Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Li, X.; Chen, H.; Li, H.; Liu, L.; Lu, Z.; Zhang, T.; Duan, W. Integration of form-stable paraffin/nanosilica phase change material composites into vacuum insulation panels for thermal energy storage. Appl. Energy 2015, 159, 601–609. [Google Scholar] [CrossRef]
- Ma, T.; Yang, H.; Zhang, Y.; Lu, L.; Wang, X. Using phase change materials in photovoltaic systems for thermal regulation and electrical efficiency improvement: A review and outlook. Renew. Sust. Energy Rev. 2015, 43, 1273–1284. [Google Scholar] [CrossRef]
- Asbik, M.; Ansari, O.; Bah, A.; Zari, N.; Mimet, A.; El-Ghetany, H. Exergy analysis of solar desalination still combined with heat storage system using phase change material (PCM). Desalination 2016, 381, 26–37. [Google Scholar] [CrossRef]
- Tian, B.; Yang, W.; Luo, L.; Wang, J.; Zhang, K.; Fan, J.; Wu, J.; Xing, T. Synergistic enhancement of thermal conductivity for expanded graphite and carbon fiber in paraffin/EVA form-stable phase change materials. Sol. Energy 2016, 127, 48–55. [Google Scholar] [CrossRef]
- Nithyanandam, K.; Pitchumani, R. Computational studies on a latent thermal energy storage system with integral heat pipes for concentrating solar power. Appl. Energy 2013, 103, 400–415. [Google Scholar] [CrossRef]
