Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks
Abstract
1. Introduction
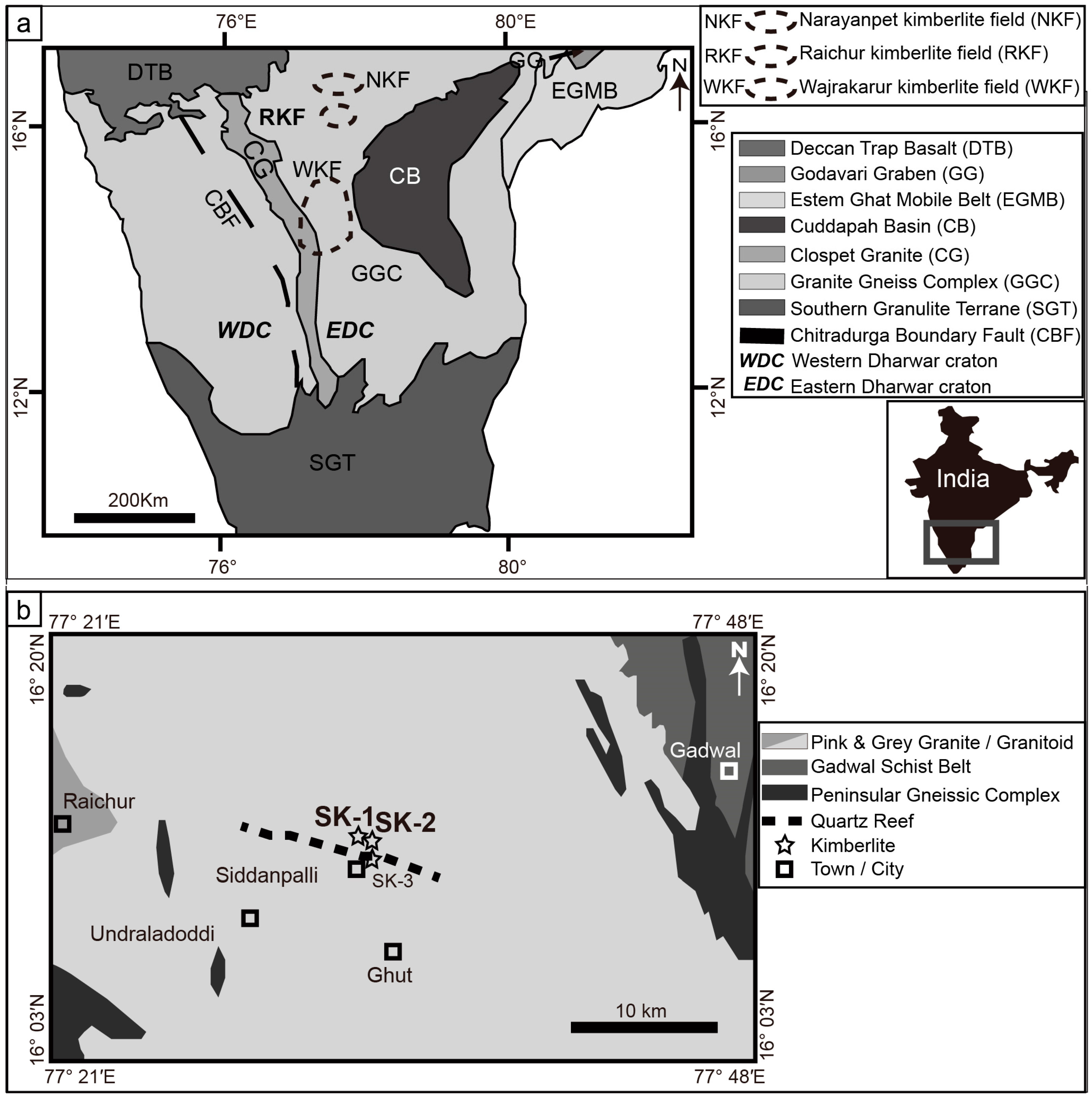
2. Geological Setting
3. Methods
4. Results
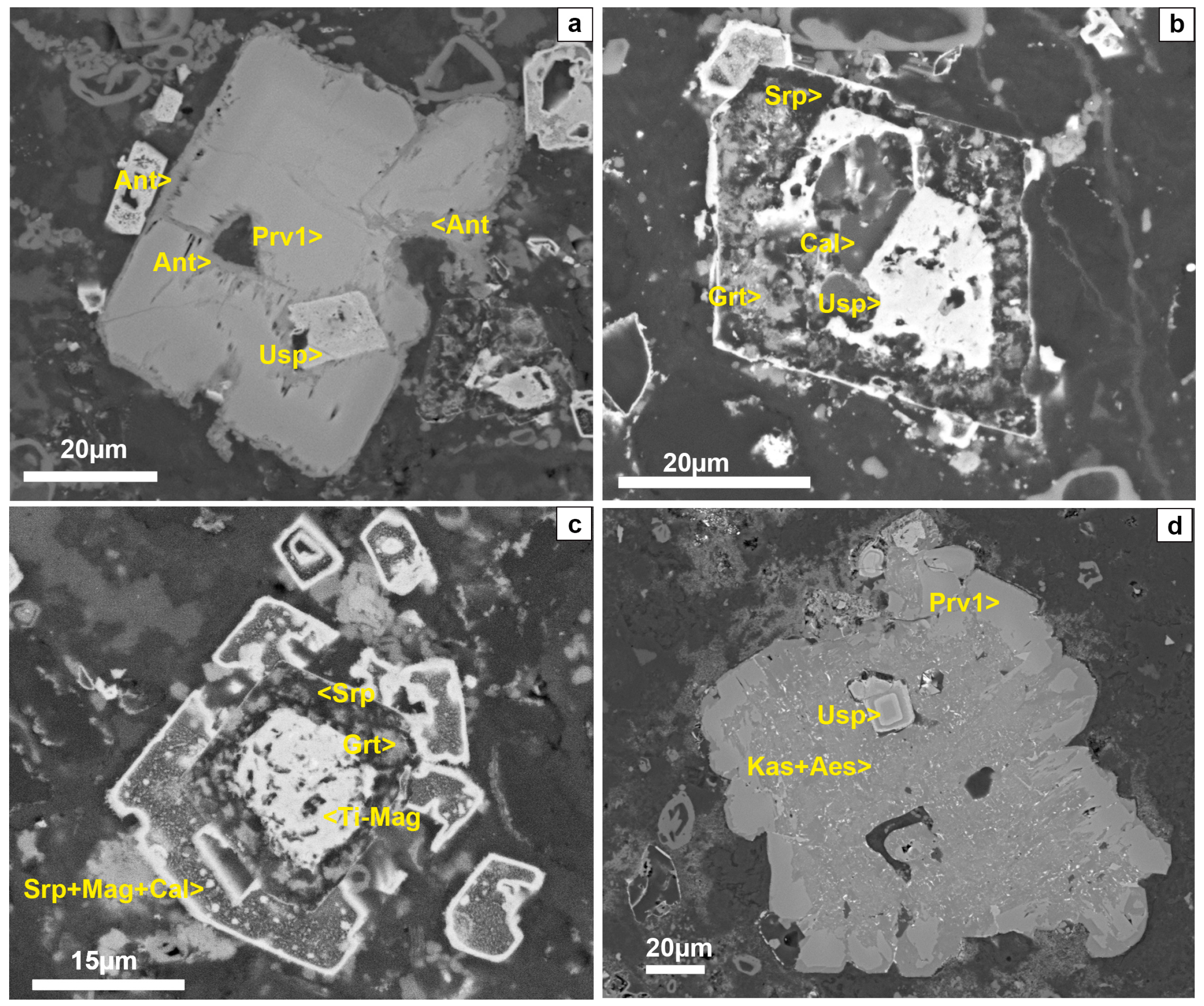
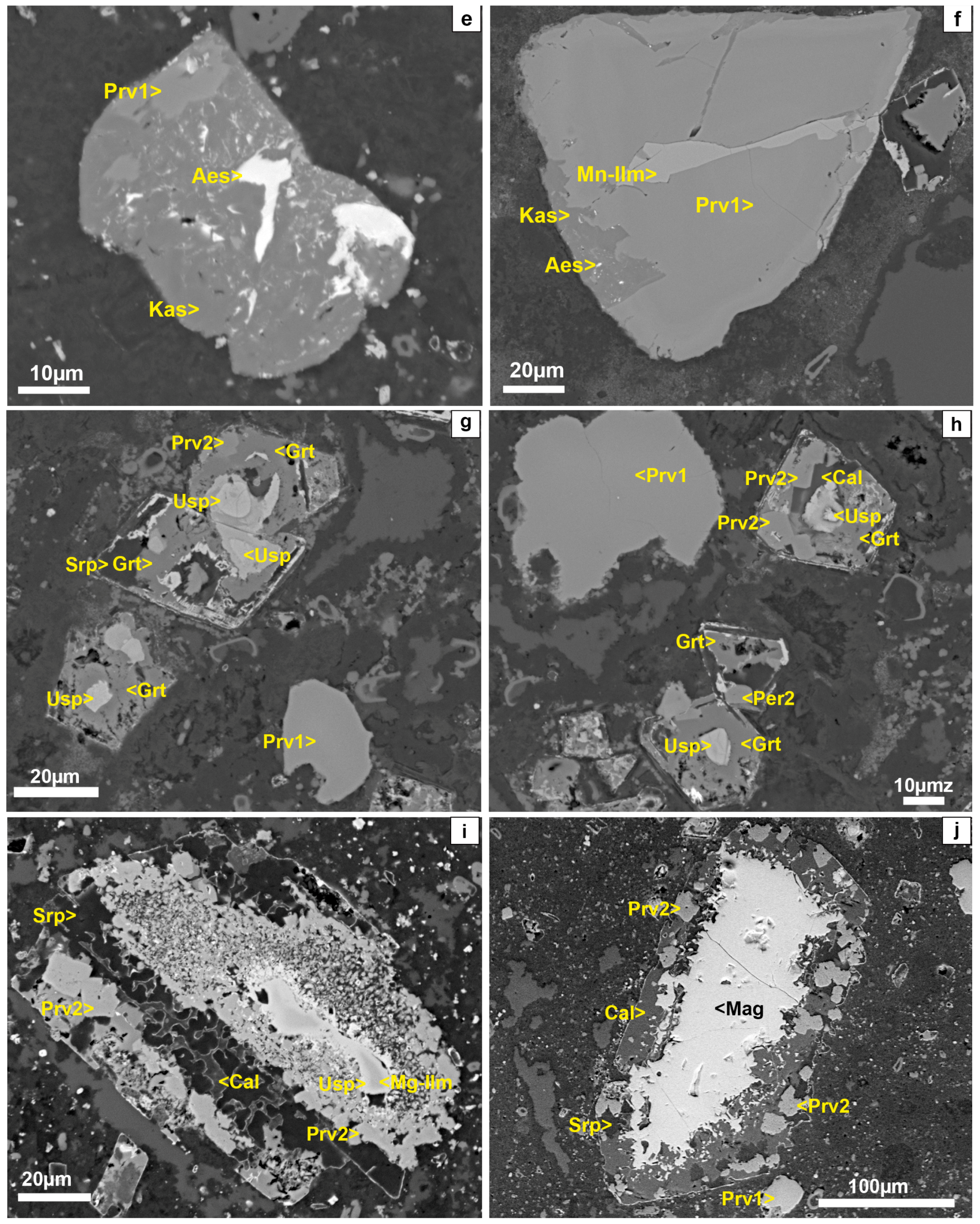
4.1. Mineral Textures
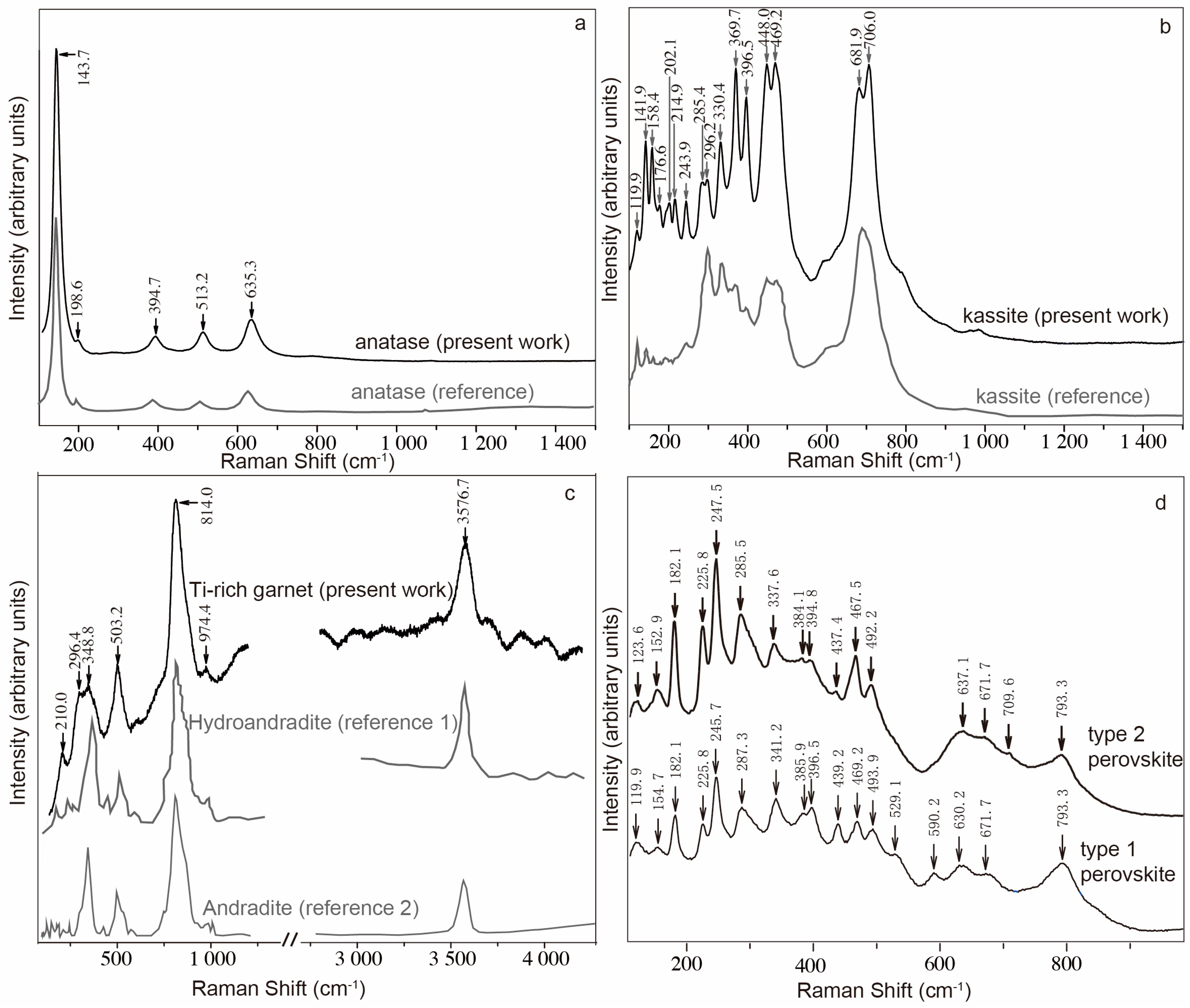
4.2. MicroRaman Study
4.3. Mineral Chemistry
5. Discussion
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rezvukhin, D.I.; Malkovets, V.G.; Sharygin, I.S.; Kuzmin, D.V.; Litasov, K.D.; Gibsher, A.A.; Pokhilenko, N.P.; Sobolev, N.V. Inclusions of Cr- and Cr-Nb-Rutile in pyropes from the Internatsionalnaya kimberlite pipe, Yakutia. Dokl. Earth Sci. 2016, 466, 173–176. [Google Scholar] [CrossRef]
- Ragozin, A.L.; Zedgenizov, D.A.; Shatskii, V.S.; Orihashi, Y.; Agashev, A.M.; Kagi, H. U-Pb age of rutile from the eclogite xenolith of the Udachnaya kimberlite pipe. Dokl. Earth Sci. 2014, 457, 861–864. [Google Scholar] [CrossRef]
- Roeder, P.L.; Schulze, D.J. Crystallization of groundmass spinel in kimberlite. J. Petrol. 2008, 49, 1473–1495. [Google Scholar] [CrossRef]
- Bellis, A.; Canil, D. Ferric Iron in CaTiO3 perovskite as an oxygen barometer for kimberlitic magmas I: Experimental calibration. J. Petrol. 2007, 48, 219–230. [Google Scholar] [CrossRef]
- Castillo-Oliver, M.; Galí, S.; Melgarejo, J.C.; Griffin, W.L.; Belousova, E.; Pearson, N.J.; Watangua, M.; O’Reilly, S.Y. Trace-element geochemistry and U-Pb dating of perovskite in kimberlites of the Lunda Norte province (NE Angola): Petrogenetic and tectonic implications. Chem. Geol. 2016, 426, 118–134. [Google Scholar] [CrossRef]
- Batumike, J.M.; Griffin, W.L.; Belousova, E.A.; Pearson, N.J.; O’Reilly, S.Y.; Shee, S.R. LAM-ICPMS U-Pb dating of kimberlitic perovskite: Eocene-Oligocene kimberlites from the Kundelungu Plateau, D.R. Congo. Earth Planet. Sci. Lett. 2008, 267, 609–619. [Google Scholar] [CrossRef]
- Tappe, S.; Kjarsgaard, B.A.; Kurszlaukis, S.; Nowell, G.M.; Phillips, D. Petrology and Nd-Hf Isotope Geochemistry of the Neoproterozoic Amon Kimberlite Sills, Baffin Island (Canada): Evidence for Deep Mantle Magmatic Activity Linked to Supercontinent Cycles. J. Petrol. 2014, 55, 2003–2042. [Google Scholar] [CrossRef]
- Malkovets, V.G.; Rezvukhin, D.I.; Belousova, E.A.; Griffin, W.L.; Sharygin, I.S.; Tretiakova, I.G.; Gibsher, A.A.; O’Reilly, S.Y.; Kuzmin, D.V.; Litasov, K.D.; et al. Cr-rich rutile: A powerful tool for diamond exploration. Lithos 2016, 265, 304–311. [Google Scholar] [CrossRef]
- Mitchell, R.H. Perovskites Modern and Ancient; Almaz Press: Thunder Bay, ON, Canada, 2002. [Google Scholar]
