A Geometallurgical Approach to Tailings Management: An Example from the Savage River Fe-Ore Mine, Western Tasmania
Abstract
:1. Introduction
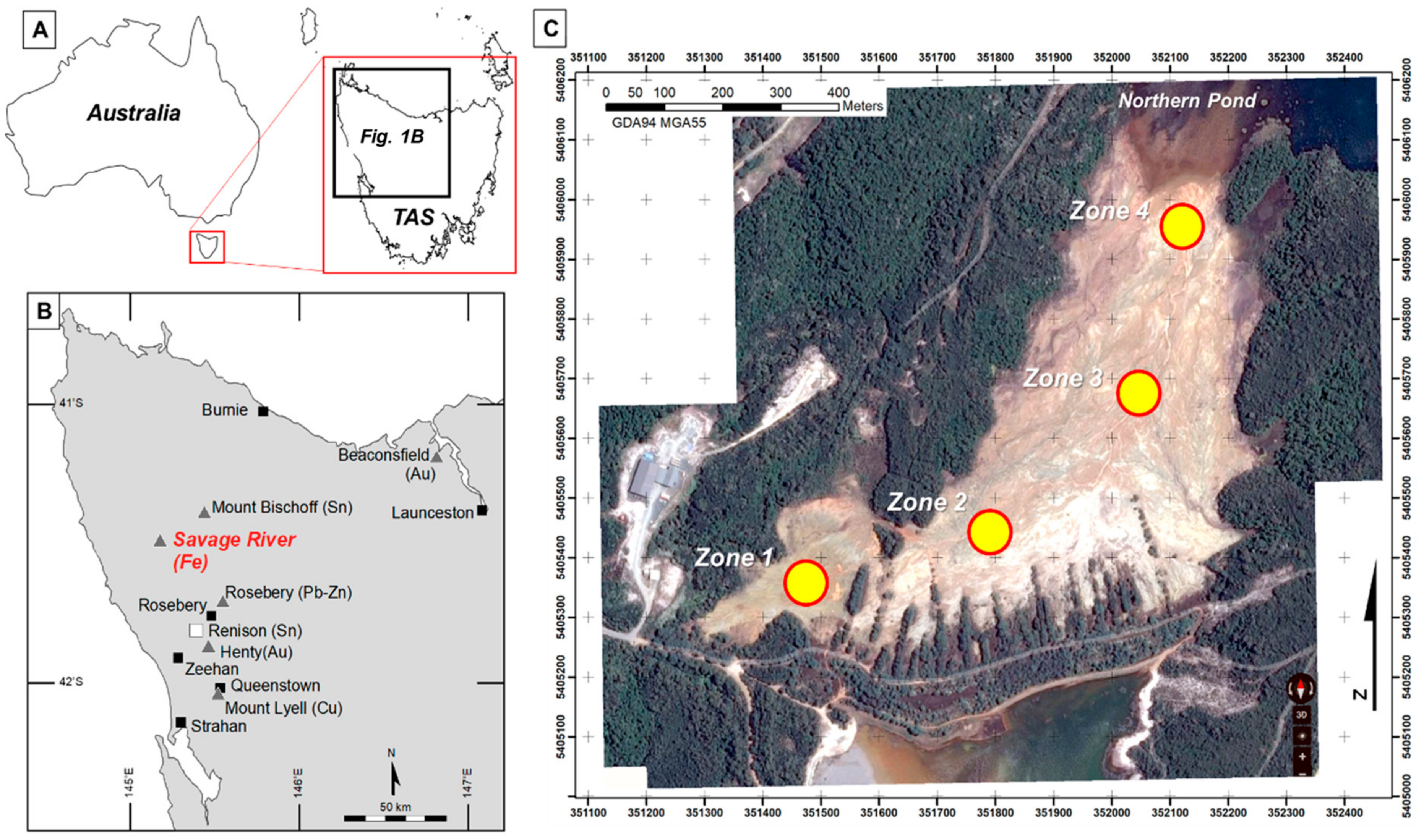
2. Site Description
2.1. Local Geology and Mineralisation
2.2. Mining and Mineral Processing
2.3. Geoenvironmental Risks
3. Materials and Methods
3.1. Tailings Collection
3.2. Bacterial Adaption
3.3. Optimization Experiments
3.4. Flotation Testwork
3.5. Iron and Cobalt Precipitation Tests
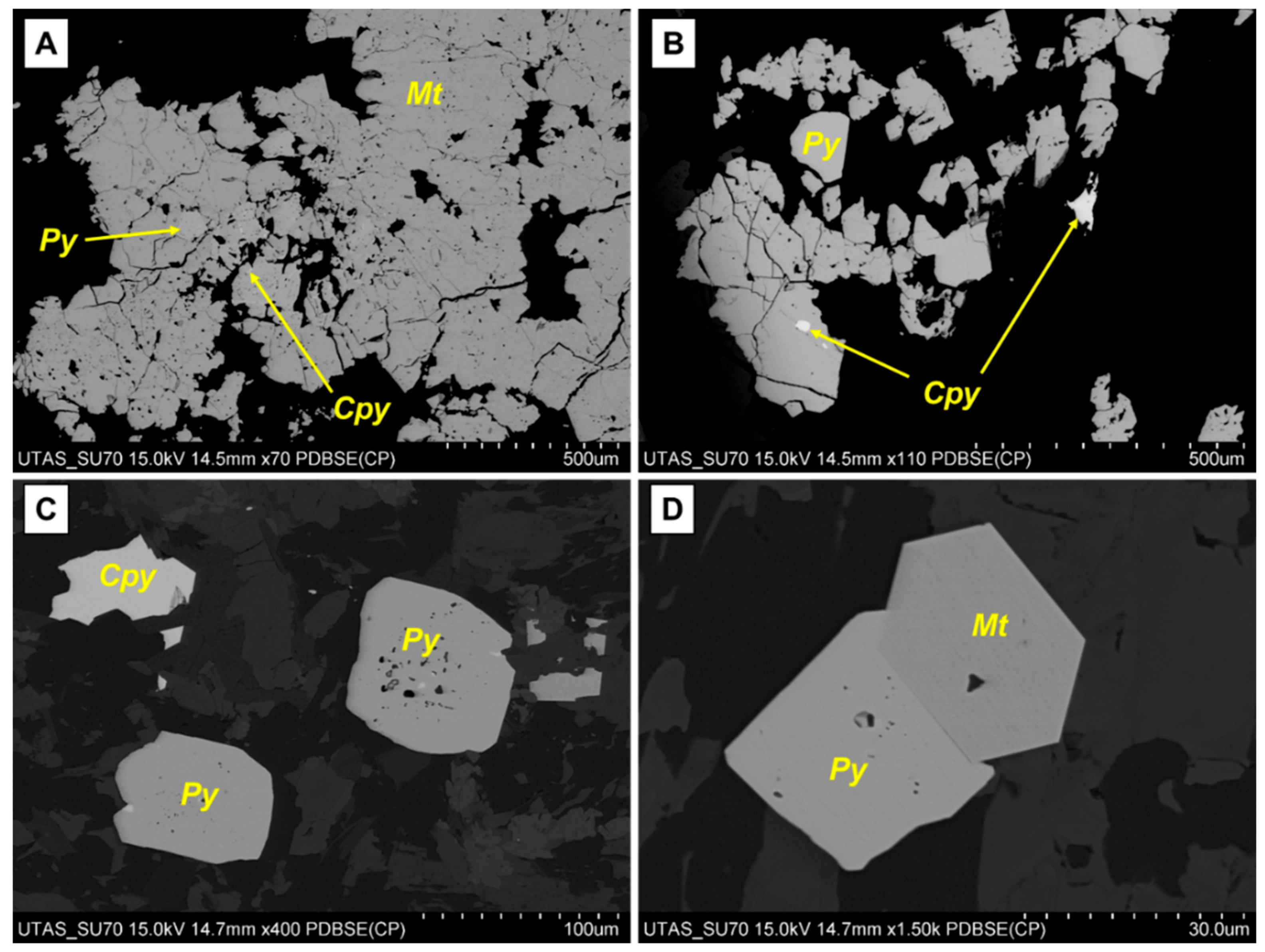
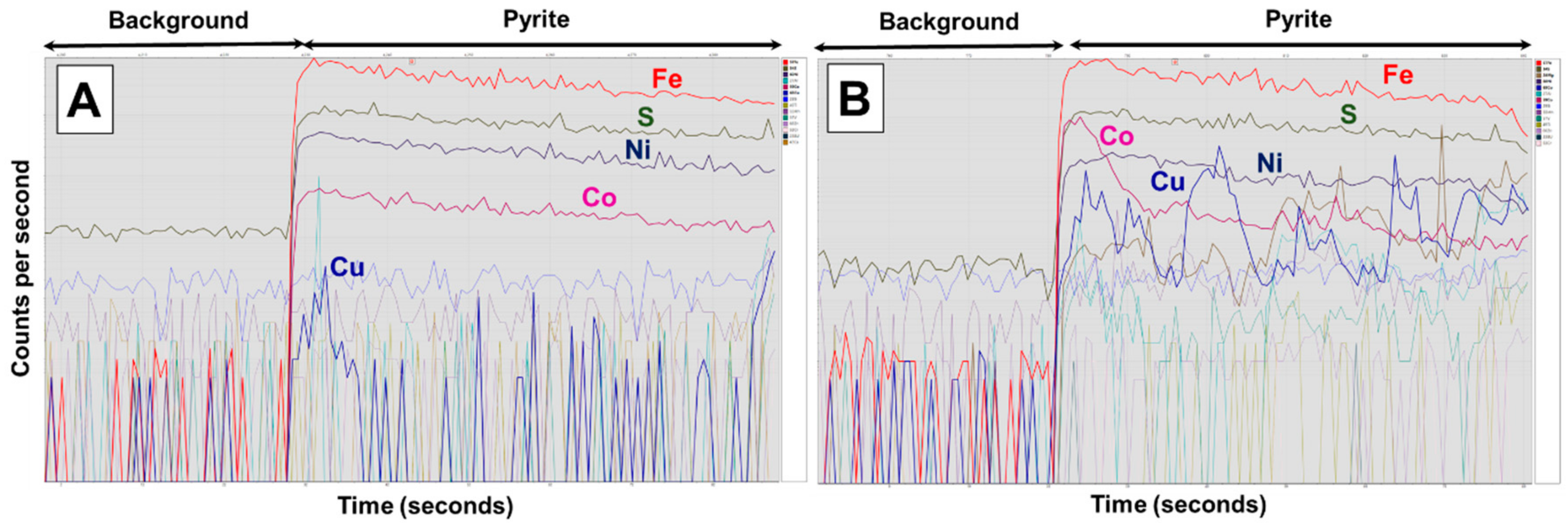
3.6. Mineralogical Characterisation
4. Results
4.1. Batch Stirred Tank Reactor Experiments
4.1.1. Tailings Feed Characteristics
4.1.2. Bacterial Adaption
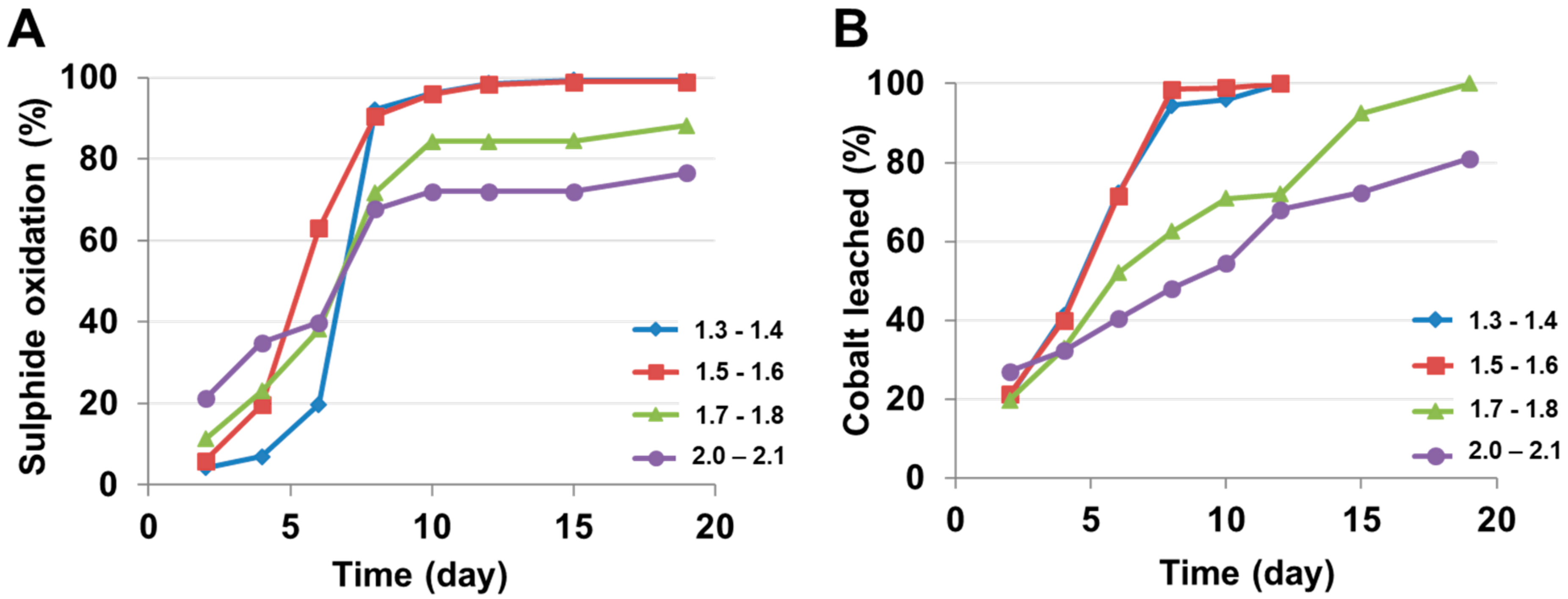
4.1.3. pH Modification
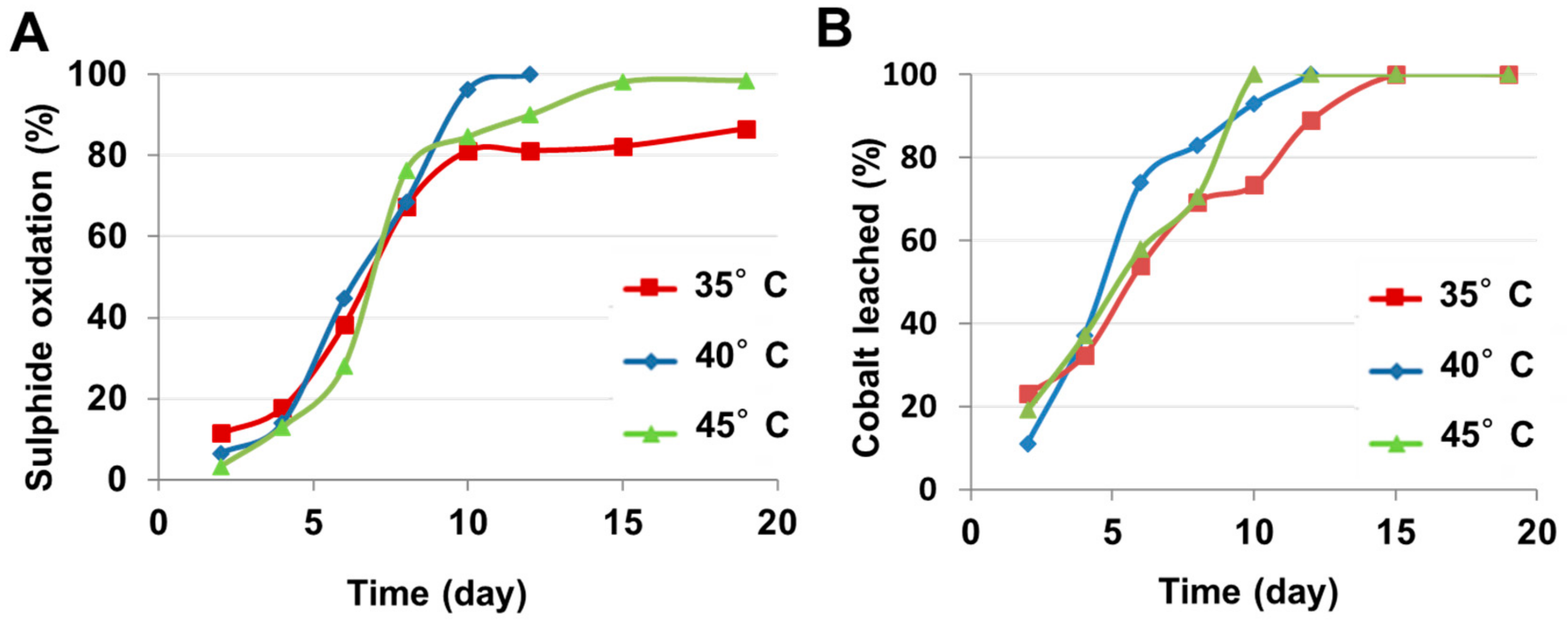
4.1.4. Temperature
4.1.5. Nutrient Medium Amendment
4.2. Continuous Stirred Tank Experiments
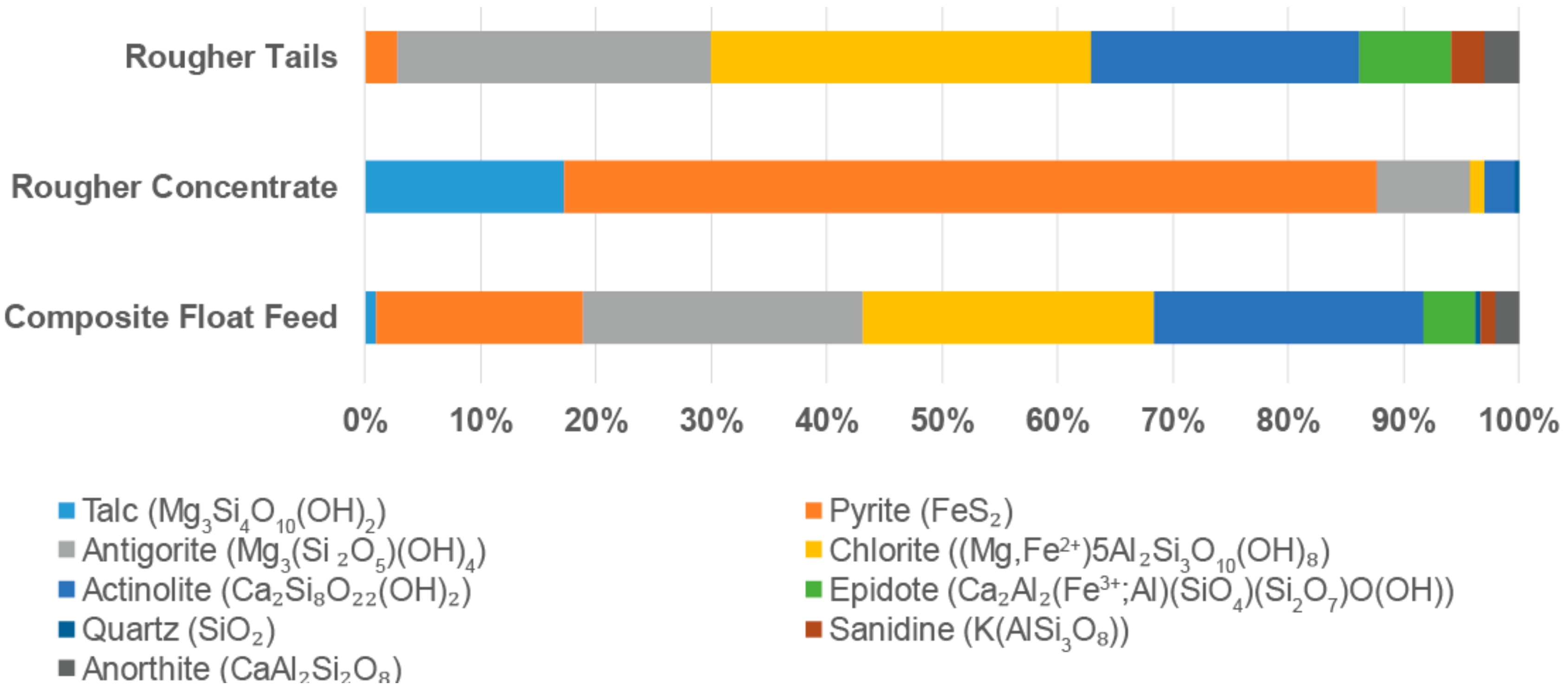
4.2.1. Sulphide Concentrate Production
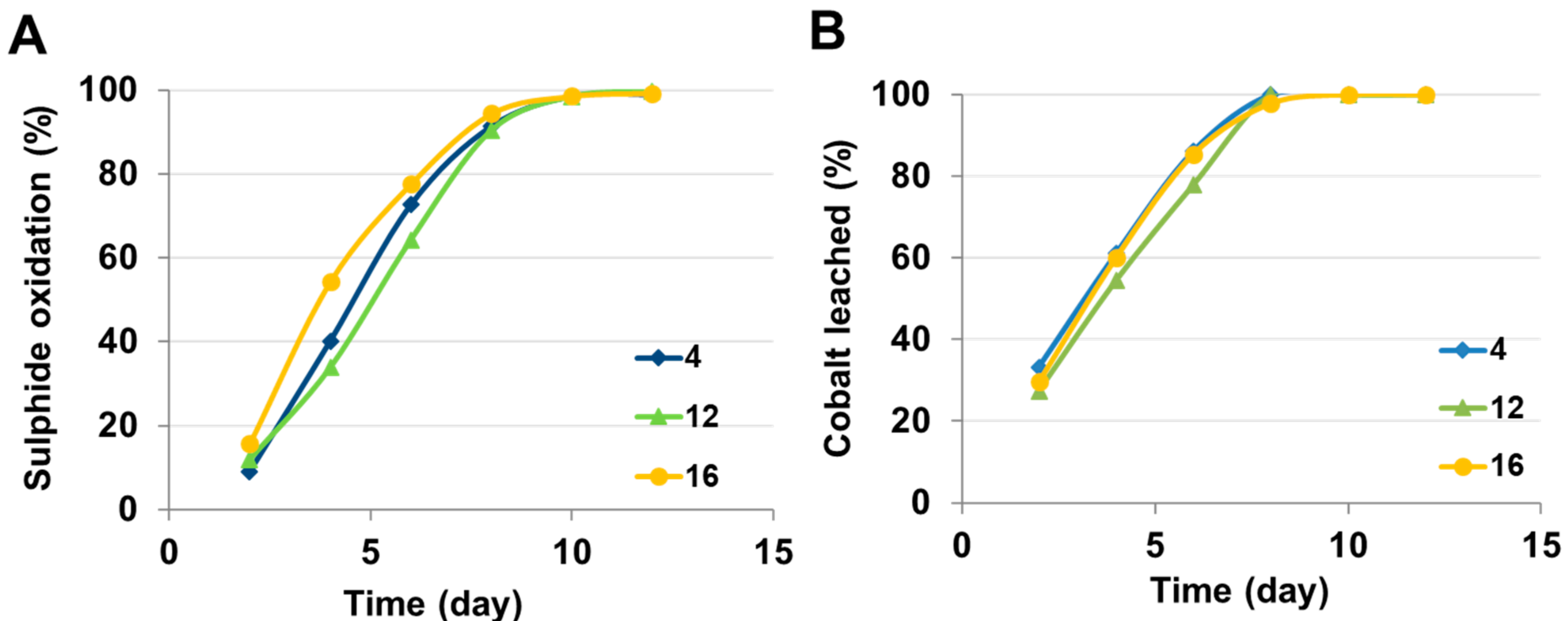
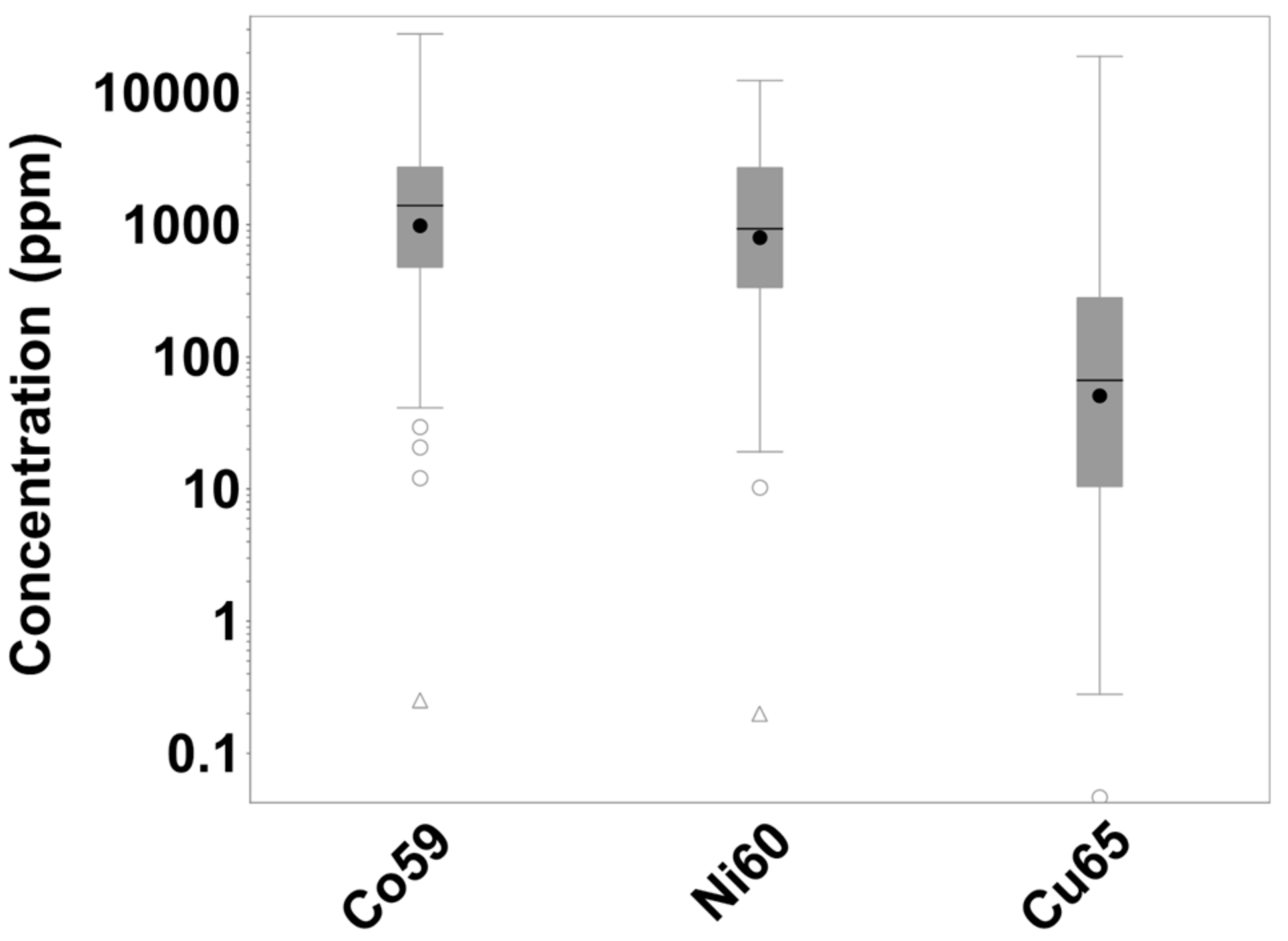
4.2.2. Cobalt Liberation
4.2.3. Iron and Cobalt Precipitation Experiments
5. Discussion
5.1. Optimal Conditions for Biooxidation
5.2. Flotation Testwork Evaluation
5.3. Metal Seperation
5.4. Future Tailings Management
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Adinansyah, J.S.; Rosano, M.; Vink, S.; Keir, G. A framework for a sustainable approach to mine tailings management: Disposal strategies. J. Clean. Prod. 2015, 108, 1050–1062. [Google Scholar] [CrossRef]
