Abstract
This study elucidated the mechanisms during the bioleaching process when optimizing the dewaterability of municipal sludge using quartz sand. The experiment was conducted with a shaking table and a series of controlled trials designed to investigate the influence of quartz sand on sludge dewaterability. Scanning electron microscopy and X-ray diffraction were applied to explore the quartz sand’s action mechanism. Results indicated that quartz sand could improve the sludge bioleaching efficiency. The optimal reaction time was between 24–48 h and 48–72 h with and without 10 g·L−1 of quartz sand, and a minimum sludge specific resistance to filtration was 1.2 × 1012 and 2.4 × 1012 m·kg−1, respectively. Quartz sand could provide nucleating sites for secondary iron minerals and overcome the unfavorable influence of a low Fe3+ supply rate in the initial bioleaching stage (0–24 h). Because it was conducive to accelerating the initial mineral precipitation, quartz sand could improve bioleaching efficiency. The X-ray diffraction spectrum showed that quartz sand induced changes in the synthesis pathway of secondary iron minerals when the concentration of Fe2+ ≥ 4 g·L−1. This then promoted the transformation of schwertmannite into jarosite during the mineralization process, which immobilizes nutrients such as K+ and NH4+ in the form of jarosite. Accordingly, bioleached sludge dewaterability and its utilization value can be improved. These results provide theoretical reference for improving bioleaching techniques in the treatment of municipal sludge.
1. Introduction
Bioleaching is a harmless treatment technology that uses an Acidithiobacillus species (including A. ferrooxidans and A. thiooxidans) to treat sewage sludge [1,2]. Numerous studies have demonstrated that bioleaching can effectively remove heavy metals in sewage sludge while simultaneously improving the sludge dewaterability. Dewaterability indicates the degree of difficulty to achieve the solid–liquid separation of sewage sludge through filter media; it is commonly characterized by specific resistance to filtration (SRF) and capillary suction time (CST) [3,4]. For instance, after being treated with a bioleaching method, the SRF decreased by nearly 80%, and after mechanical dewatering the sludge moisture content was less than 60% [5,6], which significantly reduces the sludge volume and improves subsequent sludge treatment. Liu et al. confirmed that 2.4 is the optimal pH value to promote the dewaterability of municipal sewage sludge in the bioleaching process. At this pH, H+ ions neutralize the sludge surface’s negative charge to near-neutral to enhance the sewage sludge’s coagulation and dehydration [7]. In addition to optimizing pH, the energy sources, FeSO4 and S0, need to be added in order for the Acidithiobacillus species to improve the sludge system’s bio-acidification efficiency. However, the strong hydrophobicity of S0 inhibits its full utilization by A. thiooxidans during shorter periods, resulting in large quantities of residual S0 in sewage sludge at the end of bioleaching, thus impacting the utilization of dewatered sludge [8,9]. Ultimately, there are limits to adopting bioleaching technology mediated by A. ferrooxidans and A. thiooxidans to promote the dewaterability of municipal sludge.
During the sludge bioleaching process, A. ferrooxidans can improve sludge dewaterability by oxidizing Fe2+ to biological polymeric ferric sulfate, which has a flocculation effect on sludge particles [9]. Meanwhile, hydrolyzing Fe3+ from the bio-oxidation of Fe2+ will produce secondary iron minerals and release large amounts of H+ lowering sludge pH [10,11]. The reactions can be summarized as follows:
8Fe3+ + 14H2O + SO42− → Fe8O8(OH)6SO4 (schwertmannite) + 22H+
M+ + 3Fe3+ + 2SO42− + 6H2O → MFe3(SO4)2(OH)6 (jarosite) + 6H+
Among the reactions, M+ refers to monovalent cations in sewage sludge such as K+, Na+, and NH4+. As stated above, there are two advantages to promoting the hydrolysis and mineralization of Fe3+. First, this process helps to accelerate sludge acidification so it can reach the optimum pH more quickly. Second, the increase in secondary iron minerals contributes to a decrease in the compressibility of the sludge needed to further improve sludge dewaterability [12]. In addition, the sewage sludge contains abundant agricultural nutrients such as N and K. Promoting the biosynthesis of jarosite (Reaction 2) can immobilize K+ or NH4+ ions contained in the liquid phase into the solid phase. This decreases the loss of nutrient elements and reduces the risk of water eutrophication. Therefore, using the biomineralization of A. ferrooxidans to improve traditional sludge bioleaching technology not only prevents adverse effects brought by A. thiooxidans oxidizing S0, which adds extra S0 to bioleached sludge, but also improves the utilization value of bioleached sludge by introducing nutrient elements (such as N and K) into the solid phase. In recent years, there have been several reports on the formation and characteristic of biogenic secondary iron minerals in the bioleaching system. These characteristics indicate that the secondary iron minerals absorb and coprecipitate heavy metals or metalloids in solutions, thus decreasing the leaching ratio of toxic elements in solid phases (e.g., tannery sludge and pig manure) [13,14]. When removing heavy metals in sludge through bioleaching, the secondary iron minerals that are formed can lead to negative effects. According to the Control Standards of Pollutants in Sludge for Agricultural Use (China, GB 4284-2018) [15], the heavy metal content in municipal sludge is of a low over-proof rate. For instance, by collating and counting the heavy metal content of Chinese municipal sludge reported in relevant literature (2006–2013), Guo et al. [16] concluded that the over-proof rate of the heavy metals Cu, Pb, Zn, Cd, Hg, As, Cr, and Ni were 2.3%, 0%, 5.9%, 5.5%, 2.9%, 0%, 0%, and 3.5%, respectively. Therefore, the critical objective in treating municipal sludge is to reduce the sludge volume. As mentioned previously, reducing the volume is possible using secondary iron minerals formation since it improves sludge dewaterability. However, regarding the sludge bioleaching system, there are no reports on how secondary iron mineral formation mediated by A. ferrooxidans specifically affects sludge dewaterability.
In general, the formation of biogenic secondary iron minerals can be roughly divided into three steps: Fe2+ is oxidized to Fe3+; Fe3+ is hydrolyzed and forms mineral crystal nucleus; and the nucleus gradually agglomerates and expands [17]. These show that the formation of secondary iron minerals is a new phase formation process, closely relevant to the supply rate of Fe3+ in solution [18,19]. Dutrizac found that the addition of crystal seeds (sodium, ammonium, and potassium jarosites) can accelerate the hydrolysis and mineralization of Fe3+ and raise the mineral output [20]. Wang et al. also reported that employing quartz sand or diatomite as crystal seed has little influence on the Fe2+ bio-oxidation process and can help precipitate and remove Fe3+ in simulative acid mine drainage [21]. Based on this, we assume that the inductive effects of crystal seeds can promote the quick synthesis of secondary iron minerals and accelerate the sludge acidification rate, thus increasing the sludge bioleaching efficiency. The purpose of this study can be summarized as follows: (1) explore the specific effects of secondary iron minerals on municipal sludge dewaterability and (2) study the role of quartz sand in a bioleaching system mediated by A. ferrooxidans and mechanism by which quartz sand induces secondary iron mineral synthesis. We aim to find an optimal method to improve municipal sludge dewaterability and achieve sludge volume reduction. The study may also provide a theoretical reference for improvement in the municipal sludge bioleaching technique.
2. Materials and Methods
2.1. Municipal Sewage Sludge Sample and Quartz Sand
Municipal sewage sludge used in this study was collected from the sludge thickener of a wastewater treatment plant in Wuhan City, Hubei Province, China. The physicochemical properties of the sludge are as follows: a solid content of 2.1%, a pH of 7.22, an organic matter content of 49.4%, a total N of 4.4%, a total P of 3.5%, a total K of 1.5%, and a sludge SRF of 12.4 × 1012 m·kg−1. The heavy metal content of municipal sludge was measured to be Cu 137.4, Pb 83.9, Zn 219.6, Cd 0.7, Hg 0, As 1.4, Cr 102.3, and Ni 26.2 mg/kg, respectively. No heavy metal content exceeds the criterion [15]. Municipal sewage sludge was stored in polypropylene bottles at 4 °C before use. The particle size of quartz sand used in this study was 30–40 mesh. It was firstly soaked in 1 mol·L−1 H2SO4 for 24 h and then dried for further use.
