Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characterization of the Raw Material (BR)
2.2. Experimental Method
2.2.1. Reagents
2.2.2. Instrumentation
2.2.3. Analytical Procedure
2.3. Arithmetical Methods of Data Processing
3. Results (Design Aspects)
- Mixing time, especially for values <1 h
- Acid feed concentration and the dependent pH value of the leachate
- L/S ratio, at the leaching reactor feed
- Number of cycles of the same leachate re-using onto fresh BR batches
3.1. Kinetics (Mixing Time Impact)
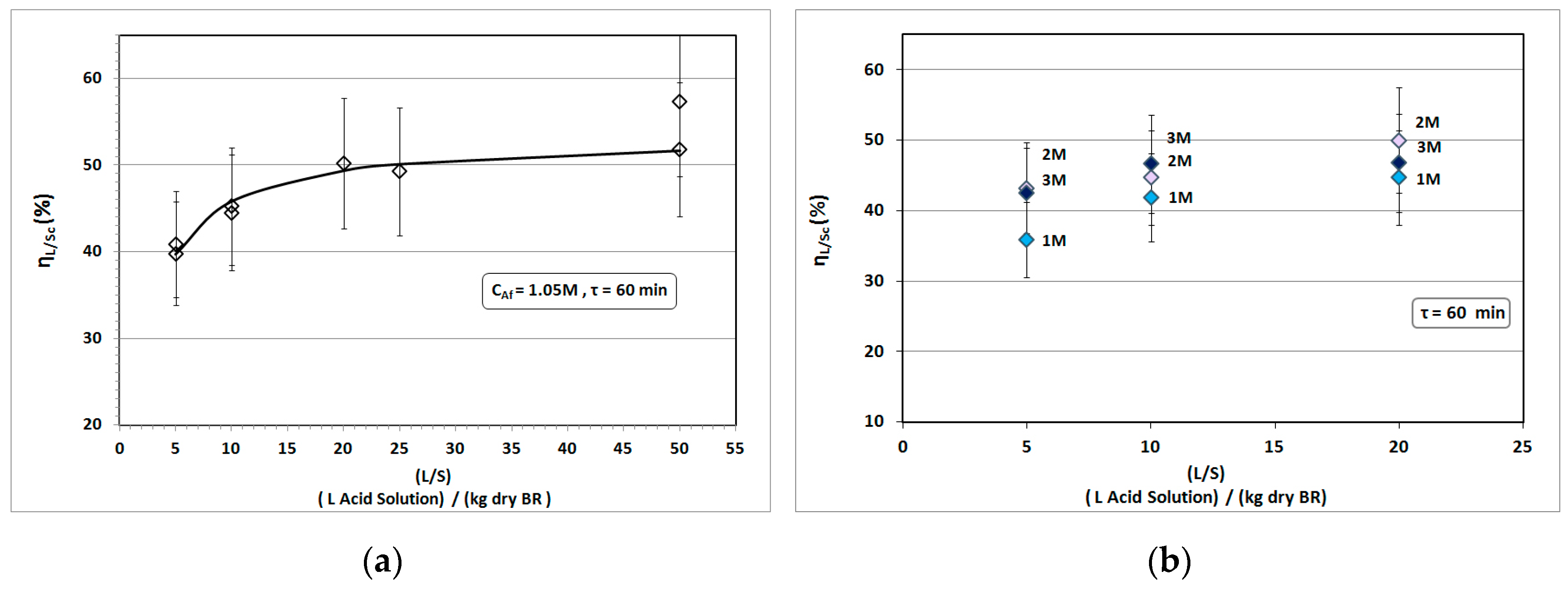