- Edraki, M.; Baumgartl, T.; Manlapig, E.; Bradshaw, D.; Franks, M.D.; Moran, C.J. Designing mine tailings for better environmental, social and economic outcomes: A review of alternative approaches. J. Clean. Prod. 2014, 84, 411–420. [Google Scholar] [CrossRef]
- Lindsay, M.B.; Moncur, M.C.; Bain, J.G.; Jambor, J.L.; Ptacek, C.J.; Blowes, D.W. Geochemical and mineralogical aspects of sulphide mine tailings. Appl. Geochem. 2015, 57, 157–177. [Google Scholar] [CrossRef]
- Dold, B. Evolution of acid mine drainage formation in sulphidic mine tailings. Minerals 2014, 4, 621–641. [Google Scholar] [CrossRef]
- Quispe, D.; Pérez-López, R.; Acero, P.; Ayora, C.; Nieto, J.M.; Tucoulou, R. Formation of a hardpan in the co-disposal of fly ash and sulphide mine tailings and its influence on the generation of acid mine drainage. Chem. Geol. 2013, 355, 45–55. [Google Scholar] [CrossRef]
- Moncur, M.C.; Ptacek, C.J.; Lindsay, M.B.; Blowes, D.W.; Jambor, J.L. Long-term mineralogical and geochemical evolution of sulphide mine tailings under a shallow water cover. Appl. Geochem. 2015, 57, 178–193. [Google Scholar] [CrossRef]
- Wang, L.; Ji, B.; Hu, Y.; Liu, R.; Sun, W. A review on in situ phytoremediation of mine tailings. Chemosphere 2017, 184, 594–600. [Google Scholar] [CrossRef] [PubMed]
- Jambor, J.L.; Dutrizac, J.E.; Groat, L.A.; Raudsepp, M. Static tests of neutralization potentials of silicate and aluminosilicate minerals. Environ. Geol. 2002, 43, 1–17. [Google Scholar]
