Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Reagents
2.2. Flotation Tests
2.3. Adsorption Tests
2.4. FTIR Measurements
2.5. Molecular Dynamics (MD) Simulations
3. Results and Discussion
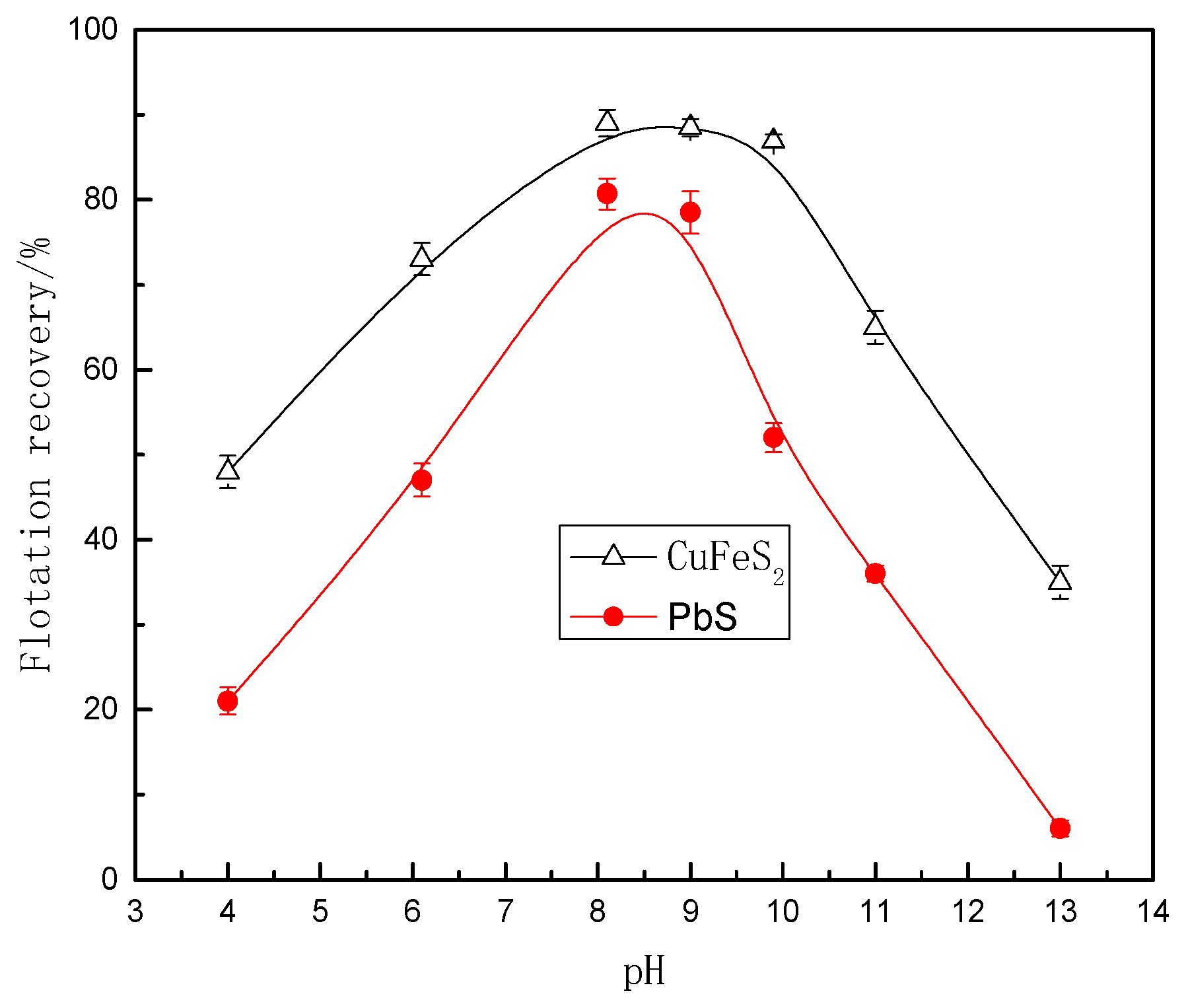
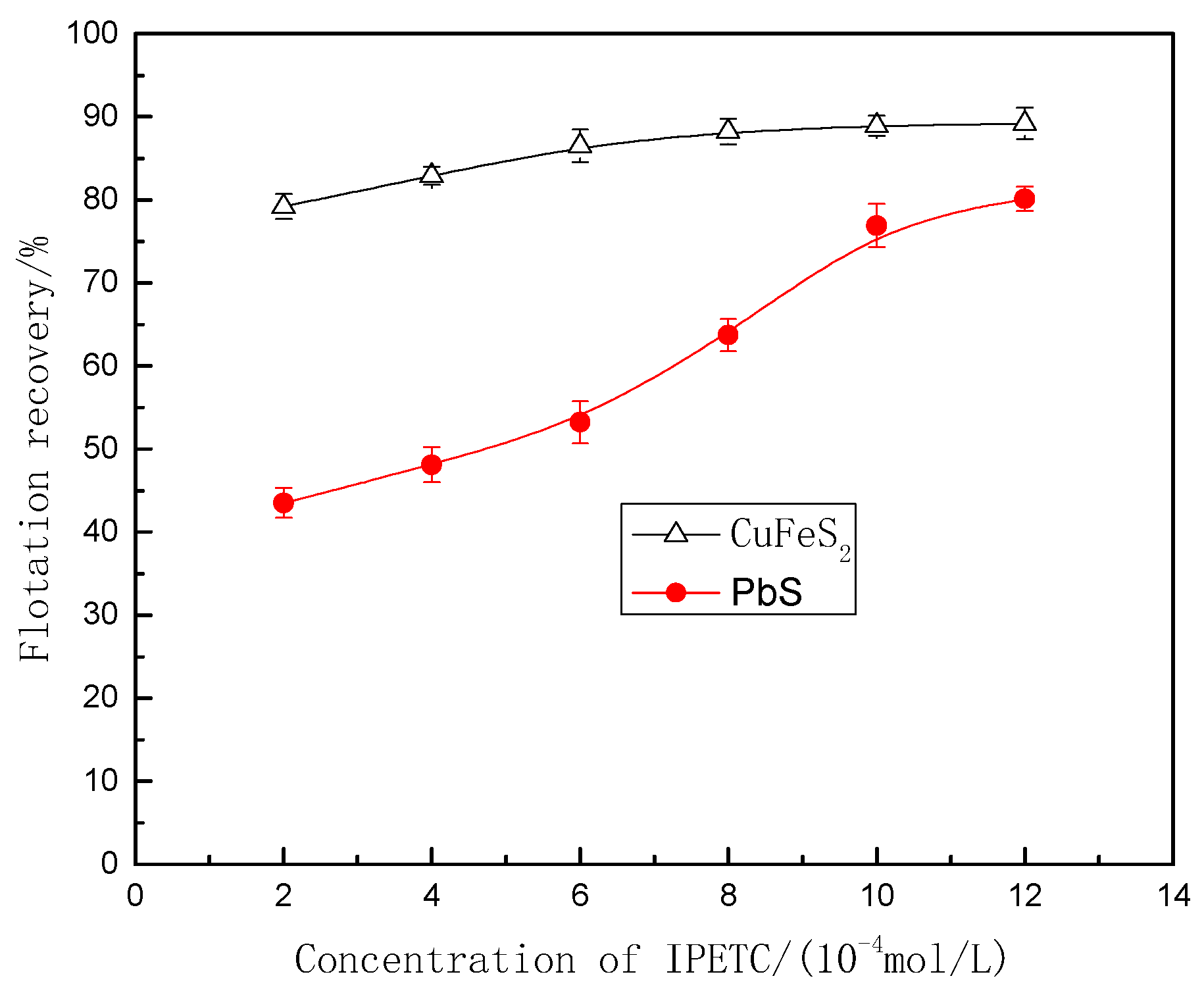
3.1. Flotation Test
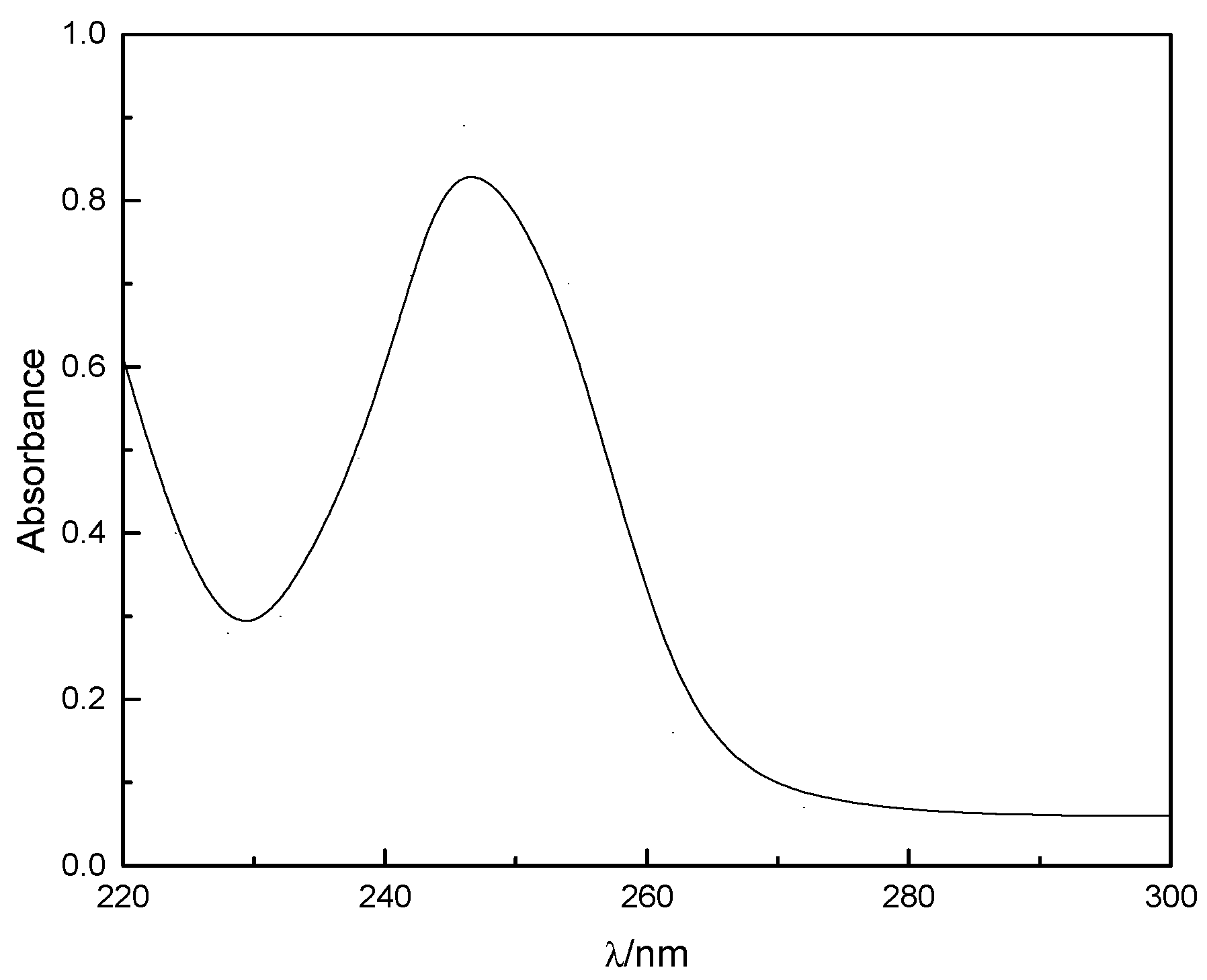
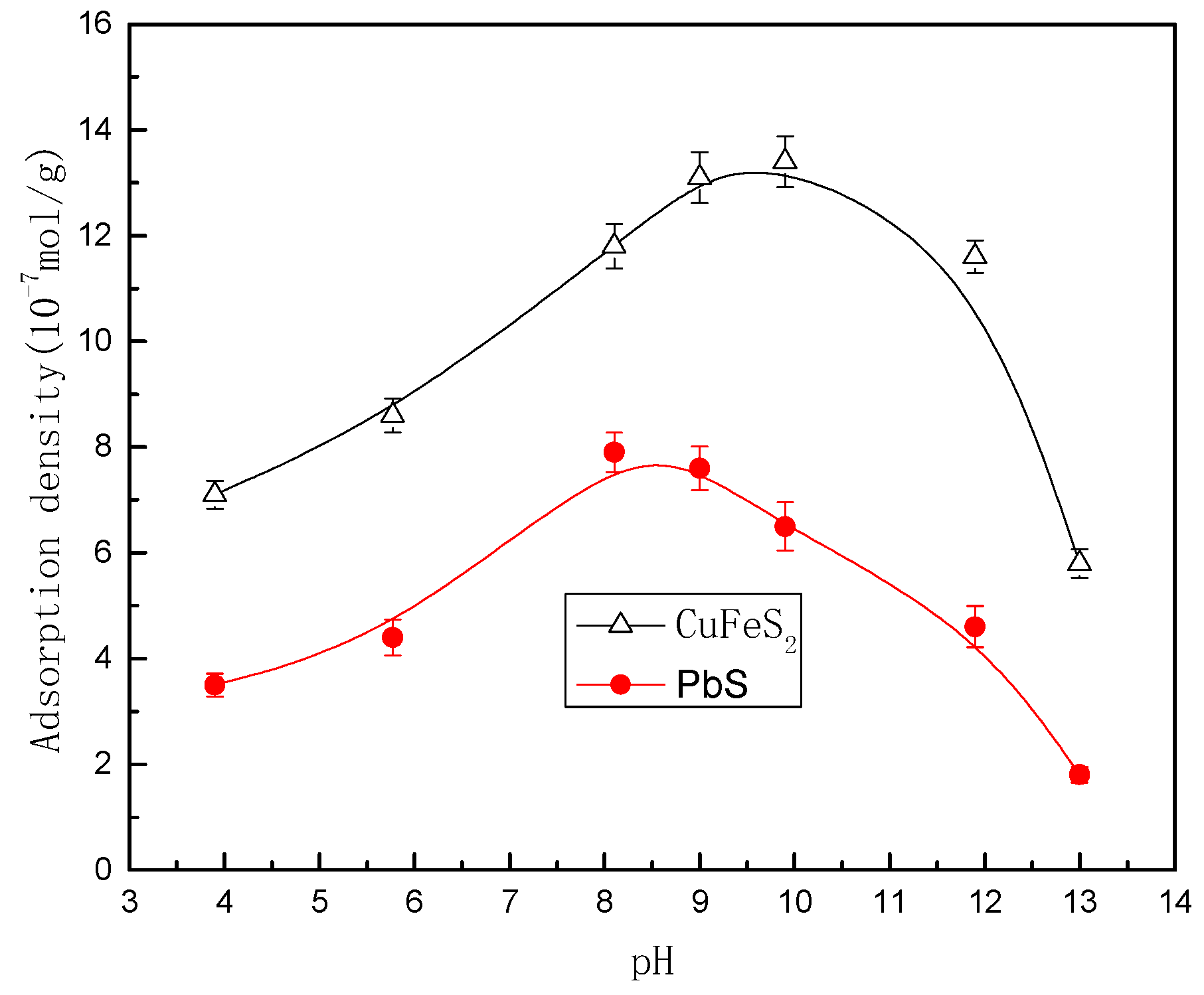
3.2. Adsorption Studies
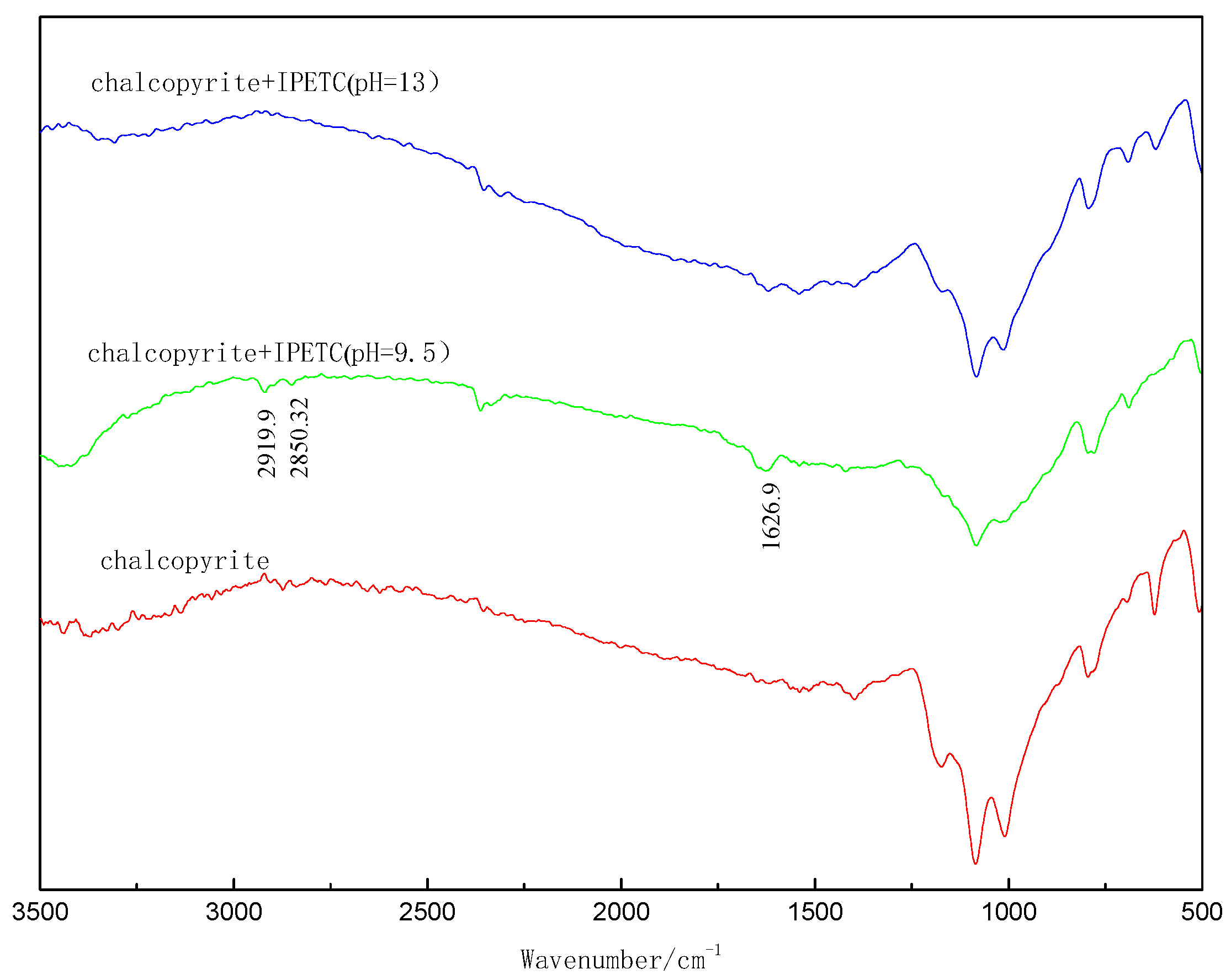
3.3. FTIR Study
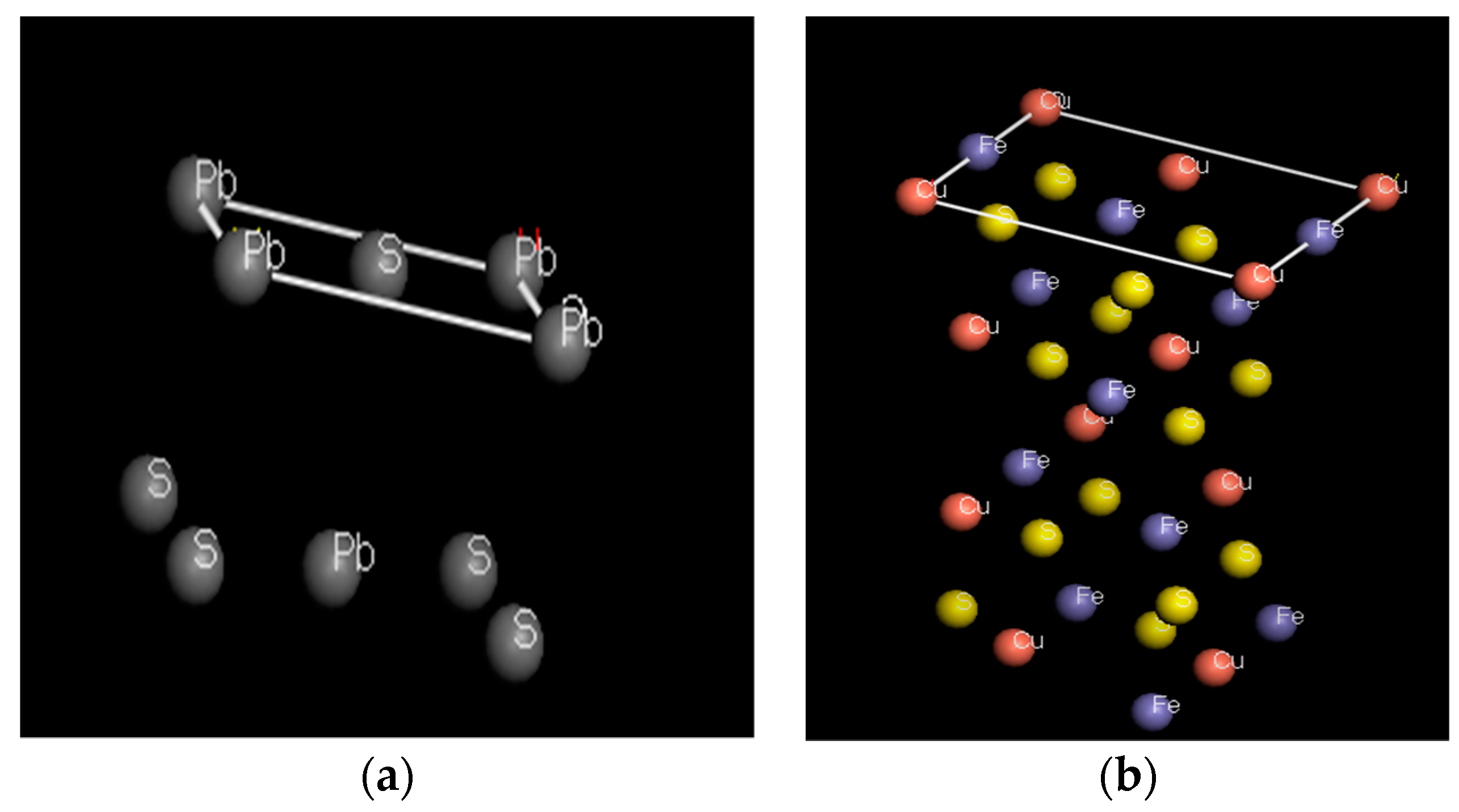
3.4. MD Simulations
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, C.; Song, N.; Zeng, G.M.; Jiang, M.; Zhang, J.C.; Hu, X.J.; Zhen, J.M. Bioaccumulation of zinc, lead, copper, and cadmium from contaminated sediments by native plant species and Acrida cinerea in South China. Environ. Monit. Assess. 2014, 186, 1735–1745. