Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge
Abstract
:1. Introduction
Chemistry, Environmental Fate, and Effects of Selenium
2. Historical Relevance of Selenium in Mining Industry
3. Selenium Treatment Technologies
3.1. Physical Treatment
3.2. Chemical Treatment
3.3. Biological Treatment
4. Overview of Selenium in Alaskan Mines
5. Future Directions
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ABMet | The Advanced Biological Metals Removal Process |
| ADEC | Alaska Department of Environmental Conservation |
| ADNR | Alaska Department of Natural Resources |
| EPA | Environmental Protection Agency |
| FBR | Fluidized Bed Reactor |
| MCL | Maximum contaminant level |
| RO | Reverse Osmosis |
| ZVI | Zero-Valent iron |
References
- Lemly, A.D. Aquatic selenium pollution is a global environmental safety issue. Ecotoxicol. Environ. Saf. 2004, 59, 44–56. [Google Scholar] [CrossRef]
- Sandy, T.; DiSante, C. Review of Available Technologies for the Removal of Selenium from Water; Technical Report; CH2M Hill: Englewood, CO, USA, June 2010; pp. 2–223. [Google Scholar]
- Sharma, V.K.; McDonald, T.J.; Sohn, M.; Anquandah, G.A.K.; Pettine, M.; Zboril, R. Biogeochemistry of selenium. A review. Environ. Chem. Lett. 2014, 13, 49–58. [Google Scholar] [CrossRef]
- Brookins, D.G. Eh-pH Diagrams for Geochemistry; Springer: Berling/Heidelberg, Germany, 1989; Volume 53, pp. 18–19. [Google Scholar]
- Watanabe, T.; Kiron, V.; Satoh, S. Trace minerals in fish nutrition. Aquaculture 1997, 151, 185–207. [Google Scholar] [CrossRef]
- Lemly, A.D. A teratogenic deformity index for evaluating impacts of selenium on fish populations. Ecotoxicol. Environ. Saf. 1997, 37, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Sappington, K.G. Development of aquatic life criteria for selenium: A regulatory perspective on critical issues and research needs. Aquat. Toxicol. 2002, 57, 101–113. [Google Scholar] [CrossRef]
- U.S. Energy Information Administration (EIA). Annual Coal Report 2015; Technical Report; U.S. Department of Energy: Washington, D.C., USA, November, 2016; p. 69.
- Seshadri, P.; Sisk, D.; Bowman, F.; Benson, S. Leachability of Arsenic and Selenium in Submicron Coal Fly Ash. In Proceedings of the World of Coal Ash Conference, University of Kentucky and American Coal Ash Association, Denver, CO, USA, 9–12 May 2011.
- Izquierdo, M.; Querol, X. Leaching behavior of elements from coal combustion fly ash: An overview. Int. J. Coal Geol. 2012, 94, 54–66. [Google Scholar] [CrossRef]
- Yan, Q.Z.; Zhang, J. Species and distribution of inorganic selenium in the Bohai Sea. Chin. J. Oceanol. Limnol. 2006, 24, 204–211. [Google Scholar]
- Nriagu, J.O.; Wong, H.K. Selenium pollution of lakes near the smelters at Sudbury, Ontario. Nature 1983, 301, 55–57. [Google Scholar] [CrossRef]
- Wasewar, K.L.; Prasad, B.; Gulipalli, S. Removal of selenium by adsorption onto granular activated carbon (GAC) and powdered activated carbon (PAC). CLEAN Soil Air Water 2009, 37, 872–883. [Google Scholar] [CrossRef]
- Mar Systems. Sorbster Removes Selenium from Coal Mine Tailings and Process Waters; Technical Report; Mar Systems: Solon, OH, USA, June 2012; pp. 1–5. [Google Scholar]
- Twidwell, L.G.; Mccloskey, J.; Joyce, H.; Dahlgren, E.; Hadden, A. Removal of Selenium Oxyanions from Mine Waters Utilizing Elemental Iron and Galvanically Coupled Metals. In Proceedings of the Jan D. Mill Symposium—Innovations in Natural Resource, Salt Lake City, UT, USA, 28 February–2 March 2005; pp. 299–313.
