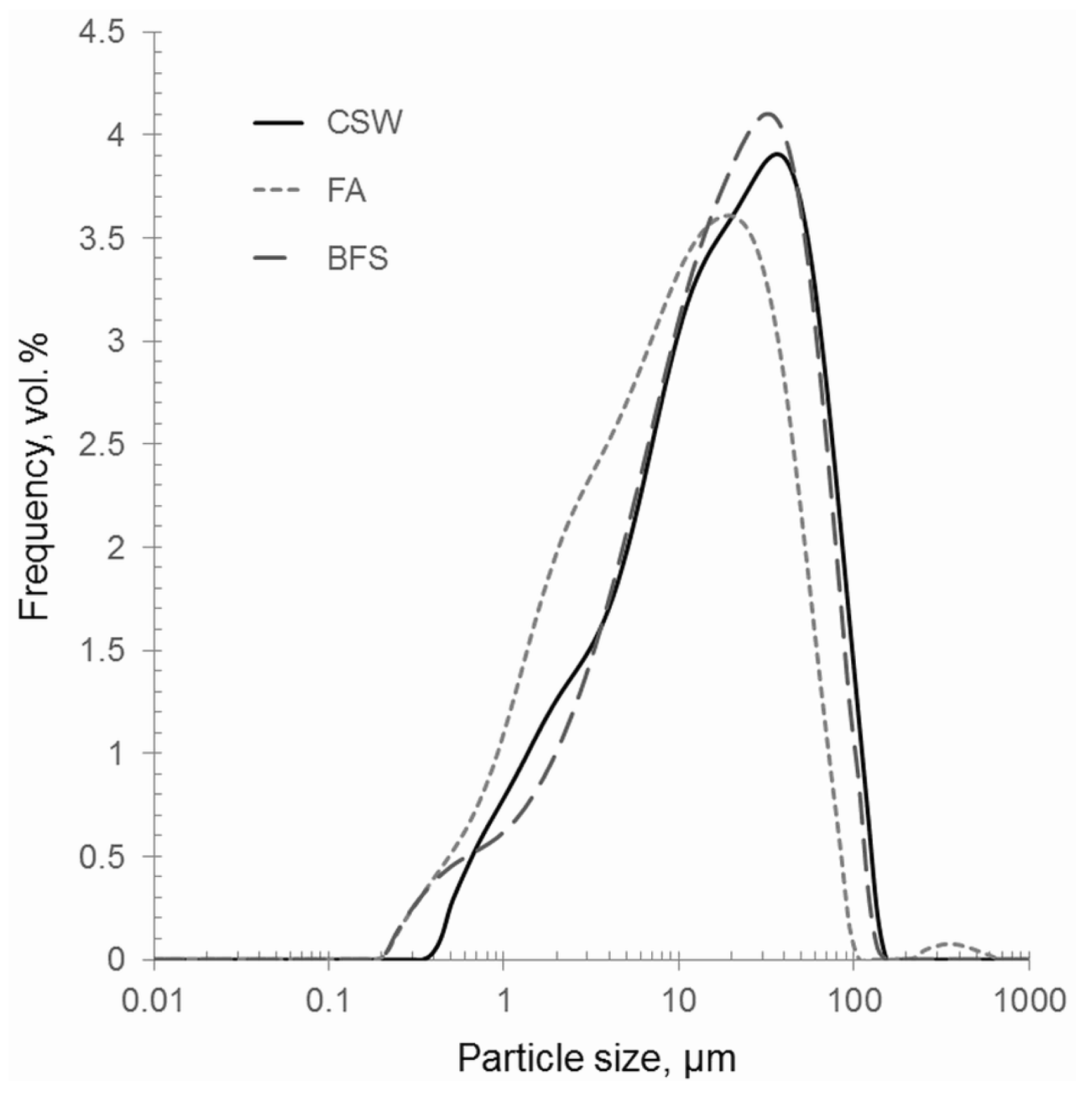
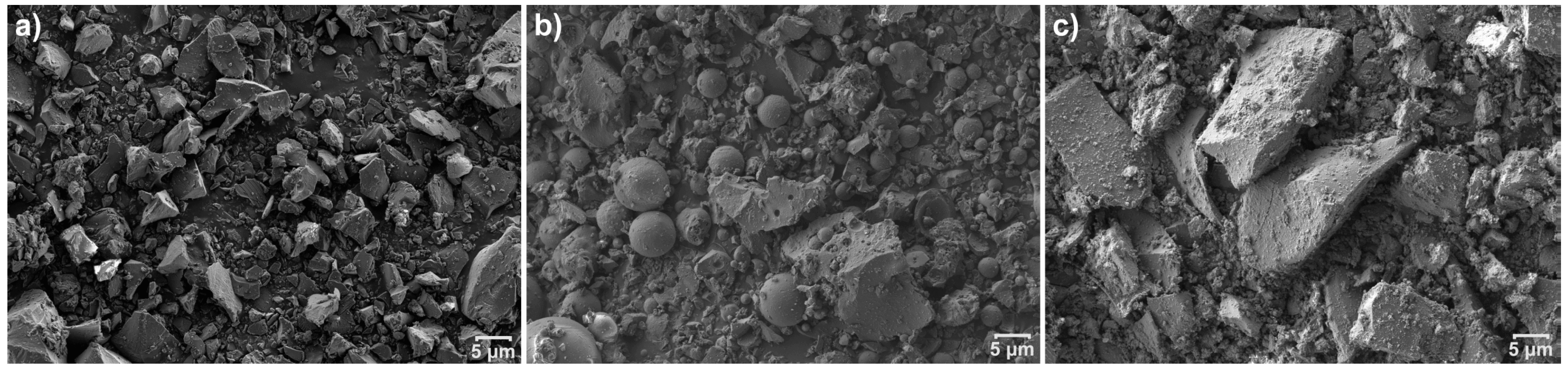
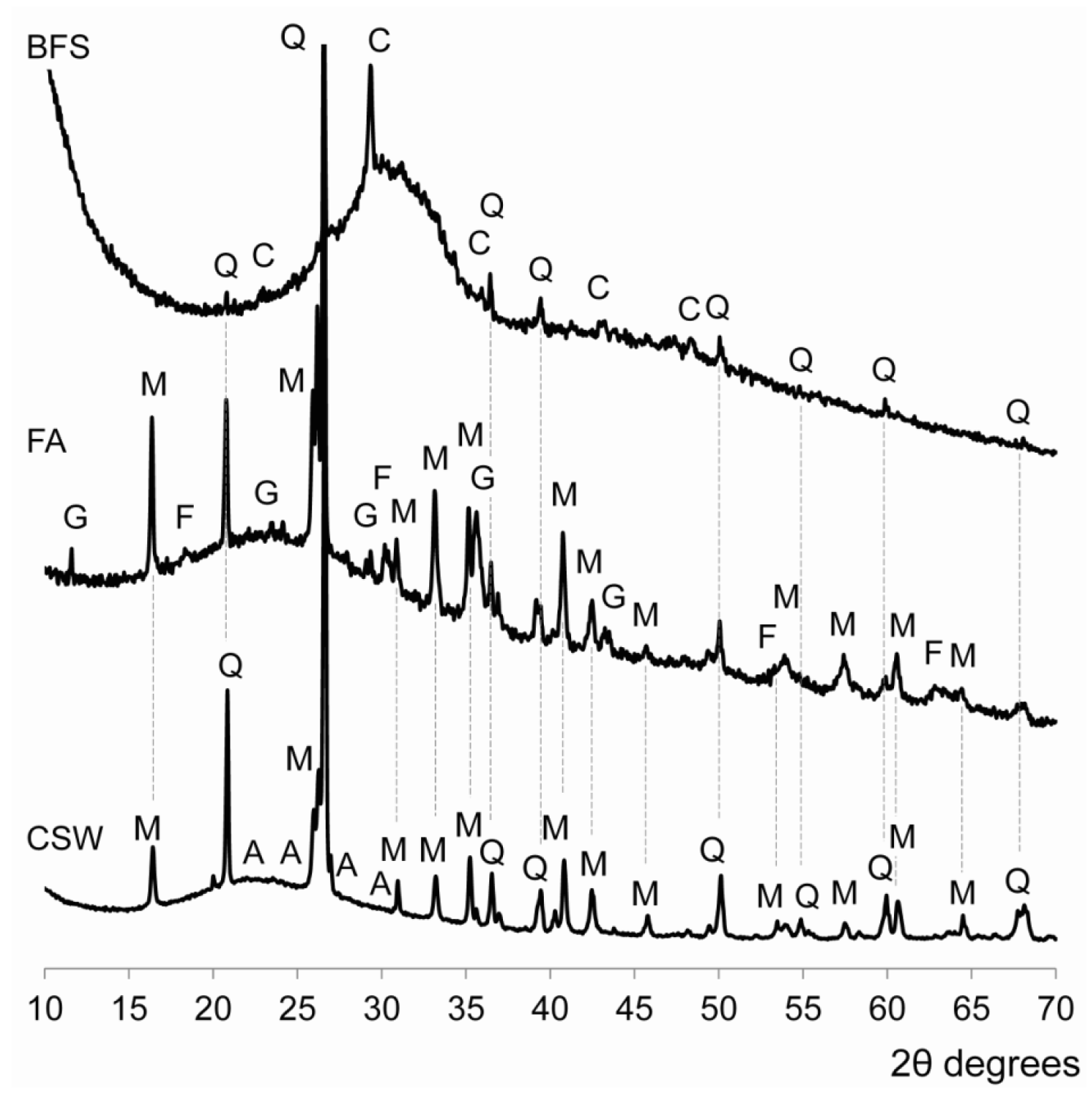
3.1. Characteristics of the Waste Materials
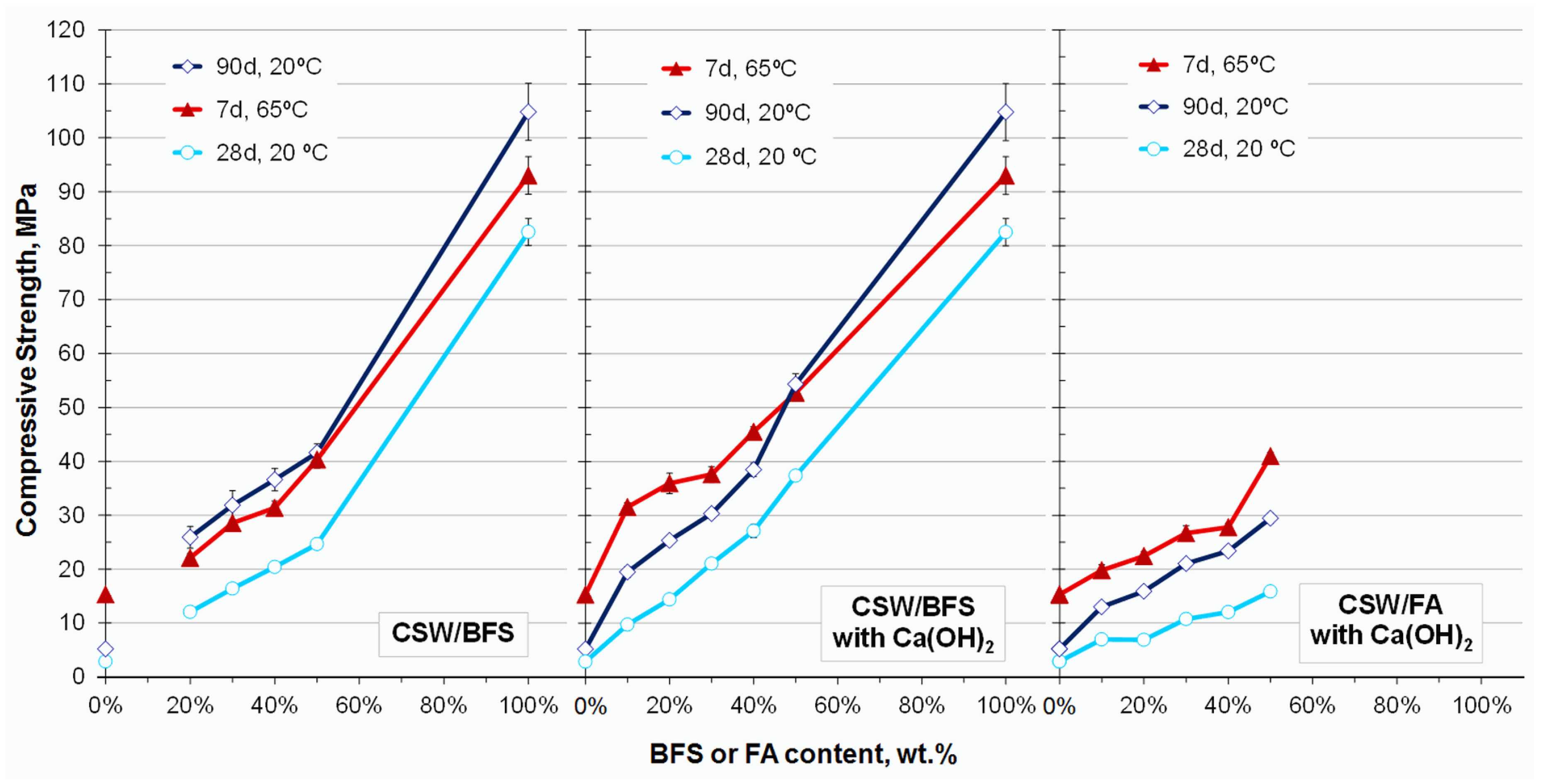
3.2. Compressive Strength
3.2.1. Further Results on the Influence of BFS and Ca(OH)2 in the Alkali-Activated CSW Blended Systems
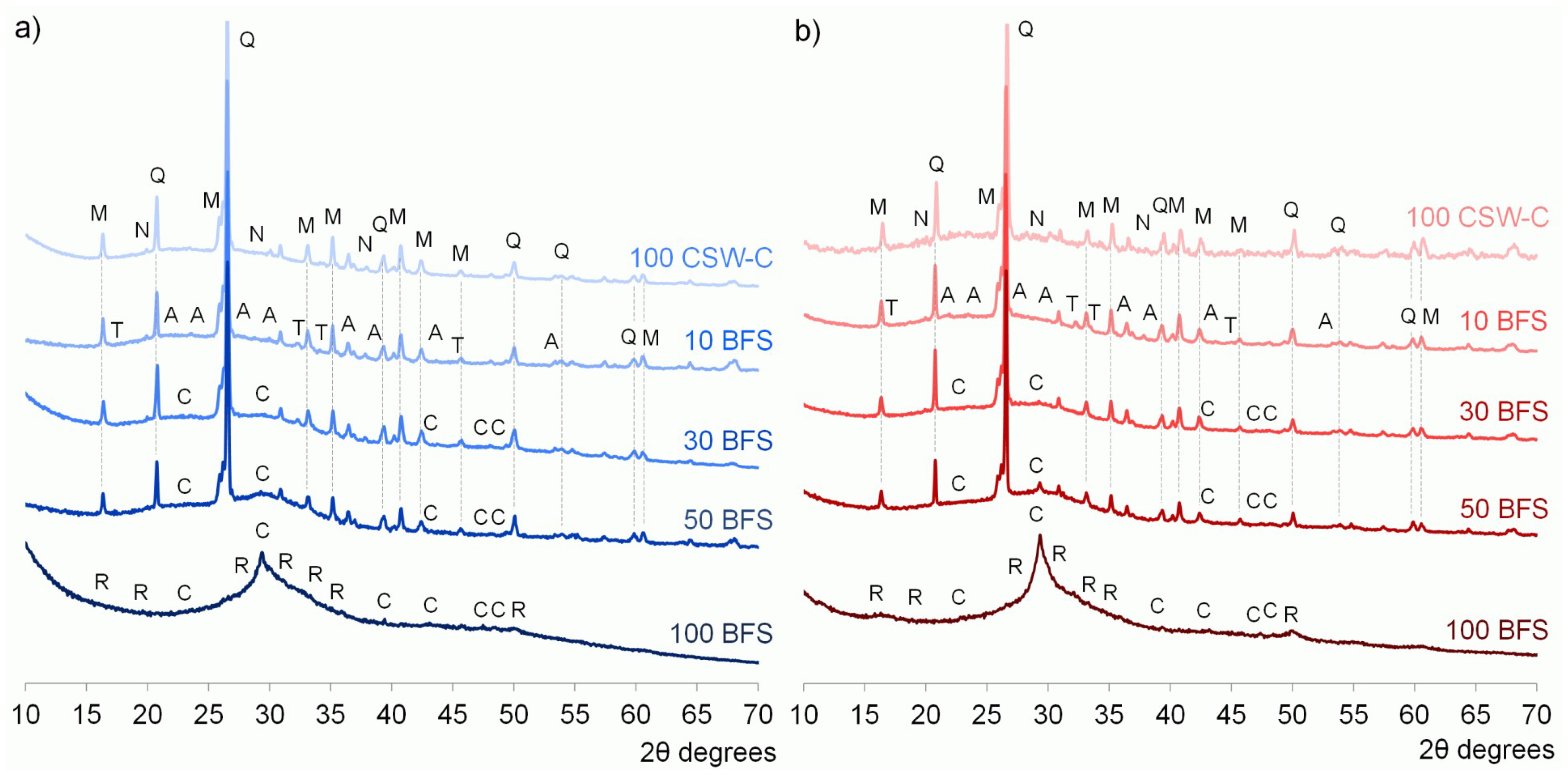
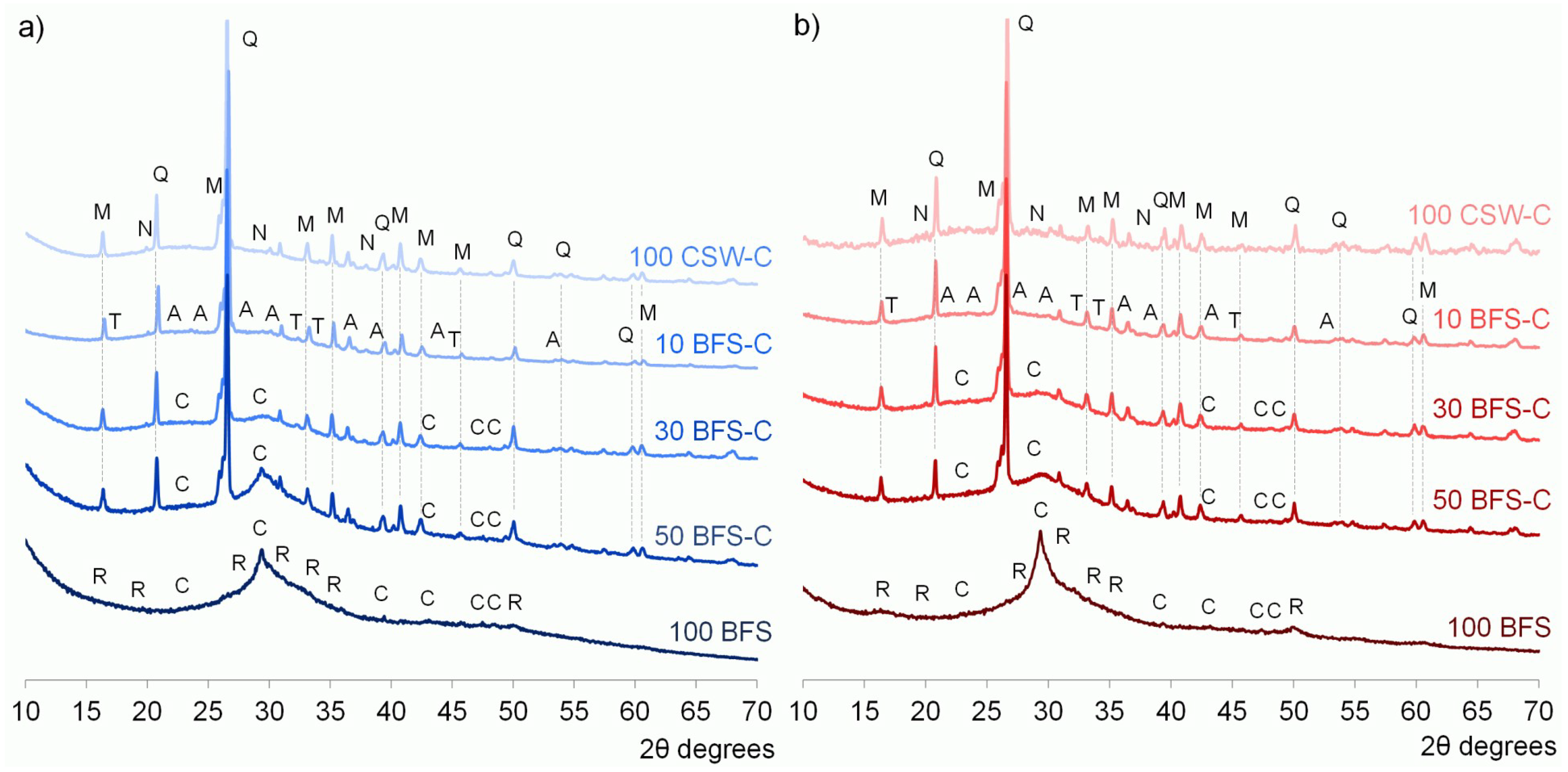
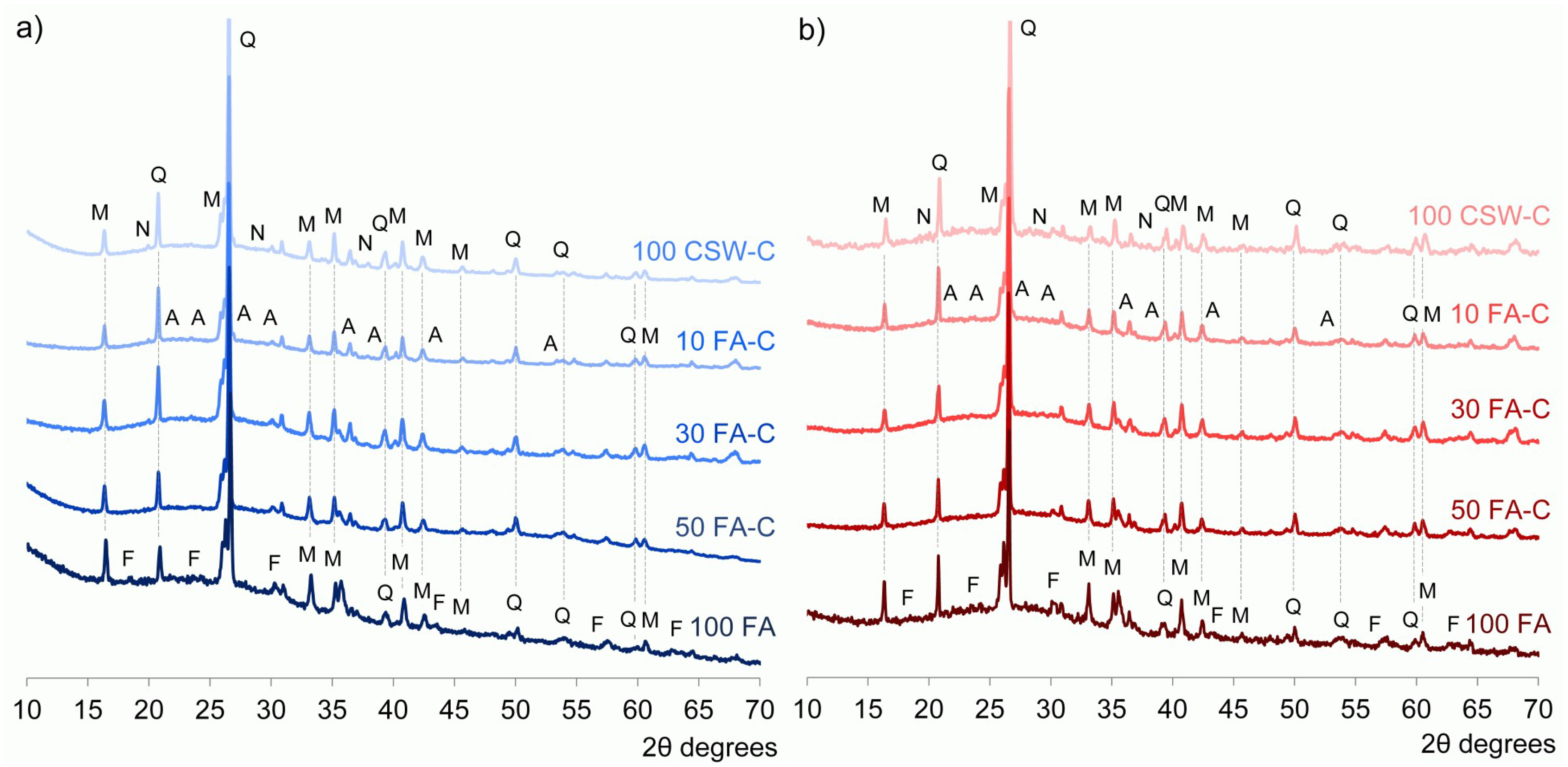
3.3. X-ray Diffraction (XRD) Studies
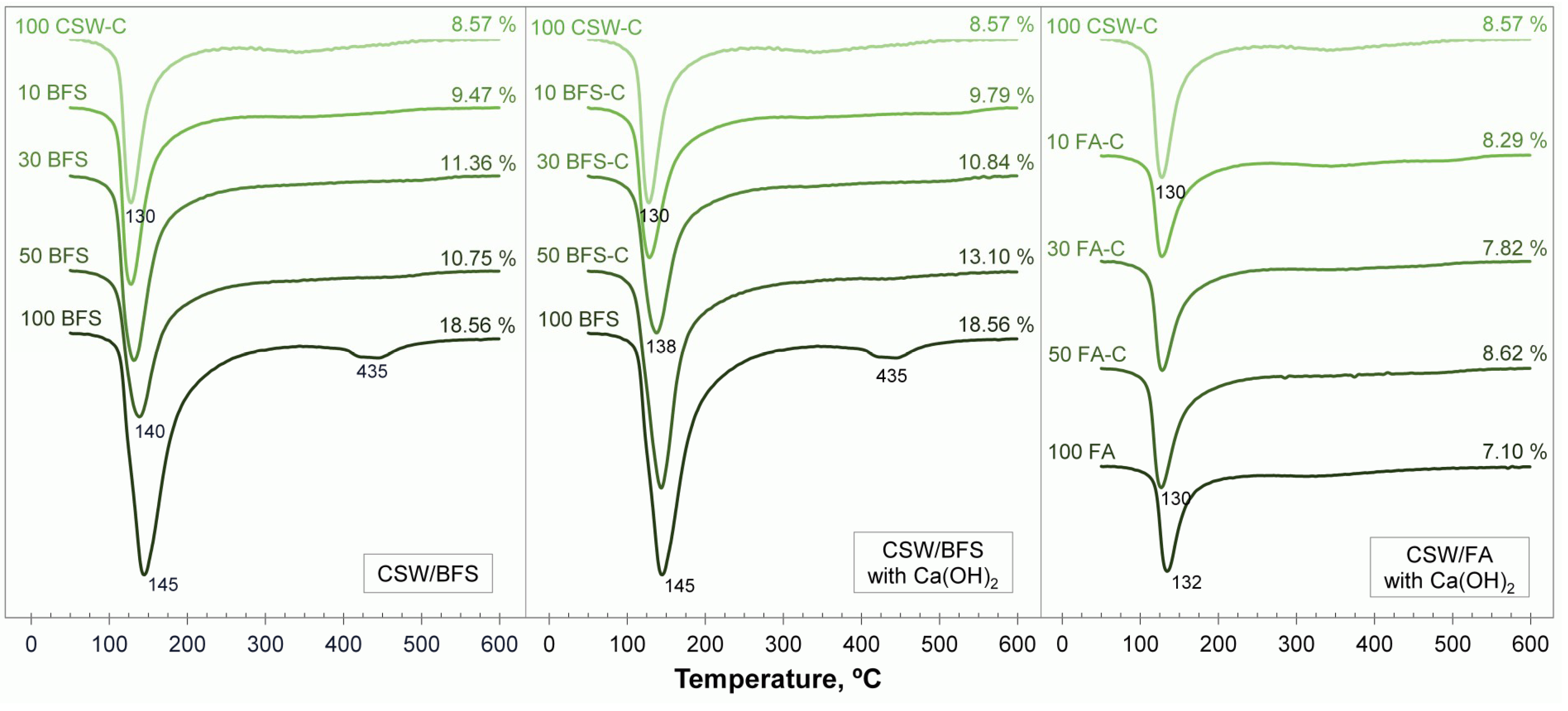
