Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Acid Treating
2.3. Experimental Procedure
2.4. Characterizations
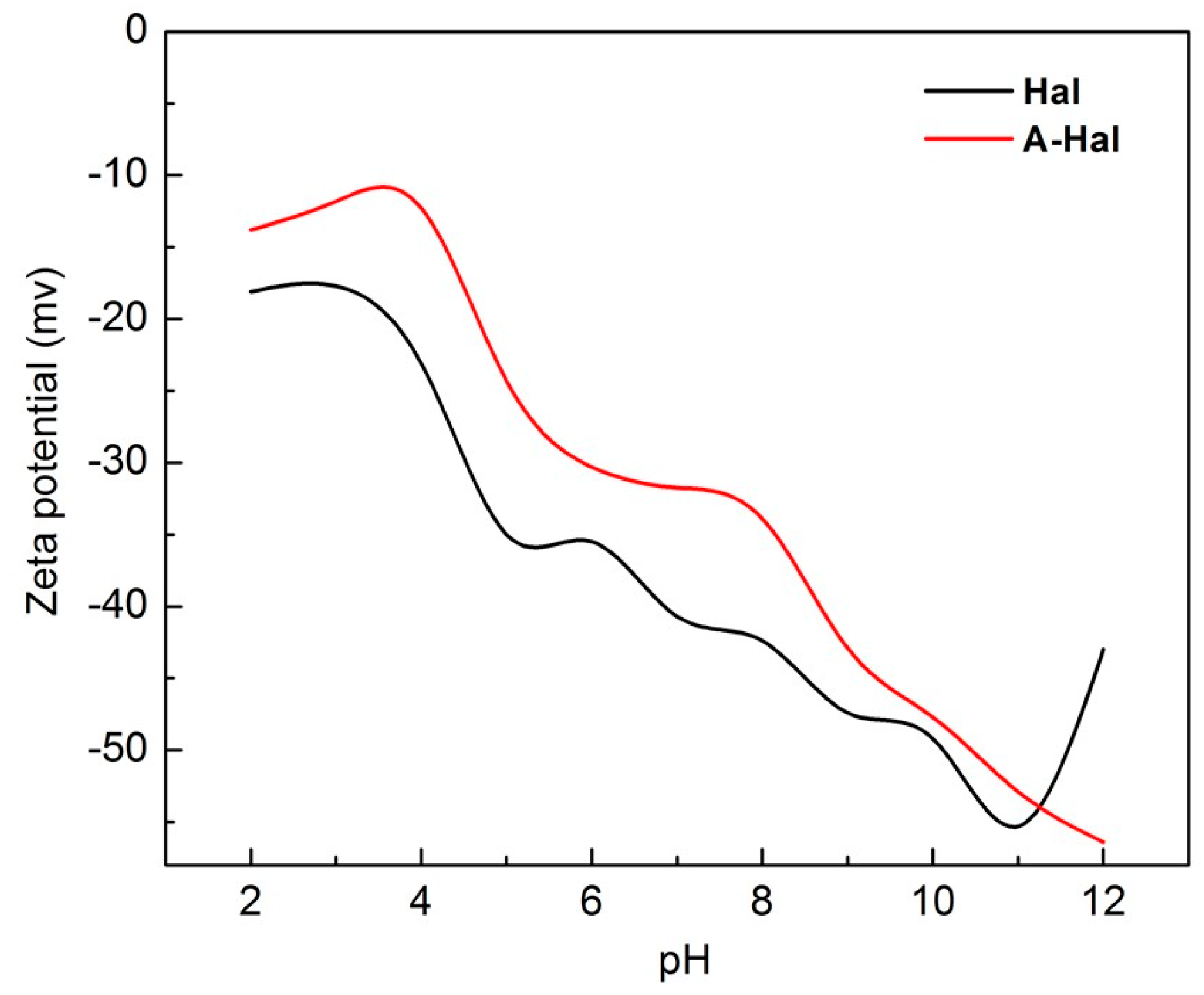
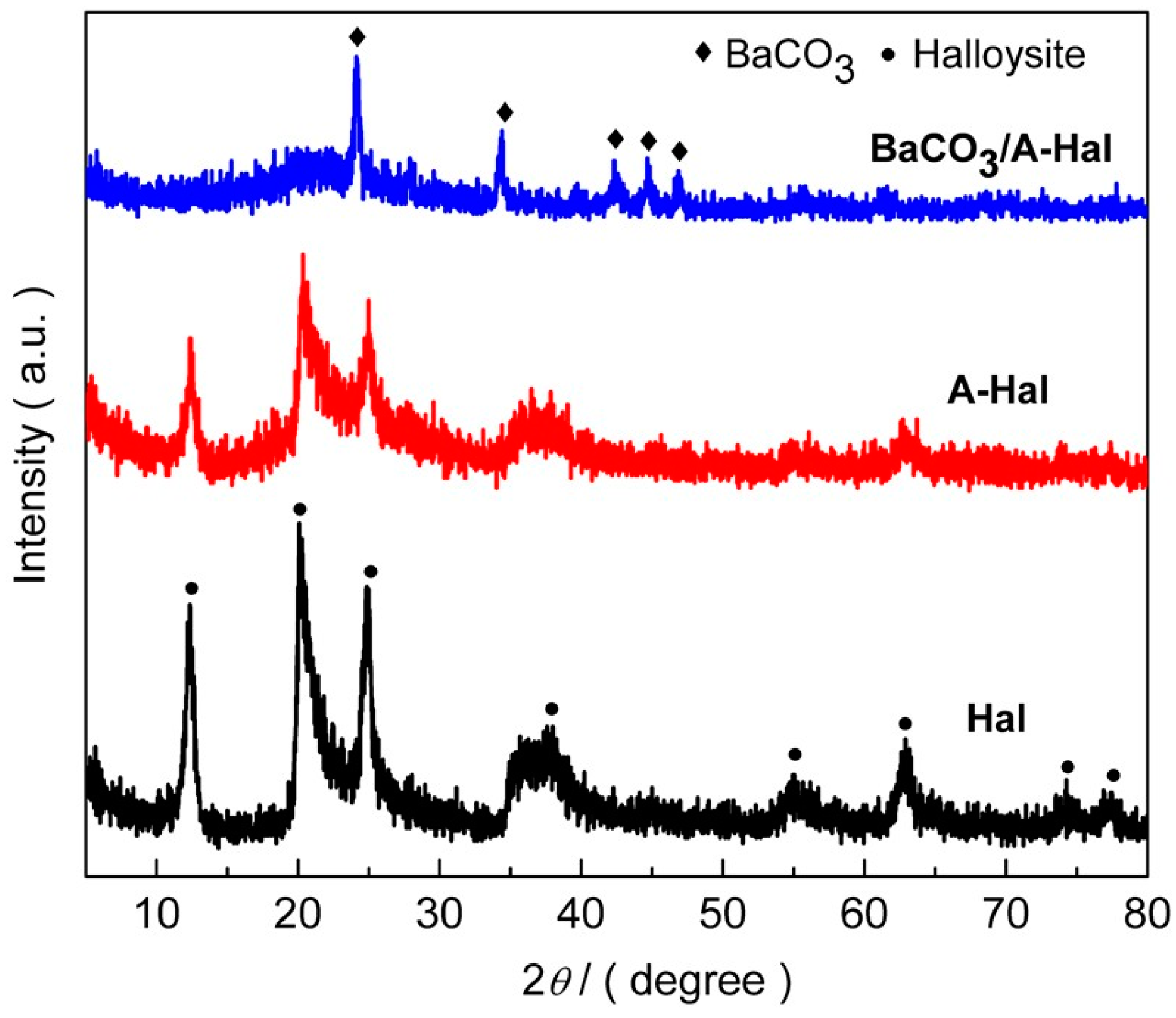
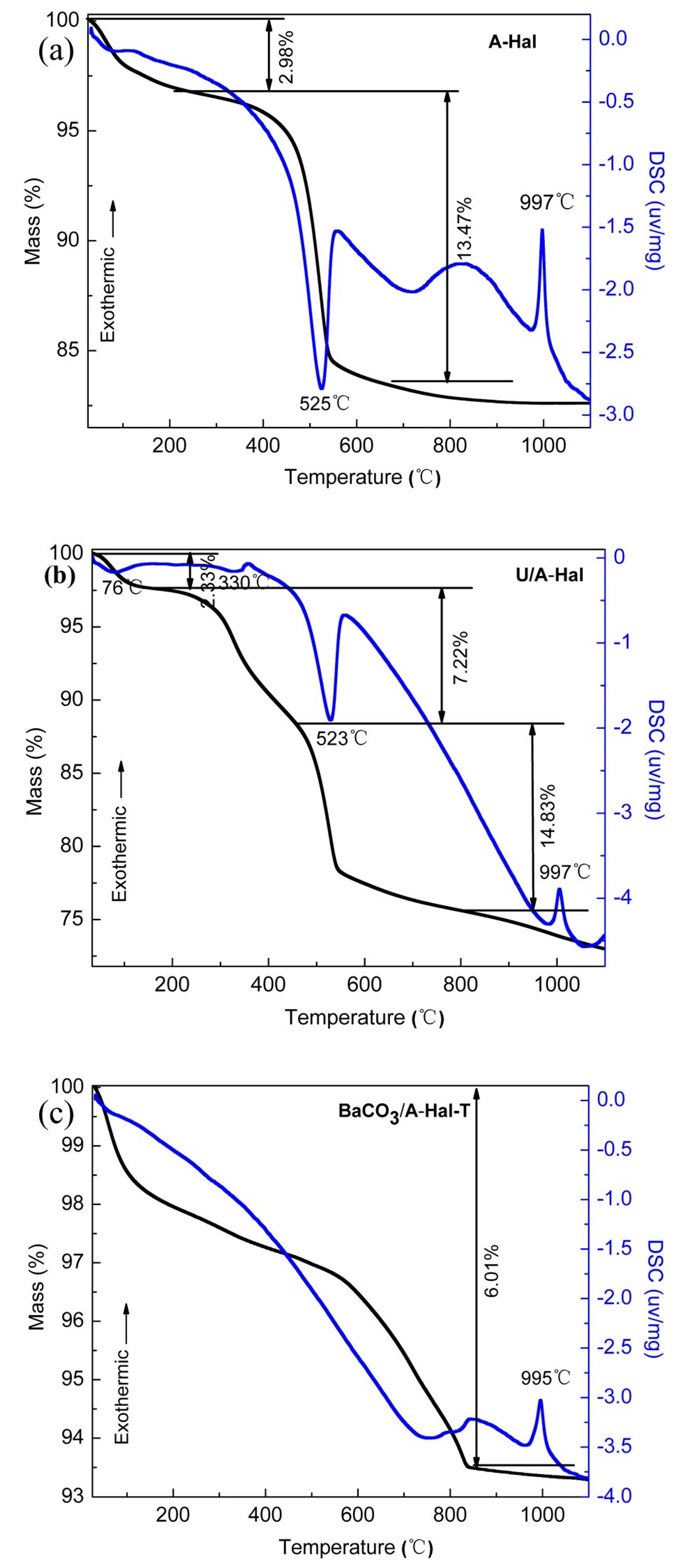
3. Results and Discussion
4. Assembly Mechanism Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, Y.; Tang, A.; Yang, H.; Ouyang, J. Applications and interfaces of halloysite nanocomposites. Appl. Clay Sci. 2016, 119, 8–17. [Google Scholar] [CrossRef]
- Yuan, P. Chapter 7–Thermal-Treatment-Induced Deformations and Modifications of Halloysite. Dev. Clay Sci. 2016, 7, 137–166. [Google Scholar]
- Vinokurov, V.A.; Stavitskaya, A.V.; Chudakov, Y.A.; Ivanov, E.V.; Shrestha, L.K.; Ariga, K.; Darrat, Y.A.; Lvov, Y.M. Formation of metal clusters in halloysite clay nanotubes. Sci. Technol. Adv. Mater. 2017, 18, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, S. Halloysite clay nanotubes as nano-bazookas for drug delivery. Polym. Int. 2017, 66, 1111–1118. [Google Scholar] [CrossRef]
- García, F.J.; García Rodríguez, S.; Kalytta, A.; Reller, A. Study of Natural Halloysite from the Dragon Mine, Utah (USA). Z. Anorg. Allg. Chem. 2009, 635, 790–795. [Google Scholar] [CrossRef]
- Massaro, M.; Cavallaro, G.; Colletti, C.G.; D’Azzo, G.; Guernelli, S.; Lazzara, G.; Pieraccini, S.; Riela, S. Halloysite nanotubes for efficient loading, stabilization and controlled release of insulin. J. Colloid Interface Sci. 2018, 524, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Zhou, Z.; Zhang, Y.; Yang, H. High morphological stability and structural transition of halloysite (Hunan, China) in heat treatment. Appl. Clay Sci. 2014, 101, 16–22. [Google Scholar] [CrossRef]