- Desborough, G.; DeWitt, E.; Jones, J. Preliminary Mineralogical and Chemical Studies Related to the Potential Mobility of Selenium and Associated Elements in Phosphoria Formation Strata, Southeastern Idaho; Open-File Report 99-129; U.S. Department of the Interior, U.S. Geological Survey: Reston, VA, USA, 1999; pp. 6–7.
- Wen, H.; Carignan, J. Reviews on atmospheric selenium: Emissions, speciation, and fate. Atmos. Environ. 2007, 41, 7151–7165. [Google Scholar] [CrossRef]
- Golder Associates. Literature Review of Treatment Technologies to Remove Selenium from Mining-Influenced Water; Technical Report; Golder Associates: Lakewood, CO, USA, July 2009; p. 40. [Google Scholar]
- Rittmann, B.E. The Membrane Biofilm Reactor is a Versatile Platform for Water and Wastewater Treatment. Environ. Eng. Res. 2007, 12, 8–11. [Google Scholar] [CrossRef]
- Tang, C.; Huang, Y.H.; Zeng, H.; Zhang, Z. Reductive removal of selenate by zero-valent iron: The roles of aqueous Fe2+ and corrosion products, and selenate removal mechanisms. Water Res. 2014, 67, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Microbial Technologies Inc. Evaluation of Treatment Options to Reduce Water-Borne Selenium at Coal Mines in West-Central Alberta; Technical Report; Microbial Technologies Inc.: Ochelata, OK, USA, 2005; p. 11. Available online: http://www3.gov.ab.ca/env/info/infocentre/publist.cfm (accessed on 22 September 2016).
- Twidwell, L.G.; Mccloskey, J.; Miranda, P.; Gale, M. Technologies and potential technologies for removing selenium from process and mine wastewater. In Proceedings of the Recycling, Waste, Treatment and Clean Technology (REWAS), San Sebastian, Spain, 5–9 September 1999; pp. 1645–1656.
- Kharaka, Y.K.; Ambats, G.; Presser, T.S.; Davis, R.A. Removal of selenium from contaminated agricultural drainage water by nanofiltration membranes. Appl. Geochem. 1996, 11, 797–802. [Google Scholar] [CrossRef]
- MSE Technology Applications Inc. Selenium Treatment/Removal Alternatives Demonstration Project: Mine Waste Technology Program Activity III, Project 20; Technical Report; MSE Technology Applications Inc.: Butte, MT, USA, June 2001; pp. 1–133. [Google Scholar]
- Drelich, J.; Hwang, J.Y.; Adams, J.; Nagaraj, D.R. Proceeding of the Water in Mineral Processing; Society for Mining, Metallurgy & Exploration (SME): Englewood, CO, USA, 2012; pp. 291–294. [Google Scholar]
- Manceau, A.; Gallup, D.L. Removal of selenocyanate in water by precipitation: Characterization of copper-selenium precipitate by X-ray diffraction, infrared, and X-ray absorption spectroscopy. Environ. Sci. Technol. 1997, 31, 968–976. [Google Scholar] [CrossRef]
- Mavrov, V.; Stamenov, S.; Todorova, E.; Chmiel, H.; Erwe, T. New hybrid electrocoagulation membrane process for removing selenium from industrial wastewater. Desalination 2006, 201, 290–296. [Google Scholar] [CrossRef]
- CH2M Hill. NAMC White Paper Report Addendum Selenium Treatment; Technical addendum; CH2M Hill: Englewood, CO, USA, 29 March 2013; pp. 1–68. [Google Scholar]
- Frisch, S.; Technologies, E.; Enegess, D.; Technologies, E. Advances in fluidized bed reactor treatment of selenium in mining waters. In Proceedings of the SME Annual Meeting 2014, Salt Lake City, UT, USA, 23–26 February 2014; pp. 1–6.