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Z.; Qin, W.Q.; Jiao, F.; Wang, X.J.; Bin, P.; Yang, Y.J.; Lai, C.H. Flotation separation of chalcopyrite from galena by sodium humate and ammonium persulfate. Trans. Nonferrous Met. Soc. China 2016, 26, 265–271. [Google Scholar] [CrossRef]
- Han, Y.X.; Zhu, Y.M.; Li, Y.J.; Liu, H.; Ma, Y.W. Flotation behaviors and mechanisms of chalcopyrite and galena after cyanide treatment. Trans. Nonferrous Met. Soc. China 2016, 26, 3245–3252. [Google Scholar]
- Chen, J.H.; Ye, C.; Li, Y.Q. Quantum-mechanical study of effect of lattice defects on surface properties and copper activation of sphalerite surface. Trans. Nonferrous Met. Soc. China 2010, 20, 1121–1130. [Google Scholar] [CrossRef]
- Liu, R.Q.; Sun, W.; Hu, Y.H.; Wang, D.Z. Effect of organic depressant lignosulfonate calcium on separation of chalcopyrite from pyrite. J. Cent. South Univ. Technol. 2009, 16, 753–757. [Google Scholar] [CrossRef]
- Yang, C.; Jiao, F.; Qin, W. Co-Bioleaching of Chalcopyrite and Silver-Bearing Bornite in a Mixed Moderately Thermophilic Culture. Minerals 2018, 8, 4. [Google Scholar] [CrossRef]
- Bruckard, W.; Sparrow, G.; Woodcock, J. A review of the effects of the grinding environment on the flotation of copper sulphides. Int. J. Miner. Process. 2011, 100, 1–13. [Google Scholar] [CrossRef]
- Wang, D.; Jiao, F.; Qin, W.; Wang, X. Effect of surface oxidation on the flotation separation of chalcopyrite and galena using sodium humate as depressant. Sep. Sci. Technol. 2018, 53, 961–972. [Google Scholar] [CrossRef]
- Reyes-Bozo, L.; Higueras, P.; Godoy-Faúndez, A.; Sobarzo, F.; Sáez-Navarrete, C.; Vásquez-Bestagno, J.; Herrera-Urbina, R. Assessment of the floatability of chalcopyrite, molybdenite and pyrite using biosolids and their main components as collectors for greening the froth flotation of copper sulphide ores. Miner. Eng. 2014, 64, 38–43. [Google Scholar] [CrossRef]
