1. Introduction
Refractory gold ores have increasingly been the main source of gold production with the exhaustion of amenable gold ores. Of the 2630 tons of total world gold production in 2011, about 25% was produced from refractory gold deposits, and of the top 20 gold operations in 2011, eight were processing refractory ores [
1]. Commonly, relatively high content of detrimental impurities like S (sulfur), As (arsenic) and C (carbon) occur in these refractory gold ores [
2,
3,
4]. Also, gold is usually disseminated as micro-fine particles which are easily encapsulated in associated minerals, resulting in unacceptable exposure levels of gold unless undergoing ultra-fine grinding with high energy consumption. As a result, proper pretreatments are necessary to improve the leaching of gold from these refractory gold ores [
5,
6].
The pretreatment of oxidative roasting has always been one of the most extensively used methods in China attributing to its mature technique, high efficiency, energy conservation, etc. [
7,
8] Effective removal of harmful elements including S, As and C can be achieved after oxidative roasting, but the sintering phenomenon occurs inevitably even when adjusting the roasting atmosphere and lowering the roasting temperature [
9]. Consequently, the oxidative roasting is often accompanied by the secondary encapsulation of gold, which leads to the unsatisfactory extraction of gold from the calcine and thus the waste of gold resources [
7,
10,
11,
12,
13].
The locked gold in calcine is commonly referred to as encapsulation by iron oxides (mainly hematite, Fe
2O
3). Currently, the pretreatment of acid pickling (i.e., commonly using dilute sulfuric acid liquors to dissolve iron oxides) has been widely used to open the encapsulation of gold in metallurgical plants of China [
14]. The acid consumption is generally high, but the damage of iron oxides is still insufficient, and so the improvement of gold extraction is rather limited [
15,
16,
17]. It has been shown that about 50% of iron could be leached from a cinder of pyrite at the optimal conditions of leaching temperature 110 °C, sulfuric acid concentration 55% (
w/
w), and leaching time 2 h [
15]. Similarly, the research of, Zhang et al. [
17] indicated that the optimal leaching ratio of iron from a pyrite cinder was only 45%. In view of crystal forms of Fe
2O
3, the dissolution rates of α-Fe
2O
3 and γ-Fe
2O
3 in sulfuric acid were also investigated and it was found that the leaching of iron from α-Fe
2O
3 and γ-Fe
2O
3 were both rather restricted, with an iron leaching ratio of 20% and 40%, respectively [
16]. Other pretreatments that include reduction roasting–acid pickling, acidic pressure pickling and sulfuric acid curing (i.e., directly using concentrated sulfuric acid to react with iron oxides under high temperatures)–water leaching were further studied to expose the locked gold [
18,
19,
20,
21,
22]. The removal of iron oxides is enhanced to some degree by using the former two methods, but the gold extractions are still not high enough for some gold calcines. By comparison, the sulfuric acid curing-water leaching is more advantageous due to the higher damage degree of iron oxides and the simple technology [
23]. The iron oxides can be transformed fully into a soluble form of rhomboclase HFe(SO
4)
2·4H
2O by this pretreatment, so the effective exposure of gold can be achieved to significantly improve gold extraction [
24,
25,
26,
27].
Additionally, the gold encapsulated in gangue minerals, especially in quartz or silicate, cannot effectively be exposed during the oxidative roasting. On the contrary, a more compact structure of the silicates is apt to form after oxidative roasting, causing another secondary encapsulation of gold by silicates [
28]. Brady et al. [
29] researched the dissolution of silicate in neutral and basic pH solutions and found that a higher pH was beneficial to the dissolution of silicates. Therefore, it is necessary to expose the gold encapsulated in silicates through pretreatment with alkali washing [
30,
31].
With respect to the simultaneous encapsulation of gold by iron oxides and silicates, however, the efficient extraction of gold is nearly impossible by only sulfuric acid curing–water leaching or alkali washing. As for the distribution of gold, Paktunc et al. [
32] found that gold tended to be distributed in fine-grained and inclusion-rich pyrite crystals rather than the coarse-grained crystals, and after roasting, the gold appeared to be confined to impervious bands of maghemite within iron oxide particles and to mimic the distribution of arsenic. Moreover, it was also reported that gold encapsulation in iron oxides occurred more easily in the relatively fine size fraction of calcine [
13,
33], but the causes for this phenomenon are still unclear. Little research has concentrated on exposing the encapsulated gold in different size fractions of the calcine.