- Quinn, N.W.T.; Leighton, T.; Lundquist, T.J.; Green, F.B.; Zárate, M.A.; Oswald, W.J. Algal-Bacterial treatment facility removes selenium from drainage water. Calif. Agric. 2008, 54, 50–56. [Google Scholar] [CrossRef]
- Cohen, R.R.H. Use of microbes for cost reduction of metal removal from metals and mining industry waste streams. J. Clean. Prod. 2006, 14, 1146–1157. [Google Scholar] [CrossRef]
- Harrison, T.; Hill, C.H.M.; Sandy, T.; Hill, C.H.M.; Leber, K.; Hill, C.H.M.; Srinivasan, R.; Hill, C.H.M.; Mchale, J.; Corp, P.C. Characterization and treatment of selenium in water discharged from surface coal mining operations in West Virginia. In Proceedings of the Society for Mining, Metallurgy & Exploration (SME) Annual Meeting, Phoenix, AZ, USA, 28 February–3 March 2010; pp. 1–5.
- Sonstegard, J.; Pickett, T.; Harwood, J.; Johnson, D. Full-Scale Operation of GE ABMet® Biological Technology for the Removal of Selenium from FGD Wastewaters. In Proceedings of the 69th Annual International Water Conference, San Antonio, TX, USA, 26–30 October 2008; pp. 343–356.
- Department of Commerce, Community, and Economic Development. Available online: https://www.commerce.alaska.gov/web/ded/DEV/MineralsDevelopment/MineralsProduction.aspx (accessed on 6 October 2016).
- Department of Environmental Conservation. Water Quality Standards. 2016. Available online: http://dec.alaska.gov/Water/wqsar/wqs/index.htm (accessed on 10 October 2016). [Google Scholar]
- Kinross. Kinross Fort Knox Annual Activity Report 2015. Available online: http://dnr.alaska.gov/mlw/mining/largemine/fortknox/annualmeetings.cfm (accessed on 15 October 2016).
- Sumitomo Metal Mining Pogo LLC. 2015 Annual Activity and Monitoring Report Sumitomo Metal Mining Pogo LLC. Available online: http://dnr.alaska.gov/mlw/mining/largemine/pogo/ (accessed on 15 October 2016).
- 2015Teck Resources Limited. Teck 2014 Annual Report. Available online: http://dnr.alaska.gov/mlw/mining/largemine/reddog/ (accessed on 18 October 2016).
- Fort Knox Mine. Available online: http://northern.org/programs/clean-water-mines/hardrock-mines-in-interior-and-arctic-alaska/fort-knox-true-north-mine-1 (accessed on 15 October 2016).
- Edgerton, D. Reconstruction of the Red Dog Zn-Pb-Ba orebody, Alaska: Implications for the vent environment during the mineralizing event. Can. J. Earth Sci. 1997, 34, 1581–1602. [Google Scholar] [CrossRef]
- Edwards, M.; Kulas, J.E.; Kuit, W.J.; Weakly, J.O.; Bloom, N.S.; Wallschlager, D. Aquatic selenium at Cominco’s Red Dog Mine: Sources, speciation, distribution, and control. In Proceedings of the Tailings and Mine Waste ’99, Fort Collins, CO, USA, 24–27 January 1999; pp. 535–542.
- Brienne, S.; Falutsu, M.; Weakley, J.; Kulas, J.; Kuit, W.; Geist, D.; Gustafson, J.; Wood, S.; Baker, L.; Rosenzweig, R.; et al. Selenium release and removal from the Red Dog Mine operation. In Proceedings of the British Columbia Mine Reclamation Symposium, Williams Lake, BC, Canada, 18–21 September 2000; pp. 194–203.
