Abstract
In this paper, mullite was adopted in order to absorb Palmitic Acid (PA) via a direct impregnation method. The prepared PA/mullite form-stable phase change materials (FSPCM) were systematically characterized by the Leakage Test (LT), Scanning Electron Microscope (SEM), Fourier Transform Infrared Spectroscopy (FTIR), X-Ray Diffraction (XRD), Differential Scanning Calorimeter (DSC), Thermogravimetry (TG) and Cooling Curve Method (CCM). The results indicated that, among these composites with different mass fractions of PA, the sample with the 32 wt % Palmitic Acid has the best properties without any leakage. The enthalpy of 32%PA/68%mullite FSPCM is 50.8 J/g for melting process, and 58.3 J/g for solidifying process. The phase change point of 32%PA/68%mullite FSPCM is 64.1 °C for melting and 58.7 °C for solidifying. The heat storage efficiency of the PA/mullite FSPCM was enhanced considerably by adding mullite. The leakage and thermal properties of PA/mullite FSPCM were discussed and the performance of the FSPCM has been apparently improved.
1. Introduction
With the rapid growth of the world’s population and economy, energy storage technology is increasingly important. Due to the high latent heat capacity and small temperature variation during phase change process, phase change materials (PCM) have lately attracted extensive interest for thermal energy storage [1,2,3,4]. PCM, which is mainly applied in thermal energy storage (TES), is usually divided into organic, inorganic and composite materials. Organic PCMs are mainly paraffin wax, fatty acid and polymer, while inorganic PCMs mainly include hydrated salt, metal, and so on [2]. Compare to other PCMs, there are almost no supercooling and phase separation problems in organic PCMs [5,6]. Hence, organic PCMs are more widely used in TES. However, the leakage and low thermal conductivity of organic materials during the phase change process have greatly hindered the application of PCM [7,8].
In order to overcome the two major challenges of organic PCMs, many scholars have paid more attention on the form-stable phase change materials (FSPCM) in the past few years, in which some kinds of porous materials were introduced to absorb PCM. Among these porous materials for fabricating FSPCM, the clay mineral-based FSPCM has emerged as an interesting candidate for the reasons below [2]. On one hand, some clay minerals have outstanding pore structure, which provides much space for organic materials to be easily absorbed. During this process, the PCM can be limited into the pore structure of clay minerals even if the PCMs become liquid when heated, so the leakage problem can be avoided [9,10,11]. On the other hand, because of the relatively high thermal conductivity of clay mineral, the overall thermal conductivity of clay mineral-based FSPCMs will be enhanced conspicuously [10,12,13]. The clay minerals, which are used as the supporting materials to prepare FSPCM, are commonly diatomite [14], attapulgite [15], perlite [16,17], vermiculite [18], kaolinite [19], montmorillonite [20], etc. This is because the porosity of diatomite is quite high, and can reach up to 90% [21], expanded vermiculite and perlite have significant pore structures [22] and attapulgite has a remarkable specific surface area (300–600 m2) [23]. Mullite, a heated product of clay mineral-kaolin, has a lot of advantages such as a nontoxic, developed pore structure; low cost, superior mechanical strength; and relatively high thermal conductivity, whose character is even better than some other clay minerals [8,10,13]. The thermal properties of the clay mineral-based FSPCM are well proved after being investigated. Therefore, mullite could be a quite great candidate for the supporting material of PCM. However, there are only a few scholars who have researched the mullite-based FSPCM in the literature [24,25,26].
Among the all PCMs, fatty acids (FAs) have high latent heat of transition, as well as very small volume change, mostly no supercooling, and are nontoxic and producible from renewable resources [27]. Due to the proper phase change point (about 60 °C), palmitic acid (PA) is quite suitable FA to fabricate the mullite-based FSPCM [28]. As far as the authors know, PA/mullite FSPCM has not been fabricated and characterized in any other literature till now.
