Abstract
Hydrometallurgical researchers, and other professionals alike, invest significant amounts of time reading scientific articles, technical notes, and other scientific documents, while looking for the most relevant information for their particular research interest. In an attempt to save the researcher’s time, this study presents an information retrieval tool using case-based reasoning. The tool was built for comparing scientific articles concerning cyanide-free leaching of gold ores/concentrates/tailings. Altogether, 50 cases of experiments were gathered in a case base. 15 different attributes related to the treatment of the raw material and the leaching conditions were selected to compare the cases. The attributes were as follows: Pretreatment, Overall method, Complexant source, Oxidant source, Complexant concentration, Oxidant concentration, Temperature, pH, Redox-potential, Pressure, Materials of construction, Extraction, Extraction rate, Reagent consumption, and Solid-liquid ratio. The resulting retrieval tool (LeachSim) was able to rank the scientific articles according to their similarity with the user’s research interest. Such a tool could eventually aid the user in finding the most relevant information, but not replace thorough understanding and human expertise.
1. Introduction
In recent years, cyanide leaching of gold ores has become politically and environmentally more controversial. New alternatives have been investigated broadly [1,2] and one cyanide-free process, thiosulfate leaching of gold, is utilized on industrial scale by Barrick Gold Corporation [3,4]. Several other cyanide-free methods have been studied, such as chloride/hypochlorite [5], cupric chloride [6], ferric chloride [7], thiourea [8], and glycine [9], along with several others [2]. This article aims to create a retrieval tool for uncovering the most relevant research information about cyanide-free gold leaching based on the leaching process, material treatment, and achieved results.
There are several research engines available for information retrieval, such as Google Scholar, and databases of peer-reviewed literature, such as Scopus, but the retrieval of articles within these tools is often based on exact matches. Therefore, the user often receives a lot of unimportant results and might miss some relevant ones simply because of difference in wording. For example, if one searches for “cyanide-free”, the articles with the phrase “non-cyanide” are likely left out from the top results.
Previous research has shown that a branch of artificial intelligence called Case-based Reasoning (CBR) is a suitable knowledge modelling method in the field of hydrometallurgical applications [10]. The main benefits of CBR in this area are the ability to function with incomplete data, and that it produces results even without exact matches, but by comparing similarities [11]. The flexible similarity comparison, without the need for exact matches, is especially useful in comparing the mineralogy of ores where each ore body is unique. Therefore, CBR was selected as the knowledge modelling method for this research. Due to the many advantages of CBR, it can be utilized in a variety of industrial applications, such as prediction of process parameters [12,13], operation adjustment [14], and machinery design [15].
The CBR method compares a new problem description to descriptions of previously solved problems and it tries to reuse the solution to the most similar previously solved problem, assuming that similar problems have similar solutions [16]. The old problem descriptions and their respective solutions form cases, while the description of the new problem is called a query case. The problem description parts of the cases hold several defining attributes, such as mass, time, temperature, or any qualitative/quantitative feature that might affect the solution of the case. In order to compare the cases with the query case, the software compares each attribute value in the query with the corresponding values in the cases in the case base. The similarity between these attribute values is the so called local similarity value, as defined by a local similarity model. Based on these local similarity values, the system calculates a global similarity value for each case according to Equation (1).
Here, Sim is the global similarity between the submitted query q and a case c, there are n attributes in each case, ωi denotes the weight assigned for attribute i, and simi represents the local similarity between attribute values qi in the query and ci in the case. The result is scaled to be within [0, 1] [17].
It is common to illustrate CBR through the CBR cycle in Figure 1. In the cycle, and in CBR generally, cases are retrieved from the case base according to the similarity calculation. Then, the most similar ones are reused for the new problem, which leads to a revised new case that can be retained in the case base.
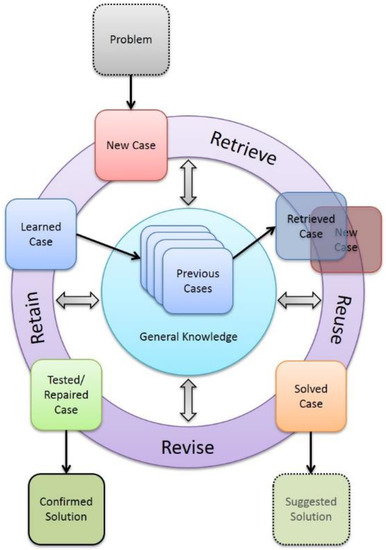
Figure 1.
The case-based reasoning cycle, redrawn after [16].
This research focuses on the retrieval part of the CBR cycle. The new problem is a research topic/interest, which is entered into the system as a query consisting of process parameters. Scientific articles with similar process parameters are retrieved from the case base. The problem description of a case includes the process parameters and the scientific article that has studied those parameters can be seen as the solution part of the case. The retrieved scientific articles can then be used, for example, as a starting point for new research, as a literature reference, or as a source for new ideas.
This study is based on a previously constructed CBR-model [18]. The preliminary knowledge model was built for comparing the cyanide-free gold leaching methods for a particular type of ore. The model included a case base of 24 cases, consisting of previously conducted cyanide-free leaching experiments. The cases were collected from published scientific articles, short communications, and technical reports. It then used a simple ore description to compare the cases with each other and returned the most similar articles to the user. However, the preliminary model had a narrow focus in the case base, and, when it came to the leaching procedure, it considered only the leaching method, not taking into account any specific leaching parameters.
This study continues to develop the initial model by expanding the case base, adding more attributes, and developing the similarity modelling concerning the leaching process and material treatment. From here on, this improved model is called the LeachSim model.
2. Methods
This section explains the different tools and techniques that were used in defining, implementing, and testing the LeachSim model.
