Graphite and Diamond Formation in the Carbide–Oxide–Carbonate Interactions (Experimental Modeling under Mantle P,T-Conditions)
Abstract
1. Introduction
2. Materials and Methods
3. Results
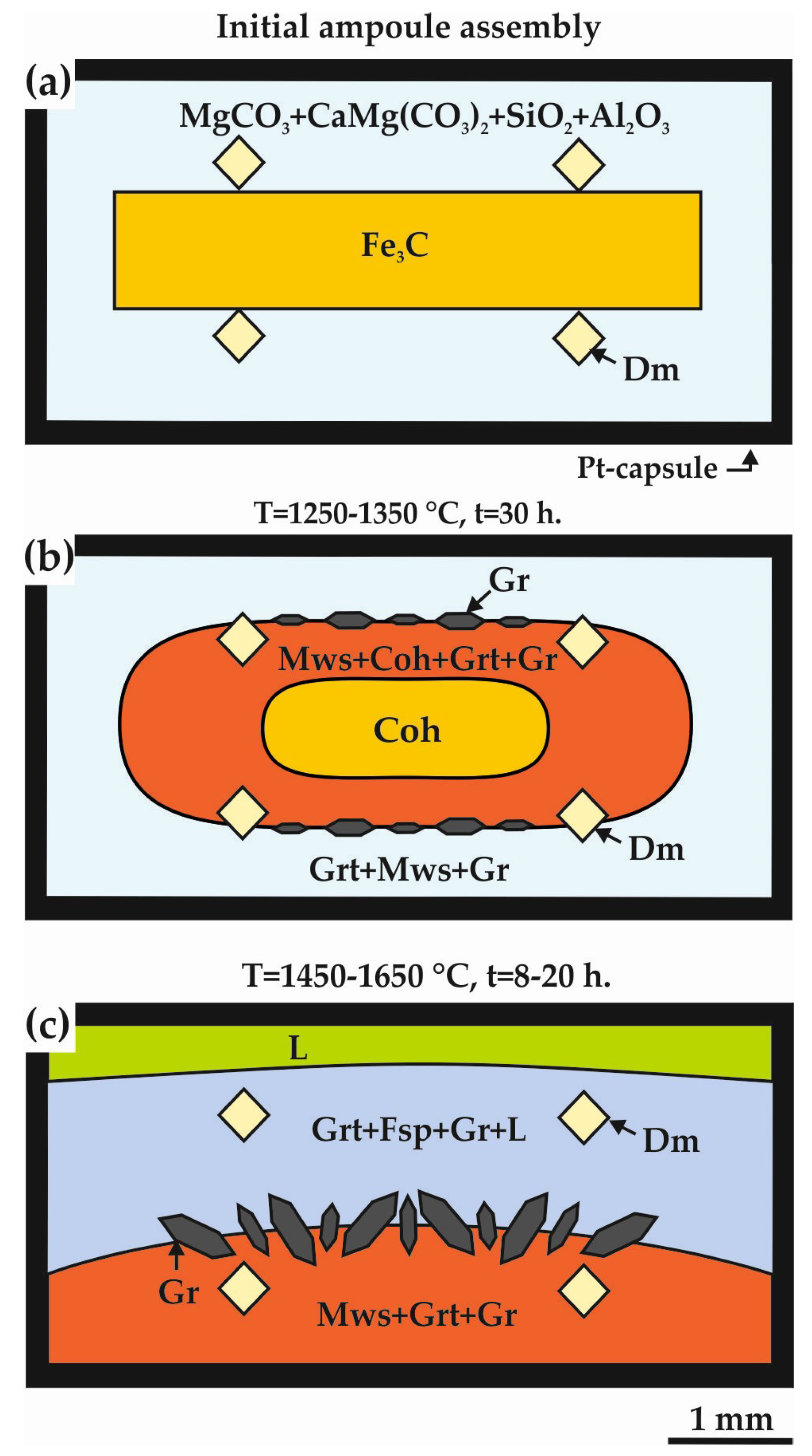
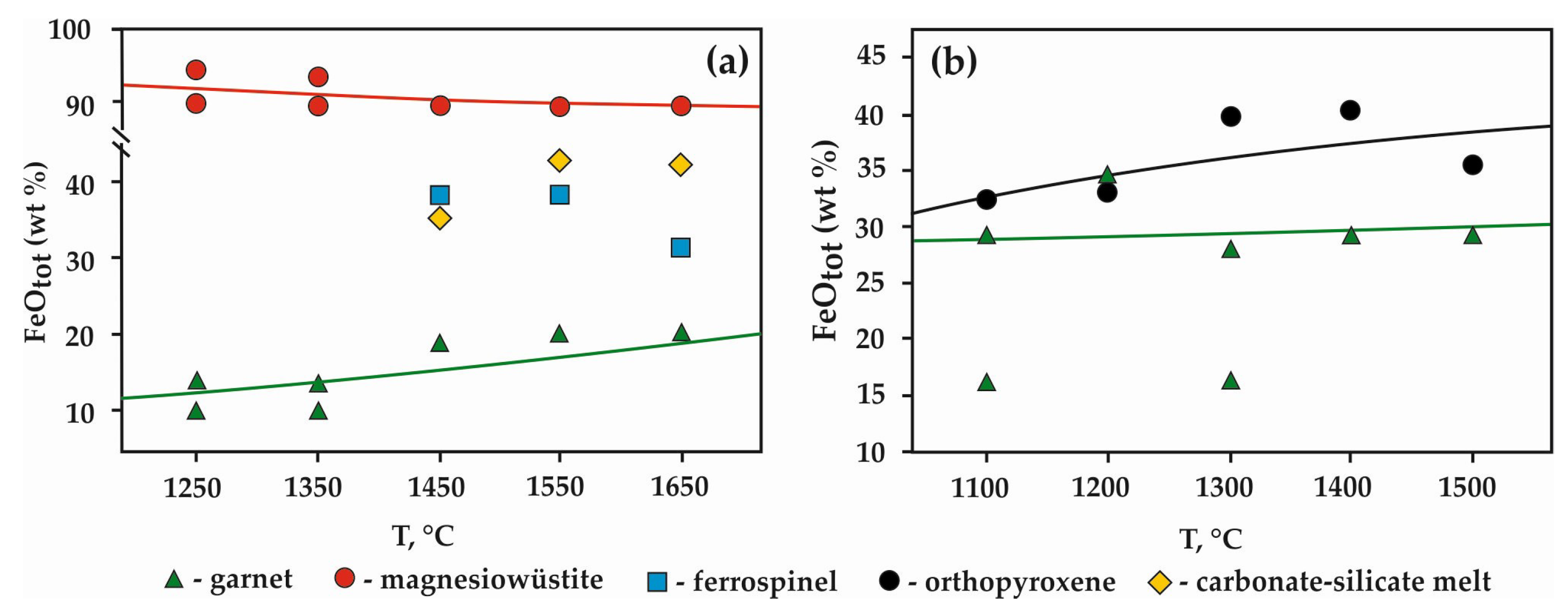
3.1. Experimental Results in the Carbide–Oxide–Carbonate System at the Pressure of 7.5 GPa (“Sandwich”-Type Experiments with ƒO2-Gradient)
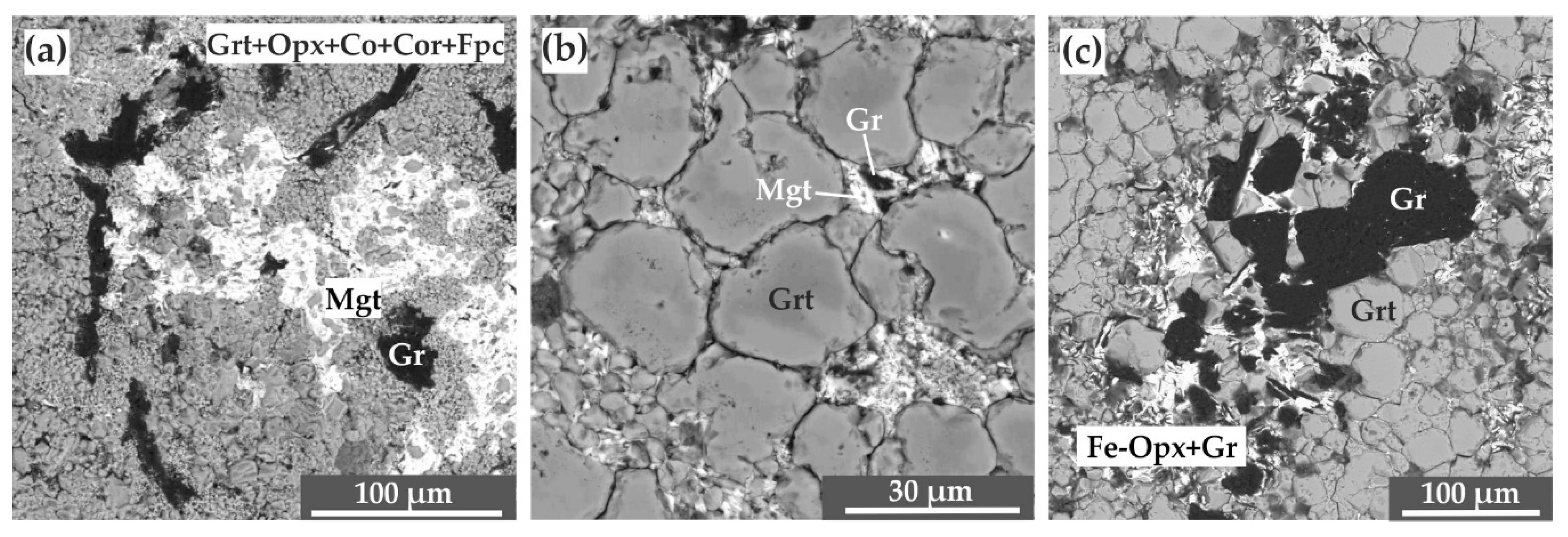
3.2. Experimental Results in the Carbide–Oxide–Carbonate System at the Pressure of 6.3 GPa (“Mixture”-Type of Experiments without ƒO2-Gradient)
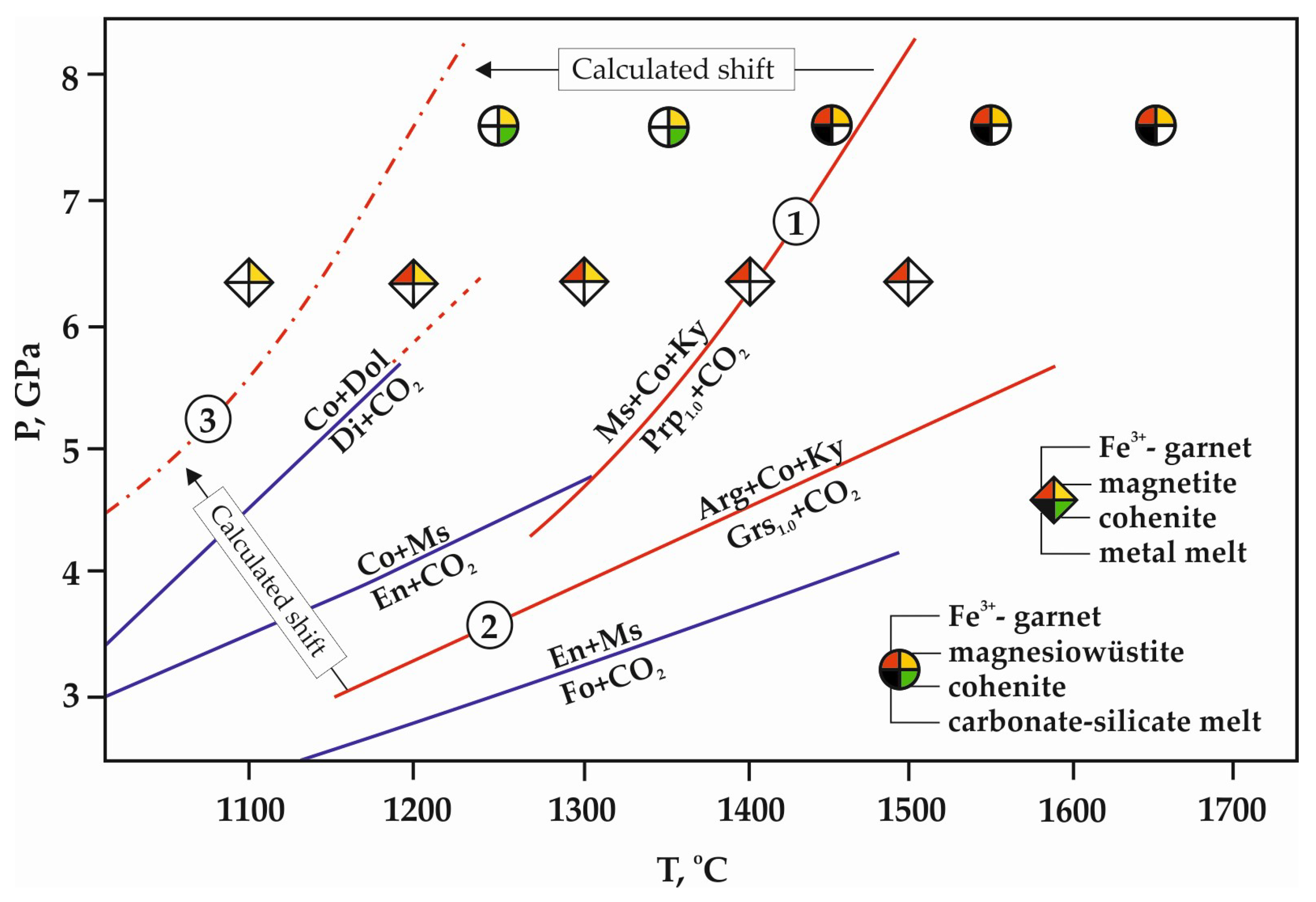
4. Discussion
4.1. General Reconstruction of Carbide–Oxide–Carbonate Interaction Processes
4.1.1. Carbide–Oxide–Carbonate System (“Sandwich”-Type Experiments, P = 7.5 GPa)
4.1.2. Carbide–Oxide–Carbonate System (“Mixture”-Type experiments)
4.2. Reconstruction of Elemental Carbon Formation Processes
Graphite Formation and Diamond Growth from Carbon of Carbide and Carbonate
4.3. Scenarios of the Cohenite–Fluid Interaction in the Presence of Silicates in Natural Deep Mantle Environments
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luth, R.W.; Virgo, D.; Boyd, F.R.; Wood, B.J. Ferric iron in mantle derived garnets: Implications for thermo-barometry and for the oxidation state of the mantle. Contrib. Mineral. Petrol. 1990, 104, 56–72. [Google Scholar] [CrossRef]
- Luth, R.W. Carbon and carbonates in mantle. In Mantle Petrology: Field Observation and High Pressure Experimentation: A Tribute to Francis R. (Joe) Boyd; Fei, Y., Bertka, M.C., Mysen, B.O., Eds.; The Geochemical Society: Washington, DC, USA, 1999; pp. 297–316. ISBN 0-941809-05-6. [Google Scholar]
- Frost, D.J.; Liebske, C.; Langenhorst, F.; McCammon, C.A. Experimental evidence for the existence of iron-rich metal in the Earth’s lower mantle. Nature 2004, 428, 409–412. [Google Scholar] [CrossRef] [PubMed]
- Frost, D.J.; McCammon, C.A. The redox state of Earth’s mantle. Annu. Rev. Earth Planet. Sci. 2008, 36, 389–420. [Google Scholar] [CrossRef]
- Woodland, A.B.; Koch, M. Variation in oxygen fugacity with depth in the upper mantle beneath the Kaapvaal craton, Southern Africa. Earth Planet. Sci. Lett. 2003, 214, 295–310. [Google Scholar] [CrossRef]
- Rohrbach, A.; Ballhaus, C.; Golla-Schindler, U.; Ulmer, P.; Kamenetsky, V.S.; Kuzmin, D.V. Metal saturation in the upper mantle. Nature 2007, 449, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Schmidt, M.W. Redox freezing and melting in the Earth’s deep mantle resulting from carbon–iron redox coupling. Nature 2007, 472, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Stagno, V.; Tange, Y.; Miyajima, N.; McCammon, C.A.; Irifune, T.; Frost, D.J. The stability of magnesite in the transition zone and the lower mantle as function of oxygen fugacity. Geophys. Res. Lett. 2011, 38, 1–5. [Google Scholar] [CrossRef]