2.2. Preparation of A. ferrooxidans LX5 Inoculum
The acidophilic chemoautotrophic bacterium Acidothiobacillus ferrooxidans LX5 (CGMCC No. 0727) was obtained from the China General Microbiological Culture Collection Center (Beijing, China) and grown in modified 9K medium. The pH value of the cultivation medium was 2.50. The inoculum with an initial cell density of 107 cells·mL−1 was cultured in a gyratory shaker at 28 °C and 180 rpm for 3–4 days until the cell density reached 108 cells·mL−1 [3,22].
2.3. The Influence of Quartz Sand on the Dewaterability of Sludge in the Bioleaching System Mediated by A. ferrooxidans LX5
Using 1000 mL Erlenmeyer flasks, the sludge treatment is listed as follows: (1) 540 mL sludge + 60 mL deionized water; (2) 540 mL sludge + 60 mL deionized water + 10 g·L−1 quartz sand; (3) 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O; (4) 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O + 10 g·L−1 quartz sand. Each treatment was tested three times. The initial cell density in every flask was 5 × 107 cells·mL−1. The flasks were incubated in a gyratory shaker at 28 °C and 180 rpm for 120 h. Periodic samples were taken to monitor the sludge pH and dewaterability (characterized by SRF) during the process. With each sample, the flasks were shaken to mix the supernatant and were taken after 5 s for full sedimentation of quartz sand. The concentrations of Fe2+ were measured and its oxidation ratio was calculated via the following equation: Fe2+ oxidation efficiency (%) = (C0 − Ct)/C0 × 100%, where C0 is the initial Fe2+ concentration (g·L−1), and Ct is the Fe2+ concentration (g·L−1) at different times.
The residual sludge in Erlenmeyer flasks was collected at the termination of culture and grinded into powder with agate mortar after dried at 55 °C. Scanning electron microscope (SEM) was applied to observe the appearance features of dried sludge samples.
2.4. The Influence of Quartz Sand on the Formation of Secondary Iron Minerals in the Bioleaching System Mediated by A. ferrooxidans LX5
FeSO4·7H2O was supplemented with an Fe2+ concentration gradient of 8, 6, 4, and 2 g·L−1 into a series of Erlenmeyer flasks each containing 270 mL of sludge leachate. Using different concentrations, each group was further divided into two groups, with one group receiving 10 g·L−1 of quartz sand. Thirty-milliliter A. ferrooxidans LX5 suspensions were inoculated at 10% (v/v), which increased the total volume of liquid to 300 mL. Each treatment was tested three times. The flasks were incubated in a gyratory shaker at 28 °C and 180 rpm for 120 h. Samples were taken periodically to measure the pH and concentrations of Fe2+, Fe3+, and total Fe (TFe). The TFe fractional precipitation efficiency as well as the cumulative precipitation efficiency in the termination of culture were calculated via the following equations: TFe fractional precipitation efficiency (%) = (C’t1 − C’t2)/C’0 × 100%, and TFe cumulative precipitation efficiency (%) = (C’0 − C’120)/C’0 × 100%, where C’0 is the initial TFe concentration (g·L−1 ), C’t1 or C’t2 is the TFe concentration (g·L−1 ) at t1 or t2 h, and C’120 is the TFe concentration (g·L−1 ) at 120 h.
The secondary iron minerals synthesized at terminal were collected by filtering using Whatman No. 4 filter paper and were then dried at 55 °C to constant weight. The mineral phases of the dried solid precipitates was determined using power X-ray diffraction (XRD).
2.5. Analytical Methods
Sludge pH was measured using a pHS-3C precise pH meter with a resolution of 0.01 pH unit. The SRF was determined by filtrating 50 mL sludge sample in a Buchner funnel with a filter paper disc (Whatman No. 4) and 0.08 MPa suction pressure. The time required for the collection of every 5 mL of the filtrate was recorded and the SRF was calculated according to the method of Murugesan et al. [9]. The solid content and organic matter content of sludge was determined by drying at 105 °C and combustion at 600 °C, respectively [23]. The concentrations of Fe2+, Fe3+, and TFe were determined using the 1,10-phenanthroline method by a spectrophotometer (721, XTZ Optical Instrument Factory, Shanghai, China) with a detection wavelength at 530 nm [23]. The morphology of minerals was observed by scanning electron microscope (SEM, SU8010, Hitachi Limited, Tokyo, Japan), and the mineral phase was determined by X-ray diffraction (XRD, Bruker D8A25, Bruker Corporation, Karlsruhe, Germany) using Cu Kα radiation (40 kV, 40 mA) [24]. According to the Control Standards of Pollutants in Sludge for Agricultural Use (GB 4284-2018) [15], the Cd and Pb in municipal sludge was measured by graphite furnace atomic absorption spectrometry (YCA-1000G, Yuwei Technology, Beijing, China). Hg and As was determined by atomic fluorescence spectrometry (AFS-9730, Haiguang Instrument, Beijing, China). Cr, Ni, Zn, and Cu was measured by flame atomic absorption spectrophotometry (Agilent 7700e, Agilent Technologies, Santa Clara, CA, USA).
2.6. Statistical Analysis
The statistical analysis was graphically carried out with Origin software version 8.0. The data shown in the figures was presented as mean values with standard deviations to indicate the reproducibility and reliability of the results.
