Non-Destructive Multi-Analytical Approach to Study the Pigments of Wall Painting Fragments Reused in Mortars from the Archaeological Site of Pompeii (Italy)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Macroscopic Features of the Samples and Colorimetric Analysis
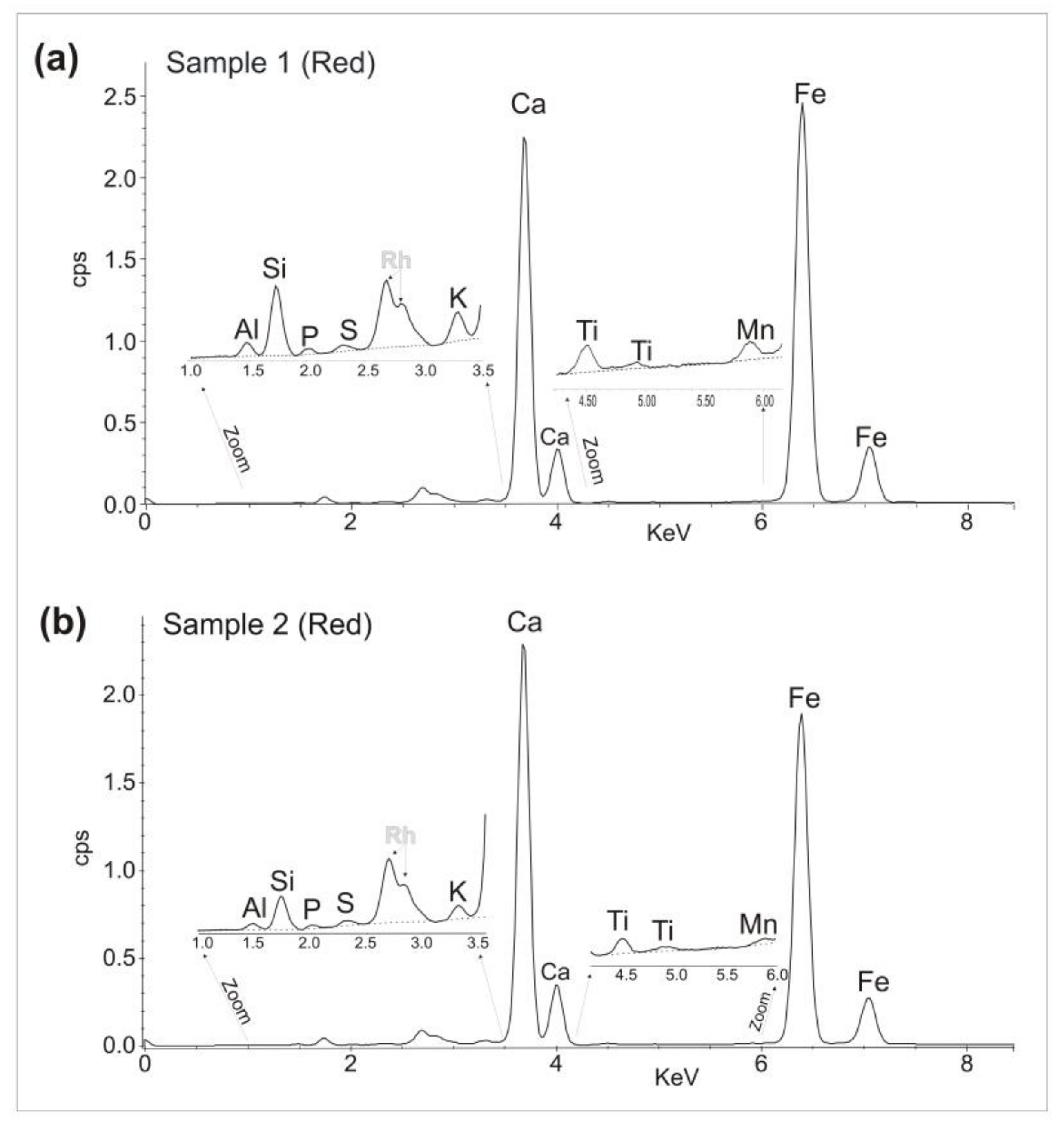
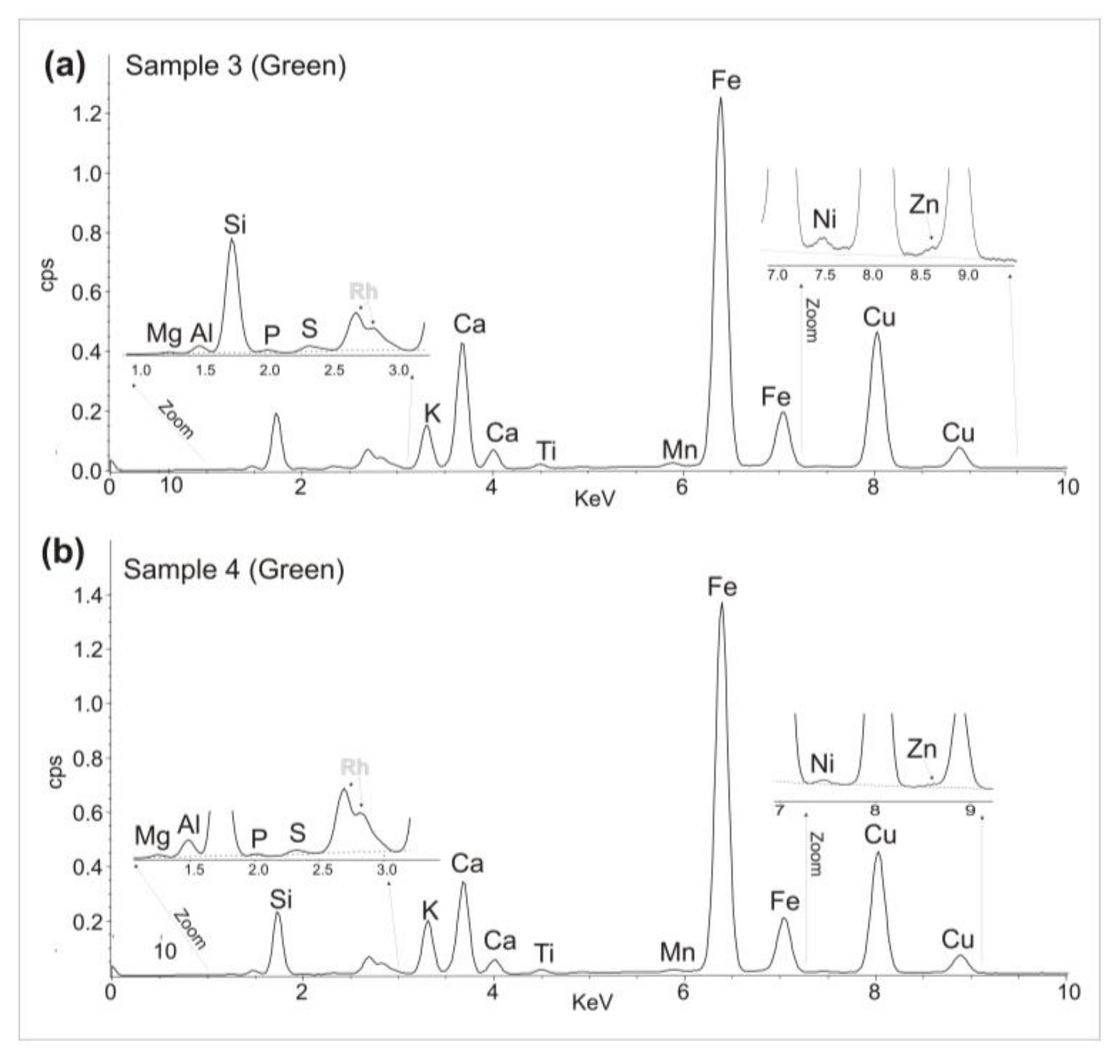
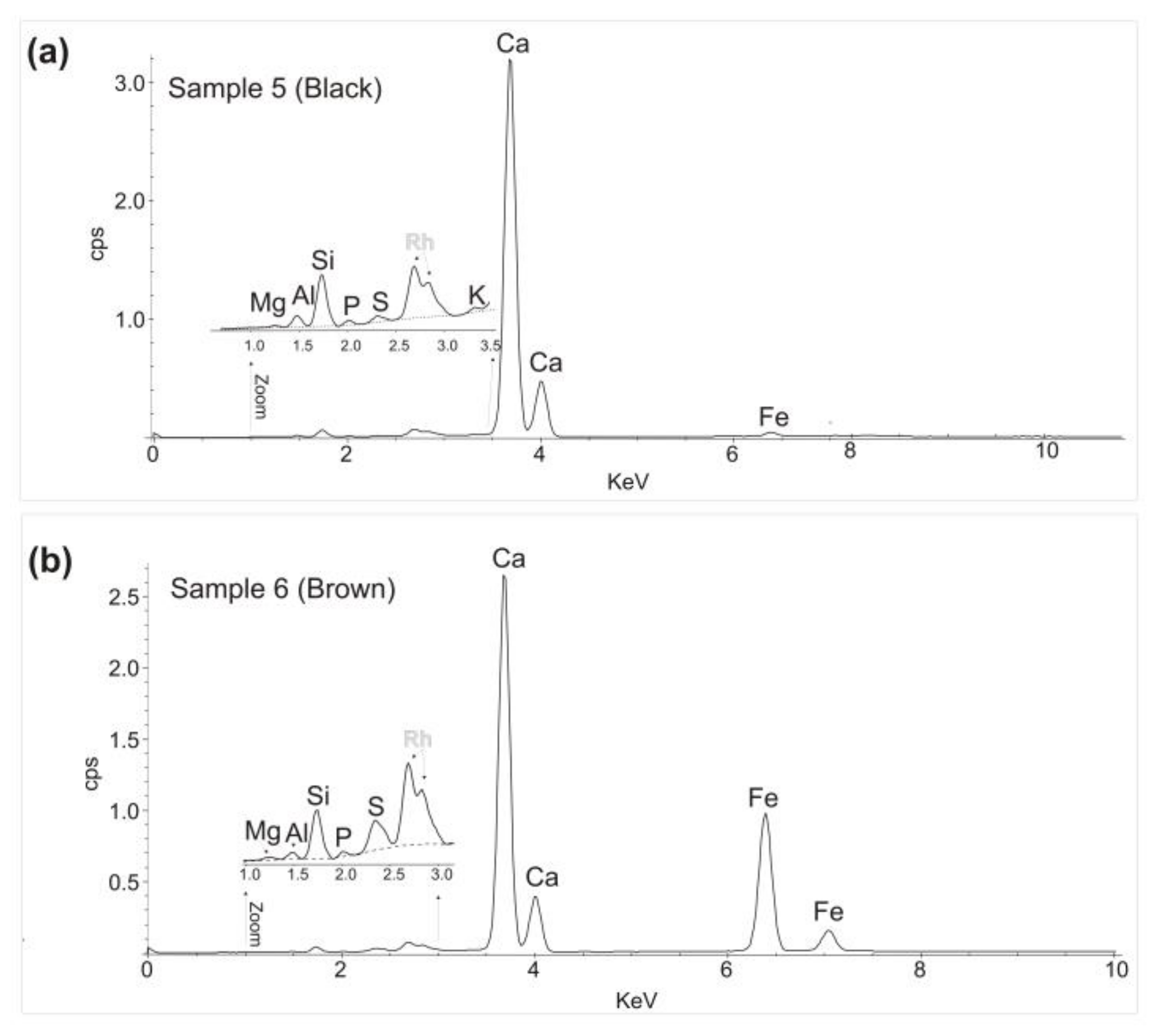
3.2. Portable X-ray Fluorescence
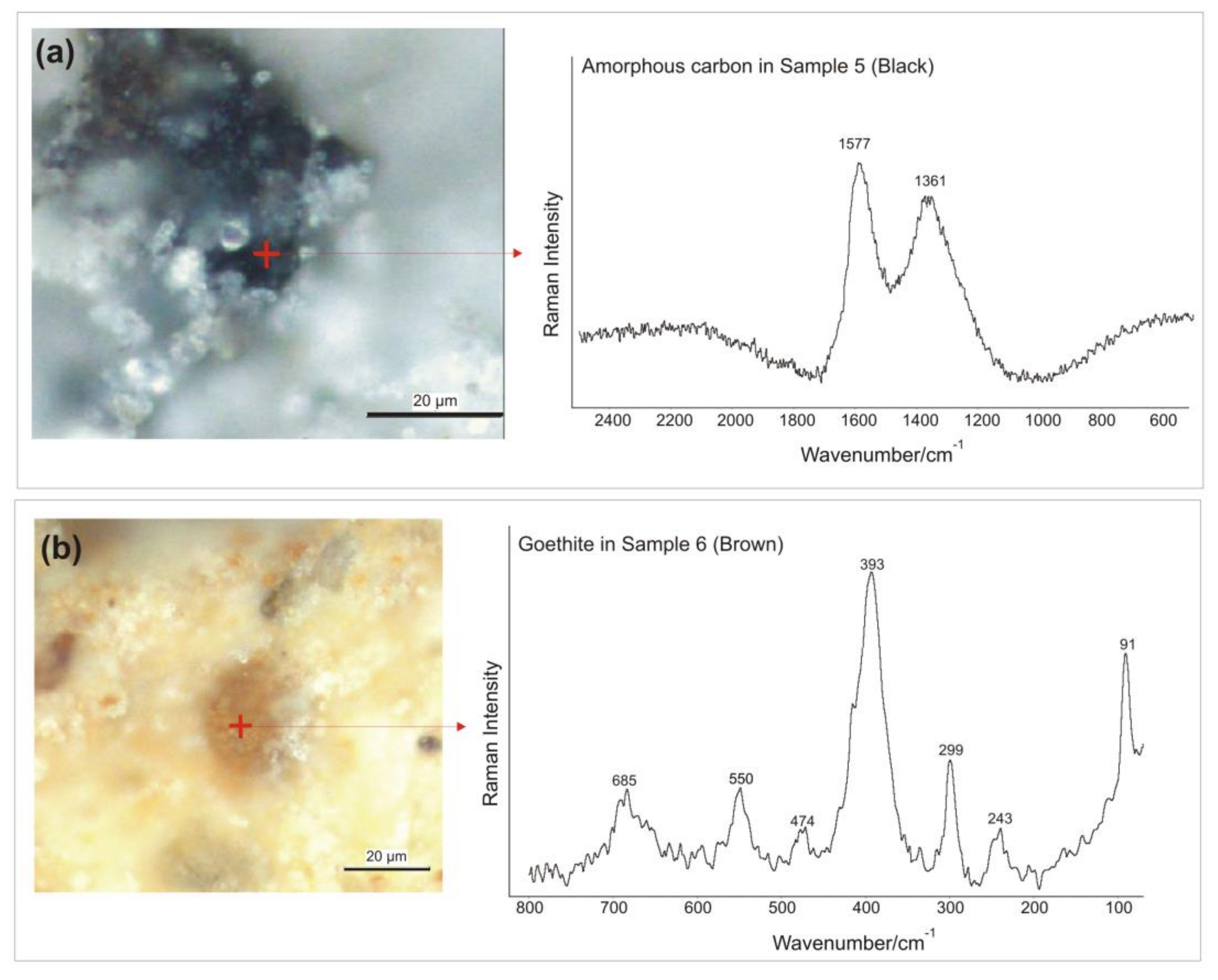
3.3. Micro-Raman Spectroscopy
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Senatore, M.R.; Ciarallo, A.; Stanley, J.D. Pompeii damaged by volcaniclastic debris flows triggered centuries prior to the 79 AD Vesuvius eruption. Geoarchaeology 2014, 29, 1–15. [Google Scholar] [CrossRef]
- Richardson, L.J.R. Pompeii: An Architectural History; Johns Hopkins University Press: Baltimore, MD, USA, 1988. [Google Scholar]
- Santini, L. Pompeii; Plurigraf: Sesto Fiorentino, Italy, 2004. [Google Scholar]
- Varone, A. Pompeii. I Misteri di una Città Sepolta-Storia e Segreti di un Luogo in Cui la Vita si è Fermata Duemila Anni fa; Newton Compton Editori: Roma, Italy, 2005. [Google Scholar]
- De Vos, A.; De Vos, M. Pompei, Ercolano e Stabia. Guide Archeologiche; Laterza: Bari, Italy, 1982. [Google Scholar]
- Castriota, M.; Cosco, V.; Barone, T.; De Santo, G.; Carafa, P.; Cazzanelli, E. Micro-Raman characterizations of Pompei’s mortars. J. Raman Spectrosc. 2008, 39, 295–301. [Google Scholar] [CrossRef]
- Kastenmeier, P.; Di Maio, G.; Balassone, G.; Boni, M.; Joachimski, M.; Mondillo, N. The source of stone building materials from the Pompeii archaeological area and its surroundings. Period. Mineral. 2010, 79, 39–58. [Google Scholar]
- Miriello, D.; Barca, D.; Bloise, A.; Ciarallo, A.; Crisci, G.M.; De Rose, T.; Gattuso, C.; Gazineo, F.; La Russa, M.F. Characterisation of archaeological mortars from Pompeii (Campania, Italy) and identification of construction phases by compositional data analysis. J. Archaeol. Sci. 2010, 37, 2207–2223. [Google Scholar] [CrossRef]
- Maguregui, M.; Knuutinen, U.; Trebolazabala, J.; Morillas, H.; Castro, K.; Martinez-Arkarazo, I.; Madariaga, J.M. Use of in situ and confocal Raman spectroscopy to study the nature and distribution of carotenoids in brown patinas from a deteriorated wall painting in Marcus Lucretius House (Pompeii). Anal. Bioanal. Chem. 2012, 402, 1529–1539. [Google Scholar] [CrossRef] [PubMed]