- Sracek, O.; Choquette, M.; Gelinas, P.; Lefebvre, R.; Nicholson, R.V. Geochemical characterisation of acid mine drainage from a waste rock pile, Mine Doyon, Quebec, Canada. J. Contam. Hydrol. 2004, 69, 45–71. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Edraki, M.; Hardie, K.; Kadletz, O.; Hall, T. Identification of acid rock drainage sources through mesotextural classification at abandoned mines of Croydon, Australia: Implications for the rehabilitation of waste rock repositories. J. Geochem. Explor. 2014, 37, 11–28. [Google Scholar] [CrossRef]
- Parvainen, A.; Mäkilä, M.; Loukola-Ruskeeniemi, K. Pre-mining acid rock drainage in the Talvivaara Ni-Cu-Zn-Co deposit (Finland): Natural peat layers as a natural analog to constructed wetlands. J. Geochem. Explor. 2014, 143, 84–95. [Google Scholar] [CrossRef]
- Buzatu, A.; Dill, H.G.; Buzgar, N.; Damian, G.; Maftei, A.E.; Apopei, A.I. Efflorescent sulfates from Baia Sprie mining area (Romania)–Acid mine drainage and climatological approach. Sci. Total Environ. 2016, 542, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Salomon, W.; Eagle, A.M. Hydrology, sedimentology and the fate and distribution of copper in mine-related discharges in the Fly River system, Papua New Guinea. Sci. Total Environ. 1990, 97, 315–334. [Google Scholar] [CrossRef]
- Ramirez-Llodra, E.; Trannum, H.C.; Evenset, A.; Levin, L.A.; Andersson, M.; Finne, T.E.; Hilario, A.; Flem, B.; Christensen, G.; Schanning, M.; et al. Submarine and deep sea mine tailing placements: A review of current practices, environmental issues, natural analogs and knowledge gaps in Norway and internationally. Mar. Poll. Bull. 2015, 97, 13–35. [Google Scholar] [CrossRef] [PubMed]