- Chakhmouradian, A.R.; Mitchell, R.H. Three compositional varieties of perovskite from kimberlites of the Lac de Gras field (Northwest Territories, Canada). Mineral. Mag. 2001, 65, 133–148. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Lehmann, B.; Mainkar, D.; Belyatsky, B. Petrogenesis of the end-Cretaceous diamondiferous Behradih orangeite pipe: Implication for mantle plume-lithosphere interaction in the Bastar craton, Central India. Contrib. Mineral. Petrol. 2011, 161, 721–742. [Google Scholar] [CrossRef]
- Chakhmouradian, A.R.; Mitchell, R.H. Occurrence, alteration patterns and compositional variation of perovskite in kimberlites. Can. Mineral. 2000, 38, 975–994. [Google Scholar] [CrossRef]
- Mitchell, R.H.; Chakhmouradian, A.R. Instability of perovskite in a CO2-rich environment: Examples from carbonatite and kimberlite. Can. Mineral. 1998, 36, 939–952. [Google Scholar]
- Pereira, V.P.; Conceicao, R.V.; Formoso, M.L.L.; Pires, A.C. Alteration of perovskite to anatase in silica-undersaturated rocks of the Catalao I carbonatite complex, Brazil; a Raman study. Rev. Bras. Geociênc. 2005, 35, 239–244. [Google Scholar]
- Martins, T.; Chakhmouradian, A.R.; Medici, L. Perovskite alteration in kimberlites and carbonatites: The role of kassite, CaTi2O4(OH)2. Phys. Chem. Miner. 2014, 41, 473–484. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A. Timing of eastern North American kimberlite magmatism: Continental extension of the Great Meteor hotspot track? Earth Planet. Sci. Lett. 2000, 178, 253–268. [Google Scholar] [CrossRef]
- Chalapathi Rao, N.V.; Wu, F.Y.; Mitchell, R.H.; Li, Q.L.; Lehmann, B. Mesoproterozoic U-Pb ages, trace element and Sr-Nd isotopic composition of perovskite from kimberlites of the Eastern Dharwar craton, southern India: Distinct mantle sources and a widespread 1.1Ga tectonomagmatic event. Chem. Geol. 2013, 353, 48–64. [Google Scholar] [CrossRef]
- Heaman, L.M.; Kjarsgaard, B.A.; Creaser, R.A. The temporal evolution of North American kimberlites. Lithos 2004, 76, 377–397. [Google Scholar] [CrossRef]
- Paton, C.; Hergt, J.M.; Phillips, D.; Woodhead, J.D.; Shee, S.R. New insights into the genesis of Indian kimberlites from the Dharwar Craton via in situ Sr isotope analysis of groundmass perovskite. Geology 2007, 35, 1011–1014. [Google Scholar] [CrossRef]
- Woodhead, J.; Hergt, J.; Phillips, D.; Paton, C. African kimberlites revisited: In situ Sr-isotope analysis of groundmass perovskite. Lithos 2009, 112, 311–317. [Google Scholar] [CrossRef]