- Tian, Y.; Zhao, C.Y. A review of solar collectors and thermal energy storage in solar thermal applications. Appl. Energy 2013, 104, 538–553. [Google Scholar] [CrossRef]
- Nomura, T.; Okinaka, N.; Akiyama, T. Impregnation of porous material with phase change material for thermal energy storage. Mater. Chem. Phys. 2009, 115, 846–850. [Google Scholar] [CrossRef]
- Zhou, D.; Zhao, C.Y.; Tian, Y. Review on thermal energy storage with phase change materials (PCMs) in building applications. Appl. Energy 2012, 92, 593–605. [Google Scholar] [CrossRef]
- Zhou, T.Y.; Darkwa, J.; Kokogiannakis, G. Thermal evaluation of laminated composite phase change material gypsum board under dynamic conditions. Renew. Energy 2015, 78, 448–456. [Google Scholar] [CrossRef]
- Liu, H.; Awbi, H.B. Performance of phase change material boards under natural convection. Build. Environ. 2009, 44, 1788–1793. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Q.; Zhao, Z.; Zhang, J.; Sun, Y.; Sun, L.; Xu, F.; Sawada, Y. Preparation and thermal performance of gypsum boards incorporated with microencapsulated phase change materials for thermal regulation. Sol. Energy Mat. Sol. Cells 2012, 102, 93–102. [Google Scholar] [CrossRef]