In this paper, the particle characteristics including the chemical compositions, chemical phase and particle morphology of different size fractions of the gold concentrate and calcine were investigated systematically. On this basis, the enhancement of gold leaching from the calcine was studied by purposefully pretreating each size fraction with sulfuric acid curing-water leaching or alkali washing. Finally, an efficient process from improving gold extraction from refractory As-, S- and C-bearing gold concentrate calcine was developed based on the different predominant encapsulation phase of gold in different size fractions of the calcine.
2. Materials and Methods
2.1. Materials and Reagents
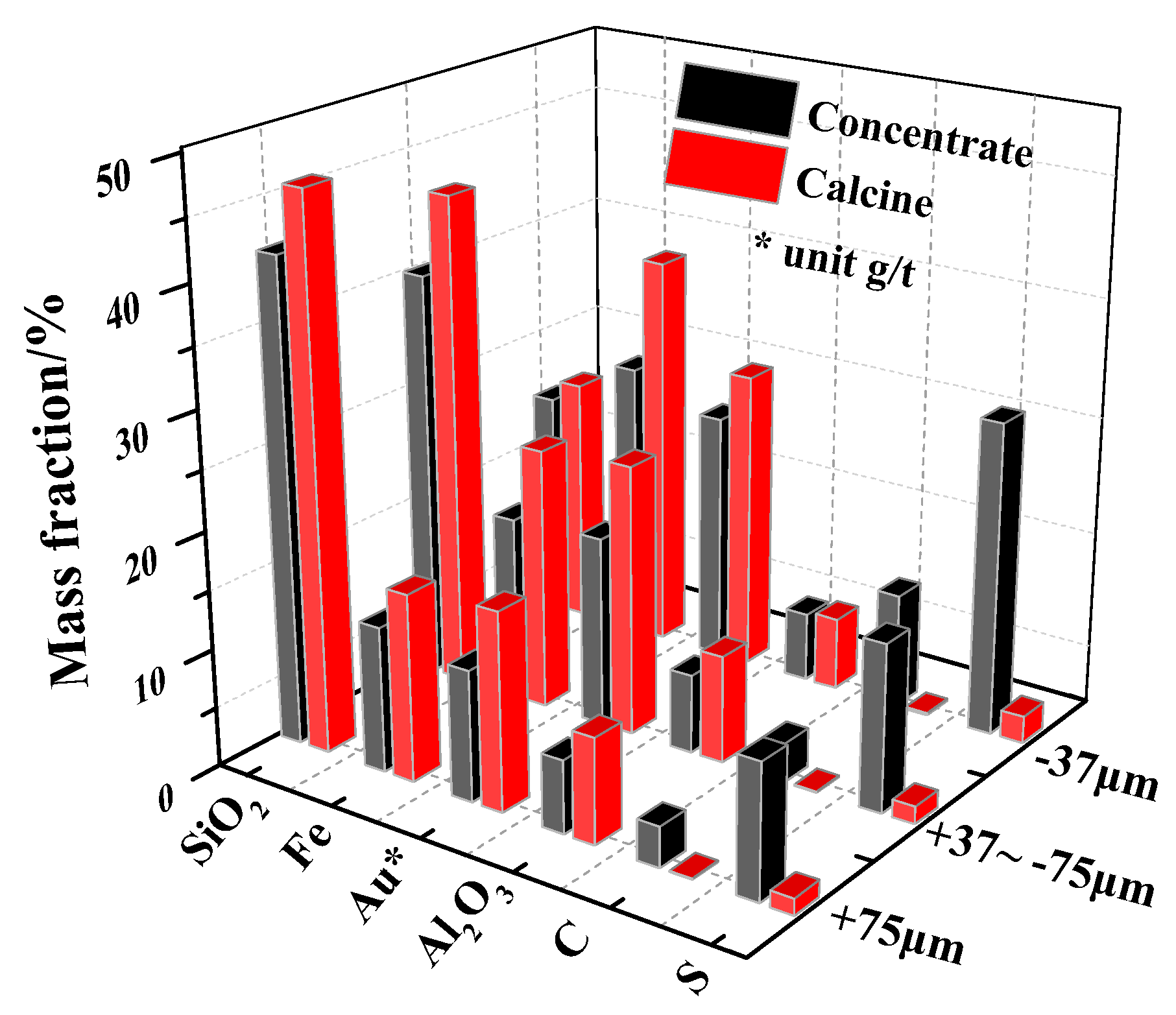
The As-, S- and C-bearing gold concentrate was a floatation concentrate from Yunnan province of China. The calcine was obtained from the gold concentrate by two-stage oxidative roasting. Mineralogical phases of the gold concentrate and calcine were determined by quantitative X-ray diffraction (XRD) analysis, and the chemical and mineralogical compositions of them are shown in
Table 1. Through optical microscopy and electron probe microanalyses and chemical analyses for different phases, the chemical phase analyses of gold in the concentrate and calcine are also presented in
Table 2.
