Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Sample Characterization
2.3. Wettability Determination
2.4. Adsorption Experiments
3. Results
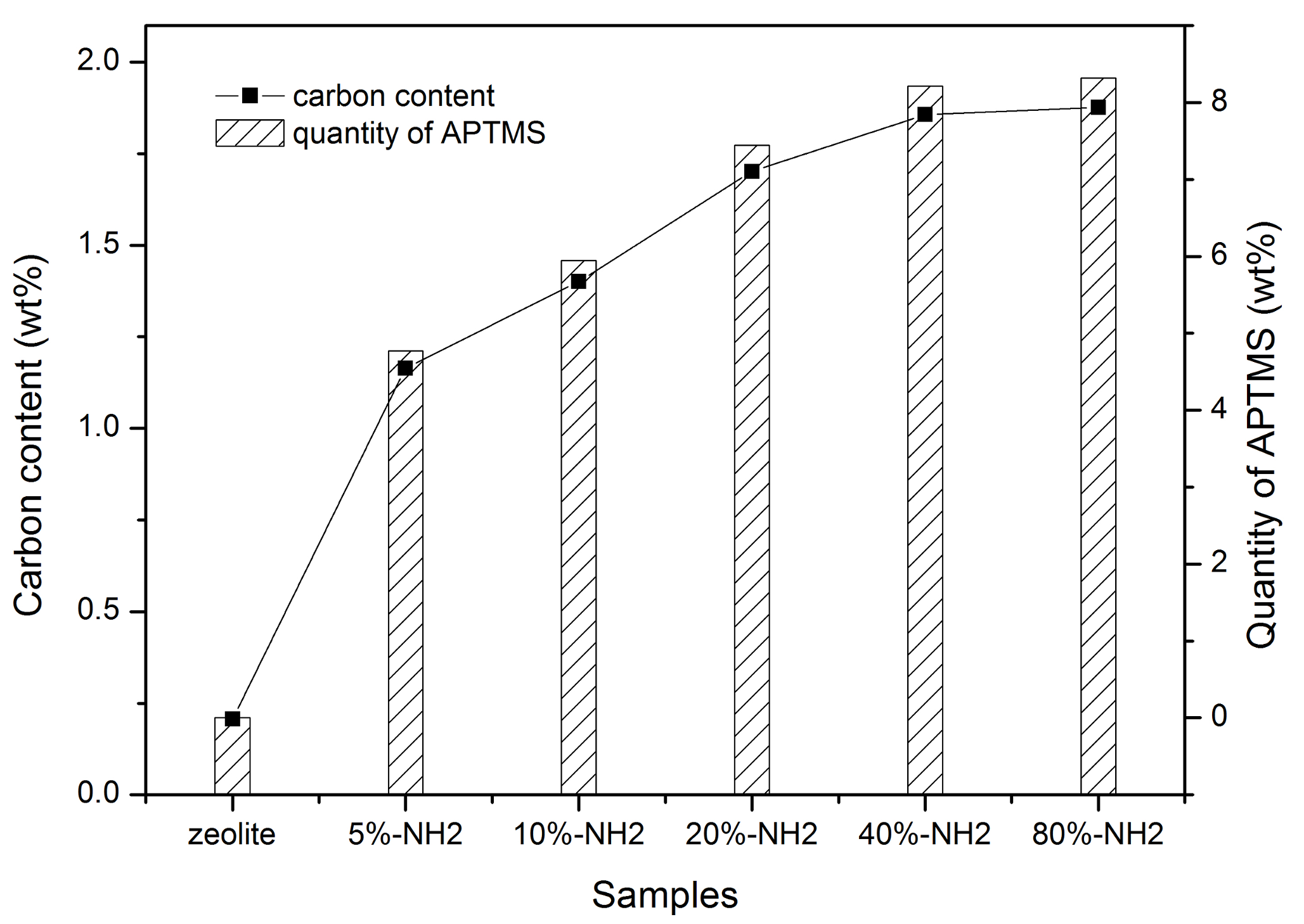
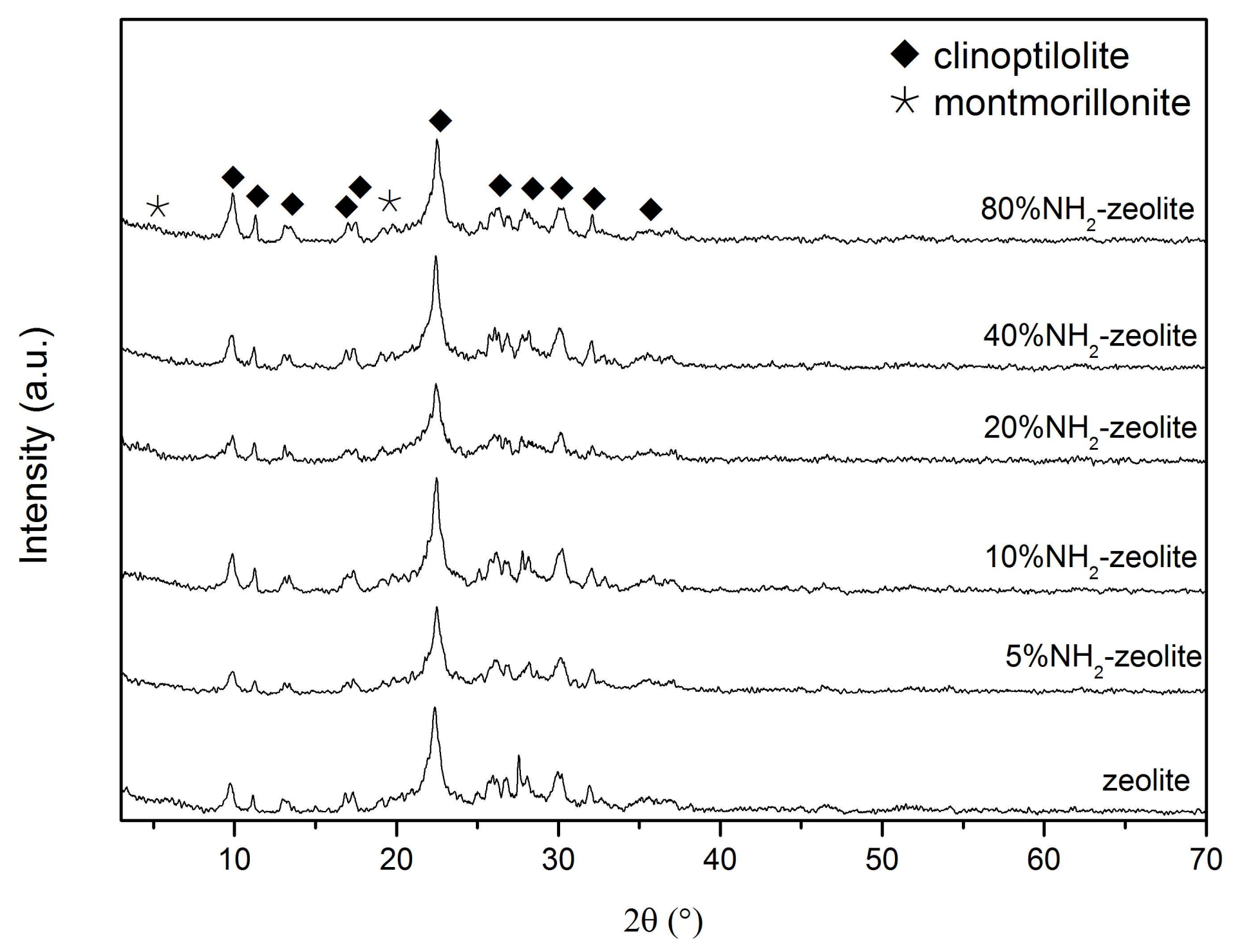
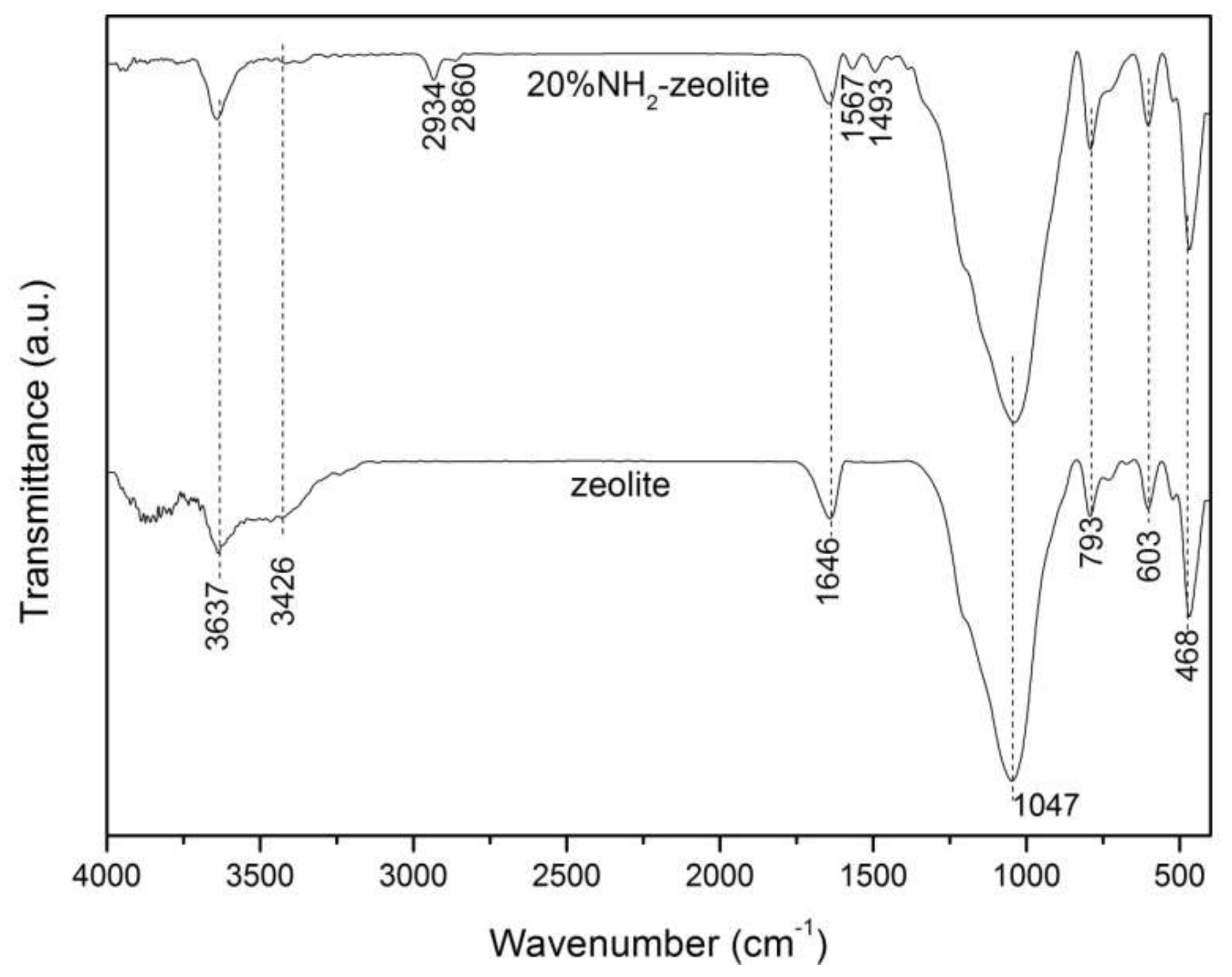
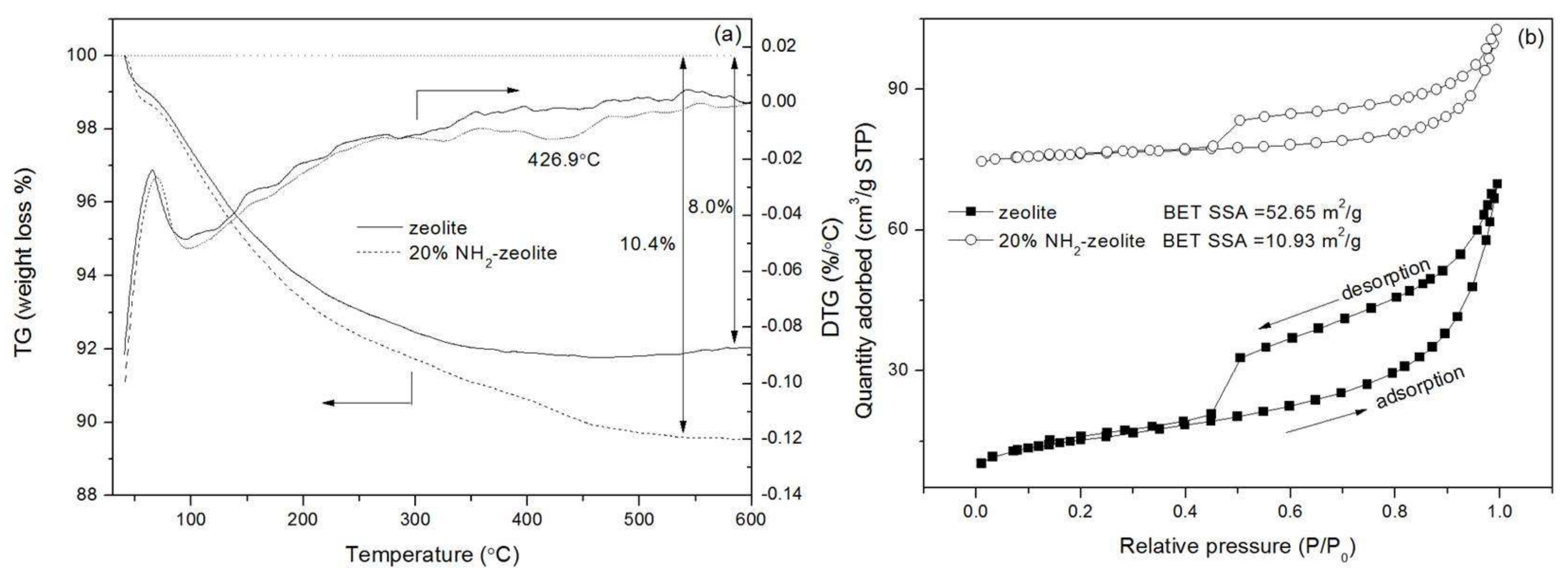
3.1. Characterization of the Samples
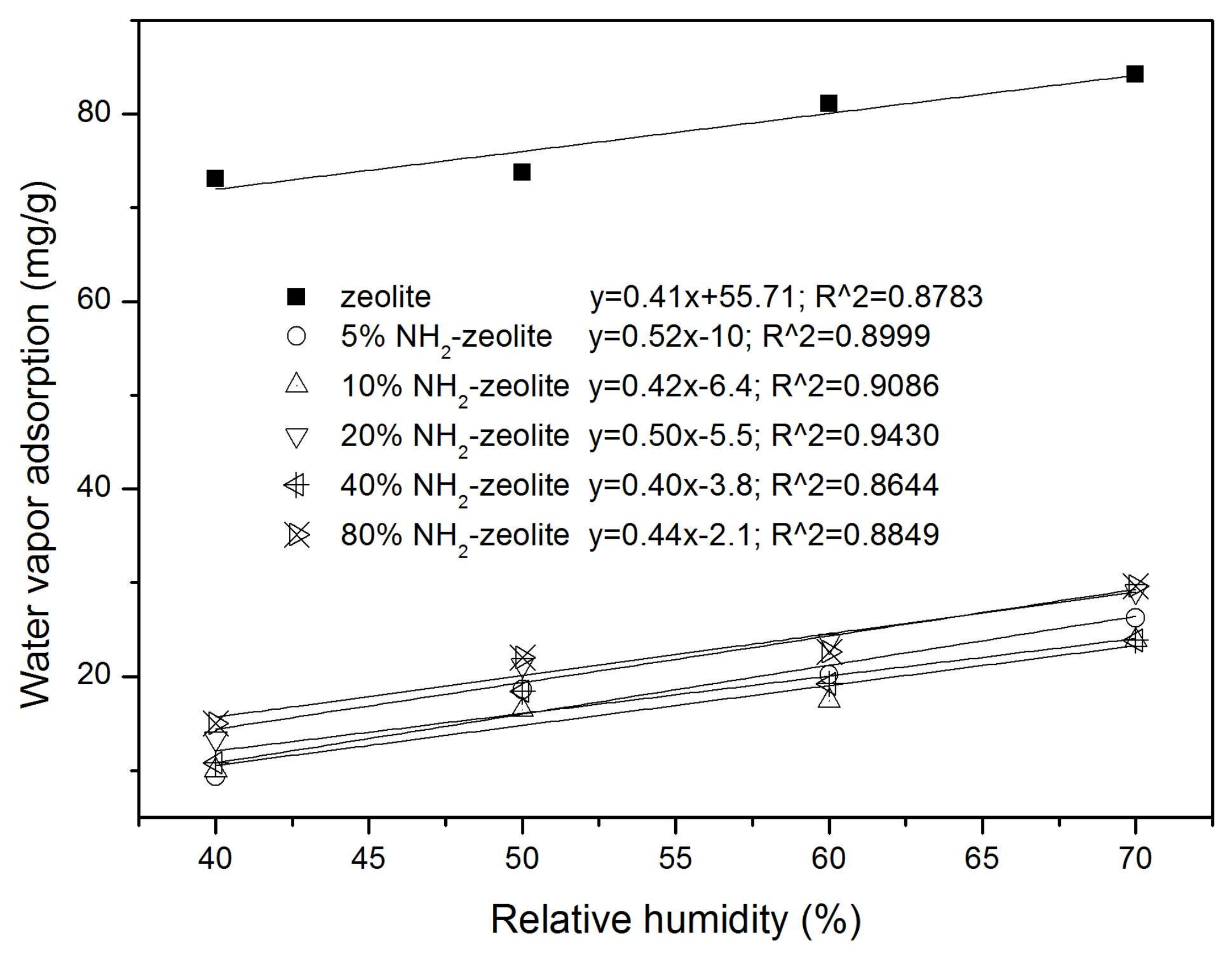
3.2. Wettability of the Samples
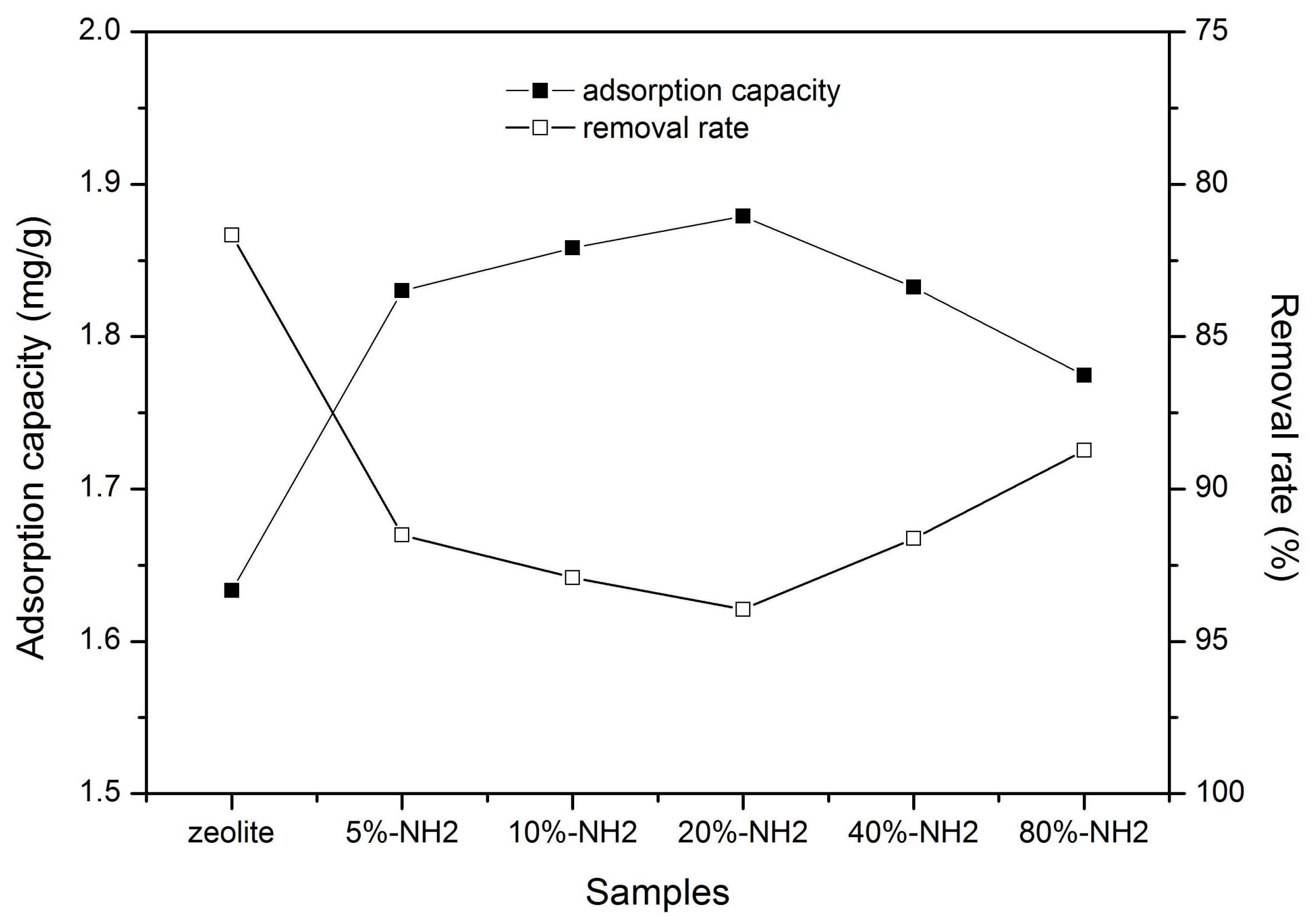
3.3. Adsorption Properties of the Samples
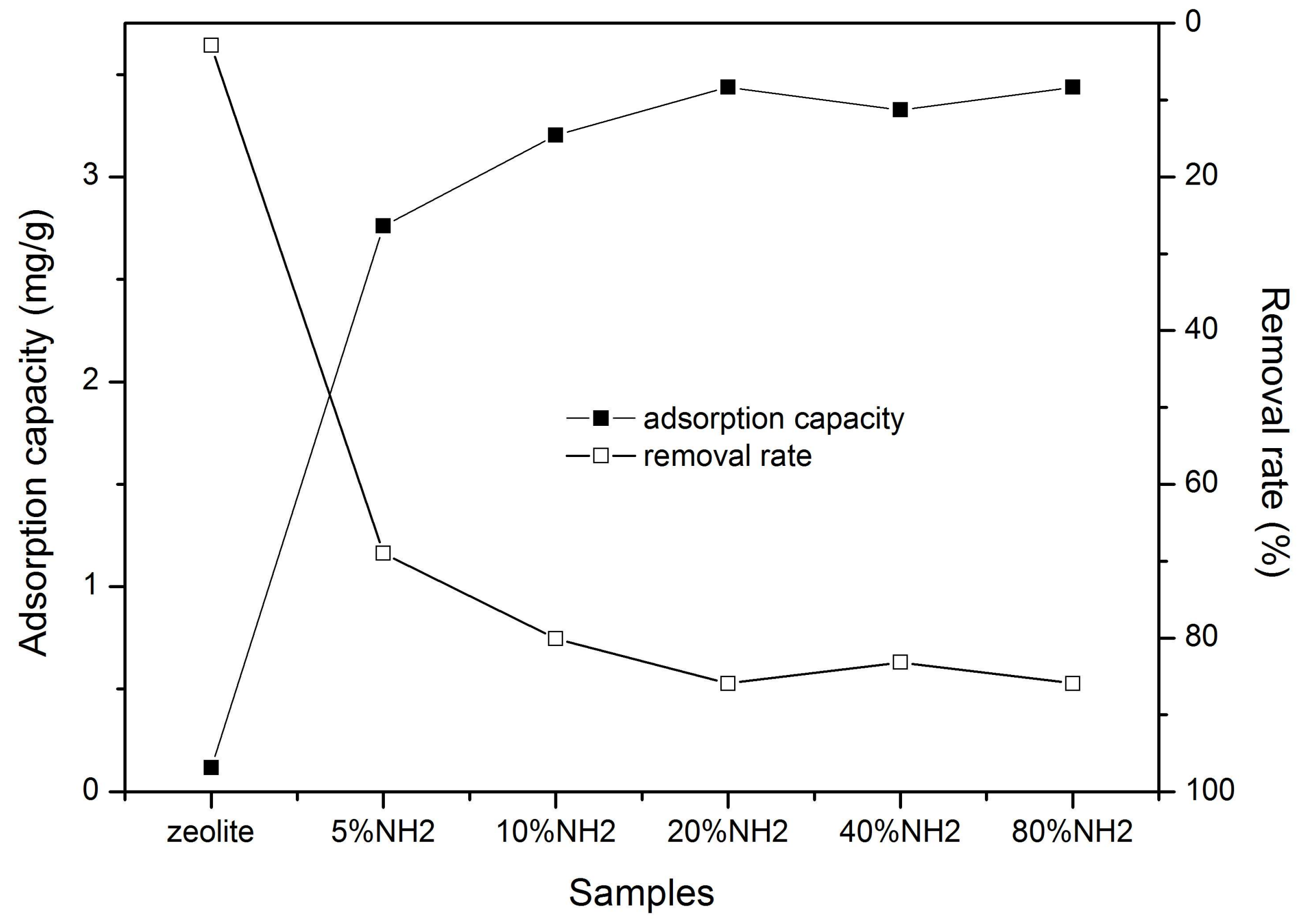
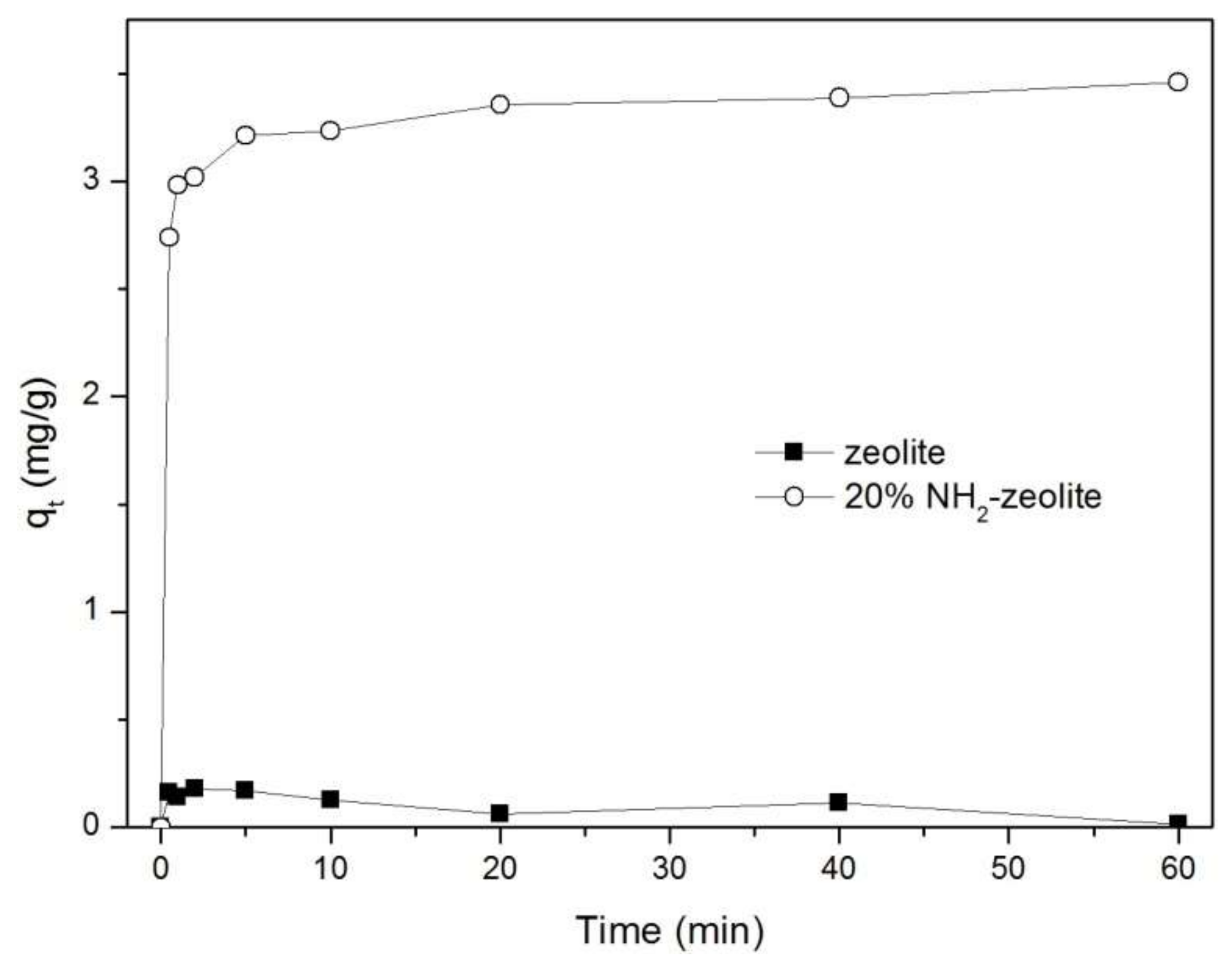
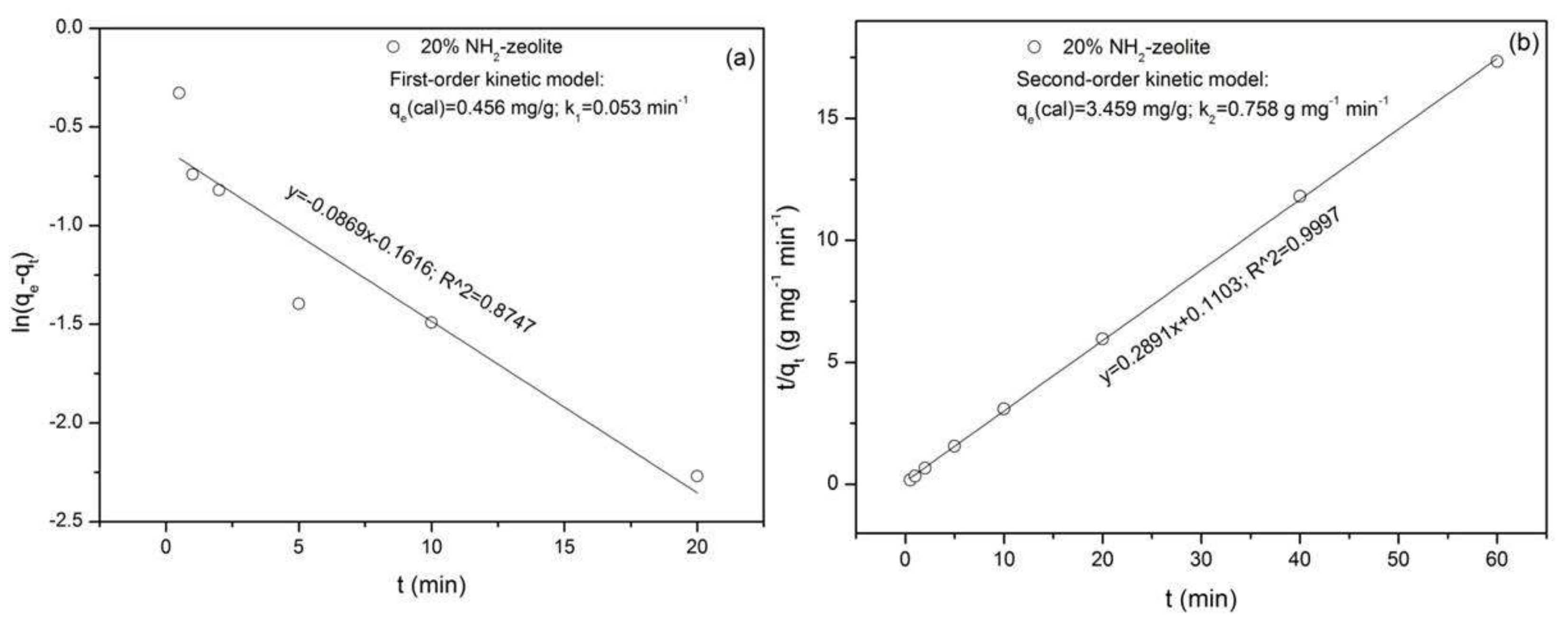
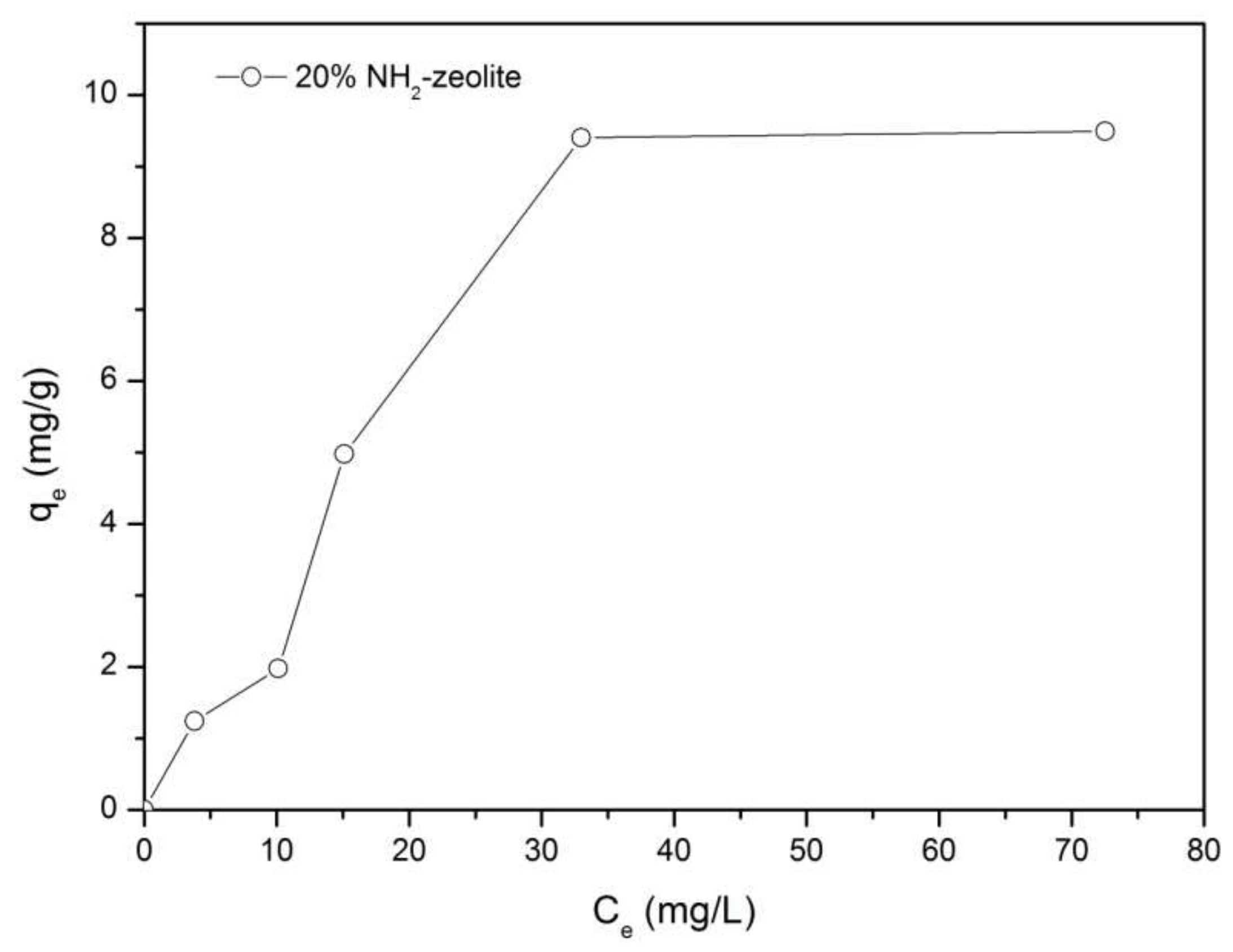
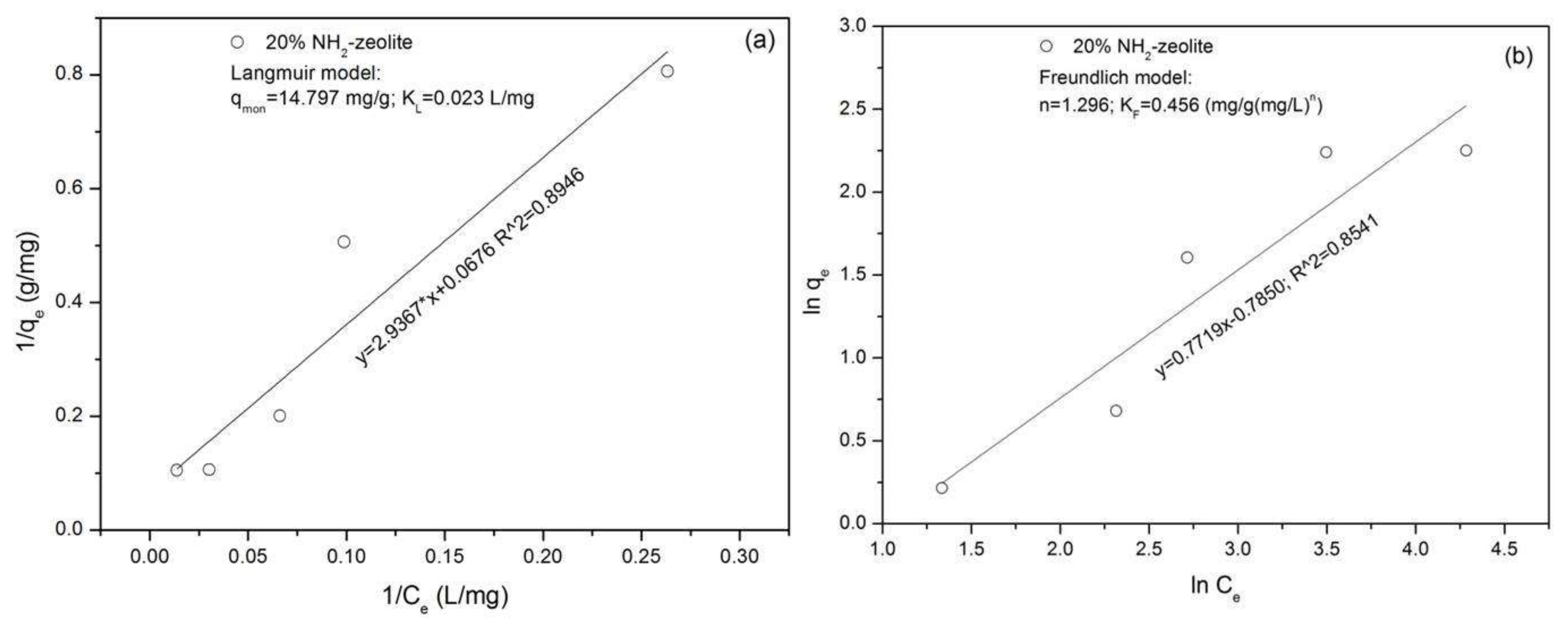
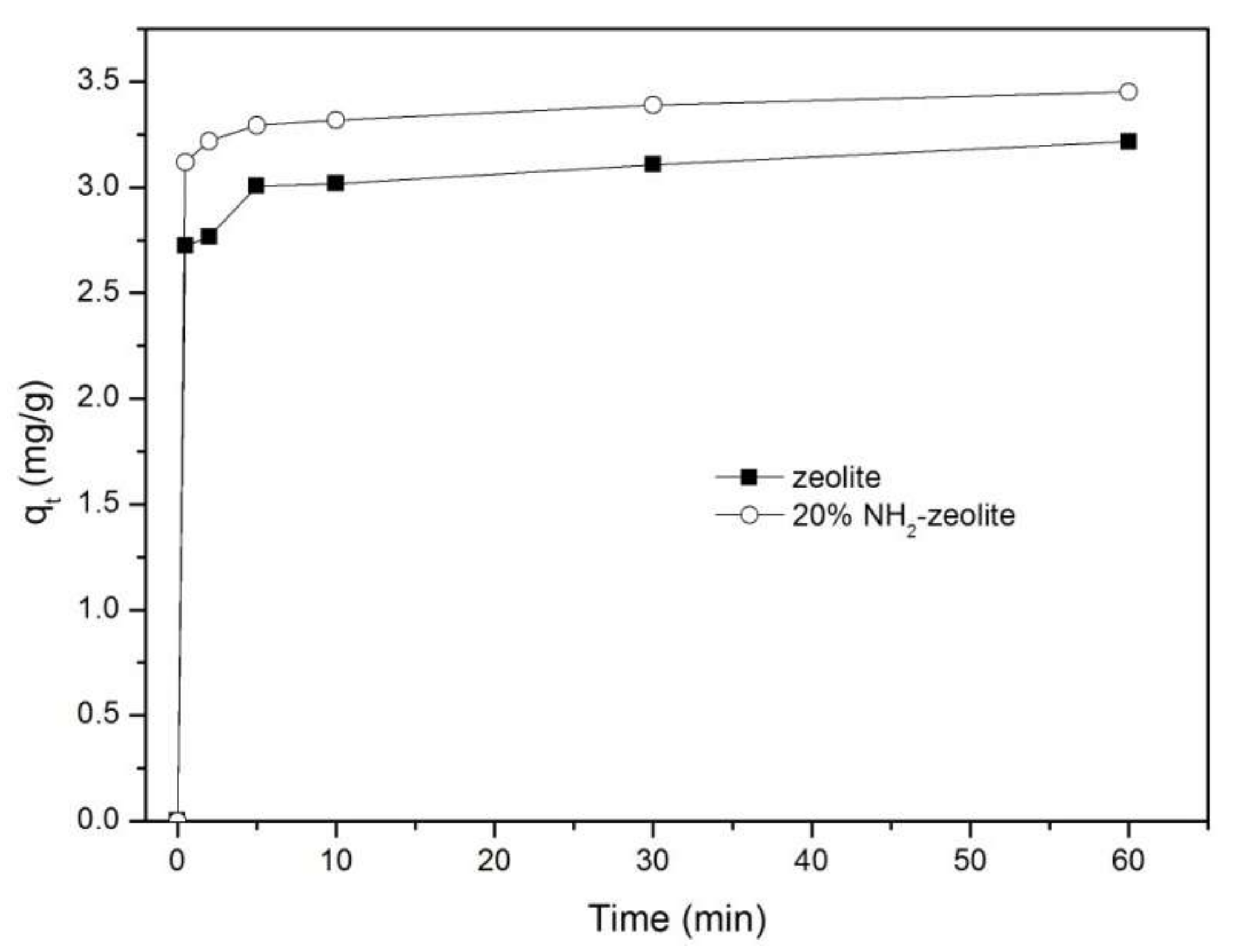
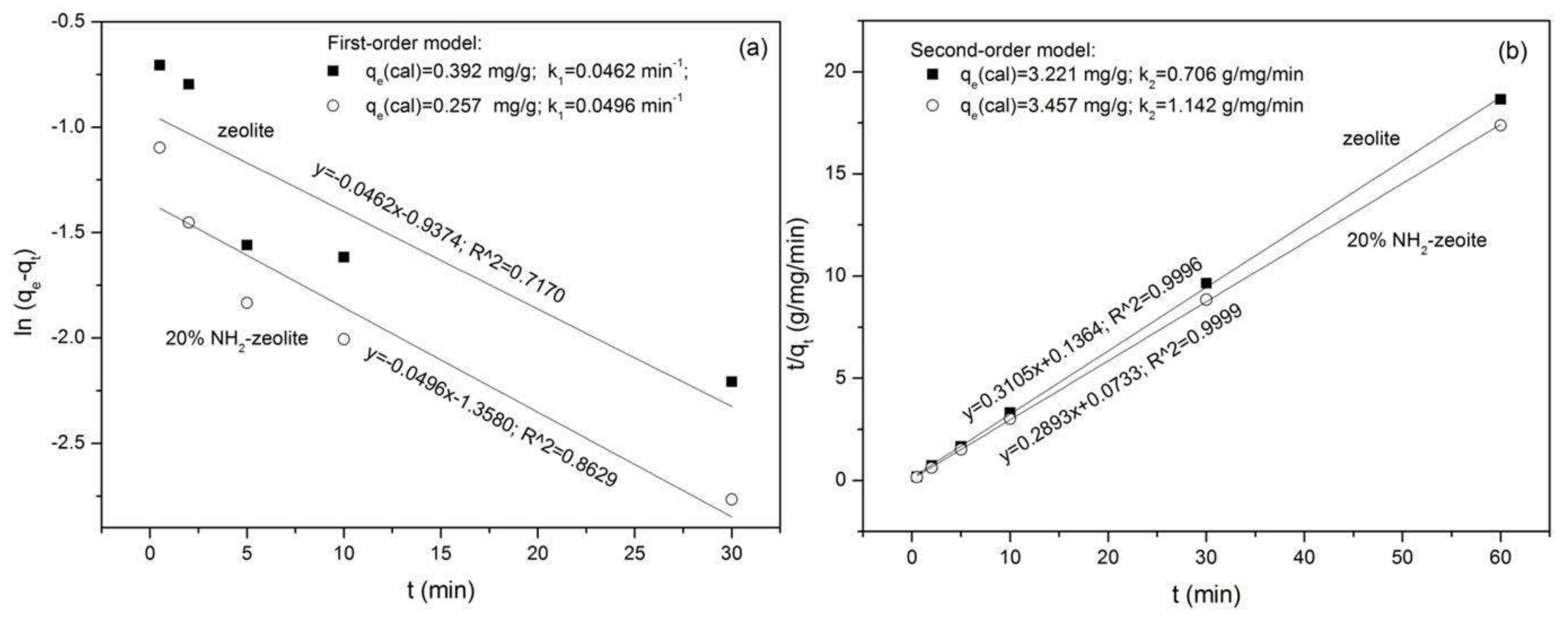
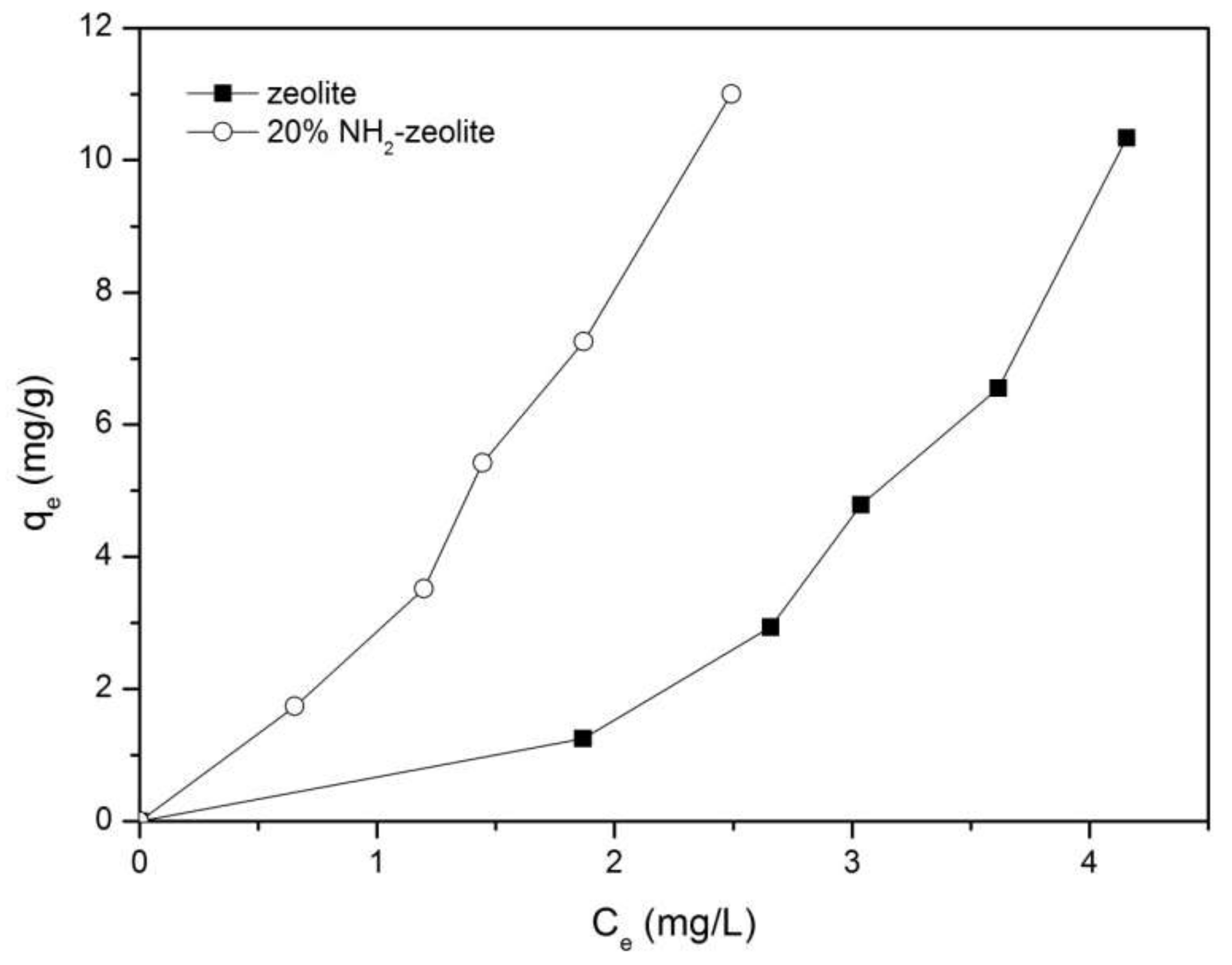