- Hunger, M.; Entrop, A.G.; Mandilaras, I.; Brouwers, H.J.H.; Founti, M. The behavior of self-compacting concrete containing micro-encapsulated Phase Change Materials. Cement Concr. Comp. 2009, 31, 731–743. [Google Scholar] [CrossRef]
- Bentz, D.P.; Turpin, R. Potential applications of phase change materials in concrete technology. Cement Concr. Comp. 2007, 29, 527–532. [Google Scholar] [CrossRef]
- Cabeza, L.F.; Castellón, C.; Nogués, M.; Medrano, M.; Leppers, R.; Zubillaga, O. Use of microencapsulated PCM in concrete walls for energy savings. Energy Build. 2007, 39, 113–119. [Google Scholar] [CrossRef]
- Chen, C.; Liu, X.; Liu, W.; Ma, M. A comparative study of myristic acid/bentonite and myristic acid/Eudragit L100 form stable phase change materials for thermal energy storage. Sol. Energy Mat. Sol. Cells 2014, 127, 14–20. [Google Scholar] [CrossRef]
- Min, L.; Wu, Z.; Kao, H.; Tan, J. Experimental investigation of preparation and thermal performances of paraffin/bentonite composite phase change material. Energy Convers. Manag. 2011, 52, 3275–3281. [Google Scholar]
