Abstract
The mid-low grade sedimentary phosphate ore, abundant in silicate and carbonate gangue minerals, exhibits a poor processability. It is conventionally enriched using high temperature flotation to remove silicate gangues with fatty acid as a collector. Cottonseed oil has been proved to be an efficient collector for achieving ambient temperature flotation of the sedimentary phosphate ore used in this study. Flotation kinetics was investigated to ascertain the excellent collecting performance of cottonseed oil, as compared with oleic acid, and the phosphate flotation fitted well with the first-order flotation model. Based on the analysis of flotation reagent effect on the direct flotation process using the response surface methodology (RSM), a closed circuit of direct-reverse flotation for stepwise removing silicate and carbonate gangues from the sedimentary phosphate ore was established. Consequently, a required high quality of phosphate concentrate containing 30.16% P2O5 was obtained, with a recovery of 90.90%. Scanning electron microscopy (SEM) and X-ray diffraction analysis (XRD) of the flotation products confirmed that the majority of silicate and carbonate gangues were effectively removed from the concentrate products.
1. Introduction
Phosphate ore is a vital nonrenewable and strategic resource widely used in manufacturing fertilizers, detergents, pesticides, animal fodder, and other phosphorous-based chemicals [1]. More than 75% of the phosphate resources in the world are distributed as sedimentary deposits [2]. Among these, the majority is a sedimentary phosphate ore characterized by a mid-low grade and a cryptocrystalline structure. They coexist with silicate and carbonate gangues such as quartz, dolomite, calcite, etc., and thus have a poor processability [3]. Therefore, the beneficiation and upgrade of this kind of sedimentary phosphate ore is of great significance to meet the marketable requirements of the phosphate industry and to further alleviate the resource scarcity in the world.
Froth flotation is considered as the most effective process for the beneficiation of the sedimentary phosphate ore, due to the high efficiency of removing silicate and carbonate gangue minerals [4,5]. Reverse flotation is effective and is usually applied to remove carbonate gangues from phosphate ores with sulfuric acid, with phosphoric acid being used as a pH regulator and depressant [6,7]. In the meantime, there are two typical flotation processes for the removal of silicate gangues from phosphate ores with different types of collectors. A reverse flotation process with cationic collectors is used to float silicate gangue minerals, while the phosphate minerals are depressed in a weak acidic medium environment [8]. However, this method always generates a froth concentrate with a high viscosity caused by the common cationic collectors, resulting in the difficulty of filtrating the float products [9,10]. Therefore, efforts have been paid to the direct flotation process with anionic collectors, which can overcome the disadvantages of the reverse flotation process and which displays a better filtration performance of the corresponding froth products.
Fatty acids are the main traditional anionic collectors used for the flotation of non-sulphide ores [11]. However, their application suffers from sensitivity to slimes and ions, an increased collector price, relatively high consumption due to their very low solubility in water, and a high temperature requirement [12]. Flotation operations are generally carried out at room temperatures, or at temperatures less than 30 °C. An increasing operation temperature would incur expensive flotation separation, especially for the separation of silicate gangues in the direct flotation process of sedimentary phosphate ores [13]. Therefore, searching for collectors which can achieve the ambient flotation of phosphate ores is a future direction for improving the flotation separation efficiency of phosphate ores.
Vegetable oils have recently been investigated as an economically and environmentally friendly alternative collector in selective flotation. Such vegetable oils include soybean oil [14,15], canola and palm oils [16], jojoba oil [17], etc. Cottonseed oil has an advantage of containing a relatively high level of unsaturated compounds, especially polyunsaturated fatty acids (linoleic acid and linolenic acid). This unique feature of cottonseed oil results in an excellent solubility in pulp, without the need to raise the flotation temperature.
This study aimed to investigate the potential application of cottonseed oil as an alternative collector in the ambient temperature flotation of sedimentary phosphate ore. A flotation kinetics study was conducted to elucidate the more advantageous properties of cottonseed oil compared to that of oleic acid, thereby establishing the most suitable flotation model. Then, batch flotation experiments were performed to probe the effects of flotation reagents on the beneficiation efficiency and to obtain the proper flotation conditions by a response surface methodology and factorial experimental analysis. Consequently, a direct-reverse flotation closed circuit has been established to simulate the actual industrial flotation process. The goal was to reach the target of the content and recovery of P2O5 in a flotation concentrate, with values above 30% and 90%, respectively. To understand the fundamental mechanisms of the process, the changes in the mineral phases and surface morphology of the phosphate ore before and after the flotation process were examined by scanning electron microscopy (SEM) and X-ray diffraction (XRD) analysis.
2. Materials and Methods
2.1. Materials
2.1.1. Sedimentary Phosphate Ore
The sedimentary phosphate ore was derived from Yichang, Hubei province, in China, and its main chemical and mineral compositions are presented in Table 1 and Table 2, respectively. Table 1 showed that the raw ore was a typical calcareous and siliceous phosphate ore characterized by a middle grade of 23.34% P2O5, with a relatively high content of SiO2 (22.15%) and MgO (2.50%). As shown in Table 2, hydroxyapatite was the main phosphate mineral, with a content of more than 67%, while muscovite, quartz, dolomite, and feldspar were the major gangue minerals accounting for about 26% of the ore.

