Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China)
Abstract
:1. Introduction
2. Materials and Methods
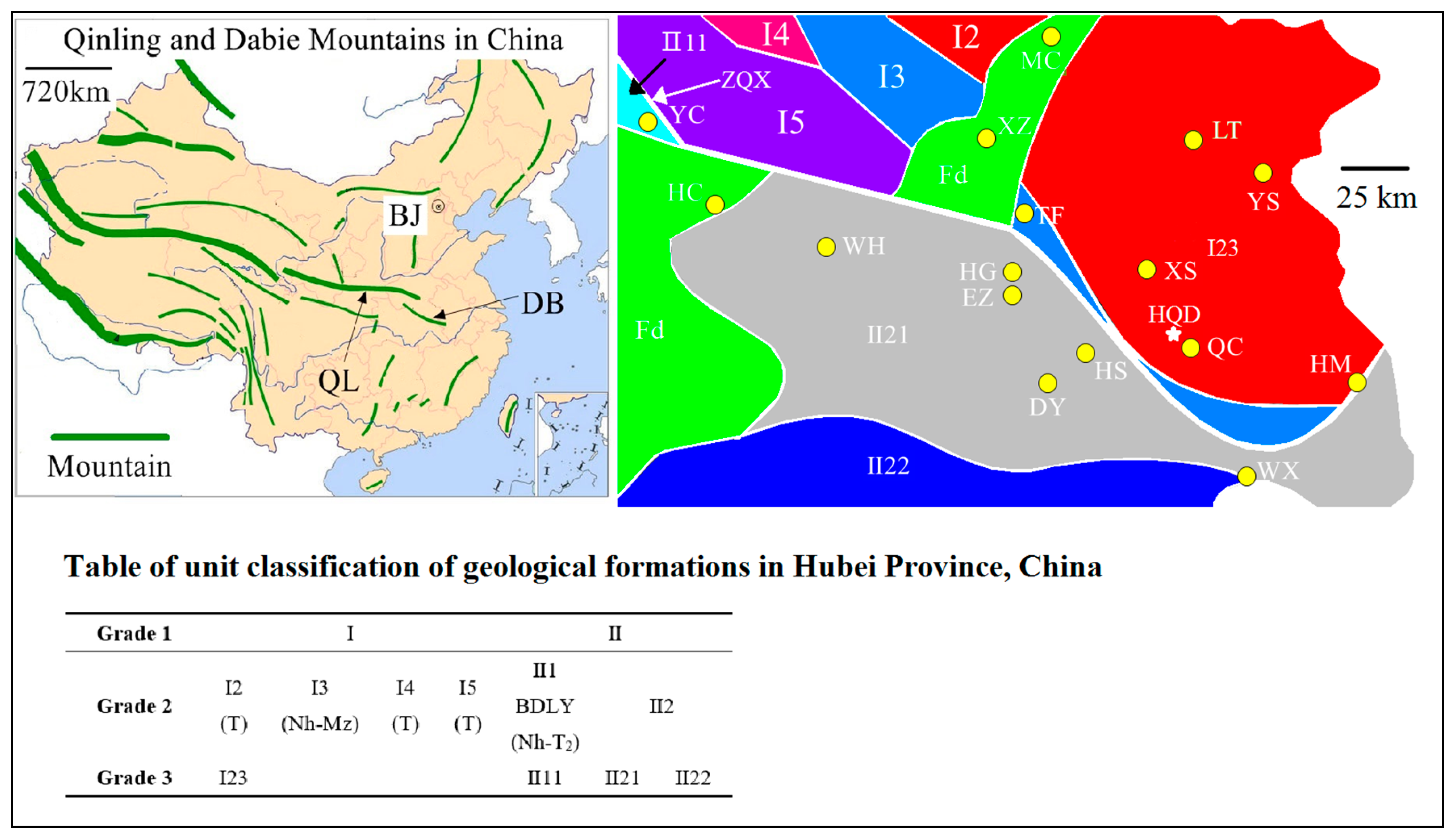
2.1. Materials and Geological Situation
2.2. Mineralogical Analysis
2.3. Chemical Analysis
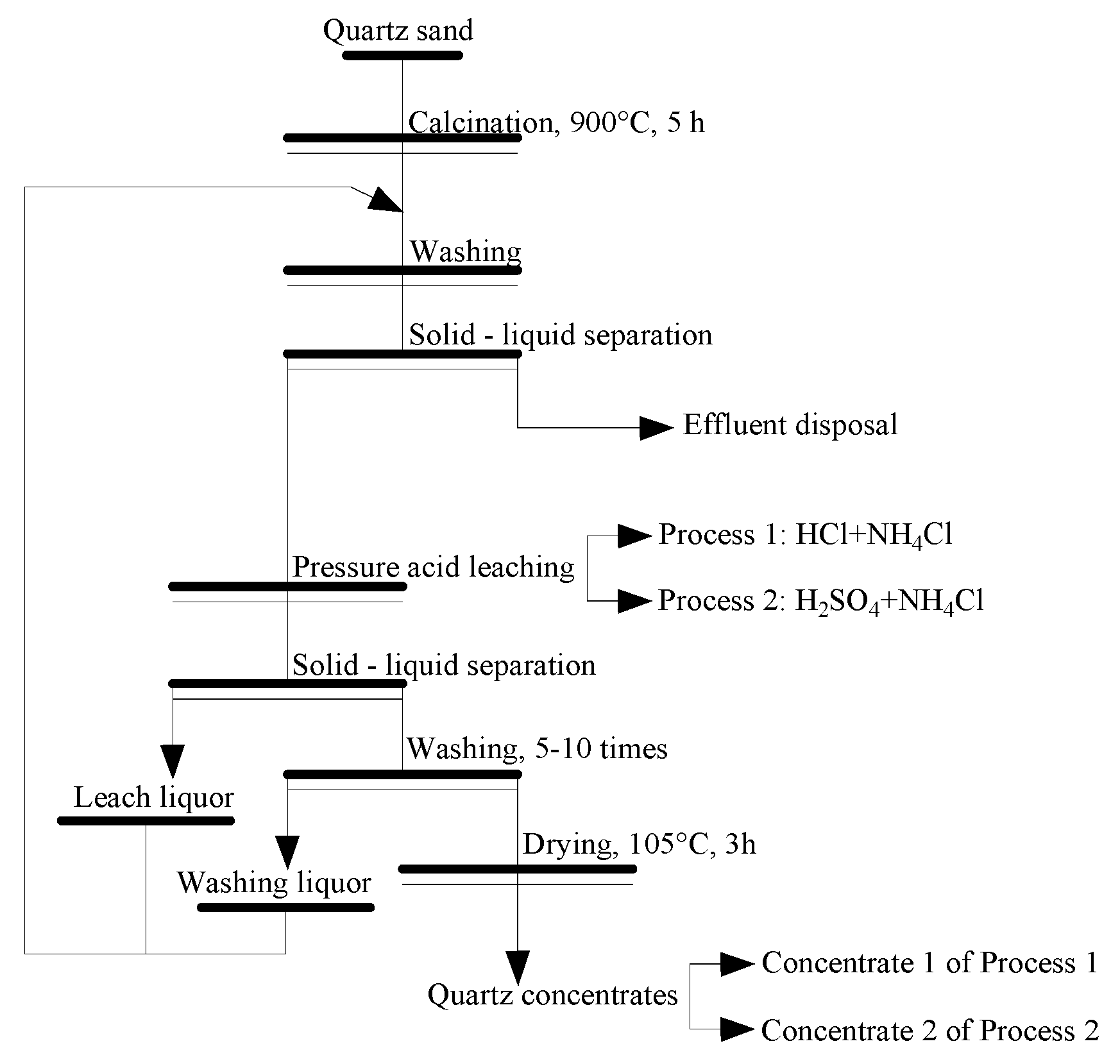
2.4. Processing and Characterization of Quartz Sand
3. Results and Discussion
3.1. Mineralogy of Hydrothermal Quartz
3.1.1. Impurity Elements in Quartz Ore
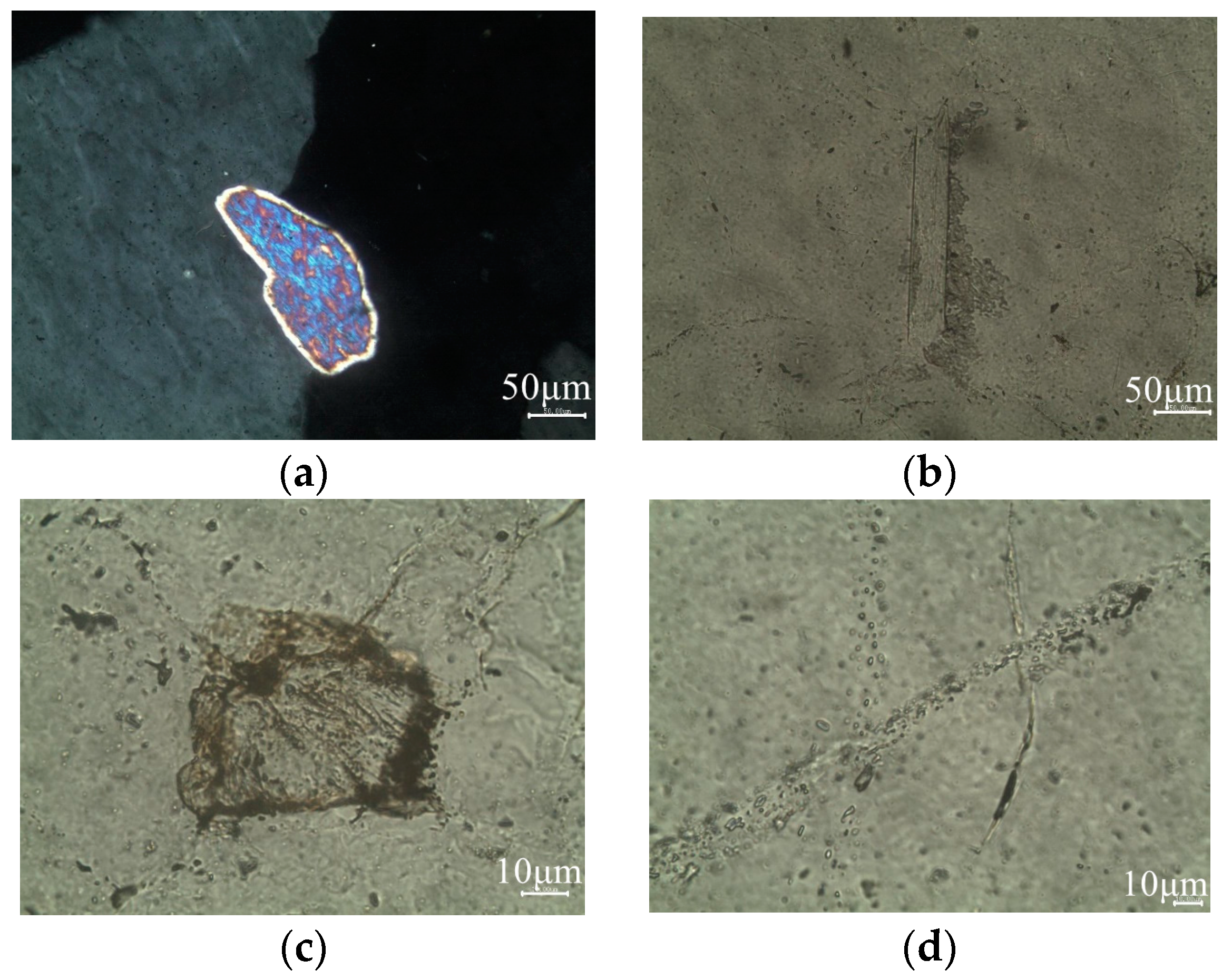
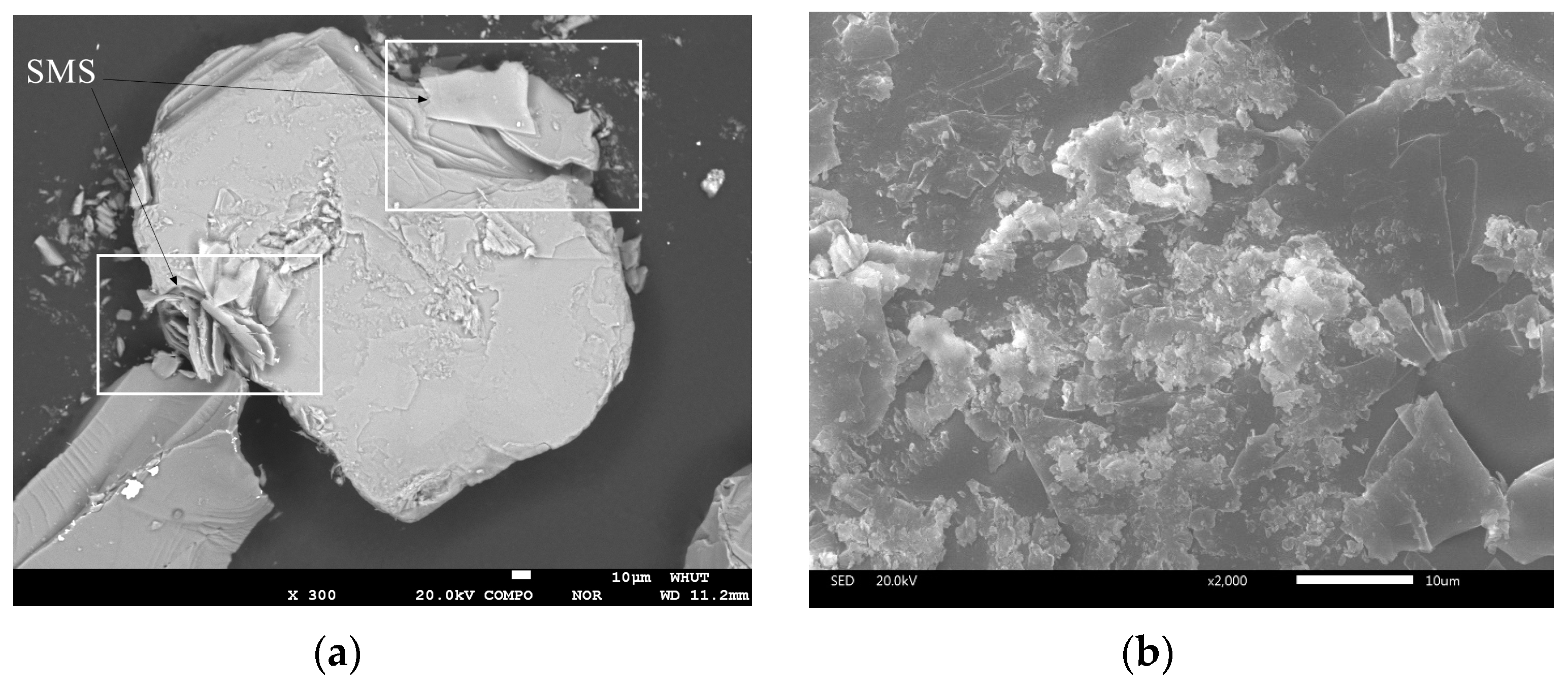
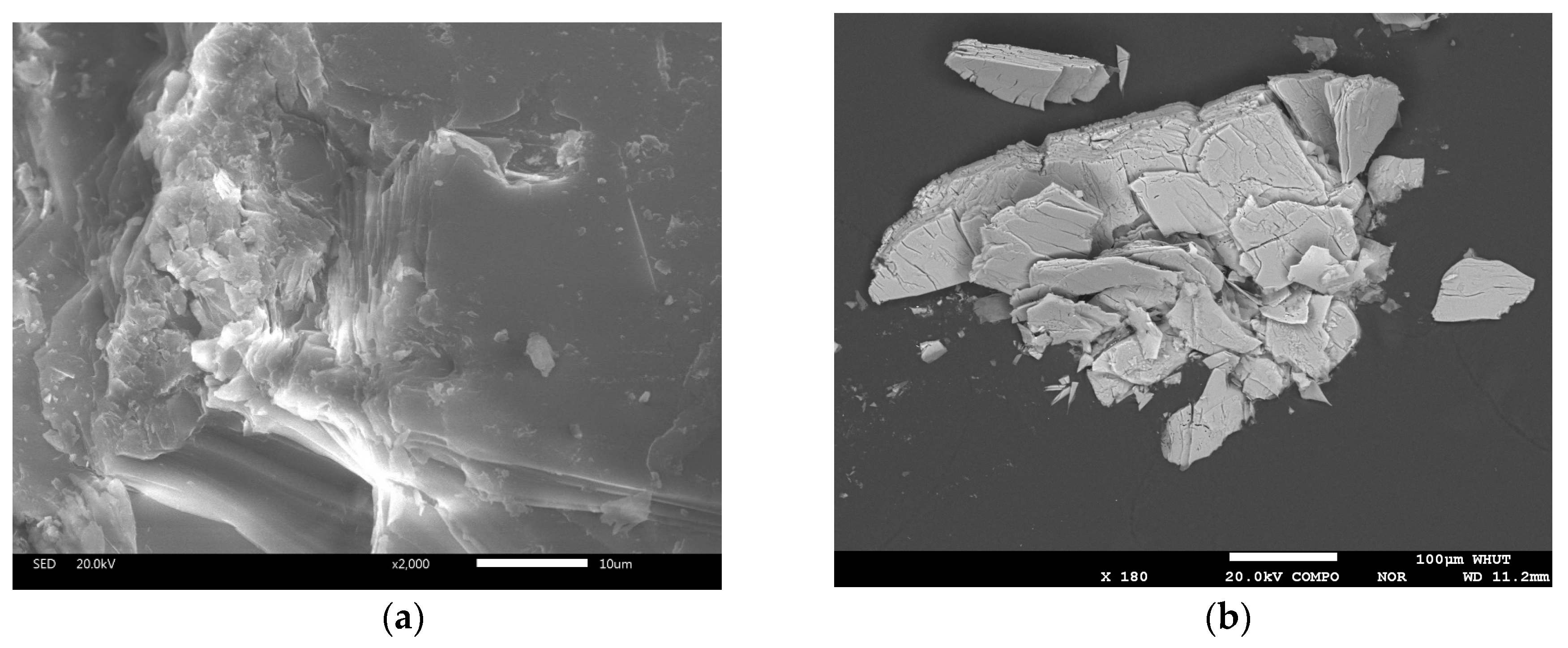
3.1.2. Optical Microscope Analysis
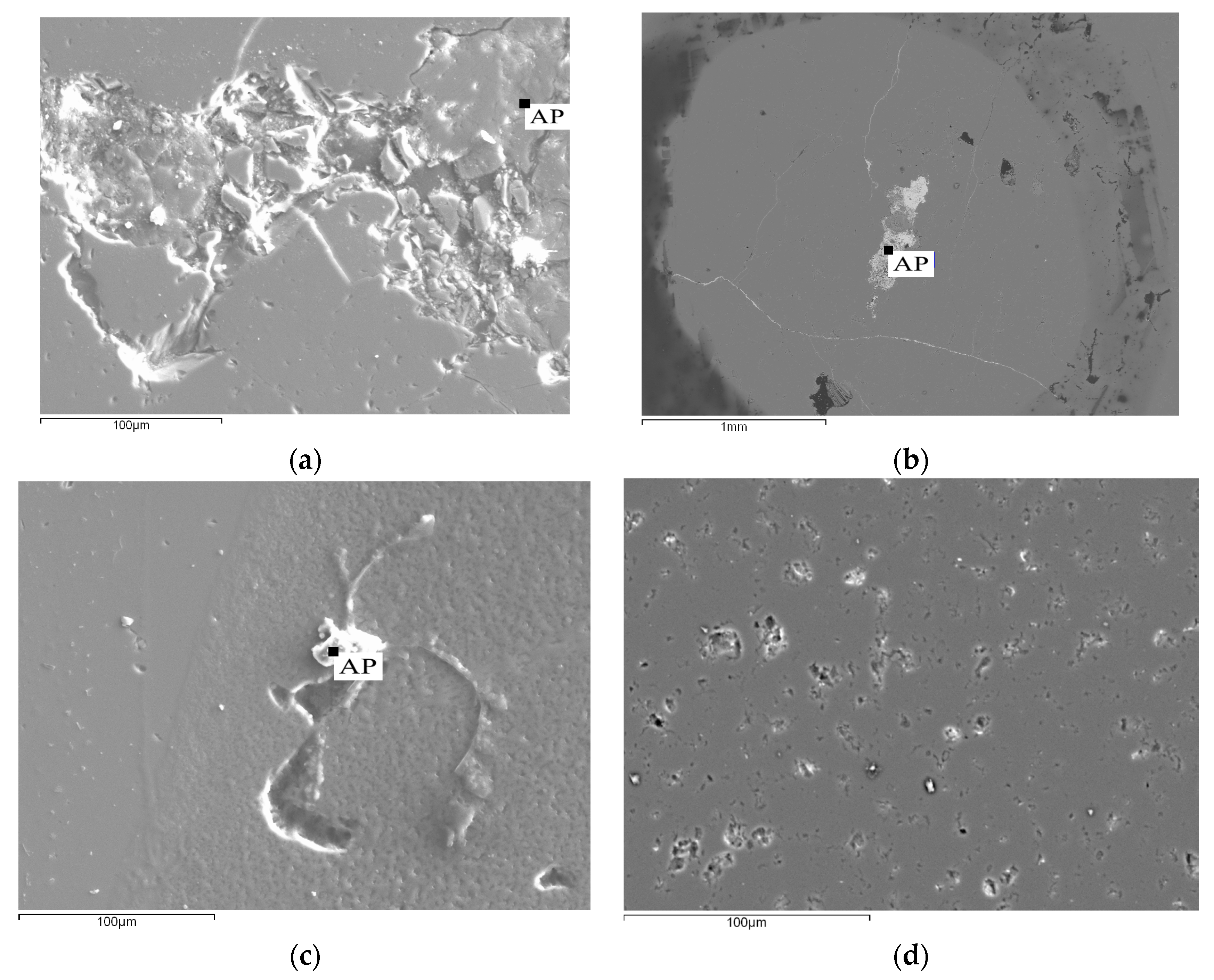
3.1.3. Electron Probe Microanalysis
3.2. Quartz Processing
3.2.1. Recommended Process
3.2.2. Effects of the Calcination Process
3.2.3. Effects of Fluoride-Free Pressure Acid Leaching
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Götze, J. Classification, mineralogy and industrial potential of SiO2, minerals and rocks. In Quartz: Deposits, Mineralogy and Analytics, 1st ed.; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–27. [Google Scholar]
- Howm, R.A. Silica: Physical behavior, geochemistry and materials applications. Mineral. Mag. 1996, 60, 390–391. [Google Scholar] [CrossRef]
- Armington, A.F.; Larkin, J.J. Purification and analysis of α-quartz. J. Cryst. Growth 1986, 75, 122–125. [Google Scholar] [CrossRef]
- Li, J.S.; Li, X.X.; Shen, Q.; Zhang, Z.Z.; Du, F.H. Further purification of industrial quartz by much milder conditions and a harmless method. Environ. Sci. Technol. 2010, 44, 7673. [Google Scholar] [CrossRef] [PubMed]