- Qin, W.Q.; Wei, Q.; Jiao, F.; Li, N.; Wang, P.P.; Ke, L.F. Effect of sodium pyrophosphate on the flotation separation of chalcopyrite from galena. Int. J. Min. Sci. Technol. 2012, 22, 345–349. [Google Scholar] [CrossRef]
- Buckley, A.N.; Hope, G.A.; Lee, K.C.; Petrovic, E.A.; Woods, R. Adsorption of O-isopropyl-N-ethyl thionocarbamate on Cu sulfide ore minerals. Miner. Eng. 2014, 69, 120–132. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhong, H.; Xia, L.Y.; Wang, S.; Dai, T.G. Effect of N-substituents on performance of thiourea collectors by density functional theory calculations. Trans. Nonferrous Met. Soc. China 2010, 20, 695–701. [Google Scholar] [CrossRef]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Solution properties of thionocarbamate collectors. Int. J. Miner. Process. 1996, 46, 137–153. [Google Scholar] [CrossRef]
- Liu, G.Y.; Zhong, H.; Dai, T.G.; Xia, L.Y. Investigation of the effect of N-substituents on performance of thionocarbamates as selective collectors for copper sulfides by ab initio calculations. Miner. Eng. 2008, 21, 1050–1054. [Google Scholar] [CrossRef]
- Fairthorne, G.; Fornasiero, D.; Ralston, J. Interaction of thionocarbamate and thiourea collectors with sulphide minerals: A flotation and adsorption study. Int. J. Miner. Process. 1997, 50, 227–242. [Google Scholar] [CrossRef]
- Wei, M. Fundamental Research on Flotation Separation of Chalcopyrite and Galena. Ph.D. Thesis, Northeastern University, Shenyang, China, 2008. [Google Scholar]
- Wang, F. Fundamental Research of Flotation Typical Sulphides /Carbonates/Oxides of Cu, Pb, Zn and Fe. Ph.D. Thesis, Northeastern University, Shenyang, China, 2008. [Google Scholar]
- Wei, R.; Peng, Y.; Seaman, D. The interaction of lignosulfonate dispersants and grinding media in copper–gold flotation from a high clay ore. Miner. Eng. 2013, 50, 93–98. [Google Scholar] [CrossRef]
