The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece †
Abstract
:1. Introduction
2. Materials and Methods
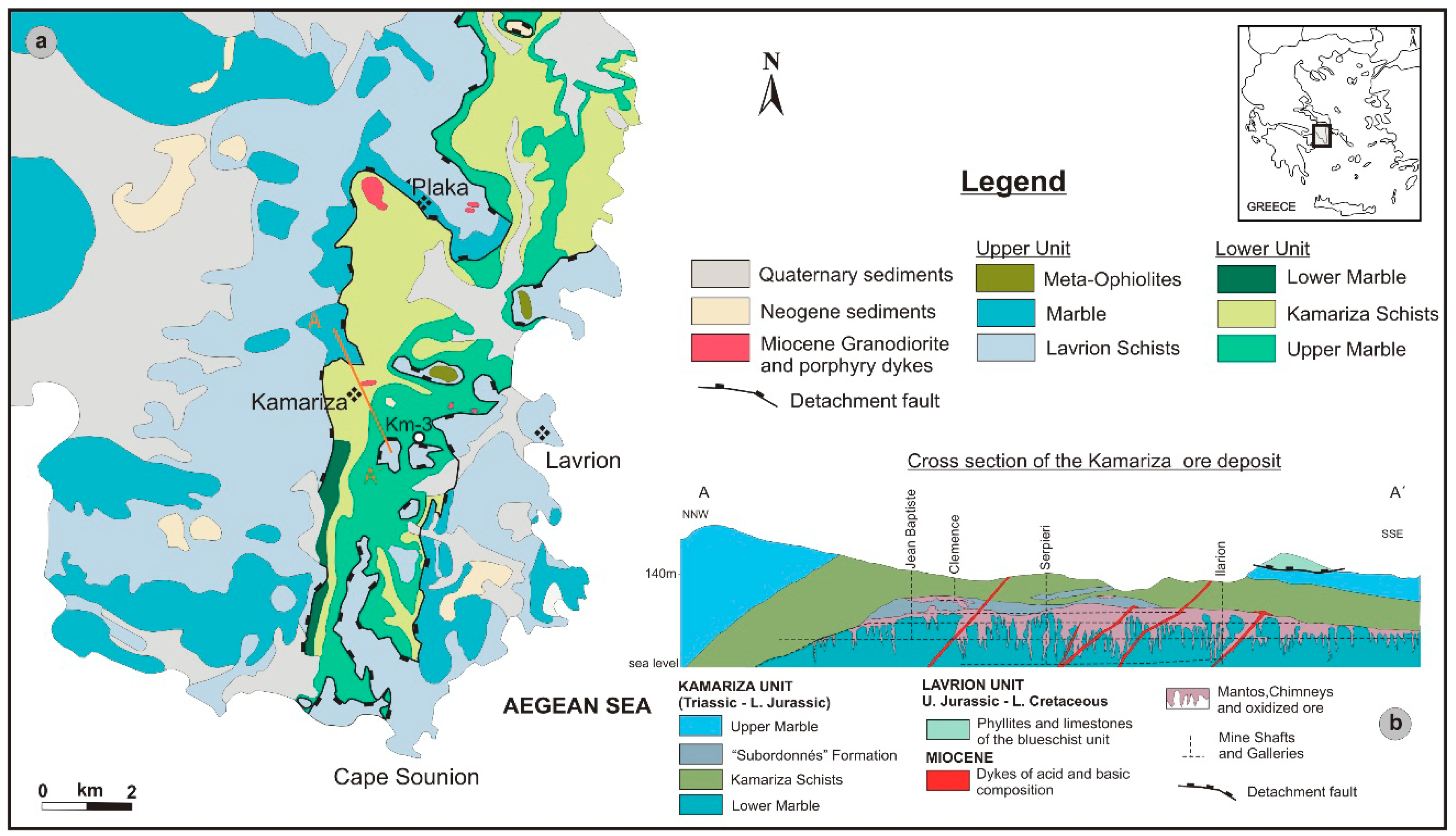
3. Geology and Mineralization
4. Bulk Ore Geochemistry
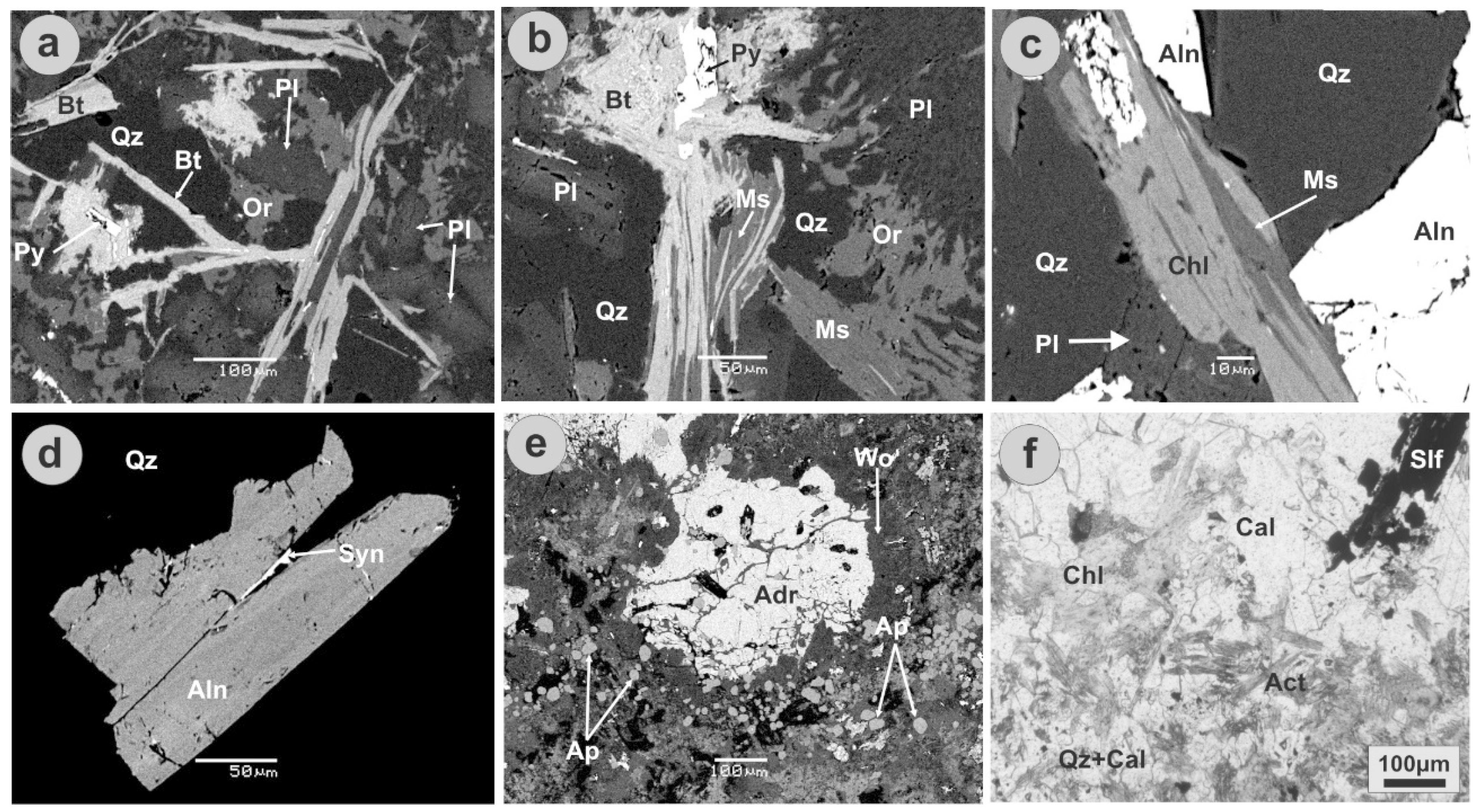
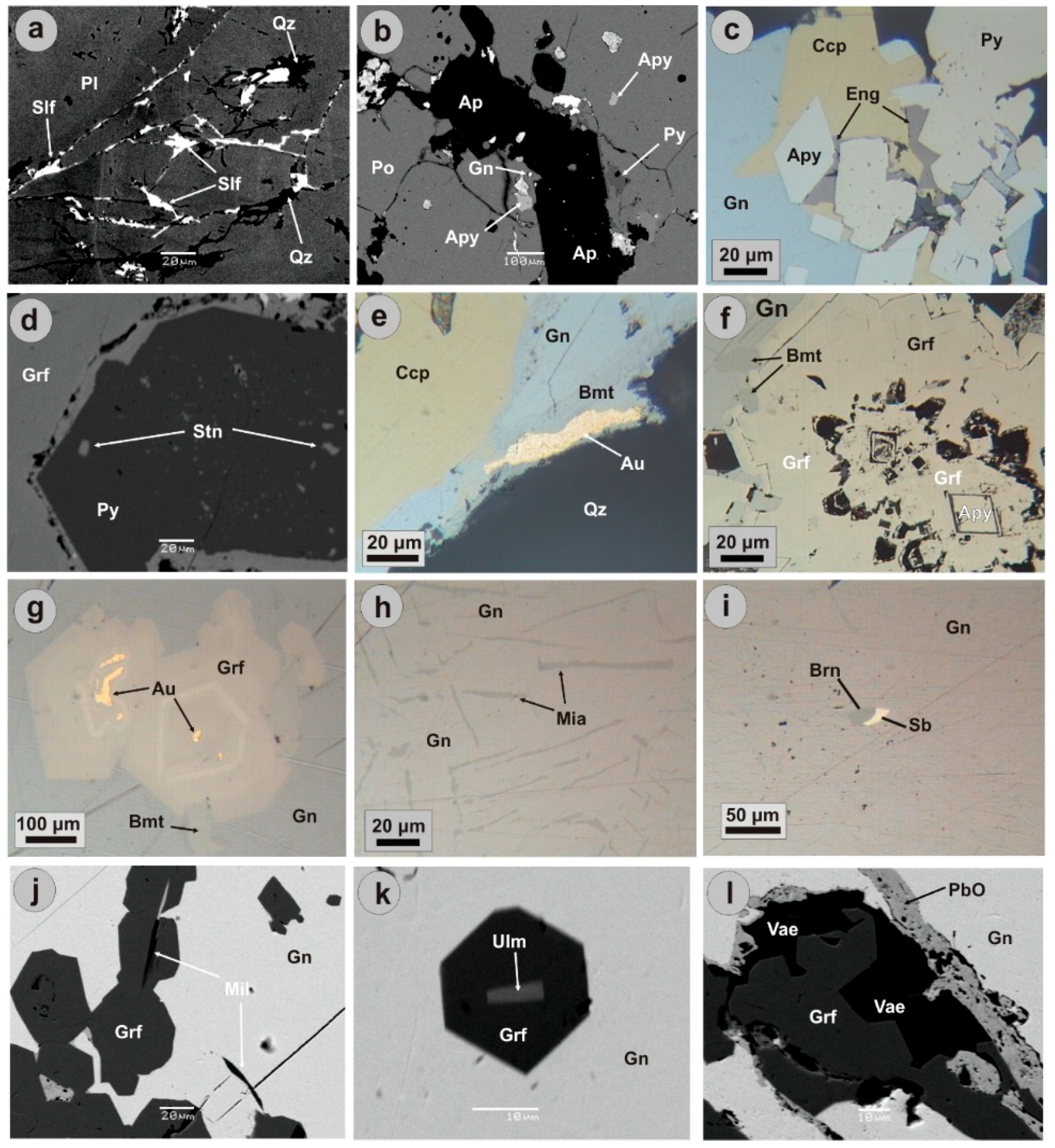
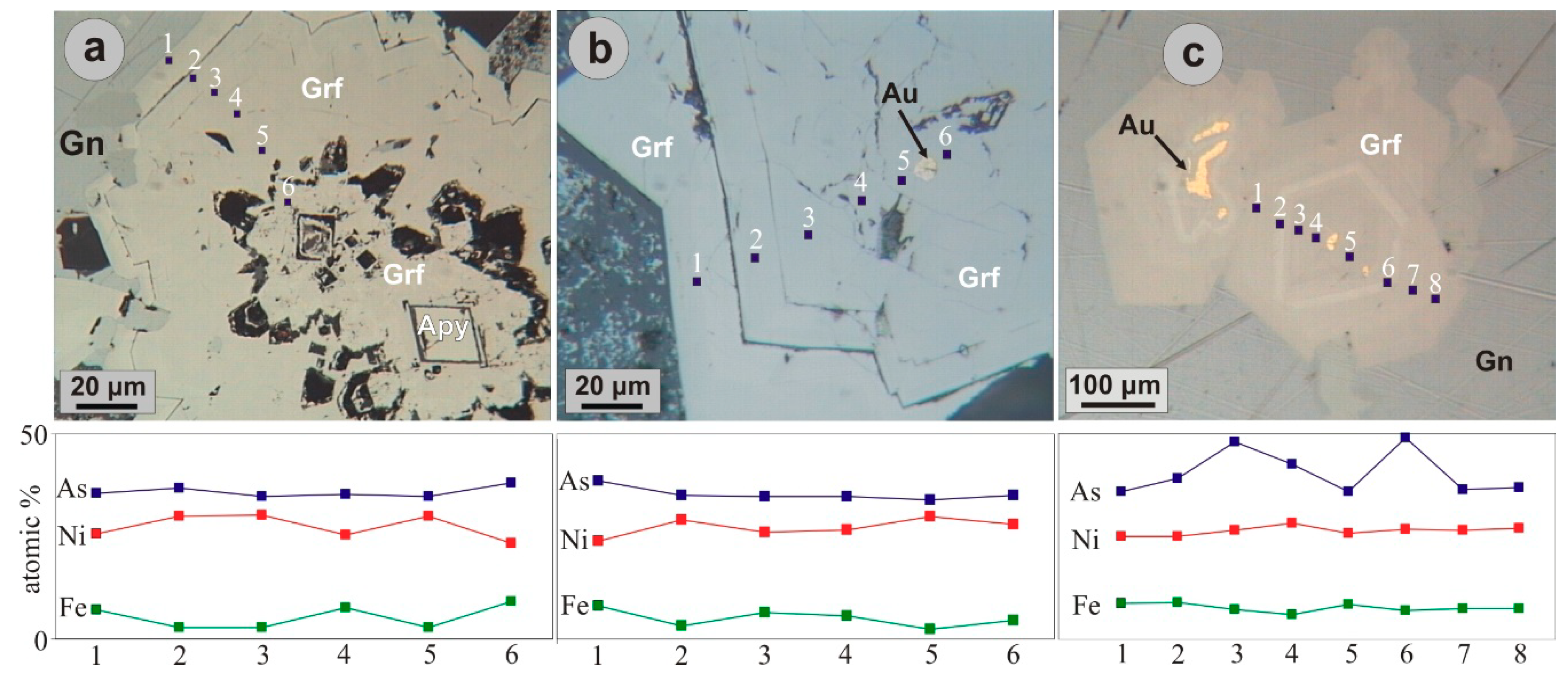
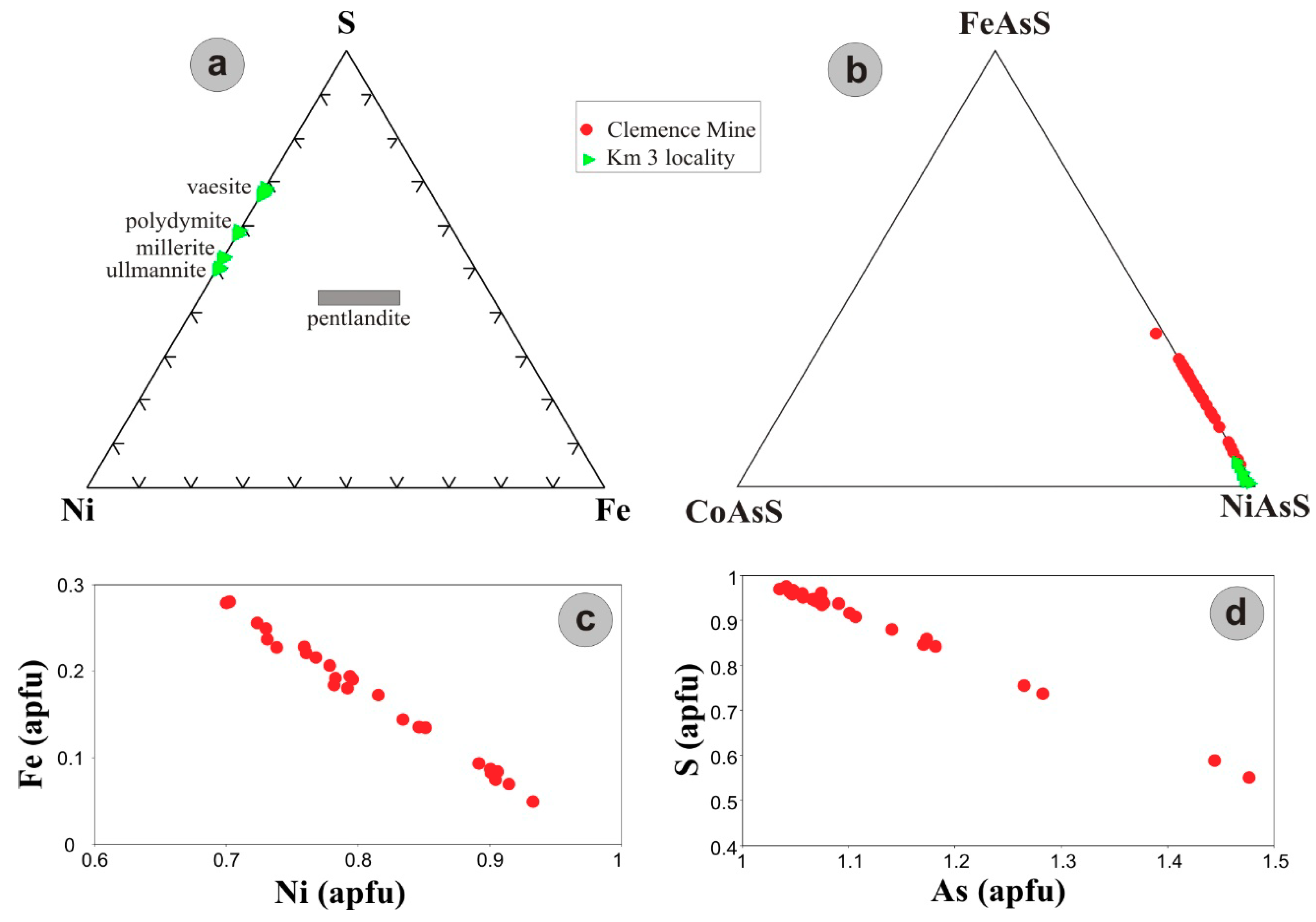
5. Ore Mineralogy
6. Discussion
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marinos, G.; Petrascheck, W.E. Laurium. Geological and geophysical research. Greece Inst. Geol. Subsurf. Res. 1956, 4, 1–247. [Google Scholar]
- Voudouris, P.; Economou-Eliopoulos, M. Mineralogy and chemistry of Cu-rich ores from the Kamariza carbonate-hosted deposit (Lavrion), Greece. In Exploration and Sustainable Development; Millpress: Rotterdam, The Netherlands, 2003; pp. 499–502. [Google Scholar]
- Solomos, C.; Voudouris, P.; Katerinopoulos, A. Mineralogical studies of a bismuth-gold-antimony mineralization in Kamariza, Lavrion. Bull. Geol. Soc. Greece 2004, 36, 387–396, (In Greek with English Abstract). [Google Scholar] [CrossRef]