- Bayaraa, B.; Greg, B.; Noriyoshi, T. Hydrothermal quartz vein formation, revealed by coupled SEM-CL imaging and fluid inclusion microthermometry: Shuteen Complex, South Gobi, Mongolia. Resour. Geol. 2010, 55, 1–8. [Google Scholar] [CrossRef]
- Johnson, G.R. History of the industrial production and technical development of single crystal cultured quartz. In Proceedings of the IEEE International Frequency Control Symposium & Exposition, Montreal, QC, Canada, 23–27 August 2004. [Google Scholar]
- Mavrogenes, J.A.; Bodnar, R.J. Hydrogen movement into and out of fluid inclusions in quartz: Experimental evidence and geologic implications. Geochim. Cosmochim. Acta 1994, 58, 141–148. [Google Scholar] [CrossRef]
- Tomlinson, E.L.; Mcmillan, P.F.; Zhang, M.; Jones, A.P.; Redfern, S.A.T. Quartz-bearing C–O–H fluid inclusions diamond: Retracing the pressure–temperature path in the mantle using calibrated high temperature IR spectroscopy. Geochim. Cosmochim. Acta 2007, 71, 6030–6039. [Google Scholar] [CrossRef]
- Sayilgan, A.; Arol, A.I. Effect of carbonate alkalinity on flotation behavior of quartz. Int. J. Miner. Process. 2004, 74, 233–238. [Google Scholar] [CrossRef]
- Xiong, K.; Lei, S.M.; Zhong, L.L.; Pei, Z.Y.; Yang, Y.Y.; Zang, F.F. Thermodynamic mechanismand purification of hot press leaching with vein quartz. China Min. Mag. 2016, 25, 129–132. [Google Scholar] [CrossRef]
- Lei, S.M.; Pei, Z.Y.; Zhong, L.L.; Ma, Q.L.; Huang, D.D.; Yang, Y.Y. Study on the technology and mechanism of reverse flotation and hot pressing leaching with vein quartz. Nonmet. Mines 2014, 37, 40–43. [Google Scholar] [CrossRef]