- Grifa, C.; De Bonis, A.; Langella, A.; Mercurio, M.; Soricelli, G.; Morra, V. A Late Roman ceramic production from Pompeii. J. Archaeol. Sci. 2013, 40, 810–826. [Google Scholar] [CrossRef]
- Piovesan, R.; Dalconi, M.C.L.; Maritan, C.; Mazzoli, C. X-ray powder diffraction clustering and quantitative phase analysis on historic mortars. Eur. J. Mineral. 2013, 25, 165–175. [Google Scholar] [CrossRef]
- Scarpelli, R.; Clark, R.J.H.; De Francesco, A.M. Archaeometric study of black-coated pottery from Pompeii by different analytical techniques. Spectrochim. Acta A 2014, 120, 60–66. [Google Scholar] [CrossRef] [PubMed]
- De Luca, R.; Miriello, D.; Pecci, A.; Domínguez-Bella, S.; Bernal-Casasola, D.; Cottica, D.; Bloise, A.; Crisci, G.M. Archaeometric study of mortars from the Garum Shop at Pompeii, Campania, Italy. Geoarchaeology 2015, 30, 330–351. [Google Scholar] [CrossRef]
- Scarpelli, R.; De Francesco, A.M.; Gaeta, M.; Cottica, D.; Toniolo, L. The provenance of the Pompeii cooking wares: Insights from LA–ICP-MS trace element analyses. Microchem. J. 2015, 119, 93–101. [Google Scholar] [CrossRef]
- Augusti, S. I Colori Pompeiani, Studi e Documentazioni I; Ministero della Pubblica Istruzione, Direzione Generale delle Antichità e Belle Arti, De Luca Editori: Roma, Italy, 1967. [Google Scholar]
- Zanella, E.; Gurioli, L.; Chiari, G.; Ciarallo, A.; Cioni, R.; De Carolis, E.; Lanza, R. Archaeomagnetic results from mural paintings and pyroclastic rocks in Pompeii and Herculaneum. Phys. Earth Planet. Inter. 2000, 118, 227–240. [Google Scholar] [CrossRef]
- Cotte, M.; Susini, J.; Metrich, N.; Moscato, A.; Gratziu, C.; Bertagnini, A.; Pagano, M. Blackening of Pompeian cinnabar paintings: X-ray microspectroscopy analysis. Anal. Chem. 2006, 78, 7484–7492. [Google Scholar] [CrossRef] [PubMed]
- Welcomme, E.; Walter, P.; Van Elslande, E.; Tsoucaris, G. Investigation of white pigments used as make-up during the Greco-Roman period. Appl. Phys. A 2006, 83, 551–556. [Google Scholar] [CrossRef]
