Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz
Abstract
:1. Introduction
2. Materials and Methods
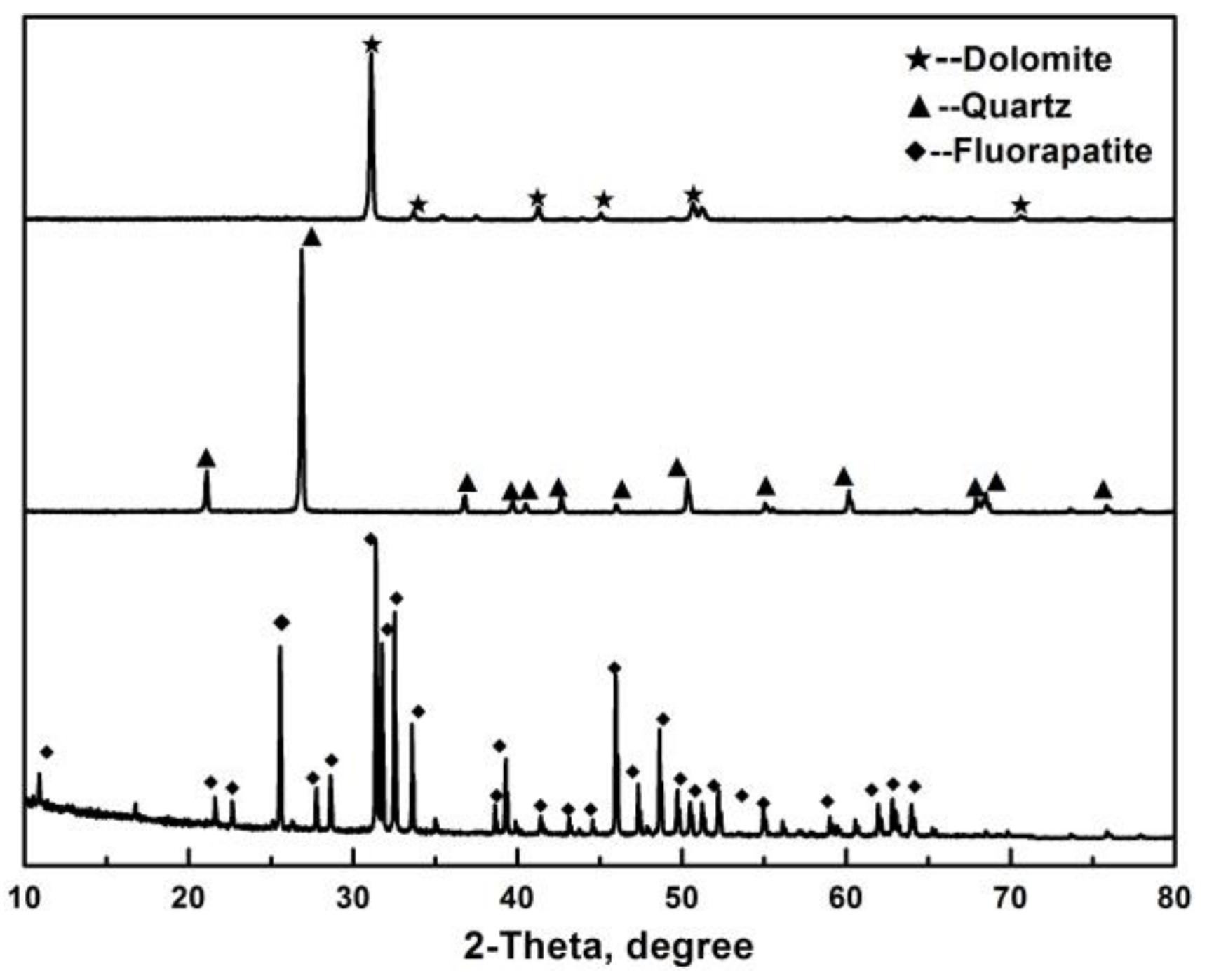
2.1. Pure Minerals
2.2. Reagents
2.3. Micro-Flotation Test
2.4. Zeta Potential Measurement
2.5. X-ray Photoelectron Spectroscopy (XPS) Analyses
3. Results and Discussion
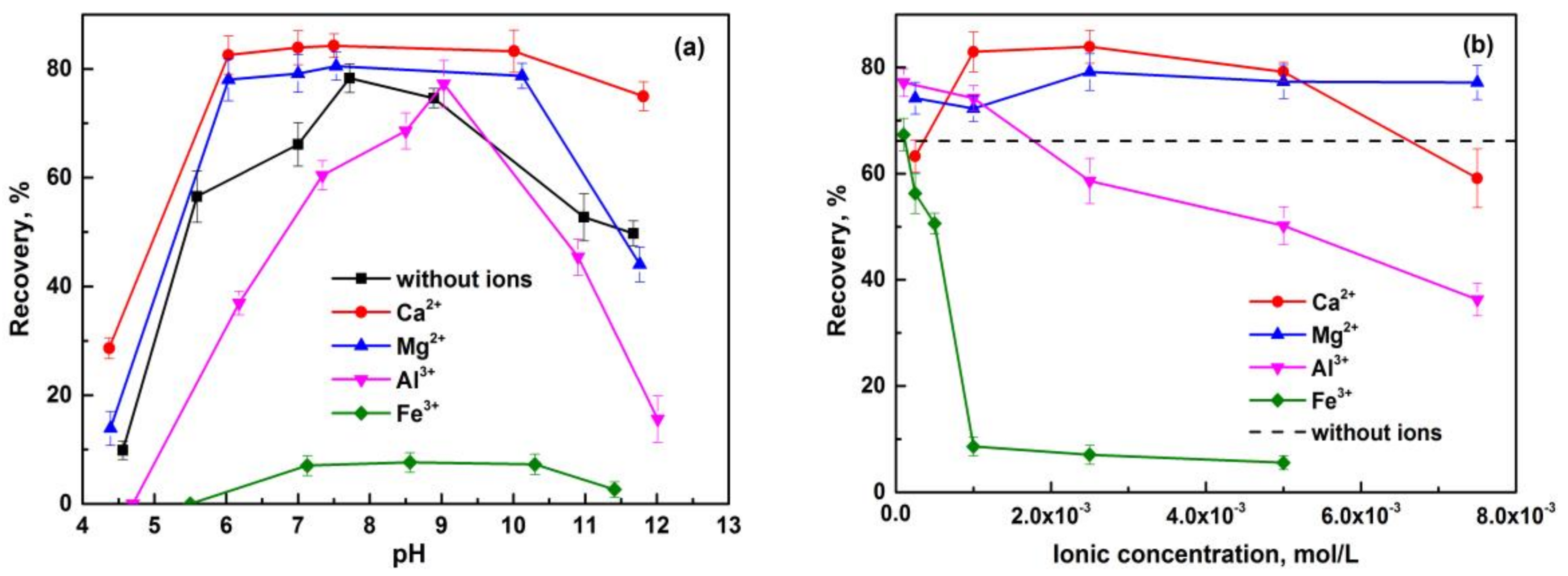