Table 1.
The chemical compositions of the phosphate ore by X-ray fluorescence analysis (wt %).

Table 2.
The modal mineralogy of the phosphate ore by mineral liberation analysis (wt %).
The size distribution and chemical analysis of the sedimentary phosphate ore are summarized in Table 3. The results showed that P2O5 was distributed almost uniformly in different size fractions, instead of being enriched in any particular fractions. Because of this reason, the sedimentary phosphate ore cannot be selectively beneficiated through physical classification, and needs further processing.

Table 3.
The size distribution and chemical analysis of the phosphate ore.
2.1.2. Reagents
Sodium carbonate (Na2CO3) and sulfuric acid (H2SO4) were used as pH regulators, while sodium silicate (Na2SiO3) and citric acid were used as depressants of silicate and phosphate minerals, respectively. Cottonseed oil (iodine value 135) was purchased from the local market. Its components were analyzed by gas chromatography and are listed in Table 4. The main components of cottonseed oil were oleic acid and linoleic acid, with a combined content of over 85%. After having been saponified by sodium hydroxide, cottonseed oil soap was used as a collector for the flotation experiments in this study. All of the reagents except the cottonseed oil were analytical grade.

Table 4.
The main components of cottonseed oil (wt %).
2.2. Flotation Experiments
The samples used in the flotation were ground to about 80%, passing 74 μm. The flotation experiments were carried out in a 0.5 L laboratory flotation cell with a pulp density of about 36% solid, at a temperature of 25 °C. The pulp was conditioned at a rotor speed of 2000 rpm, and a conditioning time of 2 min was used for each reagent (pH regulator, depressant, cottonseed oil soap). Flotation continued for 5 min. The collected concentrate and tailings were filtered, dried, and weighed. Then, the representative samples obtained from each product by quartering techniques were analyzed for the phosphate and magnesium content (to calculate the removal ratio of the carbonate minerals) by X-ray fluorescence analysis.
The grade and recovery of the valuable minerals have usually been used to evaluate the selectivity and efficiency of the separation process. However, this was not sufficient for determining the optimum conditions, due to the inverse relationship between them. In order to investigate the flotation efficiency effectively, the overall beneficiation efficiency was calculated by the Hancock formula, which is given below.
where ε and γ were the recovery of P2O5 and the yield of the phosphate concentrate, respectively. εmax was the theoretical maximum recovery of P2O5 for the phosphate ore and γ0 was the theoretical yield of the flotation concentrate.
Substituting equations ε = β·γ/α and εmax = β0·γ0/α into Equation (1), obtained an expression for the beneficiation efficiency, as follows:
where α and β were the grade of the raw ore and the flotation concentrate, respectively, and β0 was the theoretical grade of the apatite (41.36 wt % P2O5 by the chemical assaying of a single pure mineral). The theoretical yield of concentrate γ0 could be calculated by the equation γ0 = α/β0.