- Dasgupta, R.; Hirschmann, M.M. The deep carbon cycle and melting in Earth’s interior. Earth Planet. Sci. Lett. 2010, 298, 1–13. [Google Scholar] [CrossRef]
- Marty, B. The origins and concentrations of water, carbon, nitrogen and noble gases on Earth. Earth Planet. Sci. Lett. 2012, 313–314, 56–66. [Google Scholar] [CrossRef]
- Garber, J.M.; Maurya, S.; Hernandez, J.A.; Duncan, M.S.; Zeng, L.; Zhang, H.L.; Faul, U.; McCammon, C.; Montagner, J.P.; Moresi, L.; et al. Multidisciplinary constraints on the abundance of diamond and eclogite in the cratonic lithosphere. Geochem. Geophys. Geosyst. 2018, 19, 2062–2086. [Google Scholar] [CrossRef]
- Lord, O.T.; Walter, M.J.; Dasgupta, R.; Walker, D.; Clark, S.M. Melting in the Fe–C system to 70 GPa. Earth Planet. Sci. Lett. 2009, 284, 157–167. [Google Scholar] [CrossRef]
- Sharp, W.E. Pyrrhotite, a common inclusion in South African diamonds. Nature 1966, 21, 402–403. [Google Scholar] [CrossRef]
- Torsvik, T.K.; Burke, K.; Steinberger, B.; Webb, S.J.; Ashwal, L.D. Diamonds sampled by plumes from the core–mantle boundary. Nature 2010, 466, 253–357. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, F.V.; Wirth, R. Iron carbide inclusions in lower-mantle diamond from Juina, Brazil. Can. Mineral. 2011, 49, 555–572. [Google Scholar] [CrossRef]
- Smith, E.M.; Kopylova, M.G. Implications of metallic iron for diamonds and nitrogen in the sublithospheric mantle. Can. J. Earth Sci. 2014, 51, 510–516. [Google Scholar] [CrossRef]
- Smith, E.M.; Shirey, S.B.; Nestola, F.; Bullock, E.S.; Wang, J.; Richardson, S.H.; Wang, W. Large gem diamonds from metallic liquid in Earth’s deep mantle. Science 2016, 354, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Palyanov, Y.N.; Bataleva, Y.V.; Sokol, A.G.; Borzdov, Y.M.; Kupriyanov, I.N.; Reutsky, V.N.; Sobolev, N.V. Mantle–slab interaction and redox mechanism of diamond formation. Proc. Natl. Acad. Sci. USA 2013, 110, 20408–20413. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.; Ghosh, S.; Schmidt, M.W.; Wijbrans, C.H.; Klemme, S. The stability of Fe-Ni carbides in the Earth’s mantle: Evidence for a low Fe-Ni-C melt fraction in the deep mantle. Earth Planet. Sci. Lett. 2014, 388, 211–221. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Zdrokov, E.V. Iron carbide as a source of carbon for graphite and diamond formation under lithospheric mantle P-T parameters. Lithos 2017, 286–287, 151–161. [Google Scholar] [CrossRef]
- Dasgupta, R. Ingassing, storage, and outgassing of terrestrial carbon through geologic time. Rev. Mineral. Geochem. 2013, 75, 183–229. [Google Scholar] [CrossRef]
