1. Introduction
The industrial minerals sector has grown and diversified in the mining industry at both regional and global scales [
1,
2,
3,
4]. In contrast to metallic ores, industrial minerals, as a mineral or a group of minerals, are mined not for their metallic content but for their physical and chemical properties [
1,
5]. Due to their intrinsic characteristics, most industrial minerals are used in their raw form which allows wide product variability strictly tied to consumer specifications [
1].
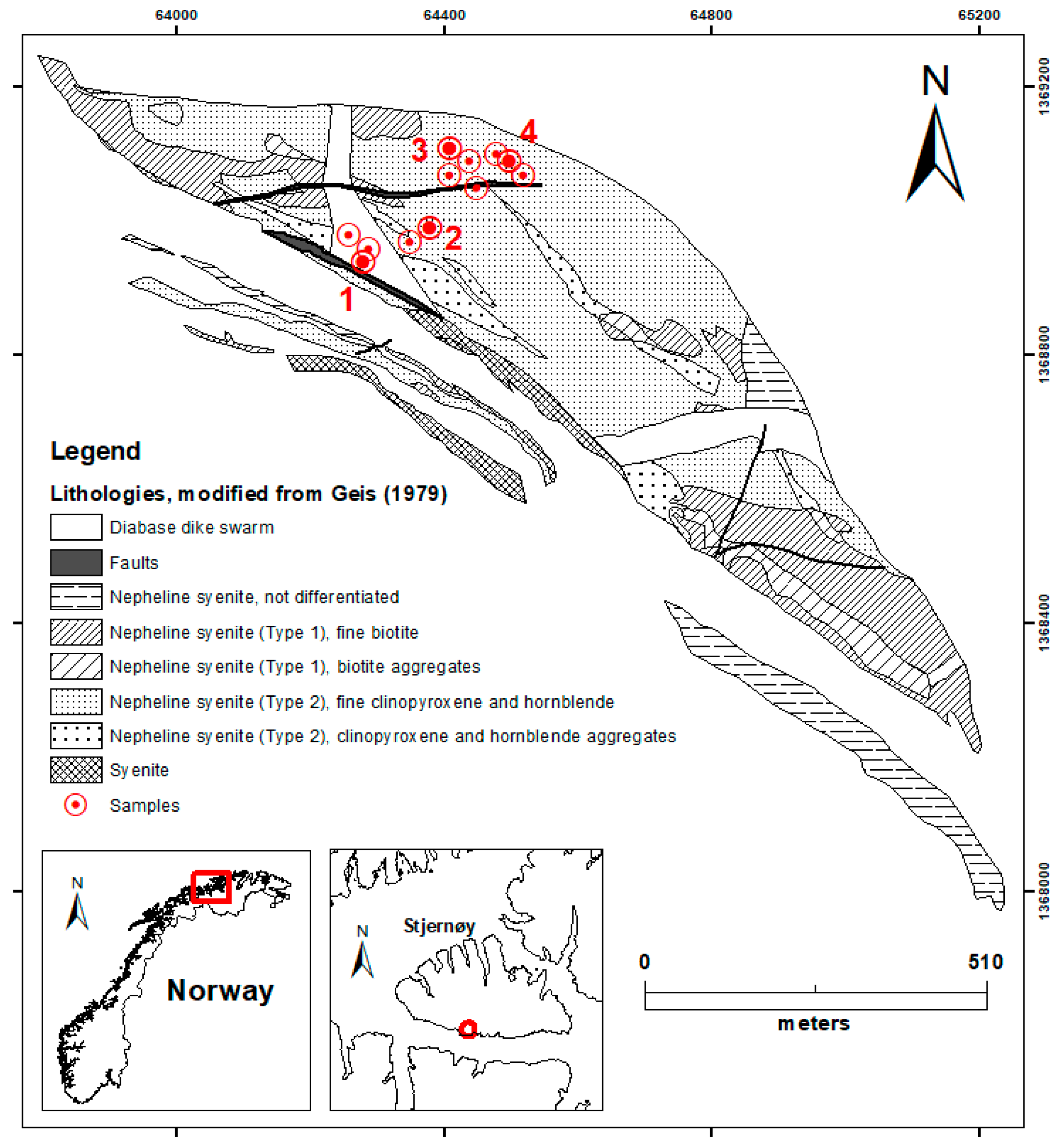
Nepheline syenite is a silica-deficient intrusive rock, which when processed into a low-Fe feldspar-nepheline may become a commercial industrial mineral. Practical applications as a raw material include glass, ceramics, flatware manufacturing, and as pigment and filler [
6]. The world leading nepheline syenite producers are Russia, Canada, and Norway with roughly 84, 10, and 6% of the world’s production, respectively [
4,
7]. The information used to define the industrial mineral quality relates to consumer specifications: minimum SiO
2 wt %, maximum Fe
2O
3 wt % or whiteness, among others [
8].