- Wang, L.; Li, C.X.; Wang, Y.; Yin, D.Q. China technologies present of high-purity quartz processing and the development propositions. J. Mineral. Petrol. 2011, 31, 110–114. [Google Scholar] [CrossRef]
- Haßler, S.; Kempe, U.; Monecke, T.; Götze, J. Trace Element Content of Quartz from the Ehrenfriedersdorf Sn-w Deposit, Germany: Results of an Acid-Wash Procedure. In Mineral Deposit Research: Meeting the Global Challenge, Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Mao, J.W., Bierlein, F.P., Eds.; Society of Economic Geologists, Inc.: Littleton, CO, USA, 2006. [Google Scholar]
- Haus, R.; Prinz, S.; Priess, C. Assessment of high purity quartz resources. In Quartz: Deposits, Mineralogy and Analytics, 1st ed.; Götze, J., Möckel, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 45–49. [Google Scholar]
- Vidyadhar, A.; Hanumantha, R.K. Adsorption mechanism of mixed cationic/anionic collectors in feldspar-quartz flotation system. J. Colloid Interface Sci. 2007, 306, 195–204. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Lee, H.A.; Lee, J.; Yoon, H.O. Fluorine distribution in soil in the vicinity of an accidental spillage of hydrofluoric acid in Korea. Chemosphere 2015, 119, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, P.K. Comment on “hydrofluoric acid in the Southern California atmosphere”. Environ. Sci. Technol. 1998, 31, 427. [Google Scholar] [CrossRef]
- Zhou, Y.H. Study on refining quartz powder by leaching in HF acid solution. J. Mineral. Petrol. 2005, 25, 23–26. [Google Scholar] [CrossRef]