2.3. Mineralogical Analysis
Mineralogical analysis for the samples including the raw material and flotation concentrate was conducted by coupling X-ray diffraction (XRD, D8 ADVANCE, Bruker, Karlsruhe, Germany) with scanning electron microscopy (SEM, JSM-5510LV, Japan Electron Optics Laboratory, Tokyo, Japan). SEM was used to investigate the surface morphology of solid samples, and XRD was applied for the mineral phase analysis.
3. Results and Discussion
3.1. Flotation Kinetics of Sedimentary Phosphate Ore
The results of the phosphate flotation as the function of the sodium oleate and cottonseed oil concentration are shown in Figure 1. It can be concluded that the equilibrium time was about 4 min at ambient temperature (25 °C), regardless of the collector type and its dosage. This value increased to 6 min as the pulp temperature rose to 40 °C.
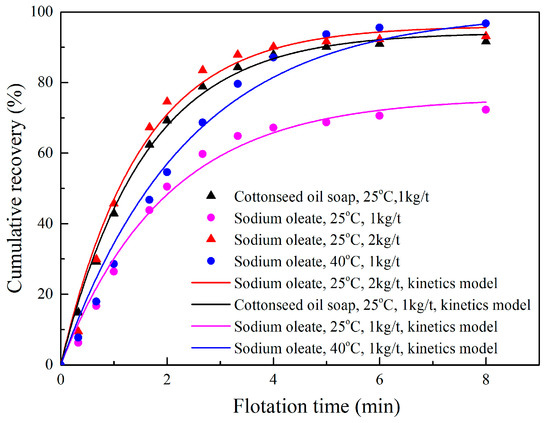
Figure 1.
Flotation kinetics of sedimentary phosphate ore under different conditions.
As can be seen from Figure 1, the recovery of P2O5 in the concentrate increased sharply with the increase of the collector dosage and pulp temperature in the first 3–4 min of flotation. The recovery, using cottonseed oil soap at a dosage of 1 kg/t and a pulp temperature of 25 °C, was much higher than that of sodium oleate under the same condition. It approached a recovery using a sodium oleate dosage of 2 kg/t at 25 °C, or sodium oleate dosage of 1 kg/t at 40 °C. It was evident that the cottonseed oil soap had a more satisfactory collecting performance at ambient temperature than sodium oleate when studying the phosphate flotation. This was probably attributed to a higher unsaturation degree of the cottonseed oil (iodine value 135) when compared with the oleic acid (iodine value 85~89), leading to a faster solubility and dispersion in the pulp.
In order to elucidate the relationship between the various parameters, the viz. collector type, pulp temperature, collector dosage, and the recovery of P2O5 in the phosphate flotation process, the data shown in Figure 1 was fitted to different flotation models, including a classical first-order model, fully mixed reactor model, and second-order kinetic model [18,19,20]. The statistical program Statgraphics Centurion (Statpoint Technologies, The Plains, VA, USA) was used to correlate data for the non-linear regression of each kinetic model [21].
Table 5 presents the results of the first-order model for each flotation condition, and the corresponding fitting curves of the models are also shown in Figure 1. According to the values of the correlation coefficient (R2) of each kinetics model in Table 5, the classical first-order model demonstrated a good fit to the experimental data, especially for the condition of using cottonseed oil soap as the collector. It should be pointed out that the model for sodium oleate (40 °C, 1 kg/t) was established when R∞ was predetermined by 100%, because the value of R∞ obtained by non-linear regression had exceeded 100%.