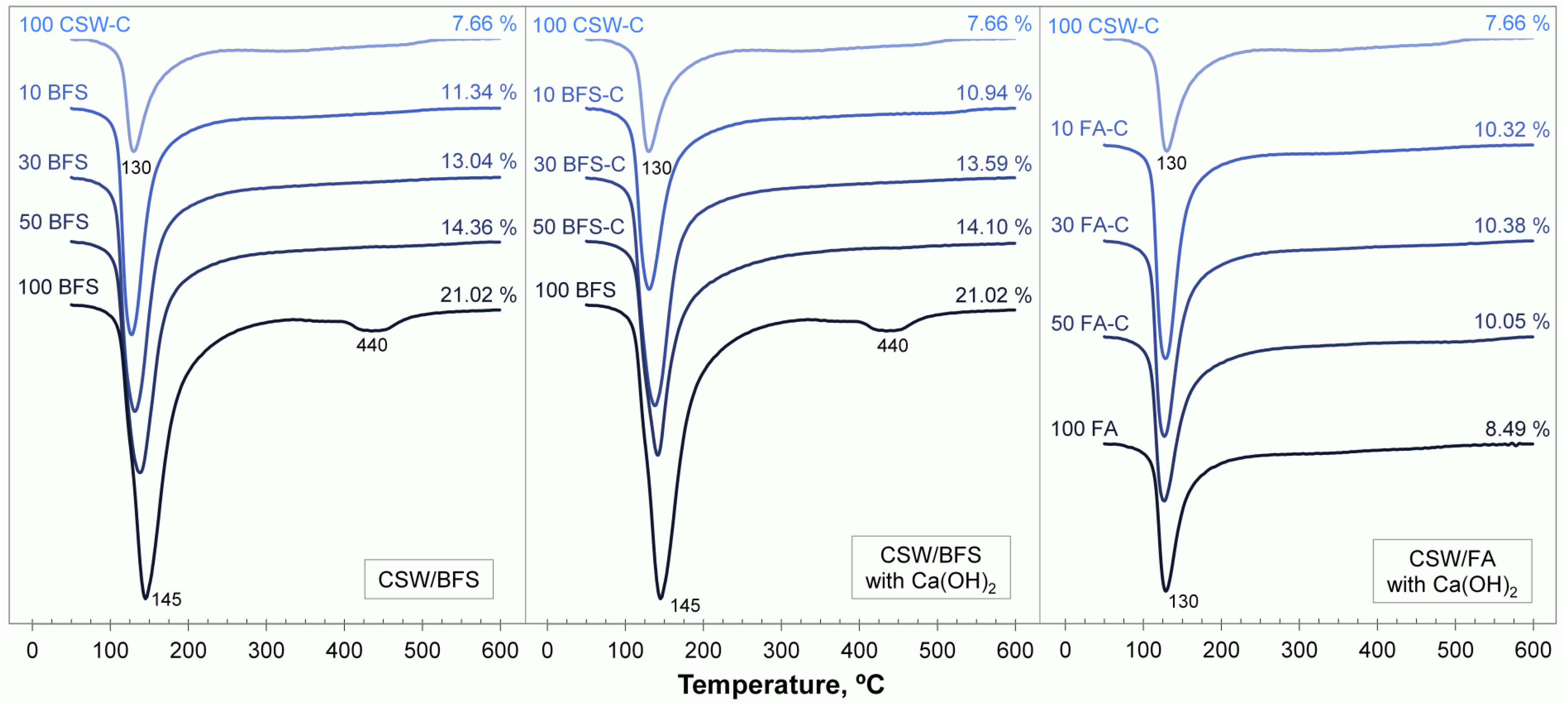
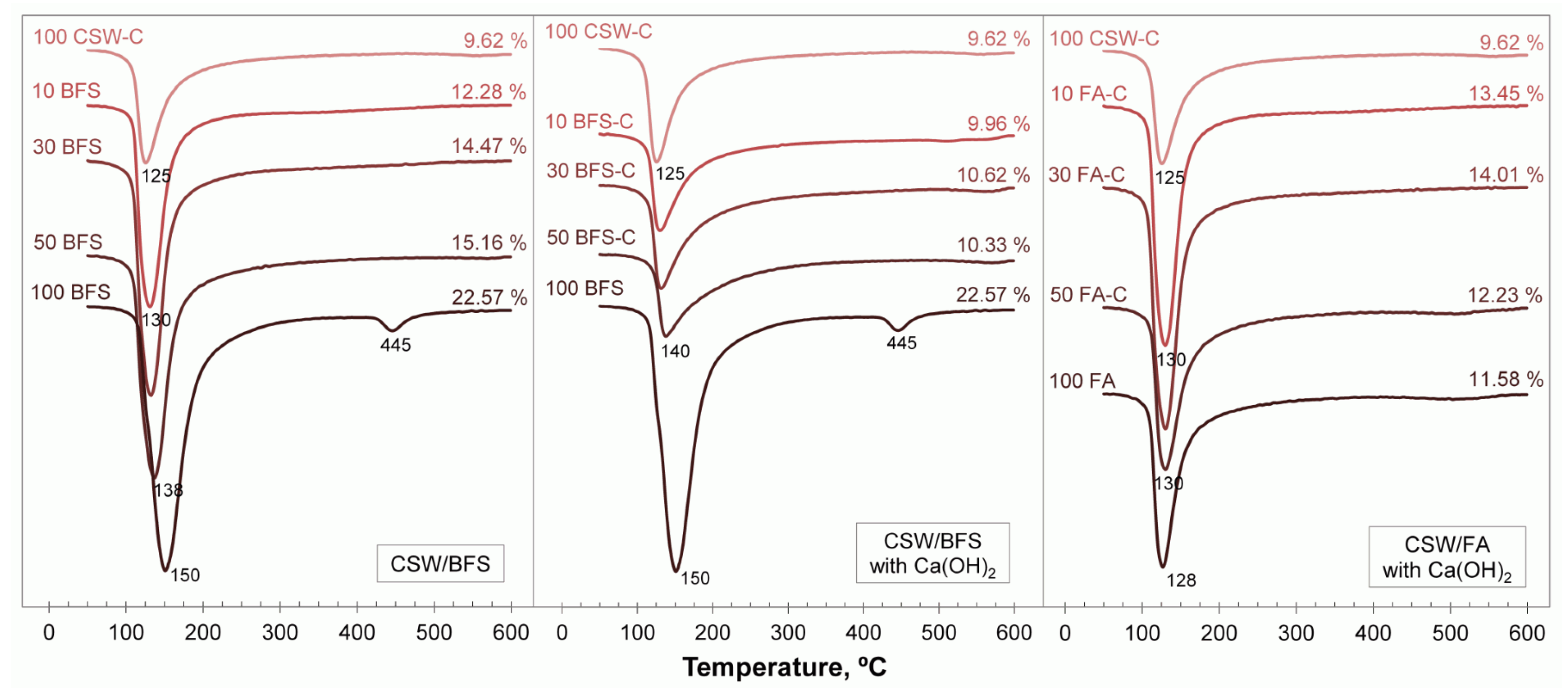
3.4. Thermogravimetric Analyses
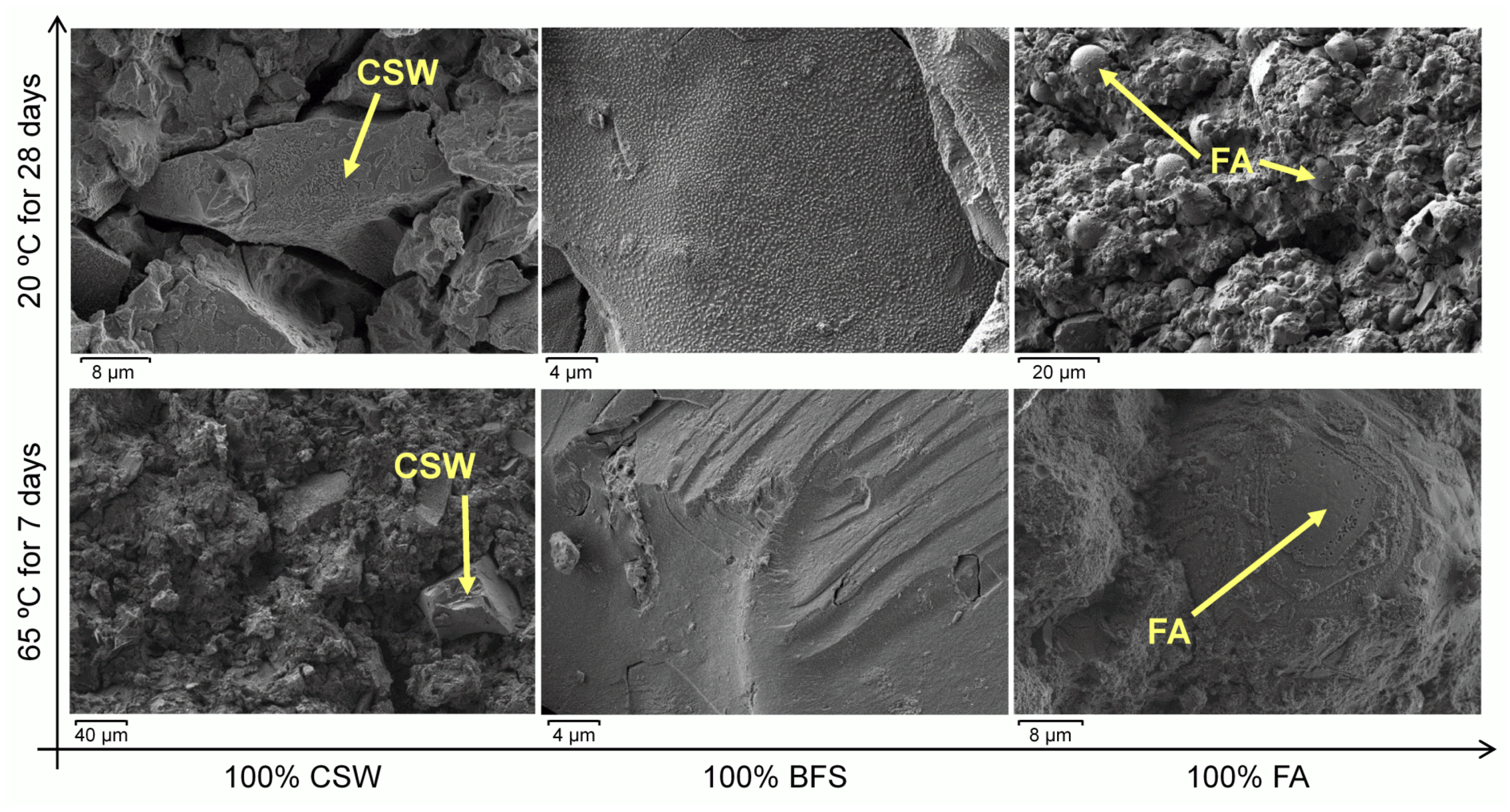
3.5. Field Emission Scanning Electron Microscopy (FESEM)
4. Discussion
4.1. Strength of the CSW/BFS and CSW/FA Blended Mortars
4.2. Types of Gels Formed
4.3. Role of Calcium in the Kinetics of the Process
4.4. Future Research
5. Conclusions
- The compressive strength of the 100 wt % CSW mortars improved with BFS or FA content. The best results were provided by the CSW/BFS blended systems with the Ca(OH)2 