Thus, in the present work, PA/mullite FSPCMs are fabricated to research the thermophysical properties. The chemical compatibility of PA/mullite composites was characterized by the Fourier Transform Infrared (FTIR) Spectroscopy and the microstructure was researched using a Scanning Electron Microscope (SEM). The thermal properties and thermal stability were subsequently tested using Differential Scanning Calorimetry (DSC), the Cooling Curve Method (CCM) and Thermal Gravimetric Analysis (TGA).
2. Experimental Section
2.1. Materials
Mullite, which was produced by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) was employed as the supporting material, while Palmitic Acid (PA, phase change point is about 60 °C), also provided by Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China), acted as PCM.
2.2. Preparation of PA/Mullite FSPCM
The PA/mullite FSPCM were prepared via direct impregnation method [13,29]. PA can be absorbed into the interlayer spacing [10,18] due to the developed interlayer spacing in the porous mullite [25,26]. In order to increase its porosity of supporting material, the mullite was preprocessed at 105 °C for 12 h in the baking oven for dehydration before the fabrication of PA/mullite FSPCM. PA was then heated to the temperature of 80 °C in the water bath, which is higher than its phase change point, and the liquid PCM was injected into preprocessed mullite to fabricate the FSPCM.
Six PA/mullite composites, whose weight fraction of PA varied from 30% to 70% with an interval of 10%, were firstly prepared in this experiment. The preparation process is exhibited in Figure 1. As shown obviously in Figure 1a–d, PA cannot be absorbed fully by mullite, and there is liquid PA left in the beaker. However, in Figure 1e, there is no leakage in the beaker even if the ambient temperature exceeds the melting point of PA. In order to obtain the optimal percentage of PA/mullite FSPCM, another four composites were then fabricated, in which the weight fraction of PA varied from 30% to 40% with a smaller interval of 2%. All the samples are marked according to Table 1 in this paper. The leakage tests of the PA/mullite composites which were heated at 80 °C for 20 min, are demonstrated in Figure 2. Figure 2a–d show the heating process of samples, while Figure 2e–h show the imprint left in the filter paper by the paper after being heated at 80 °C for 20 min.
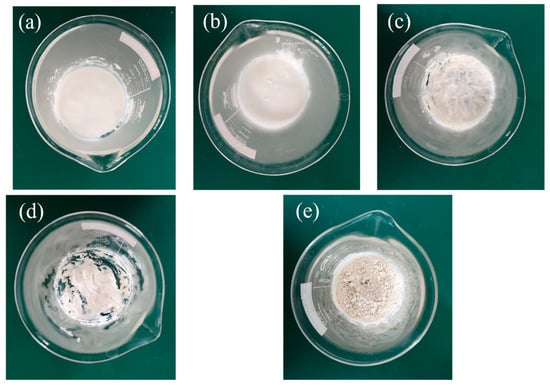
Figure 1.
The preparation picture of Palmitic Acid (PA)/mullite composites (a) S-1-2; (b) S-1-3; (c) S-1-4; (d) S-1-5; (e) S-1-6.

Table 1.
The composition ratio of the PA/mullite composite.
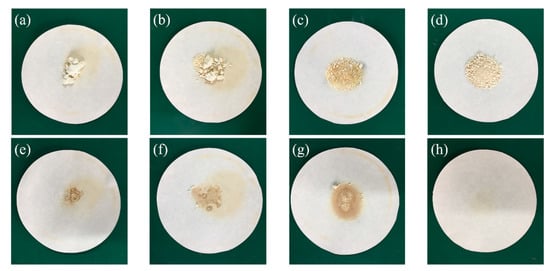
Figure 2.
The leakage test of the PA/mullite composite: (a–d) the heating process; (e–h) the imprint left in the filter paper.
As shown in Figure 2 S-2-1~S-2-3, of which the loading of PA was 38 wt %, 36 wt % and 34 wt %, showed an obvious deformation, indicating that they could not be regarded as form-stable PCMs. On the other hand, S-2-4, containing 32 wt % PA, demonstrated good stability without any leakage. Additionally, as can be seen from Figure 2h, there was no imprint left in the filter, while in Figure 2e–g there was a noticeable imprint left. The experimental phenomenon in Figure 2e–h further confirmed that 32%PA/68%mullite is the optimal percentage of the composites. Hence, we could conclude that the PA/mullite composites possess excellent form-stability when the loading of PA is 32 wt %.