3. Results and Discussion
3.1. The Influence of Quartz Sand on the pH, Oxidation Efficiency of Fe2+, SRF in Sludge Bioleaching System
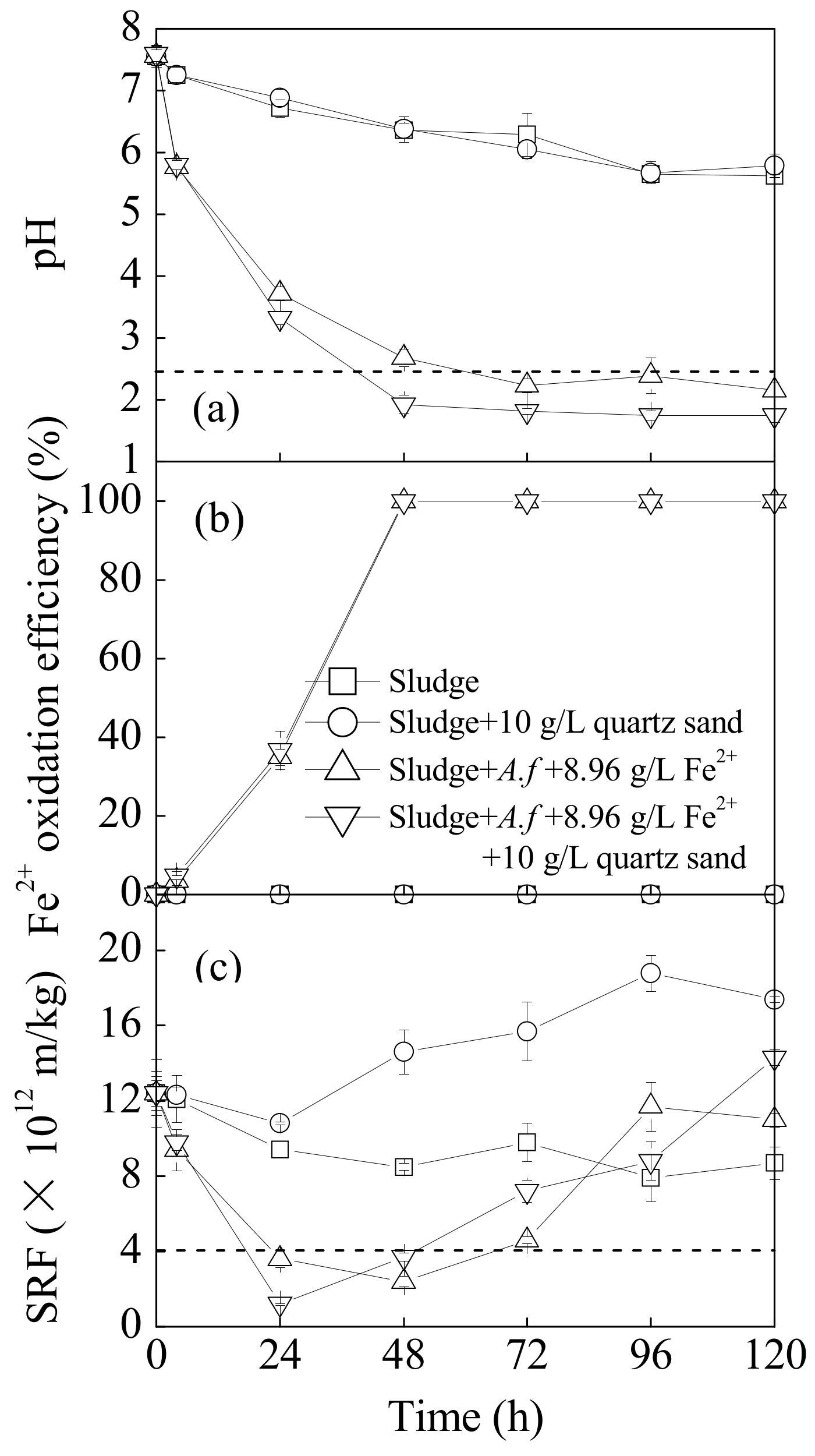
The influence of quartz sand on the pH, Fe2+ oxidation efficiency, and SRF of sludge in a bioleaching system is illustrated in Figure 1. In culture, the pH in both the original sludge system and the sludge system infused with quartz sand declined slowly. The variation curves of these two cultures essentially agreed according to the sludge nitration with the minimum pH ranging from 5.62 to 5.79. This result demonstrated that the quartz sand alone did not have a significant effect on sludge pH. Affected by the low pH of the A. ferrooxidans LX5 suspensions, the original sludge pH declined from 7.57 to 5.77 4 h post-inoculation. With the extension of the bioleaching time, A. ferrooxidans LX5 gradually adapted to the sludge system and began to use Fe2+ energy sources to supply cell metabolism. The Fe3+, oxidized from Fe2+, released H+ through hydrolysis, accelerating sludge acidification. The pH of the sludge system decreased rapidly in the initial bioleaching stage (0–72 h) and then became stable. This result occurred because the slow growth period for the A. ferrooxidans LX5 was relatively short and the cells quickly entered the logarithmic period and propagated rapidly. When the energy sources were exhausted, the A. ferrooxidans LX5 reached an invariable period, with little effect on the changes to sludge pH. As observed in Figure 1a, when sludge is inoculated and supplemented with Fe2+, introducing quartz sand significantly improves the acidification rate and degree. Based on the Fe2+ oxidation levels (Figure 1b), there were no significant effects on the Fe2+ bio-oxidizing efficiency with and without quartz sand. In the sludge system treated with only A. ferrooxidans LX5 and supplemented with both A. ferrooxidans LX5 and quartz sand, the Fe2+ oxidation efficiencies were 35% and 100% at 24 h and 48 h, respectively. The changes in the sludge pH and the Fe2+ oxidizing efficiency indicated that the quartz sand promoted the acidification effects of bioleached sludge because it accelerated the hydrolysis process of bio-oxidation products (Fe3+). Liu et al. reported that 2.4 is the optimum pH to promote municipal sewage sludge dewaterability during the bioleaching process [7]. As shown in Figure 1a,b, the inoculation treatment without quartz sand reached the optimum critical pH point for dewatering after bioleaching between 48 and 72 h, while 10 g·L−1 quartz sand could shorten the bioleaching time to 24–48 h by accelerating the hydrolysis of Fe3+ to release H+.
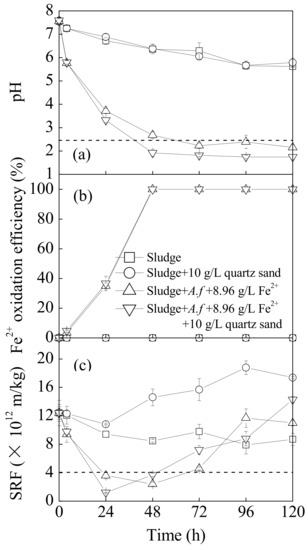
Figure 1.
Changes in sludge (a) pH, (b) Fe2+ oxidation efficiency, and (c) specific resistance to filtration (SRF) upon different treatments at 28 °C and 180 rpm for 120 h: (1) sludge: 540 mL sludge + 60 mL deionized water; (2) sludge + 10 g·L−1 quartz sand: 540 mL sludge + 60 mL deionized water + 10 g·L−1 quartz sand; (3) sludge + A.f + 8.96 g·L−1 Fe2+: 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O; (4) sludge + A.f + 8.96 g·L−1 Fe2+ + 10 g·L−1 quartz sand: 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O + 10 g·L−1 quartz sand (initial pH = 7.22, SRF = 12.4 × 1012 m·kg−1, A. ferrooxidans LX5 density = 5 × 107 cells·mL−1).
From the sludge SRF perspective, the sludge dewaterability in each treatment improved to a certain degree (Figure 1c). For instance, the SRF of the original sludge gradually decreased from 12.4 × 1012 m·kg−1 initially to a minimum of 7.9 × 1012 m·kg−1 at 96 h. After adding 10 g·L−1 of quartz sand, the pH of the original sludge did not change significantly, but the SRF index showed that the quartz sand had an adverse effect on sludge dewaterability, manifesting as a rapid rise in SRF after it declined to 10.8 × 1012 m·kg−1 at 24 h. Analysis suggested that the durable characteristics of the quartz sand changed the sludge’s physical properties so that it congealed during flocculation, then broke and dissolved due to the dynamic hydraulic shear and grinding effects of the quartz sand over a long period of time. The sludge particle size declined, which resulted in fine granules blocking the filter media, thus increasing filtration resistance and decreasing the filtration ability (verified by Figure 2). By comparison, the dewaterability of sludge inoculated with A. ferrooxidans LX5 improved significantly and the sludge SRF decreased to 3.6 × 1012 m·kg−1 at 24 h. Moreover, although the quartz sand supplement proved to adversely affect sludge dewaterability, the SRF variation trend reflected that the quartz sand supplement continued to have a positive effect on improving sludge dewaterability in the initial bioleaching stage, with the sludge SRF decreasing to a minimum of 1.2 × 1012 m·kg−1 within 24 h. Compared to the treatment without quartz sand, the bioleaching method using A. ferrooxidans LX5 and quartz sand both further improved the sludge dewaterability and shortened the bioleaching time accordingly, which is essential to adopting the bioleaching method mediated by A. ferrooxidans LX5 to treat municipal sewage sludge.