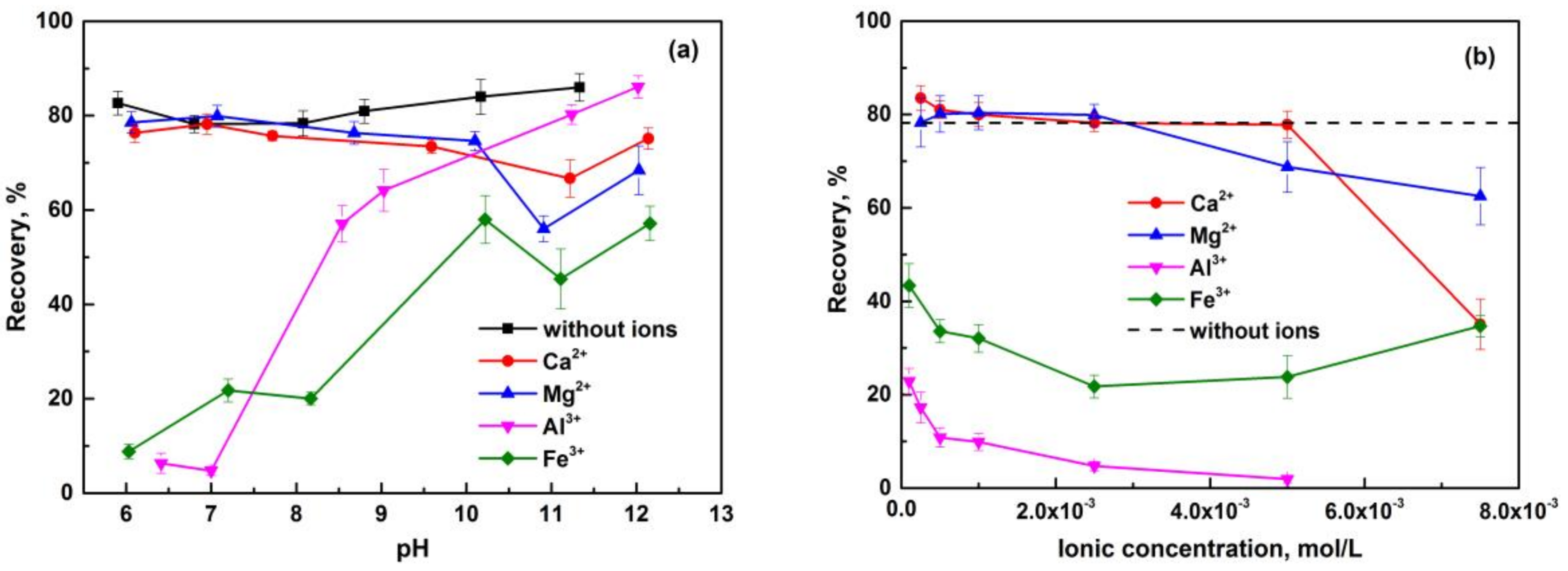
3.1. Flotation Behavior of Pure Minerals in the Presence of Metal Ions
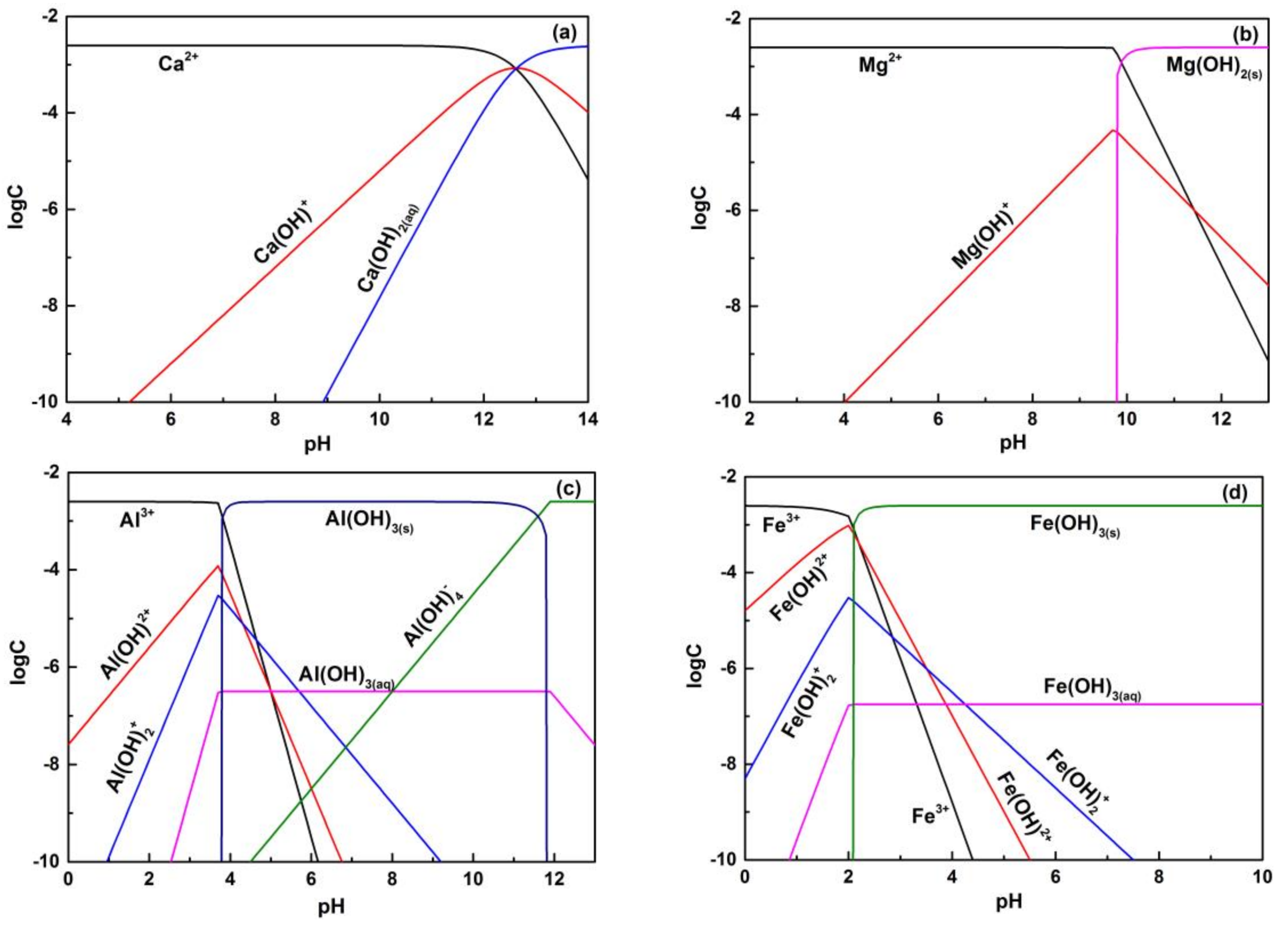
3.2. Hydrolysis Equilibria of Metal Ions
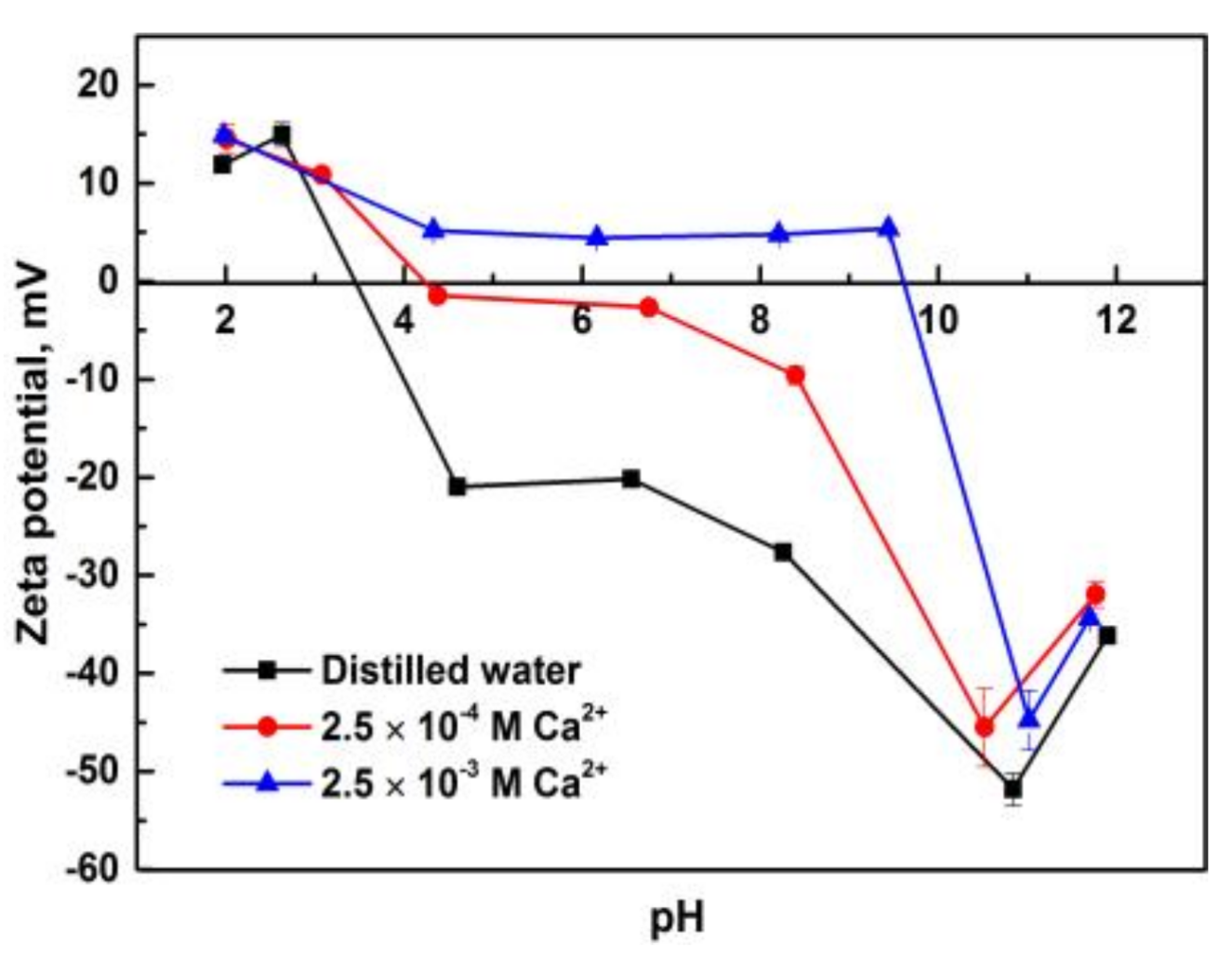
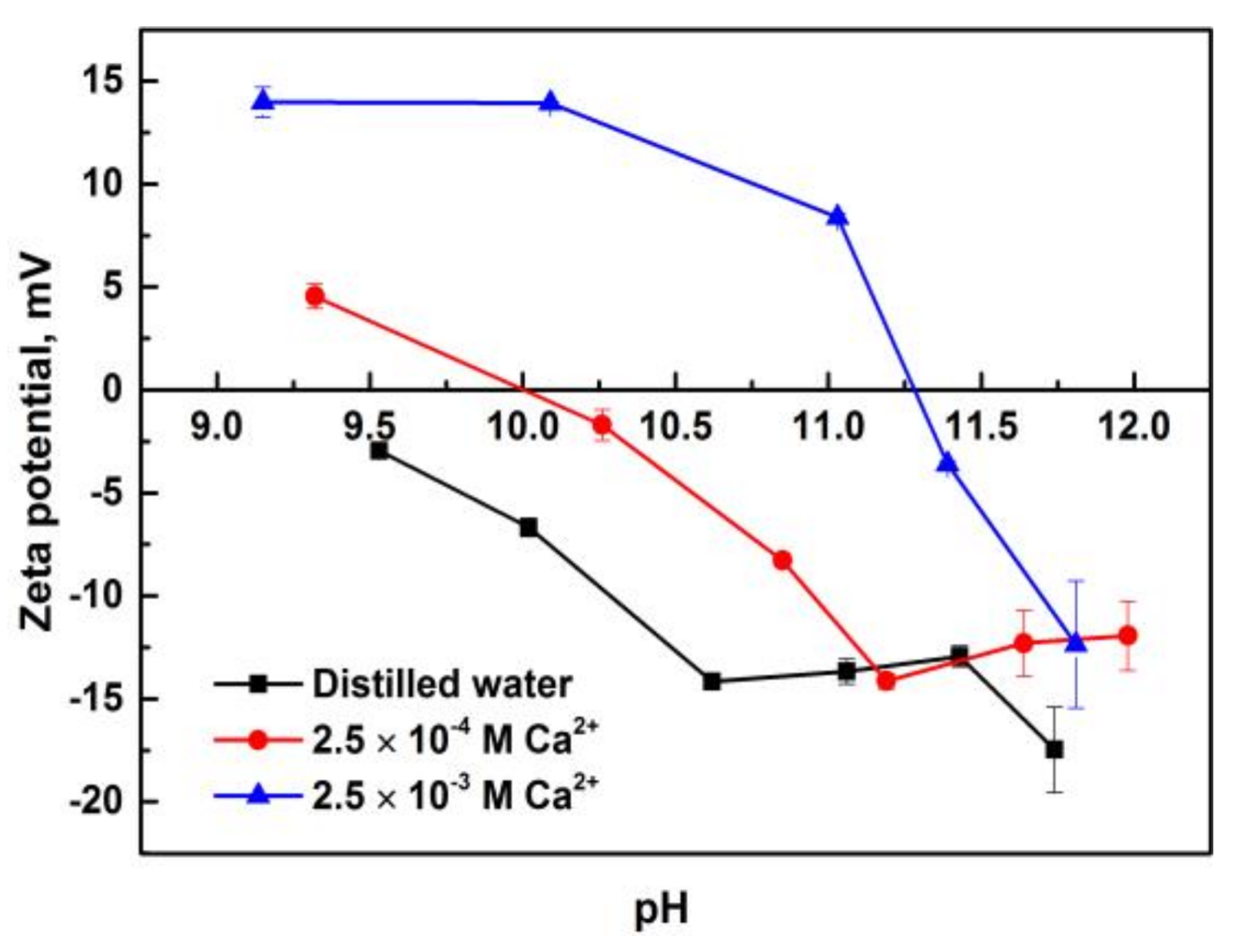
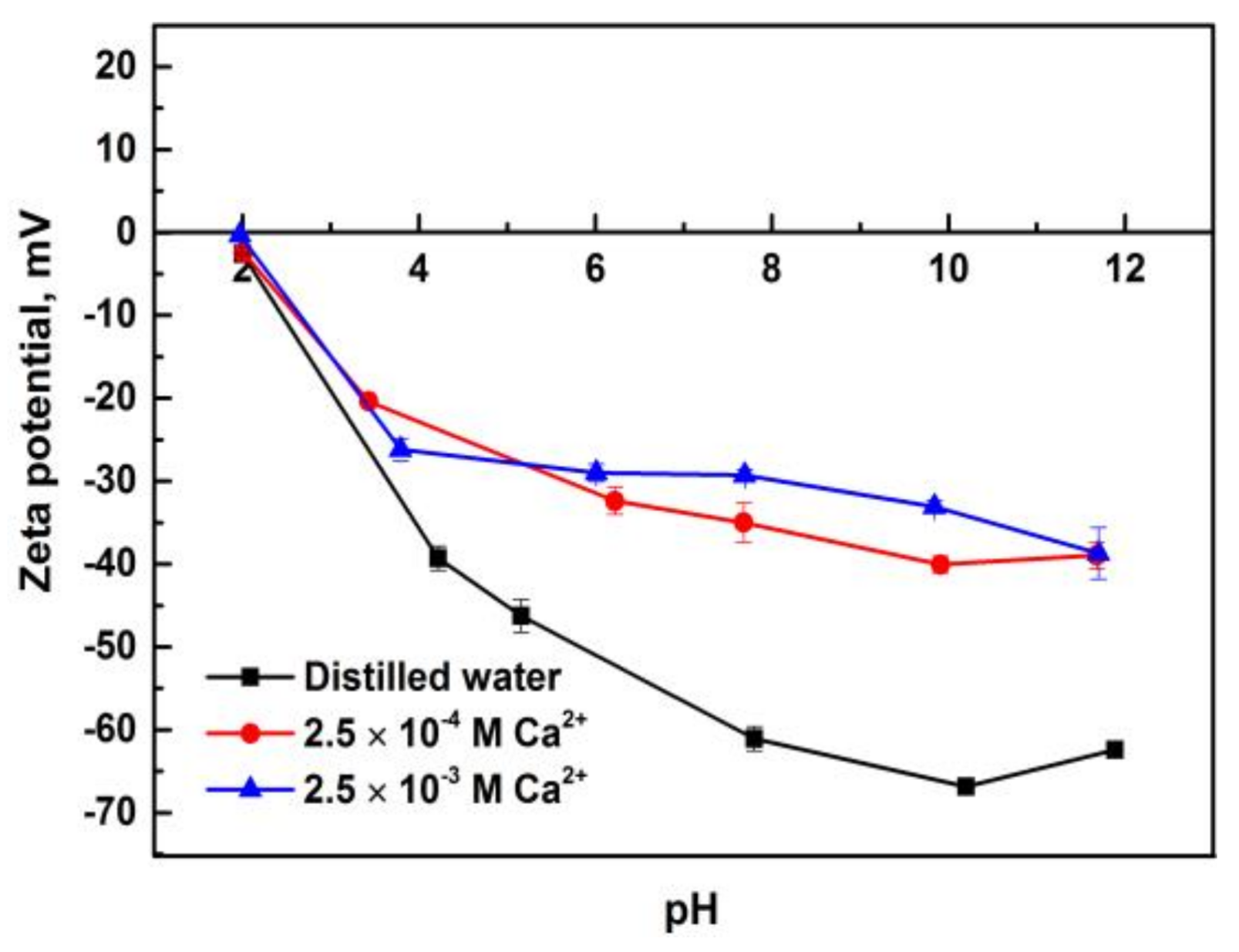
3.3. Zeta Potentials of Pure Minerals in the Presence of Calcium Ions