- Harte, B.; Richardson, S.H. Mineral inclusions in diamonds track the evolution of a Mesozoic subducted slab beneath West Gondwanaland. Gondwana Res. 2012, 21, 236–245. [Google Scholar] [CrossRef]
- Shirey, S.B.; Cartigny, P.; Frost, D.G.; Keshav, S.; Nestola, F.; Nimis, P.; Pearson, D.G.; Sobolev, N.V.; Walter, M.J. Diamonds and the geology of mantle carbon. Rev. Mineral. Geochem. 2013, 75, 355–421. [Google Scholar] [CrossRef]
- Creighton, S.; Stachel, T.; Matveev, S.; Höfer, H.; McCammon, C.; Luth, R.W. Oxidation of the Kaapvaal lithospheric mantle driven by metasomatism. Contrib. Mineral. Petrol. 2009, 157, 491–504. [Google Scholar] [CrossRef]
- Kogarko, L.N. Alkaline magmatism and enriched mantle reservoirs: Mechanisms, time, and depth of formation. Geochem. Int. 2006, 44, 3–10. [Google Scholar] [CrossRef]
- Malaspina, N.; Scambelluri, M.; Poli, S.; van Roermund, H.L.M.; Langenhorst, F. The oxidation state of mantle wedge majoritic garnet websterites metasomatised by C-bearing subduction fluids. Earth Planet. Sci. Lett. 2010, 298, 417–426. [Google Scholar] [CrossRef]
- Ryabchikov, I.D. Mechanisms of diamond formation: Reduction of carbonates or partial oxidation of hydrocarbons. Dokl. Earth Sci. 2009, 429, 1346–1349. [Google Scholar] [CrossRef]
- Ryabchikov, I.D.; Kogarko, L.N. Redox potential of mantle magmatic systems. Petrology 2010, 18, 239–251. [Google Scholar] [CrossRef]
- Jacob, D.E.; Kronz, A.; Viljoen, K.S. Cohenite, native iron and troilite inclusions in garnets from polycrystalline diamonds aggregates. Contrib. Mineral. Petrol. 2004, 146, 566–576. [Google Scholar] [CrossRef]
- Sobolev, N.V.; Efimova, E.S.; Pospelova, L.N. Native iron in diamonds of Yakutia and its paragenesis. Sov. Geol. Geophys. 1981, 22, 18–21. [Google Scholar]
- Stachel, T.; Harris, J.W.; Brey, G.P. Rare and unusual mineral inclusions in diamonds from Mwadui, Tanzania: Contrib. Mineral. Petrol. 1998, 132, 34–47. [Google Scholar] [CrossRef]
- Boulard, E.; Gloter, A.; Corgne, A.; Antonangeli, D.; Auzende, A.-L.; Perrillat, J.-P.; Guyot, F.; Fiquet, G. New host for carbon in the deep Earth. Proc. Natl. Acad. Sci. USA 2011, 10, 5184–5187. [Google Scholar] [CrossRef] [PubMed]
- Haggerty, S.E. Upper mantle mineralogy. J. Geodyn. 1995, 20, 331–364. [Google Scholar] [CrossRef]
- Harris, J.W. Diamond geology. In Properties of Natural and Synthetic Diamond; Field, J.E., Ed.; Academic Press: London, UK, 1992; pp. 345–393. ISBN 9780122553523. [Google Scholar]
- Sobolev, N.V. Deep-Seated Inclusions in Kimberlites and the Problem of the Composition of the Upper Mantle, 1st ed.; Nauka: Novosibirsk, Russia, 1974; 264p. (In Russian) [Google Scholar]
- Schrauder, M.; Navon, O. Solid carbon dioxide in natural diamond. Nature 1993, 365, 42–44. [Google Scholar] [CrossRef]