- Aliatis, I.; Bersani, D.; Campani, E.; Casoli, A.; Lottici, P.P.; Mantovan, S.; Marino, I.G.; Ospitali, F. Green pigments of the Pompeian artists’ palette. Spectrochim. Acta A 2009, 73, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Piovesan, R.; Siddall, R.; Mazzoli, C.; Nodari, L. The Temple of Venus (Pompeii): A study of the pigments and painting techniques. J. Archaeol. Sci. 2011, 38, 2633–2643. [Google Scholar] [CrossRef]
- Aliatis, I.; Bersani, D.; Campani, E.; Casoli, A.; Lottici, P.P.; Mantovan, S.; Marino, I.G. Pigments used in Roman wall paintings in the Vesuvian area. J. Raman Spectrosc. 2010, 41, 1537–1542. [Google Scholar] [CrossRef]
- Caneve, L.; Diamanti, A.; Grimaldi, F.; Palleschi, G.; Spizzichino, V.; Valentini, F. Analysis of fresco by laser induced breakdown spectroscopy. Spectrochim. Acta B 2010, 65, 702–706. [Google Scholar] [CrossRef]
- Duran, A.; Jimenez De Haro, M.C.; Perez-Rodriguez, J.L.; Franquelo, M.L.; Herrera, L.K.; Justo, A. Determination of pigments and binders in Pompeian wall paintings using synchrotron radiation—High-resolution X-ray powder diffraction and conventional spectroscopy–chromatography. Archaeometry 2010, 52, 286–307. [Google Scholar] [CrossRef]
- Gelzo, M.; Grimaldi, M.; Vergara, A.; Severino, V.; Chambery, A.; Russo, A.D.; Piccioli, C.; Corso, G.; Arcari, P. Comparison of binder compositions in Pompeian wall painting styles from Insula Occidentalis. Chem. Cent. J. 2014, 8, 65. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marcaida, I.; Maguregui, M.; Morillas, H.; García-Florentino, C.; Knuutinen, U.; Carrero, J.A.; de Vallejuelo, S.F.O.; Pitarch Martí, A.; Castro, K; Madariaga, J.M. Multispectroscopic and Isotopic Ratio Analysis to Characterize the Inorganic Binder Used on Pompeian Pink and Purple Lake Pigments. Anal. Chem. 2016, 88, 6395–6402. [Google Scholar] [CrossRef] [PubMed]
- Marcaida, I.; Maguregui, M.; de Vallejuelo, S.F.O.; Morillas, H.; Prieto-Taboada, N.; Veneranda, M.; Castro, K.; Madariaga, J.M. In situ X-ray fluorescence-based method to differentiate among red ochre pigments and yellow ochre pigments thermally transformed to red pigments of wall paintings from Pompeii. Anal. Bioanal. Chem. 2017, 409, 3853–3860. [Google Scholar] [CrossRef] [PubMed]
- Maguregui, M.; Castro, K.; Morillas, H.; Trebolazabala, J.; Knuutinen, U.; Wiesinger, R.; Schreinerd, M.; Madariaga, J.M. Multianalytical approach to explain the darkening process of hematite pigment in paintings from ancient Pompeii after accelerated weathering experiments. Anal. Methods 2014, 6, 372–378. [Google Scholar] [CrossRef]