Geometallurgy is a holistic mining-oriented discipline that incorporates information from the whole mining value chain to increase resource performance and reduce risks [
9,
10,
11]. The ultimate goal of geometallurgy is to generate quantitative georeferenced data, which can be integrated into the 3D model and production plan of the mine [
12,
13]. To assess quantitative data, prediction models can be developed for operational purposes [
14,
15,
16]. These models aim to predict multiple rock characteristics and processing behavior, and are usually restricted to the modal mineralogy of the rock. Modal mineralogy prediction is commonly based on bulk chemical data, and these methodologies known as element-to-mineral conversion (EMC).
One of the main driving forces behind the application and development of EMC is to have access to modal mineralogy data in less time and at lower costs. Despite the relevance of mineralogical data in the development of the mining industry, the time span required for its acquisition is still a shortcoming that must be overcome in the mining industry. Mineralogical and chemical data are not the only information relevant to EMC. Mineral chemistry is also a subject to keep in mind when applying this type of methodology. Mineral chemistry is the common ground between modal mineralogy and bulk chemistry.
This study compared two EMC methodologies using available X-ray fluorescence and X-ray diffraction data. The mineral chemistry of the main minerals present in the deposit was acquired with electron probe microanalysis (EPMA) to evaluate its influence on the mineralogical estimations.
5. Discussion
It is common at processing plants to use bulk chemistry data rather than modal mineralogical data. However, mineralogical data could help to reduce risks and uncertainties along the whole processing chain [
45]. Finding a cheap, fast, and reliable method to estimate modal mineralogy is of great value for the mining industry and geometallurgy.
Most of the estimated systems are underdetermined, i.e., they have more unknowns than equations. Obtaining a solution for this type of problem requires the acceptance of un-avoidable errors and assumptions. The EMC methodologies proposed in the present paper were not an exception. The methodologies used LOI and unknown as input data in the bulk chemistry, a pre-defined mineral list in the Rietveld mineralogy and a set of restrictions to each method.
The bulk chemistry and mineral list from modal mineralogy used in the EMC had their drawbacks. Regarding bulk chemistry, the addition of more elements can help in the determination of the system. The use of minor elements can definitely increase the level of detail for the samples analyzed at the expense of consuming more time and risking further sample preparation errors. This is the same for other major elements such as oxygen, carbon, and sulphur.