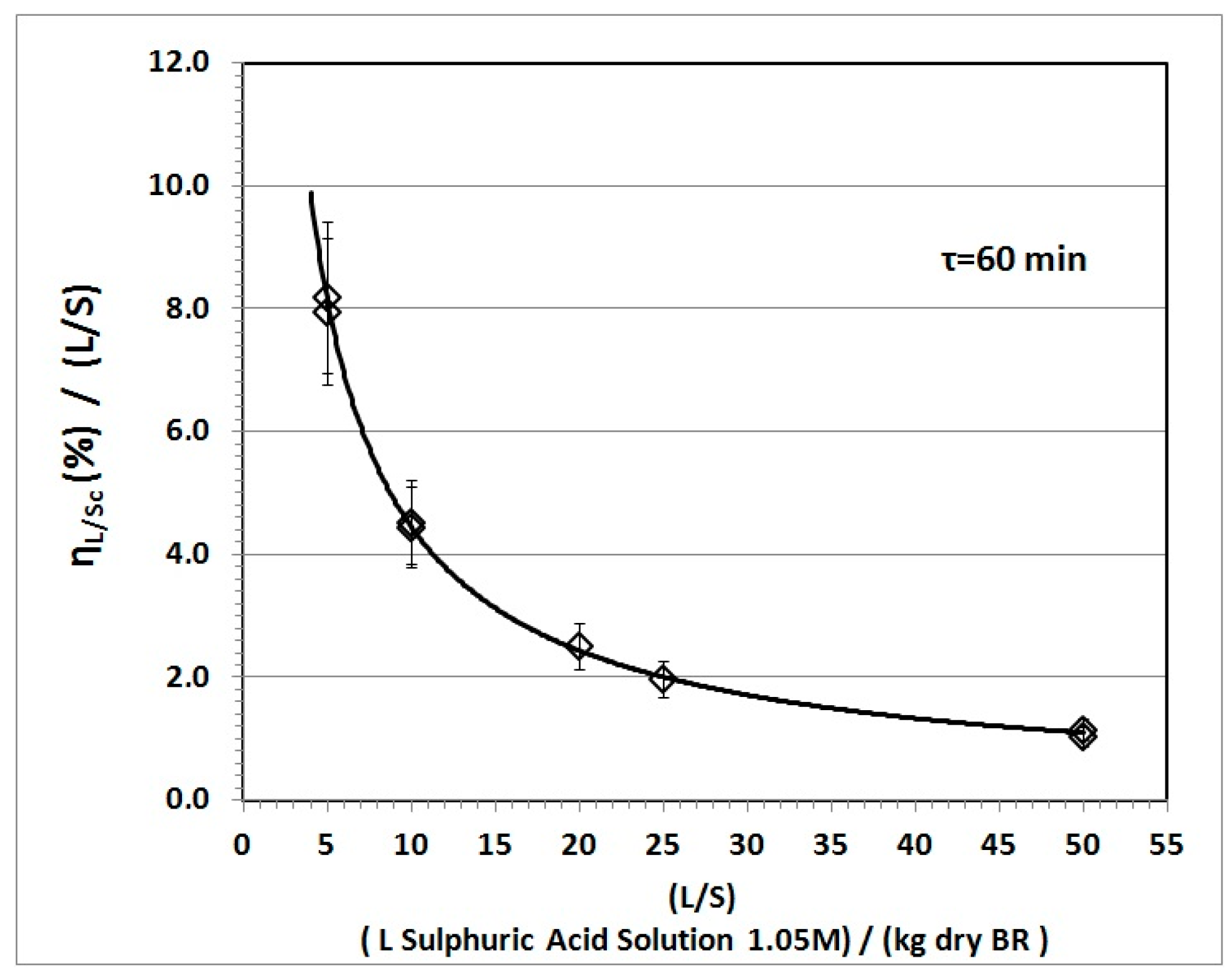
3.2. Solvent Excess (L/S) Impact
- (i)
- The diffusion coefficient k′ (in KL(=k′/b)) is taken as approximately constant [39], by only varying L/S.
- (ii)
- The ratio A/b remains constant with processing time by simultaneous proportional variation of both A and b (see Supplementary Materials, Text S2), or otherwise the product of the overall mass transfer coefficient with the specific surface area KLS = (k′/b)(A/Md) = (k′/Md)(A/b) remains constant.
- (iii)
- The mixing residence time is constant (τ = 60 min) for the entire set of Solvent Excess experiments.