Table 5.
Classical first-order model (r = R∞ × [1 − exp(−kt)]) for various conditions.
The results in Table 5 also show that increasing the collector dosage and pulp temperature contributed to the enhancement of the ultimate recovery of P2O5. The flotation rate of constant k at a pulp temperature of 25 °C was in this order: 2 kg/t sodium oleate > 1 kg/t cottonseed oil soap > 1 kg/t sodium oleate, which was identical to the flotation results shown in Figure 1. It is worth noting that the flotation rate constant at 25 °C, 1 kg/t (k = 0.4994) for oleate, was higher than that at 40 °C, 1 kg/t (k = 0.4178), as shown in Table 5. This was verified and explained in the previous literature [13], by the fact that increasing the temperature would reduce the viscosity of water and enhance the elutriation of the gangues back to the pulp, and thus caused the decrease of the flotation rate with the augment of temperature.
The grade and recovery of the phosphate concentrate in the function of sodium oleate and cottonseed oil under different conditions are shown in Figure 2. When sodium oleate was used as a collector in the phosphate flotation, the P2O5 grade of the flotation concentrate decreased gradually with the increase of the pulp temperature and collector dosage. However, the recovery of P2O5 increased dramatically, to more than 93% (Figure 2c,d), which is much higher than the 73% value seen for the dosage of 1 kg/t at ambient temperature (Figure 2b). However, a more desirable phosphate concentrate (Figure 2a) was obtained with the grade of 27.63% and recovery of 91.62%, when using cottonseed oil soap as a collector at a low dosage of 1 kg/t and a pulp temperature of 25 °C. Thus, cottonseed oil would be considered as an ideal alternative collector in the phosphate industry due to its excellent flotation performance and selectivity, even at ambient temperature.
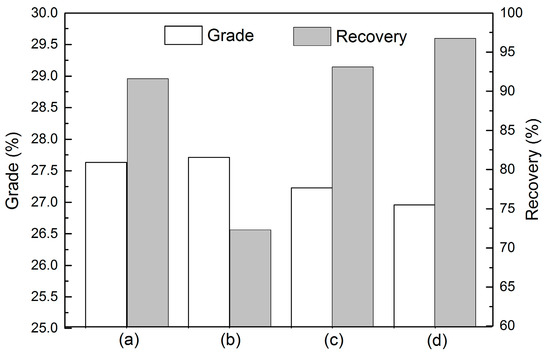
Figure 2.
Grade and P2O5 recovery of phosphate concentrates for sedimentary phosphate ore under different conditions (a) cottonseed oil soap, 25 °C, 1 kg/t; (b) sodium oleate, 25 °C, 1 kg/t; (c) sodium oleate, 25 °C, 2 kg/t; (d) sodium oleate, 40 °C, 1 kg/t.
3.2. The Direct Flotation of Sedimentary Phosphate Ore
As seen from the above section, cottonseed oil soap has a better flotation performance than the sodium oleate in the flotation of the sedimentary phosphate ores. Thus, the cottonseed oil soap was applied in the direct flotation process aiming at removing the silicate gangues from the sedimentary phosphate ore.
3.2.1. Response Surface Methodology Analysis
Using a response surface methodology (RSM) to design the experiments, the optimum flotation parameters can be obtained by the minimum number of experiments compared with the traditional factorial experiments, which demanded an investigation of all the possible combinations [22,23]. In the direct flotation process of the phosphate ore, especially the fine-particle sized one, a high pulp temperature was required to improve the solubility and activation energy of the reagents. In this case, the reagent dosage became the most significant factor for the removal of silicate minerals from the sedimentary phosphate ore when the pulp temperature was determined at 25 °C. A three-factor three-level Box-Behnken design based on the RSM was performed, and its experimental scheme and corresponding flotation results are shown in Table 6.

Table 6.
Experimental scheme and flotation results.
To verify the mathematical relationship between the three independent variables (Na2CO3 dosage, Na2SiO3 dosage, collector dosage) and the response of the beneficiation efficiency, a quadratic model was established on the basis of the data in Table 6.
where E was the beneficiation efficiency; A, B, and C represented the linear effects of sodium carbonate, sodium silicate, and the collector, respectively; AB, AC, and BC were the interaction effects of the three corresponding factors; and A2, B2, and C2 were the quadratic effects of each factor.
For the estimation of the significance of the model, the analysis of variance (ANOVA) [24] was applied using Design-Expert 8.0 software and the results are shown in Table 7. The accuracy of the model was found to be excellent, with an R-square value of 0.9659 [25]. The Model F-value of 22.05 implied that the model was significant, as the value of “Prob. > F” was less than 0.05, indicating that the model terms were significant. This meant that the main and interaction effects of all of the above variables have a great influence on the flotation process.

