Comparison of Adsorption of Phenol O-O and N-O Chelating Collectors at the Malachite/Water Interface in Flotation
Abstract
:1. Introduction
2. Experimental
2.1. Materials
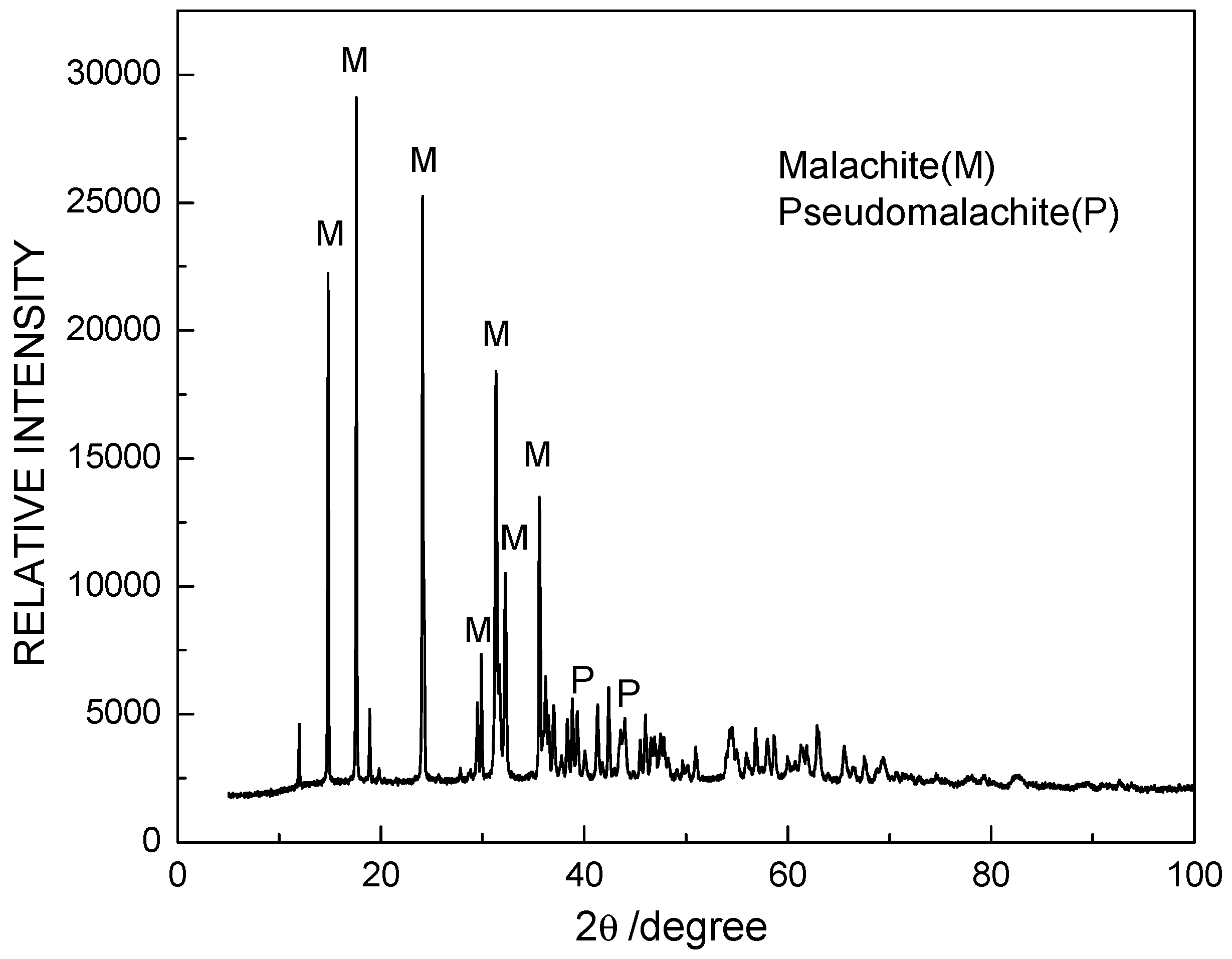
2.2. Methods
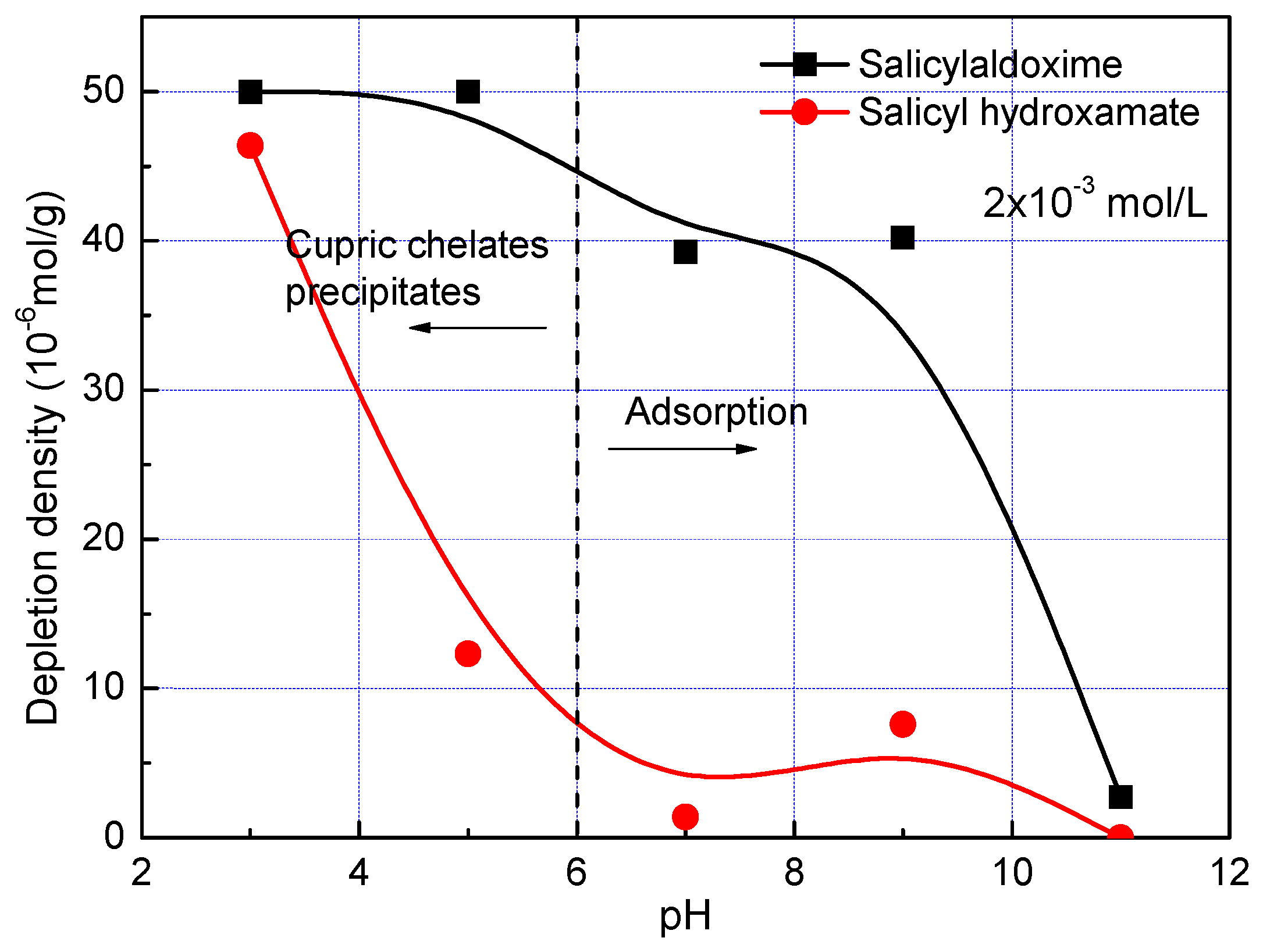
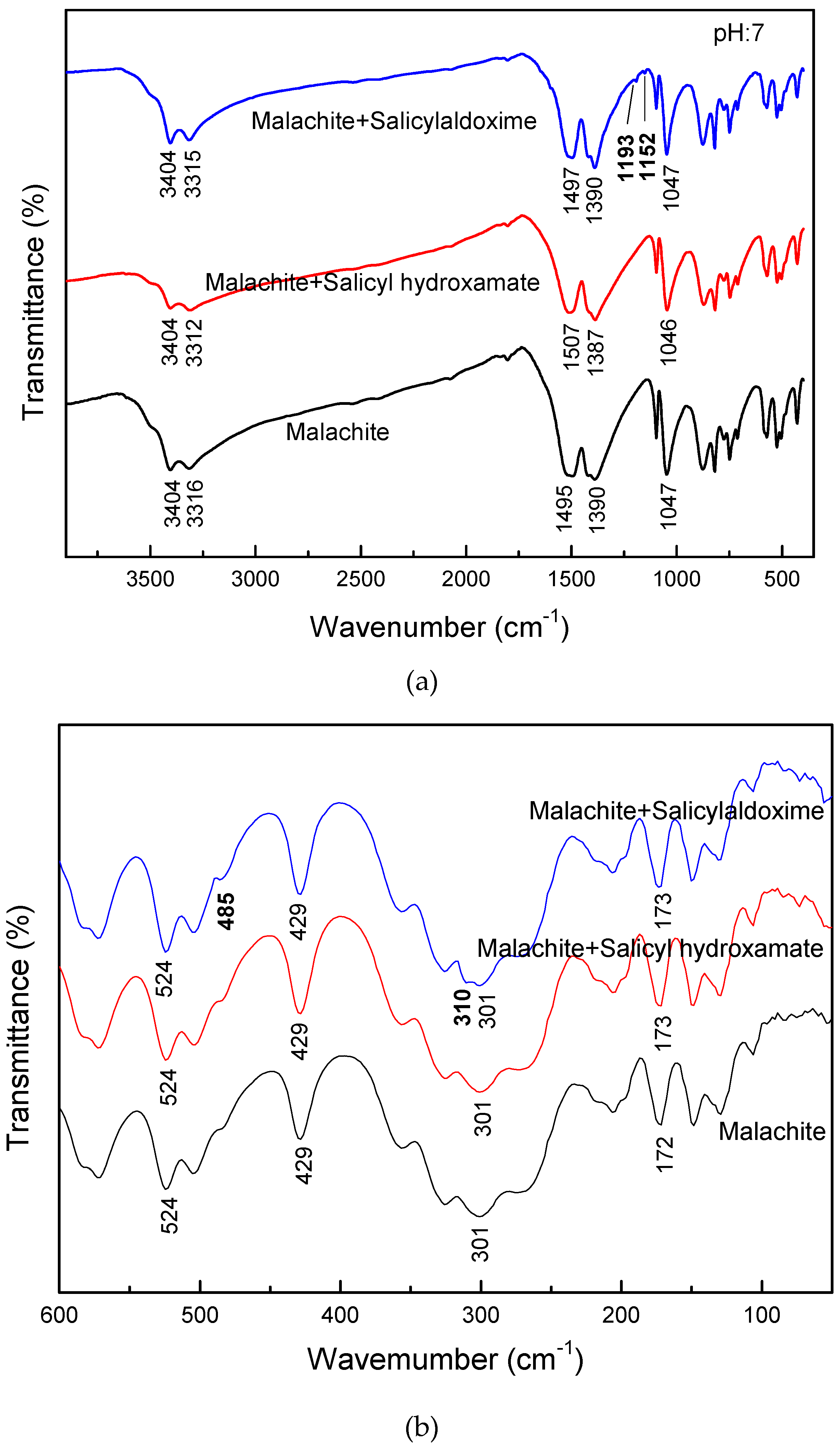
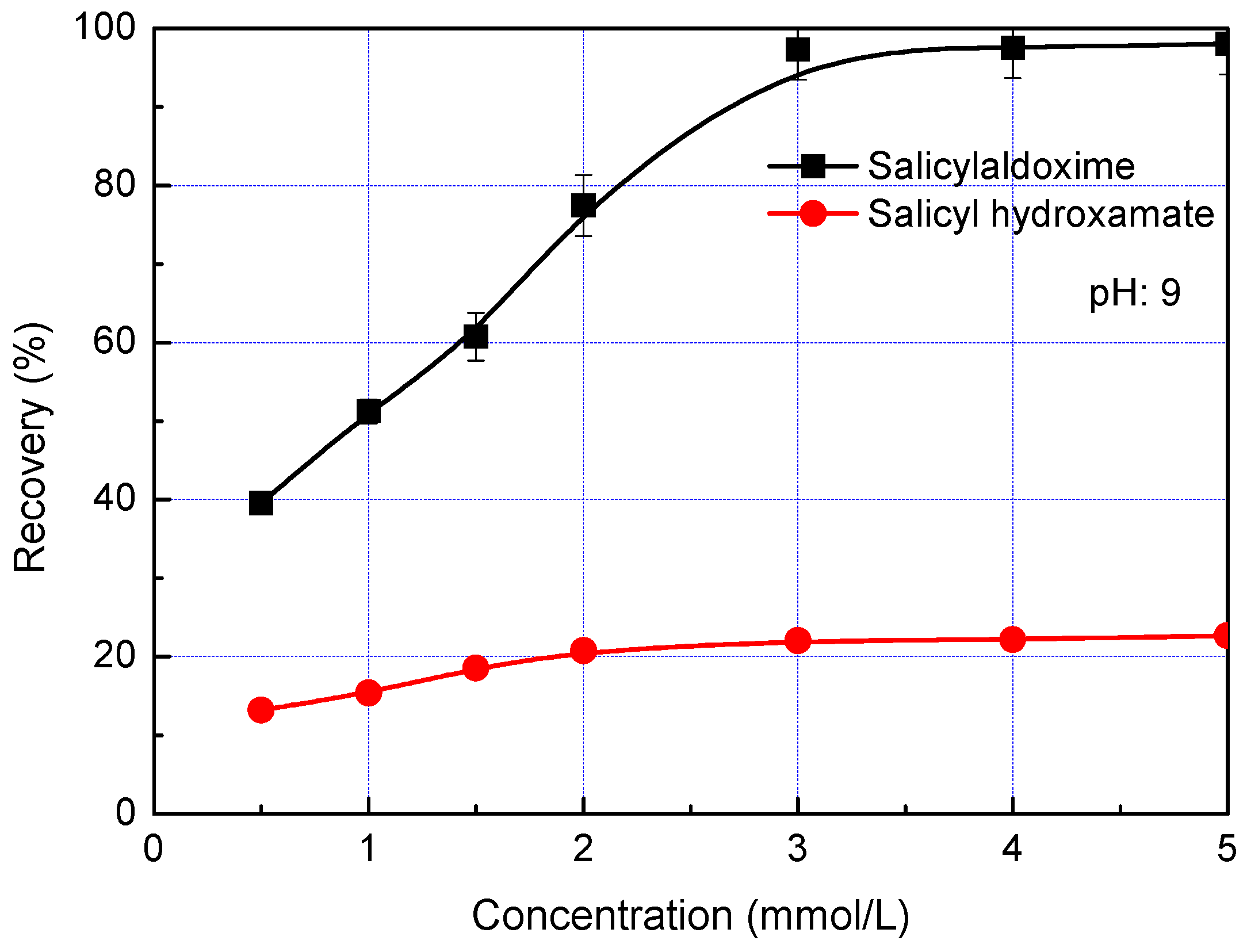
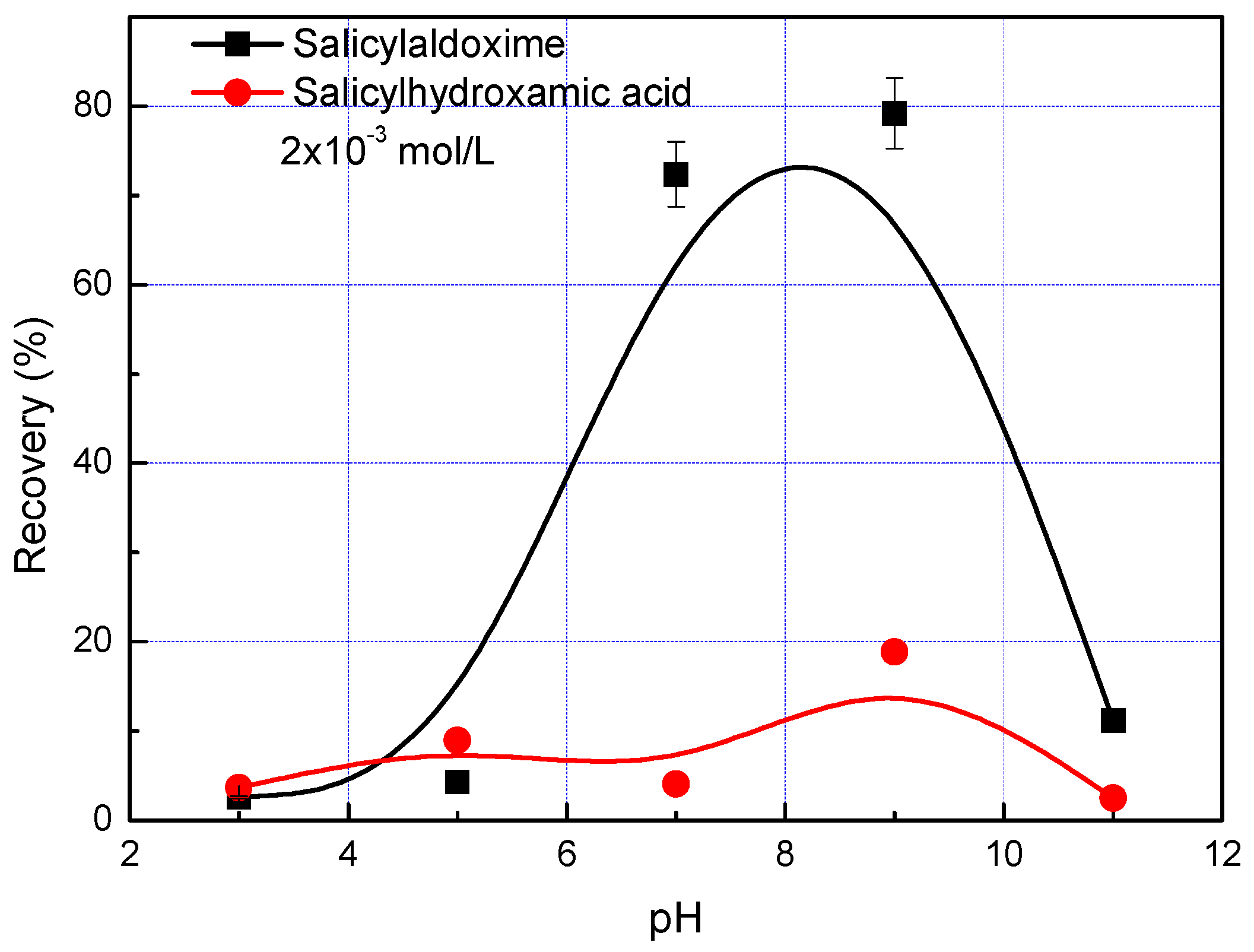
3. Results and Discussion
4. Conclusions