2.1. Utilized Software
The open source software utilized in this research, myCBR 3.0 (German Research Center for Artificial Intelligence, Kaiserslautern, Germany and University of West London, London, UK) [19], is designed for the rapid prototyping of CBR systems [17]. This particular software has been in use for a hydrometallurgical application previously [10,20,21]. myCBR Workbench has several functionalities that make it suitable for developing a metallurgical application, such as the possibility to construct several different similarity models or case bases that can be selected for each test run [21]. This gave great flexibility for preliminary testing while developing the knowledge model.
2.2. Interviewing Technique
In order to determine the most useful attributes to be included in the revised knowledge model, a series of interviews was conducted with altogether 23 professionals in the field of gold hydrometallurgy. The interviewees described their careers as industrial (6), academic (9), or both (8), represented four different continents, and possessed varying levels of expertise. The scope of targeted interviewees was set as large as possible, because the decision support system should ultimately serve both the senior and the less experienced users. The objective was to collect the most important attributes (attributes that the interviewees considered first) when considering hydrometallurgical cyanide-free leaching of a new gold ore. The interviews were performed by utilizing a semi-structured interviewing technique [22] (pp. 392–395), where the questions were predefined, asked in the same order, and no additional questions were asked. All of the questions were open questions, meaning that the interviewee could answer them in their own words [23]. The questions presented during the interviews are presented in Appendix B. The questions were the same as the ones used while compiling the initial knowledge model [18]. One way to determine an adequate sample size, i.e., the number of interviewed researchers, is based on the concept of data saturation, which is widely utilized in health sciences, to confirm content validity [24]. Data saturation is achieved when no new ideas or concepts emerge from increasing the number of interviews [25] (pp. 61–62). One suggested approach is conducting a minimum of ten interviews, after which interviews are continued until three consecutive interviews do not introduce any new information [26]. During the 23 interviews performed for this study, the last nine interviewees did not propose any attributes that had not been mentioned by former interviewees. Therefore, it was concluded that the sample size of 23 had been adequate.
The ore and process attributes mentioned by the interviewees were quantified, and the ones that were mentioned most frequently were selected to be incorporated into the knowledge model. This article discusses the creation and testing of the LeachSim model, which includes the attributes concerning process parameters and leaching results.
2.3. Testing the Improved Model
First, the LeachSim model was tested by simple queries in order to verify that the myCBR similarity calculations functioned correctly. The queries consisted of one or more attribute values and the global similarity values given by the system were compared with manual calculations to confirm the results. Second, the LeachSim’s performance in comparing the cyanide-free gold leaching process parameters was tested by artificial test queries. The aim of the performance tests was to demonstrate the functionality of the tool in retrieving scientific articles based on the user’s research interest.
3. LeachSim Model
As mentioned earlier, this research builds on the preliminary CBR model developed by the authors [18]. This section discusses how the initial model has been developed into the LeachSim model with new attributes, local similarity models that are related to the leaching process parameters, and the weighting of the attributes.
3.1. New Attributes
Table 1 presents the process attributes that arose from the interviews that were conducted with hydrometallurgical professionals. Ore attributes are not in the scope of this study and therefore, only the attributes concerning the leaching process are listed, along with the respective number of interviewees that mentioned the attribute. Altogether 26 process attributes were brought up during the interviews, but only the ones mentioned by 15% or more of the interviewees were included in the LeachSim model. A threshold needed to be defined in order to limit the scope of the research.

Table 1.
Included and excluded process attributes sorted according to the number of mentions during interviews.
The attribute Solution characteristics, is essentially composed of the attributes pH, Redox-potential, Complexant concentration, and Oxidant concentration. Therefore, these four attributes were selected for LeachSim instead of making Solution characteristics into its own attribute. Additionally, the leaching method itself and the chemicals used were presumed to be known in the interviewing questions, leading to their lacking in the interviewing results. After taking these considerations into account, altogether, 15 attributes were implemented into the LeachSim model: Pretreatment, Overall method, Complexant source, Oxidant source, Complexant concentration, Oxidant concentration, Temperature, pH, Redox-potential, Pressure, Materials of construction, Extraction, Extraction rate, Reagent consumption, and Solid-liquid ratio. The details of each attribute and the way in which they were incorporated are discussed one by one in Section 3.3.
3.2. Case Base
The case base was compiled by formalizing cyanide-free gold leaching articles that used an ore or a concentrate as their raw material. Altogether, 50 cases were extracted from 37 scientific research articles, technical notes, and short communications that are referenced in Appendix A. For clarity, the case sources are referred to as articles from here on. The articles were formalized by finding values to the selected attributes and then inserting these values into the LeachSim model.
3.3. Local Similarity Models
This section describes the development of similarity models for the selected process attributes.
3.3.1. Method
In this study, method implies the type of leaching that is used for gold extraction. There are several different sources of complexant and oxidant that can be used within the same overall method. A chloride based leaching method can use, for example, cupric ions [6], ferric ions [7], sodium hypochlorite [27], calcium hypochlorite [5], or sodium chlorate [28] as the oxidant source. It is also possible to use several different complexant sources at the same time. Similarly, different overall methods can be combined in a dual lixiviant system [29]. Therefore, the leaching method was divided into three separate attributes that can have several values at the same time:
- Overall method; possibility for several values;
- Complexant source; possibility for several values; and,
- Oxidant source; possibility for several values.
An example of the method attributes of a case could be:
- Overall method: “Chloride”;
- Complexant source: “Sodium chloride NaCl”; and,
- Oxidant source: “Calcium hypochlorite Ca(OCl)2”.