- Voudouris, P.; Melfos, P.; Spry, P.G.; Bonsall, T.A.; Tarkian, M.; Solomos, C. Carbonate-replacement Pb–Zn–Ag ± Au mineralization in the Kamariza area, Lavrion, Greece: Mineralogy and thermochemical conditions of formation. Mineral. Petrol. 2008, 94, 85–106. [Google Scholar] [CrossRef]
- Bonsall, T.A.; Spry, P.G.; Voudouris, P.; Seymour, K.S.; Tombros, S.; Melfos, V. The geochemistry of carbonate-replacement Pb-Zn-Ag mineralization in the Lavrion district, Attica, Greece: Fluid inclusion, stable isotope, and rare earth element studies. Econ. Geol. 2011, 106, 619–651. [Google Scholar] [CrossRef]
- Kolitsch, U.; Rieck, B.; Voudouris, P. Mineralogy and genesis of the Lavrion ore deposit: New insights from the study of ore and accessory minerals. Mitt. Österr. Mineral. Ges. 2015, 161, 66. [Google Scholar]
- Skarpelis, N. The Lavrion deposit (SE Attika, Greece): Geology, mineralogy and minor elements chemistry. Neues Jahrb. Miner. Abh. J. Miner. Geochem. 2007, 183, 227–249. [Google Scholar]
- Wendel, W.; Markl, G. Lavrion: Mineralogische Klassiker und Raritäten für Sammler. Lapis 1999, 24, 34–52. (In German) [Google Scholar]
- Rieck, B.; Kolitsch, U.; Voudouris, P.; Giester, G.; Tzeferis, P. More new finds from Lavrion, Greece. Mineral.-Welt 2018, 29, 32–77. (In German) [Google Scholar]