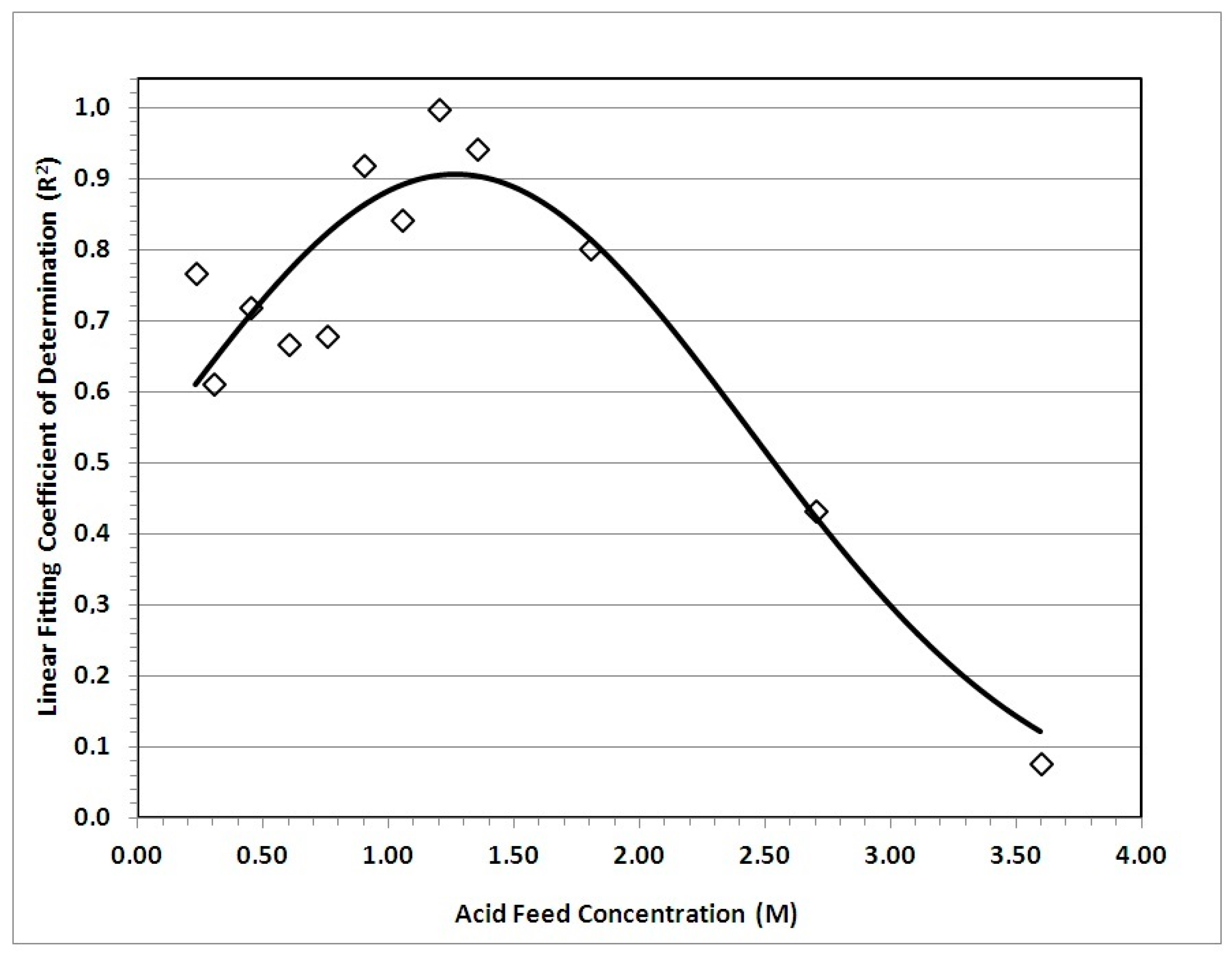
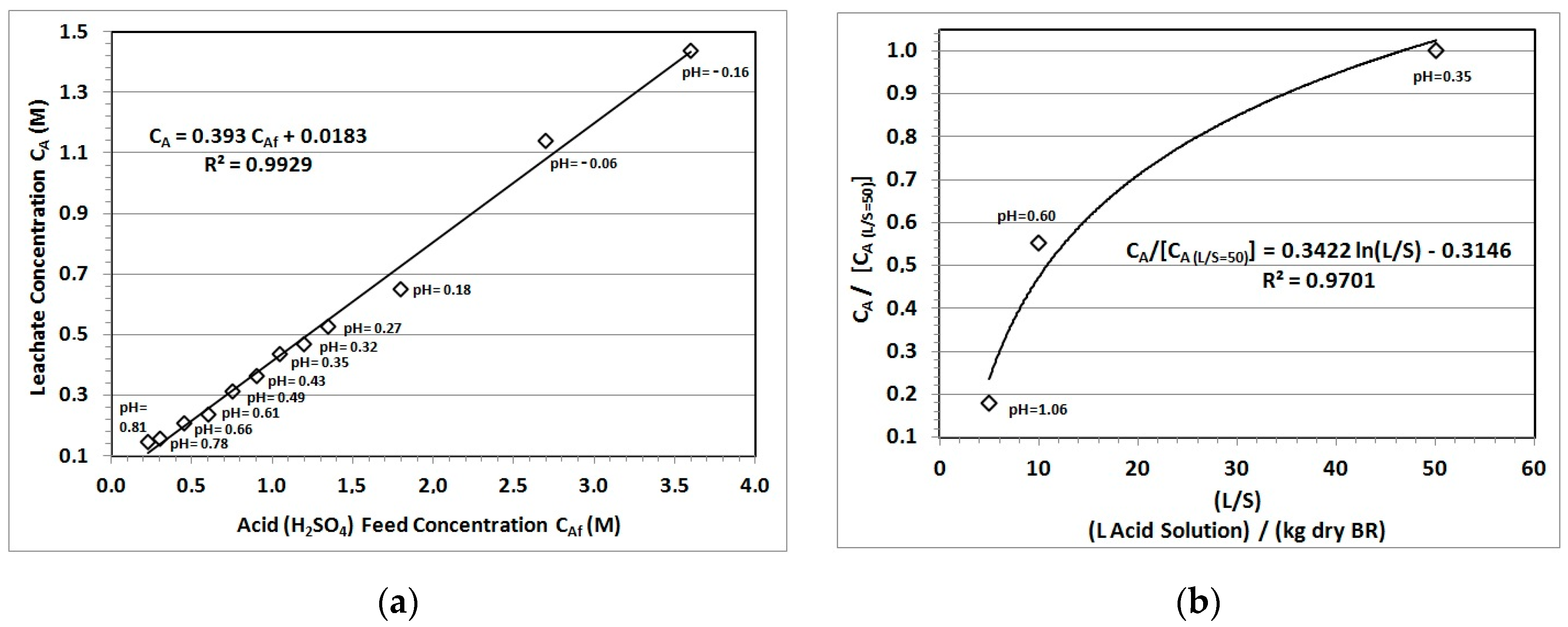
3.3. Chemical Reaction Impact (pH of Leachate)
3.4. Leachate Recycling Impact
3.4.1. Specific Recovery and Process Economics
- Market competitive operation starts when capacity exceeds a lower boundary (t BR/day)
- Economic viability is particularly sensitive to the cost of the concentrated acid
- Unit cost depends substantially on the Sc concentration in the PLS, or the specific recovery
- Sc content in the BR and water consumption have impact on process economics.