- Scott, H.S. The decrepitation method applied to minerals with fluid inclusions. Econ. Geol. 1948, 43, 637–654. [Google Scholar] [CrossRef]
- Knotter, D.M. Etching mechanism of vitreous silicon dioxide in HF-Based solutions. J. Am. Chem. Soc. 2000, 122, 4345–4351. [Google Scholar] [CrossRef]
- Su, Y.; Zhou, Y.H.; Huang, W.; Gu, Z.A. Study on reaction kinetics between silica glasses and hydrofluoric acid. J. Chin. Ceram. Soc. 2004, 32, 287–293. [Google Scholar] [CrossRef]
- Xue, N.N.; Zhang, Y.M.; Liu, T.; Huang, J.; Zheng, Q.S. Effects of hydration and hardening of calcium sulfate on muscovite dissolution during pressure acid leaching of black shale. J. Clean Prod. 2017, 149, 989–998. [Google Scholar] [CrossRef]
- Mao, X.W.; Ye, Q.; Liao, M.F.; Yang, J.X.; Zhang, H.J.; Wang, Z.Y. Division and discussion of geotectonic units in Hubei Province. Resour. Environ. Eng. 2014, 1, 6–15. [Google Scholar] [CrossRef]
- Zhang, P.C.; Liu, Y.F.; Li, J.F.; Deng, M.; Liu, S.T. Study on high-purity quartz mineral resource engineering. J. Mineral. Petrol. 2012, 32, 38–44. [Google Scholar] [CrossRef]
- China Technical Committee for Standardization of Microbeam Analysis. GB/T 15617-2002 Methods for Quantitative Analysis of Silicate Minerals by Electron Probe; Standards Press of China: Beijing, China, 2002. [Google Scholar]
- China Technical Committee for Standardization of Semiconductor Equipment and Materials. SJ/T 11554-2015 Determination of the Metals’ Concentration of Hydrofluoric Acid by ICP-OES; Standards Press of China: Beijing, China, 2015. [Google Scholar]