- Jin, J.; Zhang, Y.; Ouyang, J.; Yang, H. Halloysite nanotubes as hydrogen storage materials. Phys. Chem. Miner. 2013, 41, 323–331. [Google Scholar] [CrossRef]
- Jin, J.; Fu, L.; Yang, H.; Ouyang, J. Carbon hybridized halloysite nanotubes for high-performance hydrogen storage capacities. Sci. Rep. 2015, 5, 12429. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Weiner, M.L. Hydrogen Storage Apparatus Comprised of Halloysite. U.S. Patent 7,425,232 B2, 16 September 2008. [Google Scholar]
- Ouyang, J.; Zhao, Z.; Zhang, Y.; Yang, H. Textual properties and catalytic performances of halloysite hybrid CeO2-ZrO2 nanoparticles. J. Colloid Interface Sci. 2017, 505, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, X.; Ouyang, J.; Yang, H. Palladium nanoparticles deposited on silanized halloysite nanotubes: Synthesis, characterization and enhanced catalytic property. Sci. Rep. 2013, 3, 2948. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Wang, Q.; Wang, H.; Yang, Y. Catalytically active silver nanoparticles loaded in the lumen of halloysite nanotubes via electrostatic interactions. J. Mater. Sci. 2017, 52, 8391–8400. [Google Scholar] [CrossRef]
- Massaro, M.; Colletti, C.G.; Lazzara, G.; Milioto, S.; Noto, R.; Riela, S. Halloysite nanotubes as support for metal-based catalysts. J. Mater. Chem. A 2017, 5, 13276–13293. [Google Scholar] [CrossRef]
- Wang, P.; Lv, A.; Hu, J.; Xu, J.A.; Lu, G. In Situ Synthesis of SAPO-34 Grown onto Fully Calcined Kaolin Microspheres and Its Catalytic Properties for the MTO Reaction. Ind. Eng. Chem. Res. 2011, 50, 9989–9997. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, H. Zns/halloysite nanocomposites: Synthesis, characterization and enhanced photocatalytic activity. Funct. Mater. Lett. 2013, 6, 50013. [Google Scholar] [CrossRef]