- Li, C.; Fu, L.; Ouyang, J.; Yang, H. Enhanced performance and interfacial investigation of mineral-based composite phase change materials for thermal energy storage. Sci. Rep. 2013, 3, 1908. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, H.; Zok, F. Expanded vermiculite/paraffin composite as a solar thermal energy storage material. J. Am. Ceram. Soc. 2013, 96, 2793–2798. [Google Scholar] [CrossRef]
- Li, C.; Ouyang, J.; Yang, H. Novel sensible thermal storage material from natural minerals. Phys. Chem. Miner. 2013, 40, 681–689. [Google Scholar] [CrossRef]
- Wang, W.; Yang, X.; Fang, Y.; Ding, J. Preparation and performance of form-stable polyethylene glycol/silicon dioxide composites as solid–liquid phase change materials. Appl. Energy 2009, 86, 170–174. [Google Scholar] [CrossRef]
- Zhou, X.; Xiao, H.; Feng, J.; Zhang, C.; Jiang, Y. Pore structure modification of silica matrix infiltrated with paraffin as phase change material. Chem. Eng. Res. Des. 2010, 88, 1013–1017. [Google Scholar]
- Zhou, X.; Xiao, H.; Feng, J.; Zhang, C.; Jiang, Y. Preparation and thermal properties of paraffin/porous silica ceramic composite. Compos. Sci. Technol. 2009, 69, 1246–1249. [Google Scholar] [CrossRef]
- Żyrkowski, M.; Neto, R.C.; Santos, L.F.; Witkowski, K. Characterization of fly-ash cenospheres from coal-fired power plant unit. Fuel 2016, 174, 49–53. [Google Scholar] [CrossRef]