2.3. Characterization Techniques
The Fourier Transform Infrared Spectroscopy (FTIR) spectra were inspected by Fourier Transform Infrared Spectrometer (FTIR, Nicolet is5, Thermo Scientific, Waltham, MA, USA) to study the chemical structure of the composites, whose wavenumber region of FTIR spectra ranged from 400 cm−1 to 4000 cm−1, with a resolution of 2 cm−1 using KBr pellets. To investigate the chemical compatibility between PA and mullite, X-ray diffraction (XRD, D/max-rB, Rigaku, Tokyo, Japan) were collected in the 2θ range 4°–50° using Ni-filtered Cu Ka radiation (λ = 0.1541 nm) and operating at 40 kV and 40 mA with a scanning rate of 4°/min. The method of Scanning Electron Microscope (SEM, Phenom ProX, Eindhoven, The Netherlands, operating voltage: 3 kV) was utilized to investigate the impregnation morphology of PA, mullite and PA/mullite FSPCM. The phase change point and latent heat of PA and PA/mullite were researched by the Differential Scanning Calorimeter (DSC, Q100, TA, New Castle, DE, USA). The testing temperature was between 25 °C (room temperature) and 90 °C, while the heating rate was 10 °C per minute and the samples were under nitrogen. The temperature fluctuation was ±0.1 °C and the accuracy of DSC was 0.1 μW.
The thermal stability of PA and PA/mullite FSPCM was studied by Thermal Gravimetric Analyzer Instrument (TGA, Q600, TA, New Castle, DE, USA) and the operating temperature ranged from 25 °C (room temperature) to 600 °C with a heating rate of 20 °C/min. The cooling curve of PA/mullite composites was investigated by Paperless Recorder (PR, ECR7100, Yikong, Hangzhou, ZJ, China), which can record the temperature variations of the PA/mullite mixtures. For investigating the thermophysical properties of PA/mullite composites, 10 g composite for each sample was loaded into the centrifuge tube. In addition, the temperature ranged from 25 °C (room temperature) to 90 °C for the heating process and 90 °C to 25 °C for the freezing process, respectively. During the whole process, the temperature data were recorded by the paperless logger.
3. Results and Discussion
3.1. Chemical Compatibility of the PA/Mullite FSPCM
The chemical compatibility between PCM and the supporting material is critical for the application of the composites [30,31]. Therefore, the FTIR spectrum and the XRD patterns of PA, mullite and the selected PA/mullite FSPCM were investigated and showed in Figure 3. As shown in Figure 3a, the strong peaks at 2917 and 2860 cm−1 in the spectra of the PA are the asymmetric and symmetric vibration of –CH3 and –CH2 group, while the band at 1702 cm−1 stands for the C=O stretching vibration, the peak at 1071 cm−1 signifies the C–O stretching vibration [28]. The wide peak between 1000 and 1200 cm−1 in the spectra of mullite may be ascribed to the Al–O and Si–O stretch vibration [32]. As shown in Figure 3a, all the characteristic peaks of PA and mullite appear and no new peaks emerge in the FTIR spectra PA/mullite FSPCM. Figure 3b shows the XRD patterns of PA, mullite and PA/mullite FSPCM. We can see from the picture that PA has two sharp peaks at 21.4° and 24.0°, while the XRD pattern of mullite presents a flat peak with three characteristic peaks at 16.4°, 26.2° and 40.8°. Comparing the diffraction peak of the composite with the components, the composite does not show any new peaks and peak position movement. By combining the results of FTIR spectra and XRD patterns, we can conclude that the composites are a physical combination of PA and mullite, and the chemical compatibility of the selected FSPCM is very good.
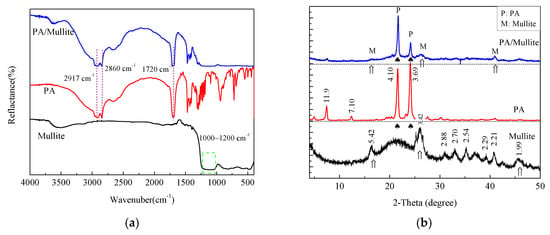
Figure 3.