- Zurevinski, S.E.; Heaman, L.M.; Creaser, R.A. The origin of Triassic/Jurassic kimberlite magmatism, Canada: Two mantle sources revealed from the Sr-Nd isotopic composition of groundmass perovskite. Geochem. Geophys. Geosyst. 2011, 12. [Google Scholar] [CrossRef]
- Kukharenko, A.A.; Orlova, M.P.; Bulakh, A.G.; Bagdasarov, E.A.; Rimski-Korsakov, O.M.; Nefedov, E.I.; Ilyinskiy, G.A.; Sergeev, A.B.; Abakumova, N.B. The Caledonian Ultramafic Alkaline and Carbonatite Complexes of the Kola Peninsula and Northern Karelia; Nedra: Moscow, Russia, 1965. [Google Scholar]
- Lepekhina, E.N.; Antonov, A.V.; Savva, E.V.; Belyatsky, B.V.; Sergeev, S.A. Perovskite from the Tiksheozero carbonatite: Age and genesis. In Perovskite from the Tiksheozero Carbonatite: Age and Genesis. Extended Abstract: Geochemistry of Magmatic Rocks—School Geochemistry of Alkaline Rocks; Vernadsky Institute of Geochemistry and Analytical Chemistry, Russian Academy of Sciences: Moscow, Russia, 2009. [Google Scholar]
- Tappe, S.; Jenner, G.A.; Foley, S.F.; Heaman, L.; Besserer, D.; Kjarsgaard, B.A.; Ryan, B. Torngat ultramafic lamprophyres and their relation to the North Atlantic Alkaline Province. Lithos 2004, 76, 491–518. [Google Scholar] [CrossRef]
- Dongre, A.N.; Viljoen, K.S.; Chalapathi Rao, N.V.; Gucsik, A. Origin of Ti-rich garnets in the groundmass of Wajrakarur field kimberlites, southern India: Insights from EPMA and Raman spectroscopy. Mineral. Petrol. 2016, 110, 295–307. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Heaman, L.M.; Kjarsgaard, B.A.; Romer, R.L.; Stracke, A.; Joyce, N.; Hoefs, J. Genesis of ultramafic lamprophyres and carbonatites at Aillik Bay, Labrador: A consequence of incipient lithospheric thinning beneath the North Atlantic Craton. J. Petrol. 2006, 47, 1261–1315. [Google Scholar] [CrossRef]
- Hammond, A.L.; Mitchell, R.H. Accessory mineralogy of orangeite from Swartruggens, South Africa. Mineral. Petrol. 2002, 76, 1–19. [Google Scholar] [CrossRef]
- Müntener, O.; Hermann, J. Titanian andradite in a metapyroxenite layer from the Malenco ultramafics (Italy): Implications for Ti-mobility and low oxygen fugacity. Contrib. Mineral. Petrol. 1994, 116, 156–168. [Google Scholar] [CrossRef]
- Amthauer, G.; Rossman, G.R. The hydrous component in andradite garnet. Am. Mineral. 1998, 83, 835–840. [Google Scholar] [CrossRef]
- Laverne, C.; Grauby, O.; Alt, J.C.; Bohn, M. Hydroschorlomite in altered basalts from Hole 1256D, ODP Leg 206: The transition from low-temperature to hydrothermal alteration. Geochem. Geophys. Geosyst. 2006, 7. [Google Scholar] [CrossRef]