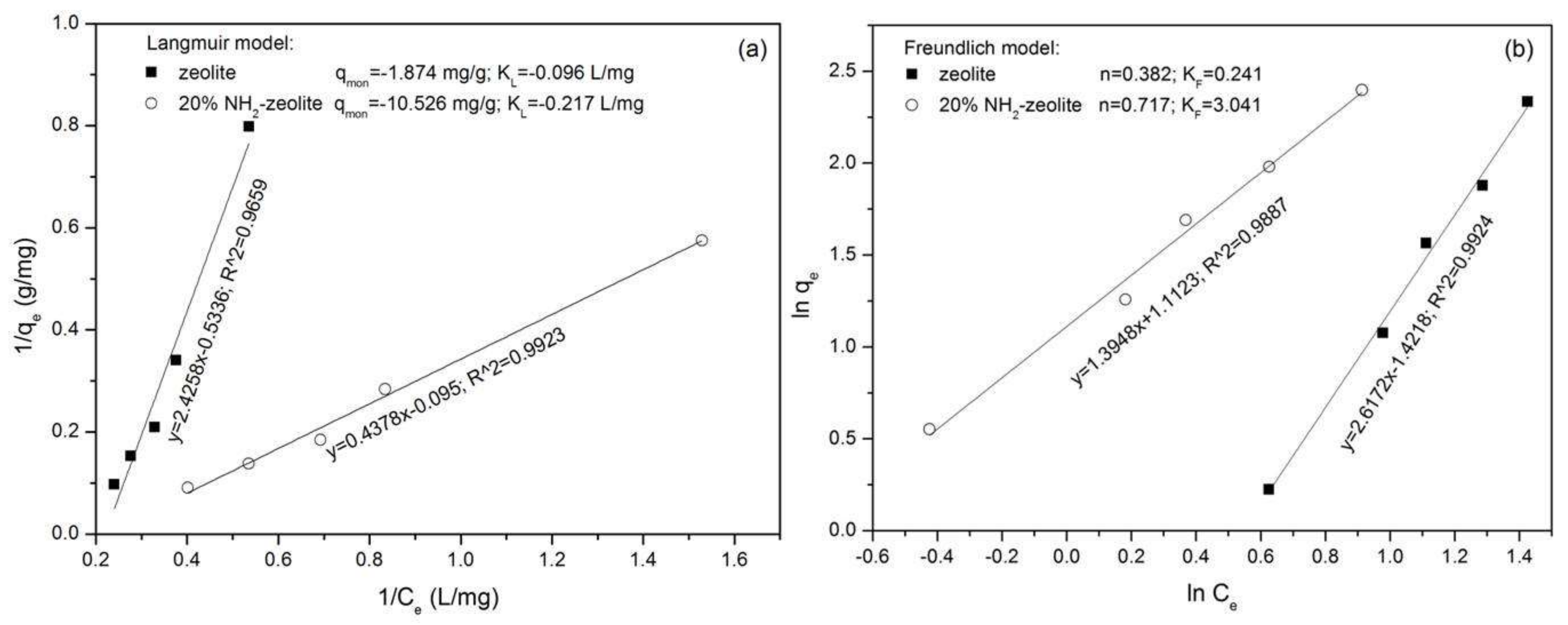
3.3.1. Adsorption of Chromate
3.3.2. Adsorption of Naphthalene
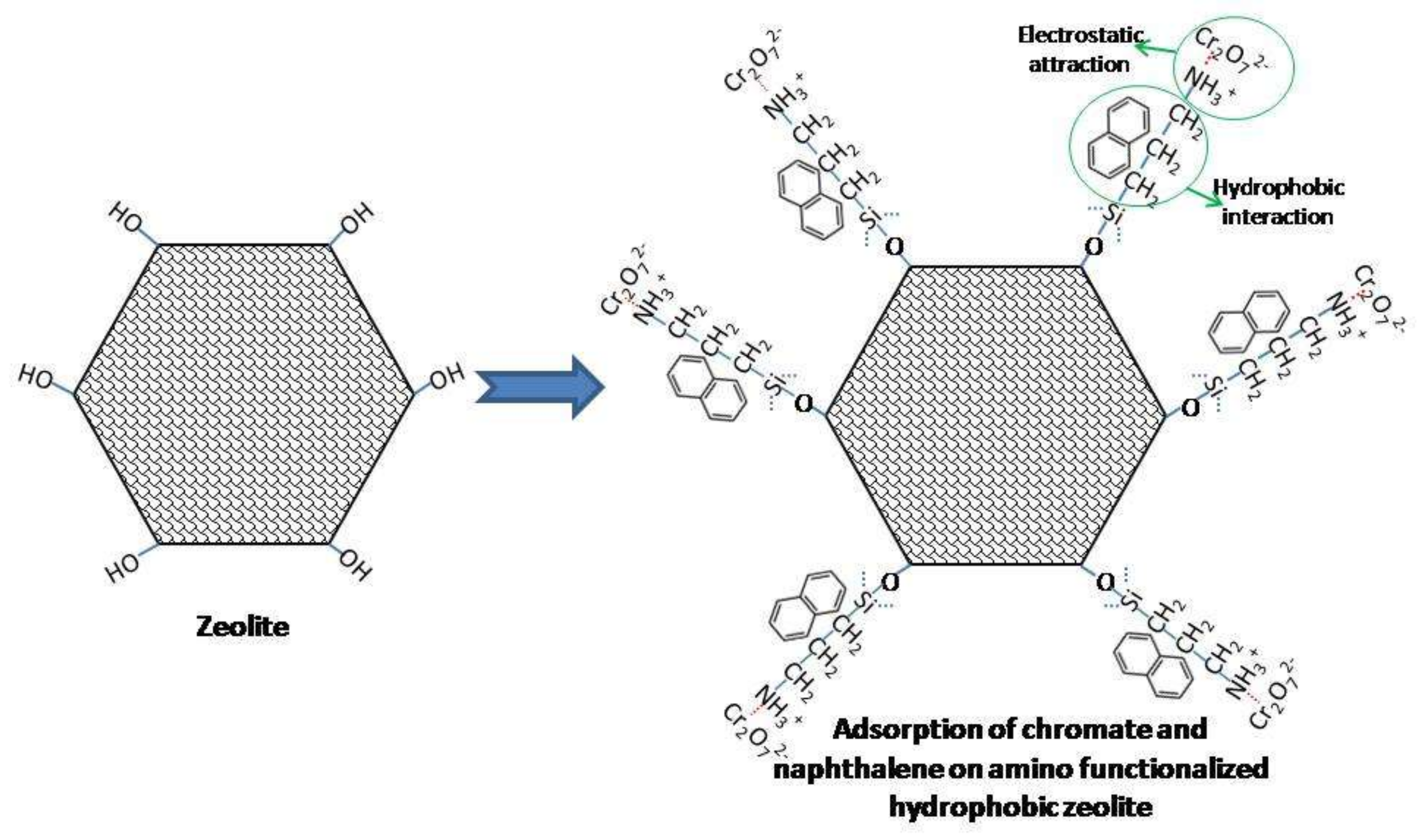
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.; Peng, Y. Natural zeolites as effective adsorbents in water and wastewater treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Misaelides, P. Application of natural zeolites in environmental remediation: A short review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Franus, W.; Wdowin, M. Removal of ammonium ions by selected natural and synthetic zeolites. Gospod. Surowcami Miner. 2010, 26, 133–148. [Google Scholar]
- Korkuna, O.; Leboda, R.; Skubiszewska-Zie, J.; Vrublevs’ka, T.; Gun’ko, V.M.; Ryczkowski, J. Structural and physicochemical properties of natural zeolites: Clinoptilolite and mordenite. Microporous Mesoporous Mater. 2006, 87, 243–254. [Google Scholar] [CrossRef]