addition, which reached almost 55 MPa after 90 curing days at 20 °C or 7 days at 65 °C.
- The BFS mortars presented better strength results than FA, especially when cured at 20 °C.
- BFS mainly reacted with the activating solution in the CSW/BFS blended cements, which led to a significant loss of strength compared to the 100 wt % BFS sample. This was attributed to excess reagents for the diluted amount of BFS in the system.
- Although 4 wt % Ca(OH)2 was consumed mainly by BFS in the CSW/BFS blended systems, the 8 wt % additions promoted the reactivity of CSW, which also conferred the binder strength.
- No significant new crystalline phases were identified in the CSW blended cements, and Ca(OH)2 reacted during the alkali-activation process.
- Although it was not possible to clearly differentiate the different types of gel that formed, N–A–S–H and low-calcium N–(C)–A–S–H gels most probably formed in the CSW/FA blended systems, and a combination of N–(C)–A–S–H/C–S–H/C–A–S–H gels formed in the CSW/BFS binary cements.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zedan, S.R.; Mohamed, M.R.; Ahmed, D.A.; Mohammed, A.H. Alkali activated ceramic waste with or without two different calcium sources. Adv. Mater. Res. 2015, 4, 133–144. [Google Scholar] [CrossRef]
- Marjanović, N.; Komljenović, M.; Baščarević, Z.; Nikolić, V.; Petrović, R. Physical-mechanical and microstructural properties of alkali-activated fly ash-blast furnace slag blends. Ceram. Int. 2015, 41, 1421–1435. [Google Scholar] [CrossRef]
- Najimi, M.; Ghafoori, N.; Sharbaf, M. Alkali-activated natural pozzolan/slag mortars: A parametric study. Constr. Build. Mater. 2018, 164, 625–643. [Google Scholar] [CrossRef]
- Chen, X.; Sutrisno, A.; Struble, L.J. Effects of calcium on setting mechanism of metakaolin-based geopolymer. J. Am. Ceram. Soc. 2018, 101, 957–968. [Google Scholar] [CrossRef]
- CEDEX, Center for Studies and Experimentation of Public Works-Ministerio de Fomento. Gobierno de España: Catálogo de Residuos. Ficha Técnica Cenizas Volantes De Carbón Y Cenizas De Hogar O Escorias. 2011. Available online: http://www.cedexmateriales.es/catalogo-de-residuos/24/diciembre-2011/ (accessed on 4 August 2018). (In Spanish).