- Schingaro, E.; Lacalamita, M.; Mesto, E.; Ventruti, G.; Pedrazzi, G.; Ottolini, L.; Scordari, F. Crystal chemistry and light elements analysis of Ti-rich garnets. Am. Mineral. 2016, 101, 371–384. [Google Scholar] [CrossRef]
- Dongre, A.N.; Jacob, D.E.; Stern, R.A. Subduction-related origin of eclogite xenoliths from the Wajrakarur kimberlite field, Eastern Dharwar craton, Southern India: Constraints from petrology and geochemistry. Geochim. Cosmochim. Acta 2015, 166, 165–188. [Google Scholar] [CrossRef]
- Ghosh, B.; Morishita, T.; Ray, J.; Tamura, A.; Mizukami, T.; Soda, Y.; Ovung, T.N. A new occurrence of titanian (hydro)andradite from the Nagaland ophiolite, India: Implications for element mobility in hydrothermal environments. Chem. Geol. 2017, 457, 47–60. [Google Scholar] [CrossRef]
- Downs, R.T. The RRUFF Project: An integrated study of the chemistry, crystallography, Raman and infrared spectroscopy of minerals. In Program and Abstracts of the 19th General Meeting of the International Mineralogical Association; International Mineralogical Association: Kobe, Japan, 2006; pp. O03–O13. [Google Scholar]
- Paschoal, C.W.A.; Moreira, R.L.; Fantini, C.; Pimenta, M.A.; Surendran, K.P.; Sebastian, M.T. Raman scattering study of RETiTaO6 dielectric ceramics. J. Eur. Ceram. Soc. 2003, 23, 2661–2666. [Google Scholar] [CrossRef]
- Tomašić, N.; Gajović, A.; Bermanec, V.; Rajić, M. Recrystallization of metamict Nb–Ta–Ti–REE complex oxides: A coupled X-Ray-Diffraction and Raman Spectroscopy study of aeschynite-(Y) and polycrase-(Y). Can. Mineral. 2004, 42, 1847–1857. [Google Scholar] [CrossRef]
- Nasir, S.; Theye, T.; Massonne, H.J. REE-Rich Aeschynite in Apatite-Dolomite Carbonatite, Eastern Oman Mountains. Open Mineral. J. 2009, 3, 17–27. [Google Scholar]
- Macdonald, R.; Bagiński, B.; Kartashov, P.M.; Zozulya, D.; Dzierżnowski, P. Hydrothermal alteration of chevkinite-group minerals. Part 2. Metasomatite from the Keivy massif, Kola Peninsula, Russia. Mineral. Mag. 2015, 79, 1039–1059. [Google Scholar] [CrossRef]
- Grew, E.S.; Locock, A.J.; Mills, S.J.; Galuskina, I.O.; Galuskin, E.V.; Hålenius, U. IMA report: Nomenclature of the garnet supergroup. Am. Mineral. 2013, 98, 785–810. [Google Scholar] [CrossRef]
- Mitchell, R.H. Kimberlites: Mineralogy, Geochemistry, and Petrology; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Cox, R.A.; Wilton, D.H.C. U–Pb dating of perovskite by LA-ICP-MS: An example from the Oka carbonatite, Quebec, Canada. Chem. Geol. 2006, 235, 21–32. [Google Scholar] [CrossRef]