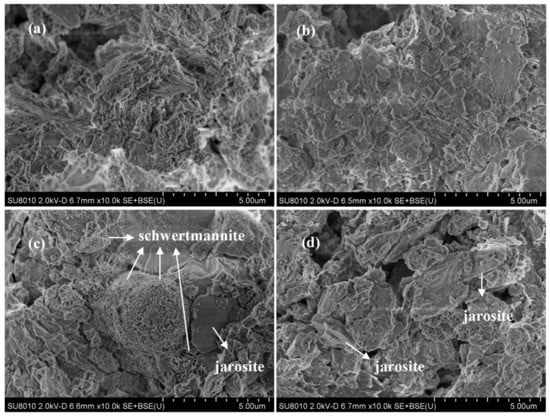
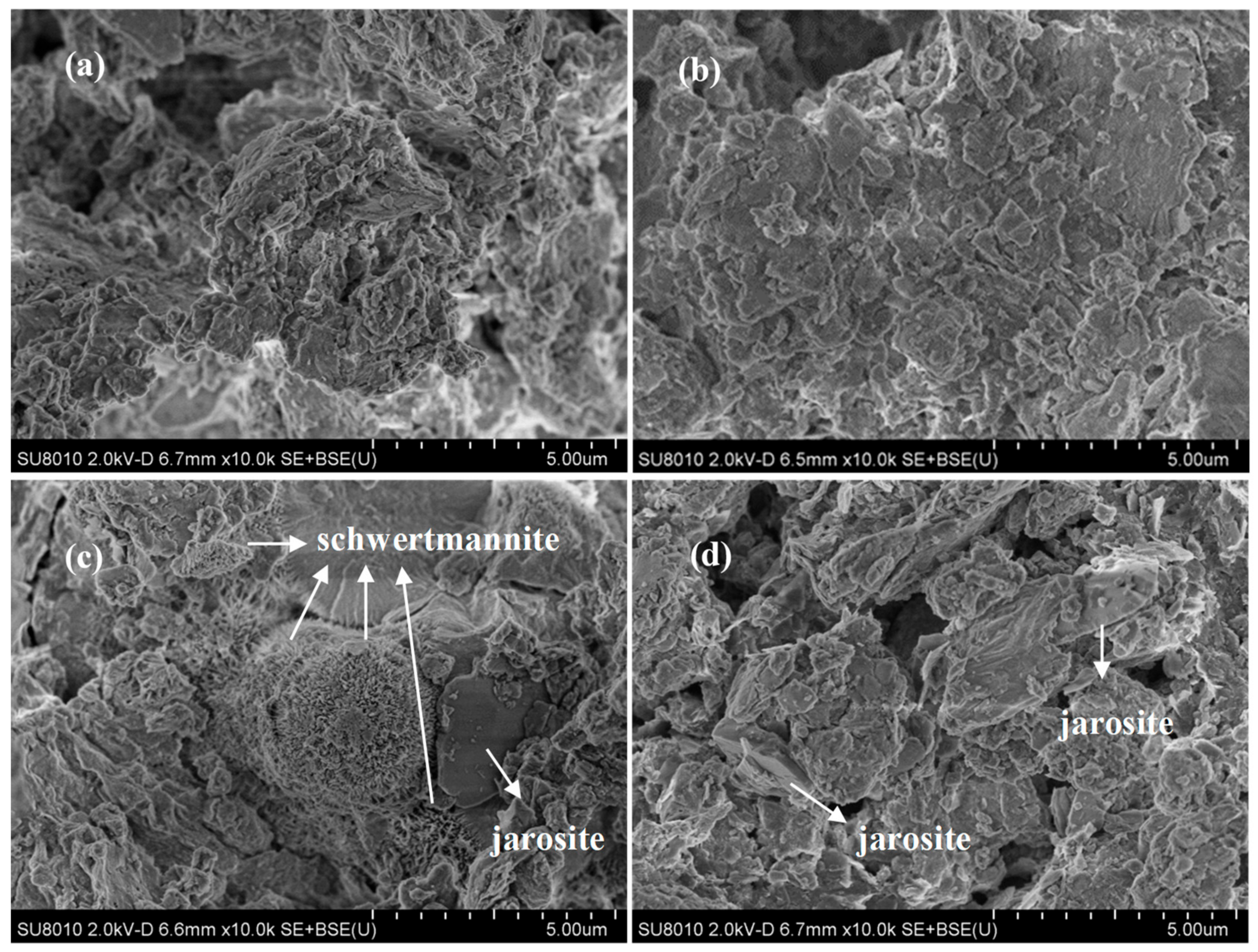
Figure 2.
The scanning electron microscope (SEM) photos of municipal sewage sludge with different treatments at 28 °C and 180 rpm for 120 h: (a) 540 mL sludge + 60 mL deionized water; (b) 540 mL sludge + 60 mL deionized water + 10 g·L−1 quartz sand; (c) 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O; (d) 540 mL sludge + 60 mL A. ferrooxidans LX5 + 44.2 g·L−1 FeSO4·7H2O + 10 g·L−1 quartz sand (initial pH = 7.22, SRF = 12.4 × 1012 m·kg−1, A. ferrooxidans LX5 density = 5 × 107 cells·mL−1).
To remove heavy metals in the solid phase, Liao et al. [13] and Zhou et al. [14] observed the occurrence of secondary iron minerals in separate bioleaching processes for tannery sludge and pig manure. The X-ray diffraction results revealed that the collected minerals were pure schwertmannite and a mixture of schwertmannite and jarosite, respectively [13,14]. The differences in the bioleaching target characteristics also affected the biosynthesis method for secondary iron minerals. In addition, previous researchers have not observed the appearance features of the derivative minerals in the municipal sewage sludge bioleaching system. As shown in Figure 2, the samples from non-treated sludge had irregular colloidal structures and formed clusters during flocculation (Figure 2a). After being treated with quartz sand, the flocculation structure of the original sludge dispersed and the sludge particles were comparatively well distributed; irregular particles in bulk could be clearly observed (Figure 2b). Compared with the original sludge, there were particles shaped like “myrica rubra” inlaid in the flocculation structure of the bioleached sludge; these particles were approximately 3 μm in diameter and covered with acerate burr (Figure 2c). Loan et al. found that schwertmannite, with the structure of a spherical “sea urchin,” would form in an acidic sulfate environment rich in Fe, the surface of which was also covered with acerate burr at a length of approximately 60–90 nm [25,26,27,28]. As inferred, the study particles that were shaped like “myrica rubra” were likely schwertmannite. In addition, smooth blocks were observed attached to sludge particles in the treated samples. According to the findings of Liao et al. [13] and Zhou et al. [14], we assume that the minerals might be jarosite. However, in the bioleaching system supplemented with quartz sand, there was no occurrence of schwertmannite’s unique spherical structure and only some jarosite could be observed in the sludge particles. Therefore, we deduced that the quartz sand might change the way in which Fe3+ is hydrolyzed and mineralized, indicating a tendency to synthesize jarosite in its synthesis process.
3.2. The Influence of Quartz Sand on the Formation of Secondary Iron Minerals in the Bioleaching System Mediated by A. Ferrooxidans LX5
It is necessary to identify the sediment mineral phase to further demonstrate whether quartz sand can change the synthesis of biogenic secondary iron minerals in sludge systems. Based on the complex composition of municipal sludge, the secondary iron minerals would combine with sludge particles and influence identification during the mineral phase. Therefore, it is extremely important to substitute sludge for sludge leachate in order to investigate the formation and properties of biogenic secondary iron minerals. In addition, it has been noted in previous experiments that, when the concentration of the energy source, FeSO4·7H2O, was 44.2 g·L−1 in the sludge system (that is, the concentration of Fe2+ was 8.9 g·L−1), adding 10 g·L−1 of quartz sand could accelerate the sludge acidification and improve sludge dewaterability, with the sludge pH declining to a minimum of 1.74. Decreasing the energy source dosage helped lower sludge treatment costs, which is essential in optimizing bioleaching technology. Nevertheless, there have been no reports on the effects of mineralizing quartz sand to induce low concentrations of Fe2+ in the sludge bioleaching system.