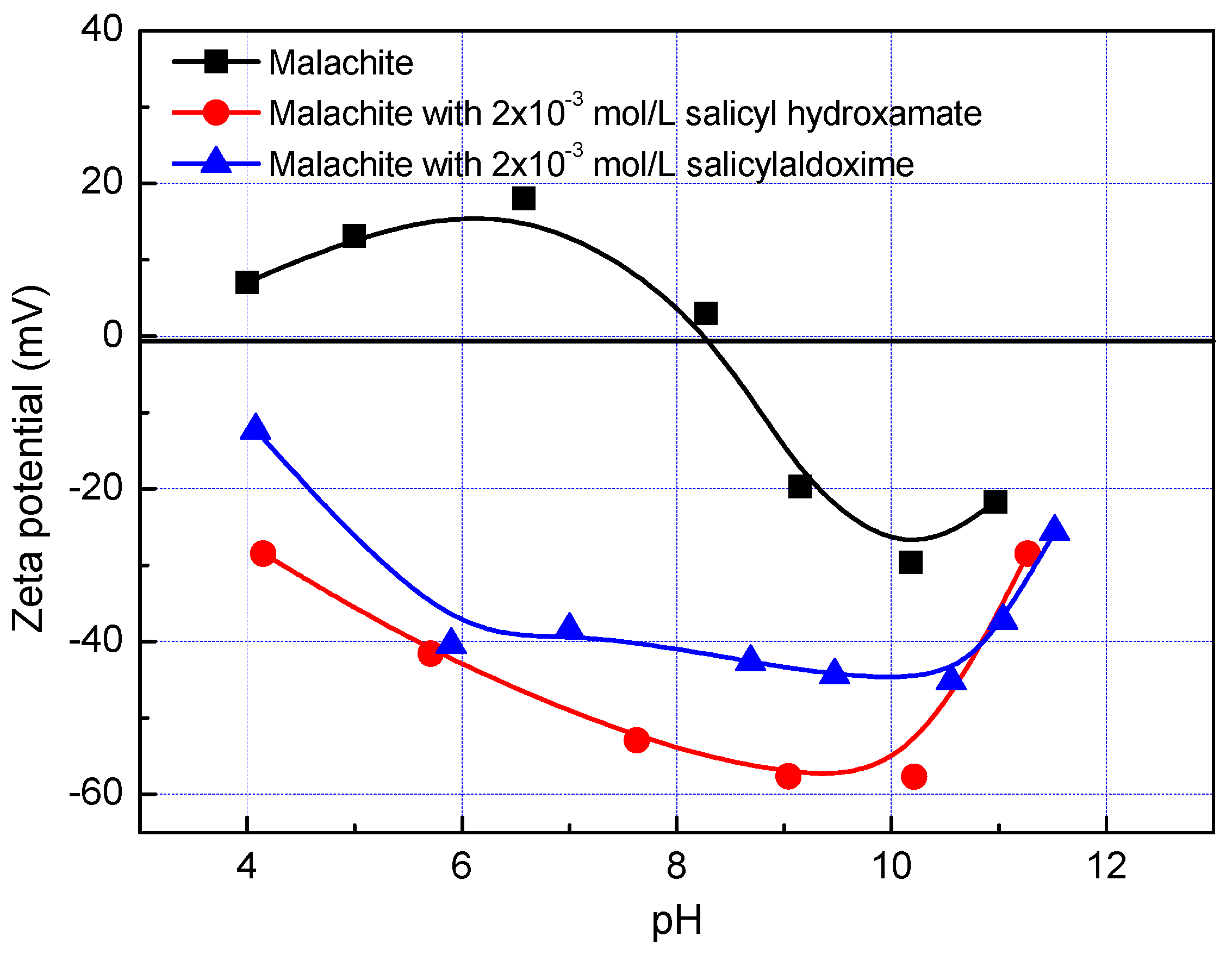
- Salicylaldoxime and salicyl hydroxamate resemble phenol chelating reagents, but salicylaldoxime induces a much higher adsorption density on the malachite surface than salicyl hydroxamate at a pH less than pH 9 because it has a higher stability constant with cupric ions. Thus, in malachite flotation at pH 7–9, the recovery rates are around 80% and 20% when using salicylaldoxime and salicyl hydroxamate as collectors respectively.