- Simonetti, A.; Heaman, L.M.; Chacko, T. Use of discrete-dynode secondary electron multipliers with Faradays—A “reduced volume” approach for in-situ U-Pb dating of accessory minerals within petrographic thin sections by LA-MC-ICP-MS. In Laser Ablation-ICP-MS in the Earth Sciences; Short Course Series 40; Ylvester, P., Ed.; Mineralogical Association of Canada: Quebec, QC, Canada, 2008; pp. 24–264. [Google Scholar]
- Yang, Y.H.; Wu, F.Y.; Wilde, S.A.; Liu, X.M.; Zhang, Y.B.; Xie, L.W.; Yang, J.H. In situ perovskite Sr-Nd isotopic constraints on the petrogenesis of the Ordovician Mengyin kimberlites in the North China Craton. Chem. Geol. 2009, 264, 24–42. [Google Scholar] [CrossRef]
- Tappe, S.; Foley, S.F.; Jenner, G.A.; Kjarsgaard, B.A. Integrating ultramafic lamprophyres into the IUGS classification of igneous rocks: Rationale and implications. J. Petrol. 2005, 46, 1893–1900. [Google Scholar] [CrossRef]
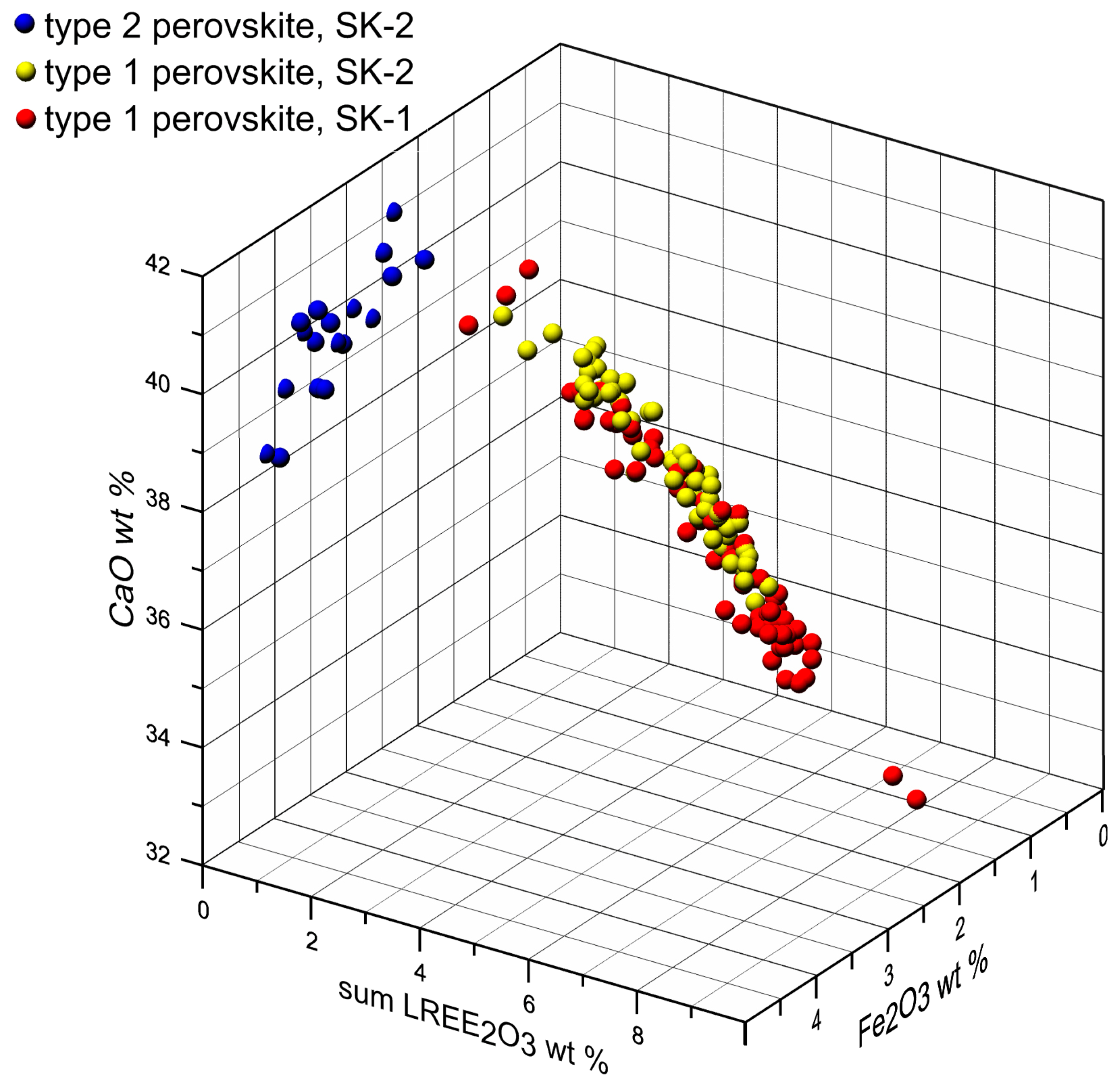
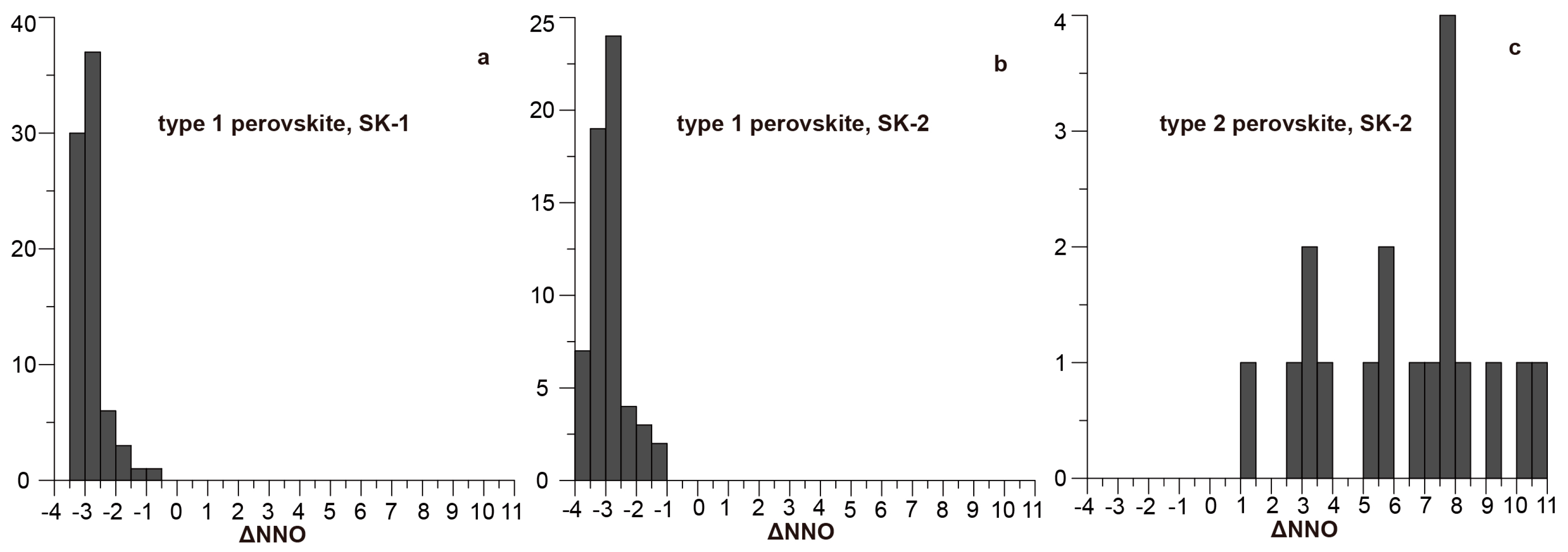
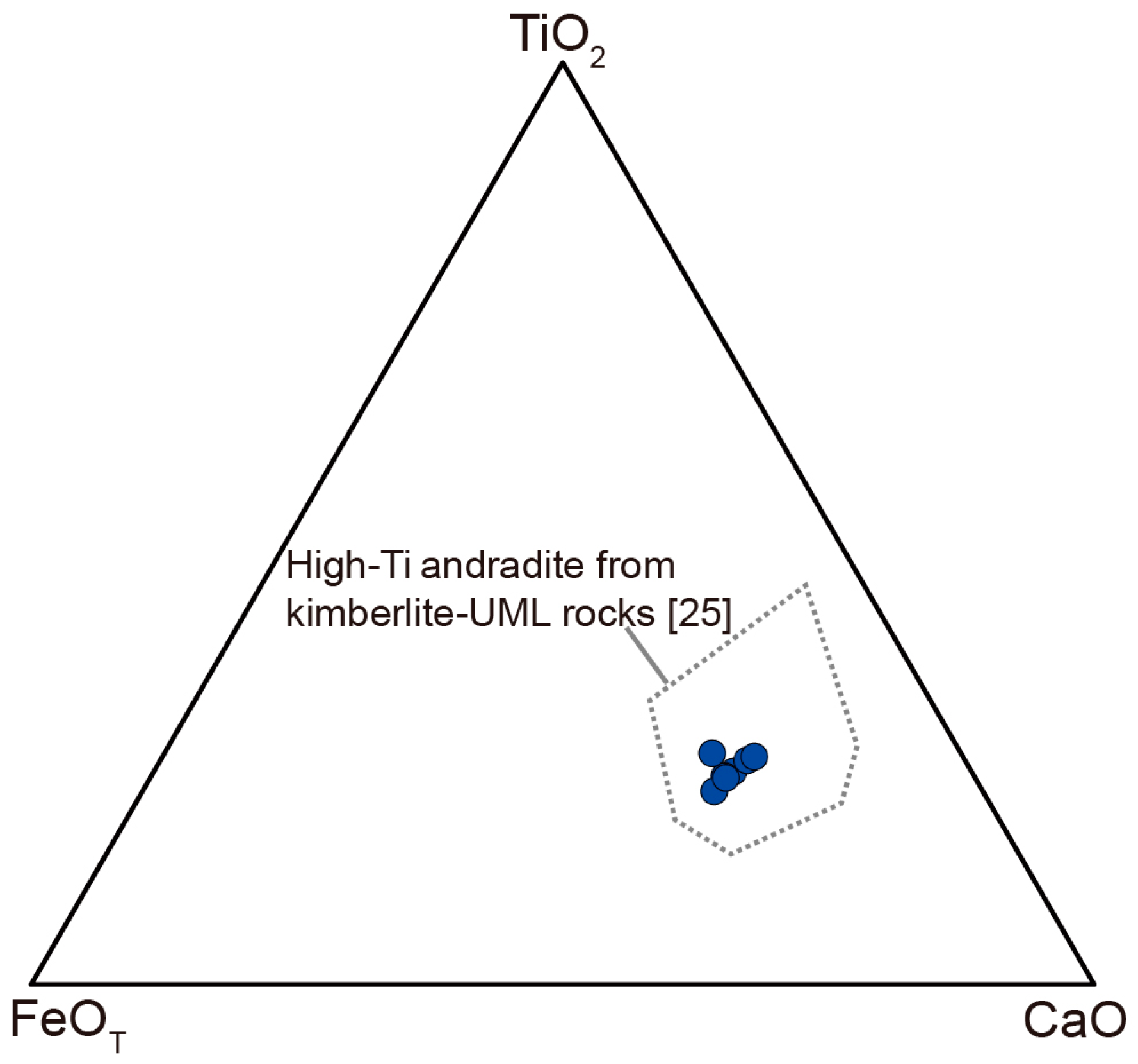
| Mineral | Type 1 Perovskite | Type 2 Perovskite | Aeschynite-(Ce) | Kassite | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SK-1 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | SK-2 | |||||||
| (wt %) | Centre | Centre | Border | Border | Centre | Centre | Border | Border | |||||||
| SrO | 0.34 | 0.18 | 0.23 | 0.25 | 0.20 | 0.19 | 0.20 | 0.24 | bdl | bdl | 0.13 | bdl | bdl | bdl | bdl |
| ZrO2 | 0.10 | 0.21 | 0.23 | 0.38 | 0.08 | 0.14 | 0.19 | 0.28 | 0.09 | 0.46 | 0.74 | 0.25 | 0.43 | 0.40 | 0.08 |
| Nb2O5 | 0.60 | 0.98 | 0.73 | 0.61 | 0.54 | 0.63 | 0.44 | 0.50 | 0.10 | bdl | 3.10 | 0.99 | 0.63 | 0.46 | 0.62 |
| CaO | 34.68 | 34.64 | 37.11 | 37.63 | 35.36 | 35.27 | 38.06 | 38.19 | 39.95 | 38.20 | 7.24 | 8.00 | 22.49 | 22.12 | 21.32 |
| ThO2 | - | - | - | - | - | - | - | - | - | - | 0.37 | bdl | - | - | - |
| Na2O | 0.57 | 0.57 | 0.35 | 0.22 | 36.53 | 0.60 | 0.28 | 0.22 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| MgO | 0.06 | bdl | 0.05 | 0.09 | 0.08 | bdl | 0.05 | 0.05 | 0.14 | 0.08 | 0.04 | 0.05 | 0.26 | 0.05 | bdl |
| Al2O3 | 0.33 | 0.30 | 0.24 | 0.31 | 0.25 | 0.29 | 0.23 | 0.28 | bdl | bdl | bdl | bdl | bdl | 0.03 | bdl |
| SiO2 | bdl | bdl | bdl | 0.04 | bdl | bdl | bdl | bdl | 0.61 | 0.60 | 0.38 | bdl | 0.05 | 0.06 | 0.07 |
| BaO | 0.19 | 0.12 | - | 0.08 | - | 0.13 | 0.18 | 0.12 | - | - | 0.46 | 0.15 | - | - | - |
| TiO2 | 54.36 | 54.26 | 54.13 | 56.30 | 54.99 | 54.75 | 57.10 | 56.67 | 55.49 | 53.48 | 48.61 | 52.20 | 62.42 | 64.74 | 65.26 |