Table 7.
Analysis of the variance (ANOVA) of the quadratic model.
3.2.2. Effects of Reagent Dosages on Phosphate Flotation
With the purpose of gaining a better understanding of the main and interaction effects of the flotation reagents on the beneficiation efficiency, three-dimension (3D) plots were formed, as shown in Figure 3. The curves of sodium carbonate and the cottonseed oil soap similarly varied as the beneficiation efficiency increased dramatically with the increase in the sodium carbonate or cottonseed oil soap dosage. Figure 3a,c displays the interaction effects between sodium silicate and the other reagents in direct flotation, and it can be seen that the beneficiation efficiency increased to a maximum and then generally decreased with the increasing of the sodium silicate dosage when that of the sodium carbonate and cottonseed oil soap was constant. Thus, it can be concluded that a high level of sodium carbonate and a collector dosage with a relatively low level of sodium silicate dosage were proved to be more favorable to improve the beneficiation efficiency of the direct flotation process.
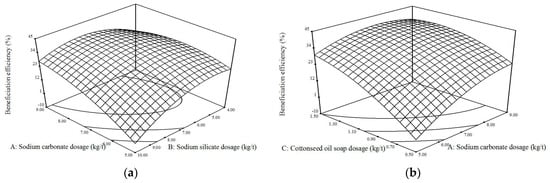

Figure 3.
Effects of the reagent dosage and their interactions on the beneficiation efficiency: (a) effects of the sodium carbonate and sodium silicate dosage; (b) effects of the sodium carbonate and cottonseed oil soap dosage; (c) effects of the sodium silicate and cottonseed oil soap dosage.
3.2.3. Optimization of Direct Flotation Parameters
Additionally, when investigating the main and interaction effects of the various parameters on the beneficiation efficiency, RSM can also be applied to find an optimum condition which was much more favorable to the separation process [26]. In this study, a maximum beneficiation efficiency of 39.32% was obtained under the condition of 7.90 kg/t sodium carbonate, 7.72 kg/t sodium silicate, and 1.49 kg/t cottonseed oil soap, which was optimized by RSM.
3.3. The Reverse Flotation of the Rougher Concentrate
To obtain a high quality phosphate concentrate with a P2O5 grade of more than 30%, a reverse flotation process for removing carbonate minerals was conducted on the rougher concentrate derived from the direct flotation process of the sedimentary phosphate ore.
3.3.1. Effect of Sulfuric Acid Dosage
Sulfuric acid mainly functioned as a pH modifier and phosphate depressant in reverse flotation. The effect of the sulfuric acid dosage on the reverse flotation with 0.5 kg/t cottonseed oil soap consumed is shown in Figure 4. It can be seen that the grade of the phosphate concentrate enhanced with the increase in the sulfuric acid dosage, while the variation tendency of the recovery of P2O5 and the beneficiation efficiency behaved differently. The recovery initially increased to a maximum value at a sulfuric acid dosage of 15 kg/t, then decreased gradually to a minimum value at a dosage of 21 kg/t, before starting to subsequently increase. It was reported that the desirable pH for the depression of the phosphate minerals was 4.5–5.5 [5,6,11], but the pH of the pulp in the function of sulfuric acid at the range of 15–21 kg/t was below 4. Therefore, it was not sufficient to depress the phosphate minerals under this condition. However, following this, phosphoric acid, which can depress phosphate minerals better than sulfuric acid, was generated by the chemical reaction between the sulfuric acid and phosphate ore, and resulted in the enhancement of P2O5 recovery. Though the beneficiation efficiency under the dosage of 24 kg/t H2SO4 seemed to be more favorable, 15 kg/t H2SO4 should be considered as the proper condition. This is because a high level dosage of H2SO4 would intensify acid corrosion to the flotation equipment and increase the cost of reagent consumption.