- Galanos, E.; Voudouris, P.; Rieck, B.; Kolitsch, U.; Mavrogonatos, C.; Melfos, V.; Zaimis, S.; Soukis, K. Oscillatory zoned gersdorffite and the Ni-Bi-Au association at Clemence Mine and Km 3 locality, Lavrion District, Greece. In Proceedings of the 1st International Electronic Conference on Mineral Science; MDPI: Basel, Switzerland, 2018. [Google Scholar]
- Garrels, R.M.; Christ, C.L. Solutions, Minerals and Equilibria; Harper and Row: New York, NY, USA, 1965; p. 450. [Google Scholar]
- Henley, R.W.; Truesdell, A.H.; Barton, P.B.; Whitney, J.A. Fluid-mineral equilibria in hydrothermal systems. Rev. Econ. Geol. 1984, 1, 1–267. [Google Scholar]
- Altherr, R.; Kreuzer, H.; Wendt, I.; Lenz, H.; Wagner, G.; Keller, J.; Harre, W.; Hohndorf, A. A late Oligocene/Early Miocene high temperature belt in the Attic-Cycladic crystalline complex (SE Pelagonian, Greece). Geol. Jahrb. 1982, 23, 97–164. [Google Scholar]
- Jolivet, L.; Brun, J.P. Cenozoic geodynamic evolution of the Aegean region. Int. J. Earth Sci. 2010, 99, 109–138. [Google Scholar] [CrossRef]
- Photiades, A.; Carras, N. Stratigraphy and geological structure of the Lavrion area (Attica, Greece). Bull. Geol. Soc. Greece 2001, 34, 103–109. [Google Scholar] [CrossRef]