- Giachi, G.; De Carolis, E.; Pallecchi, P. Raw materials in Pompeian paintings: Characterization of some colors from the archaeological site. Mater. Manuf. Processes. 2009, 24, 1015–1022. [Google Scholar] [CrossRef]
- D’Esposito, L.; Iadanza, M. Pompei per Tutti; Sirano, F., Ed.; Arte’m: Napoli, Italy, 2016; pp. 37–38. [Google Scholar]
- Cioni, R.; Gurioli, L.; Lanza, R.; Zanella, E. Temperatures of the AD 79 pyroclastic density current deposits (Vesuvius, Italy). J. Geophys. Res. 2004, 109, B02207. [Google Scholar] [CrossRef]
- Gonçalves, Í.G.; Petter, C.O.; Machado, J.L. Quantification of hematite and goethite concentrations in kaolin using diffuse reflectance spectroscopy: A new approach to Kubelka-Munk theory. Clays Clay Miner. 2012, 60, 473–483. [Google Scholar] [CrossRef]
- Liu, Q.S.; Torrent, J.; Barrón, V.; Duan, Q.Z.; Bloemendal, J. Quantification of hematite from the visible diffuse reflectance spectrum: Effects of aluminium substitution and grain morphology. Clay Miner. 2011, 46, 137–147. [Google Scholar] [CrossRef]
- Torrent, J.; Barrón, V. The visible diffuse reflectance in relation to the color and crystal properties of hematite. Clays Clay Miner. 2003, 51, 309–317. [Google Scholar] [CrossRef]
- Ji, J.; Balsam, W.; Chen, J.; Liu, L. Rapid and quantitative measurement of hematite and goethite in the Chinese loess-paleosol sequence by diffuse reflectance spectroscopy. Clays Clay Miner. 2002, 50, 208–216. [Google Scholar] [CrossRef]
- Scheinost, A.C.; Chavernas, A.; Barrón, V.; Torrent, J. Use and limitations of second-derivative diffuse reflectance spectroscopy in the visible to near-infrared range to identify and quantify Fe oxide minerals in soils. Clays Clay Miner. 1998, 46, 528–536. [Google Scholar] [CrossRef]
- Moioli, P.; Seccaroni, C. Analysis of art objects using a portable X-ray fluorescence spectrometer. X-ray Spectrom. 2000, 29, 48–52. [Google Scholar] [CrossRef]
- Seccaroni, C.; Moioli, P.; Fluorescenza, X. Prontuario per L’analisi XRF Portatile Applicata a Superfici Policrome; Nardini Editore: Firenze, Italy, 2001. [Google Scholar]