- China Technical Committee for Standardization of Semiconductor Equipment and Materials. GB/T 32650-2016 Determining the Content of Trace Elements in Arenaceous Quartz by Inductively Coupled Plasma Mass Spectrometry (ICP-MS); Standards Press of China: Beijing, China, 2016. [Google Scholar]
- Lei, S.M.; Lin, M.; Pei, Z.Y.; Wang, E.W.; Zang, F.F.; Xiong, K. Occurrence and removal of mineral impurities in quartz. China Min. Mag. 2016, 25, 79–83. [Google Scholar] [CrossRef]
- Shmulovich, K. An experimental study of phase equilibria in the systems H2O-CO2-CaCl2 and H2O-CO2-NaCl at high pressures and temperatures (500–800 °C, 0.5–0.9 GPa): Geological and geophysical applications. Contrib. Mineral. Petrol. 2004, 146, 450–462. [Google Scholar] [CrossRef]
- Yan, F.L. Distribution properties and hosting conditions and purification methods of baneful impurity elements in quartz. J. Geol. 2009, 33, 277–279. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Li, J.S.; Li, X.X.; Huang, H.Q.; Zhou, L.F.; Xiong, T.T. High efficiency iron removal from quartz sand using phosphoric acid. Int. J. Miner. Process. 2012, 114–117, 30–34. [Google Scholar] [CrossRef]
- Bai, J.X.; Li, S.Q.; Yang, C.Q.; Kong, J.W. Study on the influence of ultrasound on iron removal by acid leaching for quartz sand. Nonmet. Mines 2016, 39, 69–71. [Google Scholar] [CrossRef]
- Tang, Q.; Sun, X.M.; Xu, L.; Zhai, W.; Liang, J.L.; Liang, Y.H.; Shen, K. U-Th-Pb Chemical dating of monazite exsolutions in apatite aggregates in quartz veins of UHP rocks from the Chinese Continental Scientific Drilling (CCSD) Project. Acta Petrol. Sin. 2006, 22, 1927–1932. [Google Scholar]
