The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran: Mineralogy, Geochemistry, and Sulfur Isotope Data
Abstract
:1. Introduction
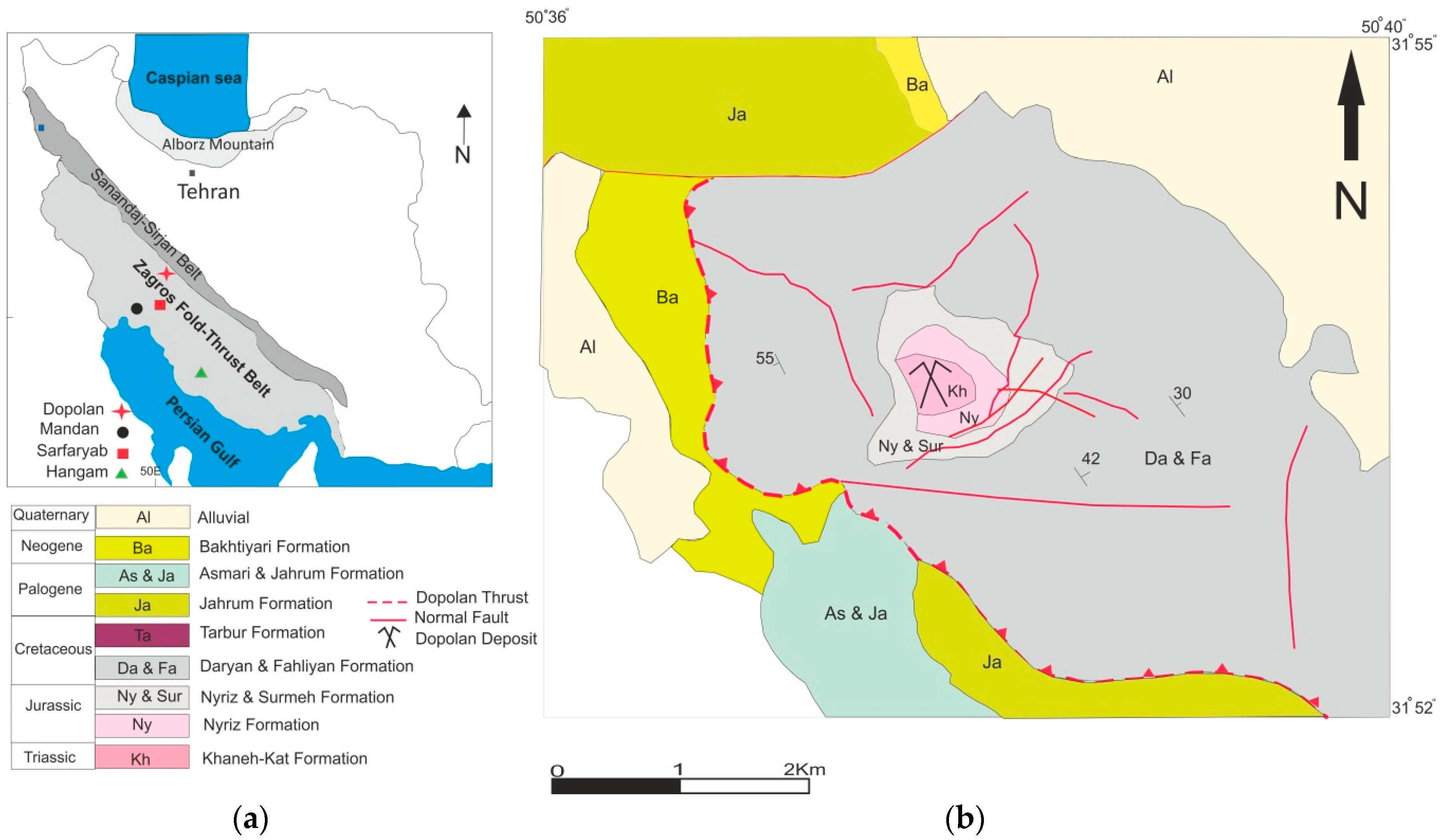
2. Geology
3. Methodology
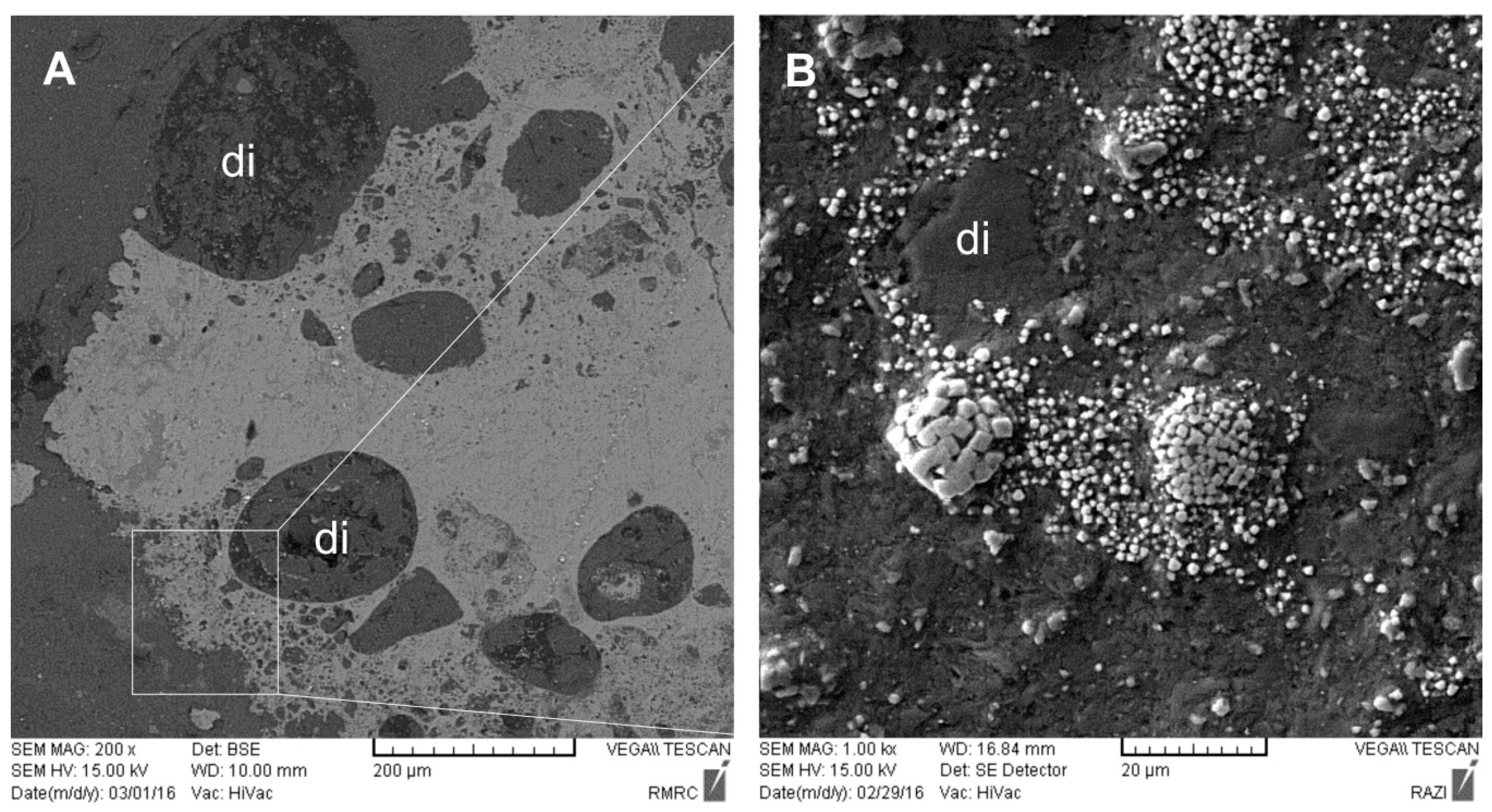
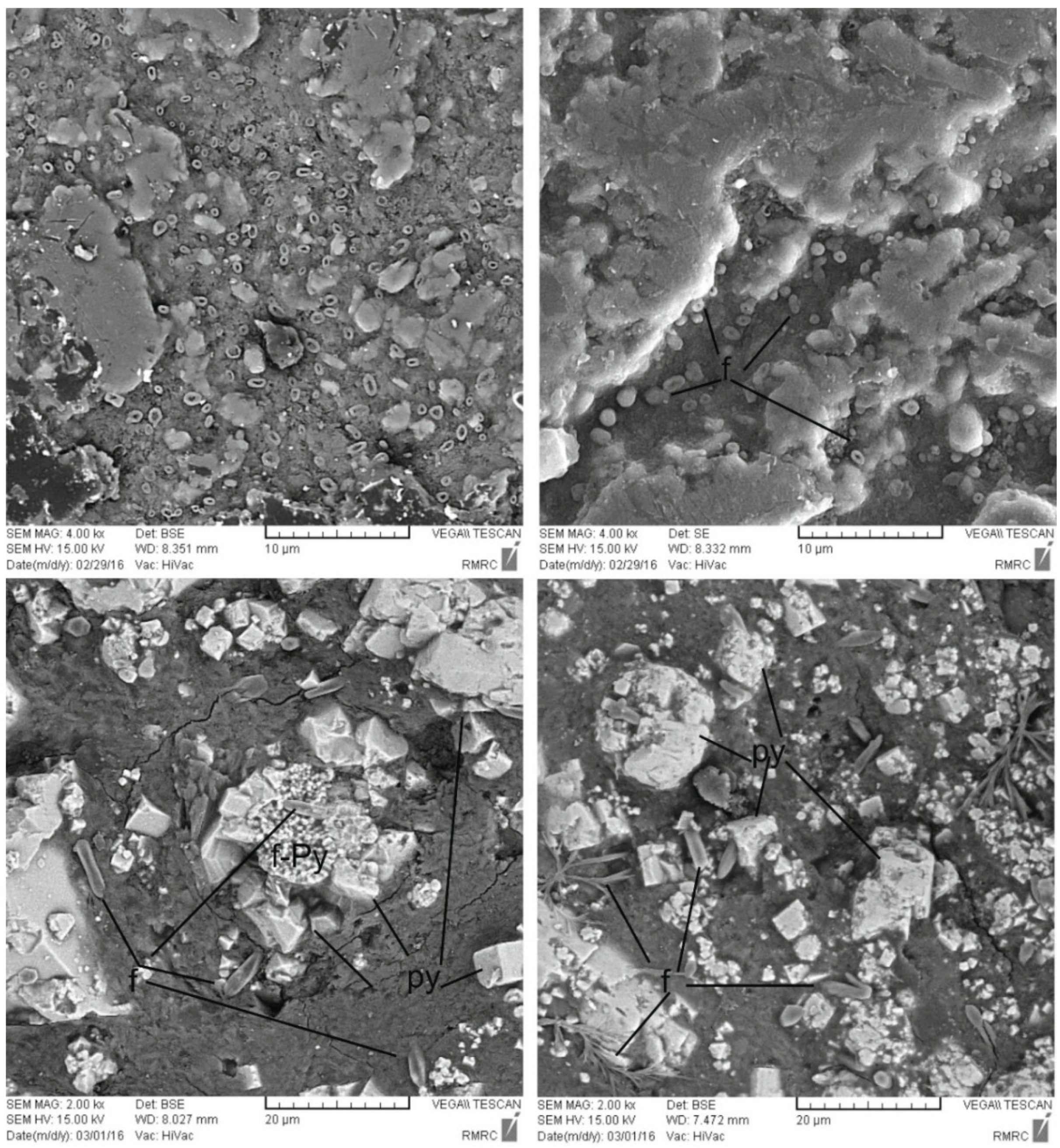
4. Mineralogical Characteristics
5. Geochemical Features
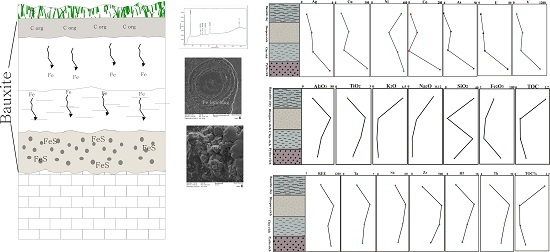
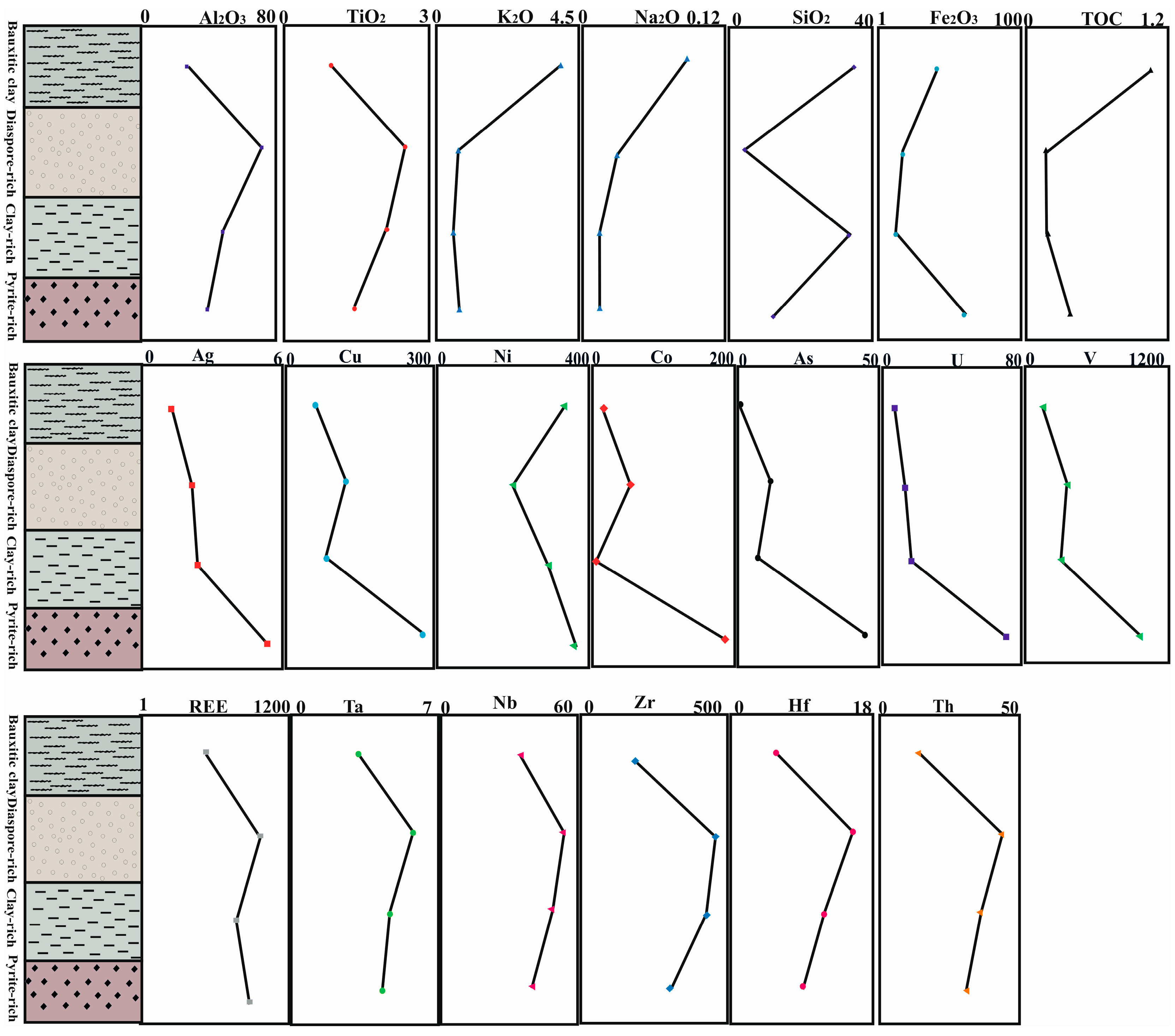
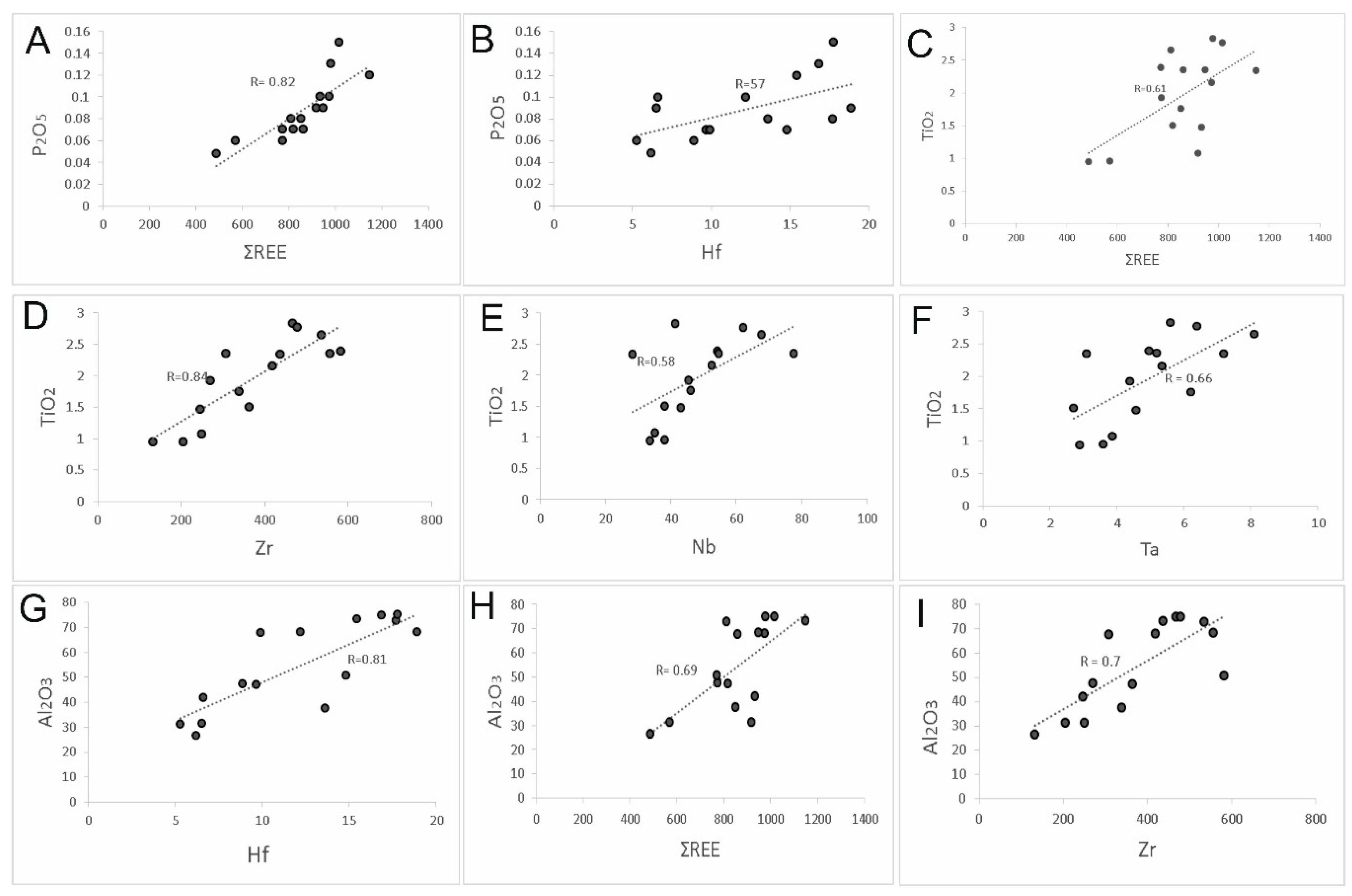