- Azhari, A.E.; Rhouijait, A.; Laarabi, E.H.M. Assessment of heavy metals and arsenic contamination in the sediments of the Moulouya River and the Hassan II Dam downstream of the abandoned mine Zeïda (High Moulouya, Morocco). J. Afr. Sci. 2016, 119, 279–288. [Google Scholar] [CrossRef]
- Lima, A.T.; Mitchell, K.; O’Connell, D.W.; Verhoeven, J.; Van Cappellen, P. The legacy of surface mining: Remediation, restoration, reclamation and rehabilitation. Environ. Sci. Policy 2016, 66, 227–233. [Google Scholar] [CrossRef]
- Harris, T.M.; Hottle, T.A.; Soratana, K.; Klane, J.; Landis, A. Life cycle assessment of sunflower cultivation on abandoned mine land for biodiesel production. J. Clean Prod. 2016, 112, 182–195. [Google Scholar] [CrossRef]
- Van Veen, E.; Lottermoser, B.G.; Parbhakar-Fox, A.; Fox, N.; Hunt, J. A new test for plant bioaccessibility in sulphidic wastes and soils: A case study from the Wheal Maid historic tailings repository in Cornwall, UK. Sci. Total Environ. 2016, 563, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Liu, C.; Jiang, Y.; Xue, J.; Li, P.; Yu, J. Green recovery of potassium and aluminum elements from alunite tailings using gradient leaching process. J. Clean Prod. 2017, 168, 1080–1090. [Google Scholar] [CrossRef]
- Lei, C.; Yan, B.; Chen, T.; Wang, X.L.; Xiao, X.M. Silver leaching and recovery of valuable metals from magnetic tailings using chloride leaching. J. Clean Prod. 2018, 181, 408–415. [Google Scholar] [CrossRef]