(a) Fourier Transform Infrared Spectroscopy (FTIR) spectra and (b) X-Ray Diffraction (XRD) patterns of the PA, mullite, and PA/mullite composite.
3.2. The Microstructure of the PA/Mullite FSPCM
Figure 4 showed the SEM images of pure PA, mullite and the selected PA/mullite FSPCM. As demonstrated in Figure 4, mullite have an irregular flake structure, and their particle sizes are heterogeneous, this feature resulted in the irregularity of thermophysical properties of composites [10,33,34]. Figure 4c shows the anomalistic morphology of PA/mullite FSPCM. As we can see from Figure 4, the FSPCMs were prepared successfully, in which the PA was adsorbed on the surface of mullite.
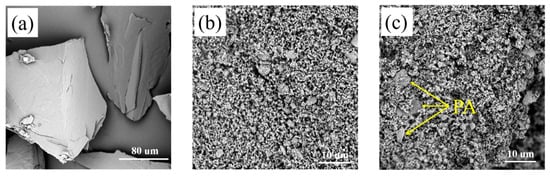
Figure 4.
Scanning Electron Microscope (SEM) images of (a) PA, (b) mullite and (c) PA/mullite.
3.3. Thermal Properties of the PA/Mullite FSPCM
The phase change point and latent heat of PA/mullite FSPCM are two key parameters for its application. Thus, the PCM selected to prepare the FSPCM should have a proper phase change point and sufficiently high enthalpy. The melting temperature and latent heat of the chosen PA were measured by DSC method as 64.4 °C and 211.8 J/g, respectively. As shown in the DSC curves in Figure 5, the other two samples have steady phase change behavior just like the pure PA. The thermal properties of the composites obtained from the DSC are also presented in Table 2.
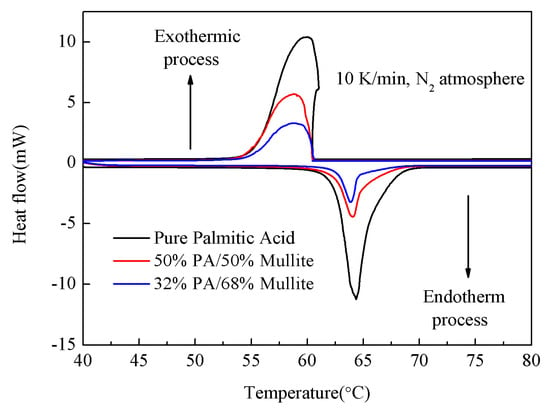
Figure 5.
Differential Scanning Calorimeter (DSC) curves of pure PA, 50%PA/50%mullite and 32%PA/68%mullite.

Table 2.
The thermal properties of the PA/mullite composites.
As the Table 2 shows, the prepared PA/mullite composites including S-1-1, S-1-4, S-2-4 melt at 64.4 °C, 64.1 °C and 64.1 °C, and stored latent heat of 211.8, 80.1 and 50.8 J/g, respectively, while the solidifying temperature and latent heat of the three samples were 60.08 °C, 58.4 °C, 58.7 °C and −211.2, −88.4, −61.3 J/g, respectively. Pure PA has a supercooling degree of 4.3 °C, while the S-1-4 and S-2-4 have the supercooling of 5.7 and 5.4, respectively. The phase change point of the fabricated PA/mullite FSPCMs makes them have a great application prospect for an indoor hot water system. Moreover, after impregnating the PA to the mullite, the prepared preferred PA/mullite composite can be form-stable, compared to the pure PA, due to capillary force and surface tension, which is also mentioned in other PCM composites by some scholars [3,8,10,35].