- Papanikolaou, D.J.; Syskakis, D. Geometry of acid intrusives in Plaka, Laurium and relation between magmatism and deformation. Bull. Geol. Soc. Greece 1991, 25, 355–368. [Google Scholar]
- Baltatzis, E. Contact metamorphism of a calc-silicate hornfels from Plaka area, Laurium, Greece. Neues Jahrb. Miner. Monatsh. 1981, 11, 481–488. [Google Scholar]
- Bassi, E.; Soukis, K.; Lekkas, S. The presence of Vari-Kirou Pira unit at Panion Hill (SE Attica, Greece). Bull. Geol. Soc. Greece 2004, 36, 1608–1617. [Google Scholar] [CrossRef]
- Photiades, A.; Saccani, E. Geochemistry and tectono-magmatic significance of HP/LT metaophiolites of the Attic-Cycladic zone in the Lavrion area (Attica, Greece). Ofioliti 2006, 31, 89–102. [Google Scholar]
- Berger, A.; Schneider, D.A.; Grasemann, B.; Stöckli, D. Footwall mineralization during Late Miocene extension along the West Cycladic Detachment System, Lavrion, Greece. Terra Nova 2013, 25, 181–191. [Google Scholar] [CrossRef]
- Scheffer, C.; Vanderhaeghe, O.; Lanari, P.; Tarantola, A.; Ponthus, L.; Photiades, A.; France, L. Syn- to post-orogenic exhumation of metamorphic nappes: Structure and thermobarometry of the western Attic-Cycladic metamorphic complex (Lavrion, Greece). J. Geodyn. 2016, 96, 174–193. [Google Scholar] [CrossRef]