It is indicated in
Table 1 that the gold concentrate had a high content of S (20.76%), As (1.62%) and C (6.95%), which are all possibly detrimental to gold leaching. Specifically, according to the chemical phase analyses of gold in the concentrate (see
Table 2), the gold concentrate belongs to a refractory sulfidic carbonaceous gold ore chiefly due to the encapsulation of gold by associated sulfides (mainly pyrite and arsenopyrite) and the “preg robbing” of gold by carbonaceous matters (mainly simple substance C). After two-stage oxidative roasting, most of the S (93.84%), As (71.40%) and C (98.27%) in the gold concentrate was removed and the content of gold increased from 18.05 g/t to 22.50 g/t. In addition, the exposed gold (cyanide leachable) increased from 1.72% in the concentrate to 64.27% in the calcine. However, the enclosed gold by iron oxides increased significantly from 10.36% in the concentrate to 21.96% in the calcine, which indicated that, except the initial gold encapsulated by iron oxides, during oxidation roasting, the secondary gold encapsulation by iron oxides occurred. Meanwhile, the distribution of gold in silicates rose markedly from 0.71% in the concentrate to 11.41% in the calcine, meaning that the secondary gold encapsulation by silicates was also generated during oxidation roasting. Actually during the roasting, solid phase reactions of FeO with SiO
2, Al
2O
3, etc. can occur at a temperature of less than 600 °C. Together with the exothermic effect of carbon combustion, an overhigh local temperature is inclined to be caused and thereby leading to the fusion of low-melting resultants of solid-solid reactions such as fayalite and aluminosilicate, resulting in the secondary encapsulation of gold by silicates [
34]. At the same time, under overhigh local temperature, the iron oxides will undergo recrystallization leading to secondary gold encapsulation by iron oxides.
The reagents used in this study, such as sodium cyanide, sodium hydroxide and sulfuric acid, were all of analytically pure grade. De-ionized water was used throughout all experiments.
2.2. Experiment Methods
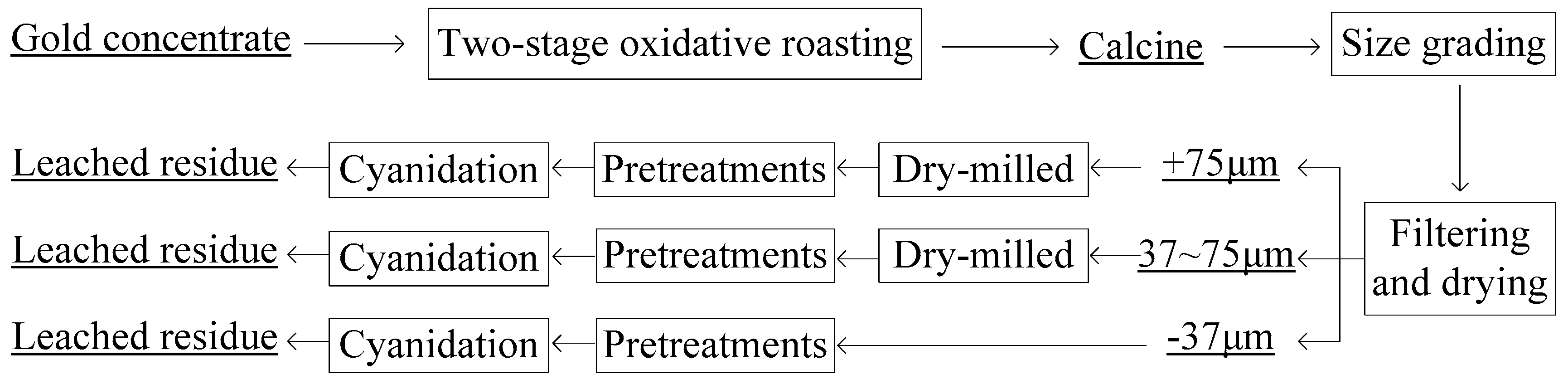
The simplified flow chart of manipulations is presented in
Figure 1. The size grading of gold concentrate or calcine was carried out in water with screens of 200 mesh and 400 mesh, thus obtaining three particle size fractions of +75 μm (coarse), +37~−75 μm (medium) and −37 μm (fine). These different size fractions were filtrated, dried and stored in air-tight plastic bags. The two-stage oxidative roasting of gold concentrate was conducted in a horizontal tube furnace under the optimum conditions that had been obtained in our previous studies [
31], aiming to remove As in the I stage and then S and C in the II stage.