- Zhou, Z.; Ouyang, J.; Yang, H.; Tang, A. Three-way catalytic performances of Pd loaded halloysite-Ce0.5Zr0.5O2 hybrid materials. Appl. Clay Sci. 2016, 121–122, 63–70. [Google Scholar] [CrossRef]
- Ouyang, J.; Zhao, Z.; Yang, H.; Zhang, Y.; Tang, A. Large-scale synthesis of sub-micro sized halloysite-composed CZA with enhanced catalysis performances. Appl. Clay Sci. 2018, 152, 221–229. [Google Scholar] [CrossRef]
- Ouyang, J.; Guo, B.; Fu, L.; Yang, H.; Hu, Y.; Tang, A.; Long, H.; Jin, Y.; Chen, J.; Jiang, J. Radical guided selective loading of silver nanoparticles at interior lumen and out surface of halloysite nanotubes. Mater. Des. 2016, 110, 169–178. [Google Scholar] [CrossRef]
- Shchukin, D.G.; Sukhorukov, G.B.; Price, R.R.; Lvov, Y.M. Halloysite nanotubes as biomimetic nanoreactors. Small 2005, 1, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Du, Y.; Ma, X. Selective fabrication of iron oxide particles in halloysite lumen. Mater. Chem. Phys. 2015, 151, 14–17. [Google Scholar] [CrossRef]
- Zahidah, K.A.; Kakooei, S.; Ismail, M.C.; Bothi Raja, P. Halloysite nanotubes as nanocontainer for smart coating application: A review. Prog. Org. Coat. 2017, 111, 175–185. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Yang, Q.; Yang, H. Halloysite Nanotubes Supported Ag and ZnO Nanoparticles with Synergistically Enhanced Antibacterial Activity. Nanoscale Res. Lett. 2017, 12, 135. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Q.; Ouyang, J.; Yang, H.; Chang, S. Chitosan modified halloysite nanotubes as emerging porous microspheres for drug carrier. Appl. Clay Sci. 2016, 126, 306–312. [Google Scholar] [CrossRef]
- Lun, H.; Ouyang, J.; Yang, H. Natural halloysite nanotubes modified as an aspirin carrier. RSC Adv. 2014, 4, 44197–44202. [Google Scholar] [CrossRef]