- Scheffer, C.; Tarantola, A.; Vanderhaeghe, O.; Rigaudier, T.; Photiades, A. CO2 flow during orogenic gravitational collapse: Syntectonic decarbonation and fluid mixing at the ductile-brittle transition (Lavrion, Greece). Chem. Geol. 2017, 450, 248–263. [Google Scholar] [CrossRef]
- Altherr, R.; Siebel, W. I-type plutonism in a continental back-arc setting: Miocene granitoids and monzonites from the central Aegean Sea, Greece. Contrib. Miner. Petrol. 2002, 143, 397–415. [Google Scholar] [CrossRef]
- Skarpelis, N.; Tsikouras, B.; Pe-Piper, G. The Miocene igneous rocks in the Basal unit of Lavrion (SE Attica Greece): Petrology and geodynamic implications. Geol. Mag. 2008, 145, 1–15. [Google Scholar] [CrossRef]
- Peccerillo, A.; Taylor, S.R. Geochemistry of Eocene calc-alkaline volcanic rocks from the Kastamonu area, northern Turkey. Contrib. Mineral. Petrol. 1976, 58, 63–81. [Google Scholar] [CrossRef]
- Middlemost, E.A.K. Magmas and Magmatic Rocks: An Introduction to Igneous Petrology; U.S. Department of Energy Office of Scientific and Technical Information: New York, NY, USA, 1985.