- Tao, H.; Dongwei, L. Presentation on mechanisms and applications of chalcopyrite and pyrite bioleaching in biohydrometallurgy—A presentation. Biotechnol. Rep. 2014, 4, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.D. Biomining goes underground. Nat. Geo 2015, 8, 165–166. [Google Scholar] [CrossRef]
- Mahmoud, A.; Cézac, P.; Hoadley, A.F.; Contamine, F.; D’Hugues, P. A review of sulphide minerals microbially assisted leaching in stirred tank reactors. Inter. Biodeterior. Biodegrad. 2017, 119, 118–146. [Google Scholar] [CrossRef]
- Sethurajan, M.; van Hullebusch, E.D.; Nancharaiah, Y.V. Biotechnology in the management and resource recovery from metal bearing solid wastes: Recent advances. J. Environ. Manag. 2018, 211, 138–153. [Google Scholar] [CrossRef] [PubMed]
- Gurung, S. Tasmanian Acid Drainage Reconnaissance–Vol. 1: Acid Drainage from Abandoned Mines in Tasmania (Tasmanian Geological Survey, Mineral Resources Tasmania); Department of Infrastructure, Energy and Resources and Natural Heritage Trust: Hobart, TAS, Australia, 2001. [Google Scholar]
- Jackson, L.M.; Parbhakar-Fox, A.K. Mineralogical and geochemical characterization of the Old Tailings Dam, Australia: Evaluating the effectiveness of a water cover for long-term AMD control. Appl. Geochem. 2016, 68, 64–78. [Google Scholar] [CrossRef]
- Parbhakar-Fox, A.; Fox, N.; Jackson, L.J. Geometallurgical evaluations of mine waste—An example from the Old Tailings Dam, Savage River, Tasmania. In Proceedings of the third AusIMM International Geometallurgy Conference, Perth, Australia, 15–16 June 2016. [Google Scholar]
- London Metals Exchange. Available online: https://www.lme.com/en-GB/Metals/Minor-metals/Cobalt (accessed on 15 August 2018).