| La2O3 | 0.97 | 1.02 | 0.67 | 0.50 | 0.60 | 0.84 | 0.62 | 0.53 | bdl | bdl | 7.79 | 11.11 | bdl | bdl | 0.15 |
| Ce2O3 | 3.07 | 3.03 | 1.79 | 0.88 | 2.18 | 2.84 | 0.89 | 0.89 | bdl | bdl | 18.34 | 17.99 | 0.23 | 0.48 | 0.58 |
| Nd2O3 | 1.56 | 1.50 | 1.08 | 0.35 | 1.18 | 1.50 | 0.34 | 0.41 | bdl | bdl | 6.88 | 3.99 | 0.24 | 0.22 | 0.28 |
| Cr2O3 | - | - | - | - | - | - | - | - | - | - | bdl | 0.05 | - | - | - |
| Pr2O3 | 0.45 | 0.43 | 0.17 | 0.19 | 0.08 | 0.36 | 0.19 | 0.17 | bdl | bdl | 1.97 | 1.45 | 0.12 | 0.08 | bdl |
| MnO | 0.02 | 0.05 | 0.04 | bdl | 0.03 | bdl | 0.02 | 0.05 | bdl | 0.03 | bdl | 0.19 | 0.15 | 0.37 | 1.28 |
| Sm2O3 | - | - | - | - | - | - | - | - | - | - | 0.51 | 0.19 | - | - | - |
| Fe2O3 | 1.28 | 1.36 | 1.74 | 1.31 | 0.99 | 1.29 | 1.16 | 1.01 | 2.00 | 4.03 | 0.59 | 0.66 | 1.29 | 0.93 | 0.51 |
| Gd2O3 | - | - | - | - | - | - | - | - | - | - | 0.24 | 0.13 | - | - | - |
| HfO2 | - | - | - | - | - | - | - | - | - | - | 0.11 | bdl | - | - | - |
| Ta2O5 | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | 0.07 | bdl | 0.06 | 0.11 | bdl | 0.04 | bdl |
| K2O | 0.04 | 0.03 | 0.02 | 0.00 | 0.03 | 0.03 | 0.02 | 0.02 | 0.00 | 0.02 | - | - | bdl | 0.01 | bdl |
| Total | 98.63 | 98.74 | 98.57 | 99.16 | 98.25 | 98.92 | 99.99 | 99.65 | 98.80 | 97.19 | 97.53 | 97.79 | 88.37 | 90.05 | 90.19 |
| ΣLREE2O3 | 6.04 | 5.98 | 3.70 | 1.93 | 4.04 | 5.54 | 2.04 | 1.99 | 0.14 | 0.15 | 35.49 | 34.73 | 0.59 | 0.81 | 1.03 |
| (apfu) | O = 3 | O = 3 | Σcations = 3 | Σcations = 3 | |||||||||||
| Sr | 0.005 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.003 | 0.000 | 0.000 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 |
| Y | - | - | - | - | - | - | - | - | - | - | 0.000 | 0.000 | - | - | - |
| Zr | 0.001 | 0.002 | 0.003 | 0.004 | 0.001 | 0.002 | 0.002 | 0.003 | 0.001 | 0.005 | 0.018 | 0.006 | 0.001 | 0.001 | 0.001 |
| Nb | 0.006 | 0.011 | 0.008 | 0.006 | 0.006 | 0.007 | 0.005 | 0.005 | 0.001 | 0.000 | 0.070 | 0.022 | 0.012 | 0.008 | 0.011 |
| Ca | 0.885 | 0.883 | 0.938 | 0.932 | 0.924 | 0.894 | 0.936 | 0.942 | 0.985 | 0.960 | 0.387 | 0.417 | 0.986 | 0.957 | 0.926 |
| Th | - | - | - | - | - | - | - | - | - | - | 0.004 | 0.000 | - | - | - |
| Na | 0.026 | 0.026 | 0.016 | 0.010 | 0.022 | 0.027 | 0.012 | 0.010 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Mg | 0.002 | 0.001 | 0.002 | 0.003 | 0.003 | 0.000 | 0.002 | 0.002 | 0.005 | 0.003 | 0.003 | 0.004 | 0.016 | 0.003 | 0.000 |
| Al | 0.009 | 0.008 | 0.007 | 0.008 | 0.007 | 0.008 | 0.006 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Si | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.014 | 0.014 | 0.019 | 0.000 | 0.002 | 0.002 | 0.003 |