- Shand, S.J. The Eruptive Rocks, 2nd ed.; John Wiley: New York, NY, USA, 1943. [Google Scholar]
- Maniar, P.D.; Piccoli, P.M. Tectonic discrimination of granitoids. Geol. Soc. Am. Bull. 1989, 101, 635–643. [Google Scholar] [CrossRef]
- Stouraiti, C.; Mitropoulos, P.; Tarney, J.; Barreiro, B.; McGrath, A.M.; Baltatzis, E. Geochemistry and petrogenesis of late Miocene granitoids, Cyclades, southern Aegean: Nature of source components. Lithos 2010, 114, 337–352. [Google Scholar] [CrossRef]
- Kissin, S.A. Five-element (Ni-Co-As-Ag-Bi) veins. Geosci. Can. 1992, 19, 113–124. [Google Scholar]
- Markl, G.; Burisch, M.; Neumann, U. Natural fracking and the genesis of five-element veins. Miner. Depos. 2016, 51, 703–712. [Google Scholar] [CrossRef]
- Choi, S.G.; Youm, S.J. Compositional variation of arsenopyrite and fluid evolution at the Ulsan deposit, southeastern Korea: A low-sulfidation porphyry system. Can. Mineral. 2000, 38, 567–583. [Google Scholar] [CrossRef]
- Henning, A.; Van der Westhuizen, W.A.; De Bruiyn, H.; Beukes, G.J. Hydrothermal Cu-Ni-Au-Ag mineralization in a granodiorite sill north of Cradock, Republic of South Africa. Miner. Depos. 1997, 32, 410–418. [Google Scholar] [CrossRef]
- Seifert, T.; Sandmann, D. Metallogeny and economic potential of tungsten deposits in the Erzgebirge, Saxony/Bohemia. In Proceedings of the 33rd International Geological Congress—MRD04 Giant Ore Deposits, Oslo, Norway, 6–14 August 2008. [Google Scholar]
- Staude, S.; Werner, W.; Mordhorst, T.; Wemmer, K.; Jacob, D.E.; Markl, G. Multi-stage Ag-Bi-Co-Ni-U and Cu-Bi vein mineralization at Wittichen, Schwarzwald, SW Germany: Geological setting, ore mineralogy, and fluid evolution. Miner. Depos. 2012, 47, 251–276. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Arai, S.; Ikenne, M. Mineralogy and paragenesis of the Co-Ni arsenide ores of Bou Azzer, Anti-Atlas, Morocco. Econ. Geol. 2009, 104, 249–266. [Google Scholar] [CrossRef]
- Marshall, D.D.; Diamond, L.W.; Skippen, G.B. Silver transport and deposition at Cobalt, Ontario, Canada: Fluid inclusion evidence. Econ. Geol. 1993, 88, 837–854. [Google Scholar] [CrossRef]
- Essarraj, S.; Boiron, M.C.; Cathelineau, M.; Tarantola, A.; Mathieu Leisen, M.; Boulvais, P.; Maacha, L. Basinal brines at the Origin of the Imiter Ag-Hg Deposit (Anti-Atlas, Morocco): Evidence from LA-ICP-MS data on fluid inclusions, halogen signatures, and stable isotoped (H, C, O). Econ. Geol. 2016, 111, 1753–1781. [Google Scholar] [CrossRef]
- Pažout, R.; Šrein, V.; Korbelová, Z. An unusual Ni–Sb–Ag–Au association of ullmannite, allargentum, Au-rich silver and Au-bearing dyscrasite from Oselské pásmo “silver” Lode of Kutná Hora Pb–Zn–Ag ore district (Czech Republic). J. Geosci. 2017, 62, 247–252. [Google Scholar] [CrossRef]
- Scheffer, C.; Tarantola, A.; Vanderhaeghe, O.; Voudouris, P.; Rigaudier, T.; Photiades, A.; Morin, D.; Alloucherie, A. The Lavrion Pb-Zn-Fe-Cu-Ag detachment-related district (Attica, Greece): Structural control on hydrothermal flow and element transfer-deposition. Tectonophysics 2017, 717, 607–627. [Google Scholar] [CrossRef]
- Plotinskaya, O.Y.; Rusinov, V.L.; Seltmann, R. Oscillatory zoning fahlores from Au–Ag epithermal deposits. Goldschmidt Conf. Abstr. 2007, A796, 910–931. [Google Scholar]
- Deditius, A.P.; Utsunomiya, S.; Renock, D.; Ewing, R.C.; Ramana, C.V.; Becker, U.; Kesler, S.E. A proposed new form of arsenian pyrite: Composition, nanostructure and geochemical significance. Geochim. Cosmochim. Acta 2008, 72, 2919–2933. [Google Scholar] [CrossRef]
- Grabezhev, A.; Voudouris, P. Rhenium distribution in molybdenite from Vosnesensk porphyry Cu ± (Mo-Au) deposit (southern Urals, Russia). Can. Mineral. 2014, 52, 671–686. [Google Scholar] [CrossRef]
- Fanlo, I.; Subías, I.; Gervilla, F.; Paniagua, A.; García, B. The composition of Co–Ni–Fe sulfarsenides, diarsenides and triarsenides from the San Juan de Plan deposit, central Pyrenees, Spain. Can. Mineral. 2004, 42, 1221–1240. [Google Scholar] [CrossRef]