- Market competitive operation starts at a minimum plant capacity (t BR/day)
- The economic viability is particularly sensitive to the cost of the consuming concentrated acid
- The unit cost depends substantially on the Sc concentration in the PLS, or the specific recovery
- The Sc content in the BR and the water consumption have, also, an impact on the process economics.
3.4.2. A Unified Mathematical Expression for the Sc Leaching Yield
- : It corrects the minor deviation from the assumption that regression parameters (cs*,q) are constant for the entire set of the investigated leaching conditions. The use of a particular feed acid concentration (C0) to the uniform values of cs* and q, introduces concentration-dependent deviation from the uniformity. The negative optimized value for the exponent p means higher deviations for lower feed acid concentrations, as a result of the long-lasting kinetic effects at this concentration zone (Figure 1 and Section 3.2).
- Δη: It allows correction of the systematic error in prediction of Sc yield. This kind of deviation is explained by possible incompatibilities between the different sets of experimental measurements exploited in Figure 6 data.
4. Discussion
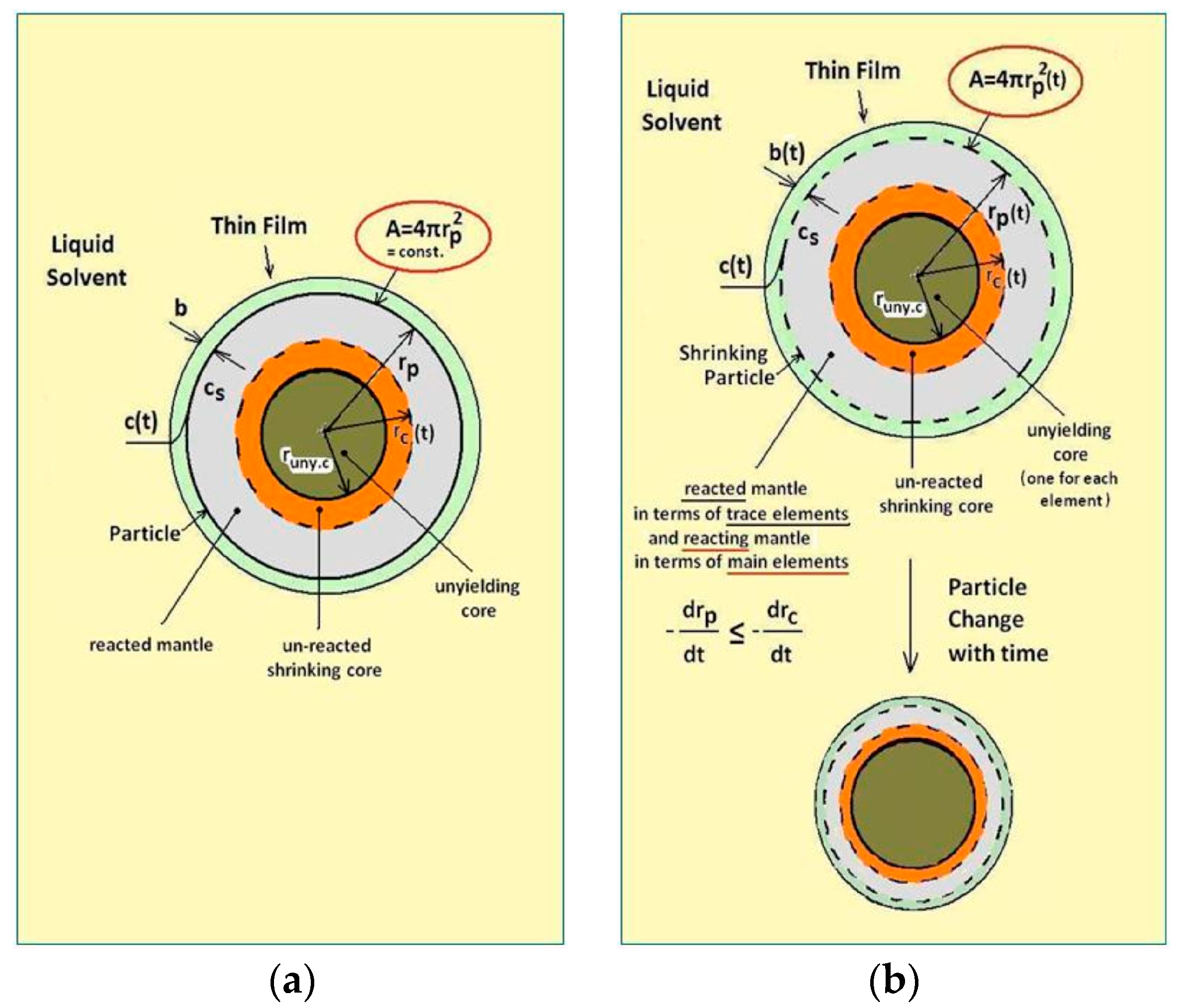
4.1. Evaluation of the Data Interpretation with the Thin Film Theory
4.2. Model Extensions
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| (a, b, μ) | regression parameters set for the general Gaussian function G(CAf) of Figure 2, (dimensionless R2, M−2, M) |
| A | total area of the solids-liquid interface in the leaching mixture, (dm2) |
| b | effective thickness of the liquid film surrounding the particles, (dm) |
| c | concentration (w/v) of the solute (here, Sc) in the bulk of the solution at time t, (mg Sc/L of acidic leachate) |
| C0 | constant feed acid concentration in the corrective factor of Equation (5), (M) |
| CA | nominal acid concentration in the leachate that is calculated from measured pH, (M) |
| average acid concentration in the leachate after n times of leachate (or, PLS) re-usage for leaching of fresh BR batches, or , (M) | |
| CAf | acid feed concentration, as a variable, (M) |
| CAf* | acid feed concentration, as a discrete value (M) |
| cs | concentration (w/v) of the solute (here, Sc) in the saturated solution in contact with the particles, during leaching kinetics effects, (mg Sc/L acidic leachate);it has the meaning of Sc concentration in a solution of the leaching solvent, where the being dissolved BR matter is at solubility levels |
| time average concentration (w/v) of the solute (here, Sc) in the saturated solution in contact with the particles, during leaching kinetics effects, (mg Sc/L acidic leachate) | |
| cs* | regression variable of Equation (3), which is corresponding to the cs parameter of Equation (2) when mixing time exceeds the period of leaching kinetic effects, (mg Sc/L acidic leachate) |
| CSc/BR | Sc concentration (w/w) in dry BR solids, (mg Sc/kg dry BR) |
| CSc/PLS | Sc concentration (w/v) in the PLS, (mg Sc/L PLS) |