- Shu, Z.; Zhang, Y.; Ouyang, J.; Yang, H. Characterization and synergetic antibacterial properties of ZnO and CeO2 supported by halloysite. Appl. Surf. Sci. 2017, 420, 833–838. [Google Scholar] [CrossRef]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.K.; Lee, S.M.; Milioto, S. Effect of Morphology and Size of Halloysite Nanotubes on Functional Pectin Bionanocomposites for Food Packaging Applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Zeng, Y.; Zhou, C.; Chen, J.; Wen, X.; Xu, S.; Cheng, J.; Lin, Y.; Pi, P. Durable underwater superoleophobic PDDA/halloysite nanotubes decorated stainless steel mesh for efficient oil–water separation. Appl. Surf. Sci. 2017, 416, 344–352. [Google Scholar] [CrossRef]
- Ganganboina, A.B.; Chowdhury, A.D.; Doong, R. New Avenue for Appendage of Graphene Quantum Dots on Halloysite Nanotubes as Anode Materials for High Performance Supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 4930–4940. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite Nanotubes: Controlled Access and Release by Smart Gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Yang, H.; Mu, D.; Pan, A.; Liang, S. Fabrication of si nanoparticles@carbon fibers composites from natural nanoclay as an advanced lithium-ion battery flexible anode. Minerals 2018, 8, 180. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Preuß, K.; Qiao, M.; Titirici, M.-M.; Varcoe, J.; Cai, Q. Halloysite-derived nitrogen doped carbon electrocatalysts for anion exchange membrane fuel cells. J. Power Sources 2017, 372, 82–90. [Google Scholar] [CrossRef]
- Niu, M.; Yang, H.; Zhang, X.; Wang, Y.; Tang, A. Amine-Impregnated Mesoporous Silica Nanotube as an Emerging Nanocomposite for CO2 Capture. ACS Appl. Mater. Interfaces 2016, 8, 17312–17320. [Google Scholar] [CrossRef] [PubMed]