5.1. Distribution of Elements
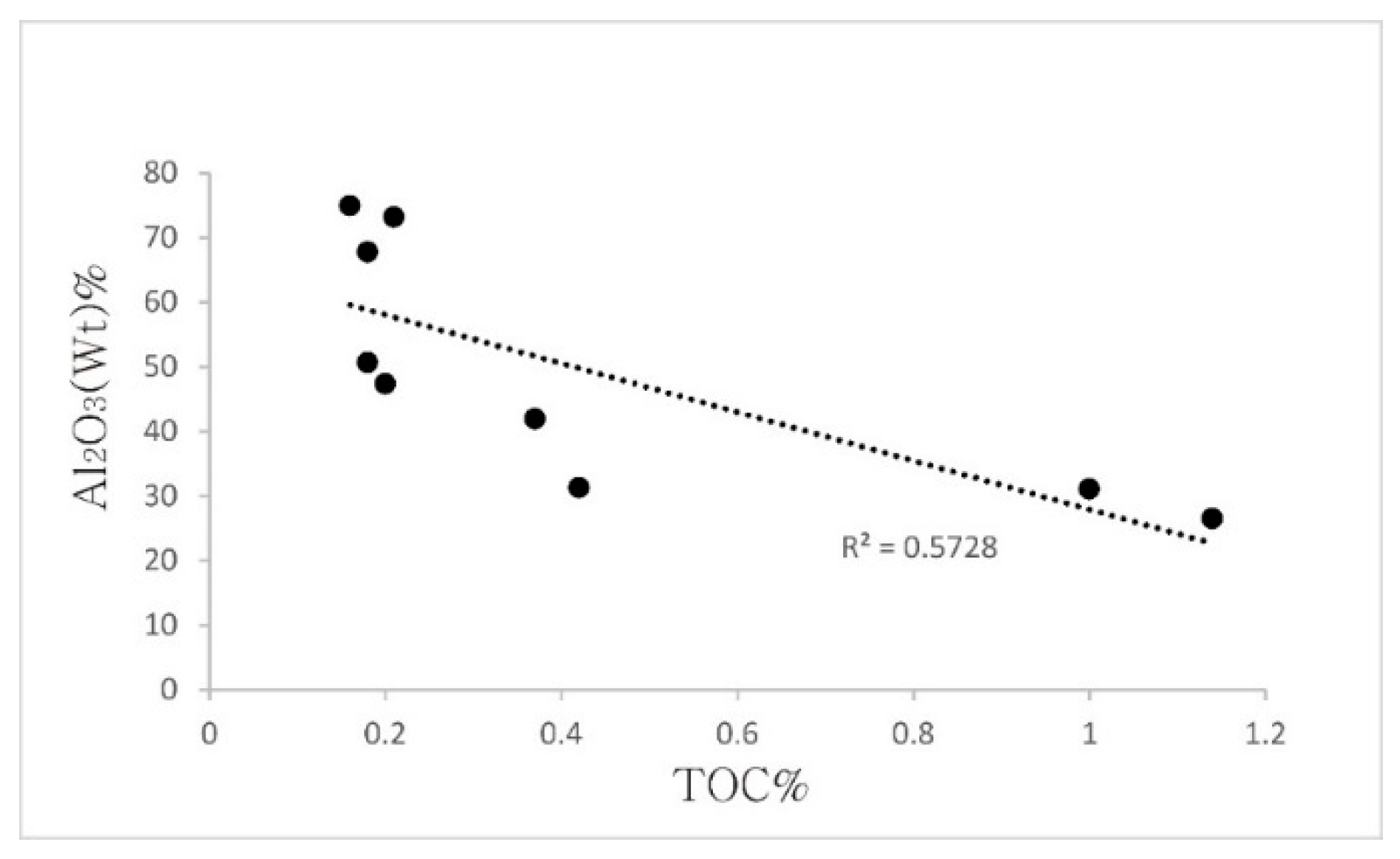
5.2. Organic Matter of Bauxite Horizons
5.3. Sulfur Isotopes
6. Discussion
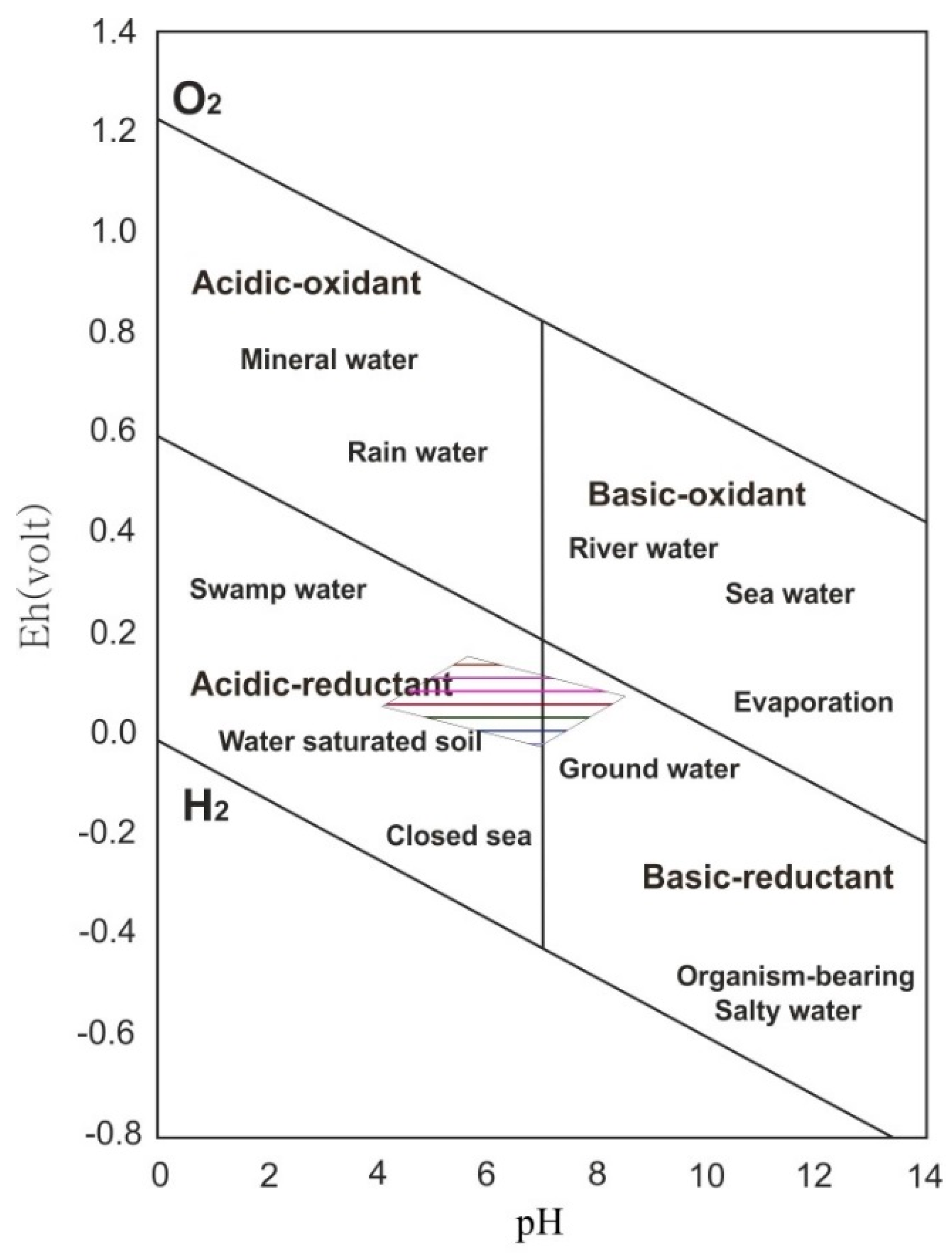
6.1. Formation of the Pyrite-Rich Horizon
6.2. Deposition Mechanisms of High-Grade Al Bauxite
6.3. Mobility of REE and Trace Elements
6.4. Sulfur Isotope Constraints
7. Conclusions
- The main minerals, such as diaspore, pyrite, kaolinite, and its association with preserved organic matter, suggest that bauxitization occurred in acidic reducing to slightly alkaline reducing conditions.
- The low Fe content of the Dopolan bauxite implies that Fe leaching under reducing conditions may have resulted from organic matter in the presence of microbial activity. Fe was leached from the upper part of the bauxite profile and was deposited as pyrite in the lower part, generating a pyrite-rich bauxite horizon.
- During the formation of the bauxite profile, less mobile elements, such as REE, Nb, Ta, Zr, and Hf, accumulated in the diaspore-rich horizon with higher alumina content, whereas redox sensitive elements such as Cr, Ni, Ag, Cu, Pb, Zn, U, and V, were concentrated in the pyrite-rich horizon.
- Sulfur isotope data reveal a wide range of negative values of δ34S for pyrite samples (from −10‰ to −34‰), suggesting the biogeochemical reduction of sulfate to sulfur.