- Wang, A.; Pasteris, J.D.; Meyer, H.O.A.; DeleDuboi, M.L. Magnesite-bearing inclusion assemblage in natural diamond. Earth Planet. Sci. Lett. 1996, 141, 293–306. [Google Scholar] [CrossRef]
- Burnham, A.D.; Thomson, A.R.; Bulanova, G.P.; Kohn, S.C.; Smith, C.B.; Walter, M.J. Stable isotope evidence for crustal recycling as recorded by superdeep diamonds. Earth Planet. Sci. Lett. 2015, 432, 374–380. [Google Scholar] [CrossRef]
- Dasgupta, R.; Buono, A.; Whelan, G.; Walker, D. High-pressure melting relations in Fe–C-S systems: Implications for formation, evolution, and structure of metallic cores in planetary bodies: Geochim. Cosmochim. Acta 2009, 73, 6678–6691. [Google Scholar] [CrossRef]
- Deng, L.; Fei, Y.; Liu, X.; Gong, Z.; Shahar, A. Effect of carbon, sulfur and silicon on iron melting at high pressure: Implications for composition and evolution of the planetary terrestrial cores. Geochim. Cosmochim. Acta 2013, 114, 220–233. [Google Scholar] [CrossRef]
- Tsuno, K.; Dasgupta, R. Fe-Ni-Cu-C-S phase relations at high pressures and temperatures—The role of sulfur in carbon storage and diamond stability at mid-to deep-upper mantle. Earth Planet. Sci. Lett. 2015, 412, 132–142. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Sobolev, N.V. Interaction of iron carbide and sulfur under P-T conditions of the lithospheric mantle. Dokl. Earth Sci. 2015, 463, 707–711. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Bayukov, O.A.; Sobolev, N.V. Conditions for diamond and graphite formation from iron carbide at the P-T parameters of lithospheric mantle. Russ. Geol. Geophys. 2016, 57, 176–189. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Khokhryakov, A.F.; Kupriyanov, I.N.; Sokol, A.G. Effect of nitrogen impurity on diamond crystal growth processes. Cryst. Growth Des. 2010, 10, 3169–3175. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F. Fluid-bearing alkaline carbonate melts as the medium for the formation of diamonds in the Earth’s mantle: An experimental study. Lithos 2002, 60, 145–159. [Google Scholar] [CrossRef]
- Sokol, A.G.; Borzdov, Y.M.; Palyanov, Y.N.; Khokhryakov, A.F. High-temperature calibration of a multi-anvil high pressure apparatus. High Press. Res. 2015, 35, 139–147. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Borzdov, Y.M.; Bataleva, Y.V.; Sokol, A.G.; Palyanova, G.A.; Kupriyanov, I.N. Reducing role of sulfides and diamond formation in the Earth’s mantle: Earth Planet. Sci. Lett. 2007, 260, 242–256. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. The role of rocks saturated with metallic iron in the formation of ferric carbonate–silicate melts: Experimental modeling under PT-conditions of lithospheric mantle. Russ. Geol. Geophys. 2015, 56, 143–154. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Borzdov, Y.M.; Kupriyanov, I.N.; Sokol, A.G. Synthesis of diamonds with mineral, fluid and melt inclusions. Lithos 2016, 265, 292–303. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Palyanova, G.A. Conditions for the origin of oxidized carbonate-silicate melts: Implications for mantle metasomatism and diamond formation. Lithos 2012, 128–131, 113–125. [Google Scholar] [CrossRef]