- Kumar, P.; Aslam, M.; Singh, N.; Mittal, S.; Bansal, A.; Jha, M.K.; Sarma, A.K. Characterization, activity and process optimization with a biomass-based thermal power plant's fly ash as a potential catalyst for biodiesel production. RSC Adv. 2015, 5, 9946–9954. [Google Scholar] [CrossRef]
- Shu, Y.; Wei, X.; Fang, Y.; Lan, B.; Chen, H. Removal of sulfuric acid mist from lead-acid battery plants by coal fly ash-based sorbents. J. Hazard. Mater. 2015, 286, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y.; Gu, Y.; Wang, J.; Liu, Z.; Zhang, Y.; Cao, Y.; Romero, C.E.; Pan, W. Using modified fly ash for mercury emissions control for coal-fired power plant applications in China. Fuel 2016, 181, 1230–1237. [Google Scholar] [CrossRef]
- Yao, Z.T.; Ji, X.S.; Sarker, P.K.; Tang, J.H.; Ge, L.Q.; Xia, M.S.; Xi, T.Q. A comprehensive review on the applications of coal fly ash. Earth-Sci. Rev. 2015, 141, 105–121. [Google Scholar] [CrossRef]
- Temimi, M.; Camps, J.P.; Laquerbe, M. Valorization of fly ash in the cold stabilization of clay materials. Resour. Conserv. Recycl. 1995, 15, 219–234. [Google Scholar] [CrossRef]
- Ilic, M.; Cheeseman, C.; Sollars, C.; Knight, J. Mineralogy and microstructure of sintered lignite coal fly ash. Fuel 2003, 82, 331–336. [Google Scholar] [CrossRef]