Due to the inherent difficulty of comparing different leaching methods, the method attributes were made discriminatory, meaning that if the user gives a value for any of the method attributes, only cases with the exact same value/s would be included in the comparison. If, however, the user does not define any value for the method attributes, then all the cases are included in the similarity comparison. Since the attributes are discriminatory, similarity values between different methods, oxidants and complexants were not needed. If there are several values for one attribute, then an extra value of “Dual” was added. For example, if the leaching method combines thiourea and thiocyanate, the Overall method attribute value would be: “Thiourea”; “Thiocyanate”; “Dual”. This type of case would be included in the retrieval for following queries:
- Overall method: “Thiourea”; “Thiocyanate”
- Overall method: “Dual”
The attribute Complexant source becomes relevant only in relation to a certain Overall method. Therefore, it was disabled unless the Overall method was first defined. In practice, the selection of the method attribute values was done through several case bases. Each case base contained cases with certain values for the attributes and the user chooses the case base which correlates with their interest.
3.3.2. Complexant and Oxidant Concentration
After the user has defined the Overall method, the Complexant source, and the Oxidant source, the concentrations of the complexant and oxidant can then be entered as molarity values (mol/L). The molarity values can be entered into the knowledge model as floating point numbers.
The concentration of each reagent affects the leaching effectivity, i.e., gold dissolution rate and final percentage of dissolved gold [30] (pp. 113–121). However, the leaching effectiveness also depends on other aspects of the solution and the process. For example, the fact that the chloride concentrations in two leaching experiments of an ore are the same does not necessarily result in similar Gold leaching results. This depends on the oxidant concentration, temperature, pH, mixing etc. that all affect the overall gold extraction and dissolution rate. Since the similarity between two molarity values is not independent from other attributes, depicting the similarity with a linear distance function was seen as the only viable option. It may not reflect the behavior of the leaching phenomena in every situation, but it is at least an approximation that can be interpreted by the user.
The linear distance function calculates the similarity sim between the query value and the case value based on their difference according to Equations (2) and (3).
Here, x is the difference between query and case values; valuequery and valuecase. It is compared to the whole range of values in the case base for the given attribute, i.e., the difference between valuemax and valuemin. The local similarity for the attribute is the resulting value of sim. Figure 2 illustrates a linear distance function. In this hypothetical example, the values for Oxidant concentration are between 0.0 mol/L and 5.0 mol/L. Therefore, the maximum difference between two attribute values is ±5 mol/L, resulting in the x-axis ranging from −5 to 5.
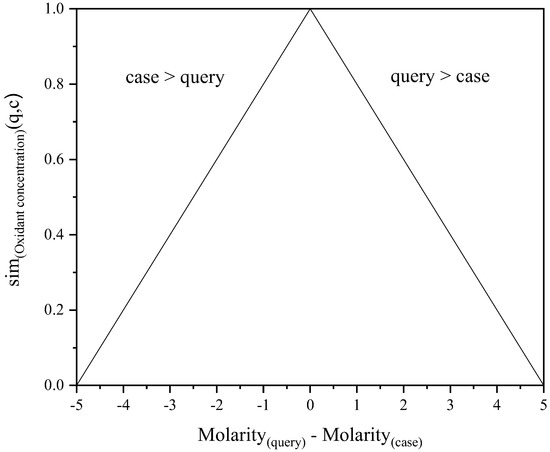
Figure 2.
An example of a possible linear distance function for Oxidant concentration.
3.3.3. pH and Redox-Potential
By definition, the pH and redox-potential of the solution define the possible compounds that are present in the solution and manipulating pH and redox-potential is related to upholding a certain chemistry within the solution. This principle is evident in a Pourbaix (potential/pH) diagram [31].
Certain leaching methods require particular conditions in order to function. As can be seen from Figure 3, the redox-potential [V vs. Standard hydrogen electrode (SHE)] and pH ranges for each gold leaching method are different [32] showing that gold can be leached at different pHs and redox-potentials, depending on the leaching media—oxidizing and complexing agent. This indicates that the pH and redox-potentials of a process cannot be compared across different leaching methods. However, the user might want to find articles where a certain range of pH or redox has been used, regardless of the method. As the three method attributes (Overall method, Complexant source, and Oxidant source) are already discriminative, the attributes pH and Redox-potential are compared within only one leaching method, if the method attribute/s are defined. If the user leaves all of the method attributes undefined, then pH and Redox-potential are compared across methods, despite the above-mentioned difficulties of this type of comparison. In this way the possibility for comparing pH and Redox-potential across methods was left as an option for the user.
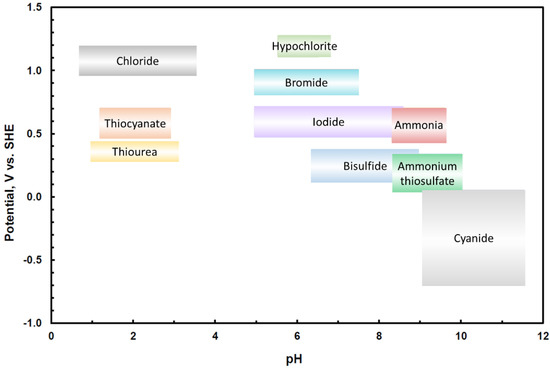
Figure 3.
Typical potential [V vs. Standard hydrogen electrode (SHE)] and pH operating regions for gold lixiviants, redrawn after [32].
Figure 3 indicates that generally speaking, the pH or redox-potential values are similar with each other when relatively close, but become very dissimilar quickly if not close to each other. When considering this fact, the similarity calculations for attributes pH and Redox-potential were modelled with the “smooth-step” distance function available in the myCBR software. The function creates a smooth drop in the similarity as distance from the query value increases. The user can define the point at which the similarity is 0.5 and the smooth curve is drawn around that threshold value. The default distances for the 0.5 similarities were defined as 1 for pH and 0.2 V vs. SHE for Redox-potential. However, the user can adjust these threshold values to suit their preferences in the calculation. As an example, the default similarity function for pH is illustrated in Figure 4.