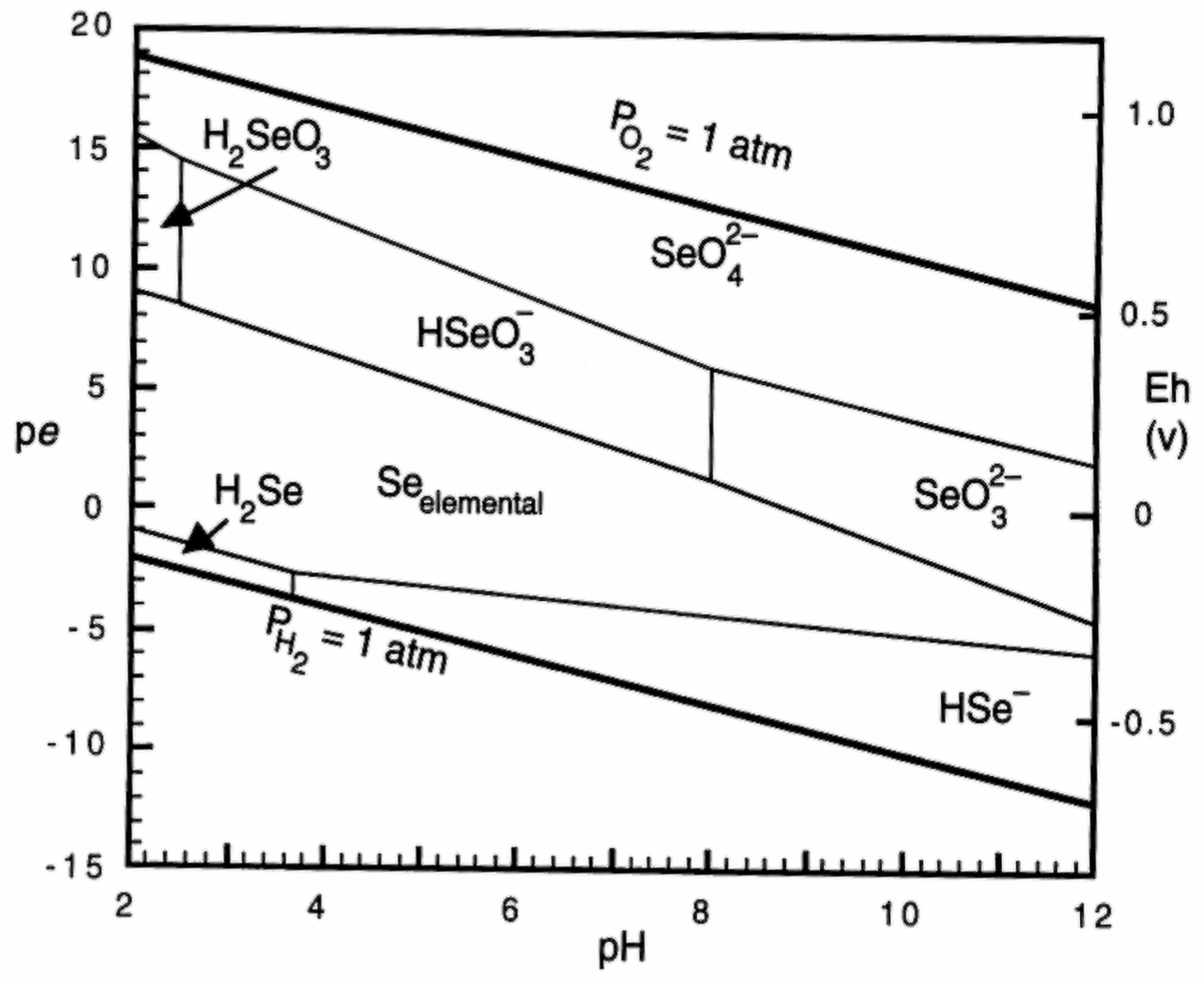
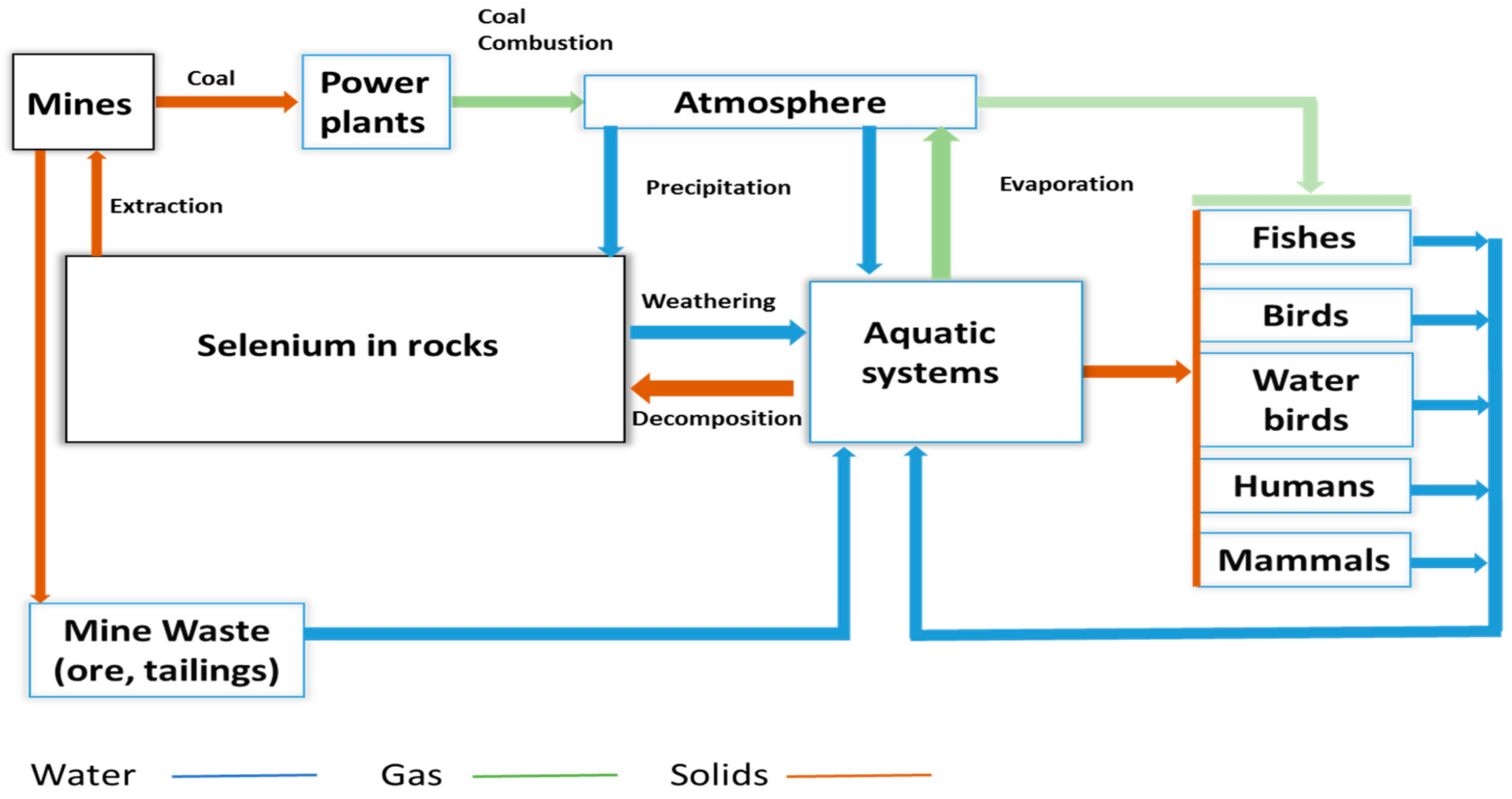
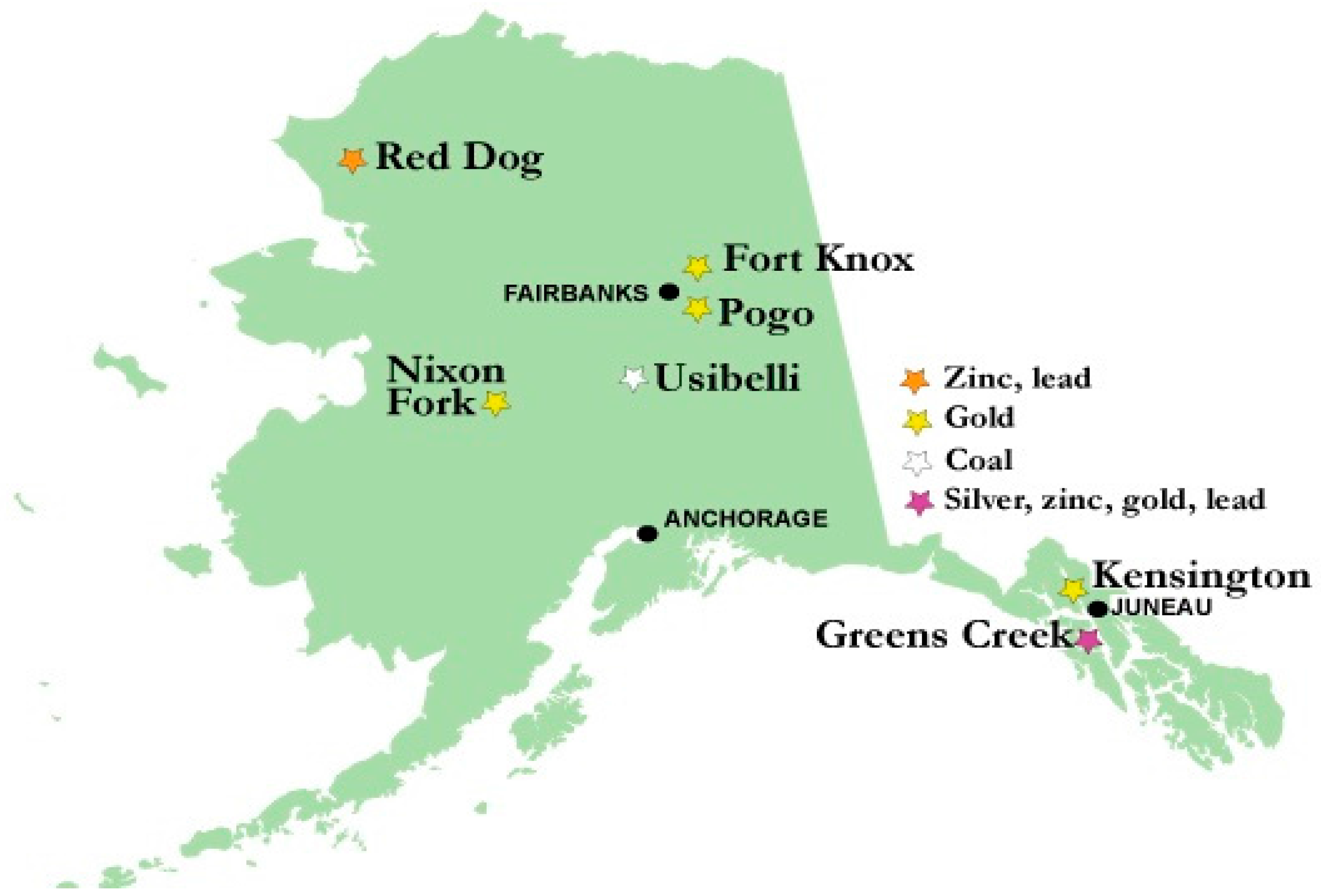
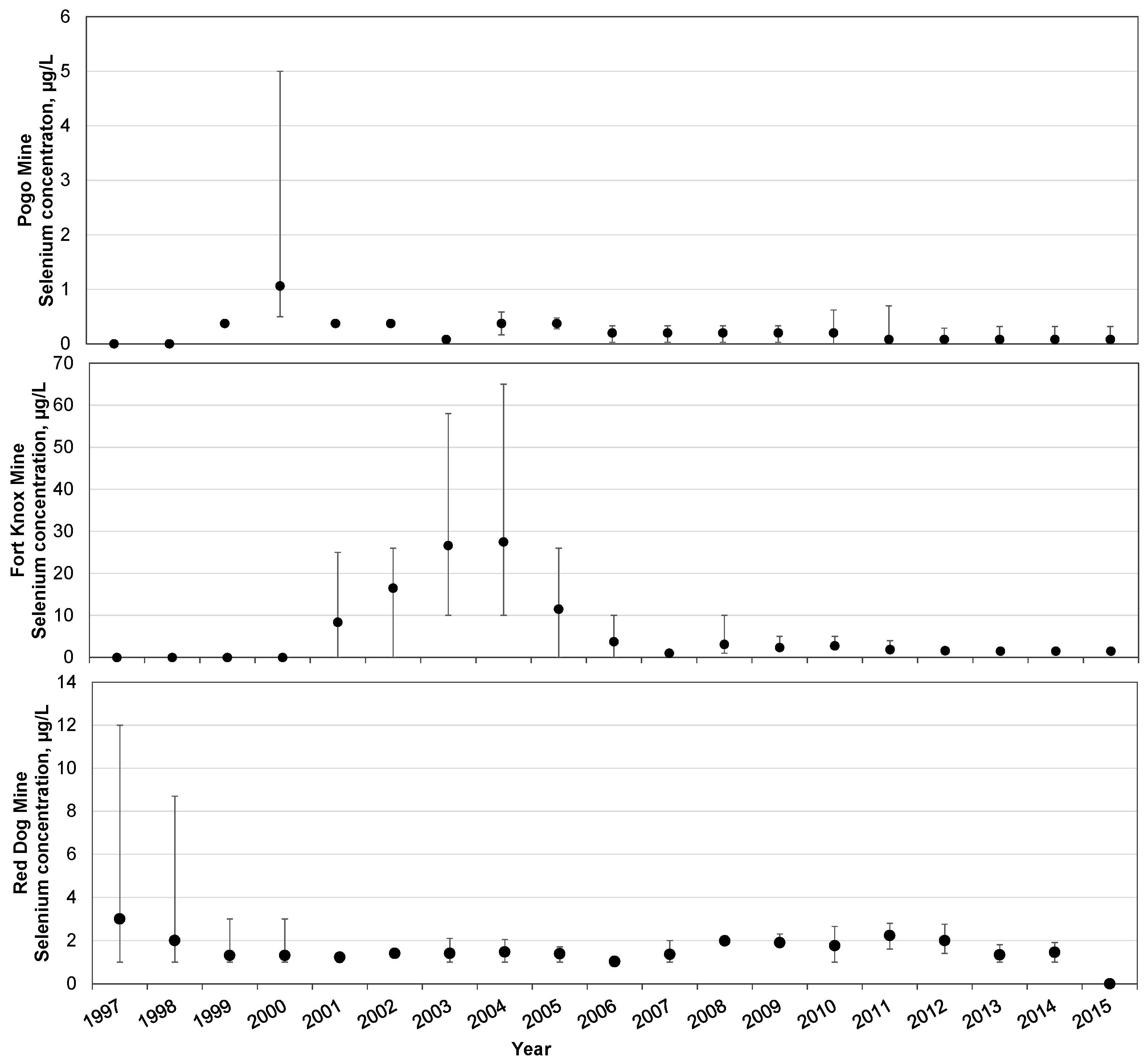
| Criteria Version | Egg-Ovary (mg/kg dw a) | Whole Body (mg/kg dw a) | Muscle (mg/kg dw a) | Water Lentic (µg/L) | Water Lotic (µg/L) |
|---|---|---|---|---|---|
| 2016 | 15.1 | 8.5 | 11.3 | 1.5 (30 day) | 3.1 (30 day) |
| Material or Waste | Se Concentration | References |
|---|---|---|
| Surface waters | 0.2 µg/L | [1] |
| Agricultural drainage water | 140–1400 µg/L | [11] |
| Copper ore | 20–82 µg/g | [12] |
| Mining wastewater | 3–12 µg/L | [13] |
| Coal | 0.4–24 µg/g | [12] |
| Coal mining pond water | 8.8–389 µg/L | [14] |
| Gold mine wastewater | 0.2–33 µg/L | [15] |
| Uranium mine wastewater | 1600 µg/L | [15] |
| Oil shale | 1.3–5.2 µg/g | [1] |
| Crude shale oils | 92–540 µg/L | [1] |
| Crude oil | 500–2200 µg/L | [1] |
| Refined oils | 5–258 µg/L | [1] |