- Kashiwakura, S.; Kubo, H.; Kumagai, Y.; Kubo, H.; Matsubae-Yokoyama, K.; Nakajima, K.; Nagasaka, T. Removal of boron from coal fly ash by washing with HCl solution. Fuel 2009, 88, 1245–1250. [Google Scholar] [CrossRef]
- Xu, D.; Yang, H. Wollastonite hybridizing stearic acid as thermal energy storage material. Funct. Mater. Lett. 2014, 7, 1440011. [Google Scholar] [CrossRef]
- Liu, S.; Yan, Z.; Fu, L.; Yang, H. Hierarchical nano-activated silica nanosheets for thermal energy storage. Sol. Energy Mat. Sol. Cells 2017, 167, 140–149. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Li, X.; Ouyang, J.; Yang, H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Shen, Q.; Ouyang, J.; Zhang, Y.; Yang, H. Lauric acid/modified sepiolite composite as a form-stable phase change material for thermal energy storage. Appl. Clay Sci. 2017, 146, 14–22. [Google Scholar]
- Yang, H.; Du, C.; Hu, Y.; Jin, S.; Yang, W.; Tang, A.; Avvakumov, E.G. Preparation of porous silica from talc by mechanochemical treatment and subsequent leaching. Appl. Clay Sci. 2006, 31, 290–297. [Google Scholar] [CrossRef]