- Mineralogical data, geochemical evidence, and sulfur isotope data in this study suggest that biological activity played an important role in Fe remobilization, Al-upgrading, and the formation of the pyrite-rich horizon in the Dopolan bauxite deposit.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozturk, H.; Hein, R.J.; Hanilci, N. Genesis of the Dog¢ankuzu and Mortas, bauxite deposits, Taurides, Turkey: Separation of Al, Fe, and Mn and implications for passive margin metallogeny. Econ. Geol. 2002, 97, 1063–1077. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Micro-organisms as fossils and present day development in Ni-laterites and bauxites of the Balkan Peninsula. In Mineral Deposits Research Meeting the Global Challenge, Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1003–1006. [Google Scholar]
- Laskou, M.; Economou-Eliopoulos, M. The role of microorganisms on the mineralogical and geochemical characteristics of the Parnassos-Ghiona bauxite deposits, Greece. J. Geochem. Explor. 2007, 93, 67–77. [Google Scholar] [CrossRef]
- Laskou, M.; Economou-Eliopoulos, M. Bio-mineralization and potential biogeochemical processes in bauxite deposits: genetic and ore quality significance. Mineral. Petrol. 2012, 73, 124–140. [Google Scholar] [CrossRef]
- Kalaitzidis, S.; Siavalas, G.; Skarpelis, N.; Carla Viviane Araujo, C.; Christanis, C. Late Cretaceous coal overlying karstic bauxite deposits in the Parnassus-Ghiona Unit, Central Greece: coal characteristics and depositional environment. Int. J. Coal Geol. 2010, 81, 211–226. [Google Scholar] [CrossRef]
- Kalatha, S.; Economou-Eliopoulos, M. Framboidal pyrite and bacterio-morphic goethite at transitional zones between-Fe-Ni-laterite and limestones: Evidence from Lokris, Greece. Ore Geol. Rev. 2015, 65, 413–425. [Google Scholar] [CrossRef]
- Lovely, D.R. Microbial reduction of iron, manganese, and other metals. Adv. Agron. 1995, 54, 175–231. [Google Scholar]
- Berthelin, J. Microbial weathering processes. In Microbial Geochemistry; Krumbein, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 223–263. [Google Scholar]
- Furrer, G.; Stumn, W. A coordination chemical approach to the kinetics of weathering. I. Dissolution of δ-Al2O3 and BeO. Geochim. Cosmochim. Acta 1986, 50, 1847–1860. [Google Scholar] [CrossRef]
- McBride, M.; Sauvé, S.; Hendershot, W. Solubility control of Cu, Zn, Cd and Pb in contaminated soils. Eur. J. Soil Sci. 1997, 48, 337–346. [Google Scholar] [CrossRef]
- Müller, B.; Axelesson, M.D.; Öhlander, B. Adsorption of trace elements on pyrite surfaces in sulfidic mine tailing from Kristenberg (Sweden) a few years after remediation. Sci. Total Environ. 2002, 298, 1–16. [Google Scholar] [CrossRef]
- Lazo, O.; Cullaj, A.; Deda, T. Arsenic in soil environments in Albania. In Arsenic in Soil and Groundwater: Biogeochemical Interactions; Bhattacharya, P., Mukherjee, A.B., Zevenhoven, R., Loeppert, R., Eds.; Elsevier Book Series; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Oliveira, F.S.; Varajao, A.F.D.; Varajao, C.A.C.; Schaefer, C.E.G.R.; Bolange, B. The role of biogical agents in the microstructural and mineralogical transformations in aluminium lateritic deposit in Central Brazil. Geoderma 2014, 226–227, 250–269. [Google Scholar] [CrossRef]
- Liaghat, S.; Hossini, M.; Zarasvandi, A. Determination of the origin and mass change geochemistry during bauxitization process at the Hangam Deposit, SE Iran. Geochem. J. 2003, 37, 627–637. [Google Scholar] [CrossRef]
- Zarasvandi, A.; Charchi, A.; Carranza, E.J.M.; Alizadeh, B. Karst bauxite deposits in the Zagros mountain belt, Iran. Ore Geol. Rev. 2008, 34, 521–532. [Google Scholar] [CrossRef]
- Zarasvandi, A.; Carranza, E.J.M.; Ellahi, S.S. Geological, geochemical, and mineralogical characteristics of the Mandan and Deh-now bauxite deposits, Zagros Fold Belt, Iran. Ore Geol. Rev. 2012, 48, 125–138. [Google Scholar] [CrossRef]
- Calagari, A.A.; Abedini, A. Geochemical investigations on Permo-Triassic bauxite horizon at Kanisheeteh, east of Bukan, West-Azarbaidjan, Iran. J. Geochem. Geoexplor. 2007, 93, 232–576. [Google Scholar] [CrossRef]
- Salamab Ellahi, S.; Taghipour, B.; Zarasvandi, A.; Bird, M.I.; Somarin, A.K. Mineralogy, Geochemistry and Stable Isotope Studies of the Dopolan Bauxite Deposit, Zagros Mountain, Iran. Minerals 2016, 6, 11. [Google Scholar]
- Ehsanbakhsh, M.H.; Rahimzadeh, F. Ardal Map 1; 100000; Geological Survey and Mineral Exploration: Tehran, Iran, 1996. [Google Scholar]
- Takin, M. Iranian geology and continental drift in the Middle East. Nature 1972, 235, 147–150. [Google Scholar] [CrossRef]
- Agard, P.; Omrani, J.; Jolivet, L.; Mouthereau, F. Convergence history across Zagros, Iran: Constraints from collisional and earlier deformation. Int. J. Earth Sci. 2005, 94, 401–419. [Google Scholar] [CrossRef]
- Walkey, A.; Black, I.A. An examination of Degtjareff method for determining soil organic matter, and proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Baskar, S.; Baskar, R.; Kaushik, A. Role of microorganisms in weathering of the Konkan-Goa laterite formation. Curr. Sci. 2003, 85, 1129–1134. [Google Scholar]
- Spatio, G. The Geochemistry of Soil; Oxford University Press: New York, NY, USA, 1989; p. 27. [Google Scholar]
- Chapelle, F.H. Ground-Water Microbiology and Geochemistry; John Wiley & Sons: New York, NY, USA, 1993. [Google Scholar]
- Berner, R.A. Biogeochemical cycles of carbon and sulfur and their effect on atmospheric oxygen over Phanerozoic time. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1989, 75, 97–122. [Google Scholar] [CrossRef]
- Jensen, M.L. Sulfur isotopes and mineral genesis. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Holt, Rinehart, and Winston: New York, NY, USA, 1967; pp. 143–165. [Google Scholar]
- Ohmoto, H.; Rye, R.O. Isotopes of sulfur and carbon. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1979; pp. 509–567. [Google Scholar]
- Ohmoto, H.; Goldhaber, M.B. Sulfur and carbon isotopes. In Geochemistry of Hydrothermal Ore Deposits; Barnes, H.L., Ed.; Wiley and Sons: New York, NY, USA, 1997; pp. 517–611. [Google Scholar]
- Seal, R. Sulfur Isotope Geochemistry of Sulfide Minerals; U.S. Geological Survey Staff-Published Research: Reston, VA, USA, 2006; p. 345. [Google Scholar]
- Roedder, E. The noncolloidal origin of “colloform” textures in sphalerite ores. Econ. Geol. 1968, 63, 451–471. [Google Scholar] [CrossRef]
- Anderson, G.M. The mixing hypothesis and the origin of Mississippi Valley-type ore deposits. GeoFluids 2008, 103, 1683–1690. [Google Scholar] [CrossRef]
- Nejadhadad, M.; Taghipour, B.; Zarasvandi, A.K.; Somarin, A. Geological, geochemical, and fluid inclusion evidences for the origin of the Ravanj Pb–Ba–Ag deposit, north of Delijan city, Markazi Province, Iran. Turk. J. Earth Sci. 2016, 25, 179–200. [Google Scholar] [CrossRef]
- Wang, H.; Morse, H.C. IRF8 regulates myeloid and B lymphoid lineage diversification. Immunol. Res. 2009, 43, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Patterson, S.H. Bauxite Reserves and Potential Aluminum Resources of the World; U.S. Geological Survey: Reston, VA, USA, 1967; pp. 1228–1258.