- Lun, H.; Ouyang, J.; Yang, H. Enhancing dispersion of halloysite nanotubes via chemical modification. Phys. Chem. Miner. 2013, 41, 281–288. [Google Scholar] [CrossRef]
- Zhang, H. Selective modification of inner surface of halloysite nanotubes: A review. Nanotechnol. Rev. 2017, 6. [Google Scholar] [CrossRef]
- Chao, C.; Liu, J.; Wang, J.; Zhang, Y.; Zhang, B.; Zhang, Y.; Xiang, X.; Chen, R. Surface modification of halloysite nanotubes with dopamine for enzyme immobilization. ACS Appl. Mater. Interfaces 2013, 5, 10559–10564. [Google Scholar] [CrossRef] [PubMed]
- Ropp, R.C. Encyclopedia of the Alkaline Earth Compounds; Elsevier: Amsterdam, The Netherlands, 2013; pp. 1179–1187. [Google Scholar]
- Brightlin, B.C.; Balamurugan, S. Magnetic, Micro-structural, and Optical Properties of Hexaferrite, BaFe12O19 Materials Synthesized by Salt Flux-Assisted Method. J. Superconduct. Nov. Magn. 2016, 30, 215–225. [Google Scholar] [CrossRef]
- Hong, T.; Chen, F.; Xia, C. Barium carbonate nanoparticle as high temperature oxygen reduction catalyst for solid oxide fuel cell. Electrochem. Commun. 2015, 51, 93–97. [Google Scholar] [CrossRef]
- Hong, T.; Chen, F.; Xia, C. Barium carbonate nanoparticle to enhance oxygen reduction activity of strontium doped lanthanum ferrite for solid oxide fuel cell. J. Power Sources 2015, 278, 741–750. [Google Scholar] [CrossRef]
- Cao, X.; Hong, T.; Yang, R.; Tian, J.-H.; Xia, C.; Dong, J.-C.; Li, J.-F. Insights into the Catalytic Activity of Barium Carbonate for Oxygen Reduction Reaction. J. Phys. Chem. C 2016, 120, 22895–22902. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Liu, T.; Huang, J. Mechanisms of Vanadium Recovery from Stone Coal by Novel BaCO3/CaO Composite Additive Roasting and Acid Leaching Technology. Minerals 2016, 6, 26. [Google Scholar] [CrossRef]