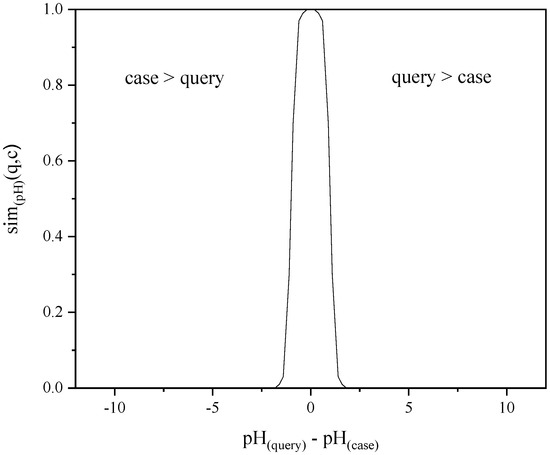
Figure 4.
Illustration of the default similarity function for pH in the LeachSim model.
Of course, the true value of the similarity depends on the solution, the leached material, the reagents, and the form of the relevant Pourbaix diagram, but this similarity function was seen as the best available technique to represent all situations when considering the nature of the pH and Redox-potential attributes.
3.3.4. Temperature
Many aspects of a leaching process depend on the used temperature, such as, leaching kinetics, type of equipment used, and material selection of the equipment. A major concern are the costs of running the process; elevated temperature generally leading to higher energy consumption and an increased cost of construction materials. The leaching kinetics of the leaching process are affected by temperature according to the well-known Arrhenius equation [30] (p. 132). Then again, other aspects depend on the temperature via different mechanisms, making the similarity assessment multidimensional. Since the intent of the user for comparing the temperatures is unknown, it was decided that the simplest similarity measure, a linear distance function as shown in Figure 2, would serve most of the users adequately.
3.3.5. Extraction
The Extraction attribute is a numerical value for the highest achieved extraction percentage for gold. As in the previous knowledge model [18], the similarity model for the extraction is a linear distance function, see Figure 2.
3.3.6. Extraction Rate
The extraction rate was not directly reported in the articles. Hence, the attribute Extraction rate needed to be a virtual attribute, meaning its value needs to be calculated from other information given in the article. Best achieved extraction percentage E% and leaching time t (min) were used according to Equation (4) in order to get a descriptive value for Extraction rate Er. However, this approach has its limitations as the extraction rate changes during the leaching period and the leaching time might not be optimized, meaning the maximum recovery has been achieved already in shorter time.
The similarity between the calculated extraction rate values was defined with a linear distance function, see Figure 2.
3.3.7. Pretreatment
Generally, the aim of applying a pretreatment to an ore is to expose the gold particles to the leaching solution and to hinder the effect of reagent consuming or preg-robbing substances [30] (p. 84). There are several ways an ore can be treated prior to the leaching step. In the cases collected for this study, three different pretreatment methods emerged; “Roasting”, “Pressure oxidation”, and “Nitric acid pretreatment”. In accordance with Marsden and House [30] (p. 148), also the following pretreatments were added to the knowledge model: “Biological oxidation”, “Preaeration”, “Acidic pressure oxidation”, and “Chlorination”. The model also needed to accommodate the more vague expression “Any”, as some articles did not specify the method of oxidation. The “Any” option can also serve the user by giving more flexibility for query options.
The Pretreatment attribute was set as a symbol type, meaning the value of the attribute is the name of the pretreatment or “None” and the similarity is defined with a similarity table relating each pretreatment with each other. In order to define the similarities between different pretreatments, they were compared with each other considering the following traits:
- hydrometallurgical vs. pyrometallurgical;
- suitability for refractory vs. mildly refractory ores;
- suitability for high sulfur content;
- suitability for high carbon content; and,
- pH: acid vs. neutral/alkaline.
In essence, the similarity of the pretreatments was assessed according to these binary attributes. The comparison was made according to the pretreatment descriptions in Marsden and House [30] (pp. 147–224). The similarity was defined by the number of similar attributes divided by the total number (5) of attributes. These considerations resulted in a similarity table for the attribute Pretreatment, as presented in Table 2. “Roasting” was seen as having a zero similarity with everything else regarding the pH. “Any” was defined to have 1.0 similarity with all other pretreatments. This feature was designed for users who want to look for leaching procedures where a pretreatment has been used, but the specific pretreatment is not relevant.

Table 2.
Similarity table for the attribute Pretreatment.
3.3.8. Solid-Liquid Ratio
The solid-liquid ratio has an effect on the leaching efficiency by influencing mass transport [30] (p. 263). If the mass of the leaching solution increases when compared to the mass of the solid material, the leaching rate, extraction percentage, or both are improved [28,33,34,35]. However, this phenomena is only true if mass transport is a limiting factor in the system.
In addition to affecting leaching kinetics, the solid-liquid ratio gives an indication of the industrial feasibility of the process. Industrial gold leaching processes operate in the range of 35–50% solids of the slurry [30] (p. 263). Although these are cyanide-based processes, the indication is that economically viable leaching processes operate at relatively high concentrations of solids. Therefore, the solid-liquid ratio that has been used in a certain research article gives the reader information about the economic potential and the scalability of the process.
When considering that the Solid-liquid ratio attribute gives the user information about both the leaching process and its economic aspects, it was seen best to model the similarity with a linear distance function, see Figure 2.
3.3.9. Pressure
Elevated pressure can be used to increase the leaching rate for some reactions, especially when oxygen gas is utilized as the oxidant source, because increased pressure raises the solubility of gases in the leaching solution [30] (p. 270). If chlorine or other hazardous gas is used, vacuum can be utilized to control the gas, but in these cases, the vacuum is relatively mild [30] (p. 190).
Utilizing a high pressurized leaching reactor affects the type of equipment that can be used, typically increasing costs. Even though the utilized oxygen pressure can vary (0.6 MPa [36]/5 MPa in Consolidated Murchison Mine [37]) the matter was seen as a binary situation, where the process is either under ambient pressure/vacuum, or it is in elevated pressure. The fact that elevated pressure is used was seen as significantly more influential than the level of that elevated pressure. Therefore, the attribute was given a binary value: “Ambient/mild vacuum” vs. “High pressure”, indicated in the sostware itself with “atm” and “pres”, respectively. The similarity is therefore either 1 for similar attribute values, or 0 for different attribute values, as depicted in Table 3.