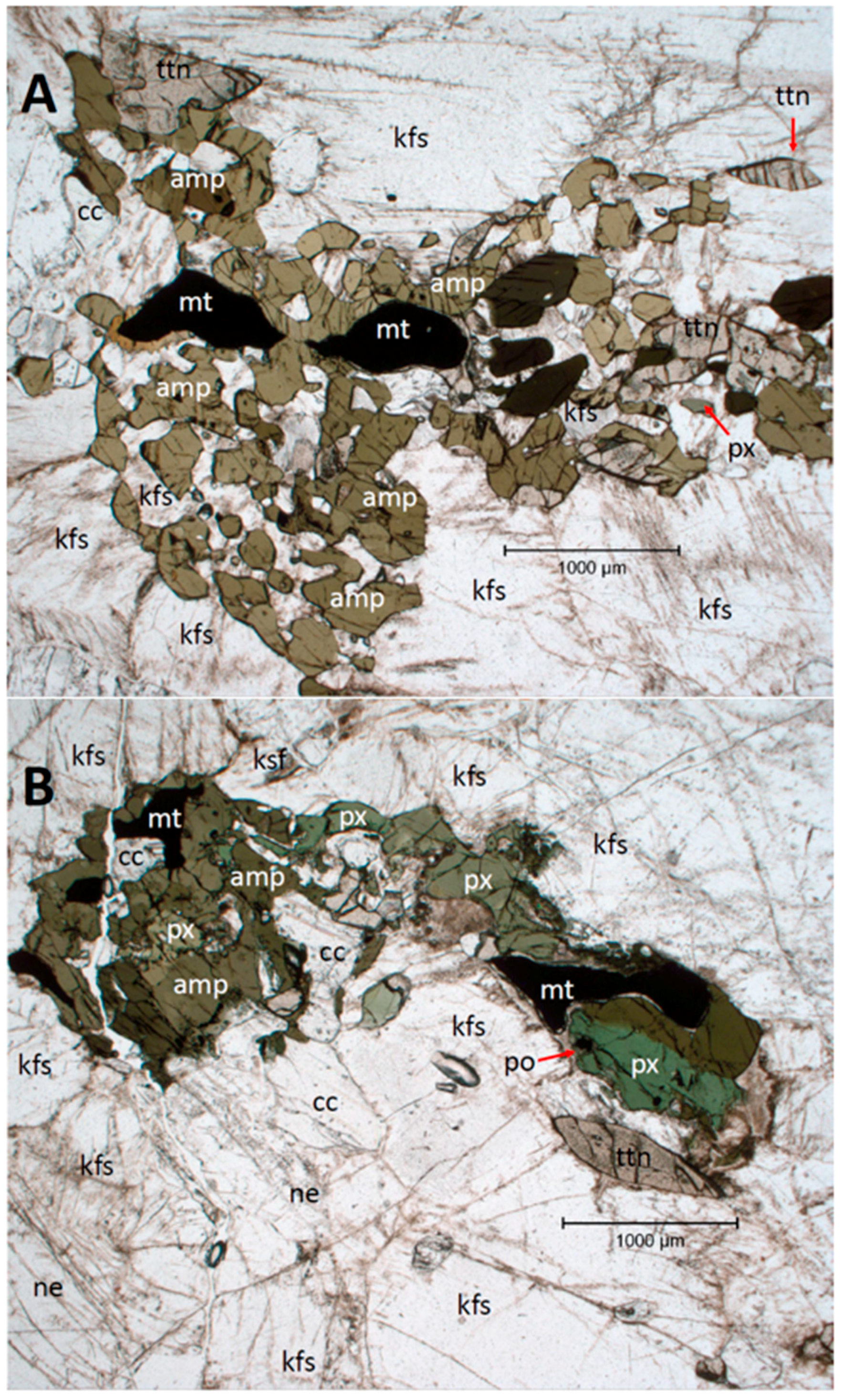
As for modal mineralogy, the mineral list used in the Rietveld XRD analyses is another pre-assumption imposed on the system. The use of a pre-defined mineral list to apply to all the analyses can have drawbacks during estimations. For example, the mineral list missed minerals known to be present in the rock such as Fe and Ti oxides or different alteration zeolites, as the ones reported by [
20]. Nonetheless, their presence could not have been detected with XRD due to their low concentration in the rock (i.e., traces) or due to the low level of alteration presented in the samples analyzed (i.e., away from exposed sites). The detection limit of XRD is not as low as other techniques like SEM, the acquisition time and cost associated to XRD acquisition makes it an acceptable option for modal mineralogical quantifications, and therefore accepting the drawbacks of the XRD data.
The use of XRD modal mineralogy as input data is one of the options for EMC estimations. EMC estimations could be improved if Rietveld modal mineralogy was improved. There are different ways to improve Rietveld modal mineralogy: by input EPMA data of the deposit and particularly from the samples analyzed input during modelling, or by running single-crystal XRD analyses for the main minerals of the deposit and then using that information in the modelling.