- Wang, Q.; Chen, J.; Han, K.; Wang, J.; Lu, C. Influence of BaCO3 on chlorine fixation, combustion characteristics and KCl conversion during biomass combustion. Fuel 2017, 208, 82–90. [Google Scholar] [CrossRef]
- Zhang, Q.; Chai, G.; Guo, Y.; Zhan, W.; Guo, Y.; Wang, L.; Wang, Y.; Lu, G. Gas-phase epoxidation of propylene by molecular oxygen over Ag-CuCl2/BaCO3 catalyst with low CuCl2 doping: Catalytic performance, deactivation and regeneration. J. Mol. Catal. A Chem. 2016, 424, 65–76. [Google Scholar] [CrossRef]
- Whittaker, M.L.; Smeets, P.J.M.; Asayesh-Ardakani, H.; Shahbazian-Yassar, R.; Joester, D. Multi-Step Crystallization of Barium Carbonate: Rapid Interconversion of Amorphous and Crystalline Precursors. Angew. Chem. Int. Ed. Engl. 2017, 56, 16028–16031. [Google Scholar] [CrossRef] [PubMed]
- Massoni, N.; Le Gallet, S. Investigation of the sintering of barytocalcite with BaCO3 as a secondary phase for immobilizing carbon-14. J. Nucl. Mater. 2016, 476, 13–19. [Google Scholar] [CrossRef]
- Zhang, H.; Kong, W.; Tan, T.; Baeyens, J. High-efficiency concentrated solar power plants need appropriate materials for high-temperature heat capture, conveying and storage. Energy 2017, 139, 52–64. [Google Scholar] [CrossRef]
- Disawal, S.; Qiu, J.; Elmore, B.B.; Lvov, Y.M. Two-step sequential reaction catalyzed by layer-by-layer assembled urease and arginase multilayers. Colloids Surf. B Biointerfaces 2003, 32, 145–156. [Google Scholar] [CrossRef]
- Zhang, Q.; Yan, Z.; Ouyang, J.; Zhang, Y.; Yang, H.; Chen, D. Chemically modified kaolinite nanolayers for the removal of organic pollutants. Appl. Clay Sci. 2018, 157, 283–290. [Google Scholar] [CrossRef]
- Fu, L.; Yang, H.; Tang, A.; Hu, Y. Engineering a tubular mesoporous silica nanocontainer with well-preserved clay shell from natural halloysite. Nano Res. 2017, 10, 2782–2799. [Google Scholar] [CrossRef]