- Götze, J. Chemistry, textures and physical properties of quartz-geological interpretation and technical application. Mineral. Mag. 2009, 73, 645–671. [Google Scholar] [CrossRef]
- Mclaren, A.C.; Cook, R.F.; Hyde, S.T.; Tobin, R.C. The mechanisms of the formation and growth of water bubbles and associated dislocation loops in synthetic quartz. Phys. Chem. Miner. 1983, 9, 79–94. [Google Scholar] [CrossRef]
- Zhou, L.G. The Basic of Ore Petrology, 3rd ed.; Metallurgical Industry Press: Beijing, China, 2007; pp. 206–309. [Google Scholar]
- Liu, C.; Lin, J. Influence of calcination temperature on dielectric constant and structure of the micro-crystalline muscovite. China Nonmet. Min. Ind. Her. 2008, 5, 38–46. [Google Scholar] [CrossRef]
- Liu, X.C.; Wu, Y.B.; Gong, H.J.; Yang, S.H.; Wang, J.; Peng, M.; Jiao, W.F. Zircon age and Hf isotopic composition of quartz veins in UHP eclogites from western Dabie Mountains. Chin. Sci. Bull. 2009, 54, 1449–1454. [Google Scholar] [CrossRef]
- Lu, H.P.; Wang, R.C.; Lu, X.X.; Xu, S.J.; Chen, J.; Gao, J.F. Study on dissolution behavior of zircon in hydrothermal solution of 180 °C. Prog. Nat. Sci. 2003, 13, 1042–1047. [Google Scholar] [CrossRef]
- Schmidt-Mumm, A. Low frequency acoustic emission from quartz upon heating from 90 to 610 °C. Phys. Chem. Miner. 1991, 17, 545–553. [Google Scholar] [CrossRef]



| Element | Al | Fe | Na | S | P | Li | K | Ca | Ti |
| Content 1 (μg·g−1) | 353 | 61.2 | 13.4 | 5.64 | 15.5 | 2.20 | 118 | 8.04 | 8.31 |
| Element | Mg | Ni | Zr | Zn | As | B | In Total | ||