- Driscoll, R.; Hageman, P.; Benzel, W.; Diehl, S.; Adams, D.; Morman, S.; Choate, L.D. Assessment of the Geoavailability of Trace Elements from Minerals in Mine Wastes: Analytical Techniques and Assessment of Selected Copper Minerals; Denver Science Publishing Network: Denver, CO, USA, 2011. [Google Scholar]
- EL-Nafaty, J.M. Geology and Trace Element Geochemistry of the Barite-Copper Mineralization in Gulani Area, NE Nigeria. Iosr. Jagg. 2017, 5, 1–16. [Google Scholar] [CrossRef]
- Burgio, L.; Clark, R.J. Library of FT-Raman spectra of pigments, minerals, pigment media and varnishes, and supplement to existing library of Raman spectra of pigments with visible excitation. Spectrochim. Acta A 2001, 57, 1491–1521. [Google Scholar] [CrossRef]
- Bouchard, M.; Smith, D.C. Catalogue of 45 reference Raman spectra of minerals concerning research in art history or archaeology, especially on corroded metals and coloured glass. Spectrochim. Acta A 2003, 59, 2247–2266. [Google Scholar] [CrossRef]
- Giarola, M.; Mariotto, G.; Ajò, D. Micro-Raman investigations on inclusions of unusual habit in a commercial tanzanite gemstone. J. Raman Spectrosc. 2012, 43, 556–558. [Google Scholar] [CrossRef]
- Veiga, A.; Teixeira, D.M.; Candeias, A.J.; Mirão, J.; Manhita, A.; Miguel, C.; Rodrigues, P.; Teixeira, J.G. Micro-analytical study of two 17th century gilded miniature portraits on copper. Microchem. J. 2015, 123, 51–61. [Google Scholar] [CrossRef]
- Caggiani, M.C.; Cosentino, A.; Mangone, A. Pigments Checker version 3.0, a handy set for conservation scientists: A free online Raman spectra database. Microchem. J. 2016, 129, 123–132. [Google Scholar] [CrossRef]
- Kawakami, M.; Karato, T.; Takenaka, T.; Yokoyama, S. Structure analysis of coke, wood charcoal and bamboo charcoal by Raman spectroscopy and their reaction rate with CO2. ISIJ Int. 2005, 45, 1027–1034. [Google Scholar] [CrossRef]
- Cohen-Ofri, I.; Weiner, L.; Boaretto, E.; Mintz, G.; Weiner, S. Modern and fossil charcoal: Aspects of structure and diagenesis. J. Archaeol. Sci. 2006, 33, 428–439. [Google Scholar] [CrossRef]
- Gil, M.; Carvalho, M.L.; Seruya, A.; Candeias, A.E.; Mirão, J.; Queralt, I. Yellow and red ochre pigments from southern Portugal: Elemental composition and characterization by WDXRF and XRD. Nucl. Instrum. Methods Phys. Res. A 2007, 580, 728–731. [Google Scholar] [CrossRef]