3.2.1. The Influence of Quartz Sand on the pH, Concentrations of Fe2+ and Fe3+ in the Bioleaching System Mediated by A. Ferrooxidans LX5
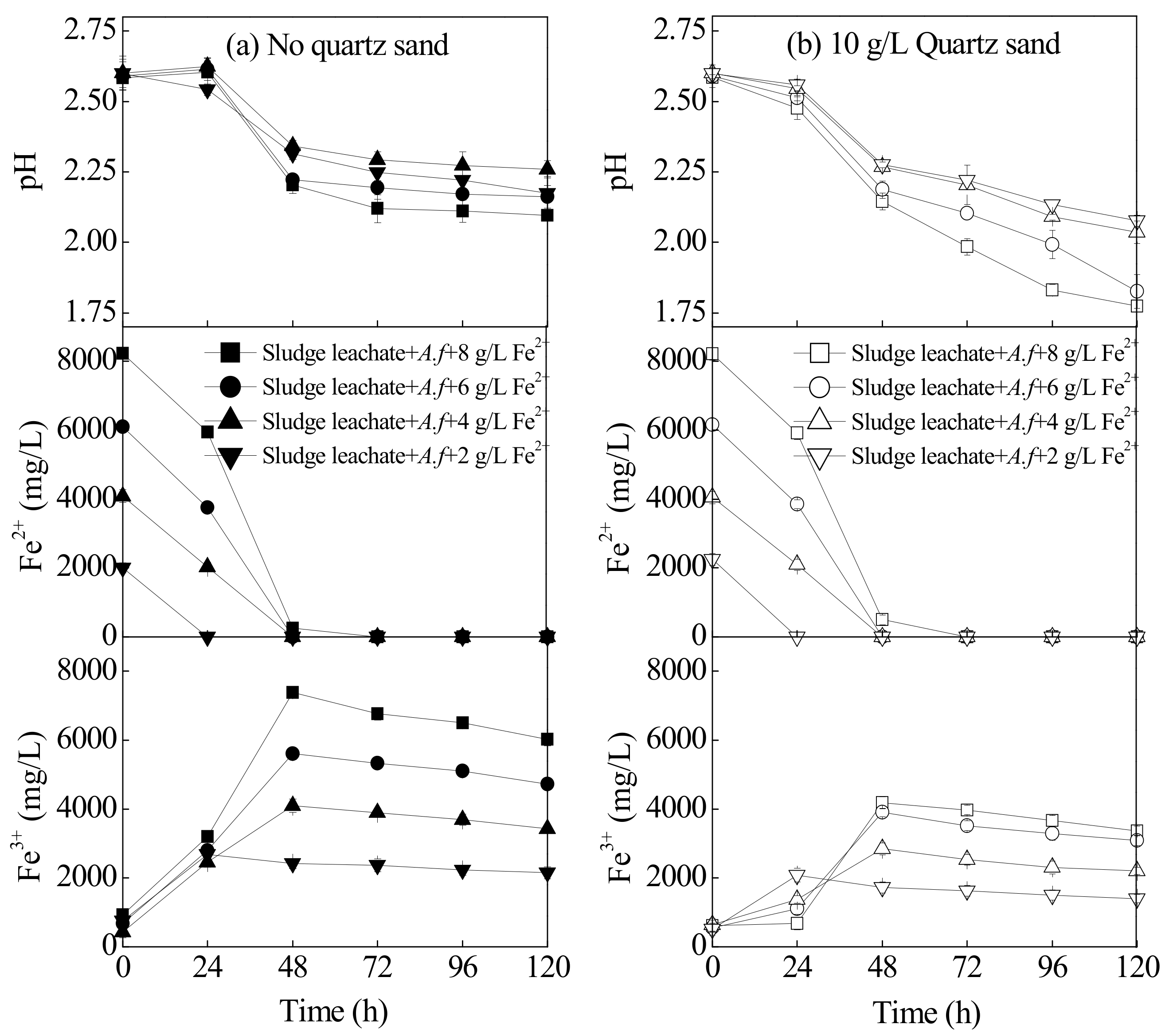
Changes in the indexes in the sludge leachate system under different concentrations of energy sources are illustrated in Figure 3. Previous studies have demonstrated that, when the solution pH > 5, the oxidation efficiency of Fe2+ is positively correlated with pH value. With one unit increments for pH, the oxidation efficiency of Fe2+ would increase 100-fold [29]. However, when pH < 3.5, the oxidation efficiency of Fe2+ was independent of pH with a low oxidation efficiency constant of 10−3.5·d−1 [30]. In an acidic environment, A. ferrooxidans can increase the oxidation efficiency of Fe2+ by 105–106 times [31]. The oxidation of Fe2+ by A. ferrooxidans LX5 included two acid-participating steps: the acid-consuming bio-oxidation process for Fe2+ and the acid-generating hydrolysis and mineralization of Fe3+. Therefore, the oxidation degree of Fe2+ and the mineralization degree of Fe3+ could be approximated based on the changing trends in the reaction system pH. As shown in Figure 3, in the treatment without quartz sand, the pH value of leachate was essentially trending upwards within 0–24 h, which indicated that the primary reaction during this period was the bio-oxidation of Fe2+. Afterwards, the pH began to rapidly decrease and then tended to decrease slightly at 48 h, suggesting that the reaction at 24–28 h was mainly the hydrolysis of Fe3+. When Fe2+ was entirely oxidized, it reduced the acid produced by the Fe3+ hydrolysis. After it was supplemented with 10 g·L−1 of quartz sand, the system pH began to decrease within 0–24 h. This result is compared with the treatment without quartz sand, despite the fact that there was a consistent Fe2+ oxidation efficiency in both treatments at different stages. This result suggested that quartz sand could accelerate the hydrolysis and mineralization of Fe3+. After being bioleached for 48 h, the Fe3+ hydrolysis and mineralization could still proceed quickly and continuously, the manifestations being that the system pH treated with 8, 6, 4, and 2 g·L−1 Fe2+ decreased to 1.77, 1.83, 2.04, and 2.08 at 120 h, respectively. Compared with the treatment without quartz sand under the same conditions, the pH decreased by 0.32, 0.33, 0.22, and 0.09, respectively. This further illustrates that quartz sand could promote the hydrolysis and mineralization of Fe3+, as confirmed by the changing trends in Fe3+ concentrations as shown in Figure 3.
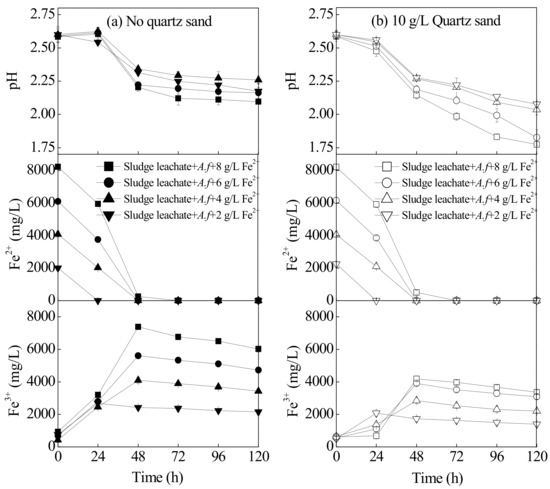
Figure 3.
The index change (pH, Fe2+ and Fe3+ concentration) in the municipal sewage sludge with different treatments at 28 °C and 180 rpm for 120 h: (a) no quartz sand; (b) 10 g·L−1 quartz sand (initial pH = 2.55, Fe2+ concentration = 2–8 g·L−1, sludge leachate volume = 270 mL, A. ferrooxidans LX5 volume = 30 mL, A. ferrooxidans LX5 density = 5 × 107 cells·mL−1).