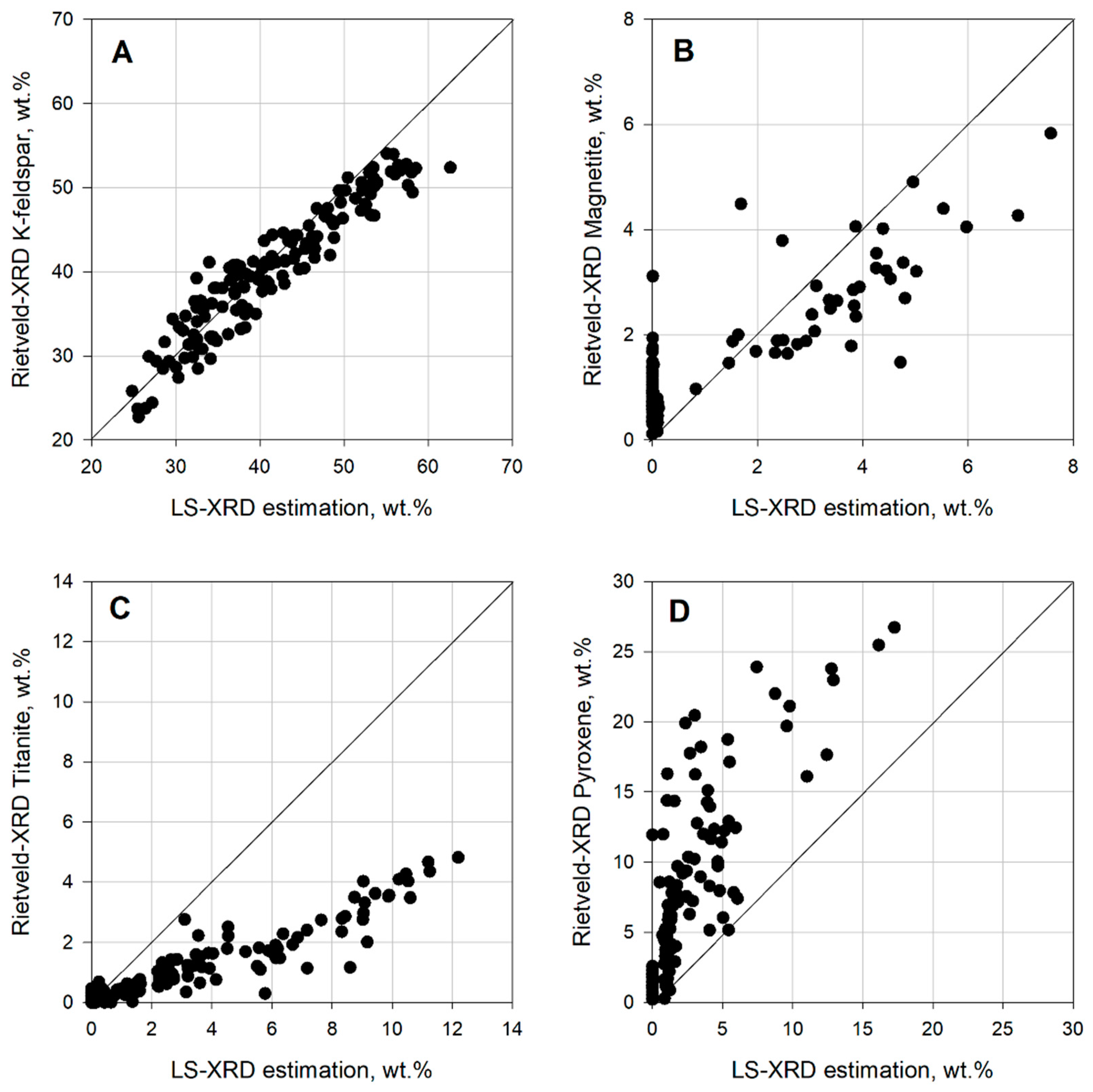
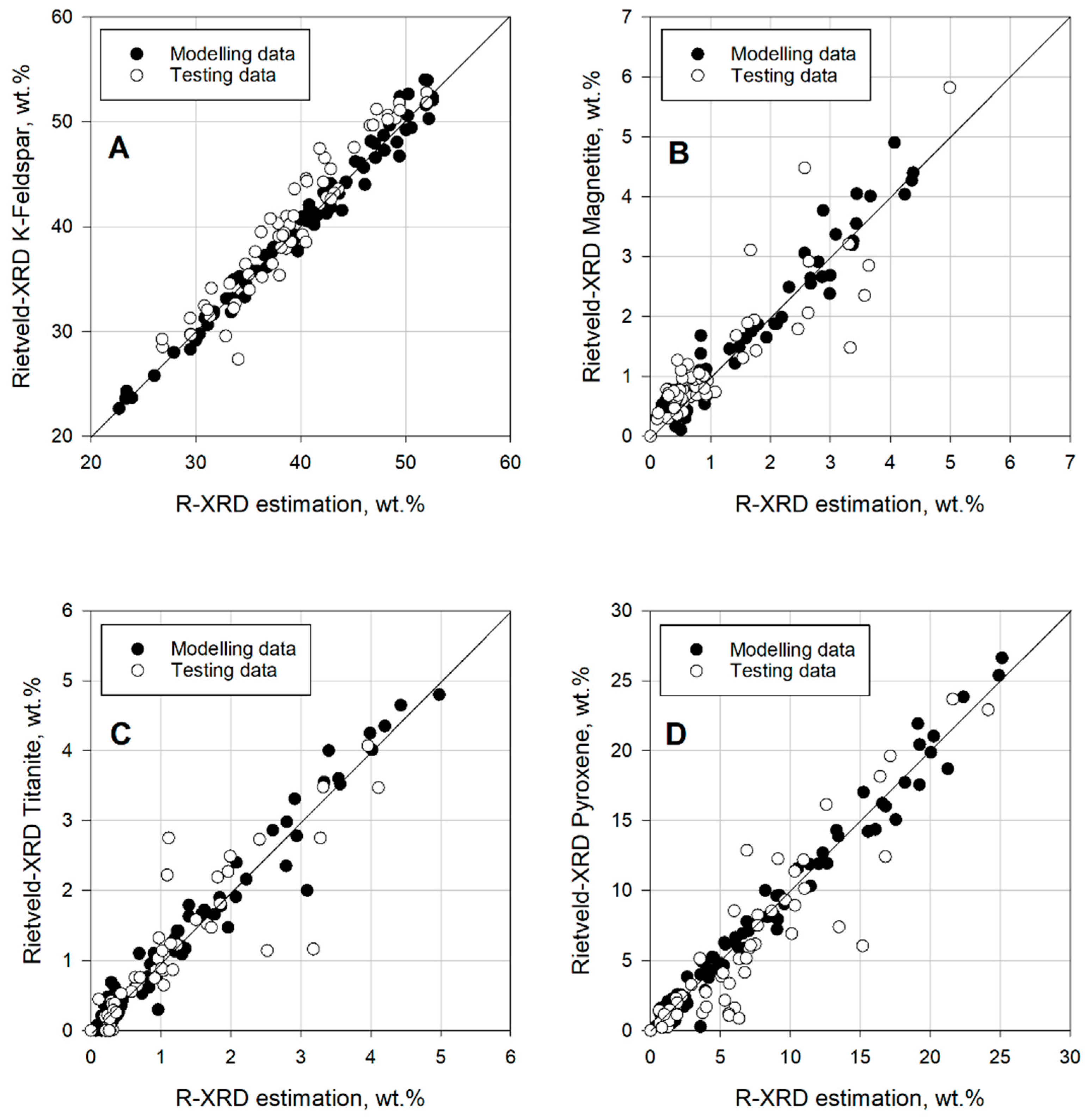
The EMC methods used showed an acceptable performance estimating abundant minerals, such as K-feldspar. The data points from K-feldspar were closer to the plot’s diagonal. Nonetheless, both methods failed to estimate correctly minerals with lower concentrations, such as titanite, magnetite, and pyroxene. Similar issues have been reported when estimating minerals in low concentration, see [
16,
23,
39]. The problems when comparing to mineralogy, in this case are the accuracy of the XRD data and the way the estimation method behaves. For example, it is possible that certain measured elements estimated to be in a particular mineral actually belong to another mineral on the list, or even worse, to a mineral not considered at all. One example of estimation method could be the mineral titanite, which was consistently estimated incorrectly in the LS-XRD method. The cases of magnetite and pyroxene, both minerals in low quantity, also show errors with the same method. The R-XRD estimations showed better results estimating modal mineralogy than the LS-method. The trends are more consistent and closer to the diagonals on each plot. It is important to keep in mind that a method such as the R-XRD will produce regression results, which will look closer to the estimation during modelling. Moreover, the R-XRD method can be applied, and used to a defined bulk chemistry (e.g., specific lithology or concentrates). The R-XRD methodology might only be valid within restricted chemical populations, thus making the method suitable for its use at operational scale only by applying it to those chemical populations.