- Kingery-Schwartz, A.; Popelka-Filcoff, R.S.; Lopez, D.A.; Pottier, F.; Hill, P.; Glascock, M. Analysis of geological ochre: Its geochemistry, use, and exchange in the US Northern Great Plains. Open J. Archaeom. 2013, 1, 15. [Google Scholar] [CrossRef]
- Bikiaris, D.; Daniilia, S.; Sotiropoulou, S.; Katsimbiri, O.; Pavlidou, E.; Moutsatsou, A.P.; Chryssoulakis, Y. Ochre-differentiation through micro-Raman and micro-FTIR spectroscopies: Application on wall paintings at Meteora and Mount Athos, Greece. Spectrochim. Acta A 2000, 56, 3–18. [Google Scholar] [CrossRef]
- Froment, F.; Tourni, A.; Colomban, P. Raman identification of natural red to yellow pigments: Ochre and iron-containing ores. J. Raman Spectrosc. 2008, 39, 560–568. [Google Scholar] [CrossRef]
- Bordignon, F.; Postorino, P.; Dorel, P.; Trojsi, G. Raman identification of green and blue pigments in Etruscan polychromes on architectural terracotta panels. J. Raman Spectrosc. 2007, 38, 255–259. [Google Scholar] [CrossRef]
- Pagès-Camagna, S.; Colinart, S.; Coupry, C. Fabrication processes of archaeological Egyptian blue and green pigments enlightened by Raman microscopy and scanning electron microscopy. J. Raman Spectrosc. 1999, 30, 313–317. [Google Scholar] [CrossRef]
- Bloise, A.; El Salam, S.A.; De Luca, R.; Crisci, G.M.; Miriello, D. Flux growth and characterization of cuprorivaite: The influence of temperature, flux, and silica source. Appl. Phys. A Mater. 2016, 122, 650. [Google Scholar] [CrossRef]
- Baraldi, P.; Baraldi, C.; Curina, R.; Tassi, L.; Zannini, P. A micro-Raman archaeometric approach to Roman wall paintings. Vib. Spectrosc. 2007, 43, 420–426. [Google Scholar] [CrossRef]
- Bruni, S.; Cariati, F.; Casadio, F.; Toniolo, L. Spectrochemical characterization by micro-FTIR spectroscopy of blue pigments in different polychrome works of art. Vib. Spectrosc. 1999, 20, 15–25. [Google Scholar] [CrossRef]
- Rosalie David, A.; Edwards, H.G.M.; Farwell, D.W.; De Faria, D.L.A. Raman spectroscopic analysis of ancient Egyptian pigments. Archaeometry 2001, 43, 461–473. [Google Scholar] [CrossRef]
- Schiegl, S.; Weiner, K.L.; El Goresy, A. The diversity of newly discovered deterioration patterns in ancient Egyptian pigments: Consequences to entirely new restoration strategies and to the Egyptological colour symbolism. MRS Proc. 1992, 267, 831. [Google Scholar] [CrossRef]