- Yousef, R.I.; El-Eswed, B.; Al-Muhtaseb, A.H. Adsorption characteristics of natural zeolites as solid adsorbents for phenol removal from aqueous solutions: Kinetics, mechanism, and thermodynamics studies. Chem. Eng. J. 2011, 171, 1143–1149. [Google Scholar] [CrossRef]
- Liang, H.J.; Gao, H.; Kong, Q.Q.; Chen, Z.X. Adsorption equilibrium and kinetics of tetrahydrofuran + water solution. J. Chem. Eng. Data 2006, 51, 119–122. [Google Scholar] [CrossRef]
- Gómez-Hortigüela, L.; Pinar, A.B.; Pérez-Pariente, J.; Sani, T.; Chebude, Y.; Díaz, I. Ion-exchange in natural zeolite stilbite and significance in defluoridation ability. Microporous Mesoporous Mater. 2014, 193, 93–102. [Google Scholar] [CrossRef]
- Jin, X.; Yu, B.; Chen, Z.; Arocena, J.M.; Thring, R.W. Adsorption of Orange II dye in aqueous solution onto surfactant-coated zeolite: Characterization, kinetic and thermodynamic studies. J. Colloid Interface Sci. 2014, 435, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Li, Y.; Shi, H.S.; Huang, J.F. Preparation and characterization of natural zeolite supported nano TiO2 photocatalysts by a modified electrostatic self-assembly method. Surf. Interface Anal. 2015, 47, 142–147. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Organosilanes functionalization of alumino-silica zeolites for water adsorption applications. Microporous Mesoporous Mater. 2016, 234, 113–119. [Google Scholar] [CrossRef]