Compare to pure PA, the enthalpy of PA/mullite FSPCM is lower than the theoretical value. On one hand, the mass fraction of PA decreases in the PA/mullite FSPCM, which lowers the enthalpy of the composites. On the other hand, the decrease in the latent heats should also be attributed to the crystallization value (CV) of PA in the composites, which are greatly influenced by the supporting material [3]. The mullite in the PA/mullite FSPCM might stop PA from crystallizing fully, therefore the CV of PA declines to some extent, which also can make the thermal properties of the composites decrease. The CV value of PA is calculated using the equation [3,8] below:
where and are the enthalpy of pure PA and the composite, respectively and stands for the mass fraction of PA. All the CV values of PA/mullite composites listed in Table 2 were calculated according to this equation. The CV of S-1-4 and S-2-4 were 83.7% and 86.3%, respectively.
In order to investigate the reliability of PA/mullite FSPCM, the 150-cycle experiment was conducted. As shown in Figure 6, no significant difference can be observed in the picture after thermal cycles, indicating the FSPCM has great thermal reliability and a long life cycle [31].
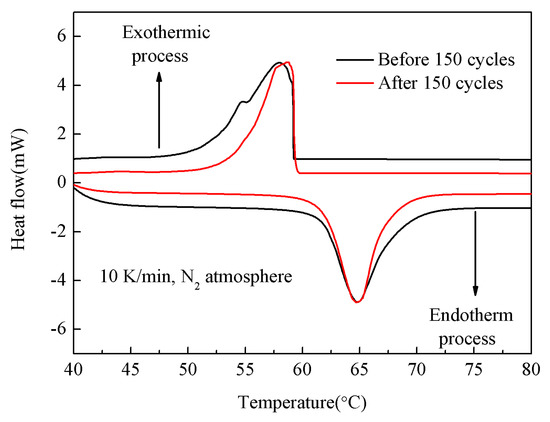
Figure 6.
DSC curve of PA/mullite Form-Stable Phase Change Materials (FSPCM) before and after 150 thermal cycles.
3.4. Thermal Stability of PA and PA/Mullite FSPCM
Thermal stability is also the critical performance to be considered for choosing PCM, which means that a newly-built FSPCM should be thermally durable when the ambient temperature exceeds its operating temperature. Therefore, fabricated PA/mullite FSPCM was investigated by Thermogravimetry (TG) analysis to test the thermal stability of the prepared PA/mullite composites. The obtained curves of mullite, pure PA and selected PA/mullite FSPCM are shown in Figure 7. As shown in Figure 7, there is about 28.19% weight loss of PA/mullite FSPCM even if the ambient temperature exceeds 600 °C, while PA almost lost all the weight when the heating temperature was up to 400 °C. In addition, when the temperature reached 200 °C, there was almost no weight loss of the composites, representing that the thermal stability of the PA/mullite FSPCM is much better than the pure PA.
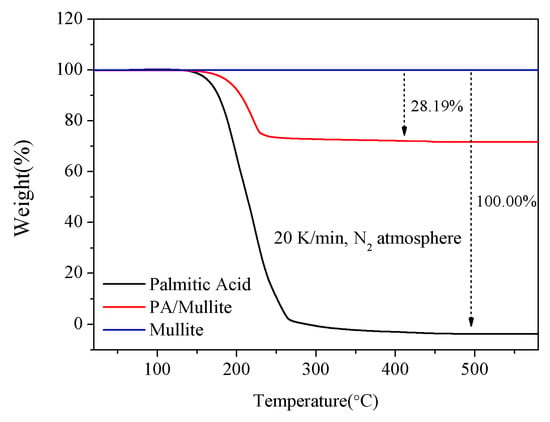
Figure 7.
Thermogravimetry (TG) curves of the PA/mullite FSPCM.
3.5. The Cooling Curve of the PA/Mullite FSPCM
The cooling curve of PA/mullite composites was researched and is demonstrated in Figure 8. The curves of Figure 8 exhibit that the melting and cooling rate of PA/mullite FSPCM was relatively high compared with pure PA. It took the pure PA about 15 min to accomplish the melting process when heated, while 50%PA/50%mullite and 32%PA/68%mullite composites only spent 6 min and 5 min for the same melting process, respectively. In the solidification process, it took pure PA about 10 min to complete this process, and while 50%PA/50%mullite and 32%PA/68%mullite composites only need 4 min and 3 min for solidifying. The results indicate that the overall thermal conductivity of PA/mullite FSPCM can be enhanced significantly because thermal conductivity of mullite is higher than pure PA.