- Wills, B.; Finch, J.A. Will’s Processing Technology—An Introduction to the Practical Aspects of Ore Treatment and Mineral Recovery, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2015; p. 498. [Google Scholar]
- Watling, H.R. The bioleaching of sulphide minerals with an emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Morin, D.; Battaglia, F.; Ollivier, P. Study of the bioleaching of a cobaltiferous pyrite concentrate. In Biohydrometallurgical Technologies; Torma, A.E., Wey, J.E., Lakshmanan, V.L., Eds.; The Minerals, Metals and Material Society: Pittsburgh, PA, USA, 1993; pp. 147–156. [Google Scholar]
- D’Hugues, P.; Cezac, P.; Cabral, T.; Battaglia, F.; Truong-Meyer, X.M.; Morin, D. Bioleaching of a cobaltiferous pyrite: A continuous laboratory-scale study at high solids concentration. Miner. Eng. 1997, 10, 507–527. [Google Scholar] [CrossRef]
- Morin, D.; D’Hugues, P. Bioleaching of a cobalt containing pyrite in stirred reactors, a case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Berlin, Germany, 2007; pp. 35–55. [Google Scholar]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Bio-processing of a saline, calcareous sulfide ore by sequential leaching. Hydrometallurgy 2018, 179, 36–43. [Google Scholar] [CrossRef]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Corbett, K.; Quilty, P.; Calver, C.R. Geological Evolution of Tasmania; Geological Society of Australia: Adelaide, South Australia, 2014; p. 639. [Google Scholar]
- Urqhart, G. Magnetite Deposits of the Savage River-Rocky River Region; Tasmanian Department of Mines: Hobart, Australia, 1966; p. 159. [Google Scholar]
- Coleman, R.J. Savage River magnetite deposit. In Economic Geology of Australia and Papua New Guinea; Knight, C.L., Ed.; I. Metals Australasian Institute of Mining and Metallurgy: Victoria, Australia, 1975; pp. 598–604. [Google Scholar]
- Parbhakar-Fox, A.; Fox, N.; Hill, R.; Ferguson, T.; Maynard, B. Improved mine waste characterisation through static blended test work. Miner. Eng. 2018, 116, 132–142. [Google Scholar] [CrossRef]
- Grange Resources. Available online: www.grangeresources.com.au (accessed on 15 August 2018).
- Savage River Rehabilitation Program. Available online: https://epa.tas.gov.au/epa/water/remediation-programs/savage-river-rehabilitation (accessed on 15 August 2018).
- Kent, S. Development Proposal and Environmental Management Plan, South Deposit Tailings Storage Facility, HB13485; Caloundra Environmental: Golden Beach, Australia, 2015. [Google Scholar]
- Silverman, M.; Lundgren, D.G. Studies on the chemoautotrophic iron bacterium Ferrobacillus ferrooxidans. I. An improved medium and a harvesting procedure for securing high cell yields. J. Bacteriol. 1959, 77, 642–647. [Google Scholar] [PubMed]
- Broadhurst, J.L. Neutralisation of arsenic bearing BIOX® liquors. Miner. Eng. 2004, 7, 1029–1038. [Google Scholar] [CrossRef]
- Van Aswegen, P.C.; Nierkerk, J.V.; Oliver, W. The BIOX® process for the treatment of refractory gold concentrates. In Biomining; Rawlings, D., Johnson, D.B., Eds.; Springer: Berlin, Germany, 2007; pp. 1–6. [Google Scholar]
- Van Hille, R.P.; Dawson, E.; Edward, C.; Harrison, S.T.L. Effect of thiocyanate on BIOX® organisms: Inhibition and adaption. Miner. Eng. 2015, 75, 110–115. [Google Scholar] [CrossRef]