- Faiza, B.G.L. Surface and Interface Chemistry of Clay Minerals. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 147–150. [Google Scholar]
- Li, Y.; Zhang, Y.; Zhang, Y.; Sun, J.; Wang, Z. Thermal behavior analysis of halloysite–dimethylsulfoxide intercalation complex. J. Therm. Anal. Calorim. 2017, 129, 985–990. [Google Scholar] [CrossRef]
- Zhai, R.; Zhang, B.; Liu, L.; Xie, Y.; Zhang, H.; Liu, J. Immobilization of enzyme biocatalyst on natural halloysite nanotubes. Catal. Commun. 2010, 12, 259–263. [Google Scholar] [CrossRef]
- Ouyang, J.; Mu, D.; Zhang, Y.; Yang, H. Mineralogy and Physico-Chemical Data of Two Newly Discovered Halloysite in China and Their Contrasts with Some Typical Minerals. Minerals 2018, 8, 108. [Google Scholar] [CrossRef]
- Vial, S.; Forano, C.; Shan, D.; Mousty, C.; Barhoumi, H.; Martelet, C.; Jaffrezic, N. Nanohybrid-layered double hydroxides/urease materials: Synthesis and application to urea biosensors. Mater. Sci. Eng. C 2006, 26, 387–393. [Google Scholar] [CrossRef]
- Yuan, P.; Tan, D.; Aannabi-Bergaya, F.; Yan, W.; Fan, M.; Liu, D.; He, H. Changes in structure, morphology, porosity, and surface activity of mesoporous halloysite nanotubes under heating. Clays Clay Miner. 2012, 60, 561–573. [Google Scholar] [CrossRef]
- György, E.; Sima, F.; Mihailescu, I.N.; Smausz, T.B.; Hopp, D.; Predoi, L.E.; Sima, S.M. Biomolecular urease thin films grown by laser techniques for blood diagnostic applications. Mater. Sci. Eng. C 2010, 30, 537–541. [Google Scholar]
- Abdullayev, E.; Joshi, A.; Wei, W.; Zhao, Y.; Lvov, Y. Enlargement of halloysite clay nanotube lumen by selective etching of aluminum oxide. ACS Nano 2012, 6, 7216–7226. [Google Scholar] [CrossRef] [PubMed]