- Yang, H.; Tang, A.; Ouyang, J.; Li, M.; Mann, S. From natural attapulgite to mesoporous materials: Methodology, characterization and structural evolution. J. Phys. Chem. B 2010, 114, 2390–2398. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Yang, H. Investigation of the physicochemical aspects from natural kaolin to Al-MCM-41 mesoporous materials. J. Colloid Interf. Sci. 2012, 369, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Yang, H. Simple synthesis and characterization of nanoporous materials from talc. Clays Clay Miner. 2009, 57, 290–301. [Google Scholar] [CrossRef]
- Wang, C.; Feng, L.; Li, W.; Zheng, J.; Tian, W.; Li, X. The influence of the pore structure of the carbon materials. Sol. Energy Mat. Sol. Cells 2012, 105, 21–26. [Google Scholar] [CrossRef]
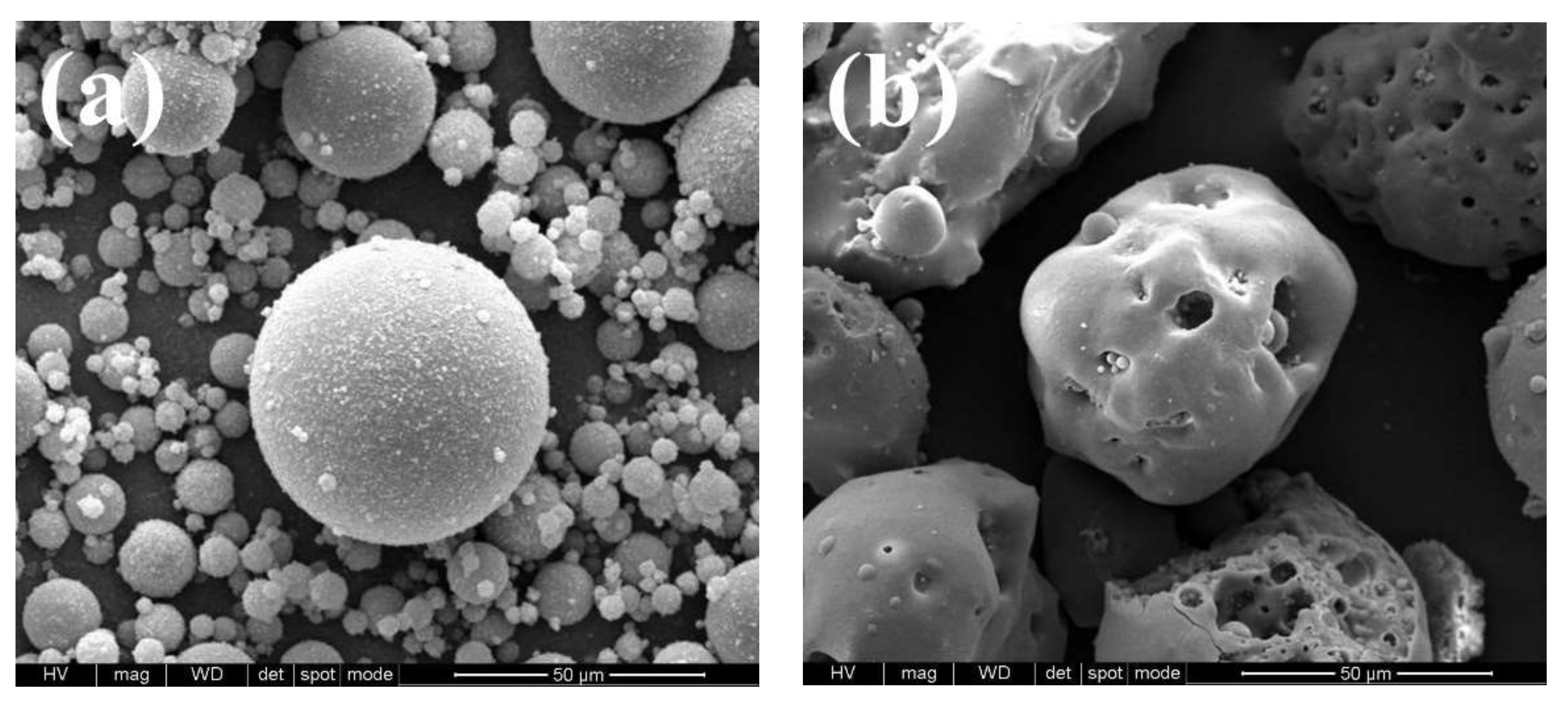

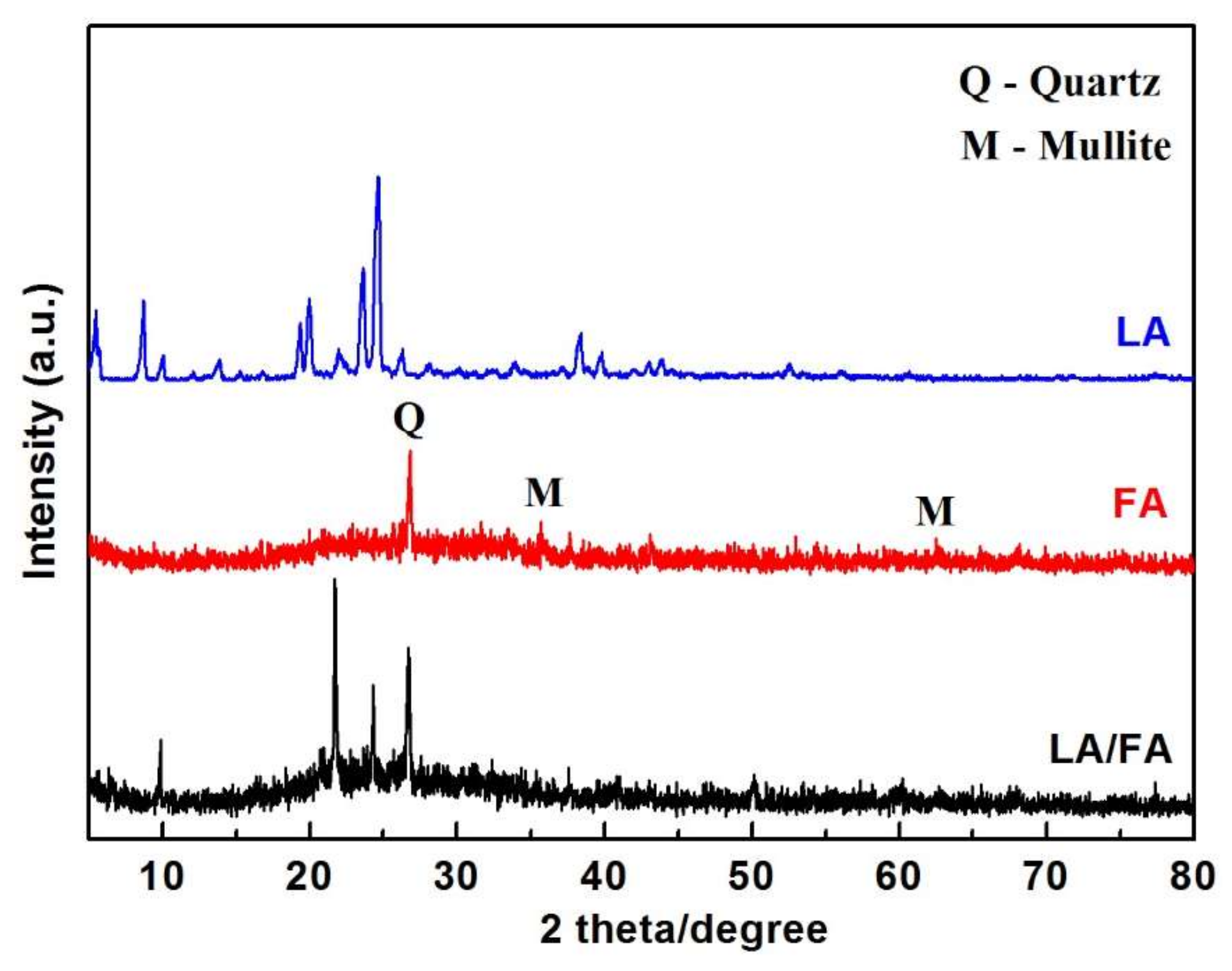
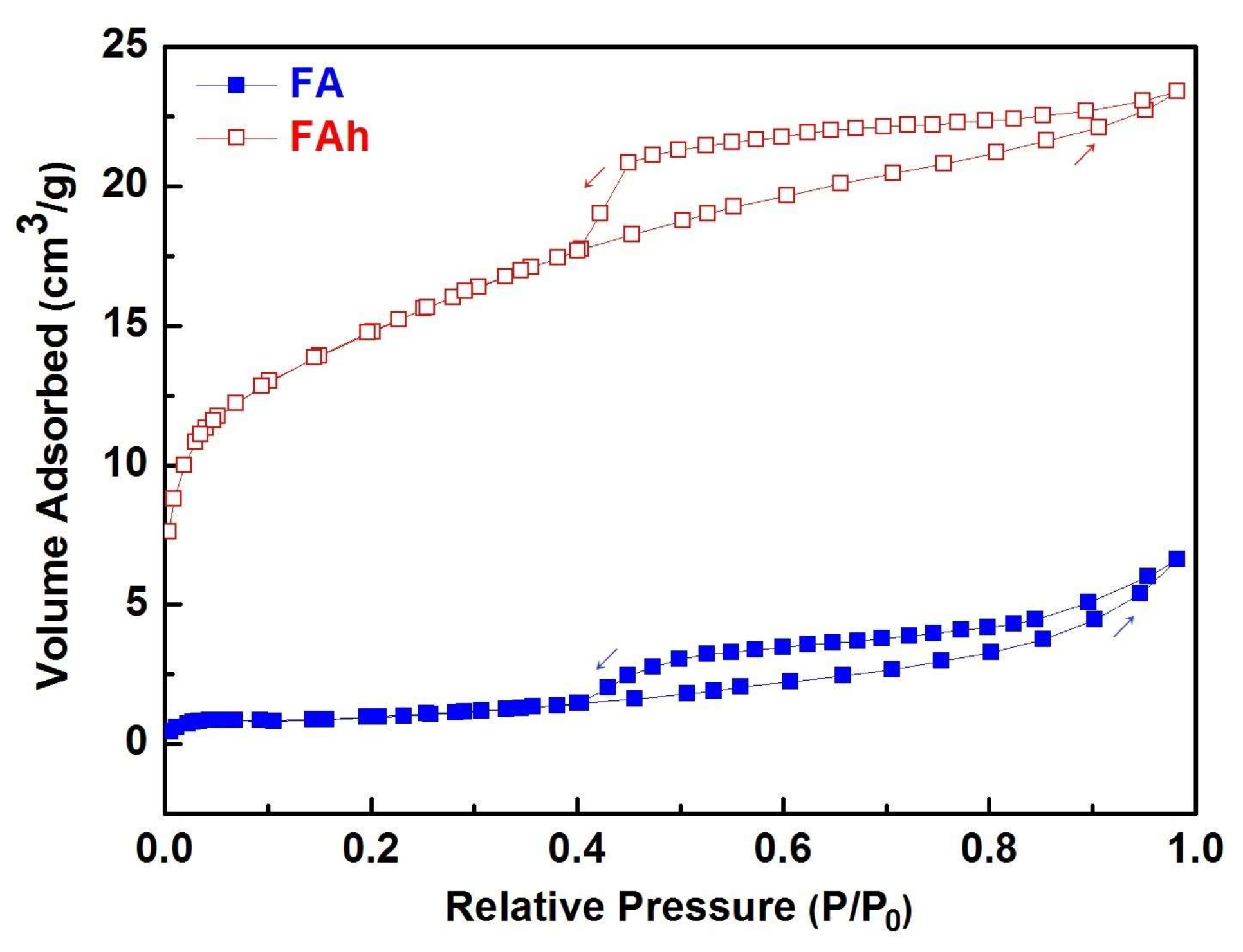
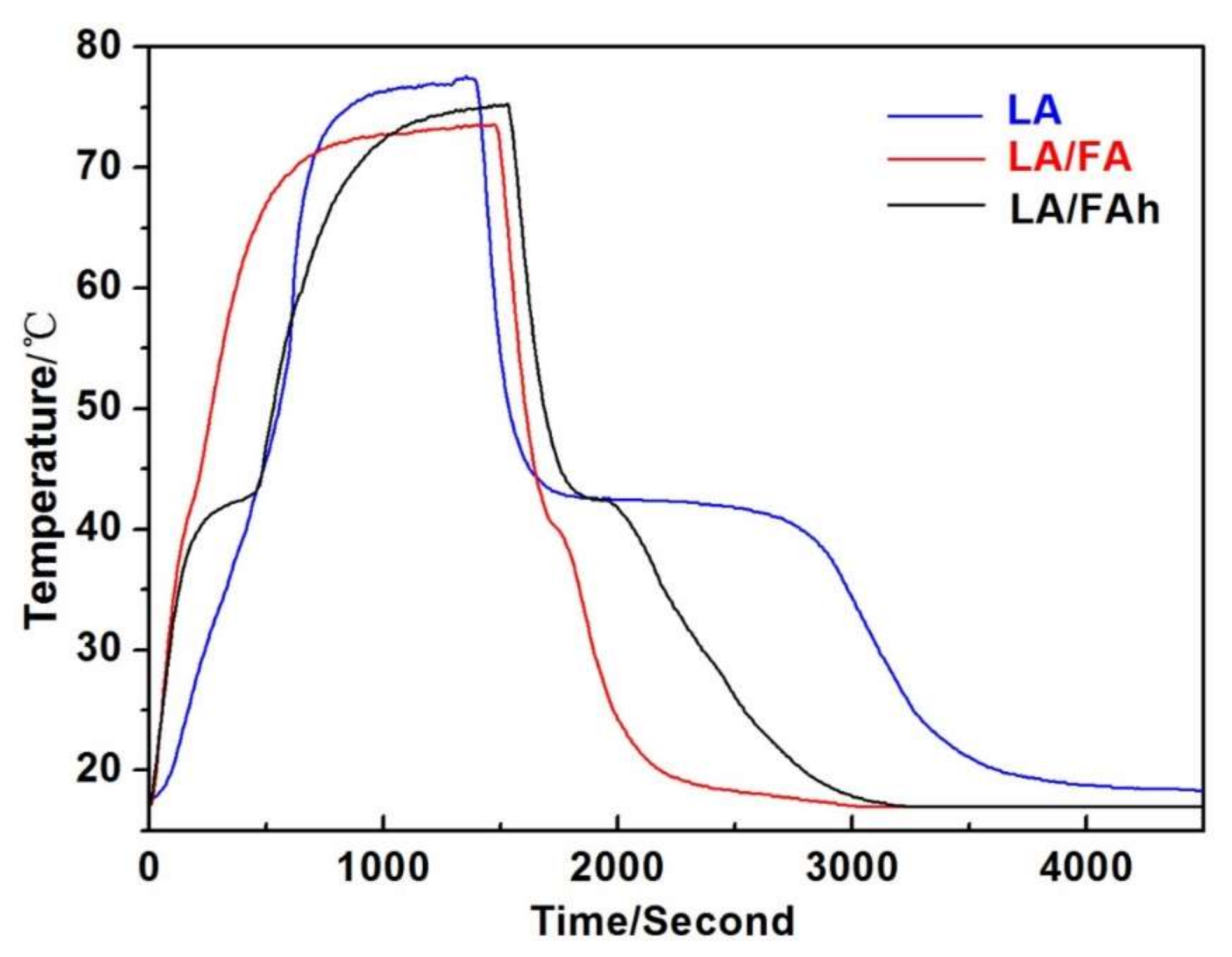
| Samples | SiO2 | Al2O3 | CaO | MgO | Fe2O3 | TiO2 | Na2O | K2O | Others |
|---|---|---|---|---|---|---|---|---|---|
| FA | 53.63 | 17.78 | 13.47 | 2.36 | 6.08 | 0.82 | 1.90 | 1.09 | 2.87 |
| FAh | 70.07 | 14.69 | 2.23 | 0.81 | 7.32 | 0.90 | 1.79 | 0.99 | 1.29 |
| Samples | BET Surface Area SBET (m2/g) | Total Pore Volume (cm3/g) |
|---|---|---|
| FA | 3.958 | 0.010 |
| FAh | 52.961 | 0.036 |
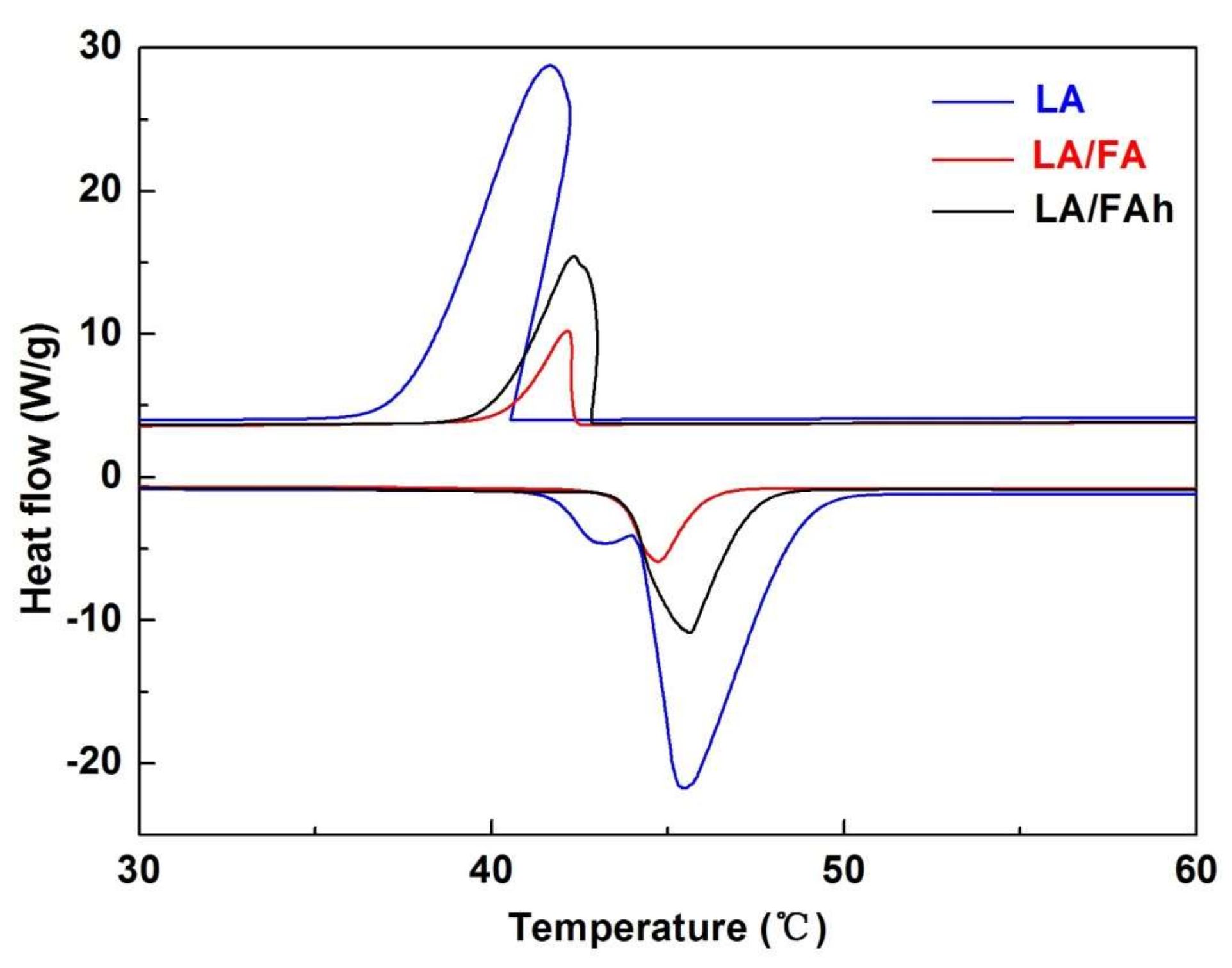