| Content (μg·g−1) | 11.8 | 1.01 | 6.46 | 0.567 | 3.16 | 10.8 | 619 |
| Element | Na | Mg | Al | Si | P | S | K | Ca | Mn | Cr | Fe | O |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Figure 3a (wt %) | 0.31 | 19.16 | 24.69 | 8.40 | 0.28 | 47.17 | ||||||
| RSD 1 (%) | 2.43 | 1.10 | 0.96 | 1.22 | 1.96 | |||||||
| Figure 3b (wt %) | 2.24 | 2.48 | 0.63 | 2.89 | 65.91 | 25.85 | ||||||
| RSD (%) | 2.23 | 1.32 | 2.64 | 1.08 | 0.68 | |||||||
| Figure 3c (wt %) | 3.31 | 5.06 | 1.57 | 6.63 | 10.38 | 2.01 | 0.87 | 14.50 | 0.91 | 14.52 | 40.24 | |
| RSD (%) | 2.88 | 2.09 | 2.11 | 1.16 | 1.67 | 1.34 | 2.89 | 1.44 | 1.57 | 0.83 |
| Leaching Process | Temperature (°C) | Acid Concentration (HCl or H2SO4) (mol·dm−3) | NH4Cl Concentration (mol·dm−3) | Liquid/Solid Ratio (cm3·g−1) | Leaching Time (h) |
|---|---|---|---|---|---|
| HCl + NH4Cl | 280 | 0.8 | 0.8 | 10 | 6 |
| H2SO4 + NH4Cl | 250 | 0.3 | 0.45 | 5 | 7 |
| Element | Ore (μg·g−1) | Concentrate 1 1 (μg·g−1) | Removal Rate 1 (wt %) | Concentrate 2 1 (μg·g−1) | Removal Rate 2 (wt %) |
|---|---|---|---|---|---|
| Al | 353 | 41.5 | 88.2 | 44.1 | 87.5 |
| Fe | 61.2 | 1.14 | 98.1 | 1.12 | 98.2 |
| Na | 13.4 | 11.3 | 15.7 | 12.2 | 8.96 |
| S | 5.64 | — | — | — | — |
| P | 15.5 | 5.00 | 67.7 | 5.39 | 65.2 |
| Li | 2.20 | 2.19 | 0.455 | 2.04 | 7.27 |
| K | 118 | 1.21 | 99.0 | 2.24 | 98.1 |
| Ca | 8.04 | 4.54 | 43.5 | 4.40 | 45.3 |
| Ti | 8.31 | 4.89 | 41.2 | 5.38 | 35.3 |
| Mg | 11.8 | 4.88 | 58.6 | 7.15 | 39.4 |
| Ni | 1.01 | - | - | - | - |
| Zr | 6.46 | 6.45 | 0.155 | 6.45 | 0.155 |
| Zn | 0.567 | - | - | -; | - |