- Bianchetti, P.; Talarico, F.; Vigliano, M.G.; Ali, M.F. Production and characterization of Egyptian blue and Egyptian green frit. J. Cult. Herit. 2000, 1, 179–188. [Google Scholar] [CrossRef]
- Mazzocchin, G.A.; Rudello, D.; Bragato, C.; Agnoli, F. A short note on Egyptian blue. J. Cult. Herit. 2004, 5, 129–133. [Google Scholar] [CrossRef]
- De Faria, D.L.A.; Venâncio Silva, S.; De Oliveira, M.T. Raman microspectroscopy of some iron oxides and oxyhydroxides. J. Raman Spectrosc. 1997, 28, 873–878. [Google Scholar] [CrossRef]
- Oh, S.J.; Cook, D.C.; Townsend, H.E. Characterization of iron oxides commonly formed as corrosion products on steel. Hyperfine Inter. 1998, 112, 59–66. [Google Scholar] [CrossRef]
- Ospitali, F.; Bersani, D.; Di Lonardo, G.; Lottici, P.P. ‘Green earths’: Vibrational and elemental characterization of glauconites, celadonites and historical pigments. J. Raman Spectrosc. 2008, 39, 1066–1073. [Google Scholar] [CrossRef]
- Pomies, M.P.; Morin, G.; Vignaud, C. XRD study of the goethite-hematite transformation: Application to the identification of heated prehistoric pigments. Eur. J. Solid State Inorg. Chem. 1998, 35, 9–25. [Google Scholar] [CrossRef]
- Pomiès, M.P.; Menu, M.; Vignaud, C. Red palaeolithic pigments: Natural hematite or heated goethite? Archaeometry 1999, 41, 275–285. [Google Scholar] [CrossRef]
- Omarini, S. Notes on colours and pigments in the ancient world. JAIC 2012, 8, 61. [Google Scholar]

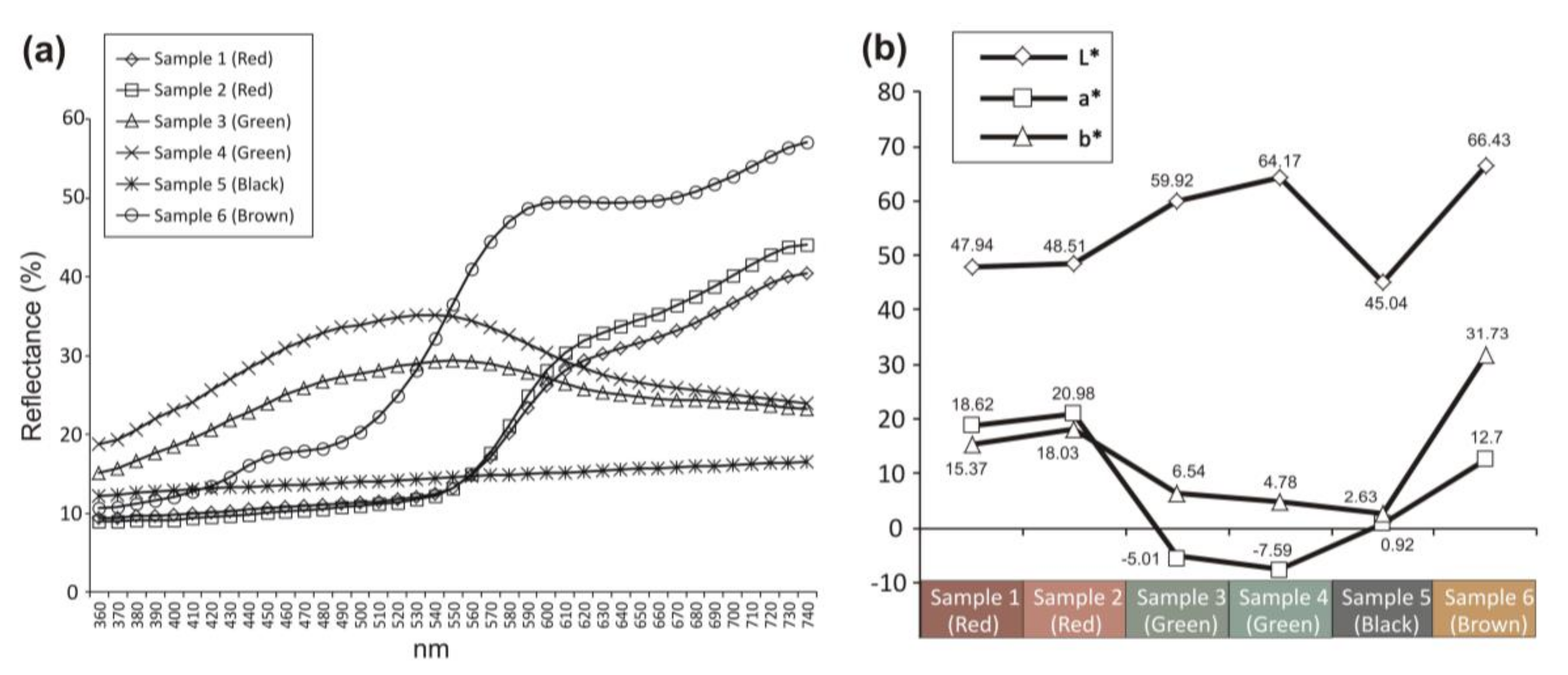
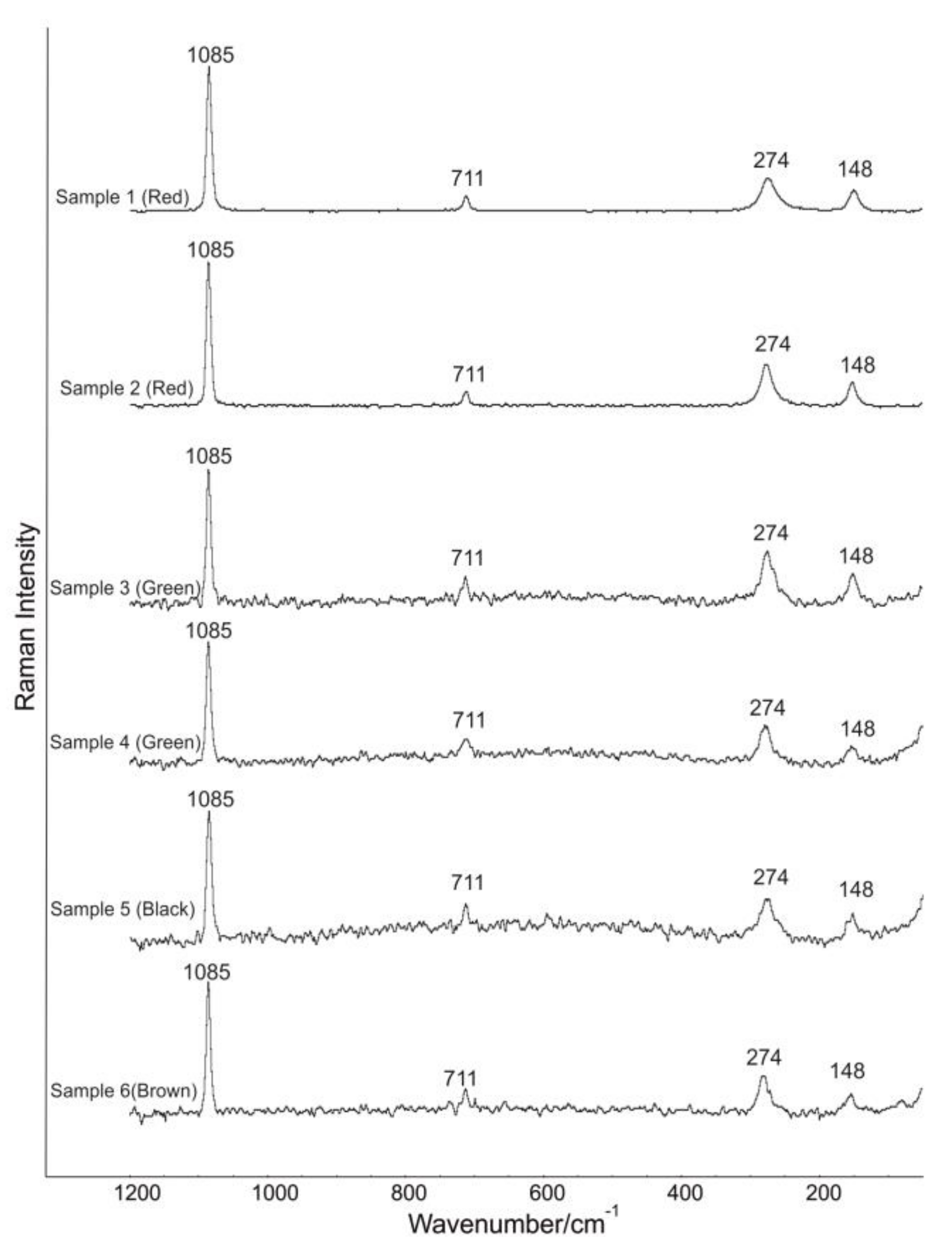
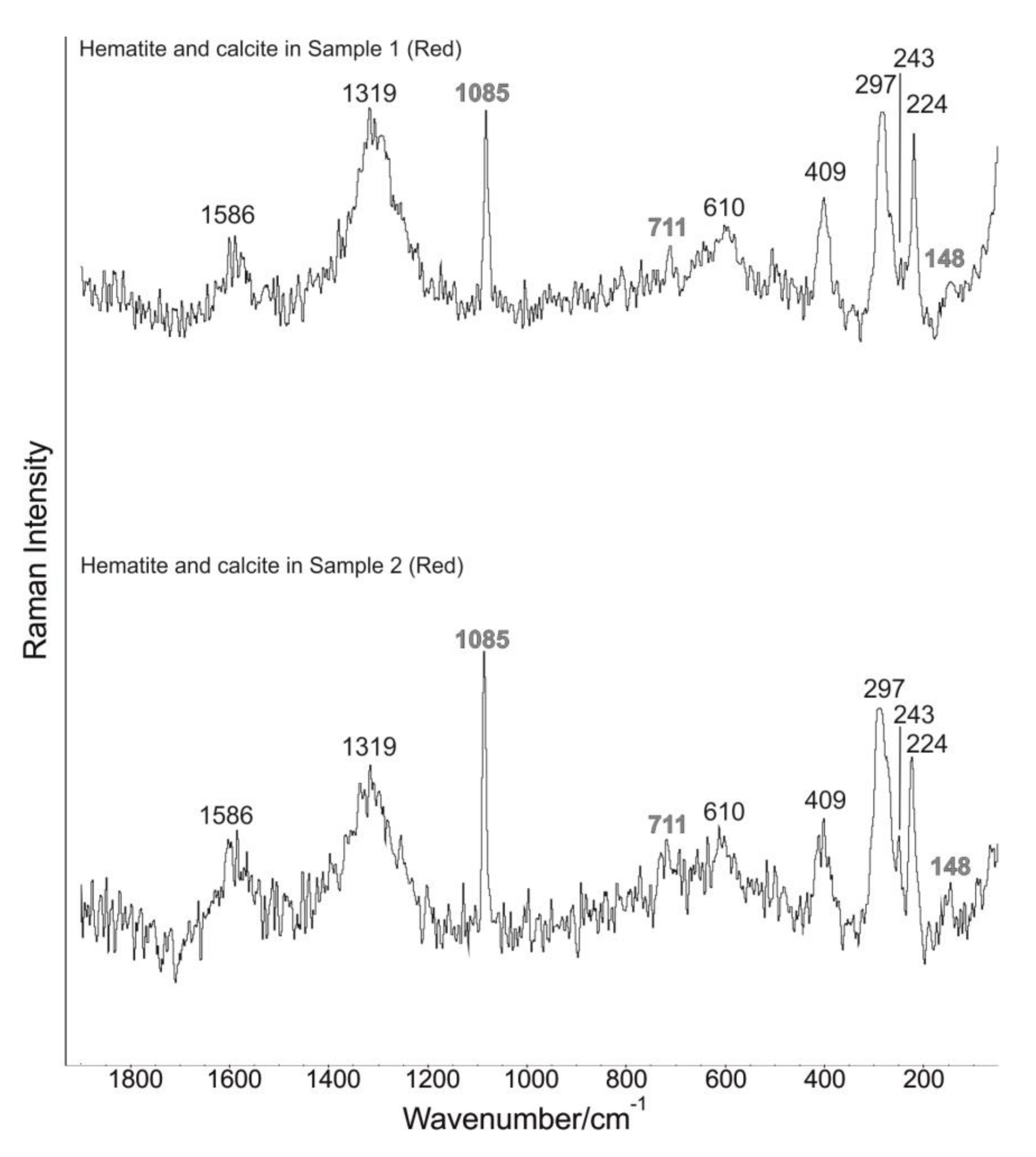
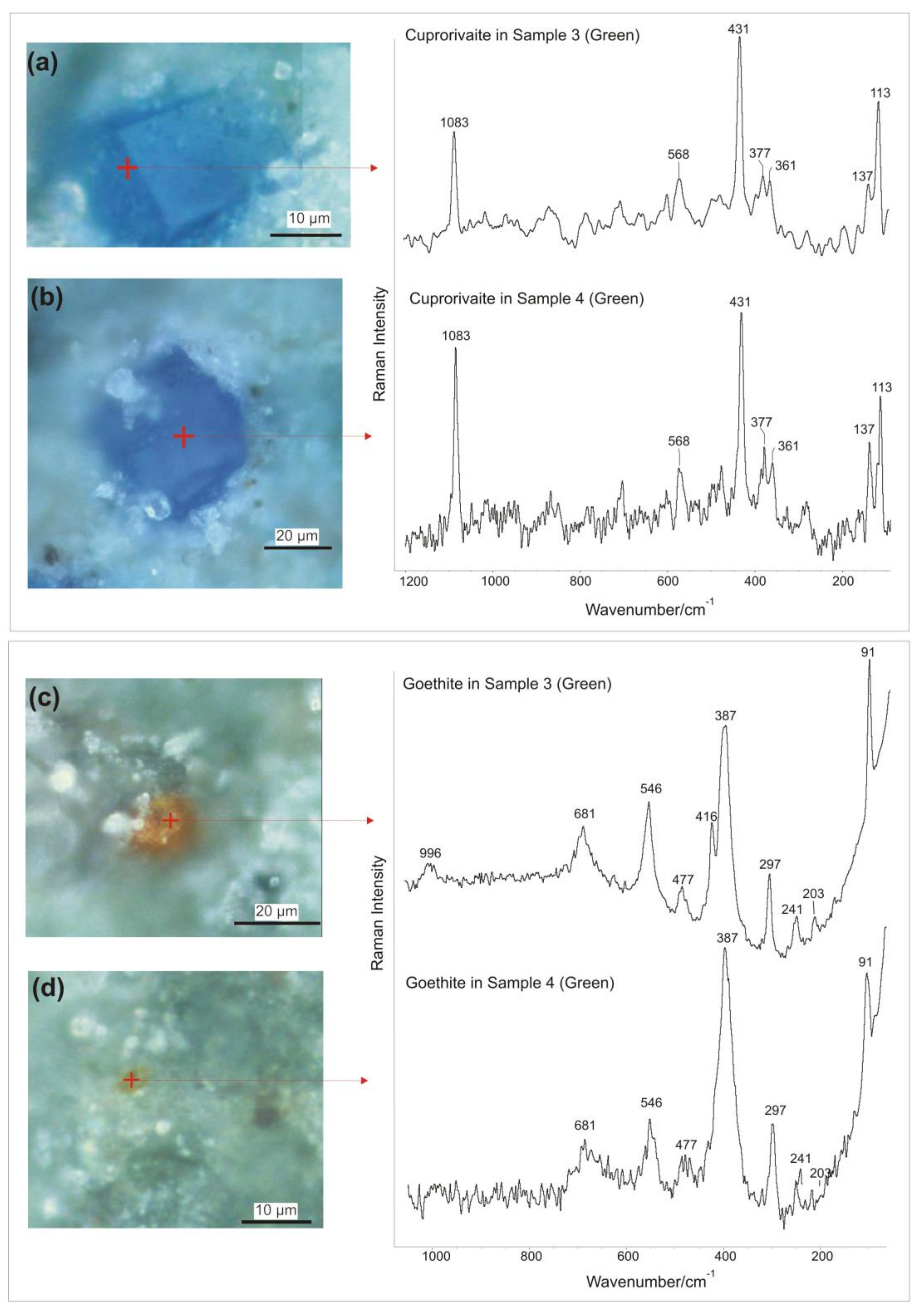
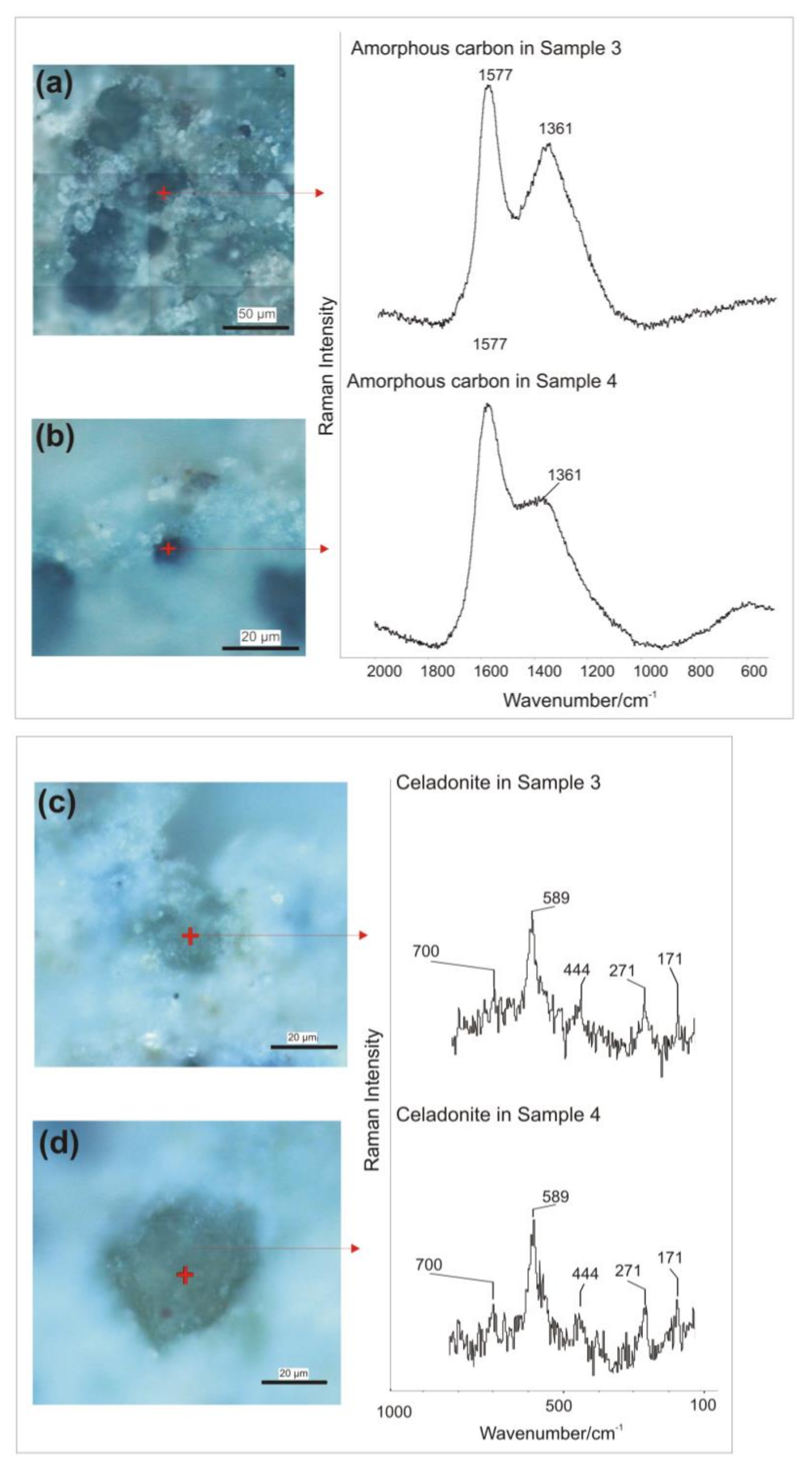
| Band Wavenumber/cm−1 and Compounds Identification by Micro-Raman Spectroscopy | P-XRF | |||||||
|---|---|---|---|---|---|---|---|---|
| Sample | Color | Calcite: 148, 274, 711, 1085 cm−1 [40]. Power output of the laser beam: 7 mW | Hematite: 224, 243, 297, 409, 610, 1319 cm−1 [41,42,43,44]. Power output of the laser beam: 2.6 mW | Cuprorivaite: 113, 137, 361, 377, 431, 568, 1083 cm−1 [40,51,52,53,54,55,56]. Power output of the laser beam: 5 mW | Goethite: 91, 203, 241, 297, 387, 416, 477, 546, 681 cm−1 [41,60,61]. Power output of the laser beam: 1.8 mW | Celadonite: 171, 271, 444, 589, 700 cm−1 [62]. Power output of the laser beam: 1.8 mW | Amorphous carbon: 1361, 1577 cm−1 [45,46]. Power output of the laser beam: 6 mW | Chemical elements identified by p-XRF |