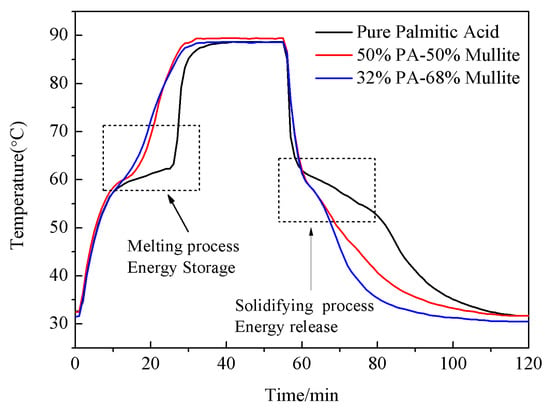
Figure 8.
The cooling curves of PA and PA/mullite composites.
4. Conclusions
The PA/mullite FSPCM has been fabricated by the direct impregnation method. The chemical compatibility, morphology and thermophysical properties of PA/mullite FSPCM have been systematically investigated by FTIR, XRD, SEM, DSC, TGA, CCM. The FTIR and XRD results demonstrate that the chemical compatibility of PA/mullite FSPCM is quite good because there are only physical interactions existing between PA and mullite. The melting and solidifying temperatures of the optimal PA/mullite FSPCM were 64.1 °C and 58.7 °C, respectively. The enthalpy of PA/mullite FSPCM reached 50.8 J/g for melting process and 58.3 J/g for solidifying process. The thermal cycle test indicated the FSPCM has excellent thermal reliability and a long life cycle. There are about 28.19% weight losses of PA/mullite FSPCM even if the ambient temperature exceeds 600 °C, while PA almost lost all the weight when the heating temperature was up to 400 °C., indicating that the thermal stability of the PA had improved significantly after the PA was absorbed into the porous mullite. The PA/mullite FSPCM had a good thermal stability. Due to the relatively high thermal conductivity of mullite, the thermal conductivity of PA/mullite FSPCM has been enhanced considerably. Based on all the results, the PA/Mullite FSPCM has a good application prospect in the field of indoor hot water systems, due to its good thermophysical properties.
Author Contributions
Conceptualization, X.G.; formal analysis, X.G. and L.P.; funding acquisition, X.G. and L.B.; investigation, P.L.; methodology, X.G. and L.B.; project administration, X.G.; validation, L.P. and H.H.; visualization, X.B.; writing—original draft, X.G. and P.L.; writing—review and editing, X.G., L.B., L.P., Y.L. and H.H. All authors discussed the results and commented on the manuscript.
Funding
This work is supported by the National Natural Science Foundation of China (41872039 and 41831285), the Open Project of State Key Laboratory Cultivation Base for Nonmetal Composites and Functional Materials [17kffk13], the One-Thousand-Talents Scheme in Sichuan Province, Sichuan Science and Technology Program [2018JY0462], Hebei Outstanding Young Scholars, Longshan Fund of Southwest University of Science and Technology (17QR004), the Opening Project of Material Corrosion and Protection Key Laboratory of Sichuan province [2018CL20], Hebei Key Technology R&D Program of the Agency of Hebei province [17214016], and PhD Research Startup Foundation of Hebei GEO University [BQ2017020, BQ2017021].