Before the pretreatments and cyanidation of calcines, the coarse and medium size fractions were previously dry-milled in a planetary ball mill to be −37 μm over 90%. The pretreatment of calcines by acid pickling or alkali washing were conducted in 1-L jacketed glass reactors each equipped with overhead mechanical stirrer (IKA EURO-STPCUS25), condenser and thermometer, and the reactors were connected with thermostatic water baths to control reaction temperature. The reaction conditions were liquid-solid ratio 3:1, temperature 80 °C, stirring speed 300 rpm, H2SO4 15% (mass fraction)/NaOH 2% (mass fraction) and time 1 h. The sulfuric acid curing-water leaching of calcines was firstly carried out in a ceramic crucible at a muffle furnace, and then the reaction product was leached by water in a 1-L beaker where pulp was stirred by a mechanical agitator. The reaction conditions of sulfuric acid curing were H2SO4 75% (mass fraction), excess coefficient of H2SO4 1.4 (ratio of the actual amount to the theoretical amount), temperature 250 °C and time 1 h. The conditions of water leaching were liquid–solid ratio 3:1, stirring speed 300 rpm, temperature 25 °C and time 2 h. Gold cyanide leaching was implemented under the conditions of liquid–solid ratio 2.5:1, temperature 25 (±0.5) °C, stirring speed 600 rpm, NaCN 0.4% (mass fraction), time 36 h and pH 11, and the pH was adjusted by the careful addition of 1.0 M NaOH solution. When the pretreatment or gold leaching test was completed, the pulp was filtrated by a vacuum filter and the obtained filter residue was washed adequately.
2.3. Analytical Methods
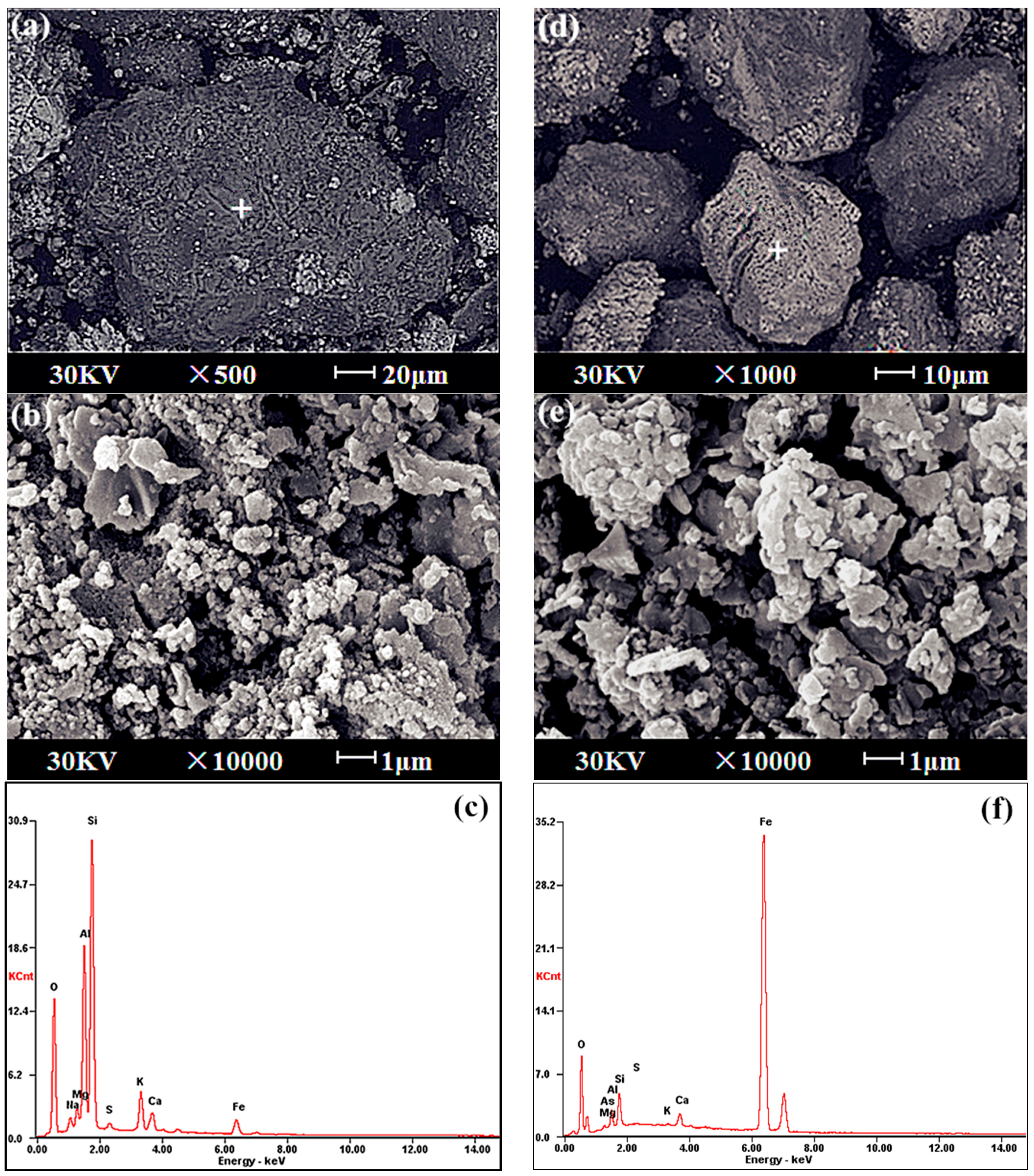
S and C contents in the gold concentrate and calcine were determined using a high frequency IR carbon and sulfur analyzer (HW2000B, Wuxi Yingzhicheng, Wuxi, China). The other elements were all analyzed using acid digestion and an atomic absorption spectrometer (AA-6800, Shimadzu, Kyoto, Japan). The specific surface areas of mineral particles were detected by the laser particle size analyzer (Mastersize2000, Malvern Instruments Ltd., Malvern, UK). Morphological studies on the calcine were carried out with Scanning Electron Microscope coupled with Energy Dispersive Spectrometer (JSM-6360LV, JEOL Ltd., Tokyo, Japan).