3.2.2. The Influence of Quartz Sand on the Precipitation of TFe in the Bioleaching System Mediated by A. Ferrooxidans LX5
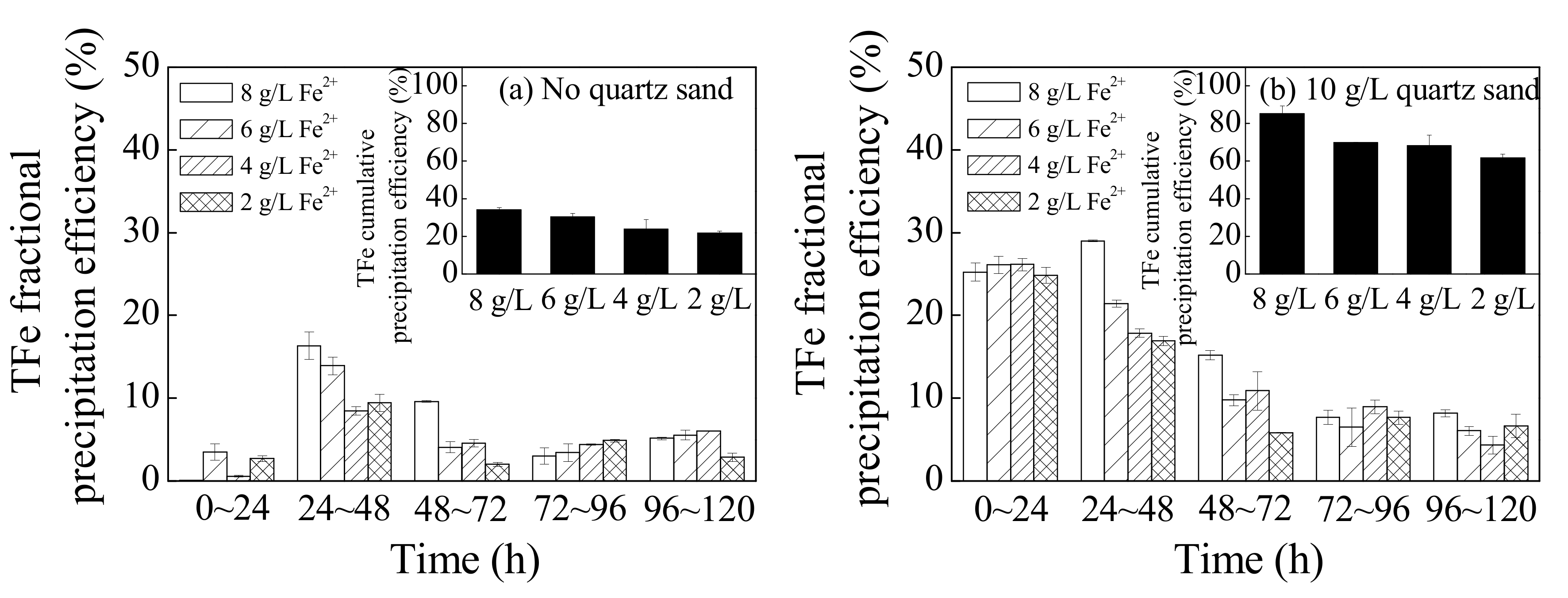
Figure 4 shows a comparison of the TFe fractional precipitation efficiency and accumulated precipitation efficiency with different treatments during different reaction periods. Essentially, in this process, the Fe3+ in the solutions was transferred from the liquid phase to the solid phase as it was hydrolyzed into secondary iron minerals during the bio-oxidation of Fe2+ [32]. Overall, introducing quartz sand or increasing the concentration of the energy source, Fe2+ in the bioleaching system improved the hydrolysis and sedimentation of Fe3+. For example, 10 g·L−1 of quartz sand could improve the TFe precipitation efficiency of system treated with 8, 6, 4, or 2 g·L−1 Fe2+ from 34.2%, 30.5%, 24.0%, and 21.9% to 85.3%, 69.9%, 68.3%, and 61.9%, respectively. In general, high concentrations of Fe3+ benefit the secondary iron mineral synthesis [33]. The results of this study indicated that a large number of secondary iron minerals could form in low Fe3+ concentrations when quartz sand is supplemented as crystal seed.
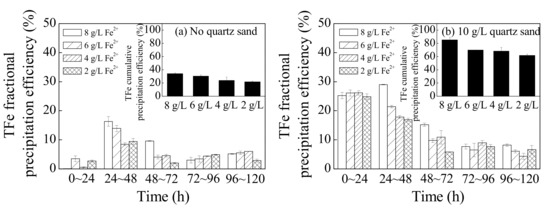
Figure 4.
The changes in total Fe (TFe) precipitation efficiency in the municipal sewage sludge leachate with different treatments at 28 °C and 180 rpm for 120 h: (a) no quartz sand; (b) 10 g·L−1 quartz sand (initial pH = 2.55, Fe2+ concentration = 2–8 g·L−1, sludge leachate volume = 270 mL, A. ferrooxidans LX5 volume = 30 mL, A. ferrooxidans LX5 density = 5 × 107 cells·mL−1).
In terms of the fractional TFe deposition efficiency, the TFe precipitation for all batches treated without quartz sand primarily occurred between 24 and 48 h, accounting for 40–50% of the cumulative TFe deposition efficiencies, among which the worst precipitation effects occurred between 0 and 24 h. Introducing quartz sand mainly improved the TFe deposition efficiencies between 0 and 48 h and the TFe deposition efficiency during this period accounted for more than 60% of the cumulative efficiency. As shown in Figure 1 and Figure 3, the supply rate of Fe3+ was low between 0 and 24 h resulting from the relatively slow Fe2+ oxidation efficiency, which then inhibited the formation of secondary iron minerals [15,34]. Employing quartz sand as crystal seed could overcome the adverse effects of low Fe3+ concentrations and significantly promote the hydrolysis and mineralization of Fe3+. Dutrizac demonstrated the following: the formation of secondary iron minerals is a new phase formation process; the initial TFe precipitation efficiency is slow due to the existence of an induction period; and crystal seed could provide nucleating sites for secondary iron minerals, accelerate the initial precipitation of minerals, and shorten the reaction stability time [20]. When Fe2+ was entirely oxidized and the induction period ended (48–120 h), the effect of quartz sand as crystal seed slightly decreased. However, as observed in Figure 1, the optimum reaction time to promote the dewaterability of bioleached sludge mediated by A. ferrooxidans LX5 was 24–48 h, after which the hydrolysis and mineralization effects had little influence on sludge dewaterability.