| Oil refinery wastewater | 15–75 µg/L | [1] |
| Phosphate ore | 2–20 µg/g | [16] |
| Category | Treatment Technology | Test Scale | Treatment Performance | Location of Test | References |
|---|---|---|---|---|---|
| Physical | Reverse osmosis | Full scale | Effective levels below 5 µg/L; Reduced concentration from 12–22 µg/L to 2 µg/L | Barrick’s Richmond Hill Mine, SD, USA | [21] |
| Activated Alumina Adsorption | Lab Scale | Selenium was adsorbed over pH range 3–7 for concentrations of 100–200 µg/L | Municipal Environmental Research Lab, OH, USA | [22] | |
| Nanofiltration | Lab and field scale | Up to 95% selenium removal from highly contaminated agricultural drainage (up to 1000 µg/L) | Los Banos, California, USA (Lab scale), Kennecott South, UT, USA (Field Scale) | [23] | |
| Ferrihydrite adsorption | Full scale | Reduction from 1950 µg/L to 90 µg/L | KUCC Garfield Wetlands-Kessler Springs, UT, USA | [24] | |
| Ion-exchange (physicochemical) | Lab scale | Reduction from 1 g/L to 0.1 mg/L | Kennecott Mining Company, Boyle Engineering Corp, USA | [18] | |
| Chemical | Precipitation | Full scale | Reduction from 0.213 mg/L to 0.014 mg/L | Cameco’s Key Lake Operation, Canada | [25] |
| Cupric co-precipitation | Lab scale | Precipitation of Selenocyanate from petroleum refinery process | University of Grenoble, France and Unocal Corporation, CA, USA | [26] | |
| Electrocoagulation | Lab scale | Reduction from 2.32 mg/L to 0.03 mg/L | University of Mining and Geology, Bulgaria and Saarland University, Germany | [27] | |
| Zero- valent iron | Full scale | Reduction of influent selenium drop from 19 to 4.7 µg/L | West Virginia coal mines, Phosphate mine in Idaho, USA | [28] | |
| Fixed bed adsorption | Pilot scale and full scale | Reduction from 0.07–0.86 mg/L to 1–11 µg/L | Cameco Resources Smith Ranch-Highland operation, WY, USA | [29] | |