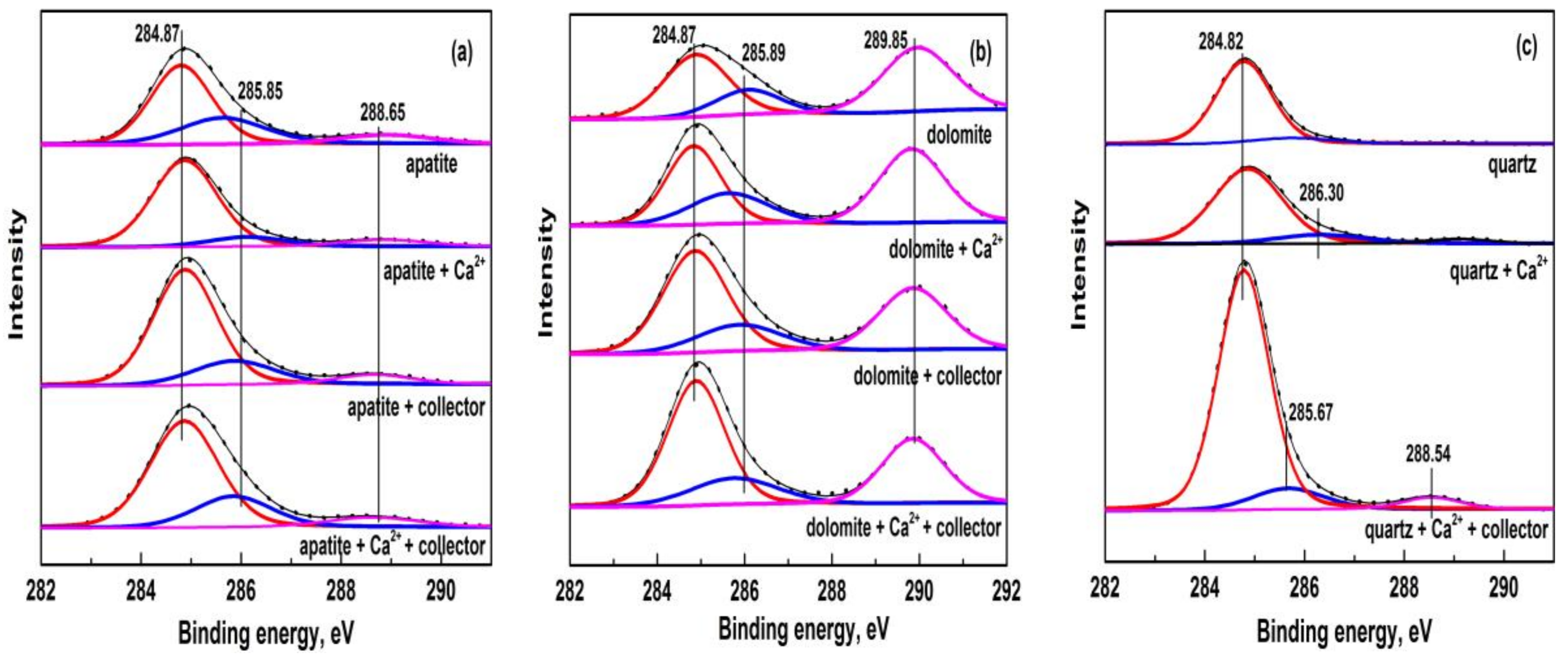
3.4. XPS Analyses and High-Resolution Spectra of Pure Minerals
4. Conclusions
- The flotation behaviors of apatite, dolomite, and quartz, in the presence of metal ions, were investigated through micro-flotation tests. The beneficial effect of Ca2+ and Mg2+ with an appropriate concentration on anionic flotation of apatite was confirmed, and was proved negligible on that of dolomite. Excessive amounts of Ca2+ can reduce the floatability of apatite and dolomite at neutral pH. Both Al3+ and Fe3+ exhibited a depression effect on these calcium-bearing minerals. Quartz can be activated by metal ions, and its floatability varied under different pH conditions and metal ion species.
- Solution chemistry and zeta potential measurement indicate that the influence of metal ions on the flotation of apatite, dolomite and quartz are attributed to the adsorption of various hydrolysis species of metal ions on the mineral surfaces. It was inferred that Ca2+ and Mg2+ were mainly adsorbed on the apatite and dolomite surface in the form of divalent ions while Ca(OH)+ and Mg(OH)+ were responsible for the activation of quartz.
- Various adsorption characteristics of collector on apatite, dolomite, and quartz surfaces in the absence and presence of calcium ions were revealed by XPS analyses, which was in agreement with the flotation results of these minerals under the same ionic concentration.