3.2.3. The Influence of Quartz Sand on the Synthesis of Secondary Minerals in the Bioleaching System Mediated by A. Ferrooxidans LX5
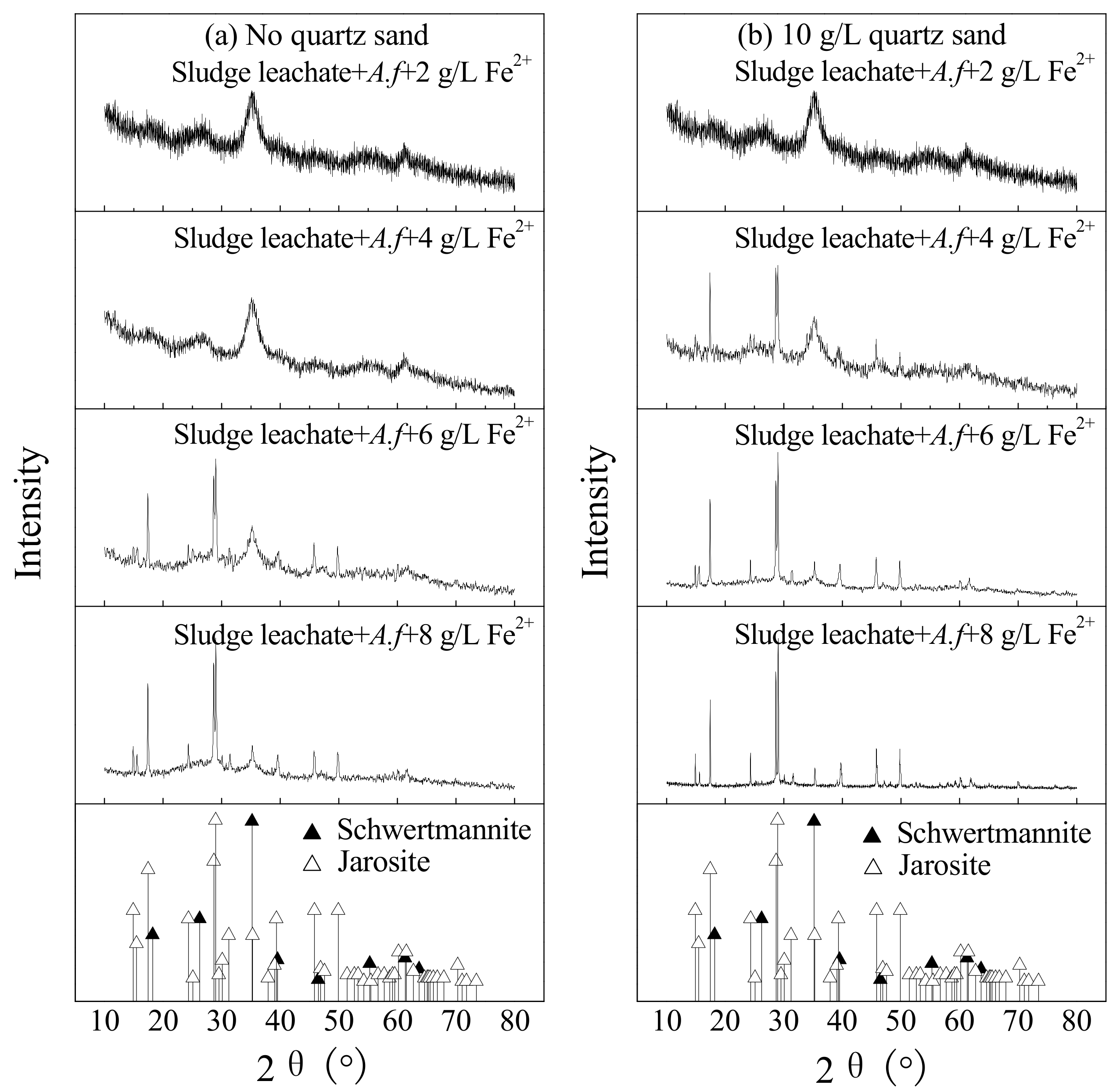
Figure 5 shows the XRD spectrum of secondary iron minerals collected at the termination of culture in sludge leachate under different treatment. Referring to the spectrum of JCPDS crystalline jarosite (No: 22-0827) and amorphous schwertmannite (No: 47-1775) [26,35], improving concentrations of energy sources is known to benefit jarosite formation. For instance, when the concentration of Fe2+ rose from 2 to 8 g·L−1, the secondary iron minerals gradually transformed from schwertmannite to a mixture of schwertmannite and jarosite, and gradually intensive diffraction peaks of jarosite were observed. Regenspurg et al. attributed this phenomenon to the high Fe3+ supply rate in the system [34]. By comparison, when Fe2+ ≥ 4 g·L−1, adding 10 g·L−1 quartz sand can induce and promote the transformation of the secondary iron minerals synthesis process, making it easier to obtain jarosite. We learned from Reactions (1) and (2) that the jarosite mass generated per Fe3+ unit should be 1.5 times more than schwertmannite, if the secondary iron minerals were synthesized through a single path. If so, this process makes it possible to produce more secondary iron minerals, thus improving the dewaterability of bioleached sludge. Moreover, when it is easier to form jarosite, the nutrients in the liquid phase can be immobilized, which increases the utilization value of the sludge. There was no sign of diffraction peaks of ammonium jarosite in this study, which might result from a low concentration of NH4+ in the sludge leachate.
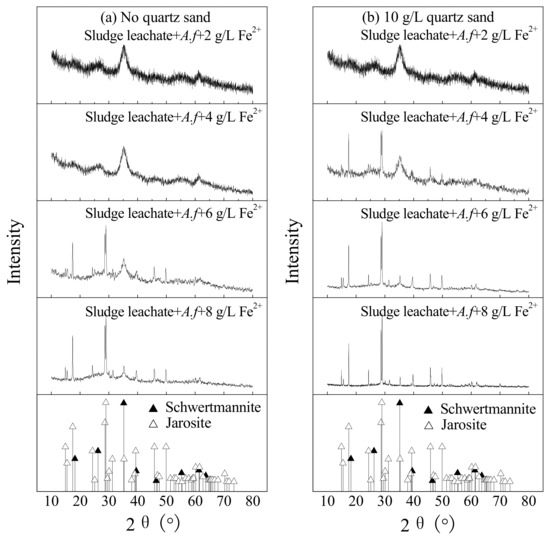
Figure 5.
The XRD pattern of biogenic secondary iron minerals under different treatments at 28 °C and 180 rpm for 120 h: (a) no quartz sand; (b) 10 g·L−1 quartz sand (initial pH = 2.55, Fe2+ concentration = 2–8 g·L−1, sludge leachate volume = 270 mL, A. ferrooxidans LX5 volume = 30 mL, A. ferrooxidans LX5 density = 5 × 107 cells·mL−1).
4. Conclusions
This study investigated the utilization and mechanism of quartz sand to improve sludge dewaterability during the bioleaching process. The results showed that quartz sand could improve sludge bioleaching efficiency and promote the sludge acidification degree. We found that the optimal reaction time was 24–48 h and 48–72 h with and without 10 g·L−1 quartz sand, respectively, and that the minimum sludge SRF was measured to be 1.2 × 1012 m·kg−1 and 2.4 × 1012 m·kg−1, respectively. Our analysis indicated that quartz sand could overcome the unfavorable influence of a low Fe3+ supply rate in the initial bioleaching stage (0–24 h), accelerate the initial mineral precipitation, shorten the reaction time, and improve bioleaching efficiency. When the concentration of Fe2+ ≥ 4 g·L−1, adding 10 g·L−1 quartz sand could induce changes to the synthesis pathway of secondary iron minerals, which promotes the transformation of schwertmannite into jarosite during the mineralization process. In conclusion, comprehensively considering the elements of the sludge acidification rate, SRF, TFe precipitation efficiency, and the secondary iron minerals phase, when supplemented with 10 g·L−1 quartz sand, 24–48 h was the optimum reaction time for municipal sewage sludge bioleaching mediated by A. ferrooxidans LX5, and the best concentration of Fe2+ energy sources was 4–6 g·L−1.
Author Contributions
Y.S. conceived and designed this study, conducted the data collation and analysis, and wrote the manuscript. Y.M., Y.W., and Z.G. performed the experiments, conducted the data collation and analysis, and wrote the manuscript. H.W. contributed to revision of the manuscript. All read and approved the final manuscript.
Funding
This research was funded by the Natural Science Foundation of Hubei Province, China (Grant No. 2016CFB289), and the Talent Introduction Foundation of Zhongnan University of Economics and Law (Grant No. 31541711302).