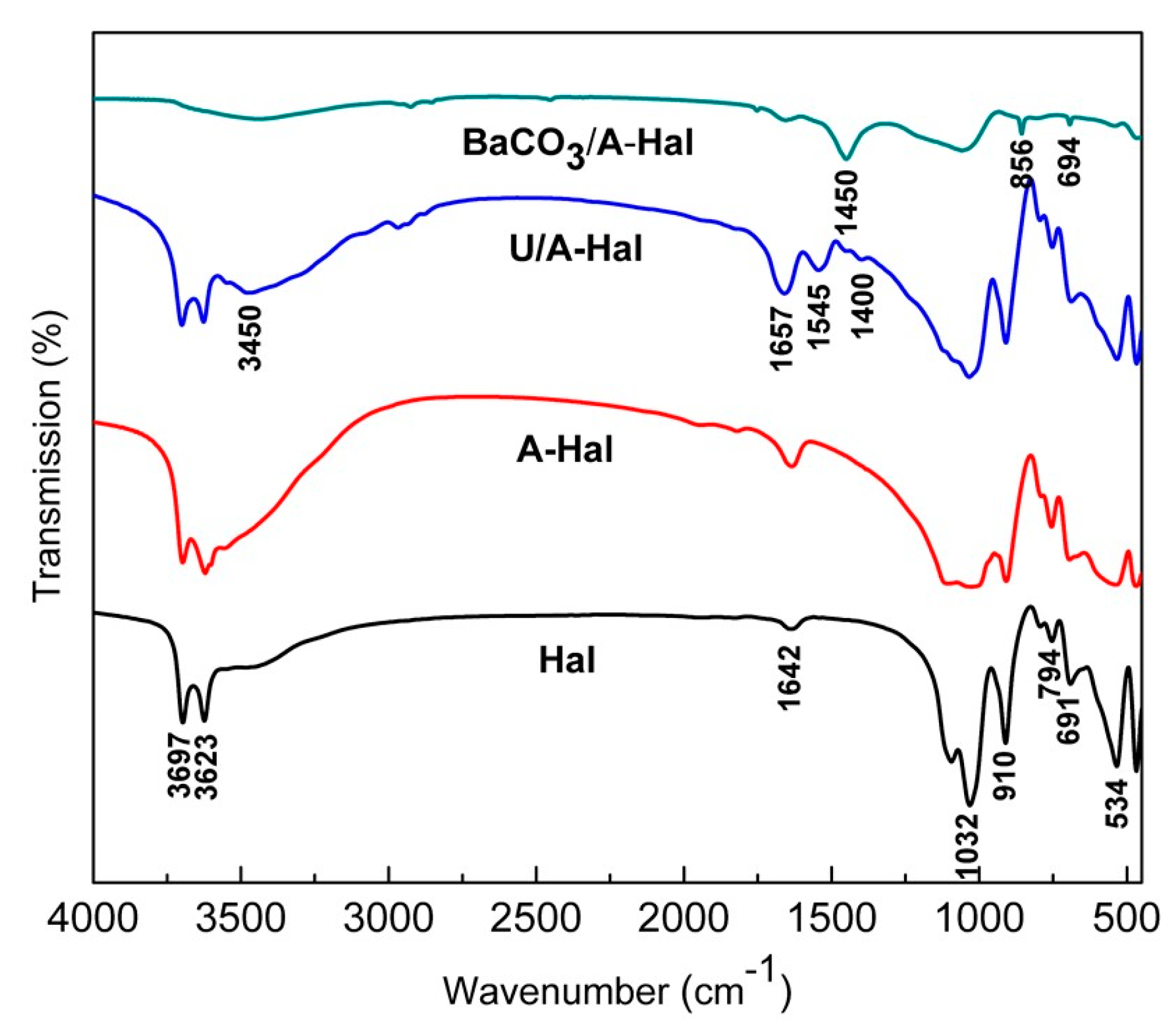
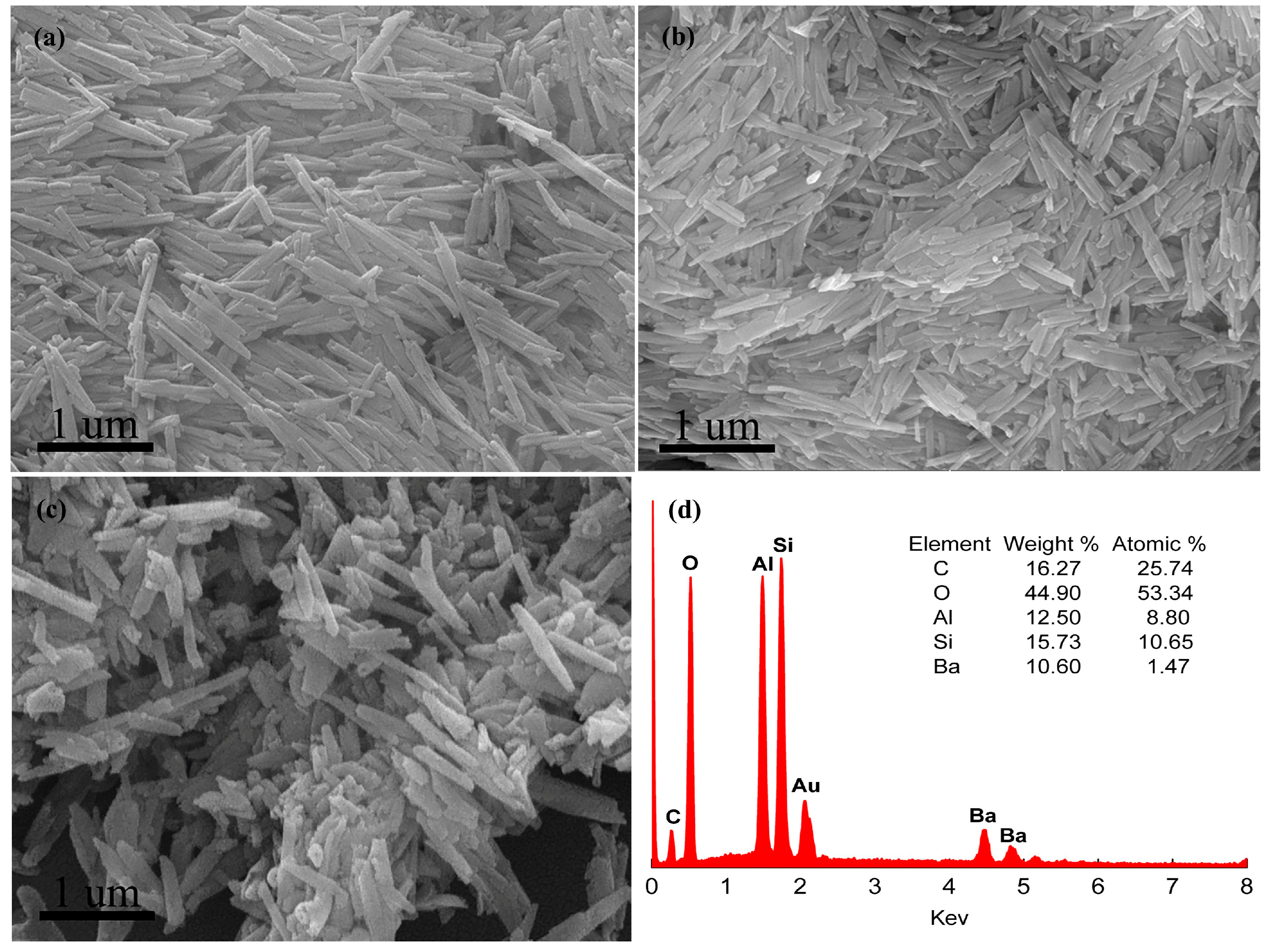
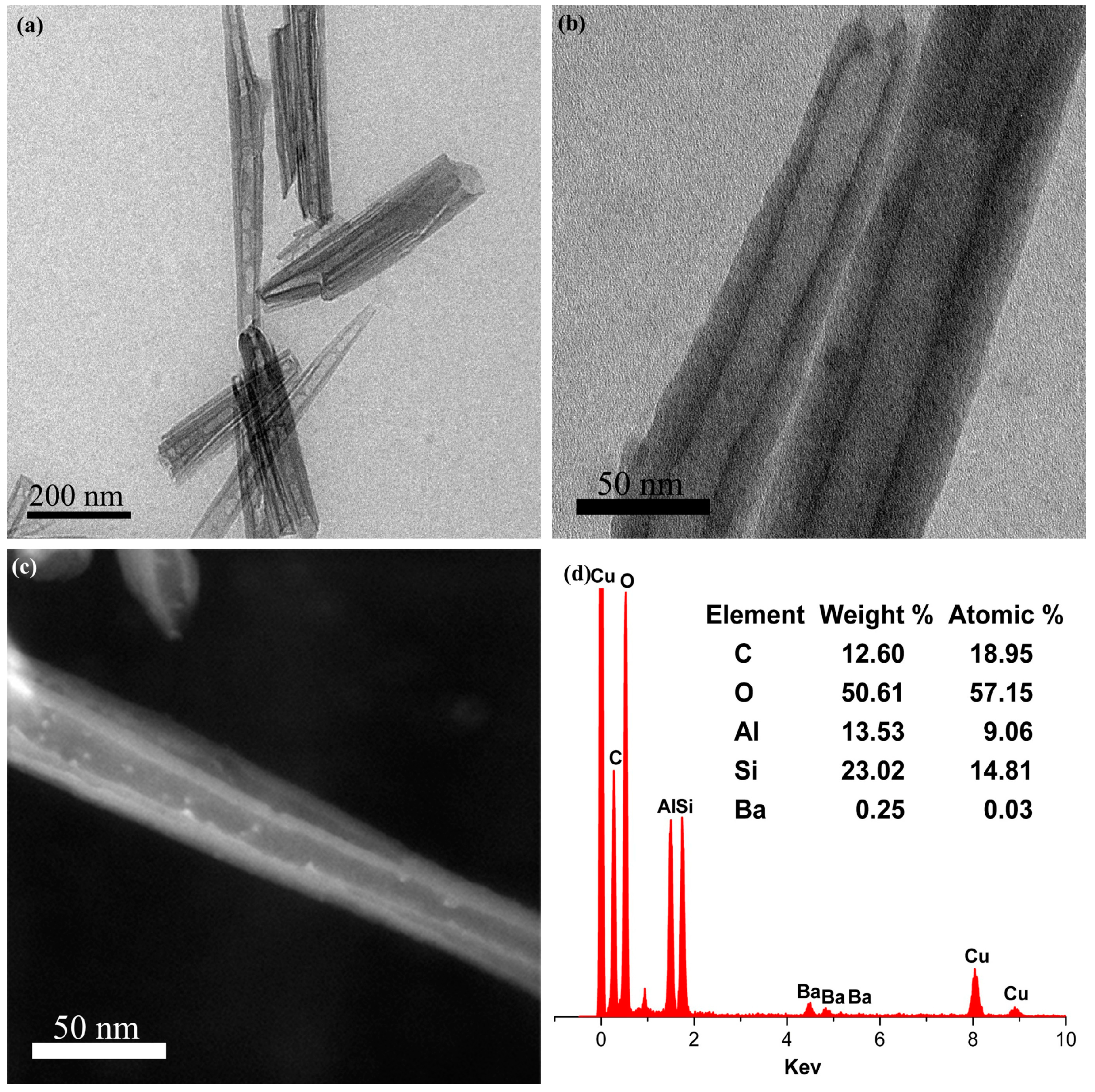
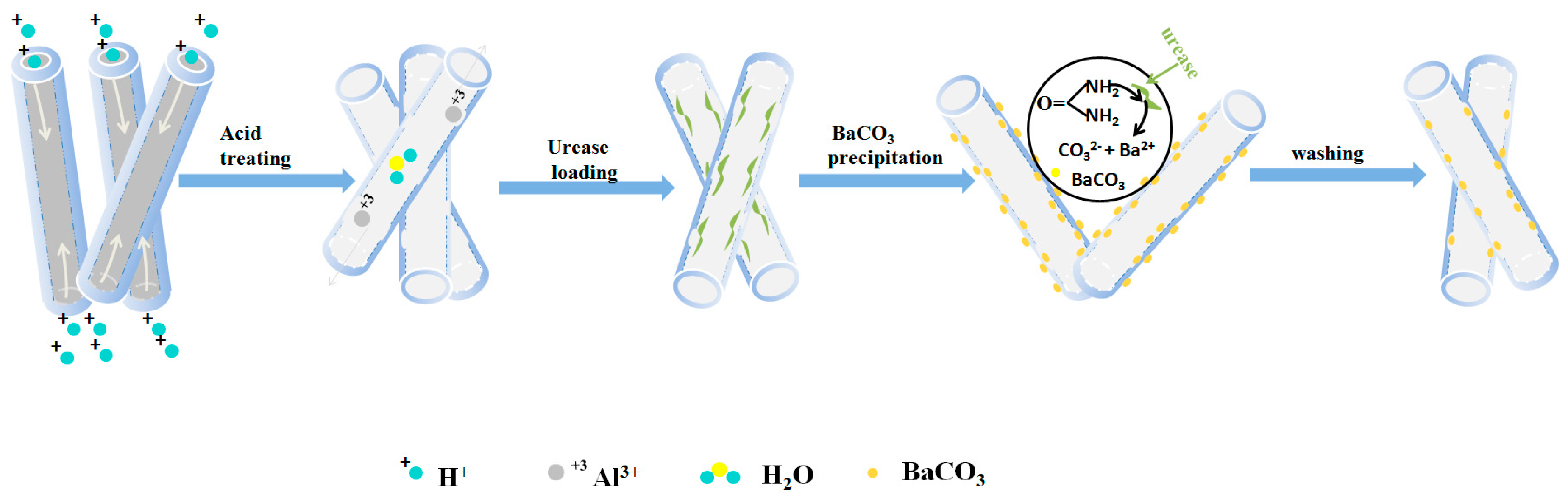
| Position/cm−1 | Assignments |
|---|---|
| 3739 | –O–H stretching of adsorbed water |
| 3695 | stretching of perpendicular surface –O–H |
| 3624 | –O–H stretching of inner hydroxyls |
| 3450 | stretching vibration peaks of O–H in water molecules |
| 1642 | bending of –OH in adsorbed water |
| 1040 | (Broad band) Si–O stretching |
| 911 | –O–H deformation of inner hydroxyls |
| 691 | perpendicular Si–O stretching |
| 535 | bending deformation of Al–O–Si |
| 1657 | stretching and deformation mode of C=O (amide I) |
| 1545 | stretching and deformation mode of N–H (amide II) |
| 1400 | C–H vibrations of the CH2 group |
| 1450 | non-stretching vibration of CO32− |
| 856 | out-of-plane bending vibration of CO32− |
| 794 | symmetric stretching of Si–O |
| 694 | in-plane plane bending vibration of CO32− |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouyang, J.; Mu, D.; Zhang, Y.; Yang, H.; Suib, S.L. Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes. Minerals 2018, 8, 296. https://doi.org/10.3390/min8070296
Ouyang J, Mu D, Zhang Y, Yang H, Suib SL. Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes. Minerals. 2018; 8(7):296. https://doi.org/10.3390/min8070296
Chicago/Turabian StyleOuyang, Jing, Dawei Mu, Yi Zhang, Huaming Yang, and Steven L. Suib. 2018. "Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes" Minerals 8, no. 7: 296. https://doi.org/10.3390/min8070296
APA StyleOuyang, J., Mu, D., Zhang, Y., Yang, H., & Suib, S. L. (2018). Selective Fabrication of Barium Carbonate Nanoparticles in the Lumen of Halloysite Nanotubes. Minerals, 8(7), 296. https://doi.org/10.3390/min8070296