| Samples | Loadage (β, %) | Melting Temperature (Tm, °C) | Freezing Temperature (Tf, °C) | Latent Heat of Melting (ΔHm, J/g) | Latent Heat of Freezing (ΔHf, J/g) | Crystallinity of LA (Fc, %) | Extent of Supercooling (Tm − Tf, °C) |
|---|---|---|---|---|---|---|---|
| LA | 100 | 40.10 | 40.76 | 235.90 | 206.00 | 100 | −0.66 |
| LA/FA | 19.30 | 41.34 | 42.75 | 34.09 | 32.97 | 74.88 | −1.41 |
| LA/FAh | 38.12 | 42.34 | 43.50 | 80.94 | 77.39 | 90.00 | −1.16 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, D.; Yang, H.; Ouyang, J.; Zhang, Y.; Fu, L.; Chen, D. Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage. Minerals 2018, 8, 161. https://doi.org/10.3390/min8040161
Xu D, Yang H, Ouyang J, Zhang Y, Fu L, Chen D. Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage. Minerals. 2018; 8(4):161. https://doi.org/10.3390/min8040161
Chicago/Turabian StyleXu, Dawei, Huaming Yang, Jing Ouyang, Yi Zhang, Liangjie Fu, and Deliang Chen. 2018. "Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage" Minerals 8, no. 4: 161. https://doi.org/10.3390/min8040161
APA StyleXu, D., Yang, H., Ouyang, J., Zhang, Y., Fu, L., & Chen, D. (2018). Lauric Acid Hybridizing Fly Ash Composite for Thermal Energy Storage. Minerals, 8(4), 161. https://doi.org/10.3390/min8040161