- Runge, K.C.; Tabosa, E.; Jankovic, A. Particle size distribution effects that should be considered when performing flotation geometallurgical testing. In Proceedings of the Second AusIMM International Geometallurgy Conference, Brisbane, Australia, 30 September–2 October 2013. [Google Scholar]
- Yang, S.; Xie, B.; Lu, Y.; Li, C. Role of magnesium-bearing silicates in the flotation of pyrite in the presence of serpentine slimes. Powder Technol. 2018, 332, 1–7. [Google Scholar] [CrossRef]
- Rawlings, D.; Johnson, D.B. The microbiology of biomining: Developing and optimization of mineral-oxidising microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, S.; Joulian, C.; Graupner, T.; Schippers, A.; Gwénaëlle Guezennec, A. Enhanced chalcopyrite dissolution in stirred tank reactors by temperature increase during bioleaching. Hydrometallurgy 2018, 179, 125–131. [Google Scholar] [CrossRef]
- Gwénaëlle Guezennec, A.; Joulian, C.; Jacob, J.; Archane, A.; Ibarra, D.; Buyer, R.D.; Bodenan, F.; d’Hughes, P. Influence of dissolved oxygen on the bioleaching efficiency under oxygen enriched atmosphere. Miner. Eng. 2017, 106, 64–70. [Google Scholar] [CrossRef]
- Tuovinen, O.H.; Niemela, S.I.; Gyllenberg, H.G. Tolerance of Thiobacillus ferrooxidans to some metals. Antonie van Leeuwenhoek 1971, 37, 489–496. [Google Scholar] [CrossRef] [PubMed]
- Trevors, J.T.; Oddie, K.M.; Belliveau, B.H. Metal resistance in bacteria. FEMS Microbio Rev. 2006, 32, 39–54. [Google Scholar] [CrossRef]
- Boseker, K. Bioleaching: Metal solubilisation by microorganisms. FEMS Microbio Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Dopson, M.; Baker-Austin, C.; Koppineedi, P.R.; Bond, P.L. Growth in sulfidic mineral environments: Metal resistance mechanisms in acidophilic micro-organisms. Microbio 2003, 149, 1959–1970. [Google Scholar] [CrossRef] [PubMed]
- Batty, J.D.; Rorke, G.V. Development and commercial demonstration of the BioCOPTM termophile process. Hydrometallurgy 2006, 83, 83–89. [Google Scholar] [CrossRef]
- Abbot, M.S.; Harvey, A.P.; Valenta Perez, G.; Theodorou, M.K. Biological processing in oscillatory baffle reactors: Operation, advantages and potential. Interf. Focus 2012, 3. [Google Scholar] [CrossRef]
- Beattie, D.A.; Huynh, L.; Kaggawa, B.N.; Ralston, J. The effect of polysaccharides and polyacrylamides on the depression of talc and the flotation of sulphide minerals. Miner. Eng. 2006, 19, 598–608. [Google Scholar] [CrossRef]
- Deng, W.; Longhua, X.; Tian, J.; Hu, Y.; Han, Y. Flotation and adsorption of a new polysaccharide depressant on pyrite and talc in the presence of a pre-adsorbed xanthate collector. Minerals 2017, 7, 40. [Google Scholar] [CrossRef]
- Swartz, B.; Donegan, S.; Amos, S. Processing considerations for cobalt recovery from Congolese copperbelt ores. In Proceedings of the Hydrometallurgy Conference, Cape Town, South Africa, 6–7 April 2009. [Google Scholar]
- Vaughan, J.; Dieters, C.; Fu, W.; Byrne, K. Properties of Lewatit® TP272, a commercial solvent impregnated cation exchange resin for cobalt recovery. Miner. Eng. 2016, 88, 2–8. [Google Scholar] [CrossRef]
- Moodley, I.; Sheridan, C.M.; Kappelmeyer, U.; Akcil, A. Environmentally sustainable acid mine drainage remediation: Research developments with a focus on waste/by products. Miner. Eng. 2018, 126, 207–220. [Google Scholar] [CrossRef]
- Zammit, C.M.; Brugger, J.; Southam, G.; Reith, F. In-situ recovery of uranium- the microbial influence. Hydrometallurgy 2014, 150, 236–244. [Google Scholar] [CrossRef]