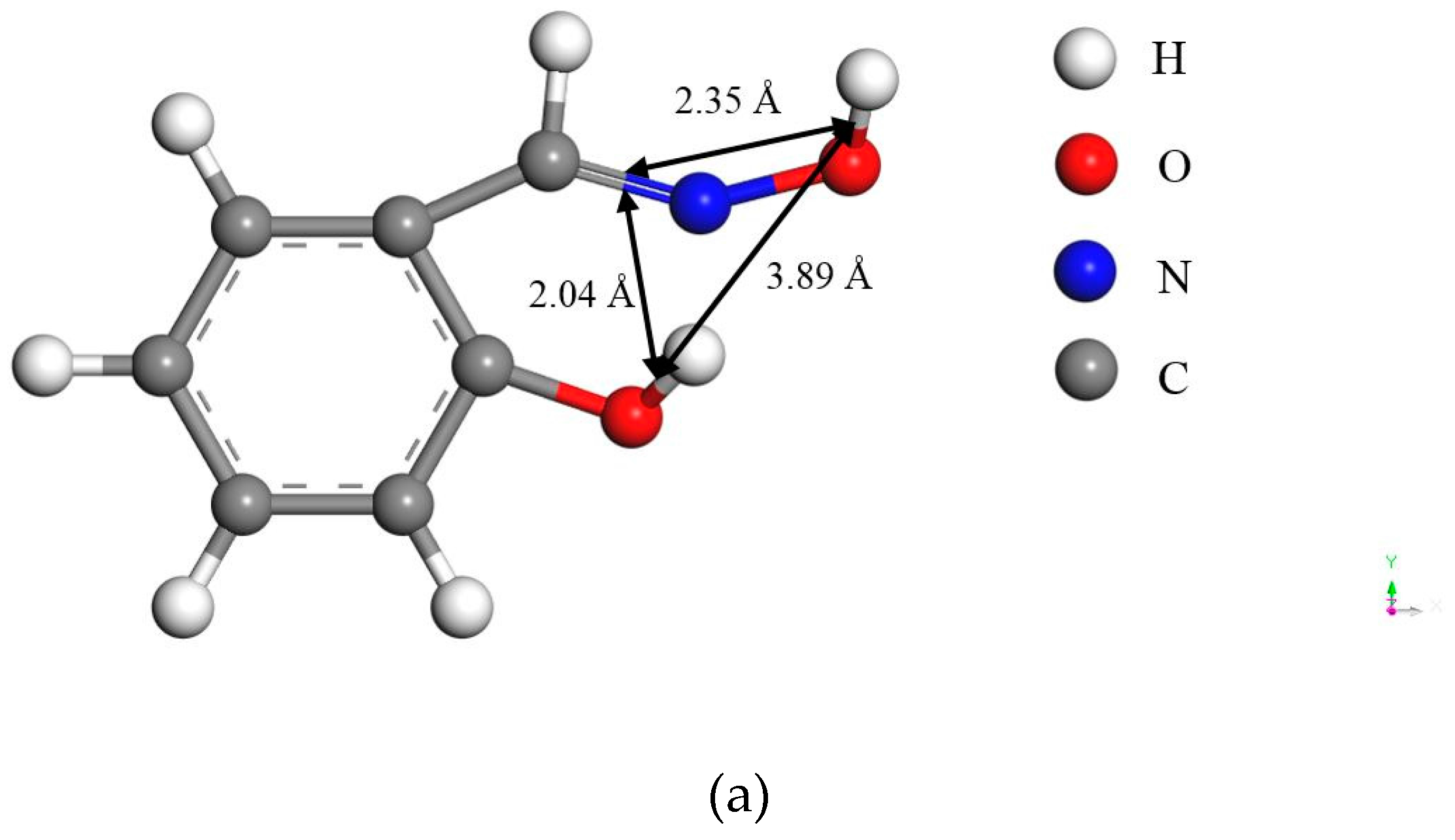
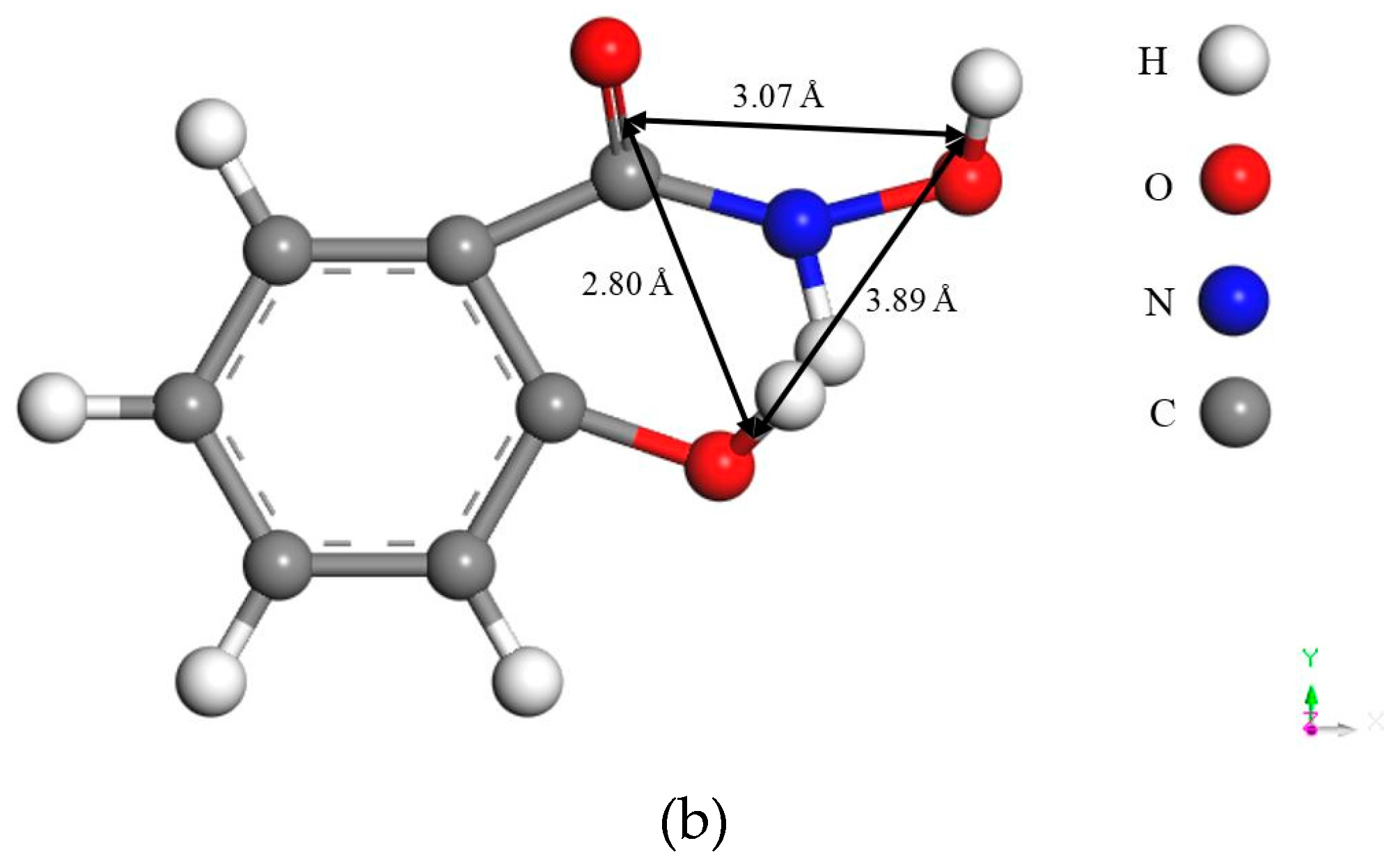
- The large bond distances of ligands in salicyl hydroxamate make its chelating reaction on the malachite surface form a ring structure by two donor atoms with one extra donor oxygen (O−) carrying a negative charge. However, in the case of salicylaldoxime, the interactions of N- and