- Toniolo, N.; Boccaccini, A.R. Fly ash-based geopolymers containing added silicate waste. A review. Ceram. Int. 2017, 43, 14545–14551. [Google Scholar] [CrossRef]
- Ranjbar, N.; Kuenzel, C. Cenospheres: A review. Fuel 2017, 207, 1–12. [Google Scholar] [CrossRef]
- CEDEX, Center for Studies and Experimentation of Public Works-Ministerio de Fomento. Gobierno de España: Catálogo de Residuos. Ficha Técnica Escorias De Horno Alto. 2011. Available online: http://www.cedexmateriales.es/catalogo-de-residuos/39/escorias-de-horno-alto/ (accessed on 4 August 2018). (In Spanish).
- World Steel Association, World Steel in Figures 2017. Available online: https://www.worldsteel.org (accessed on 4 August 2018).
- US Geological Survey, Mineral Resources Program, Iron and Steel Slag. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/iron_&_steel_slag/mcs-2017-fesla.pdf (accessed on 4 August 2018).
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review. Part 2. About materials and binders manufacture. Constr. Build. Mater. 2008, 22, 1315–1322. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. An overview of geopolymers derived from industrial by-products. Constr. Build. Mater. 2016, 127, 183–198. [Google Scholar] [CrossRef]
- Mellado, A.; Catalán, C.; Bouzón, N.; Borrachero, M.V.; Monzó, J.; Payá, J. Carbon footprint of geopolymeric mortar: Study of the contribution of the alkaline activating solution and assessment of alternative route. RSC Adv. 2014, 4, 23846–23852. [Google Scholar] [CrossRef]
- Mohammed, S. Processing, effect and reactivity assessment of artificial pozzolans obtained from clays and clay wastes: A review. Constr. Build. Mater. 2017, 140, 10–19. [Google Scholar] [CrossRef]
- Halicka, A.; Ogrodnik, P.; Zegardlo, B. Using ceramic sanitary ware waste as concrete aggregate. Constr. Build. Mater. 2013, 48, 295–305. [Google Scholar] [CrossRef]
- Bernasconi, A.; Diella, V.; Pagani, A.; Pavese, A.; Francescon, F.; Young, K.; Stuart, J.; Tunnicliffe, L. The role of firing temperature, firing time and quartz grain size on phase-formation, thermal dilatation and water absorption in sanitary-ware vitreous bodies. J. Eur. Ceram. Soc. 2011, 31, 1353–1360. [Google Scholar] [CrossRef]
- Baraldi, L. World sanitaryware production and exports. Ceram. World Rev. 2015, 114, 56–65. [Google Scholar]
- Reig, L.; Borrachero, M.V.; Monzó, J.; Savastano, H.; Tashima, M.M.; Payá, J. Use of ceramic sanitaryware as an alternative for the development of new sustainable binders. Key Eng. Mater. 2016, 668, 172–180. [Google Scholar] [CrossRef]
- Robayo-Salazar, R.A.; de Gutiérrez, M.; Puertas, F. Study of synergy between a natural volcanic pozzolan and a granulated blast furnace slag in the production of geopolymeric pastes and mortars. Constr. Build. Mater. 2017, 157, 151–160. [Google Scholar] [CrossRef]
- Pan, Z.; Tao, Z.; Cao, Y.F.; Wuhrer, R.; Murphy, T. Compressive strength and microstructure of alkali-activated fly ash/slag binders at high temperature. Cem. Concr. Compos. 2018, 86, 9–18. [Google Scholar] [CrossRef]
- Tashima, M.M.; Reig, L.; Santini, M.A.; B Moraes, J.C.; Akasaki, J.L.; Payá, J.; Borrachero, M.V.; Soriano, L. Compressive Strength and Microstructure of Alkali-Activated Blast Furnace Slag/Sewage Sludge Ash (GGBS/SSA) Blends Cured at Room Temperature. Waste Biomass Valorization 2017, 8, 1441–1451. [Google Scholar] [CrossRef]
- Perná, I.; Hanzlíček, T. The setting time of a clay-slag geopolymer matrix: The influence of blast-furnace-slag addition and the mixing method. J. Clean. Prod. 2016, 112, 1150–1155. [Google Scholar] [CrossRef]
- El-Naggar, M.R.; Amin, M. Impact of alkali cations on properties of metakaolin and metakaolin/slag geopolymers: Microstructures in relation to sorption of 134Cs radionuclide. J. Hazard. Mater. 2018, 344, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Reig, L.; Soriano, L.; Tashima, M.M.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of calcium additions on the compressive strength and microstructure of alkali-activated ceramic sanitary-ware. J. Am. Ceram. Soc. 2018, 101, 3094–3104. [Google Scholar] [CrossRef]
- García-Lodeiro, I.; Fernández-Jiménez, A.; Palomo, A. Variation in hybrid cements over time. Alkaline activation of fly ash-portland cement blends. Cem. Concr. Res. 2013, 52, 112–122. [Google Scholar] [CrossRef]
- Cosa, J.; Soriano, L.; Borrachero, M.; Reig, L.; Payá, J.; Monzó, J. Influence of Addition of Fluid Catalytic Cracking Residue (FCC) and the SiO2 Concentration in Alkali-Activated Ceramic Sanitary-Ware (CSW) Binders. Minerals 2018, 8, 123. [Google Scholar] [CrossRef]
- Reig, L.; Soriano, L.; Borrachero, M.V.; Monzó, J.; Payá, J. Influence of the activator concentration and calcium hydroxide addition on the properties of alkali-activated porcelain stoneware. Const. Build. Mater. 2014, 63, 214–222. [Google Scholar] [CrossRef]
- Temuujin, J.; Williams, R.P.; van Riessen, A. Effect of mechanical activation of fly ash on the properties of geopolymer cured at ambient temperature. J. Mater. Process. Technol. 2009, 209, 5276–5280. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; San Nicolas, R.; Hamdan, S.; Van Deventer, J.S.J. Modification of phase evolution in alkali-activated blast furnace slag by the incorporation of fly ash. Cem. Concr. Compos. 2014, 45, 125–135. [Google Scholar] [CrossRef]
- Džunuzović, N.; Komljenović, M.; Nikolić, V.; Ivanović, T. External sulfate attack on alkali-activated fly ash-blast furnace slag composite. Constr. Build. Mater. 2017, 157, 737–747. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, A.; Sobrados, I.; Sanz, J. The role played by the reactive alumina content in the alkaline activation of fly ashes. Microporous Mesoporous Mater. 2006, 91, 111–119. [Google Scholar] [CrossRef]
- De Moraes, J.C.B.; Tashima, M.M.; Melges, J.L.P.; Akasaki, J.L.; Monzó, J.; Borrachero, M.V.; Soriano, L.; Payá, J. Optimum Use of Sugar Cane Straw Ash in Alkali-Activated Binders Based on Blast Furnace Slag. J. Mater. Civ. Eng. 2018, 30, 4018084. [Google Scholar] [CrossRef]
- Rashad, A.M. A comprehensive overview about the influence of different admixtures and additives on the properties of alkali-activated fly ash. Mater. Des. 2014, 53, 1005–1025. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Comparative performance of alkali activated slag/metakaolin cement pastes exposed to high temperatures. Cem. Concr. Compos. 2017, 84, 157–166. [Google Scholar] [CrossRef]
- Hidalgo, A.; García, J.L.; Alonso, M.C.; Fernández, L.; Andrade, C. Microstructure development in mixes of calcium aluminate cement with silica fume or fly ash. J. Therm. Anal. Calorim. 2009, 96, 335–345. [Google Scholar] [CrossRef]
- Khan, M.Z.N.; Shaikh, F.; uddin Ahmed Shaikh, F.; Hao, Y.; Hao, H. Synthesis of high strength ambient cured geopolymer composite by using low calcium fly ash. Constr. Build. Mater. 2016, 125, 809–820. [Google Scholar] [CrossRef]
- Rodríguez, E.D.; Bernal, S.A.; Provis, J.L.; Paya, J.; Monzo, J.M.; Borrachero, M.V. Effect of nanosilica-based activators on the performance of an alkali-activated fly ash binder. Cem. Concr. Compos. 2013, 35, 1–11. [Google Scholar] [CrossRef]
- Dwivedi, A.; Jain, M.K. Fly ash–waste management and overview: A Review. Recent Res. Sci. Technol. 2014, 6, 30–35. [Google Scholar]
- Ministry of Public Works. RC-16. Instrucción para la Recepción de Cementos (CementReceptionInstruction); Ministry of Public Works: Madrid, Spain, 2016.