- Zhao, G.; Peng, J.; Zhong, H.; Wang, S.; Liu, G.Y. Synthesis of Novel Ether Thionocarbamates and Study on Their Flotation Performance for Chalcopyrite. Minerals 2016, 6, 97. [Google Scholar] [CrossRef]
- Wang, D. Interaction between Minerals and Reagents, in Flotation Reagents: Applied Surface Chemistry on Minerals Flotation and Energy Resources Beneficiation; Volume 1: Functional Principle; Springer: Singapore, 2016; pp. 9–38. [Google Scholar]
- Mary, Y.S.; Raju, K.; Yildiz, I.; Temiz-Arpaci, O.; Nogueira, H.I.; Granadeiro, C.M.; Van Alsenoy, C. FT-IR, FT-Raman, SERS and computational study of 5-ethylsulphonyl-2-(o-chlorobenzyl) benzoxazole. Spectrochim. Acta Part A 2012, 96, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Suryanarayanan, C.; Prasannan, A.; Hong, P.D.; Sambathkumar, B.; Somanathan, N. Variable temperature studies on mesogenic polythiophenes using 2D-IR and WAXS. Mater. Chem. Phys. 2014, 143, 1352–1363. [Google Scholar] [CrossRef]
- Manea, M.M.; Moise, I.V.; Virgolici, M.; Negut, C.D.; Barbu, O.H.; Cutrubinis, M.; Ponta, C.C. Spectroscopic evaluation of painted layer structural changes induced by gamma radiation in experimental models. Radiat. Phys. Chem. 2012, 81, 160–167. [Google Scholar] [CrossRef]
- Dlapa, P.; Bodí, M.B.; Mataix-Solera, J.; Cerdà, A.; Doerr, S.H. FT-IR spectroscopy reveals that ash water repellency is highly dependent on ash chemical composition. Catena 2013, 108, 35–43. [Google Scholar] [CrossRef]
- Wang, L.; Sun, W.; Hu, Y.H.; Xu, L.H. Adsorption mechanism of mixed anionic/cationic collectors in Muscovite–Quartz flotation system. Miner. Eng. 2014, 64, 44–50. [Google Scholar] [CrossRef]
- Xu, H.; Zhou, M.; Fang, Y.; Teng, H.H. Effect of Mica and Hematite (001) Surfaces on the Precipitation of Calcite. Minerals 2018, 8, 17. [Google Scholar] [CrossRef]

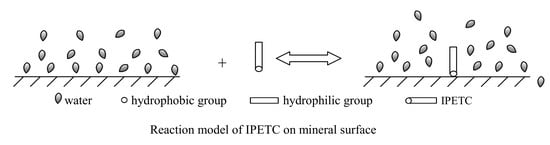
| Mineral | Reagent | ||
|---|---|---|---|
| IPETC | H2O | OH− | |
| Chalcopyrite | −29.7338 | −5.8816 | −4.0217 |
| Galena | −47.3861 | −11.3905 | −7.5328 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bu, Y.; Hu, Y.; Sun, W.; Gao, Z.; Liu, R. Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals 2018, 8, 115. https://doi.org/10.3390/min8030115
Bu Y, Hu Y, Sun W, Gao Z, Liu R. Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals. 2018; 8(3):115. https://doi.org/10.3390/min8030115
Chicago/Turabian StyleBu, Yongjie, Yuehua Hu, Wei Sun, Zhiyong Gao, and Runqing Liu. 2018. "Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector" Minerals 8, no. 3: 115. https://doi.org/10.3390/min8030115
APA StyleBu, Y., Hu, Y., Sun, W., Gao, Z., & Liu, R. (2018). Fundamental Flotation Behaviors of Chalcopyrite and Galena Using O-Isopropyl-N-Ethyl Thionocarbamate as a Collector. Minerals, 8(3), 115. https://doi.org/10.3390/min8030115