| k′ | diffusion coefficient in the leaching mixture (it is approximately equal to the product of the liquid-phase diffusivity DL and the ratio of the total molar concentration of solvent and solute to the logarithmic mean value of the solute concentration at each side of the thin film), (dm2/min) |
| KL(=k′/b) | overall mass transfer coefficient for diffusion of Sc ions from BR particles to the liquid phase, (dm/min) |
| L/S | Liquid to dry Solids ratio (v/w) for the leaching process, either at the leaching reactor feed, or inside the reactant leaching mixture, since the volume of the liquid part of the reactant leaching mixture has been experimentally found to be approximately equal to the volume of the fed acid, L/S = V/Md, (L acid/kg dry BR) |
| (L/S)* | a modified L/S for cases of solvent recycling, which is equal to (L/S) / (times of acidic solvent re-usage), or (L/S)* = (L/S)/n, (L acid/ kg dry BR) |
| M | mass of the solute (here, Sc) transferred in time t to the liquid solvent, (mg Sc) |
| Md | total mass of dry BR particles in the leaching mixture, (kg) |
| n | times of leachate (or, PLS) re-usage for leaching of fresh BR batches, (dimensionless) |
| N | number of experimental data in a series |
| p | exponent in the corrective factor of Equation (5), (dimensionless) |
| q | regression variable of Equation (3), that is corresponding to the term e−KLSτ of Equation (2), and where −lnq = KLSτ has units (L/kg) |
| R2 | the linear fitting coefficient of determination, R2 = 1 − (linear SSres)/SStot, (dimensionless) |
| R2 | the general fitting coefficient of determination, R2 = 1−SSres/SStot, (dimensionless) |
| s | coefficient that is dependent on SStot of each kinetic experiment of Figure 2 (=(SStot)0.5/N), (mg Sc in the leachate/mg Sc in the dry BR feed) |
| S | specific surface area of dry BR particles participating in the leaching process, which is S(=A/Md), if we define in the leaching process as mass exchange area the total surface area of a particle, external and internal (i.e., including pores surface area), (dm2/kg) |
| SSres | the statistical residual sum of squares, for predicted values by a given correlation, (mg Sc in the leachate/mg Sc in the dry BR feed)2 |
| SStot | the total sum of squares, for the experimental data set, (mg Sc in the leachate/mg Sc in the dry BR feed)2 |
| t | mixing residence time of the leaching process, as a variable, (min) |
| V | the volume of acid fed in the leaching reactor, or approximately the liquid volume of the leaching mixture, (L) |
| Greek Letters | |
| Δη | corrective addend of Equation (5), (mg Sc in the leachate/mg Sc in the dry BR feed) |
| root mean square of deviations between predicted and experimental values of a data series | |
| ηL/Sc | leaching fractional yield for Sc; cumulative yield for multi-stage leaching, (mg Sc in the leachate/ mg Sc in the dry BR feed) |
| ηL/Sc–max. | the maximum of ηL/Sc in Equation (1) for certain value of acid feed concentration (CAf); it represents the experimental yield value for infinite mixing period, and it is assumed here to be 1% more than the experimental value for 60 min mixing time, for logarithmization purposes, (mg Sc in the leachate/mg Sc in the dry BR feed) |