- Djobo, J.N.Y.; Tchakouté, H.K.; Ranjbar, N.; Elimbi, A.; Tchadjié, L.N.; Njopwouo, D. Gel Composition and Strength Properties of Alkali-Activated Oyster Shell-Volcanic Ash: Effect of Synthesis Conditions. J. Am. Ceram. Soc. 2016, 99, 3159–3166. [Google Scholar] [CrossRef]
- Silva, P.D.; Sagoe-Crenstil, K.; Sirivivatnanon, V. Kinetics of geopolymerization: Role of Al2O3 and SiO2. Cem. Concr. Res. 2007, 37, 512–518. [Google Scholar] [CrossRef]

| System 1. BFS without Ca(OH)2 | System 2. FA with Ca(OH)2 | System 3. BFS with Ca(OH)2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Mat. | CSW Replac. wt % | Ca(OH)2 Addition wt % | Sample | Mat. | CSW Replac. wt % | Ca(OH)2 Addition wt % | Sample | Mat. | CSW Replac. wt % | Ca(OH)2 Addition wt % |
| 100CSW-C | Blast Furnace Slag (BFS) | 0 | 4 | 100CSW-C | Fly Ash (FA) | 0 | 4 | 100CSW-C | Blast Furnace Slag (BFS) | 0 | 4 |
| BFS/10 | 10 | - | C-FA/10 | 10 | 4 | C-BFS/10 | 10 | 4 | |||
| BFS/20 | 20 | - | C-FA/20 | 20 | 4 | C-BFS/20 | 20 | 4 | |||
| BFS/30 | 30 | - | C-FA/30 | 30 | 4 | C-BFS/30 | 30 | 4 | |||
| BFS/40 | 40 | - | C-FA/40 | 40 | 4 | C-BFS/40 | 40 | 4 | |||
| BFS/50 | 50 | - | C-FA/50 | 50 | 4 | C-BFS/50 | 50 | 4 | |||
| BFS/100 | 100 | - | FA/100 | 100 | - | BFS/100 | 100 | - | |||
| w/b (a) | Na2O mol·kg−1 | SiO2 mol·kg−1 | SiO2/Na2O | Na2O mol·kgbinder−1 | SiO2 mol·kgbinder−1 | Curing Conditions |
|---|---|---|---|---|---|---|
| 0.45 | 3.75 | 7.28 | 1.94 | 1.69 | 3.28 | 7 days at 65 °C 28 and 90 days 20 °C |
| Waste Materials | d10, µm | d50, µm | d90, µm | Mean Diameter, µm |
|---|---|---|---|---|
| CSW | 2.92 | 22.38 | 73.32 | 31.24 |
| BFS | 2.78 | 20.60 | 66.16 | 28.54 |
| FA | 2.09 | 11.52 | 47.75 | 21.07 |
| Waste Materials | Al2O3 | SiO2 | CaO | Fe2O3 | K2O | MgO | Na2O | SO3 | Other | LOI 1 | Amorph. Content |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CSW | 23.60 | 66.00 | 1.20 | 1.30 | 2.80 | 0.70 | 2.40 | 0.10 | 1.80 | 0.20 | 45.6 |
| FA | 25.80 | 49.91 | 3.84 | 13.94 | 2.47 | 1.06 | - | 1.00 | 0.01 | 1.97 | 71.4 |
| BFS | 10.60 | 30.04 | 40.35 | 1.30 | 0.57 | 7.47 | 0.87 | 1.94 | 1.30 | 5.56 | 98.6 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosa, J.; Soriano, L.; Borrachero, M.V.; Reig, L.; Payá, J.; Monzó, J.M. The Compressive Strength and Microstructure of Alkali-Activated Binary Cements Developed by Combining Ceramic Sanitaryware with Fly Ash or Blast Furnace Slag. Minerals 2018, 8, 337. https://doi.org/10.3390/min8080337
Cosa J, Soriano L, Borrachero MV, Reig L, Payá J, Monzó JM. The Compressive Strength and Microstructure of Alkali-Activated Binary Cements Developed by Combining Ceramic Sanitaryware with Fly Ash or Blast Furnace Slag. Minerals. 2018; 8(8):337. https://doi.org/10.3390/min8080337
Chicago/Turabian StyleCosa, Juan, Lourdes Soriano, María Victoria Borrachero, Lucía Reig, Jordi Payá, and José María Monzó. 2018. "The Compressive Strength and Microstructure of Alkali-Activated Binary Cements Developed by Combining Ceramic Sanitaryware with Fly Ash or Blast Furnace Slag" Minerals 8, no. 8: 337. https://doi.org/10.3390/min8080337
APA StyleCosa, J., Soriano, L., Borrachero, M. V., Reig, L., Payá, J., & Monzó, J. M. (2018). The Compressive Strength and Microstructure of Alkali-Activated Binary Cements Developed by Combining Ceramic Sanitaryware with Fly Ash or Blast Furnace Slag. Minerals, 8(8), 337. https://doi.org/10.3390/min8080337