- Kiefer, S.; Majzlan, J.; Chovan, M.; Števko, M. Mineral compositions and phase relations of the complex sulfarsenides and arsenides from Dobšiná (Western Carpathians, Slovakia). Ore Geol. Rev. 2017, 89, 894–908. [Google Scholar] [CrossRef]
- Radosavljević, S.A.; Stojanović, J.N.; Vuković, N.S.; Radosavljević-Mihajlović, A.S.; Kašić, V.D. Low-temperature Ni-As-Sb-S mineralization of the Pb(Ag)-Zn deposits within the Rogozna ore field, Serbo-Macedonian Metallogenic Province: Ore mineralogy, crystal chemistry and paragenetic relationships. Ore Geol. Rev. 2015, 65, 213–227. [Google Scholar] [CrossRef]
- Shore, M.; Fowler, A.D. Oscillatory zoning in minerals: A common phenomenon. Can. Mineral. 1996, 34, 1111–1126. [Google Scholar]
- Kretschmar, U.; Scott, S.D. Phase relations involving arsenopyrite in the system Fe–As–S and their application. Can. Mineral. 1976, 14, 364–386. [Google Scholar]
- Sharp, Z.D.; Essene, E.J.; Kelly, W.C. A re-examination of the arsenopyrite geothermometer: Pressure considerations and applications to natural assemblages. Can. Mineral. 1985, 23, 517–534. [Google Scholar]
- Scott, S.D.; Barnes, H.L. Sphalerite geothermometry and geobarometry. Econ. Geol. 1971, 66, 653–669. [Google Scholar] [CrossRef]
- Barton, P.B.J.; Skinner, B.J. Sulfide mineral stabilities. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley Interscience: New York, NY, USA, 1979; pp. 278–403. [Google Scholar]
- Rieck, B. Seltene Arsenate aus der Kamariza und weitere Neufunde aus Lavrion. Lapis 1999, 24, 68–76. [Google Scholar]



| Sample | S8 | IL 1 | S6 |
|---|---|---|---|
| SiO2 | 69.29 | 69.61 | 69.41 |
| TiO2 | 0.29 | 0.28 | 0.30 |
| Al2O3 | 14.87 | 14.62 | 15.01 |
| Fe2O3 | 2.29 | 2.15 | 1.86 |
| MnO | 0.02 | 0.03 | 0.02 |
| MgO | 0.81 | 0.70 | 0.76 |
| CaO | 3.18 | 2.87 | 2.98 |
| Na2O | 3.65 | 3.52 | 3.44 |
| K2O | 3.00 | 3.31 | 2.87 |
| P2O5 | 0.09 | 0.09 | 0.10 |
| H2O | 1.41 | 1.25 | 1.89 |
| SO3 | 0.04 | 0.08 | 0.08 |
| Total | 98.94 | 98.51 | 98.72 |
| Ba | 917 | 913 | 895 |
| Ce | 50 | 56 | 67 |
| Cu | 2 | 3 | 6 |
| La | 25 | 25 | 37 |
| Nd | 24 | 29 | 29 |
| Pb | 12 | 17 | 24 |
| Rb | 85 | 85 | 94 |
| Sr | 442 | 413 | 419 |
| Zn | 12 | 12 | 166 |
| Zr | 156 | 162 | 157 |
| Mo | Cu | Au | Ag | Pb | Zn | As | Sb | Ni | Co | Te | Sn | Bi | Cd | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CLM-1 | 0.26 | 5869 | 0.04 | >100 | >10,000 | 536 | >10,000 | >2000 | 3.1 | 14.7 | 1.63 | 28.4 | 93.9 | 12.1 |
| CLM-2 | 0.25 | 3485 | 0.10 | >100 | >10,000 | 1129 | >10,000 | >2000 | 11.6 | 42.7 | 1.48 | 27 | 85.2 | 9.35 |
| CLM-3 | 0.11 | 323 | 0.04 | >100 | >10,000 | >10,000 | 4952 | 1554 | 1.1 | 83.9 | 1.51 | 25.1 | 12.4 | 304 |
| CLM-4 | 1.90 | 45.1 | bd | 7.97 | 1441 | 324 | 1338 | 62 | 8.9 | 92.2 | 0.03 | 0.9 | 0.74 | 3.87 |
| CLM-6 | 0.31 | 2503 | >100 | >100 | >10,000 | 326 | >10,000 | >2000 | 9686 | 118 | 1.26 | 16.4 | >2000 | 14.6 |
| CLM-6B | 0.26 | >10,000 | 3.80 | >100 | >10,000 | >10,000 | 7983 | >2000 | 388 | 80.4 | 1.34 | >100 | 133 | 171 |
| Km3-1 | 4.31 | 933 | 0.08 | >100 | >10,000 | 4195 | >10,000 | >2000 | >10,000 | 207 | 1.73 | 8.3 | 1.29 | 15.5 |
| Km3-2 | 35.8 | 45.2 | 0.02 | >100 | >10,000 | 1591 | >10,000 | >2000 | >10,000 | 175 | 2.10 | 5.7 | 0.81 | 4.09 |
| Km3-3 | 11.7 | 88.9 | 0.08 | >100 | >10,000 | 864 | >10,000 | >2000 | >10,000 | 231 | 2.25 | 8.1 | 1.18 | 5.06 |
| S-1 | 5.62 | 175 | 0.02 | 0.07 | 10.7 | 145 | 431 | 1.21 | 0.4 | 20.6 | 1.76 | 3.1 | 4.38 | 0.43 |
| S-3 | 0.19 | 29.8 | 0.03 | 0.04 | 15 | 79.6 | 410 | 0.72 | 1.3 | 21.3 | 0.24 | 0.3 | 0.26 | 0.13 |
| S-5 | 0.49 | 15.4 | 0.03 | 1.22 | 831 | 26.2 | 512 | 14.4 | 219 | 41.7 | 0.08 | 0.4 | 0.14 | 0.19 |
| S-8b | 2.82 | 18.8 | 0.01 | 0.03 | 110 | 69.3 | 8.8 | 12.8 | 0.8 | 68.5 | <0.02 | 0.7 | 0.17 | 0.41 |
| S-9 | 0.88 | 30.8 | 0.03 | 0.09 | 38.4 | 136 | 15.2 | 0.76 | 6.2 | 51.9 | <0.02 | 0.4 | 0.11 | 0.19 |
| S-11 | 5.37 | 74.2 | 0.05 | 0.25 | 32.8 | 166 | 121 | 0.77 | 2.9 | 15.7 | 0.14 | 3.7 | 0.87 | 0.18 |