| ηmax. | experimental leaching yield for Sc from Solvent Excess Series when CAf = 1.05 M, τ = 60 min, and L/S = 50, which is multiplied by 1.01, and used in Equations (4) and (5), (mg Sc in the leachate/mg Sc in the dry BR feed) |
| τ | mixing residence time of the leaching process, as a discrete value, (min) |
References
- Mineral Prices, Rare Earth Metals. Available online: http://mineralprices.com/default.aspx#rar (accessed on 23 January 2018).
- Duyvesteyn, W.P.C.; Putnam, G.F. EMC Metals Corporation, White Paper. SCANDIUM: A Review of the Element, Its Characteristics, and Current and Emerging Commercial Applications. May 2014. Available online: http://www.scandiummining.com/i/pdf/Scandium-White-PaperEMC-Website-June-2014-.pdf (accessed on 23 January 2018).
- Wang, Q.; Lu, Y.; Mishin, S.; Oshmyansky, Y.; Horsley, D.A. Design, fabrication, and characterization of scandium aluminum nitride-based piezoelectric micromachined ultrasonic transducers. J. Microelectromech. Syst. 2017, 26, 1132–1139. [Google Scholar] [CrossRef]
- UC RUSAL Sustainability Report 2016. Scientific and Technological Development. Available online: http://sr.rusal.com/company-management-system/scientific-and-technical-development.php (accessed on 23 January 2018).
- Project SCALE: Production of Scandium Compounds and Scandium Aluminum Alloys from European Metallurgical by-Products. European Community’s Horizon 2020 Programme. Available online: http://cordis.europa.eu/project/rcn/206331_en.html (accessed on 23 January 2018).
- Slipenyuk, A.; Lotsko, D.; Milman, Y.; Kuprin, V.; Yefimov, M.; Danylenko, M. Influence of Scandium on Amorphization of Aluminum Alloys. In Metallic Materials with High Structural Efficiency. NATO Science Series II: Mathematics, Physics and Chemistry; Senkov, O.N., Miracle, D.B., Firstov, S.A., Eds.; Springer: Dordrecht, The Netherlands, 2004; Volume 146. [Google Scholar] [CrossRef]
- Røyset, J.; Ryum, N. Scandium in aluminium alloys. Int. Mater. Rev. 2005, 50, 19–44. [Google Scholar] [CrossRef]
- Riva, S.; Yusenko, K.V.; Lavery, N.P.; Jarvis, D.J.; Brown, S.G.R. The scandium effect in multicomponent alloys. Int. Mater. Rev. 2016, 61, 203–228. [Google Scholar] [CrossRef]
- Awd, M.; Tenkamp, J.; Hirtler, M.; Siddique, S.H.; Bambach, M.; Walther, F. Comparison of microstructure and mechanical properties of scalmalloy® produced by selective laser melting and laser metal deposition. Materials 2018, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Pranolo, Y.; Cheng, C.Y. Metallurgical processes for scandium recovery from various resources: A review. Hydrometallurgy 2011, 108, 100–108. [Google Scholar] [CrossRef]
- European Commission: Critical Raw Materials. Third List of Critical Raw Materials for the EU of 2017. Available online: https://ec.europa.eu/growth/sectors/raw-materials/specific-interest/critical_en (accessed on 26 January 2018).
- U.S. Geological Survey. Mineral Commodity Summaries, SCANDIUM. January 2016. Available online: https://minerals.usgs.gov/minerals/pubs/commodity/scandium/mcs-2016-scand.pdf (accessed on 23 January 2018).