Acknowledgments
The authors express their sincere gratitude to the Longwangzui Wastewater Treatment Plant in Wuhan City for kindly providing samples.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhou, L.X.; Fang, D.; Wang, S.M.; Wong, J.W.C.; Wang, D.Z. Bioleaching of Cr from tannery sludge: the effects of initial acid addition and recycling of acidified bioleached sludge. Environ. Technol. 2005, 26, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, R.D.; Sreekrishnan, T.R.; Blais, J.F.; Surampalli, R.Y.; Campbell, P.G.C. Effect of dissolved oxygen on sludge acidification during the SSDML process. Water Air Soil Pollut. 1998, 102, 139–155. [Google Scholar] [CrossRef]
- Zhou, L.X.; Zhou, S.G.; Wang, S.M.; Fang, D.; Wang, D.Z. Cr removal and improving the settling and dehydrating capability from tannery sludge simultaneously through bioleaching approach. Acta Sci. Circum. 2004, 6, 1014–1020. [Google Scholar]
- Wang, D.Z.; Zhou, L.X.; He, F. Studies on the enhancement of dehydration property of tannery sludge by bioleaching technique. China Environ. Sci. 2006, 26, 67–71. [Google Scholar]
- Song, X.W.; Zhou, L.X. The influence of bioleaching on dewaterability of municipal sewage sludge. Acta Sci. Circum. 2008, 28, 2012–2017. [Google Scholar]
- Chen, H.; Zhou, L.X.; Li, C. The removal of Cr from tannery sludge by bioleaching in air-lift reactor: A pilot study. Environ. Sci. 2007, 28, 2046–2051. [Google Scholar]
- Liu, F.W.; Zhou, L.X.; Zhou, J.; Song, X.W.; Wang, D.Z. Improvement of sludge dewaterability and removal of sludge-borne metals by bioleaching at optimum pH. J. Hazard. Mater. 2012, 221, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zheng, G.; Wong, J.W.; Zhou, L. Degradation of inhibitory substances in sludge by Galactomyces sp. Z3 and the role of its extracellular polymeric substances in improving bioleaching. Bioresour. Technol. 2013, 132, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, K.; Ravindran, B.; Selvam, A.; Kurade, M.B.; Yu, S.M.; Wong, J.W. Enhanced dewaterability of anaerobically digested sewage sludge using Acidithiobacillus ferrooxidans culture as sludge conditioner. Bioresour. Technol. 2014, 169, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.W.C.; Xiang, L.; Gu, X.Y.; Zhou, L.X. Bioleaching of heavy metals from anaerobically digested sewage sludge using FeS2 as an energy source. Chemosphere 2004, 55, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Chan, L.C.; Wong, J.W.C. Removal of heavy metals from anaerobically digested sewage sludge by isolated indigenous iron-oxidizing bacteria. Chemosphere 2000, 41, 283–287. [Google Scholar] [CrossRef]
- Wang, H.M.; Min, X.B.; Chai, L.Y.; Shu, Y.D. Biological preparation and application of poly-ferric sulfate flocculant. Trans. Nonferrous Met. Soc. China 2011, 21, 2542–2547. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, L.; Bai, S.; Liang, J.; Wang, S. Occurrence of biogenic schwertmannite in sludge bioleaching environments and its adverse effect on solubilization of sludge-borne metals. Appl. Geochem. 2009, 24, 1739–1746. [Google Scholar] [CrossRef]
- Zhou, J.; Zhou, L.; Liu, F.; Zheng, C.; Deng, W. Transformation of heavy metals and the formation of secondary iron minerals during pig manure bioleaching by the co-inoculation acidophilic thiobacillus. Environ. Technol. 2012, 33, 2553–2560. [Google Scholar] [CrossRef] [PubMed]
- Control Standards of Pollutants in Sludge for Agricultural Use; GB 4284-2018; Inspection and Quarantine of the People’s Republic of China: Beijing, China, 2018.
- Guo, G.H.; Chen, T.B.; Yang, J.; Zheng, G.; Ding, G. Regional distribution characteristics and variation of heavy metals in sewage sludge of China. Acta Sci. Circum. 2014, 34, 2455–2461. [Google Scholar]
- Sasaki, K.; Konno, H. Morphology of jarosite-group compounds precipitated from biologically and chemically oxidized Fe ions. Can. Mineral. 2000, 38, 45–56. [Google Scholar] [CrossRef]
- Karamanev, D.G. Model of the biofilm structure of Thiobacillus ferrooxidans. J. Biotechnol. 1991, 20, 51–64. [Google Scholar] [CrossRef]
- Jensen, A.B.; Webb, C. Ferrous sulphate oxidation using Thiobacillus ferrooxidans: A review. Process Biochem. 1995, 30, 225–236. [Google Scholar] [CrossRef]
- Dutrizac, J.E. The effect of seeding on the rate of precipitation of ammonium jarosite and sodium jarosite. Hydrometallurgy 1996, 42, 293–312. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, L.X. The removal of soluble ferrousiron in acid mine drainage (AMD) through the formation of biogenic iron oxyhydrosulfate precipitates facilitated by diatomite, quartz sand and potassium. Acta Petrol. Mineral. 2011, 30, 1031–1038. [Google Scholar]
- Tichý, R.; Janssen, A.; Grotenhuis, J.T.C.; Lettinga, G.; Rulkens, W.H. Possibilities for using biologically-produced sulphur for cultivation of Thiobacillus with respect to bioleaching processes. Bioresour. Technol. 1994, 48, 221–227. [Google Scholar] [CrossRef]
- American Public Health Association. APHA: Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Liu, F.W.; Gao, S.Y.; Wang, M.; Yu, H.Y.; Cui, C.H.; Zhou, L.X. Effect of KOH on the formation of biogenic secondary iron minerals in iron- and sulfate-rich acidic environment. Acta Sci. Circum. 2015, 35, 476–483. [Google Scholar]
- Loan, M.; Richmond, W.R.; Parkinson, G.M. On the crystal growth of nanoscale schwertmannite. J. Cryst. Growth 2005, 275, 1875–1881. [Google Scholar] [CrossRef]
- Jönsson, J.; Persson, P.; Sjöberg, S.; Lövgren, L. Schwertmannite precipitated from acid mine drainage: phase transformation, sulfate release and surface properties. Appl. Geochem. 2005, 20, 179–191. [Google Scholar] [CrossRef]
- Šubrt, J.; Boháček, J.; Štengl, V.; Grygar, T.; Bezdička, P. Uniform particles with a large surface area formed by hydrolysis of Fe2(SO4)3 with Urea. Mater. Res. Bull. 1999, 34, 905–914. [Google Scholar] [CrossRef]
- Hongfu, S.; Fenghua, Z.; Zhiyuan, C. The mineral Schwertmannite found in China and its characteristics. Acta Mineral. Sin. 2006, 26, 38–42. [Google Scholar]
- Singer, P.C.; Stumm, W. Acidic mine drainage: The rate determining step. Science 1970, 167, 1121–1123. [Google Scholar] [CrossRef] [PubMed]
- Umita, T. Biological mine drainage treatment. Resour. Conserv. Recycl. 1996, 16, 179–188. [Google Scholar] [CrossRef]
- Bosecker, K. Bioleaching: Metal solubilization by microorganisms. FEMS. Microbiol. Rev. 1997, 20, 591–604. [Google Scholar] [CrossRef]
- Harahuc, L.; Lizama, H.M.; Suzuki, I. Selective inhibition of the oxidation of ferrous iron or sulfur in Thiobacillus ferrooxidans. Appl. Environ. Microbl. 2000, 66, 1031–1037. [Google Scholar] [CrossRef]
- Bai, S.Y.; Liang, J.R.; Zhou, L.X. Effects of iron/potassium molar ratio on mass of biogenic Fe(III) hydroxysulfate precipitates in the FeSO4-K2SO4-H2O system and their environmental implications. Acta Sci. Circum. 2010, 30, 1601–1607. [Google Scholar]
- Regenspurg, S.; Brand, A.; Peiffer, S. Formation and stability of schwertmannite in acid mining lakes 1. Geochim. Cosmochim. Acta 2004, 68, 1185–1197. [Google Scholar] [CrossRef]
- International Center for Diffraction Data. JCPDS-Mineral Powder Diffraction Files; International Center for Diffraction Data: Swarthmore, PA, USA, 2002. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).