Table 3.
Binary similarity table for the attribute Pressure.
3.3.10. Reagent Consumption
The attribute Reagent consumption refers to the amount of complexing agent that is spent during the leaching process. The amount depends greatly on the ore type as some minerals, also other than gold, react during the leaching process, consuming the reagent. Therefore, high reagent consumption leads to high costs, which depend on the current market situation and the price of each reagent. As the reagents and their prices vary, the related cost factors are not straightforward to compare.
It seems that the scientific culture of reporting reagent consumption is not consistent, as less than half (21/50) of the cases reported any value for it. However, the attribute was still included in the knowledge model, and compared with a linear distance function, see Figure 2. Similarly, as for pH and Redox-potential, the option of comparing the reagent consumption between leaching method was left open for the user.
3.3.11. Materials of Construction
The attribute Materials of construction was seen as merely an informative addition in the solution part of the cases. In other words, the user would see the value of this attribute in the retrieved cases, but they will not be able to conduct the retrieval based on the required materials of construction. Therefore, the attribute did not need a similarity model. The possible values of the attribute were “Inexpensive”, “Mediocre”, and “Expensive”. The values were assigned based on the type of equipment used in the leaching experiments. It needs to be noted that the materials used in laboratory scale leaching experiments are not comparable to the type of equipment that is needed in full scale industrial processes. Glass reactors are very typically used in the laboratory scale experiments, whereas, in industrial metal processing, they are rare and metallic and plastic materials such as construction steel, stainless steel, titanium, polypropylene, and fiber-reinforced plastic with different lining options are commonly applied.
3.4. Attribute Weights
The discussed similarity models for each attribute define the local similarity between the attribute values in the query and the case. These values are then weighted according to their perceived importance for the global similarity calculation (ωi in Equation (1)). In other words, the greater the weight of an attribute, the more the local similarity value of that attribute affects the global similarity value.
In previous research, it was noted that the weighing of the attribute Method would increase the accuracy of the similarity model in the case of comparing cyanide-free gold leaching articles [18]. In the current LeachSim model, this demand was met by making the attributes Overall method, Complexant source, and Oxidant source totally discriminative, i.e., if the user defines a value for the attribute, only cases with the same value are considered for the similarity calculation and assessment.
Any other weights can be determined by the user. It was seen as essential to allow the user to determine the weights for themselves, based on their own research interests. For the testing of the model, the default weights were kept as simple as possible. They were based on the interview results, so that more mentions during the interviews led to a higher weighting factor. In practice, if the attribute got less than 10 mentions during the interviews, the weighting factor was set at 1, and if the attribute got 10 or more mentions, the weight was set at 2. This led to attributes Complexant concentration, Temperature, and Extraction having a weight of 2 and attributes Pretreatment, Oxidant concentration, Temperature, pH, Redox-potential, Pressure, Extraction rate, Reagent consumption, and Solid-liquid ratio having a weighing factor of 1.
4. Testing the LeachSim Model
4.1. Preliminary Tests
The initial test queries confirmed that the software calculated the global similarity values according to the defined similarity models. All of the attributes were used in the queries individually, in pairs, and in queries of multiple attributes. The resulting similarity values matched with the manually calculated ones. An image of the query phase and the retrieval results view within the myCBR software is provided in Figure 5. Selection of the case base on the top left determines the Method attributes, the query fields define the values used for retrieval, top results are visible below the query, and all the results are in the table on the right side of the figure.
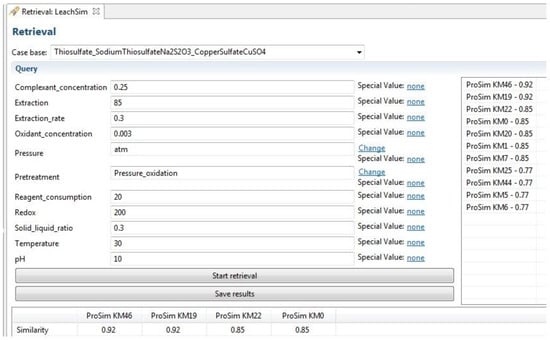
Figure 5.
An image of the retrieval function in myCBR with the LeachSim model in place.
4.2. Test Queries
The actual test queries that are presented in Table 4 were then used to test the knowledge model’s functionality in retrieving relevant research articles. These hypothetical queries (Query 1–Query 5) were formulated to represent different situations where the user has a certain objective/interest. The five test queries were formulated according to the following scenarios:

Table 4.
Queries 1–5 for testing the LeachSim model.
- Similar Leaching Methods—A researcher has conducted chloride leaching tests on an ore and is now looking for articles that have used a similar leaching process [5].
- Similar Articles—A researcher has found a particularly interesting research article, in this case bromide leaching of gold. This article’s information is used as a query to find similar articles. [36].
- Pretreatment and Thiourea—A researcher is looking for articles where the gold ore has been pretreated and leached with a thiourea solution, leading to successful results in extraction percentage (equal weights were used for the attributes Pretreatment and Extraction).
- Dual Lixiviant—A researcher is looking for any dual lixiviant systems operating at an acidic pH and at room temperature.
- Industrially Attractive—A researcher wants to find the most industrially attractive thiosulfate articles by minimizing Reagent consumption and Temperature, and by maximizing Solid-liquid ratio, Extraction, and Extraction rate.
4.3. Results and Discussion
The Queries 1–5 were entered into the LeachSim model, implemented in myCBR. The weighs of the attributes were kept at default values, except for Query 3, where the weighting of Pretreatment and Extraction were both set to 1. After the queries were made, the resulting similarity values were once more calculated manually to ensure correct computation. myCBR was shown to compute all of the similarities according to the defined similarity models.