Figure 4.
Effect of sulfuric acid dosage on the reverse flotation of the rougher concentrate.
3.3.2. Effect of Citric Acid Dosage
Citric acid proved to be an efficient depressant for phosphate minerals [27], and was thus used synergistically to depress the flotation of the phosphate minerals in the reverse flotation. The effect of the citric acid dosage on the reverse flotation process was probed, while the sulfuric acid dosage and cottonseed oil soap dosage were fixed at 15 and 0.5 kg/t, respectively.
As seen from Figure 5, it was found that citric acid had a positive effect on the selective depression of phosphate minerals due to the better flotation indexes achieved in comparison with the result of just 15 kg/t H2SO4 (grade 30.79%, P2O5 recovery 76.40%, beneficiation efficiency 40.91%). This could be attributed to the synergistic effect of the citric acid and sulfuric acid occurring in the flotation system. As evidenced from this figure, the citric acid dosage of 0.9–1.2 kg/t was proper for the removal of carbonates. Taking into consideration the beneficiation efficiency values and citric acid consumption, 0.9 kg/t should be chosen as the proper condition under which a satisfactory phosphate concentrate with a grade of 31.30%, P2O5 recovery of 79.55%, and beneficiation efficiency of 46.27%, was obtained.
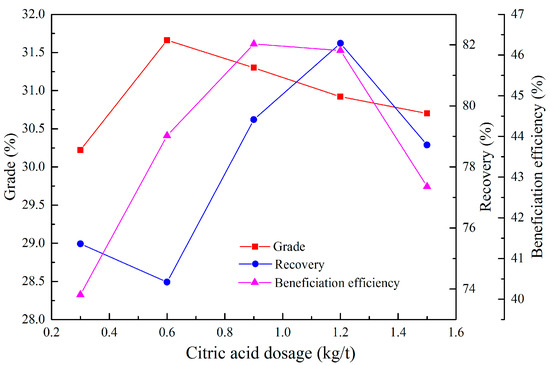
Figure 5.
Effect of the citric acid dosage on the reverse flotation of the rougher concentrate.
3.3.3. Effect of Cottonseed Oil Soap Dosage
Cottonseed oil soap was continuously used as the collector for the separation of carbonate gangues from the sedimentary phosphate ore in the reverse flotation process. Figure 6 presented the effect of the cottonseed oil soap dosage on the reverse flotation of the rougher concentrate when the sulfuric acid dosage and citric acid dosage were fixed at 15 and 0.9 kg/t, respectively.

Figure 6.
Effect of the cottonseed oil soap dosage on the reverse flotation of the rougher concentrate.
The grade of the phosphate concentrate increased slightly and the recovery of P2O5 decreased dramatically with the increasing dosage of the cottonseed oil soap. This was mainly due to the removal of unliberated phosphate minerals associated with carbonate minerals. Considering both the beneficiation efficiency and collector consumption shown in Figure 6, a cottonseed oil soap dosage of 0.5 kg/t is determined as the ideal condition for reverse flotation.
3.4. Closed Circuit Experiment and Mineralogical Analysis
According to the optimum conditions of the above flotation processes, a closed circuit that consisted of the direct flotation for the removal of silicates and the reverse flotation for removing carbonate minerals was carried out. The flowsheet and parameters of the closed circuit flotation were shown in Figure 7 and Table 8, respectively, and the corresponding results are shown in Table 9.

Figure 7.
Closed circuit flotation experiment for the removal of silicates and carbonates.

Table 8.
Flotation parameters in a closed circuit flotation experiment.

Table 9.
Experimental results of closed circuit flotation.
As seen from Table 9, a phosphate concentrate with a grade of 30.16% and a P2O5 recovery of 90.90% could be obtained through the closed circuit flotation experiment, and the overall removal ratio of magnesium has reached 60%. It revealed that the separation of silicates and carbonates from the sedimentary phosphate ore can be successfully achieved by the direct-reverse flotation process after a series of parameters have been optimized.
SEM and XRD analysis of the raw ore and the flotation products were performed to identify the morphology change and mineral phase transformation occurring in the flotation process. As seen from Figure 8, there were a lot of obvious impurities which existed on the surface of the raw ore (Figure 8a). The majority of these impurities were successfully removed after the direct and reverse flotation as the surface of the final concentrate became relatively smooth (Figure 8b), which can be explained by the XRD patterns of the flotation products below.

Figure 8.
SEM analysis of flotation samples, (a) raw ore; (b) final concentrate.
The XRD patterns of the raw ore and final concentrate are shown in Figure 9. It reveals that after the removal of most silicate and carbonate gangue minerals, the diffractions of fluorapatite in the final concentrate were intensified (Figure 9b). The intensity of the quartz decreased remarkably and the diffraction peak of dolomite disappeared, which was probably due to its low content. Figure 9c,d showed that dolomite and quartz were the predominant gangue minerals in the tailing products, and only a small percent of fluorapatite and whitlockite coexisted in the flotation tailings. These results confirmed that most of the phosphate minerals in the sedimentary phosphate ore were transferred into the final concentrate, while the main gangue minerals, quartz and dolomite, were effectively rejected into the tailing products through froth flotation.