| Sample 1 | Red | Yes | Yes | No | No | No | Yes | Al, Si, P, K, Ca, Ti, Mn, Fe. |
| Sample 2 | Red | Yes | Yes | No | No | No | Yes | Al, Si, P, K, Ca, Ti, Mn, Fe. |
| Sample 3 | Green | Yes | No | Yes | Yes | Yes | Yes | Mg, Al, Si, P, S, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn. |
| Sample 4 | Green | Yes | No | Yes | Yes | Yes | Yes | Mg, Al, Si, P, S, K, Ca, Ti, Mn, Fe, Ni, Cu, Zn. |
| Sample 5 | Black | Yes | No | No | No | No | Yes | Mg, Al, Si, P, S, K Ca, Fe. |
| Sample 6 | Brown | Yes | No | No | Yes | No | No | Mg, Al, Si, P, S, K, Ca, Fe. |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miriello, D.; Bloise, A.; Crisci, G.M.; De Luca, R.; De Nigris, B.; Martellone, A.; Osanna, M.; Pace, R.; Pecci, A.; Ruggieri, N. Non-Destructive Multi-Analytical Approach to Study the Pigments of Wall Painting Fragments Reused in Mortars from the Archaeological Site of Pompeii (Italy). Minerals 2018, 8, 134. https://doi.org/10.3390/min8040134
Miriello D, Bloise A, Crisci GM, De Luca R, De Nigris B, Martellone A, Osanna M, Pace R, Pecci A, Ruggieri N. Non-Destructive Multi-Analytical Approach to Study the Pigments of Wall Painting Fragments Reused in Mortars from the Archaeological Site of Pompeii (Italy). Minerals. 2018; 8(4):134. https://doi.org/10.3390/min8040134
Chicago/Turabian StyleMiriello, Domenico, Andrea Bloise, Gino M. Crisci, Raffaella De Luca, Bruno De Nigris, Alberta Martellone, Massimo Osanna, Rossella Pace, Alessandra Pecci, and Nicola Ruggieri. 2018. "Non-Destructive Multi-Analytical Approach to Study the Pigments of Wall Painting Fragments Reused in Mortars from the Archaeological Site of Pompeii (Italy)" Minerals 8, no. 4: 134. https://doi.org/10.3390/min8040134
APA StyleMiriello, D., Bloise, A., Crisci, G. M., De Luca, R., De Nigris, B., Martellone, A., Osanna, M., Pace, R., Pecci, A., & Ruggieri, N. (2018). Non-Destructive Multi-Analytical Approach to Study the Pigments of Wall Painting Fragments Reused in Mortars from the Archaeological Site of Pompeii (Italy). Minerals, 8(4), 134. https://doi.org/10.3390/min8040134