| Cementation | Lab scale | Reduction from 1950 µg/L to below 5 µg/L | University of Montana, MT, USA | [24] | |
| Biological | Algal-bacterial | Lab scale | Reduction from 300–500 ppb to 0–100 ppb | University of California, Berkeley, USA | [30] |
| Algal volatilization | Lab scale | Approximately reduction by 23% | University of California, Riverside, USA | [30] | |
| Microbial reduction | Full scale | Reduction from 70 µg/L to 4.6 µg/L | Golder, Closed mine in South Dakota, USA | [31] | |
| Fluidized bed reactor treatment | Pilot scale | Achieved less than 4.7 µg/L in effluent | West Virginia, USA | [32] | |
| ABMet | Pilot scale and full scale | Reduction from 20–300 µg/L (influent Selenium) to 0.7–2.0 µg/L (effluent Selenium) | British Columbia, Canada (pilot scale), West Virginia, USA (full scale) | [28] | |
| Biochemical reactor | Full scale | Inlet concentrations of 180 µg/L decreased to effluent concentrations from 3 to 33 µg/L | Alberta, Canada | [18] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khamkhash, A.; Srivastava, V.; Ghosh, T.; Akdogan, G.; Ganguli, R.; Aggarwal, S. Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals 2017, 7, 46. https://doi.org/10.3390/min7030046
Khamkhash A, Srivastava V, Ghosh T, Akdogan G, Ganguli R, Aggarwal S. Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals. 2017; 7(3):46. https://doi.org/10.3390/min7030046
Chicago/Turabian StyleKhamkhash, Aibyek, Vaibhav Srivastava, Tathagata Ghosh, Guven Akdogan, Rajive Ganguli, and Srijan Aggarwal. 2017. "Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge" Minerals 7, no. 3: 46. https://doi.org/10.3390/min7030046
APA StyleKhamkhash, A., Srivastava, V., Ghosh, T., Akdogan, G., Ganguli, R., & Aggarwal, S. (2017). Mining-Related Selenium Contamination in Alaska, and the State of Current Knowledge. Minerals, 7(3), 46. https://doi.org/10.3390/min7030046