- Bataleva, Y.V.; Palyanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Bayukov, O.A. Wüstite stability in the presence of a CO2-fluid and a carbonate-silicate melt: Implications for the graphite/diamond formation and generation of Fe-rich mantle metasomatic agents. Lithos 2016, 244, 20–29. [Google Scholar] [CrossRef]
- Newton, R.C.; Sharp, W.E. Stability of forsterite + CO2 and its bearing on the role of CO2 in the mantle. Earth Planet. Sci. Lett. 1975, 26, 239–244. [Google Scholar] [CrossRef]
- Knoche, R.; Sweeney, R.J.; Luth, R.W. Carbonation and decarbonation of eclogites: The role of garnet. Contrib. Mineral. Petrol. 1999, 135, 332–339. [Google Scholar] [CrossRef]
- Berman, R.G. Thermobarometry using multi-equilibrium calculations: A new technique with petrologic applications. Can. Mineral. 1991, 29, 833–855. [Google Scholar]
- Pal’yanov, Y.N.; Sokol, A.G.; Borzdov, Y.M.; Khokhryakov, A.F.; Sobolev, N.V. Diamond formation through carbonate-silicate interaction. Am. Mineral. 2002, 87, 1009–1013. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Tomilenko, A.A.; Sobolev, N.V. Conditions of diamond formation through carbonate-silicate interaction. Eur. J. Mineral. 2005, 17, 207–214. [Google Scholar] [CrossRef]
- Navon, O.; Hutcheon, I.D.; Rossman, G.R.; Wasserburg, G.J. Mantle-derived fluids in diamond micro-inclusions. Nature 1988, 335, 784–789. [Google Scholar] [CrossRef]
- Kopylova, M.; Navon, O.; Dubrovinsky, L.; Khachatryan, G. Carbonatitic mineralogy of natural diamond-forming fluids. Earth Planet. Sci. Lett. 2010, 291, 126–137. [Google Scholar] [CrossRef]
- Oganov, A.R.; Hemley, R.J.; Hazen, R.M.; Jones, A.P. Structure, bonding and mineralogy of carbon at extreme conditions. Rev. Mineral. Geochem. 2013, 75, 47–77. [Google Scholar] [CrossRef]
- Akaishi, M.; Kumar, M.S.D.; Kanda, H.; Yamaoka, S. Formation process of diamond from supercritical H2O-CO2 fluid under high pressure and high temperature conditions: Diam. Relat. Mater. 2000, 9, 1945–1950. [Google Scholar] [CrossRef]
- Pal’yanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Pal’yanova, G.A.; Borzdov, Y.M.; Sobolev, N.V. Diamond and graphite crystallization in COH fluid at PT parameters of the natural diamond formation. Dokl. Earth Sci. 2000, 375, 1395–1398. [Google Scholar]