| wt.% | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ni | 24.12 | 26.81 | 32.04 | 28.67 | 25.00 | 27.59 | 25.04 | 25.22 | 34.55 | 32.65 | 46.19 | 45.93 | 63.18 | 62.48 | 56.42 | 56.67 | 28.00 | 27.71 |
| Fe | 9.10 | 7.59 | 2.23 | 5.70 | 8.06 | 4.46 | 5.54 | 5.40 | 0.49 | 1.63 | 0.37 | 0.05 | 0.54 | 0.20 | 0.16 | 0.38 | 0.02 | 0.11 |
| Co | bd | bd | bd | bd | bd | bd | bd | bd | 0.13 | bd | 0.18 | 0.35 | 0.06 | 0.26 | bd | bd | bd | bd |
| Au | 0.12 | bd | 0.07 | 0.08 | 0.06 | 0.25 | 0.29 | 0.29 | na | na | na | na | na | na | na | na | na | na |
| As | 50.12 | 47.55 | 46.26 | 47.84 | 51.61 | 54.07 | 58.96 | 59.95 | 46.99 | 47.55 | 0.97 | 0.09 | 0.45 | 0.16 | bd | bd | 8.41 | 8.88 |
| Sb | 0.08 | bd | 0.78 | 0.10 | bd | 0.13 | 0.04 | 0.02 | na | na | na | na | na | na | bd | bd | 47.93 | 46.72 |
| Bi | bd | bd | 0.18 | bd | bd | 0.05 | bd | bd | na | na | na | na | na | na | na | na | na | na |
| S | 16.48 | 18.40 | 18.46 | 18.06 | 15.66 | 13.22 | 10.21 | 9.51 | 18.71 | 19.21 | 52.65 | 53.60 | 36.38 | 36.32 | 43.47 | 42.58 | 15.24 | 15.15 |
| Se | 0.16 | 0.17 | 0.19 | 0.15 | 0.14 | 0.20 | 0.20 | 0.18 | na | na | na | na | na | na | na | na | na | na |
| Total | 100.18 | 100.54 | 100.26 | 100.64 | 100.59 | 100.01 | 100.34 | 100.60 | 100.88 | 100.82 | 100.36 | 100.02 | 100.61 | 99.42 | 99.97 | 99.40 | 101.11 | 98.84 |
| apfu | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 2 | 2 | 7 | 7 | 3 | 3 |
| Ni | 0.700 | 0.759 | 0.915 | 0.816 | 0.730 | 0.835 | 0.782 | 0.792 | 0.975 | 0.918 | 0.962 | 0.955 | 0.967 | 0.963 | 2.902 | 2.943 | 0.977 | 0.977 |
| Fe | 0.278 | 0.226 | 0.067 | 0.170 | 0.247 | 0.142 | 0.182 | 0.178 | 0.016 | 0.048 | 0.008 | 0.001 | 0.008 | 0.003 | 0.009 | 0.021 | 0.000 | 0.000 |
| Co | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | Bd | 0.000 | 0.003 | 0.000 | 0.003 | 0.002 | 0.001 | 0.004 | 0.000 | 0.000 | 0.000 | 0.000 |
| Au | 0.001 | 0.000 | 0.001 | 0.001 | 0.001 | 0.002 | 0.003 | 0.003 | - | - | - | - | - | - | - | - | - | - |
| As | 1.140 | 1.056 | 1.035 | 1.067 | 1.181 | 1.282 | 1.443 | 1.475 | 1.039 | 1.048 | 0.016 | 0.002 | 0.006 | 0.001 | 0.000 | 0.000 | 0.230 | 0.245 |
| Sb | 0.001 | 0.000 | 0.011 | 0.001 | 0.000 | 0.002 | 0.001 | 0.000 | - | - | - | - | - | - | 0.000 | 0.000 | 0.806 | 0.796 |
| Bi | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | - | - | - | - | - | - | - | - | - | - |
| S | 0.876 | 0.955 | 0.965 | 0.941 | 0.837 | 0.732 | 0.584 | 0.547 | 0.967 | 0.989 | 2.009 | 2.037 | 1.018 | 1.027 | 4.094 | 4.048 | 0.973 | 0.996 |
| Se | 0.003 | 0.004 | 0.004 | 0.003 | 0.003 | 0.004 | 0.005 | 0.004 | - | - | - | - | - | - | - | - | - | - |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voudouris, P.; Mavrogonatos, C.; Rieck, B.; Kolitsch, U.; Spry, P.G.; Scheffer, C.; Tarantola, A.; Vanderhaeghe, O.; Galanos, E.; Melfos, V.; et al. The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece. Minerals 2018, 8, 531. https://doi.org/10.3390/min8110531
Voudouris P, Mavrogonatos C, Rieck B, Kolitsch U, Spry PG, Scheffer C, Tarantola A, Vanderhaeghe O, Galanos E, Melfos V, et al. The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece. Minerals. 2018; 8(11):531. https://doi.org/10.3390/min8110531
Chicago/Turabian StyleVoudouris, Panagiotis, Constantinos Mavrogonatos, Branko Rieck, Uwe Kolitsch, Paul G. Spry, Christophe Scheffer, Alexandre Tarantola, Olivier Vanderhaeghe, Emmanouil Galanos, Vasilios Melfos, and et al. 2018. "The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece" Minerals 8, no. 11: 531. https://doi.org/10.3390/min8110531
APA StyleVoudouris, P., Mavrogonatos, C., Rieck, B., Kolitsch, U., Spry, P. G., Scheffer, C., Tarantola, A., Vanderhaeghe, O., Galanos, E., Melfos, V., Zaimis, S., Soukis, K., & Photiades, A. (2018). The Gersdorffite-Bismuthinite-Native Gold Association and the Skarn-Porphyry Mineralization in the Kamariza Mining District, Lavrion, Greece. Minerals, 8(11), 531. https://doi.org/10.3390/min8110531