| Ba | 0.002 | 0.001 | 0.000 | 0.001 | 0.000 | 0.001 | 0.002 | 0.001 | - | - | 0.009 | 0.003 | - | - | - |
| Ti | 0.974 | 0.970 | 0.960 | 0.979 | 0.977 | 0.974 | 0.985 | 0.981 | 0.960 | 0.943 | 1.823 | 1.908 | 1.922 | 1.967 | 1.990 |
| La | 0.008 | 0.008 | 0.005 | 0.004 | 0.005 | 0.007 | 0.005 | 0.004 | 0.000 | 0.000 | 0.132 | 0.184 | 0.000 | 0.000 | 0.002 |
| Ce | 0.027 | 0.026 | 0.015 | 0.007 | 0.019 | 0.025 | 0.007 | 0.007 | 0.000 | 0.000 | 0.335 | 0.320 | 0.004 | 0.007 | 0.009 |
| Nd | 0.013 | 0.013 | 0.009 | 0.003 | 0.010 | 0.013 | 0.003 | 0.003 | 0.000 | 0.000 | 0.123 | 0.069 | 0.003 | 0.003 | 0.004 |
| Cr | - | - | - | - | - | - | - | - | - | - | 0.000 | 0.002 | - | - | - |
| Pr | 0.004 | 0.004 | 0.001 | 0.002 | 0.001 | 0.003 | 0.002 | 0.001 | 0.000 | 0.000 | 0.036 | 0.026 | 0.002 | 0.001 | 0.000 |
| Mn | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.006 | 0.004 | 0.010 | 0.036 |
| Sm | - | - | - | - | - | - | - | - | - | - | 0.009 | 0.003 | - | - | - |
| Fe | 0.023 | 0.024 | 0.031 | 0.023 | 0.018 | 0.023 | 0.020 | 0.017 | 0.035 | 0.071 | 0.022 | 0.024 | 0.040 | 0.028 | 0.016 |
| Gd | - | - | - | - | - | - | - | - | - | - | 0.004 | 0.002 | - | - | - |
| Hf | - | - | - | - | - | - | - | - | - | - | 0.002 | 0.000 | - | - | - |
| Ta | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 | 0.001 | 0.000 | 0.000 | 0.000 |
| K | 0.001 | 0.001 | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 | - | - | 0.000 | 0.000 | 0.000 |
| ΣLREE | 0.052 | 0.051 | 0.031 | 0.016 | 0.034 | 0.047 | 0.017 | 0.016 | 0.001 | 0.001 | 0.512 | 0.533 | 0.009 | 0.012 | 0.015 |
| (wt %) | #1 | #2 | #4 | #5 | #15 | #17 | #19 | #20 | #21 |
|---|---|---|---|---|---|---|---|---|---|
| SiO2 | 20.20 | 25.44 | 21.62 | 21.86 | 21.51 | 23.18 | 20.69 | 21.24 | 20.00 |
| Al2O3 | 0.63 | 0.69 | 0.98 | 0.98 | 0.85 | 0.89 | 0.85 | 0.84 | 0.92 |
| Cr2O3 | 0.32 | 0.83 | 1.23 | 0.77 | 2.81 | 0.37 | 2.63 | 1.25 | 2.22 |
| TiO2 | 26.33 | 23.39 | 14.80 | 14.60 | 15.50 | 13.30 | 15.64 | 14.46 | 16.02 |
| MgO | 0.80 | 1.46 | 0.08 | 0.20 | 0.17 | 0.47 | 0.14 | 0.10 | 0.68 |
| Na2O | bdl | 0.04 | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| BaO | 0.10 | 0.11 | bdl | bdl | bdl | bdl | bdl | 0.10 | 0.11 |
| MnO | 0.13 | 0.09 | 0.07 | 0.09 | 0.06 | 0.08 | 0.10 | 0.08 | 0.22 |
| FeO | 13.62 | 11.88 | 14.34 | 15.16 | 13.07 | 16.04 | 12.42 | 15.13 | 14.95 |
| SrO | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl | bdl |