- Yamaoka, S.; Akaishi, M.; Kanda, H.; Osawa, T. Crystal growth of diamond in the system of carbon and water under very high pressure and temperature. J. Cryst. Growth 1992, 125, 375–377. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Sokol, A.G.; Khokhryakov, A.F.; Kruk, A.N. Conditions of diamond crystallization in kimberlite melt: Experimental data. Russ. Geol. Geophys. 2015, 56, 196–210. [Google Scholar] [CrossRef]
- Palyanov, Y.N.; Shatsky, V.S.; Sokol, A.G.; Tomilenko, A.A.; Sobolev, N.V. Crystallization of metamorphic diamond: An experimental modeling. Dokl. Earth Sci. 2001, 381, 935–938. [Google Scholar]
- Palyanov, Y.N.; Khokhryakov, A.F.; Borzdov, Y.M.; Kupriyanov, I.N. Diamond growth and morphology under the influence of impurity adsorption. Cryst. Growth Des. 2013, 13, 5411–5419. [Google Scholar] [CrossRef]
- Brenker, F.E.; Vollmer, C.; Vincze, L.; Vekemans, B.; Szymanski, A.; Janssens, K.; Szaloki, I.; Nasdala, L.; Joswig, W.; Kaminsky, F. Carbonates from the lower part of transition zone or even the lower mantle. Earth Planet. Sci. Lett. 2007, 260, 1–9. [Google Scholar] [CrossRef]
- Martin, A.M.; Hammouda, T. Role of iron and reducing conditions on the stability of dolomite + coesite between 4.25 and 6 GPa—A potential mechanism for diamond formation during subduction. Eur. J. Mineral. 2011, 23, 5–16. [Google Scholar] [CrossRef]



| Experimental Series | P, GPa | Starting Materials, mg | ||||
|---|---|---|---|---|---|---|
| Fe3C | SiO2 | Al2O3 | MgCO3 | CaMg(CO3)2 | ||
| “Mixture-type” | 6.3 | 24.6 | 29.5 | 16.8 | 11.0 | 2.8 |
| “Sandwich-type” | 7.5 | 72.2 | 33.7 | 18.9 | 37.8 | 9.5 |
| Experimental Series | P, GPa | Mass Concentrations, wt % | Molar Concentrations, mol % | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Si | Al | Fe | Mg | Ca | C | O | Si | Al | Fe | Mg | Ca | C | O | ||
| “Mixture-type” | 6.3 | 15.6 | 10.1 | 26.1 | 4.5 | 1.5 | 4.6 | 37.6 | 12.8 | 8.6 | 10.7 | 4.3 | 0.9 | 8.7 | 54.0 |
| “Sandwich-type” | 7.5 | 8.6 | 5.5 | 36.7 | 7.4 | 2.5 | 7.0 | 32.3 | 7.4 | 4.9 | 15.8 | 7.4 | 1.5 | 14.2 | 48.8 |
| Run N | P, GPa | T, °C | t, h | Final Phases | Diamond Growth on Seeds |
|---|---|---|---|---|---|
| ”Mixture-type” experiments | |||||
| CG-01 | 6.3 | 1100 | 40 | Grt, Opx, Mgt, Cor, Co, Fpc, Gr | - |
| CG-02 | 6.3 | 1200 | 30 | Grt, Opx, Mgt, Cor, Co, Fpc, Gr | - |
| CG-03 | 6.3 | 1300 | 20 | Grt, Opx, Gr, Mgt | - |
| CG-04 | 6.3 | 1400 | 20 | Grt, Opx, Gr | - |
| CG-05 | 6.3 | 1500 | 20 | Grt, Opx, Gr | + |
| ”Sandwich-type” experiments | |||||
| ST-01 | 7.5 | 1250 | 30 | Coh, Grt, Mws, Gr | + |
| ST-02 | 7.5 | 1350 | 20 | Coh, Grt, Mws, Gr | + |
| ST-03 | 7.5 | 1450 | 20 | Grt, Mws, Fsp, Gr, Lcarb-sil | + |
| ST-04 | 7.5 | 1550 | 20 | Grt, Mws, Fsp, Gr, Lcarb-sil | + |
| ST-05 | 7.5 | 1650 | 8 | Grt, Mws, Fsp, Gr, Lcarb-sil | + |