| As | 3.16 | - | - | - | - |
| B | 10.8 | 8.77 | 18.8 | 8.77 | 18. 8 |
| In total | 619 | 91.9 | 85.2 | 99.2 | 84.0 |
| Element | Ore (μg·g−1) | C and PHAL (μg·g−1) | PHAL 1 (μg·g−1) | C and PSAL (μg·g−1) | PSAL 1 (μg·g−1) |
|---|---|---|---|---|---|
| Al | 353 | 41.5 | 167 | 44.1 | 188 |
| Fe | 61.2 | 1.14 | 7.42 | 1.12 | 13.4 |
| Na | 13.4 | 11.3 | 13.5 | 12.2 | 12.9 |
| S | 5.64 | - | 4.00 | - | 4.52 |
| P | 15.5 | 5.00 | 13.6 | 5.39 | 10.4 |
| Li | 2.20 | 2.19 | 2.20 | 2.04 | 2.19 |
| K | 118 | 1.21 | 42.6 | 2.24 | 45.9 |
| Ca | 8.04 | 4.54 | 7.50 | 4.40 | 8.01 |
| Ti | 8.31 | 4.89 | 6.07 | 5.38 | 5.91 |
| Mg | 11.8 | 4.88 | 8.60 | 7.15 | 8.12 |
| Ni | 1.01 | - | - | - | - |
| Zr | 6.46 | 6.45 | 6.46 | 6.45 | 6.46 |
| Zn | 0.567 | - | - | - | - |
| As | 3.16 | - | 1.47 | - | 2.15 |
| B | 10.8 | 8.77 | 9.80 | 8.77 | 8.47 |
| In total | 619 | 91.9 | 290 | 99.2 | 316 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, M.; Pei, Z.; Lei, S. Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals 2017, 7, 161. https://doi.org/10.3390/min7090161
Lin M, Pei Z, Lei S. Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals. 2017; 7(9):161. https://doi.org/10.3390/min7090161
Chicago/Turabian StyleLin, Min, Zhenyu Pei, and Shaomin Lei. 2017. "Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China)" Minerals 7, no. 9: 161. https://doi.org/10.3390/min7090161
APA StyleLin, M., Pei, Z., & Lei, S. (2017). Mineralogy and Processing of Hydrothermal Vein Quartz from Hengche, Hubei Province (China). Minerals, 7(9), 161. https://doi.org/10.3390/min7090161