The three most similar and the most dissimilar results for each Query are presented in Table 5, Table 6, Table 7, Table 8 and Table 9 (when applicable). For Query 1, in Table 5, the similarity values are fairly high for all of the results (Sim ≥ 0.81). Still, it can be seen that the most similar case differs significantly in only Temperature, while the dissimilarity of the articles increases towards the bottom. The least similar article clearly differs from Query 1 regarding the attributes Oxidant concentration, Redox-potential (“Unknown”), Solid-liquid ratio, and Temperature. The intended functionality of the knowledge model in ranking the articles from similar to dissimilar was shown to have succeeded.

Table 5.
Query 1 (Similar Leaching Methods) with the three most similar and the most dissimilar article retrieved from the case base.

Table 6.
Query 2 (Similar Articles) and all the retrieved articles according to similarity.

Table 7.
Query 3 (Pretreatment and Thiourea) with the three most similar and the most dissimilar article retrieved from the case base.

Table 8.
Query 4 (Dual Lixiviant) and all the retrieved articles according to similarity.

Table 9.
Query 5 (Industrially Attractive) with the three most similar articles retrieved from the case base.
The results for Query 2, in Table 6, show a slightly wider range (sim ≥ 0.7). Only three cases were retrieved from the case base, since there are only three Bromine-bromide cases included. The first result is closer to Query 2 in several attributes than the second result, which is more similar only regarding the Redox-potential. The least similar result has three “Unknown” values, which has dropped its ranking significantly.
It needs to be noted that for both Query 1 and Query 2 all of the presented results are sourced from articles by the same first authors. This might be due to two reasons. Firstly, the particular research field is so narrow that only one research group has conducted cyanide-free gold leaching experiments from ore/concentrate with that particular solution type. Secondly, the case base does not cover the whole research area, which is also very likely, since the case base was compiled manually. Automating the case creation process would increase the number of cases and improve the comprehensiveness of the case base.
Query 3 produced a wide similarity range from 0.96 to 0.00 shown in Table 7. Since only two attributes, Pretreatment and Extraction, were used for the similarity calculation, getting a zero similarity becomes more likely. It should be noted that the attribute Overall method defines the set of cases that are considered, but it does not contribute to the similarity calculation. The more attributes are used in the query, the more likely it becomes that at least one of the attributes has some similarity above zero. Here, because the Extraction value of the least similar case is “Unknown” and no pretreatment was applied, the overall similarity amounts to zero.
All three cases with the Overall method, including the value “Dual” were retrieved with Query 4, shown in Table 8. Since the case base only holds these three dual lixiviant cases, the comparison with only these attributes is not so informative, but the model does differentiate the third result due to its “Unknown” pH and slightly higher Temperature.
Query 5 and the acquired results are in Table 9. The query itself was not realistic, since it was designed to minimize and maximize certain attributes. No realistic process achieves 100% extraction with zero reagent consumption, at 0 °C, and in 20 min. Therefore, the similarity values were all under 0.76, but, nonetheless, the results have been ranked according to their similarity with the query. The scenario behind query 5 was to find the articles having high solid-liquid ratio, low reagent consumption, low temperature, high extraction, and high extraction rate, so as to indicate articles that could have industrially attractive research conditions. The most similar case has a significantly lower Reagent consumption and higher Extraction than the second result. Even though the Solid-liquid ratio of the first result is not as good as some of the other cases, its good qualities in other aspects has led to the highest score.
The different types of scenarios that were used for the test Queries are only examples of the possibilities of such a retrieval tool. When looking at the overall performance of the model, it can be stated, that the LeachSim model has succeeded in its primary task, which is to act as a fast information retrieval tool for researchers who are looking for scientific articles in the field of cyanide-free gold leaching from ores and concentrates. It ranked the articles according to the defined similarity models and the results are satisfactory.
5. Conclusions
In this research, scientific articles in the field of cyanide-free gold leaching were formalized so their findings can be compared by an AI application named LeachSim. The methodology of case-based reasoning and a software called myCBR were used for building the knowledge model and defining the similarity calculation.
The main findings of this study were:
- Scientific articles in the specific field of cyanide-free gold leaching are suitable to be compared through narrow AI.
- Case-based reasoning is a viable methodology for constructing a knowledge model that compares the scientific articles in a very specific field.
- The LeachSim model is able to sort through and organize scientific articles that are based on the user’s interests/research problem in its given field.
- Similarity assessment and sorting is possible even with incomplete and not exactly matching input data.
The next step is to extend the knowledge model by adding a set of ore attributes into it. This way the user can also use mineralogy, gold content, particle size, etc. to search for the most relevant articles. A continuation to this would be to incorporate other gold containing materials, such as urban ores (e.g., printed circuit boards and other WEEE materials) into the case base.
In order for this type of information retrieval tool to be useful and practical, the process of acquiring the cases and defining the similarity models should be sped up considerably. Subsequent development in this matter could be semi-automatic case extraction from the sources. This would require a natural language processing tool to automatically locate and interpret the findings in a research paper. It would take considerable effort to achieve fully automated case extraction, since the language that is used in scientific articles is quite complex.
Future work in the direction of intelligent article retrieval would be to develop similar knowledge models for other fields of scientific research, such as leaching of other metals than gold and new recovery methods from leach solutions. While this research was focused on the leaching step of the cyanide-free methods, other important aspects within the field would be, for example, recycling of reagents and the environmental impacts of the novel processes.
To conclude, the LeachSim model that was built in this study should ultimately aid the user by conveniently sorting through large numbers of research articles while returning the most relevant ones. Whatever the specific field of research, such a tool, once compiled, could benefit multiple researchers through saving their valuable time. However, the objective is to support the user, not to automate the role of the researcher or replace human consideration.