| ZrO2 | 0.79 | 0.77 | 0.20 | 0.28 | 0.18 | 0.30 | 0.54 | 0.40 | 0.35 |
| K2O | 0.01 | 0.01 | 0.02 | 0.01 | 0.01 | 0.01 | 0.01 | bdl | bdl |
| CaO | 30.61 | 30.24 | 34.92 | 34.82 | 35.19 | 34.12 | 35.24 | 34.94 | 32.91 |
| Total | 93.86 | 95.22 | 88.26 | 88.77 | 89.35 | 88.77 | 88.25 | 88.53 | 88.39 |
| Recalculated Analyses | |||||||||
| Fe2O3 | 15.14 | 13.20 | 15.94 | 16.85 | 14.52 | 17.83 | 13.80 | 16.81 | 16.61 |
| FeO | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| Total | 95.12 | 96.28 | 89.88 | 90.47 | 90.81 | 90.55 | 89.66 | 90.26 | 90.06 |
| (apfu) | Cation for 3(Ca + K + Na + Sr + Ba + Mn + Mg) | ||||||||
| Si | 1.7730 | 2.1941 | 1.7241 | 1.7392 | 1.6971 | 1.8626 | 1.6309 | 1.6883 | 1.6432 |
| Al | 0.0655 | 0.0704 | 0.0921 | 0.0920 | 0.0793 | 0.0842 | 0.0786 | 0.0788 | 0.0895 |
| Cr | 0.0222 | 0.0563 | 0.0778 | 0.0485 | 0.1753 | 0.0238 | 0.1639 | 0.0784 | 0.1442 |
| Ti | 1.7385 | 1.5175 | 0.8878 | 0.8738 | 0.9199 | 0.8039 | 0.9273 | 0.8646 | 0.9901 |
| Mg | 0.1049 | 0.1878 | 0.0097 | 0.0240 | 0.0201 | 0.0562 | 0.0159 | 0.0119 | 0.0838 |
| Na | 0.0000 | 0.0063 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Ba | 0.0035 | 0.0037 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0030 | 0.0036 |
| Mn | 0.0097 | 0.0063 | 0.0048 | 0.0060 | 0.0042 | 0.0057 | 0.0066 | 0.0052 | 0.0151 |
| Fe3+ | 0.9996 | 0.8567 | 0.9562 | 1.0085 | 0.8622 | 1.0777 | 0.8186 | 1.0056 | 1.0271 |
| Sr | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Zr | 0.0340 | 0.0325 | 0.0077 | 0.0109 | 0.0068 | 0.0118 | 0.0208 | 0.0156 | 0.0141 |
| K | 0.0011 | 0.0013 | 0.0015 | 0.0010 | 0.0009 | 0.0008 | 0.0009 | 0.0000 | 0.0000 |
| Ca | 2.8784 | 2.7942 | 2.9833 | 2.9679 | 2.9744 | 2.9373 | 2.9759 | 2.9754 | 2.8968 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Melgarejo, J.C.; Castillo-Oliver, M. Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks. Minerals 2018, 8, 51. https://doi.org/10.3390/min8020051
Xu J, Melgarejo JC, Castillo-Oliver M. Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks. Minerals. 2018; 8(2):51. https://doi.org/10.3390/min8020051
Chicago/Turabian StyleXu, Jingyao, Joan Carles Melgarejo, and Montgarri Castillo-Oliver. 2018. "Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks" Minerals 8, no. 2: 51. https://doi.org/10.3390/min8020051
APA StyleXu, J., Melgarejo, J. C., & Castillo-Oliver, M. (2018). Styles of Alteration of Ti Oxides of the Kimberlite Groundmass: Implications on the Petrogenesis and Classification of Kimberlites and Similar Rocks. Minerals, 8(2), 51. https://doi.org/10.3390/min8020051





