Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Minerals
2.1.2. Reagents
2.2. Experimental
2.2.1. Micro-Flotation Tests
2.2.2. Adsorption Studies
2.2.3. Zeta Potential Measurements
2.2.4. XPS Analysis
3. Results and Discussion
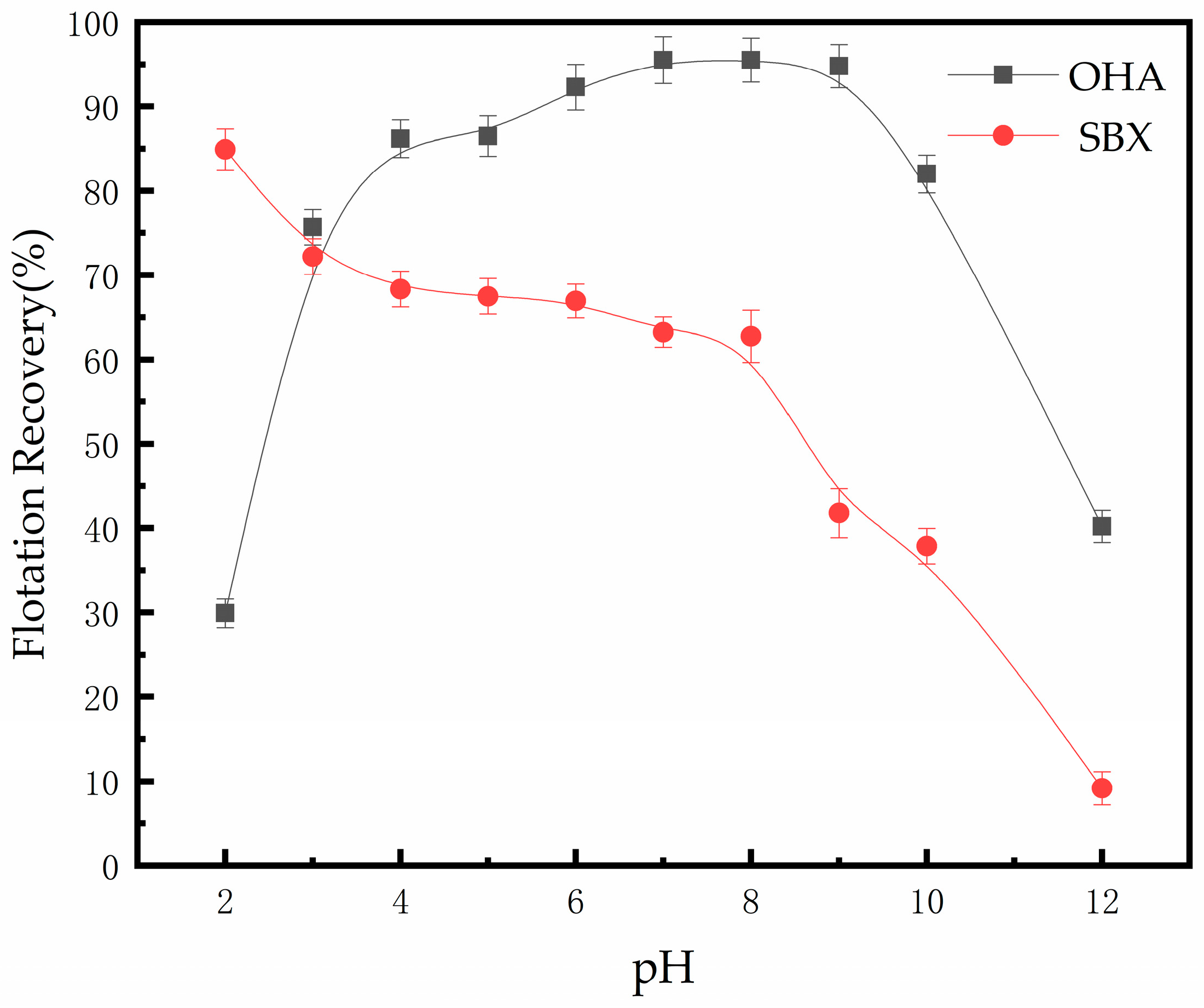
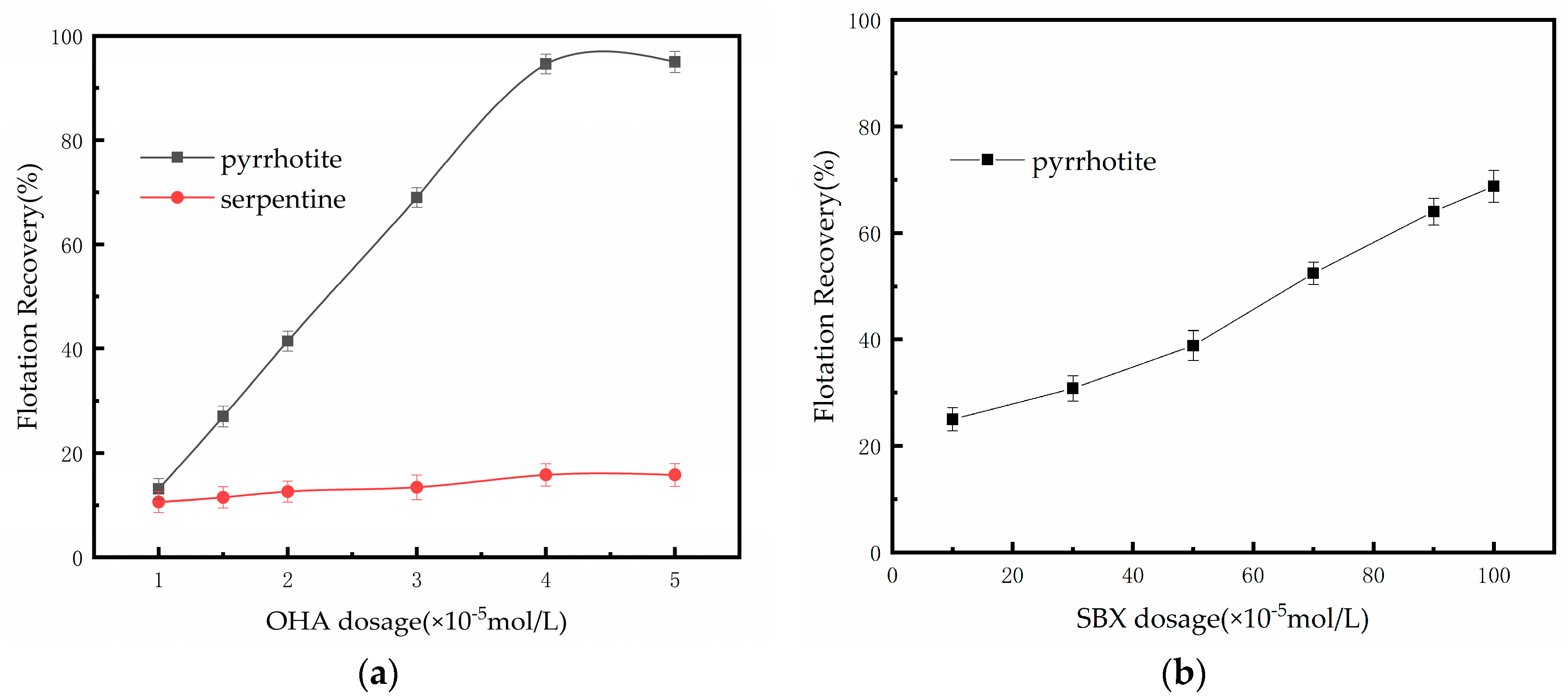
3.1. Flotation of Pyrrhotite and Serpentine with Two Collectors
3.2. Separation of Pyrrhotite from Pyrrhotite–Serpentine Mixture
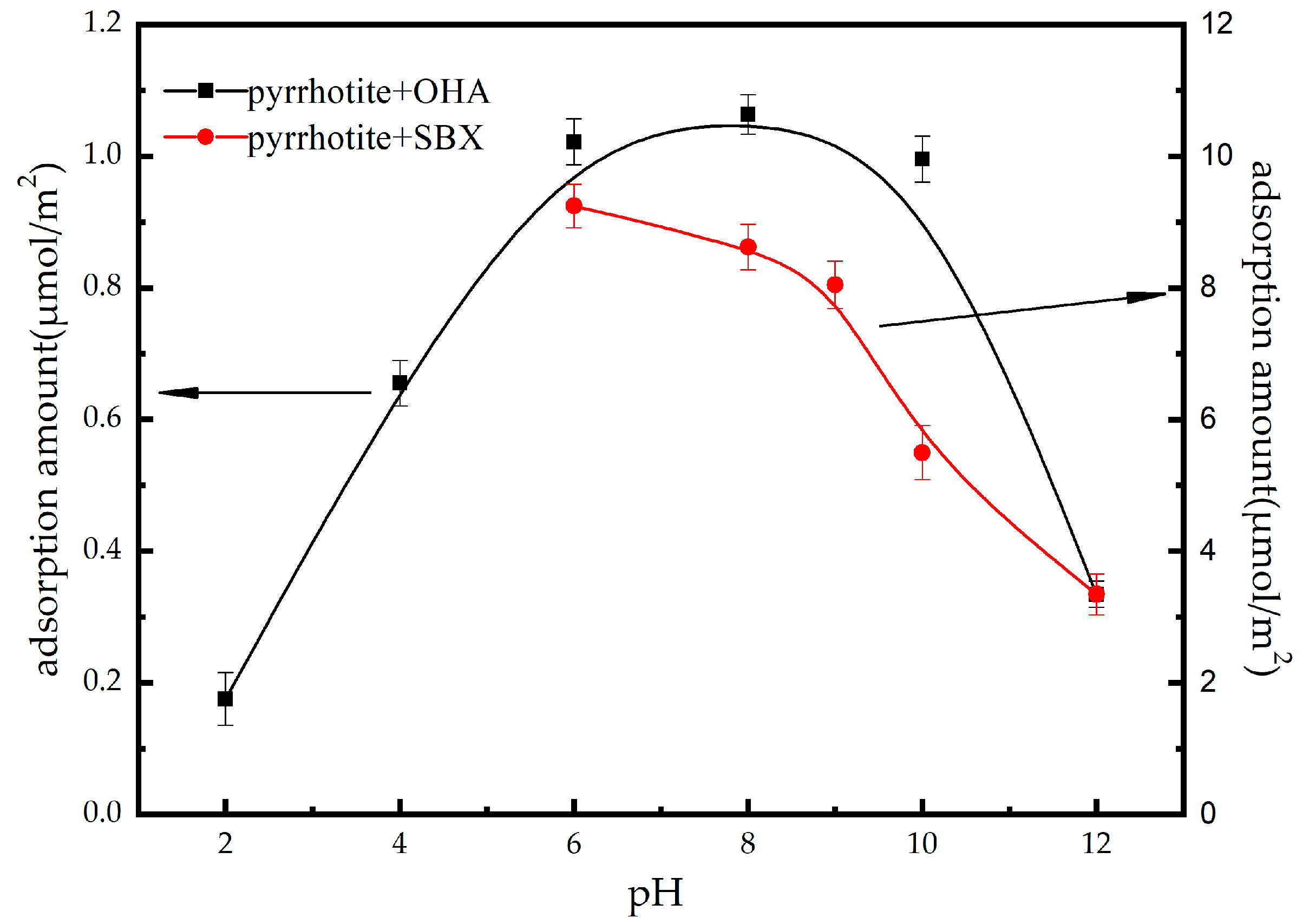