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Abokersh, M.; Osman, M.; El-Baz, O.; El-Morsi, M.; Sharaf, O. Review of the phase change material (PCM) usage for solar domestic water heating systems (SDWHS). Int. J. Energy Res. 2018, 42, 329–357. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Review on clay mineral-based form-stable phase change materials: Preparation, characterization and applications. Renew. Sustain. Energy Rev. 2017, 68, 707–726. [Google Scholar] [CrossRef]
- Gu, X.; Qin, S.; Wu, X.; Li, Y.; Liu, Y. Preparation and thermal characterization of sodium acetate trihydrate/expanded graphite composite phase change material. J. Therm. Anal. Calorim. 2016, 125, 831–838. [Google Scholar] [CrossRef]
- Sharma, A.; Tyagi, V.; Chen, C.; Buddhi, D. Review on thermal energy storage with phase change materials and applications. Renew. Sustain. Energy Rev. 2009, 13, 318–345. [Google Scholar] [CrossRef]
- Zalba, B.; Marin, J.; Cabeza, L.; Mehling, H. Review on thermal energy storage with phase change: Materials, heat transfer analysis and applications. Appl. Therm. Eng. 2003, 23, 251–283. [Google Scholar] [CrossRef]
- Ibrahim, N.; Al-Sulaiman, F.; Rahman, S.; Yilbas, B.; Sahin, A. Heat transfer enhancement of phase change materials for thermal energy storage applications: A critical review. Renew. Sustain. Energy Rev. 2017, 74, 26–50. [Google Scholar] [CrossRef]
- Sarı, A. Fabrication and thermal characterization of kaolin-based composite phase change materials for latent heat storage in buildings. Energy Build. 2015, 96, 193–200. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Stearic acid hybridizing coal–series kaolin composite phase change material for thermal energy storage. Appl. Clay Sci. 2014, 101, 277–281. [Google Scholar] [CrossRef]
- Karaipekli, A.; Biçer, A.; Sarı, A.; Tyagi, V. Thermal characteristics of expanded perlite/paraffin composite phase change material with enhanced thermal conductivity using carbon nanotubes. Energy Convers. Manag. 2017, 134, 373–381. [Google Scholar] [CrossRef]
- Lv, P.; Liu, C.; Rao, Z. Experiment study on the thermal properties of paraffin/kaolin thermal energy storage form-stable phase change materials. Appl. Energy 2016, 182, 475–487. [Google Scholar] [CrossRef]
- Fu, X.; Liu, Z.; Xiao, Y.; Wang, J.; Lei, J. Preparation and properties of lauric acid/diatomite composites as novel form-stable phase change materials for thermal energy storage. Energy Build. 2015, 104, 244–249. [Google Scholar] [CrossRef]
- Peng, K.; Fu, L.; Li, X.; Ouyang, J.; Yang, H. Stearic acid modified montmorillonite as emerging microcapsules for thermal energy storage. Appl. Clay Sci. 2017, 138, 100–106. [Google Scholar] [CrossRef]
- Li, C.; Fu, L.; Ouyang, J.; Tang, A.; Yang, H. Kaolinite stabilized paraffin composite phase change materials for thermal energy storage. Appl. Clay Sci. 2015, 115, 212–220. [Google Scholar] [CrossRef]
- Xu, B.; Li, Z. Performance of novel thermal energy storage engineered cementitious composites incorporating a paraffin/diatomite composite phase change material. Appl. Energy 2014, 121, 114–122. [Google Scholar] [CrossRef]
- Li, M.; Wu, Z.; Kao, H. Study on preparation, structure and thermal energy storage property of capric–palmitic acid/attapulgite composite phase change materials. Appl. Energy 2011, 88, 3125–3132. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Assessing the feasibility of integrating form-stable phase change material composites with cementitious composites and prevention of PCM leakage. Mater. Lett. 2017, 192, 88–91. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Wang, X.; Sanjayan, J.; Wilson, J. Thermal energy storage enhancement of lightweight cement mortars with the application of phase change materials. Procedia Eng. 2017, 180, 1170–1177. [Google Scholar] [CrossRef]
- Xu, B.; Ma, H.; Lu, Z.; Li, Z. Paraffin/expanded vermiculite composite phase change material as aggregate for developing lightweight thermal energy storage cement-based composites. Appl. Energy 2015, 160, 358–367. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Zhang, Y.; Chen, S.; Li, Q.; Guo, Y.; Xiong, C. Lauric acid/intercalated kaolinite as form-stable phase change material for thermal energy storage. Energy 2014, 76, 385–389. [Google Scholar] [CrossRef]