Figure 9.
XRD patterns of raw ore and flotation products (a) raw ore; (b) final concentrate; (c) carbonate tailing; (d) silicate tailing.
4. Conclusions
- The ambient temperature flotation separation of the sedimentary phosphate ore for the removal of the silicate gangue was successfully achieved by using cottonseed oil as a collector. The flotation kinetics study revealed that the flotation performance and selectivity of the cottonseed oil was better than that of oleic acid and the phosphate flotation fitted the classical first-order flotation model very well.
- The analysis of the response surface method and single-factor experimental analysis proved the significant effects of the flotation reagents on the flotation process of the sedimentary phosphate ore. The optimum conditions were obtained at a sodium carbonate dosage of 7.90 kg/t, sodium silicate dosage of 7.72 kg/t, and cottonseed oil soap dosage of 1.49 kg/t in the direct flotation process, and sulfuric acid dosage of 15.00 kg/t, citric acid dosage of 0.90 kg/t, and cottonseed oil soap dosage of 0.50 kg/t in the reverse flotation process.
- Based on the above optimum conditions, a closed circuit of the direct-reverse flotation process was successfully established at ambient temperature to obtain a qualified phosphate concentrate assaying 30.16% P2O5 with a recovery of 90.90%. SEM and XRD analysis of flotation samples confirmed that silicate and carbonate gangue minerals, mainly quartz and dolomite, have been successfully removed from the concentrate products.
- The experimental results suggest that cottonseed oil is a promising alternative collector with advantages of excellent selectivity and flotation performance, and that its application in phosphate flotation would be helpful to reduce the production costs of the reagent and energy consumption.
Acknowledgments
Authors would like to acknowledge the financial support from National Science Foundation of China (51404171).
Author Contributions
R.C., Z.Z., and H.L. conceived and designed the experiments; Y.R. performed the experiments; C.X. and Y.R. analyzed the data; F.Z. contributed reagents/materials/analysis tools; Y.R. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gharabaghi, M.; Noaparast, M.; Irannajad, M. Selective leaching kinetics of low-grade calcareous phosphate ore in acetic acid. Hydrometallurgy 2009, 95, 341–345. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M. Physical and thermal treatment of phosphate ores—An overview. Int. J. Miner. Process. 2008, 85, 59–84. [Google Scholar] [CrossRef]
- Luo, H.H. Discussion on beneficiation for a mid-low grade collophanite ore of Hubei Yichang. Min. Metall. 2007, 16, 10–13. (In Chinese) [Google Scholar]
- Khoshjavan, S.; Rezai, B. Beneficiation of refractory rock phosphate by calcination and flotation. Miner. Metall. Process. 2011, 28, 187–192. [Google Scholar]
- Abdel-Khalek, N.A. Evaluation of flotation strategies for sedimentary phosphates with siliceous and carbonates gangues. Miner. Eng. 2000, 13, 789–793. [Google Scholar] [CrossRef]
- Elgillani, D.A.; Abouzeid, A.Z.M. Flotation of carbonates from phosphate ores in acidic media. Int. J. Miner. Process. 1993, 38, 235–256. [Google Scholar] [CrossRef]
- Abouzeid, A.Z.M.; Negm, A.T.; Elgillani, D.A. Upgrading of calcareous phosphate ores by flotation: Effect of ore characteristics. Int. J. Miner. Process. 2009, 90, 81–89. [Google Scholar] [CrossRef]
- Mohammadkhani, M.; Noaparast, M.; Shafaei, S.Z.; Amini, A.; Amini, E.; Abdollahi, H. Double reverse flotation of a very low grade sedimentary phosphate rock, rich in carbonate and silicate. Int. J. Miner. Process. 2011, 100, 157–165. [Google Scholar] [CrossRef]