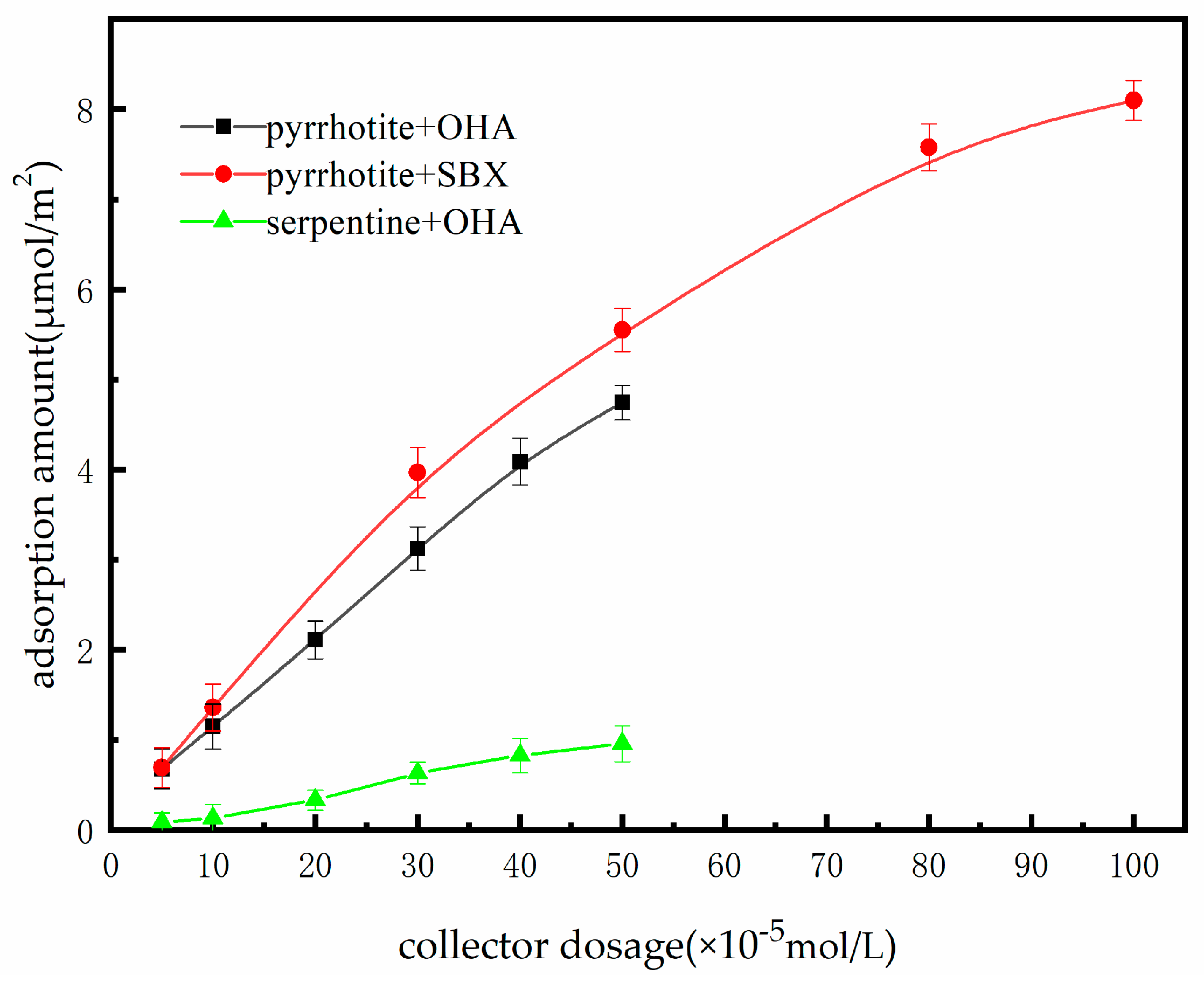
3.3. Adsorption Studies
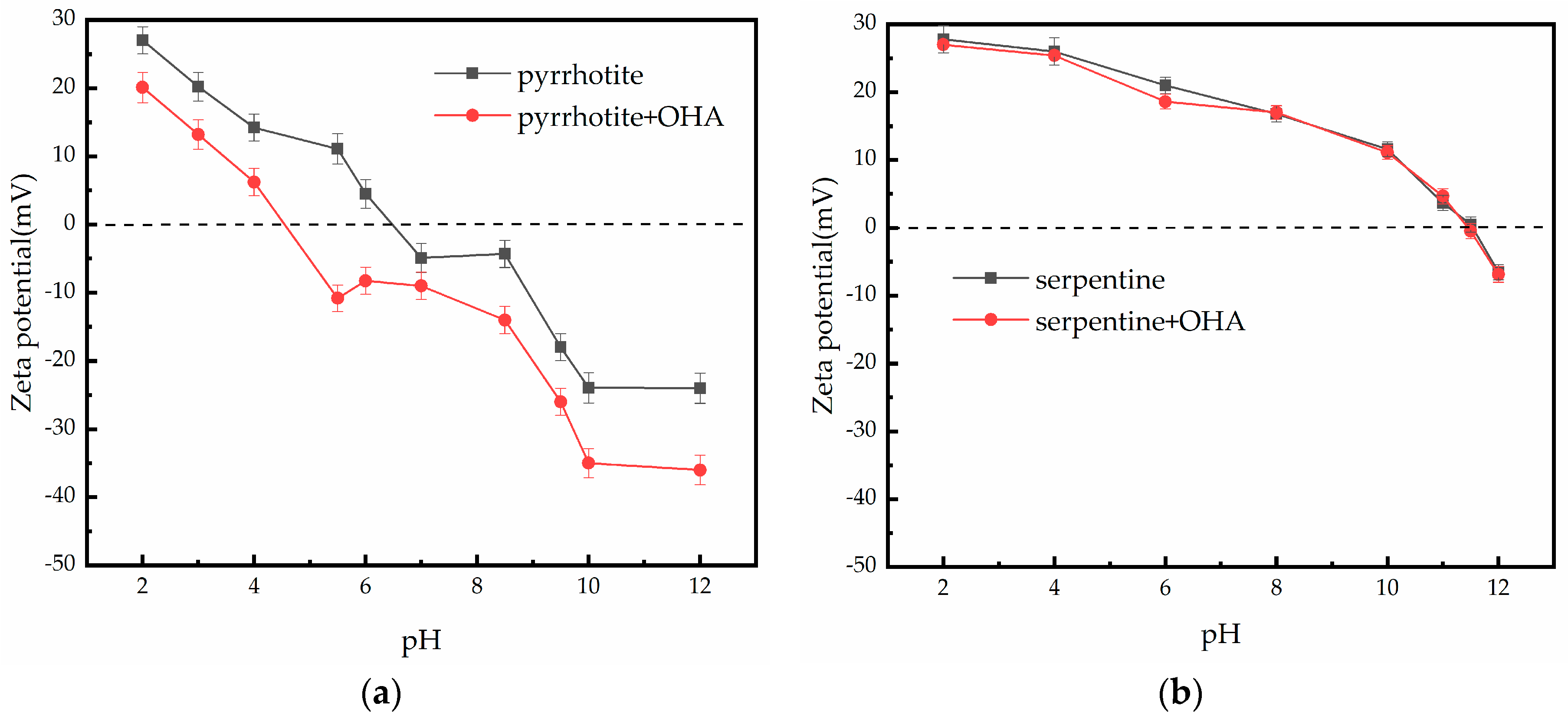
3.4. Zeta Potential Measurements
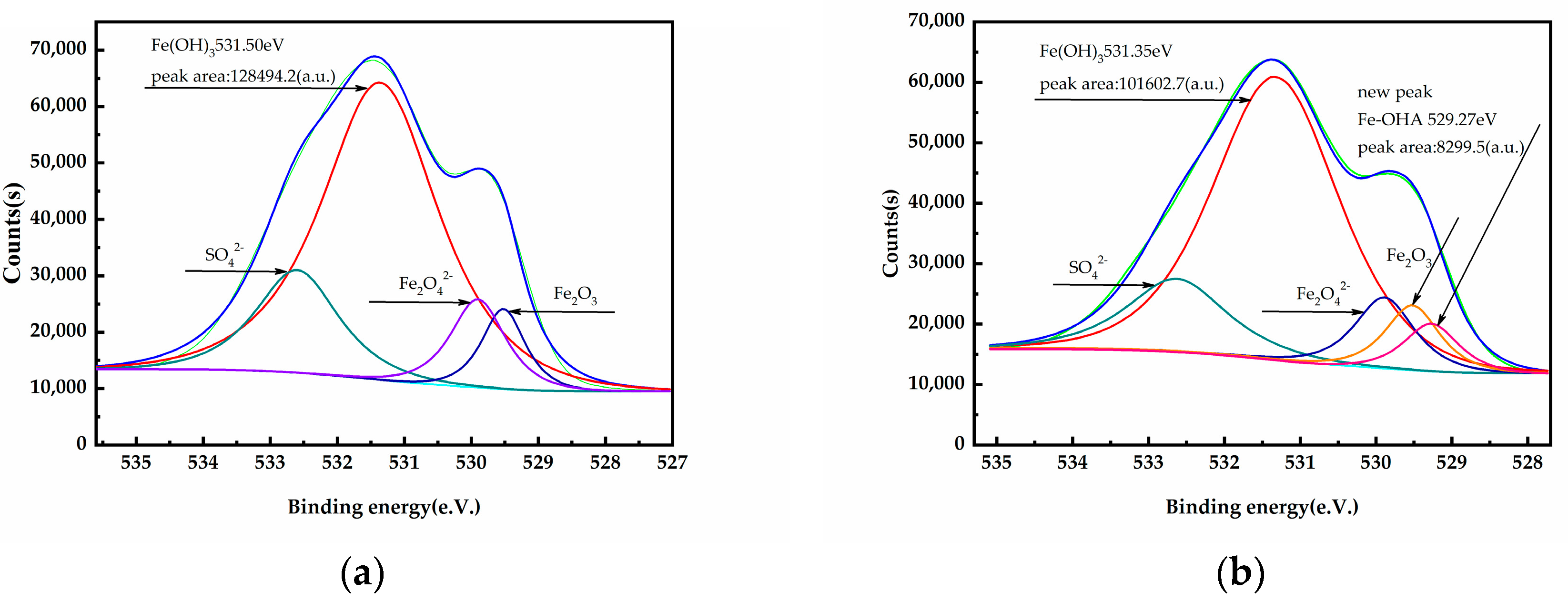
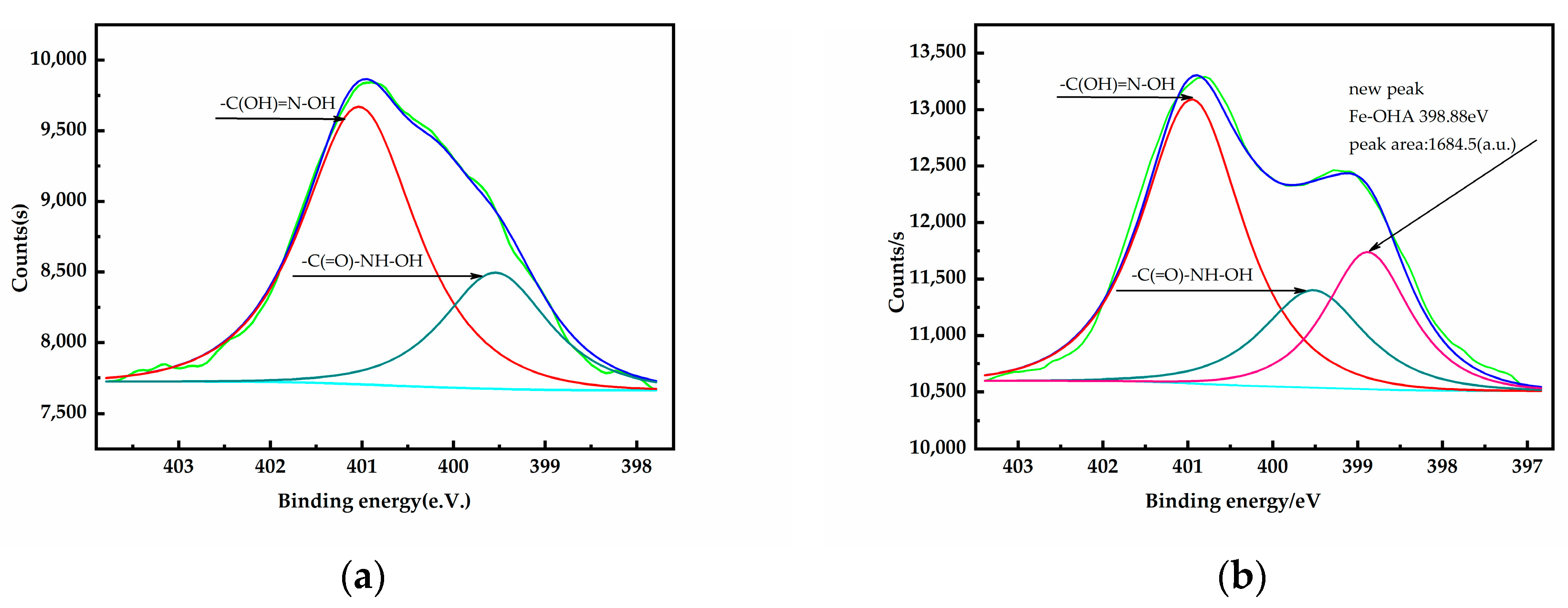
3.5. XPS Analysis
4. Conclusions
- This is a feasible way to find a suitable collector that can directly react with oxidation products on the surface of pyrrhotite. OHA can efficiently reclaim oxidized pyrrhotite and achieve the selective separation of pyrrhotite-serpentine artificially mixed minerals in a weak alkaline environment.
- Adsorption tests, zeta potential measurements, and XPS analyses show that the selective adsorption of OHA on the surface of oxidized pyrrhotite (ET = 24 h) and serpentine is obvious.