- Wang, X.; Plackowski, C.A.; Nguyen, A.V. X-ray photoelectron spectroscopic investigation into the surface effects of sulphuric acid treated natural zeolite. Powder Technol. 2016, 295, 27–34. [Google Scholar] [CrossRef]
- Wang, C.; Cao, L.Y.; Huang, J.F. Influences of acid and heat treatments on the structure and water vapor adsorption property of natural zeolite. Surf. Interface Anal. 2017, 49, 1249–1255. [Google Scholar] [CrossRef]
- Barquist, K.; Sarah, C.L. Chromate adsorption on amine-functionalized nanocrystalline silicalite-1. Microporous Mesoporous Mater. 2008, 116, 365–369. [Google Scholar] [CrossRef]
- Barquist, K.; Sarah, C.L. Chromate adsorption on bifunctional, magnetic zeolite composites. Microporous Mesoporous Mater. 2010, 130, 197–202. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.S.; Li, Y. Synthesis and characteristics of natural zeolite supported Fe3+-TiO2 photocatalysts. Appl. Surf. Sci. 2011, 257, 6873–6877. [Google Scholar] [CrossRef]
- Majoul, N.; Aouida, S.; Bessaïs, B. Progress of porous silicon APTES-functionalization by FTIR investigations. Appl. Surf. Sci. 2015, 331, 388–391. [Google Scholar] [CrossRef]
- Sing, K.S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Muir, B.; Bajda, T. Organically modified zeolites in petroleum compounds spill cleanup-Production, efficiency, utilization. Fuel Process. Technol. 2016, 149, 153–162. [Google Scholar] [CrossRef]
- Bandura, L.; Kolodyńska, D.; Franus, W. Adsorption of BTX from aqueous solutions by Na-P1 zeolite obtained from fly ash. Process Saf. Environ. Prot. 2017, 109, 214–223. [Google Scholar] [CrossRef]