- Zhou, B.; Xu, W.; Zhu, J. Experimental research on double reverse collophanite flotation on Guizhou. Ind. Miner. Process. 2016, 8, 7–8. (In Chinese) [Google Scholar]
- Wu, X.; Cao, Y.; Duan, Y. Effect of new cationic collector DHPA on froth characteristics and flotation performance of iron ore. Min. Metall. Eng. 2012, 32, 33–36. (In Chinese) [Google Scholar]
- Boulos, T.R.; Yehia, A.; Ibrahim, S.S.; Yassin, K.E. A modification in the flotation process of a calcareous-siliceous phosphorite that might improve the process economics. Miner. Eng. 2014, 69, 97–101. [Google Scholar] [CrossRef]
- Sis, H.; Chander, S. Reagents used in the flotation of phosphate ores: A critical review. Miner. Eng. 2003, 16, 577–585. [Google Scholar] [CrossRef]
- Su, F.W.; Rao, K.H.; Forssberg, K.S.E.; Samskog, P.O. The influence of temperature on the kinetics of apatite flotation from magnetite fines. Int. J. Miner. Process. 1998, 54, 134–138. [Google Scholar] [CrossRef]
- Guimarães, R.C.; Araujo, A.C.; Peres, A.E.C. Reagents in igneous phosphate ores flotation. Miner. Eng. 2005, 18, 199–204. [Google Scholar] [CrossRef]
- Santana, R.C.; Duarte, C.R.; Ataíde, C.H.; Barrozo, M.A.S. Flotation selectivity of phosphate ore: Effects of particle size and reagent concentration. Sep. Sci. Technol. 2011, 46, 1511–1518. [Google Scholar] [CrossRef]
- Owusu, C.; Quast, K.; Addai-Mensah, J. The use of canola oil as an environmentally friendly collector in sulphide mineral processing. Miner. Eng. 2016, 98, 127–136. [Google Scholar] [CrossRef]
- Santos, E.P.; Dutra, A.J.B.; Oliveira, J.F. The effect of jojoba oil on the surface properties of calcite and apatite aiming at their selective flotation. Int. J. Miner. Process. 2015, 143, 34–38. [Google Scholar] [CrossRef]
- Bayat, O.; Ucurum, M.; Poole, C. Effects of size distribution on flotation kinetics of Turkish sphalerite. Miner. Process. Extr. Metall. 2004, 113, 53–59. [Google Scholar] [CrossRef]
- Stoica, L.; Oproiu, G.C.; Cosmeleata, R.; Dinculescu, M. Kinetics of Cu2+ separation by flotation. Sep. Sci. Technol. 2003, 38, 613–632. [Google Scholar] [CrossRef]
- Hernainz, F.; Calero, M.; Blazquez, G. Kinetic considerations in the flotation of phosphate ore. Adv. Powder Technol. 2005, 16, 347–361. [Google Scholar] [CrossRef]
- Yuan, X.M.; Palsson, B.I.; Porssberg, K.S.E. Statistical interpretation of flotation kinetics for a complex sulphide ore. Miner. Eng. 1996, 9, 429–442. [Google Scholar] [CrossRef]
- Dehghani, A.; Azizi, A.; Mojtahedzadeh, S.H.; Gharibi, K.H. Optimizing rougher flotation parameters of the Esfordi phosphate ore. Miner. Process. Extr. Metall. Rev. 2012, 33, 260–268. [Google Scholar] [CrossRef]
- Mehrabani, J.V.; Noaparast, M.; Mousavi, S.M.; Dehghan, R.; Ghorbani, A. Process optimization and modelling of sphalerite flotation from a low-grade Zn-Pb ore using response surface methodology. Sep. Purif. Technol. 2010, 72, 242–249. [Google Scholar] [CrossRef]
- Aslan, N.; Cifci, F.; Yan, D. Optimization of process parameters for producing graphite concentrate using response surface methodology. Sep. Purif. Technol. 2008, 59, 9–16. [Google Scholar] [CrossRef]
- Chattopadhyay, U.S.; Kalyani, V.K.; Venugopal, R.; Charan, T.G. Application of response surface methodology in effective recovery of settling pond coal fines by froth flotation. Int. J. Coal Prep. Util. 2015, 35, 206–215. [Google Scholar] [CrossRef]
- Aslan, N.; Fidan, R. Optimization of Pb flotation using statistical technique and quadratic programming. Sep. Purif. Technol. 2008, 62, 160–165. [Google Scholar] [CrossRef]
- Zheng, X.; Smith, R.W. Dolomite depressants in the flotation of apatite and collophane from dolomite. Miner. Eng. 1997, 10, 537–545. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).