- Schluter, R.; Mischo, H. Potential and applications of underground in-situ bioleaching. In Proceedings of the AusIMM Future Mining Conference, Sydney, Australia, 4–6 November 2015. [Google Scholar]
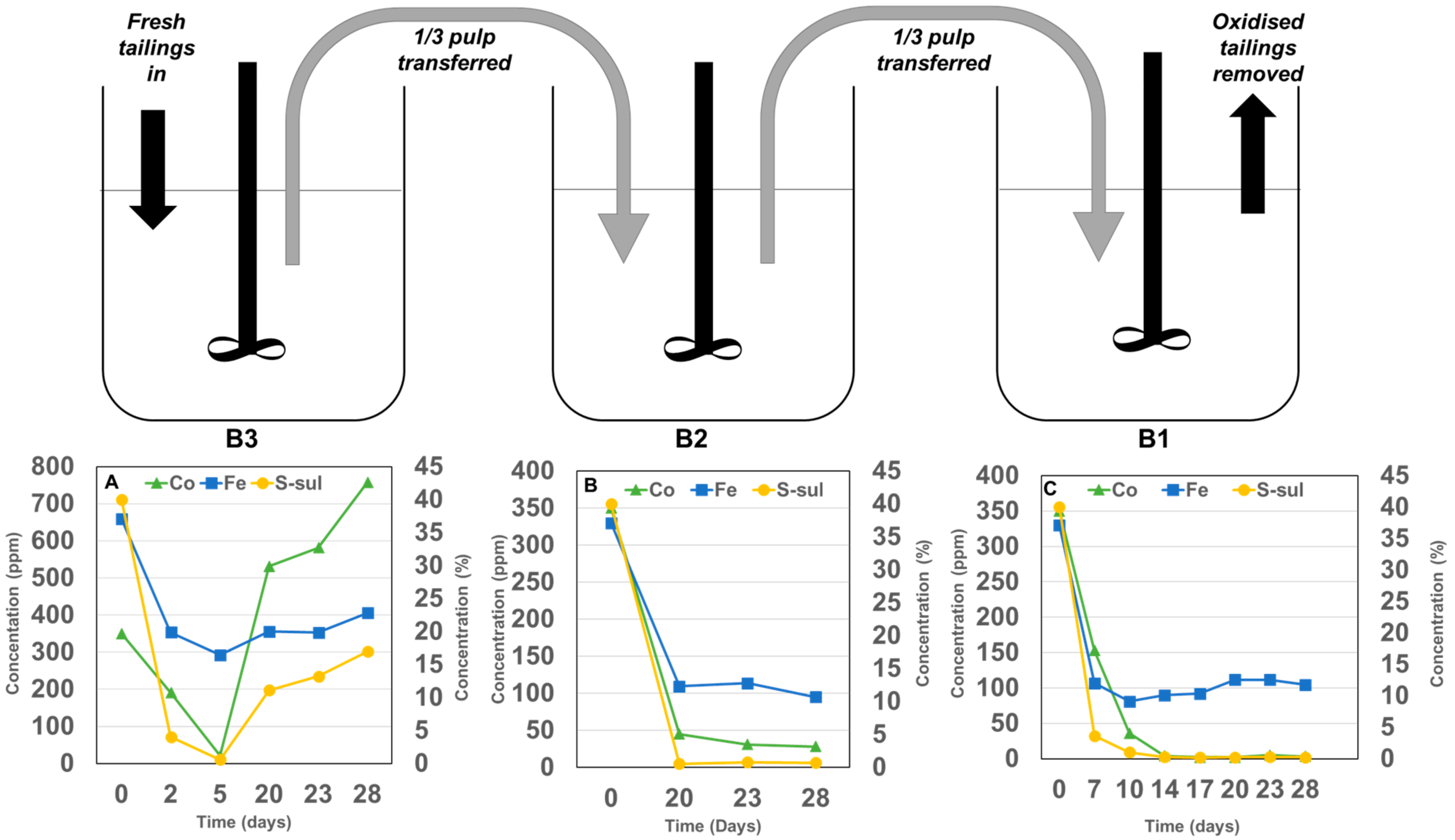
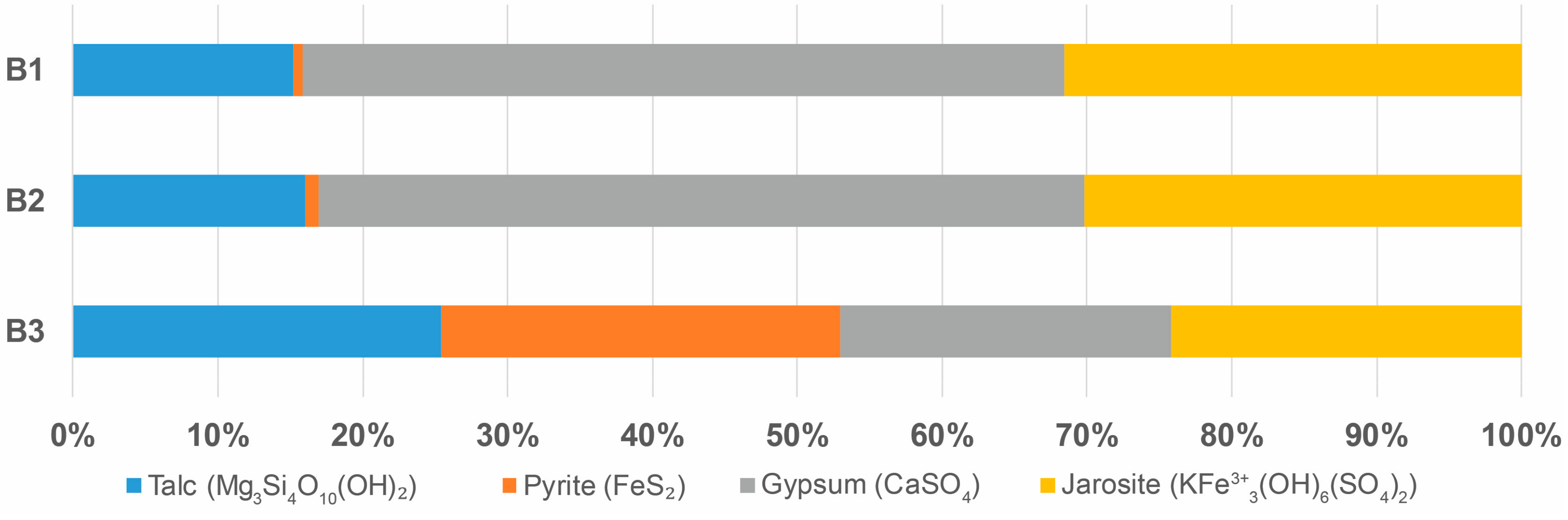
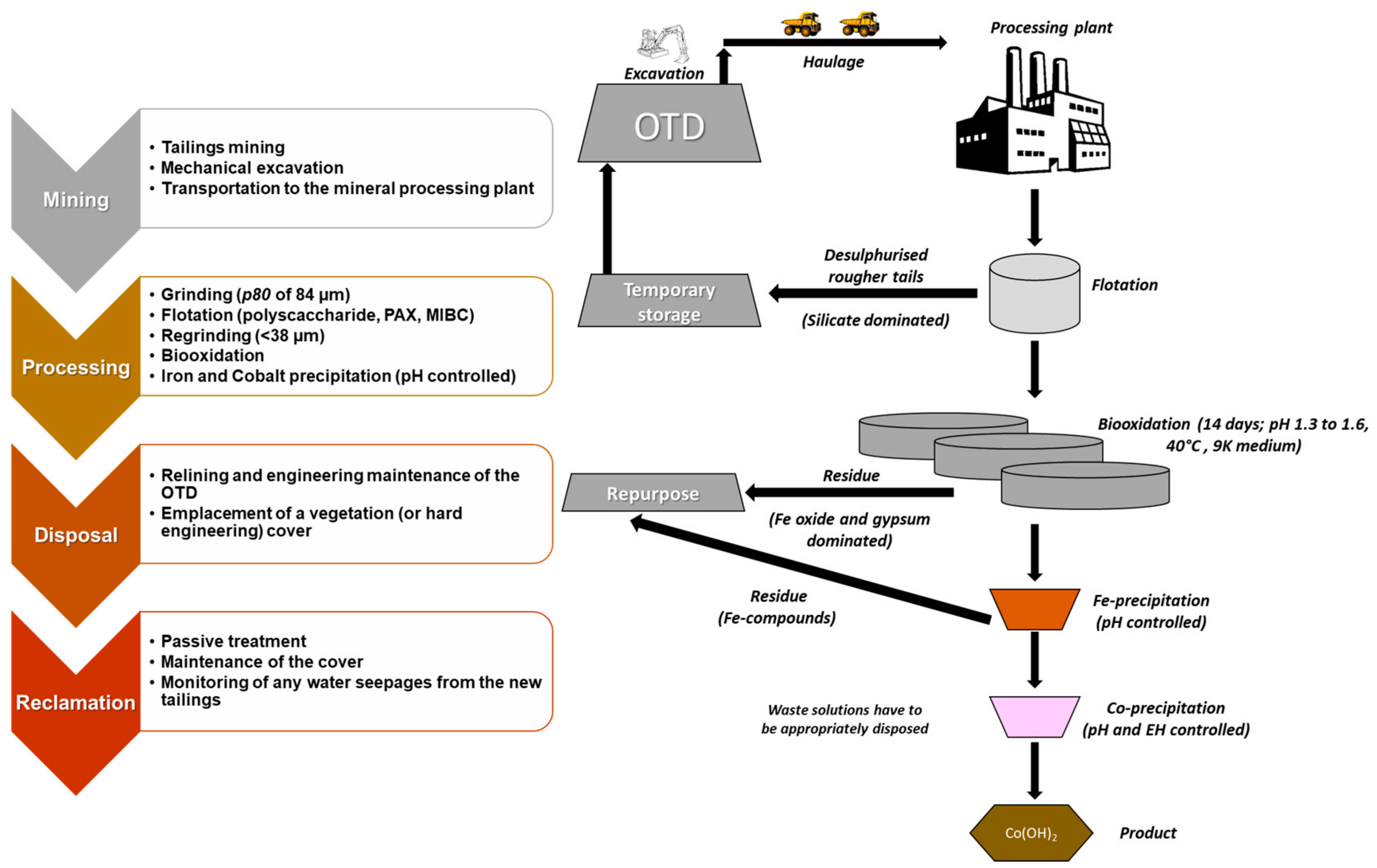
| C | Corg | S | S2− | Fe | Cu | Co | Zn | Ni | Pb | |
|---|---|---|---|---|---|---|---|---|---|---|
| % | % | % | % | % | ppm | ppm | ppm | ppm | ppm | |
| Zone 1 Head | <0.03 | <0.03 | 0.7 | 0.6 | 9.4 | 98 | 60 | 32 | 75 | 15 |
| Zone 2 Head | 0.06 | <0.03 | 15.9 | 14.2 | 18.1 | 2120 | 580 | 88 | 595 | 25 |
| Zone 3 Head | 0.03 | <0.03 | 10.6 | 9.54 | 14.1 | 1480 | 440 | 102 | 375 | 30 |
| Zone 4 Head | <0.03 | <0.03 | 9.5 | 8.6 | 13.6 | 1640 | 380 | 76 | 305 | 35 |
| Composite Head | 0.06 | <0.03 | 9.16 | 8.2 | 14 | 1400 | 360 | 74 | 325 | 30 |
| Parameter | Unit | Tailings Feed | Rougher Concentrate | Rougher Tailings |
|---|---|---|---|---|
| Fe | % | 14 | 37 | 9.5 |
| Cu | ppm | 1100 | 5910 | 452 |
| Co | ppm | 350 | 1840 | 70 |
| Ni | ppm | 350 | 1385 | 170 |
| Pb | ppm | 30 | 80 | 5 |
| Zn | ppm | 114 | 166 | 80 |
| S | % | 7.53 | 44.9 | 1.09 |
| Neutralising characteristics | ||||
| Fizz Rating | - | 1 | 0 | 1 |
| ANC (Sobek) | Kg H2SO4/t | 14 | 0 | 22 |
| Acid generating characteristics | ||||
| MPA | Kg H2SO4/t | 230 | 1374 | 33 |
| NAPP | Kg H2SO4/t | 216 | 1374 | 11 |
| NAG | Kg H2SO4/t | 45 | 115 | 15 |
| NAGpH | - | 2.3 | 2.1 | 2.6 |
| ARD Classification | PAF | PAF | PAF | |
| pH | Fe | Co | Cu | Ni | As | |
|---|---|---|---|---|---|---|
| Experiment | mg/L | mg/L | mg/L | mg/L | mg/L | |
| Test A | 2.18 | 9730 | 135.5 | 275 | 96.7 | 3.6 |
| Test B | 3 | 124 | 135.5 | 218 | 96.3 | 1.7 |
| Test C | 3.8 | 93.6 | 126 | 38.2 | 86.2 | 1.8 |
| Test D | 4.86 | 20.6 | 64.9 | 0.9 | 30 | 0.7 |
| Test E | 6.3 | 0.80 | 1 | <0.2 | 0.6 | 3.5 |
| Feed Liquor | - | 19,950 | 127 | 267 | 90.8 | 6.8 |
| Variable | Experiment | Co (%) Leached at 4 Days | Final Co (%) Leached |
|---|---|---|---|
| pH (40 °C, 9 K fixed) | 1.3–1.4 | 39 | 100 (Day 12) |
| 1.5–1.6 | 39 | 100 (Day 12) | |
| 1.7–1.8 | 32 | 100 (Day 19) | |
| 2.0–2.1 | 32 | 81 (Day 19) | |
| Temperature (pH 1.5–1.6, 9 K fixed) | 35 °C | 32 | 100 (Day 15) |
| 40 °C | 36 | 100 (Day 10) | |
| 45 °C | 36 | 100 (Day 12) | |
| Nutrient medium (pH 1.5–1.6; 40 °C fixed) | 4 K | 60 | 100 (Day 9) |
| 9 K | 37 | 100 (Day 12) | |
| 12 K | 54 | 100 (Day 9) | |
| 16 K | 60 | 100 (Day 10) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parbhakar-Fox, A.; Glen, J.; Raimondo, B. A Geometallurgical Approach to Tailings Management: An Example from the Savage River Fe-Ore Mine, Western Tasmania. Minerals 2018, 8, 454. https://doi.org/10.3390/min8100454
Parbhakar-Fox A, Glen J, Raimondo B. A Geometallurgical Approach to Tailings Management: An Example from the Savage River Fe-Ore Mine, Western Tasmania. Minerals. 2018; 8(10):454. https://doi.org/10.3390/min8100454
Chicago/Turabian StyleParbhakar-Fox, Anita, John Glen, and Bonita Raimondo. 2018. "A Geometallurgical Approach to Tailings Management: An Example from the Savage River Fe-Ore Mine, Western Tasmania" Minerals 8, no. 10: 454. https://doi.org/10.3390/min8100454
APA StyleParbhakar-Fox, A., Glen, J., & Raimondo, B. (2018). A Geometallurgical Approach to Tailings Management: An Example from the Savage River Fe-Ore Mine, Western Tasmania. Minerals, 8(10), 454. https://doi.org/10.3390/min8100454