- Brimhall, G.H.; Lewis, C.J.; Ague, J.J.; Dietrich, W.E.; Hampel, J.; Teague, T.; Rix, P. Metal enrichment in bauxites by deposition of chemically-mature aeolian dust. Nature 1988, 333, 819–824. [Google Scholar] [CrossRef]
- Ehrlich, H.L.; Wickert, L.M.; Noteboom, D.; Doucet, J. Weathering Pisolitic Bauxite by Heterotrophic Bacteria; University of Chile: Santiago, Chile, 1995; Volume I, pp. 395–403. [Google Scholar]
- McLean, E.D. Chemistry of soil aluminum. Commun. Soil Sci. Plant Anal. 1976, 7, 619–636. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and Soil Acidity. In Methods of Soil Analysis: Chemical Methods; Soil Sciences Society of America/American Society of Agronomy: Madison, WI, USA, 1996; Part 3; pp. 475–496. [Google Scholar]
- Liu, X.; Wang, Q.; Feng, Y.; Cai, Z. Genesis of the Guangou karstic bauxite deposit in western Henan, China. Ore Geol. Rev. 2013, 55, 162–175. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhou, L.; Li, Y.; Wu, C.; Zheng, C. The “coal–bauxite iron” structure in the ore-bearing rock series as a prospecting indicator for southeastern Guizhou bauxite mines. Ore Geol. Rev. 2013, 53, 145–158. [Google Scholar] [CrossRef]
- Buchanan, S.J.; So, H.B.; Kopittk, P.M.; Menzies, N.W. Influence of texture in bauxite residues on void ratio, water holding characteristics, and penetration resistance. Geoderma 2010, 158, 421–426. [Google Scholar] [CrossRef]
- Garrels, R.; Christ, L.C. Solutions, Minerals, and Equilibria; John Wiley: New York, NY, USA, 1965; 450p. [Google Scholar]
- Maksimovic, Z.; Panto, G. Contribution to the geochemistry, of rare earth elements in the karst-bauxite deposits of Yugoslavia and Greece. Geoderma 1991, 51, 93–109. [Google Scholar] [CrossRef]
- Carrillo-González, R.; González-Chávez, M. Metal accumulation in wild plants surrounding mining wastings. Environ. Pollut. 2006, 144, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Meshram, R.R.; Randiv, K.R. Geochemical study of laterites of the Jamnagar district, Gujarat, India: implications on parent rock, mineralogy and tectonics. J. Asian Earth Sci. 2011, 42, 1271–1287. [Google Scholar] [CrossRef]
- Mordberg, L.E.; Spratt, J. Alteration of zircons: The evidence of Zr mobility during bauxitic weathering. In Proceedings of the Goldschmidt Conference Toulouse, Toulouse, France, 30 August–3 September 1998; pp. 1021–1022. [Google Scholar]
- Hill, I.G.; Worden, R.H.; Meighan, I.G. Geochemical evolution of a palaeolaterite: The Interbasaltic Formation, Northern Ireland. Chem. Geol. 2000, 166, 65–84. [Google Scholar] [CrossRef]
- Karadag, M.M.; Kupeli, S.; Aryk, F.; Ayhan, A.; Zedef, V.; Doyen, A. Rare earth element (REE) geochemistry and genetic implications of the Mortas bauxite deposit (Seydisehir/Konya-Southern Turkey). Chemie der Erde 2009, 69, 143–159. [Google Scholar] [CrossRef]
- Laufer, F.; Yariv, S.; Steinberg, M. The adsorption of quadrivalent cerium by kaolinite. Clay Miner. 1984, 19, 137–149. [Google Scholar] [CrossRef]
- Mongelli, G. Ce-anomalies in the textural components of Upper Cretaceous karst bauxites from the Apulian carbonate platform (southern Italy). Chem. Geol. 1997, 14, 69–79. [Google Scholar] [CrossRef]
- Roaldest, E. Mineralogical and Chemical Changes during Weathering, Transportation and Sedimentation in Different Environments. Ph.D. Thesis, University of Oslo, Oslo, Norway, 1978. [Google Scholar]
- Forstner, U. Metal speciation–general concepts and applications. Int. J. Environ. Anal. Geochem. 1993, 51, 5–23. [Google Scholar] [CrossRef]
- Kersten, M.; Forstner, U. Geochemical characterization of the potential trace metal mobility in cohesive sediments. Geo-Mar. Lett. 1991, 11, 184–187. [Google Scholar] [CrossRef]
- Nealson, K.H.; Stahl, D.A. Microorganisms and biogeochemical cycles: What can we learn from layered microbial communities. Rev. Mineral. 1997, 35, 5–34. [Google Scholar]
- Ehrlich, H.J. Geomicrobiology, 4th ed.; Marcel Dekker: New York, NY, USA, 2002; p. 800. [Google Scholar]
- Strauss, H.; Scchieber, J. A sulfur isotope study of pyrite genesis: The Mid-Protezoic Newland Formation, Belt supergroupe, Montana. Geochim. Cosmochim. Acta 1990, 54, 197–204. [Google Scholar] [CrossRef]
- Goldhaber, M.B. Sulfur-Rich Sediments. Treatise on Geochemistry: Sediments, Diagenesis, and Sedimentary Rocks; Elsevier: Amsterdam, The Netherlands, 2003; Volume 7, pp. 257–288. [Google Scholar]