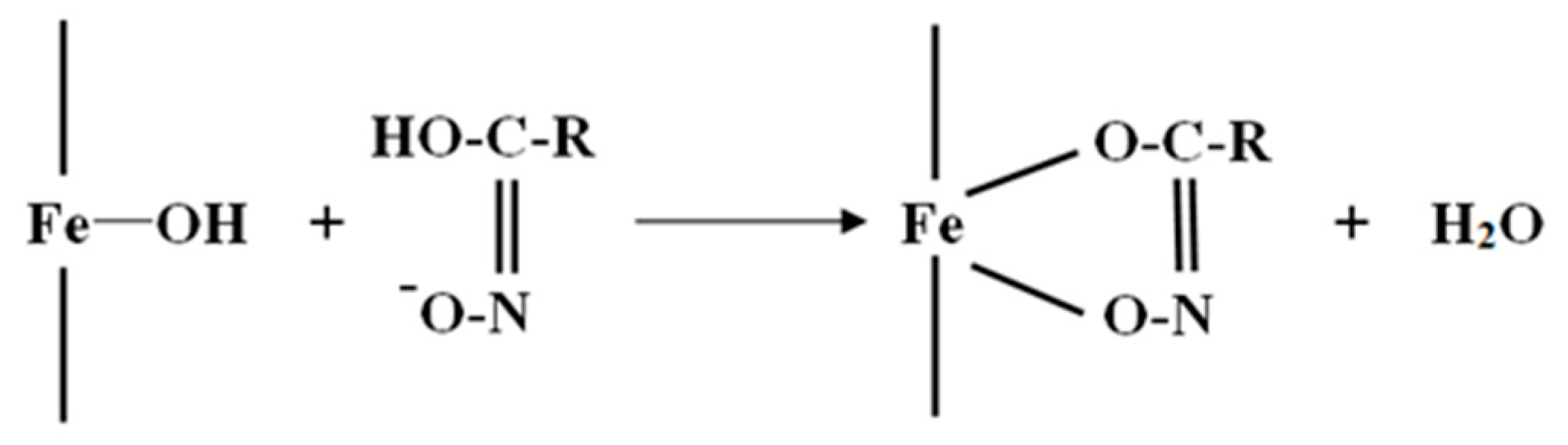
- The XPS analyses indicate that the OHA collector can chelate with Fe(OH)3 on the surface of oxidized pyrrhotite and form an “O, O” five-membered ring, and at the same time, the OHA collector may compete with the hydroxyl groups of hydrophilic substances on the mineral surface to produce hydrophobic products and reduce the hydrophilic substances on the mineral surface.
Author Contributions
Funding
Conflicts of Interest
References
- Yin, W.; Xue, J.; Li, D.; Sun, Q.; Yao, J.; Huang, S. Flotation of heavily oxidized pyrite in the presence of fine digenite particles. Miner. Eng. 2018, 115, 142–149. [Google Scholar] [CrossRef]
- Newell, A.J.H.; Bradshaw, D.J.; Harris, P.J. The effect of heavy oxidation upon flotation and potential remedies for Merensky type sulfides. Miner. Eng. 2006, 19, 675–686. [Google Scholar] [CrossRef]
- Xian, Y.; Wang, Y.; Wen, S.; Nie, Q.; Deng, J. Floatability and oxidation of pyrite with different spatial symmetry. Miner. Eng. 2015, 72, 94–100. [Google Scholar] [CrossRef]
- Ejtemaei, M.; Nguyen, A.V. Characterisation of sphalerite and pyrite surfaces activated by copper sulphate. Miner. Eng. 2017, 100, 223–232. [Google Scholar] [CrossRef]
- Nanthakumar, B.; Kelebek, S.; Katsabanis, P.D. Impact of oxidation on flotation of Ni–Cu sulphide ore with respect to grinding. Miner. Process. Extr. Metall. 2013, 116, 197–206. [Google Scholar] [CrossRef]
- Tayebi-Khorami, M.; Manlapig, E.; Forbes, E.; Edraki, M.; Bradshaw, D. Effect of surface oxidation on the flotation response of enargite in a complex ore system. Miner. Eng. 2018, 119, 149–155. [Google Scholar] [CrossRef]
- Peng, Y.; Zhao, S. The effect of surface oxidation of copper sulfide minerals on clay slime coating in flotation. Miner. Eng. 2011, 24, 1687–1693. [Google Scholar] [CrossRef]
- Jacques, S.; Greet, C.J.; Bastin, D. Oxidative weathering of a copper sulphide ore and its influence on pulp chemistry and flotation. Miner. Eng. 2016, 99, 52–59. [Google Scholar] [CrossRef]
- Fullston, D.; Fornasiero, D.; Ralston, J. Zeta potential study of the oxidation of copper sulfide minerals. Colloids Surf. A Phys. Eng. Asp. 1999, 146, 113–121. [Google Scholar] [CrossRef]
- Raichur, A.M.; Wang, X.H.; Parekh, B.K. Quantifying pyrite surface oxidation kinetics by contact angle measurements. Colloids Surf. A Phys. Eng. Asp. 2000, 167, 245–251. [Google Scholar] [CrossRef]
- Murphy, R.; Strongin, D.R. Surface reactivity of pyrite and related sulfides. Surf. Sci. Rep. 2009, 64, 1–45. [Google Scholar] [CrossRef]
- Mavros, P.; Matis, K.A. Innovations in Flotation Technology; Springer: Heidelberg, Germany, 1992; Volume 208, p. 538. [Google Scholar]
- Legrand, D.L.; Bancroft, G.M.; Nesbitt, H.W. Surface characterization of pentlandite, (Fe,Ni)9S8, by X-ray photoelectron spectroscopy. Int. J Miner. Process. 1997, 51, 217–228. [Google Scholar] [CrossRef]