- Şener, S.; Özyılmaz, A. Adsorption of naphthalene onto sonicated talc from aqueous solutions. Ultrason. Sonochem. 2010, 17, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, N.; Bektaş, T.E. Nitrate removal from aqueous solution by adsorption onto various materials. J. Hazard. Mater. 2004, 112, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Sayilkan, H.; Erdemoğlu, S.; Sener, S.; Sayilkan, F.; Akarsu, M.; Erdemoğlu, M. Surface modification of pyrophyllite with amino silane coupling agent for the removal of 4-nitrophenol from aqueous solutions. J. Colloid Interface Sci. 2004, 275, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Bouvy, C.; Marine, W.; Sporken, R.; Su, B.L. Nanosized ZnO confined inside a Faujasite X zeolite matrix: Characterization and optical properties. Colloid Surf. A 2007, 300, 145–149. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, C.; Leng, S.; Xu, Y.; Tian, Q.; Zhang, X.; Cao, L.; Huang, J. Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene. Minerals 2018, 8, 145. https://doi.org/10.3390/min8040145
Wang C, Leng S, Xu Y, Tian Q, Zhang X, Cao L, Huang J. Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene. Minerals. 2018; 8(4):145. https://doi.org/10.3390/min8040145
Chicago/Turabian StyleWang, Cheng, Shaozheng Leng, Yuan Xu, Qinyue Tian, Xuemeng Zhang, Liyun Cao, and Jianfeng Huang. 2018. "Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene" Minerals 8, no. 4: 145. https://doi.org/10.3390/min8040145
APA StyleWang, C., Leng, S., Xu, Y., Tian, Q., Zhang, X., Cao, L., & Huang, J. (2018). Preparation of Amino Functionalized Hydrophobic Zeolite and Its Adsorption Properties for Chromate and Naphthalene. Minerals, 8(4), 145. https://doi.org/10.3390/min8040145