- Cai, Y.; Song, L.; He, Q.; Yang, D.; Hu, Y. Preparation, thermal and flammability properties of a novel form-stable phase change materials based on high density polyethylene/poly (ethylene-co-vinyl acetate)/organophilic montmorillonite nanocomposites/paraffin compounds. Energy Convers. Manag. 2008, 49, 2055–2062. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, Y.; Liu, X.; Sun, J.; Sha, G.; Yang, J.; Meng, C. A novel pathway for the synthesis of ordered mesoporous silica from diatomite. Mater. Lett. 2014, 119, 150–153. [Google Scholar] [CrossRef]
- Karaipekli, A.; Sarı, A. Preparation, thermal properties and thermal reliability of eutectic mixtures of fatty acids/expanded vermiculite as novel form-stable composites for energy storage. J. Ind. Eng. Chem. 2010, 16, 767–773. [Google Scholar] [CrossRef]
- Song, S.; Dong, L.; Chen, S.; Xie, H.; Xiong, C. Stearic–capric acid eutectic/activated-attapulgiate composite as form-stable phase change material for thermal energy storage. Energy Convers. Manag. 2014, 81, 306–311. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; Su, W.; Zhao, H.; Wang, C. Impregnation of porous mullite with Na2SO4 phase change material for thermal energy storage. Sol. Energy Mater. Sol. Cells. 2015, 134, 268–274. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Porous ceramic stabilized phase change materials for thermal energy storage. RSC Adv. 2016, 6, 4833–4842. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, Y.; Wu, J.; Hu, C.; Tang, Z. Preparation and performance study of cordierite/mullite composite ceramics for solar thermal energy storage. Int. J. Appl. Ceram. Technol. 2017, 14, 162–172. [Google Scholar] [CrossRef]
- Sarı, A.; Bicer, A.; Al-Ahmed, A.; Al-Sulaiman, F.; Zahir, M.; Mohamed, S. Silica fume/capric acid-palmitic acid composite phase change material doped with CNTs for thermal energy storage. Sol. Energy Mater. Sol. Cells 2018, 179, 353–361. [Google Scholar] [CrossRef]
- Zeng, J.; Sun, S.; Zhou, L.; Chen, Y.; Shu, L.; Yu, L.; Zhu, L.; Song, L.; Cao, Z.; Sun, L. Preparation, morphology and thermal properties of microencapsulated palmitic acid phase change material with polyaniline shells. J. Therm. Anal. Calorim. 2017, 129, 1583–1592. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, N.; Peng, J.; Fang, X.; Gao, X.; Fang, Y. Preparation and thermal energy storage properties of paraffin/expanded graphite composite phase change material. Appl. Energy 2012, 91, 426–431. [Google Scholar] [CrossRef]
- Li, M.; Guo, Q.; Nutt, S. Carbon nanotube/paraffin/montmorillonite composite phase change material for thermal energy storage. Sol. Energy 2017, 146, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Meng, Z.; Zhu, H.; Wang, Y.; Peng, S. Melting heat transfer characteristics of a composite phase change material fabricated by paraffin and metal foam. Appl. Energy 2017, 185, 1971–1983. [Google Scholar] [CrossRef]
- Khorchani, I.; Hafef, O.; Reinosa, J.; Matoussi, A.; Fernandez, J. AC electrical conduction mechanisms and dielectrical studies of DD3 kaolin sintered at high temperature. Mater. Chem. Phys. 2018, 212, 187–195. [Google Scholar] [CrossRef]
- Liu, S.; Yang, H. Composite of Coal-Series Kaolinite and Capric-Lauric Acid as Form-Stable Phase-Change Material. Energy Technol. 2015, 3, 77–83. [Google Scholar] [CrossRef]
- Ali, M.; Yiu, L.; Shi, X.; Barbhuiya, S.; Cui, H. Preparation, characterization and thermal properties of Lauryl alcohol/Kaolin as novel form-stable composite phase change material for thermal energy storage in buildings. Appl. Therm. Eng. 2013, 59, 336–347. [Google Scholar]
- Min, X.; Fang, M.; Huang, Z.; Huang, Y.; Wen, R.; Qian, T.; Wu, X. Enhanced thermal properties of novel shape-stabilized PEG composite phase change materials with radial mesoporous silica sphere for thermal energy storage. Sci. Rep. 2015, 5, 12964. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).