The obtained EMC estimations can be compared with the estimated modal mineralogy and bulk chemistry, after calculating it back from the modal mineralogy. When comparing bulk chemistry differences, the LS-XRD method yields better results than the R-XRD method. Although the results of the latter are closer to the original differences between measured sets, thus similar error between both XRF data and Rietveld mineralogy. Smaller bulk chemistry errors indicate better results towards known calibrated data i.e., XRF measurements. Nonetheless, closer results do not necessarily mean better results. There is a bias when attempting such a comparison. This bias can be the assumptions made during quantification (e.g., pre-defined mineral list) and estimation (e.g., restrictions used).
When comparing modal mineralogy differences, the R-XRD estimation showed better results. Again, this does not necessarily mean that the method itself is better. It is necessary to keep in mind that the modal mineralogy comparison is uncalibrated; the diffraction patterns acquired and quantified by the Rietveld method are standard-less. This means that regardless of the quantification method used, the true modal mineralogy of the sample will remain unknown because the sample analyzed is unknown. This is not an issue if the analyzed samples have a known mineral composition, which is the case with synthetic samples as shown by [
42].
Normalizing mineral chemistries gave a more realistic idea of the mineral chemistry of the minerals present in the deposit. Normalization takes into consideration the atom-site distribution of the main atoms in the structure and its substitutions. Normalizing mineral chemistries refines the composition by adding or removing elements that otherwise would not be present in the mineral structures measured by EPMA (e.g., H2O wt % or C wt %) and are required in EMC estimations.
Mineral chemistry is not the main influence in EMC estimations. The use of different mineral chemistries showed no strong improvement on the mineralogical estimation. In
Figure 7, the Reference 2 case showed an improvement when using mineral chemistry from that sample instead of the average mineral chemistry for the deposit in the LS-XRD estimation. Nonetheless, in all other cases, mineral chemistry did not show a significant change in mineralogical estimation.
The use of mineral chemistries from the minerals present in the deposit is necessary not only from a geological point of view, but also for processing and mineral estimations.
The use of average mineral chemistry values, obtained from different points in the deposit, for EMC estimations generally gave good results. The use of mineral chemistry leads to the idea of a new data layer to be included in mine planning and in a geometallurgical model: spatial mineral chemistry variations for the implementation of EMC estimations. Understanding and quantifying elemental variations of the minerals is not only useful geological information. Those mineral variations can be implemented in mine planning by quantification campaigns at the mine. Additionally, they can be used in estimating mineralogy for an improved mineralogical estimation that can be implemented as a routine at operational scale, thereby reducing uncertainties in a processing point-of-view.
The use of EMC method is a time efficient and low cost technique to estimate mineralogy based on bulk chemistry. It is time effective and low cost because virtually no extra analysis is required besides that needed to apply the methodology.