Chemical phase analysis of gold is extensively adopted in China for research on gold-bearing materials, and the steps of analysis are as follows: firstly, the occurrence of gold in different phases was found out by optical microscopy and electron probe microanalyses, and then the content of gold in each phase was determined by the selective dissolving of each phase with certain chemical reagents [
35,
36]. The exposed gold represents that gold is leachable by direct cyanidation while the gold in sulfides, iron oxides and silicates means that gold is not cyanide leachable due to being separately locked in those associated minerals.
4. Conclusions
The total gold leaching ratio of a refractory As-, S- and C-bearing gold concentrate calcine by direct cyanidation was only 70.2%, but there was a significant variation in the gold leaching ratio based on size fraction, in the following order: medium size fraction (82.6%) > coarse size fraction (72.3%) > fine size fraction (59.7%). Due to the grindability difference of minerals and the intimate occurrence of Au with S and Fe in the raw gold ore before flotation, segregation of composition occurred—the coarse and medium size fractions always had much higher SiO2 content whilst Fe and Au were prone to being concentrated in finer size fractions. As a result, the encapsulation of gold by iron oxides was apt to occur in finer calcine particles, whilst the gold encapsulation by silicates occurred more easily in coarser particles because the porosity of particles were blocked by melted silicates.
The enhanced gold leaching tests showed that alkali washing was more effective for exposing the gold encapsulated in the coarse and medium size fractions due to effectively damaging the silicates structure and sulfuric acid curing-water leaching was more favorable to open the encapsulation of gold in the fine size fraction attributing to remarkable dissolution of iron oxides via forming the soluble rhomboclase. Hence, via the pretreatments of alkali washing for the relatively coarse size fraction (+37 μm) and sulfuric acid curing-water leaching for the relatively fine size fraction (−37 μm), the total gold leaching ratio of calcine by cyanidation was improved from 70.2% to 93.6%, which is substantial. Based on the composition segregation associated with particle size, this is an efficient way of intensifying gold extraction for this kind of refractory As-, S- and C-bearing gold concentrate calcines.