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ejtemaei, M.; Irannajad, M.; Gharabaghi, M. Role of dissolved mineral species in selective flotation of smithsonite from quartz using oleate as collector. Int. J. Miner. Process. 2012, 114–117, 40–47. [Google Scholar] [CrossRef]
- Bulut, G.; Yenial, Ü. Effects of major ions in recycled water on sulfide minerals flotation. Miner. Metall. Process. 2016, 33, 137–143. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Wang, W.; Zhang, J.; Yan, W.; Deng, J.; Feng, Q.; Huang, Y. The effects of Ca(II) and Mg(II) ions on the flotation of spodumene using NaOL. Miner. Eng. 2015, 79, 40–46. [Google Scholar] [CrossRef]
- Jeldres, R.I.; Arancibia-Bravo, M.P.; Reyes, A.; Aguirre, C.E.; Cortes, L.; Cisternas, L.A. The impact of seawater with calcium and magnesium removal for the flotation of copper-molybdenum sulphide ores. Miner. Eng. 2017, 109, 10–13. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Grimm, D.; Pawlik, M. Anionic flotation of high-iron phosphate ores—Control of process water chemistry and depression of iron minerals by starch and guar gum. Int. J. Miner. Process. 2009, 92, 49–57. [Google Scholar] [CrossRef]
- Choi, J.; Choi, S.Q.; Park, K.; Han, Y.; Kim, H. Flotation behavior of malachite in mono- and di-valent salt solutions using sodium oleate as a collector. Int. J. Miner. Process. 2016, 146, 38–45. [Google Scholar] [CrossRef]
- Liu, D.; Somasundaran, P.; Vasudevan, T.V.; Harris, C.C. Role of pH and dissolved mineral species in Pittsburgh No. 8 coal flotation system—1. Floatability of coal. Int. J. Miner. Process. 1994, 41, 201–214. [Google Scholar] [CrossRef]
- Huang, P.; Fuerstenau, D.W. The effect of the adsorption of lead and cadmium ions on the interfacial behavior of quartz and talc. Colloids Surf. A 2001, 177, 147–156. [Google Scholar] [CrossRef]
- Scott, J.L.; Smith, R.W. Calcium ion effects in amine flotation of quartz and magnetite. Miner. Eng. 1993, 6, 1245–1255. [Google Scholar] [CrossRef]
- Abouzeid, A.-Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 66. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Adsorption and contact angel of single and binary mixtures of surfactants on apatite. Miner. Eng. 2003, 16, 841–847. [Google Scholar] [CrossRef]
- Santos, M.A.; Santana, R.C.; Capponi, F.; Ataíde, C.H.; Barrozo, M.A.S. Effect of ionic species on the performance of apatite flotation. Sep. Purif. Technol. 2010, 76, 15–20. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Al-Zoubi, H. Purification of phosphate beneficiation wastewater: Separation of phosphate from Eshydia Mine (Jordan) by column-DAF flotation process. Int. J. Miner. Process. 2012, 110–111, 21–23. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abuozeid, A.-Z.M. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 239–246. [Google Scholar] [CrossRef]
- Al-Wakeel, M.I.; Lin, C.L.; Miller, J.D. Significance of liberation characteristics in the fatty acid flotation of Florida phosphate rock. Miner. Eng. 2009, 22, 244–253. [Google Scholar] [CrossRef]
- Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Ambient temperature flotation of sedimentary phosphate ore using cottonseed oil as a collector. Minerals 2017, 7, 65. [Google Scholar] [CrossRef]
- Horta, D.; Monte, M.B.M.; Leal Filho, L.S. The effect of dissolution kinetics on flotation response of apatite with sodium oleate. Int. J. Miner. Process. 2016, 146, 97–104. [Google Scholar] [CrossRef]
- Tohry, A.; Dehghani, A. Effect of sodium silicate on the reverse anionic flotation of a siliceous-phosphorus iron ore. Sep. Purif. Technol. 2016, 164, 31–32. [Google Scholar] [CrossRef]
- Zhang, G.F.; Feng, Y.; Zhu, Y.G.; Tang, P.H. Influence of Ca2+, Mg2+ on apatite and dolomite flotation. Ind. Miner. Process. 2011, 7, 1–4. (In Chinese) [Google Scholar]
- Araújo, A.C.A.; Lima, R.M.F. Influence of cations Ca2+, Mg2+ and Zn2+ on the flotation and surface charge of smithsonite and dolomite with sodium oleate and sodium silicate. Int. J. Miner. Process. 2017, 167, 35–41. [Google Scholar] [CrossRef]
- Ananthapadmanabhan, K.P.; Somasundaran, P. Surface precipitation of inorganics and surfactants and its role in adsorption and flotation. Colloids Surf. 1985, 13, 151–167. [Google Scholar] [CrossRef]
- Somasundaran, P.; Wang, D.Z. Solution Chemistry: Minerals and Reagents, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 45–57. [Google Scholar]
- Fuerstenau, M.C.; Lopez-Valdivieso, A.; Fuerstenau, D.W. Role of hydrolyzed cations in the natural hydrophobicity of talc. Int. J. Miner. Process. 1988, 23, 161–170. [Google Scholar] [CrossRef]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42+ ions on the zeta potential of calcite and dolomite particles aged with stearic acid. Colloids Surf. A 2015, 482, 293–298. [Google Scholar] [CrossRef]