| Run N | T, °C | Phase | NA | Mass Concentrations, wt % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | FeO * | Fe2O3 * | MgO | CaO | Total | ||||
| ST-01 | 1250 | Grt P | 14 | 41.6 (7) | 23.1 (5) | 8.9 (8) | 9 | - | 16.9 (5) | 9.5 (4) | 100.0 |
| Mws P | 16 | - | - | 89.7 (7) | 84 | 6 | 9.5 (8) | 0.33 (6) | 99.6 | ||
| Grt C | 15 | 40.9 (3) | 21.8 (1) | 13.4 (3) | 13 | - | 15.2 (3) | 8.5 (4) | 99.8 | ||
| Mws C | 14 | - | - | 94.7 (3) | 95 | - | 4.6 (2) | 0.03 (1) | 99.4 | ||
| ST-02 | 1350 | Grt P | 17 | 42.3 (3) | 23.1 (3) | 10.3 (6) | 23 | - | 20.7 (7) | 3.3 (7) | 99.8 |
| Mws P | 11 | - | - | 90.7 (5) | 84 | 6 | 8.8 (5) | 0.03 (1) | 99.5 | ||
| Grt C | 14 | 41.0 (1) | 21.7 (1) | 13.2 (1) | 22 | - | 15.1 (2) | 8.6 (4) | 99.7 | ||
| Mws C | 16 | - | - | 93.4 (9) | 93 | - | 5.8 (9) | 0.02 (0) | 99.3 | ||
| ST-03 | 1450 | Grt | 17 | 40.2 (4) | 22.5 (1) | 18.2 (3) | 14 | 4 | 14.9 (6) | 3.57 (6) | 99.5 |
| Mws | 14 | - | - | 89.9 (2) | 90 | - | 9.41 (6) | 0.04 (1) | 99.3 | ||
| Fsp | 5 | 1.9 (1) | 47.9 (1) | 37.3 (2) | 30 | 7 | 12.0 (1) | - | 99.1 | ||
| Lcarb-sil | 30 | 11.0 (8) | 0.1 (1) | 36 (1) | 30 | 6 | 16 (1) | 7 (1) | 70.1 | ||
| ST-04 | 1550 | Grt | 20 | 40.4 (4) | 21.4 (4) | 20.3 (2) | 16 | 4 | 14.7 (2) | 2.5 (2) | 99.4 |
| Mws | 15 | - | - | 91.7 (4) | 92 | - | 7.88 (1) | 0.03 (1) | 99.7 | ||
| Fsp | 7 | 2.0 (1) | 48.5 (2) | 37.6 (3) | 31 | 7 | 11.9 (6) | - | 100.0 | ||
| Lcarb-sil | 31 | 12.8 (9) | 2.0 (4) | 43 (1) | 34 | 10 | 11.5 (9) | 5 (1) | 74.2 | ||
| ST-05 | 1650 | Grt | 21 | 40.2 (3) | 21.3 (4) | 20.7 (3) | 16 | 5 | 14.4 (2) | 3.0 (2) | 99.6 |
| Mws | 16 | - | - | 91.2 (7) | 91 | - | 8.2 (2) | 0.03 (1) | 99.4 | ||
| Fsp | 6 | 1.8 (1) | 53.0 (3) | 31.8 (3) | 27 | 5 | 13.9 (9) | - | 100.4 | ||
| Lcarb-sil | 30 | 15.0 (9) | 3.5 (7) | 42.7 (8) | 33 | 10 | 11.0 (8) | 4.1 (9) | 76.3 | ||
| Run N | P, GPa | T, °C | Sample | Phase | Iron Valence | A, % | Fe3+/∑Fe |
|---|---|---|---|---|---|---|---|
| ST-01 | 7.5 | 1250 | Central (reduced) part | Coh | Fe0 | 18 | - |
| Mws | Fe2+ | 76 | - | ||||
| Grt | Fe2+ (8) | 6 | - | ||||
| Peripheral (oxidized) part | Mws | Fe2+ | 74 | - | |||
| Fe3+ (4) | 6 | 0.075 | |||||
| Grt | Fe2+ (8) | 20 | - | ||||
| ST-04 | 7.5 | 1550 | Quenched carbonate–silicate melt | Carb * | Fe2+ (6) | 62 | 0.18 (in melt, bulk) |
| Mgt * | Fe3+ (4) | 18 | |||||
| Fe2+ (8) | 5 | ||||||
| Opx * | Fe2+ (6) | 12 | |||||
| Grt * | Fe2+ (8) | 4 | |||||
| Garnet aggregate with interstitial melt | Grt | Fe2+ (8) | 55 | 0.23 | |||
| Fe3+ (6) | 16 | ||||||
| Carb * | Fe2+ (6) | 12 | 0.14 (in melt, bulk) | ||||
| Mgt * | Fe3+ (4) | 4 | |||||
| Fe2+ (8) | 2 | ||||||
| Opx * | Fe2+ (6) | 11 | |||||
| CG-03 | 6.3 | 1300 | Bulk sample | Grt | Fe2+ (8) | 49 | 0.17 |
| Fe3+ (6) | 10 | ||||||
| Opx | Fe2+ (6) | 41 | - | ||||
| CG-04 | 6.3 | 1400 | Bulk sample | Grt | Fe2+ (8) | 52 | 0.24 |