- Tomilo, Z.M.; Molchan, P.V.; Shestak, A.S.; Finskaya, V.M.; Prytkova, N.A.; Ustinovich, S.N. Influence of annealing on the superconductivity of ScNi2B2C. Phys. C Supercond. 2001, 361, 95–98. [Google Scholar] [CrossRef]
- Geissler, U.; Thomas, S.; Schneider-Ramelow, M.; Mukhopadhyay, B.; Lang, K.-D. Aluminum-scandium: A material for semiconductor packaging. J. Electr. Mater. 2016, 45, 5456–5467. [Google Scholar] [CrossRef]
- Schmidtke, K.; Palma, F.; Hawkins, A.; Emmelmann, C. Process and mechanical properties: Applicability of a scandium modified al-alloy for laser additive manufacturing. Phys. Procedia 2011, 12, 369–374. [Google Scholar] [CrossRef]
- Schoop, U.; Goharkhay, K.; Klimscha, J.; Zagler, M.; Wernisch, J.; Georgopoulos, A.; Sperr, W.; Moritz, A. The use of the erbium, chromium: Yttrium-scandium-gallium-garnet laser in endodontic treatment: The results of an in vitro study. J. Am. Dent Assoc. 2007, 138, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Scandium Investing. Why Scandium Could Be a Huge Opportunity: Commercially Viable Scandium Deposits Are Rare, but There Is Indeed Opportunity in this Market. Investing News Network. 12 April 2017. Available online: http://investingnews.com/daily/resource-investing/critical-metals-investing/scandium-investing/scandium-production-the-problem-and-the-opportunity/ (accessed on 23 January 2018).
- Zhang, B.; Xue, X.; Huang, X.; Yang, H.; Han, J. Study on Leaching Valuable Elements from Bayan Obo Tailings. In Proceedings of the 3rd Pan American Materials Congress; The Minerals, Metals & Materials Series; Meyers, M.A., Benavides, H.A.C., Brühl, S.P., Colorado, H.A., Dalgaard, E., Elias, C.N., Figueiredo, R.B., Garcia-Rincon, O., Kawasaki, M., Langdon, T.G., et al., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Kynický, J.; Cihlářová, H.; Smith, M.; Xu, C.; Chakhmouradian, A.; Reguir, K.; Kempe, U.; Galiová, V. Giant Bayan Obo deposit in China, Part I: The main types of ore REE-Fe-Nb-Sc. Geosci. Res. Rep. 2012, 45, 226–231. (accessed on 25 January 2018). [Google Scholar]
- Kalashnikov, A.; Yakovenchuk, V.N.; Pakhomovsky, Y.A.; Bazai, A.V.; Sokharev, V.A.; Konopleva, N.; Mikhailova, J.A.; Goryainov, P.M.; Ivanyuk, G. Scandium of the Kovdor magnetite-apatite-baddeleyite deposit (Murmansk Region, Russia): Mineralogy, spatial distribution, and potential resource. Ore Geol. Rev. 2016, 72, 532–537. [Google Scholar] [CrossRef]
- Scandium International Mining Corp. Nyngan Scandium Project. Available online: http://www.scandiummining.com/s/nyngan.asp (accessed on 25 January 2018).
- Australian Mines Limited. Sconi Project (Cobalt-Nickel-Scandium). Available online: https://australianmines.com.au/sconi (accessed on 25 January 2018).
- Suss, A.; Kuznetsova, N.V.; Kozyrev, A.; Panov, A.; Gorbachev, S. Specific Features of Scandium Behavior during Sodium Bicarbonate Digestion of Red Mud. Travaux 46. In Proceedings of the 35th International ICSOBA Conference, Hamburg, Germany, 2–5 October 2017; (accessed on 25 January 2018). [Google Scholar]
- REE Mineralisation in circum-Mediterranean Bauxites. Project EURARE. Available online: http://www.eurare.eu/countries/mediterraneanBauxites.html (accessed on 23 January 2018).
- Ochsenkühn-Petropoulou, M.; Lyberopulu, T.; Parissakis, G. Direct determination of lanthanides yttrium and scandium in bauxites and red mud from alumina production. Anal. Chim. Acta 1994, 296, 305–313. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, Μ.; Lyberopulu, T.; Parissakis, G. Selective separation and determination of scandium from yttrium and lanthanides in red mud by a combined ion exchange/solvent extraction method. Anal. Chim. Acta 1995, 315, 231–237. [Google Scholar] [CrossRef]
- Ochsenkühn-Petropulu, M.; Lyberopulu, T.; Ochsenkühn, K.M.; Parissakis, G. Recovery of lanthanides and yttrium from red mud by selective leaching. Anal. Chim. Acta 1996, 319, 249–254. [Google Scholar] [CrossRef]
- Lyberopulu, T. Determination and Recovery of Rare Earths from Bauxites and Red Mud. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 1996. Available online: https://www.didaktorika.gr/eadd/handle/10442/8817 (accessed on 23 January 2018). [CrossRef]
- Tsakanika, L.-A. Separation and Recovery of Lanthanides from Red Mud by Use of Selective Extraction and Chromatographic Techniques. Ph.D. Thesis, National Technical University of Athens, Athens, Greece, 2013. [Google Scholar]
- Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Ochsenkühn-Petropoulou, M. Optimization of Mineral Acids Leaching Process for the Recovery of Rare Earth Elements from Greek Red Mud. In Proceedings of the Book of Abstracts of ERES 2017 Conference (European Rare Earth Resources 2017), Santorini, Greece, 28–31 May 2017; pp. 182–184. (accessed on 23 January 2018). [Google Scholar]
- Ochsenkühn-Petropoulou, M.T.; Hatzilyberis, K.S.; Mendrinos, L.N.; Salmas, C.E. Pilot plant investigation of the leaching process for the recovery of scandium from red mud. Ind. Eng. Chem. Res. 2002, 41, 5794–5801. [Google Scholar] [CrossRef]
- Aluminium of Greece. Member of Mytilineos Group. Available online: http://www.alhellas.com/ (accessed on 23 January 2018).