Author Contributions
M.L. (Maria Leikola) defined the similarity models in collaboration with J.A. and L.R., designed the testing procedure, implemented the model in myCBR, conducted preliminary and actual testing, and wrote the first draft of the manuscript. M.L. (Maria Leikola) and C.S. produced the visualizations and C.S. alone solved all software issues. M.L. (Maria Leikola), L.R., C.S., and M.L. (Mari Lundström) were involved in the analysis of the results. All authors participated in the finalization of the written manuscript. M.L. (Mari Lundström) acted as the supervisor of the project and acquired all necessary funding.
Funding
This research was funded by Emil Aaltonen Foundation, project name “Ympäristöystävällistä kultaa”.
Acknowledgments
RawMatTERS Finland Infrastructure (RAMI), supported by Academy of Finland, is greatly acknowledged.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Appendix A
Scientific articles, technical reports, and short communications that were used as case sources and the number of cases extracted from them.
| Reference to Case Source | No. of Cases |
| 1 |
| 3 |
| 1 |
| 1 |
| 1 |
| 2 |
| 1 |
| 1 |
| 1 |
| 2 |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 1 |
| 3 |
| 1 |
| 2 |
| 2 |
| 1 |
| 1 |
| 2 |
| 1 |
| 2 |
| 2 |
| 1 |
| 1 |
| 2 |
| 1 |
| 1 |
| 2 |
| 1 |
| 1 |
Appendix B
The following questions (1–4) were asked during interviews to determine which attributes should be included in the knowledge model:
- Imagine you were designing an experiment series for chloride leaching. If all information from previous research articles was thoroughly organized, what knowledge and parameters would you compare for:
- (i)
- free-milling ore?
- (ii)
- refractory ore?
- What parameters would you like to use for excluding cases from the comparison?
- If you were designing a thiosulfate experiment instead, would it change your answers to questions 1 and 2?
- There is a preliminary model that compares previous research cases based on attributes in this example:
Method Mineral 1 Mineral 2 Gold Content (g/t) Chloride Ankerite Muscovite 1.5 Thiosulfate Arsenopyrite Pyrite 56 Thiosulfate Pyrite 94.63
What attributes would you add to the list, in order to better describe the ore?
References
- Hilson, G.; Monhemius, A. Alternatives to cyanide in the gold mining industry: What prospects for the future? J. Clean. Prod. 2006, 14, 1158–1167. [Google Scholar] [CrossRef]
- Senanayake, G. Gold leaching in non-cyanide lixiviant systems: Critical issues on fundamentals and applications. Miner. Eng. 2004, 17, 785–801. [Google Scholar] [CrossRef]
- Choi, Y.; Baron, J.Y.; Wang, Q.; Langhans, J.; Kondos, P. Thiosulfate processing—From lab curiosity to commercial application. In Proceedings World Gold 2013; The Australasian Institute of Mining and Metallurgy: Melbourne, Australia, 2013; pp. 45–50. [Google Scholar]
- Fleming, C.; McMullen, J.; Thomas, K.; Wells, J. Recent advances in the development of an alternative to the cyanidation process: Thiosulfate leaching and resin in pulp. Miner. Metall. Process. 2003, 20, 1–9. [Google Scholar]
- Ghobeiti Hasab, M.; Rashchi, F.; Raygan, S. Chloride–hypochlorite leaching and hydrochloric acid washing in multi-stages for extraction of gold from a refractory concentrate. Hydrometallurgy 2014, 142, 56–59. [Google Scholar] [CrossRef]
- Lampinen, M.; Seisko, S.; Forsström, O.; Laari, A.; Aromaa, J.; Lundström, M.; Koiranen, T. Mechanism and kinetics of gold leaching by cupric chloride. Hydrometallurgy 2017, 169, 103–111. [Google Scholar] [CrossRef]
- Seisko, S.; Lampinen, M.; Aromaa, J.; Laari, A.; Koiranen, T.; Lundström, M. Kinetics and mechanisms of gold dissolution by ferric chloride leaching. Miner. Eng. 2018, 115, 131–141. [Google Scholar] [CrossRef]
- Li, J.; Miller, J.D. A review of gold leaching in acid thiourea solutions. Miner. Process. Extr. Metall. Rev. 2006, 27, 177–214. [Google Scholar] [CrossRef]
- Oraby, E.; Eksteen, J. The leaching of gold, silver and their alloys in alkaline glycine–peroxide solutions and their adsorption on carbon. Hydrometallurgy 2015, 152, 199–203. [Google Scholar] [CrossRef]
- Rintala, L.; Leikola, M.; Sauer, C.; Aromaa, J.; Roth-Berghofer, T.; Forsén, O.; Lundström, M. Designing gold extraction processes: Performance study of a case-based reasoning system. Miner. Eng. 2017, 109, 42–53. [Google Scholar] [CrossRef]
- Rintala, L.; Lillkung, K.; Aromaa, J. The use of decision and optimization methods in selection of hydrometallurgical unit process alternatives. Physicochem. Probl. Miner. Process. 2011, 46, 229–242. [Google Scholar]
- Liang, Y.; Wang, H.; Xu, A.; Tian, N. A Two-step Case-based Reasoning Method Based on Attributes Reduction for Predicting the Endpoint Phosphorus Content. ISIJ Int. 2015, 55, 1035–1043. [Google Scholar] [CrossRef]
- Feng, K.; He, D.; Xu, A.; Wang, H. End temperature prediction of molten steel in RH based on case-based reasoning with optimized case base. J. Iron Steel Res. Int. 2015, 22, 68–74. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, D.; Feng, Y.; Yuan, Z.; Chen, B.; Qin, W.; Zou, S.; Qin, S.; Han, J. A strategy for enhancing the operational agility of petroleum refinery plant using case based fuzzy reasoning method. Comput. Chem. Eng. 2018, 111, 27–36. [Google Scholar] [CrossRef]