- Han, K.N.; Healy, T.W.; Fuerstenau, D.W. The mechanism of adsorption of fatty acids and other sufactants at the oxide-water interface. J. Colloid Interface Sci. 1973, 44, 407. [Google Scholar] [CrossRef]
- Amankonah, J.O.; Somasundaran, P. Effects of dissolved mineral species on the electrokinetic behavior of calcite and apatite. Colloids Surf. 1985, 15, 335–353. [Google Scholar] [CrossRef]
- Vucinic, D.R.; Radulovic, D.S.; Deusic, S.D. Electrokinetic properties of hydroxyapatite under flotation conditions. J. Colloid Interface Sci. 2010, 343, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Schott, J.; Thomas, F. Dolomite surface speciation and reactivity in aquatic systems. Geochim. Cosmochim. Acta 1999, 63, 3137. [Google Scholar] [CrossRef]
- Kou, J.; Xu, S.; Sun, T.; Guo, Y.; Wang, C. A study of sodium oleate adsorption on Ca2+ activated quartz surface using quartz crystal microbalance with dissipation. Int. J. Miner. Process. 2016, 154, 26–27. [Google Scholar] [CrossRef]
- Hu, Y.; Xu, Z. Interactions of amphoteric amino phosphoric acid with calcium-containing minerals and selective flotation. Int. J. Miner. Process. 2003, 72, 91. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. Effect of solution chemistry on flotability of magnesite and dolomite. Int. J. Miner. Process. 2004, 74, 354–356. [Google Scholar] [CrossRef]
- Gence, N.; Ozbay, N. pH dependence of electrokinetic behavior of dolomite and magnesite in aqueous electrolyte solutions. Appl. Surf. Sci. 2006, 252, 8059–8060. [Google Scholar] [CrossRef]
- Vidyadhar, A.; Rao, K.H. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 198. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Yin, W.; Gong, E. Depressing effect of fine hydrophilic particles on magnesite reverse flotation. Int. J. Miner. Process. 2016, 149, 88. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Wang, X.; Wei, D.; Wang, B. Utilization of novel surfactant N-dodecyl-isopropanolamine as collector for efficient separation of quartz from hematite. Sep. Purif. Technol. 2016, 162, 192. [Google Scholar] [CrossRef]
- Buckley, A.N. A survey of the application of X-ray photoelectron spectroscopy to flotation research. Colloids Surf. A 1994, 93, 168–169. [Google Scholar] [CrossRef]
- Sahoo, H.; Rath, S.S.; Das, B.; Mishra, B.K. Flotation of quartz using ionic liquid collectors with different functional groups and varying chain lengths. Miner. Eng. 2016, 95, 108–110. [Google Scholar] [CrossRef]
- Gouzman, I.; Dubey, M.; Carolus, M.D.; Schwartz, J.; Bernasek, S.L. Monolayer vs. multilayer self-assembled alkylphosphonate films: X-ray photoelectron spectroscopy studies. Surf. Sci. 2006, 600, 776–777. [Google Scholar] [CrossRef]
- Chen, P.; Zhai, J.; Sun, W.; Hu, Y.; Yin, Z. The activation mechanism of lead ions in the flotation of ilmenite using sodium oleate as a collector. Miner. Eng. 2017, 111, 100–107. [Google Scholar] [CrossRef]

| Metal Ions | Stability Constants | pKsp, M(OH)m | |||
|---|---|---|---|---|---|
| logβ1 | logβ2 | logβ3 | logβ4 | ||
| Ca2+ | 1.40 | 2.77 | / | / | 5.22 |
| Mg2+ | 2.58 | 1.00 | / | / | 11.15 |
| Al3+ | 9.01 | 18.70 | 27.00 | 33.00 | 33.50 |
| Fe3+ | 11.81 | 22.30 | 32.05 | 34.30 | 38.80 |
| Sample | Elements (Atomic %) | ||||
|---|---|---|---|---|---|
| O1s | C1s | Ca2p | P2p | F1s | |
| Apatite | 45.89 | 24.95 | 15.30 | 10.13 | 3.73 |
| Apatite + Ca2+ | 45.01 | 25.43 | 15.82 | 9.90 | 3.84 |
| Apatite + collector | 41.19 | 33.35 | 13.21 | 9.65 | 2.60 |
| Apatite + Ca2+ + collector | 38.62 | 36.07 | 13.22 | 8.89 | 3.20 |
| Sample | Elements (Atomic %) | |||
|---|---|---|---|---|
| O1s | C1s | Ca2p | Mg1s | |
| Dolomite | 51.66 | 34.14 | 9.26 | 4.94 |
| Dolomite + Ca2+ | 50.27 | 36.45 | 9.48 | 3.80 |
| Dolomite + collector | 44.87 | 43.51 | 8.55 | 3.07 |
| Dolomite + Ca2+ + collector | 43.64 | 45.29 | 8.50 | 2.57 |
| Sample | Elements (Atomic %) | |||
|---|---|---|---|---|
| O1s | C1s | Ca2p | Si2p | |
| quartz | 55.04 | 14.16 | - | 30.80 |
| quartz + Ca2+ | 57.78 | 14.50 | 0.17 | 27.55 |
| quartz + Ca2+ + collector | 29.38 | 53.96 | 1.48 | 15.18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruan, Y.; Zhang, Z.; Luo, H.; Xiao, C.; Zhou, F.; Chi, R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals 2018, 8, 141. https://doi.org/10.3390/min8040141
Ruan Y, Zhang Z, Luo H, Xiao C, Zhou F, Chi R. Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals. 2018; 8(4):141. https://doi.org/10.3390/min8040141
Chicago/Turabian StyleRuan, Yaoyang, Zeqiang Zhang, Huihua Luo, Chunqiao Xiao, Fang Zhou, and Ruan Chi. 2018. "Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz" Minerals 8, no. 4: 141. https://doi.org/10.3390/min8040141
APA StyleRuan, Y., Zhang, Z., Luo, H., Xiao, C., Zhou, F., & Chi, R. (2018). Effects of Metal Ions on the Flotation of Apatite, Dolomite and Quartz. Minerals, 8(4), 141. https://doi.org/10.3390/min8040141