- Liu, Z.; Li, H. Metallurgical process for valuable elements recovery from red mud—A review. Hydrometallurgy 2015, 155, 29–43. [Google Scholar] [CrossRef]
- Borra, C.R.; Pontikes, Y.; Binnemans, K.; Van Gerven, T. Leaching of rare earths from bauxite residue (red mud). Miner. Eng. 2015, 76, 20–27. [Google Scholar] [CrossRef]
- Makanyire, T.; Sanchez-Segado, S.; Jha, A. Separation and recovery of critical metal ions using ionic liquids. Adv. Manuf. 2016, 4, 33–46. [Google Scholar] [CrossRef]
- Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkuehn, K.; Georgiou, P.; Ochsenkuehn-Petropoulou, M. Design Aspects of a Selective Leaching Process for Scandium Recovery from Bauxite Residue. In Proceedings of the Book of Abstracts of ERES 2017 Conference (European Rare Earth Resources 2017), Santorini, Greece, 28–31 May 2017; pp. 188–189. [Google Scholar]
- Vaggelatos, I.V. Utilization of Red Mud in the Cement Industry. Ph.D. Thesis, University of Patras, Patras, Greece, 2008. [Google Scholar] [CrossRef]
- Gamaletsos, P.N.; Godelitsas, A.; Kasama, T.; Kuzmin, A.; Lagos, M.; Mertzimekis, T.J.; Göttlicher, J.; Steininger, R.; Xanthos, S.; Pontikes, Y.; et al. The role of nano-perovskite in the negligible thorium release in seawater from Greek bauxite residue (red mud). Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coulson, J.M.; Richardson, J.F. Chemical Engineering, 2nd ed.; Pergamon Press: Oxford, UK, 1968; Volume 2, p. §10.2. ISBN 10 0080131859. [Google Scholar]
- Vossenkaul, D.; Stopic, S.; Friedrich, B. Gel Avoidance in Leaching of High Silica Containing Ores—A Case Study for Eudialyte Concentrates. In Proceedings of the Book of Abstracts of ERES 2017 Conference (European Rare Earth Resources 2017), Santorini, Greece, 28–31 May 2017; pp. 28–31. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; John Wiley & Sons, Inc.: New York, NY, USA, 1999; p. 390. ISBN 0-471-25424-X. [Google Scholar]


| For single use of acidic solvent |
|
|
| For multiple use of acidic solvent |
|
|
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hatzilyberis, K.; Lymperopoulou, T.; Tsakanika, L.-A.; Ochsenkühn, K.-M.; Georgiou, P.; Defteraios, N.; Tsopelas, F.; Ochsenkühn-Petropoulou, M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid. Minerals 2018, 8, 79. https://doi.org/10.3390/min8030079
Hatzilyberis K, Lymperopoulou T, Tsakanika L-A, Ochsenkühn K-M, Georgiou P, Defteraios N, Tsopelas F, Ochsenkühn-Petropoulou M. Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid. Minerals. 2018; 8(3):79. https://doi.org/10.3390/min8030079
Chicago/Turabian StyleHatzilyberis, Konstantinos, Theopisti Lymperopoulou, Lamprini-Areti Tsakanika, Klaus-Michael Ochsenkühn, Paraskevas Georgiou, Nikolaos Defteraios, Fotios Tsopelas, and Maria Ochsenkühn-Petropoulou. 2018. "Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid" Minerals 8, no. 3: 79. https://doi.org/10.3390/min8030079
APA StyleHatzilyberis, K., Lymperopoulou, T., Tsakanika, L.-A., Ochsenkühn, K.-M., Georgiou, P., Defteraios, N., Tsopelas, F., & Ochsenkühn-Petropoulou, M. (2018). Process Design Aspects for Scandium-Selective Leaching of Bauxite Residue with Sulfuric Acid. Minerals, 8(3), 79. https://doi.org/10.3390/min8030079