- Feng, B.; Peng, J.; Zhang, W.; Luo, G.; Wang, H. Removal behavior of slime from pentlandite surfaces and its effect on flotation. Miner. Eng. 2018, 125, 150–154. [Google Scholar] [CrossRef]
- Multani, R.S.; Williams, H.; Johnson, B.; Li, R.; Waters, K.E. The effect of superstructure on the zeta potential, xanthate adsorption, and flotation response of pyrrhotite. Colloids Surf. A Phys. Eng. Asp. 2018, 551, 108–116. [Google Scholar] [CrossRef]
- Miller, J.D.; Li, J.; Davidtz, J.C.; Vos, F. A review of pyrrhotite flotation chemistry in the processing of PGM ores. Miner. Eng. 2005, 18, 855–865. [Google Scholar] [CrossRef]
- Khan, A.; Kelebek, S. Electrochemical Aspects of Pyrrhotite and Pentlandite in Relation to their Flotation with Xanthate. Part-I: Cyclic Voltammetry and Rest Potential Measurements. J. Appl. Electrochem. 2004, 34, 849–856. [Google Scholar] [CrossRef]
- Bunkholt, I.; Kleiv, R.A. Flotation of pyrrhotite and pyrite in saturated CaCO3 solution using a quaternary amine collector. Miner. Eng. 2015, 70, 55–63. [Google Scholar] [CrossRef]
- Bunkholt, I.; Kleiv, R.A. Pyrrhotite oxidation and its influence on alkaline amine flotation. Miner. Eng. 2015, 71, 65–72. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, C.; Feng, Q.; Chen, Y. Utilization of N-carboxymethyl chitosan as selective depressants for serpentine on the flotation of pyrite. Int. J. Miner. Process. 2017, 163, 45–47. [Google Scholar] [CrossRef]
- Bremmell, K.E.; Fornasiero, D.; Ralston, J. Pentlandite-lizardite interactions and implications for their separation by flotation. Colloids Surf. A Phys. Eng. Asp. 2005, 252, 207–212. [Google Scholar] [CrossRef]
- Buswell, A.M.; Nicol, M.J. Some aspects of the electrochemistry of the flotation of pyrrhotite. J. Appl. Electrochem. 2002, 32, 1321–1329. [Google Scholar] [CrossRef]
- Buckley, A.N.; Woods, R. Surface composition of pentlandite under flotation-related conditions. Surf. Interface Anal. 1991, 17, 675–680. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotation performance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Natarajan, R.; Sharma, J.; Nirdosh, I. Adsorption of N-hydrocinnamoyl-N-phenylhydroxylamine on pure minerals. Adsorption 2010, 16, 541–548. [Google Scholar] [CrossRef]
- Chen, G.; Grano, S.; Sobieraj, S.; Ralston, J. The effect of high intensity conditioning on the flotation of a nickel ore, part 2: Mechanisms. Miner. Eng. 1999, 12, 1359–1373. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhu, J.G. Selective flotation of cassiterite with benzohydroxamic acid. Miner. Eng. 2006, 19, 1410–1417. [Google Scholar] [CrossRef]
- Rashchi, F.; Finch, J.A. Polyphosphates: A review, their chemistry and application with particular reference to mineral processing. Miner. Eng. 2000, 10–11, 1019–1035. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, Q.; Zhang, G.; Liu, D.; Liu, R. Effect of Sodium Pyrophosphate on the Reverse Flotation of Dolomite from Apatite. Minerals 2018, 8, 278. [Google Scholar] [CrossRef]