- Qi, J.; Hu, J.; Peng, Y. A modularized case adaptation method of case-based reasoning in parametric machinery design. Eng. Appl. Artif. Intell. 2017, 64, 352–366. [Google Scholar] [CrossRef]
- Aamodt, A.; Plaza, E. Case-based reasoning: Foundational issues, methodological variations, and system approaches. AI Commun. 1994, 7, 39–59. [Google Scholar] [CrossRef]
- Stahl, A.; Roth-Berghofer, T.R. Rapid prototyping of CBR applications with the open source tool myCBR. In Advances in Case-Based Reasoning: Proceedings of the European Conference on Case-Based Reasoning, Trier, Germany, 1–4 September 2008; Althoff, K., Bergmann, R., Minor, M., Hanft, A., Eds.; Lecture Notes in Computer Science 5239; Springer: Berlin/Heidelberg, Germany, 2008; pp. 615–629. [Google Scholar]
- Leikola, M.; Rintala, L.; Sauer, C.; Roth-Berghofer, T.; Lundström, M. Applicability of Case-Based Reasoning for Selection of Cyanide-Free Gold Leaching Methods. In Case-Based Reasoning Research and Development, Proceedings of the 24th International Conference on Case-Based Reasoning (ICCBR 2016), Atlanta, GA, USA, 31 October–2 November 2016; Goel, A., Díaz-Agudo, M.B., Roth-Berghofer, T., Eds.; Lecture Notes in Computer Science 9969; Springer: Berlin/Heidelberg, Germany, 2016; pp. 249–264. [Google Scholar]
- myCBR 3 Workbench. Available online: http://mycbr-project.net/download.html (accessed on 3 May 2018).
- Sauer, C.S.; Rintala, L.; Roth-Berghofer, T. Two-phased Knowledge Formalisation for Hydrometallurgical Gold Ore process recommendation and validation. Künstliche Intell. 2014, 28, 283–295. [Google Scholar] [CrossRef]
- Rintala, L. Development of a Process Selection Method for Gold Ores Using Case-Based Reasoning; Aalto University Publication Series Doctoral Dissertations 161/2015; Aalto University: Helsinki, Finland, 2015; ISBN 978-952-60-6483-3. [Google Scholar]
- Rogers, Y.; Sharp, H.; Preece, J. Interaction Design: Beyond Human Computer Interaction; John Wiley & Sons: New York, NY, USA, 2002; pp. 392–395. ISBN 978-0-471-49278-8. [Google Scholar]
- Burgess, T.F. Guide to the Design of Questionnaires: A General Introduction to the Design of Questionnaires for Survey Research; Information System Services, University of Leeds: Leeds, UK, 2001. [Google Scholar]
- Guest, G.; Bunce, A.; Johnson, L. How many interviews are enough? An experiment with data saturation and variability. Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Glaser, B.; Strauss, A. The Discovery of Grounded Theory: Strategies for Qualitative Research; Aldine Transaction: New Brunswick, NJ, USA, 2006; pp. 61–62. ISBN 0-202-30260-1. [Google Scholar]
- Francis, J.J.; Johnston, M.; Robertson, C.; Glidewell, L.; Entwistle, V.; Eccles, M.P.; Grimshaw, J.M. What is an adequate sample size? Operationalising data saturation for theory-based interview studies. Psychol. Health 2010, 25, 1229–1245. [Google Scholar] [CrossRef] [PubMed]
- Baghalha, M. Leaching of an oxide gold ore with chloride/hypochlorite solutions. Int. J. Miner. Process. 2007, 82, 178–186. [Google Scholar] [CrossRef]
- Cheng, Y.; Shen, S.; Zhang, J.; Chen, S.; Xiong, L.; Liu, J. Fast and Effective Gold Leaching from a Desulfurized Gold Ore Using Acidic Sodium Chlorate Solution at Low Temperature. Ind. Eng. Chem. Res. 2013, 52, 16622–16629. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, S.; Cheng, Y.; Lan, H.; Hu, X.; Wang, F. Dual lixiviant leaching process for extraction and recovery of gold from ores at room temperature. Hydrometallurgy 2014, 144–145, 114–123. [Google Scholar] [CrossRef]
- Marsden, J.; House, I. The Chemistry of Gold Extraction, 2nd ed.; Society for Mining, Metallurgy and Exploration, Inc.: Littleton, CO, USA, 2006; pp. 84, 113–121, 132, 147–224, 263, 270. ISBN 978-0-87335-240-6. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 1st ed.; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Aylmore, M.G. Alternative lixiviants to cyanide for leaching gold. In Advances in Gold Ore Processing; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 501–539. ISBN 978-0-444-51730-2. [Google Scholar]
- Kim, E.; Kim, M.; Lee, J.; Pandey, B. Selective recovery of gold from waste mobile phone PCBs by hydrometallurgical process. J. Hazard. Mater. 2011, 198, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Navarro, P.; Vargas, C.; Villarroel, A.; Alguacil, F. On the use of ammoniacal/ammonium thiosulphate for gold extraction from a concentrate. Hydrometallurgy 2002, 65, 37–42. [Google Scholar] [CrossRef]
- Ubaldini, S.; Fornari, P.; Massidda, R.; Abbruzzese, C. An innovative thiourea gold leaching process. Hydrometallurgy 1998, 48, 113–124. [Google Scholar] [CrossRef]
- Parga, J.R.; Valenzuela, J.L.; Francisco, C.T. Pressure cyanide leaching for precious metals recovery. JOM 2007, 59, 43–47. [Google Scholar] [CrossRef]
- Adams, M.D. Summary of gold plants and processes. In Advances in Gold Ore Processing; Adams, M.D., Wills, B.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; Volume 15, pp. 994–1013. ISBN 978-0-444-51730-2. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).