- Hoefs, J. Stable Isotope Geochemistry, 6th ed.; Springer: Berlin, Germany, 2009; p. 285. [Google Scholar]
- Smock, A.M.; Bottcher, M.E.; Cypionka, H. Fractionation of sulfur isotopes during thiosulfate reduction by Desulfovibrio desulfuricans. Arch. Microbiol. 1998, 169, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.W.; Rittenberg, S.C. On the sulfur-source requirement for growth of Thiobacillus intermedius. Arch. Microbiol. 1974, 100, 65–71. [Google Scholar] [CrossRef] [PubMed]
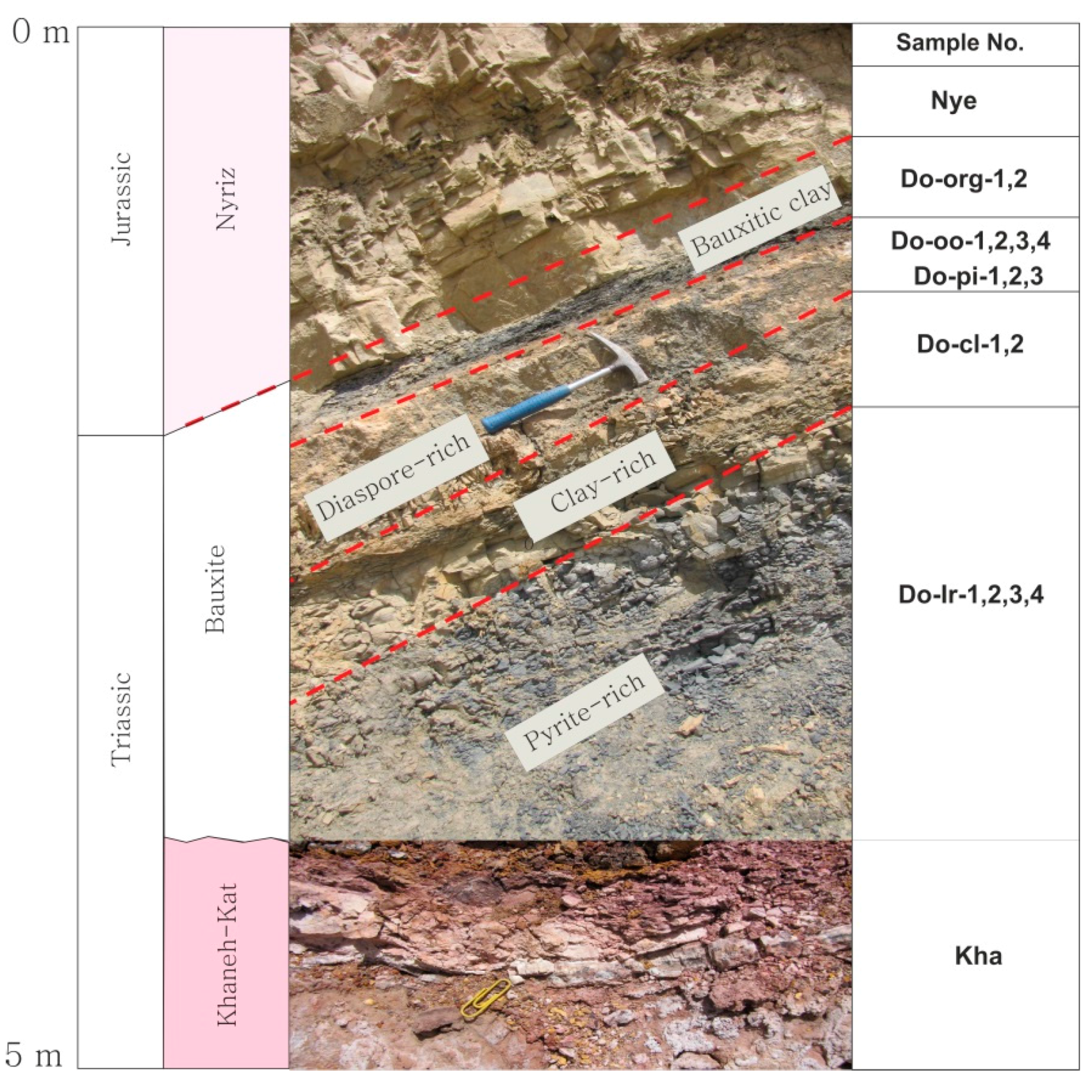

| Sample No. | Bauxite Layers | Major Phases | Minor Phases |
|---|---|---|---|
| Do-org | bauxitic kaolinite | nacrite, kaolinite, pyrite | anatase, rutile |
| Do-oo | diaspore-rich | diaspore, nacrite | anatase, muscovite, rutile |
| Do-pi | diaspore-rich | diaspore | anatase, nacrite, muscovite, rutile |
| Do-cl | clay bauxite | kaolinite, diaspore | böhmite |
| Do-ir | pyrite-rich | kaolinite, pyrite, nacrite | anatase, böhmite, muscovite, rutile |
| Kha | Khaneh-kat dolomite | calcite, dolomite, montmorillonite | - |
| Horizon | Pyrite-Rich Horizon | Clay-Rich Bauxite | Diaspore-Rich Bauxite | Black Bauxitic Kaolinite | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples | Do-IR-1 | Do-IR-2 | Do-IR-3 | Do-IR-4 | Do-cl-1 | Do-cl-2 | Do-oo-1 | Do-oo-2 | Do-oo-3 | Do-oo-4 | Do-pi-1 | Do-pi-2 | Do-pi-3 | Do-org-1 | Do-org-2 |
| SiO2 | 7.21 | 16.14 | 14.62 | 11.25 | 35.28 | 31.87 | 7.44 | 5.68 | 5.63 | 3.20 | 4.67 | 5.45 | 3.11 | 34.84 | 35.44 |
| Al2O3 | 42.02 | 31.34 | 37.58 | 47.1 | 47.39 | 50.69 | 68.11 | 67.78 | 68.23 | 72.89 | 74.98 | 73.25 | 75.09 | 31.1 | 26.55 |
| Fe2O3 | 19.68 | 21.08 | 16.42 | 11.18 | 25.9 | 1.02 | 3.53 | 2.47 | 4.55 | 0.92 | 2.26 | 2.33 | 1.36 | 5.52 | 7.45 |
| CaO | 0.06 | 0.10 | 0.04 | 0.13 | 0.06 | 0.05 | 0.07 | 0.11 | 0.04 | 0.03 | 0.04 | 0.08 | 0.07 | 0.90 | 0.13 |
| K2O | 0.51 | 1.16 | 0.61 | 0.85 | 0.80 | 0.36 | 0.30 | 0.65 | 0.80 | 1.21 | 0.25 | 1.64 | 0.29 | 4.06 | 3.83 |
| MnO | 0.01 | 0.02 | 0.01 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.02 | 0.03 | 0.00 |
| Na2O | 0.01 | 0.01 | 0.01 | 0.06 | 0.01 | 0.01 | 0.01 | 0.02 | 0.01 | 0.07 | 0.01 | 0.04 | 0.06 | 0.05 | 0.12 |
| P2O5 | 0.10 | 0.09 | 0.08 | 0.07 | 0.06 | 0.07 | 0.10 | 0.07 | 0.09 | 0.08 | 0.13 | 0.12 | 0.15 | 0.06 | 0.05 |
| MgO | 0.28 | 0.58 | 0.36 | 0.42 | 0.10 | 0.28 | 0.05 | 0.41 | 0.10 | 0.24 | 0.02 | 0.03 | 0.34 | 0.32 | 0.91 |
| TiO2 | 1.47 | 1.07 | 1.75 | 1.50 | 1.92 | 2.38 | 2.15 | 2.35 | 2.35 | 2.65 | 2.83 | 2.34 | 2.77 | 0.95 | 0.94 |
| LOI | 26.3 | 25.9 | 28.13 | 26.32 | 11.27 | 12.62 | 17.35 | 20.36 | 16.89 | 17.82 | 15.42 | 15.67 | 16.56 | 22.67 | 23.24 |
| TOC | 0.42 | 0.37 | - | - | 0.18 | 0.2 | - | 0.18 | - | - | 0.016 | 0.21 | - | 1.00 | 1.14 |
| Sum | 97.64 | 97.48 | 99.62 | 98.88 | 99.47 | 99.35 | 99.12 | 99.89 | 98.69 | 99.11 | 100.62 | 100.96 | 99.82 | 100.50 | 98.66 |
| Bi | 0.89 | 0.75 | 0.9 | 0.75 | 0.82 | 0.7 | 1.4 | 0.55 | 1.82 | 0.69 | 1.01 | 0.53 | 0.43 | 0.89 | 0.53 |
| As | 29 | 48 | 51 | 49 | 7.5 | 6 | 1 | 4.6 | 27.3 | 30 | 5.2 | 5.9 | 4.8 | 1 | 1 |
| Cd | 1.4 | 2.21 | 2.37 | 2.25 | 0.59 | 0.33 | 0.89 | 0.64 | 1.24 | 1.75 | 0.27 | 0.05 | 0.66 | 0.3 | 0.13 |
| Ni | 340 | 394 | 345 | 382 | 296 | 290 | 236 | 150 | 111 | 273 | 220 | 258 | 170 | 407 | 270 |
| Co | 203 | 179 | 155 | 197 | 5.2 | 8.3 | 6.4 | 5.7 | 43.1 | 172 | 143 | 4.6 | 2.7 | 17.9 | 11 |
| Cr | 429 | 286 | 577 | 381 | 359 | 255 | 486 | 442 | 389 | 329 | 132 | 139 | 303 | 747 | 1088 |
| Cs | 0.18 | 0.26 | 0.15 | 0.36 | 0.11 | 0.28 | 0.05 | 0.13 | 0.22 | 8.3 | 0.15 | 0.33 | 0.03 | 3.39 | 3.21 |
| Cu | 403 | 184 | 389 | 144 | 92 | 63 | 155 | 102 | 228 | 143 | 151 | 70.7 | 9 | 65.2 | 50.2 |
| Pb | 212 | 189 | 180 | 130 | 43 | 28 | 30 | 101 | 77 | 25.5 | 56 | 44 | 5 | 44 | 35 |
| Hf | 6.63 | 6.54 | 13.6 | 9.66 | 8.89 | 14.82 | 12.2 | 9.93 | 18.9 | 17.72 | 16.87 | 15.46 | 17.78 | 5.29 | 6.21 |
| Ag | 4.02 | 3.41 | 8.59 | 5.50 | 2.1 | 2.7 | 3.1 | 2.37 | 2.95 | 3.11 | 1.48 | 0.96 | 0.33 | 1.63 | 0.94 |
| La | 8.7 | 17 | 9.9 | 18.5 | 1 | 3.5 | 9.9 | 1.8 | 23.8 | 24 | 8.9 | 0.3 | 1.2 | 44.2 | 62.12 |
| Ce | 28.9 | 47.9 | 38.6 | 54 | 2.01 | 0.88 | 10.1 | 4.47 | 56 | 198 | 12.8 | 0.6 | 30.04 | 87.9 | 98 |