- Wang, P.; Qin, W.; Ren, L.; Wei, Q.; Liu, R.; Yang, C.; Zhong, S. Solution chemistry and utilization of alkyl hydroxamic acid in flotation of fine cassiterite. Trans. Nonferr. Met. Soc. China 2013, 23, 1789–1796. [Google Scholar] [CrossRef]
- Sreenivas, T.; Padmanabhan, N.P.H. Surface chemistry and flotation of cassiterite with alkyl hydroxamates. Colloids Surf. A Phys. Eng. Asp. 2002, 205, 47–59. [Google Scholar] [CrossRef]
- Tian, M.; Zhang, C.; Han, H.; Liu, R.; Gao, Z.; Chen, P.; Wang, L.; Li, Y.; Ji, B.; Hu, Y.; et al. Effects of the preassembly of benzohydroxamic acid with Fe (III) ions on its adsorption on cassiterite surface. Miner. Eng. 2018, 127, 32–41. [Google Scholar] [CrossRef]
- Griffith, D.M.; Szőcs, B.; Keogh, T.; Suponitsky, K.Y.; Farkas, E.; Buglyó, P.; Marmion, C.J. Suberoylanilide hydroxamic acid, a potent histone deacetylase inhibitor; its X-ray crystal structure and solid state and solution studies of its Zn(II), Ni(II), Cu(II) and Fe(III) complexes. J. Inorg. Biochem. 2011, 105, 763–769. [Google Scholar] [CrossRef] [PubMed]
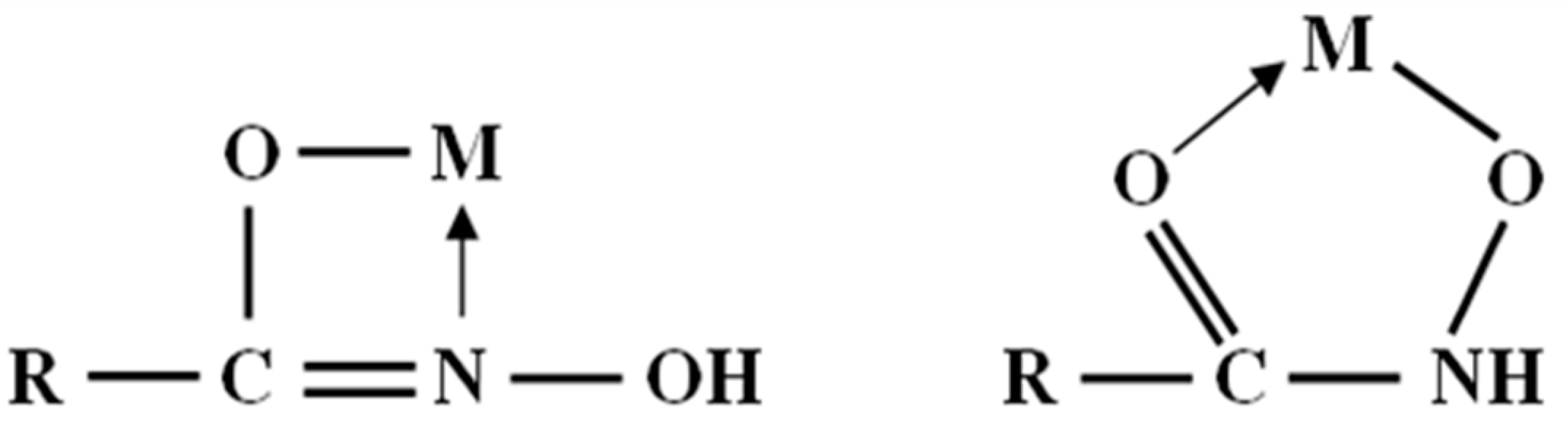
| TFe | S | P | SiO2 | MgO | CaO | Cu |
|---|---|---|---|---|---|---|
| 58.68 | 39.34 | 0.001 | 0.41 | 0.05 | 0.21 | 0.06 |
| Sample | d10 | d50 | d90 | Average Size |
|---|---|---|---|---|
| Serpentine | 1.71 | 15.10 | 48.73 | 20.70 |
| MgO | SiO2 | Fe2O3 | NiO | Al2O3 | CaO | K2O | Others |
|---|---|---|---|---|---|---|---|
| 32.92 | 37.10 | 7.39 | 0.37 | 0.80 | 0.21 | 0.06 | 21.15 |
| Number | SHMP (mg/L) | Yield (%) | S grade (%) | MgO Grade (%) | Recovery of Pyrrhotite (%) | Recovery of Serpentine (%) |
|---|---|---|---|---|---|---|
| 1 | 0 | 48.51 | 31.27 | 5.99 | 77.12 | 16.64 |
| 2 | 5 | 50.00 | 33.49 | 5.34 | 85.13 | 15.29 |
| 3 | 8 | 49.47 | 34.72 | 4.60 | 87.32 | 13.03 |
| 4 | 10 | 48.43 | 34.95 | 4.10 | 86.05 | 11.37 |
| 5 | 15 | 44.44 | 35.29 | 4.01 | 79.73 | 10.21 |
| 6 | 20 | 41.02 | 35.91 | 3.66 | 74.95 | 8.60 |
| Electronic Orbital | Pyrrhotite | Serpentine | Pyrrhotite + OHA | Serpentine + OHA |
|---|---|---|---|---|
| Binding Energy | Binding Energy | Binding Energy ΔE | Binding Energy ΔE | |
| C1s | 284.80 | 284.80 | 284.80 0 | 284.80 0 |
| O1s | 531.50 | 531.41 | 531.35 0.15 | 531.41 0 |
| Fe2p | 710.68 | - | 710.50 0.18 | - |
| Mg1s | - | 1303.00 | - | 1303.04 0.04 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Lu, Y.; Mao, G. Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine. Minerals 2018, 8, 472. https://doi.org/10.3390/min8100472
Zhou J, Lu Y, Mao G. Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine. Minerals. 2018; 8(10):472. https://doi.org/10.3390/min8100472
Chicago/Turabian StyleZhou, Jian, Yiping Lu, and Guozhi Mao. 2018. "Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine" Minerals 8, no. 10: 472. https://doi.org/10.3390/min8100472
APA StyleZhou, J., Lu, Y., & Mao, G. (2018). Separation of Oxidized Pyrrhotite from Fine Fraction Serpentine. Minerals, 8(10), 472. https://doi.org/10.3390/min8100472