| Fe3+ (6) | 16 | ||||||
| Opx | Fe2+ (6) | 26 | - | ||||
| CG-05 | 6.3 | 1500 | Bulk sample | Grt | Fe2+ (8) | 57 | 0.24 |
| Fe3+ (6) | 18 | ||||||
| Opx | Fe2+ (6) | 26 | - |
| Run N | T, °C | Phase | NA | Mass Concentrations, wt % | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | FeO | FeO * | Fe2O3 * | MgO | CaO | Total | ||||
| CG-01 | 1100 | Grt | 14 | 40 (2) | 25 (3) | 17 (3) | 17 (3) | - | 14 (3) | 4 (2) | 99.7 |
| 9 | 38.5 (5) | 21.3 (8) | 30 (3) | 30 (3) | - | 6 (1) | 4 (2) | 100.1 | |||
| Opx | 12 | 50.7 (8) | - | 33 (1) | 33 (1) | - | 14.4 (8) | 0.7 (3) | 99.4 | ||
| Mgt | 5 | - | - | 91.1 (2) | 20 | 71 | 7.0 (2) | - | 98.1 | ||
| Fpc | 6 | - | - | 30.7 (9) | 31 | - | 68.4 (8) | - | 99.1 | ||
| CG-02 | 1200 | Grt | 10 | 38.3 (1) | 18.8 (8) | 33.9 (1) | 31 | 3 | 6.1 (9) | 2.5 (9) | 99.6 |
| Opx | 10 | 50.9 (6) | 0.4 (3) | 33 (1) | - | - | 14.5 (8) | 0.6 (2) | 99.7 | ||
| Mgt | 6 | - | - | 91.5 (2) | 20 | 71 | 7.5 (1) | - | 98.0 | ||
| CG-03 | 1300 | GrtC | 12 | 37.5 (1) | 22.5 (5) | 28.6 (8) | 23 | 5 | 8.9 (6) | 2.1 (1) | 99.5 |
| GrtR | 16 | 39.0 (5) | 25.8 (4) | 16 (2) | 15 (2) | ~1 | 14 (1) | 4.2 (7) | 99.5 | ||
| Opx | 1 | 49.4 (3) | - | 40.4 (5) | 40 | - | 10.2 (5) | - | 99.9 | ||
| Mgt | 6 | - | - | 90.8 (2) | 20 | 71 | 7.6 (2) | - | 98.4 | ||
| CG-04 | 1400 | Grt | 15 | 38.6 (4) | 21.1 (6) | 28.7 (5) | 22 | 7 | 9.4 (2) | 1.7 (4) | 99.5 |
| Opx | 7 | 49.2 (3) | - | 41.0 (7) | 41 | - | 9.2 (6) | - | 99.5 | ||
| CG-05 | 1500 | Grt | 15 | 39.3 (4) | 21.1 (3) | 28 (1) | 22 | 7 | 10.0 (6) | 1.7 (4) | 99.9 |
| Opx | 12 | 50 (1) | 0.4 (3) | 35.4 (7) | - | - | 5.6 (5) | 7.5 (10) | 99.5 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataleva, Y.; Palyanov, Y.; Borzdov, Y.; Novoselov, I.; Bayukov, O. Graphite and Diamond Formation in the Carbide–Oxide–Carbonate Interactions (Experimental Modeling under Mantle P,T-Conditions). Minerals 2018, 8, 522. https://doi.org/10.3390/min8110522
Bataleva Y, Palyanov Y, Borzdov Y, Novoselov I, Bayukov O. Graphite and Diamond Formation in the Carbide–Oxide–Carbonate Interactions (Experimental Modeling under Mantle P,T-Conditions). Minerals. 2018; 8(11):522. https://doi.org/10.3390/min8110522
Chicago/Turabian StyleBataleva, Yuliya, Yuri Palyanov, Yuri Borzdov, Ivan Novoselov, and Oleg Bayukov. 2018. "Graphite and Diamond Formation in the Carbide–Oxide–Carbonate Interactions (Experimental Modeling under Mantle P,T-Conditions)" Minerals 8, no. 11: 522. https://doi.org/10.3390/min8110522
APA StyleBataleva, Y., Palyanov, Y., Borzdov, Y., Novoselov, I., & Bayukov, O. (2018). Graphite and Diamond Formation in the Carbide–Oxide–Carbonate Interactions (Experimental Modeling under Mantle P,T-Conditions). Minerals, 8(11), 522. https://doi.org/10.3390/min8110522