| Pr | 3.23 | 5.64 | 4.35 | 4.2 | 0.32 | 0.2 | 1.94 | 0.71 | 4.62 | 23.97 | 2.09 | 0.08 | 0.4 | 10.3 | 16.69 |
| Nd | 14.1 | 25 | 19.9 | 14.71 | 1.09 | 12.7 | 5.48 | 2.76 | 14 | 94.0 | 6.69 | 0.28 | 0.25 | 38.3 | 10.56 |
| Sm | 2.86 | 7.29 | 3.96 | 9.04 | 0.36 | 0.1 | 1.06 | 0.76 | 2.84 | 7.76 | 1.13 | 0.09 | 0.03 | 6.73 | 2.48 |
| Eu | 0.57 | 2.18 | 0.95 | 1.53 | 0.11 | 1.98 | 0.28 | 0.2 | 0.52 | 1.4 | 0.21 | 0.05 | 1.44 | 1.35 | 3.51 |
| Gd | 4.25 | 18.3 | 5.83 | 7.32 | 0.7 | 0.38 | 1.62 | 0.99 | 3.58 | 5.66 | 1.43 | 0.16 | 6.7 | 8.87 | 16.25 |
| Dy | 4.59 | 28.4 | 5.79 | 13.95 | 0.86 | 10.95 | 1.47 | 1.43 | 3.4 | 8.36 | 1.68 | 0.23 | 1.85 | 6.02 | 8.04 |
| Ho | 0.98 | 5.73 | 1.16 | 3.21 | 0.2 | 2.24 | 0.29 | 0.28 | 0.73 | 1.81 | 0.32 | 0.05 | 0.2 | 1.13 | 2.64 |
| Er | 2.86 | 14.9 | 3.75 | 9.9 | 0.63 | 0.74 | 0.79 | 0.87 | 2.27 | 5.48 | 0.99 | 0.2 | 0.8 | 3.38 | 7.34 |
| Tm | 0.47 | 2.02 | 0.63 | 1.64 | 0.12 | 1.1 | 0.15 | 0.15 | 0.43 | 0.92 | 0.15 | 0.05 | 0.1 | 0.48 | 1.2 |
| Yb | 2.8 | 10.4 | 4 | 11.1 | 0.7 | 7.4 | 1 | 1 | 2.6 | 6.34 | 0.8 | 0.3 | 0.5 | 3 | 7.41 |
| Lu | 0.41 | 1.5 | 0.62 | 1.71 | 0.12 | 1.15 | 0.14 | 0.14 | 0.41 | 0.89 | 0.12 | 0.03 | 0.78 | 0.47 | 0.9 |
| Th | 24.5 | 25.7 | 34.3 | 36.16 | 21.54 | 49.64 | 44.52 | 28.41 | 46.35 | 51.92 | 38.57 | 38.75 | 54.66 | 10.6 | 17.34 |
| Zn | 96 | 101 | 197 | 122 | 144 | 123 | 145 | 97.2 | 117 | 120 | 69.5 | 51.5 | 5 | 48.2 | 21 |
| Zr | 246 | 250 | 340 | 364 | 271 | 583 | 419 | 308 | 557 | 535.8 | 467.2 | 437.8 | 478.4 | 206 | 132 |
| Mo | 4.09 | 8.44 | 3.93 | 6.74 | 25.9 | 20.5 | 15.4 | 21 | 32.3 | 28 | 3.83 | 13.9 | 7 | 1.35 | 1.4 |
| Nb | 43.1 | 35.3 | 46.2 | 38.34 | 45.6 | 54.43 | 52.6 | 54.7 | 77.7 | 67.9 | 41.4 | 28.4 | 62.4 | 38.2 | 33.8 |
| S | >5 | >5 | >5 | >5 | 1 | 2.1 | 1.4 | 1.5 | 2.5 | 1.75 | 1.2 | 1.4 | 1.2 | 2.2 | 1.7 |
| Sb | 10.6 | 4.44 | 11.7 | 9.65 | 3.89 | 2.52 | 2.74 | 2.34 | 6.53 | 4.33 | 2.84 | 2.51 | 1.99 | 0.36 | 0.23 |
| Ta | 4.58 | 3.87 | 6.21 | 2.72 | 4.4 | 4.97 | 5.35 | 5.19 | 7.2 | 8.1 | 5.6 | 3.1 | 6.4 | 3.6 | 2.9 |
| Tl | 0.19 | 1.12 | 0.2 | 0.56 | 0.18 | 0.1 | 0.12 | 0.14 | 0.37 | 0.25 | 0.09 | 0.21 | 0.38 | 0.18 | 0.22 |
| U | 67.4 | 70.4 | 111 | 35.05 | 6.8 | 27.12 | 9.2 | 10.2 | 23.4 | 17.4 | 11.1 | 2.3 | 11.6 | 2.6 | 9.4 |
| V | 1250 | 759 | 1441 | 472 | 272 | 310 | 186 | 330 | 365 | 485 | 277 | 304 | 442 | 107 | 163 |
| Y | 17.1 | 147 | 17.8 | 55 | 3.2 | 4.6 | 6 | 4.6 | 13.9 | 41.06 | 5.5 | 1.2 | 56.34 | 29.9 | 55.4 |
| Sample No. | Horizon | Description | δ34S |
|---|---|---|---|
| S-1 | pyrite-rich | whole rock | −34.59 |
| S-2 | pyrite-rich | separated pyrite | −34.47 |
| S-3 | pyrite-rich | separated pyrite | −10.12 |
| S-4 | bauxitic kaolinite | separated pyrite | −26.77 |
| S-5 | bauxitic kaolinite | whole rock | −33.58 |
| S-6 | bauxitic kaolinite | whole rock | −28.63 |
| Deposit | Main Minerals | Al2O3 (wt %) | SiO2 (wt %) | Fe2O3 (wt %) | Reference |
|---|---|---|---|---|---|
| Dopolan | diaspore–kaolinite–pyrite–nacrite | 38–71 | 5–35 | 2–17 | [18] |
| Sarfaryab | böhmite > gibbsite–calcite–kaolinite–hematite | 18–63 | 6–14 | 2–18 | [15] |
| Dehnow | böhmite–calcite–kaolinite–hematite | 34–64 | 4–8 | 3–21 | [16] |
| Mandan | böhmite–calcite–kaolinite–hematite | 12–56 | 3–30 | 1–24 | [16] |
| Hangam | böhmite–kaolinite–hematite–goethite | 17–45 | 15–19 | 16–20 | [14] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamab Ellahi, S.; Taghipour, B.; Nejadhadad, M. The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran: Mineralogy, Geochemistry, and Sulfur Isotope Data. Minerals 2017, 7, 97. https://doi.org/10.3390/min7060097
Salamab Ellahi S, Taghipour B, Nejadhadad M. The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran: Mineralogy, Geochemistry, and Sulfur Isotope Data. Minerals. 2017; 7(6):97. https://doi.org/10.3390/min7060097
Chicago/Turabian StyleSalamab Ellahi, Somayeh, Batoul Taghipour, and Mostafa Nejadhadad. 2017. "The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran: Mineralogy, Geochemistry, and Sulfur Isotope Data" Minerals 7, no. 6: 97. https://doi.org/10.3390/min7060097
APA StyleSalamab Ellahi, S., Taghipour, B., & Nejadhadad, M. (2017). The Role of Organic Matter in the Formation of High-Grade Al Deposits of the Dopolan Karst Type Bauxite, Iran: Mineralogy, Geochemistry, and Sulfur Isotope Data. Minerals, 7(6), 97. https://doi.org/10.3390/min7060097