O-ligands are detected by FTIR measurements. Therefore, compared with salicylaldoxime, salicyl hydroxamate modifies the malachite surface more negatively by a lower adsorption amount.
- Salicyl hydroxamate possesses many similarities with two strong malachite collectors, namely salicylaldoxime and octyl hydroxamate, but its collecting ability is very low. This might provide useful clues for the future design of novel collectors in oxide flotations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lee, K.; Archibald, D.; Mclean, J.; Reuter, M.A. Flotation of mixed copper oxide and sulphide minerals with xanthate and hydroxamate collectors. Miner. Eng. 2009, 22, 395–401. [Google Scholar] [CrossRef]
- Miller, J.D.; Abdel Khalek, N.; Basilio, C.; El-Shall, H.; Fa, K.; Forssberg, K.S.E.; Fuerstenau, M.C.; Mathur, S.; Nalaskowski, J.; Rao, K.H.; et al. Flotation Chemistry and Technology of Nonsulfide Minerals. In Froth Flotation: A Century of Innovation; Fuerstenau, M.C., Jameson, G., Yoon, R.H., Eds.; SME Inc.: Littleton, CO, USA, 2007; pp. 465–553. [Google Scholar]
- Castro, S.; Goldfarb, J.; Laskowski, J. Sulphidizing reactions in the flotation of oxidized copper minerals, I. Chemical factors in the sulphidization of copper oxide. Int. J. Miner. Process. 1974, 1, 141–149. [Google Scholar] [CrossRef]
- Zhou, R.; Chander, S. Kinetics of sulfidization of malachite in hydrosulfide and tetrasulfide solutions. Int. J. Miner. Process. 1993, 37, 257–272. [Google Scholar] [CrossRef]
- Barbaro, M.; Urbina, R.H.; Cozza, C.; Fuerstenau, D.W.; Marabini, A. Flotation of oxidized minerals of copper using a new synthetic chelating reagent as collector. Int. J. Miner. Process. 1997, 50, 275–287. [Google Scholar] [CrossRef]
- Fuerstenau, D.W.; Herrera-Urbina, R.; Mcglashan, D.W. Studies on the applicability of chelating agents as universal collectors for copper minerals. Int. J. Miner. Process. 2000, 58, 15–33. [Google Scholar] [CrossRef]
- Gutzeit, G. Chelate-forming organic compounds as flotation reagents. Trans. Am. Inst. Min. Eng. 1946, 169, 272–286. [Google Scholar]
- Lenormand, J.; Salman, T.; Yoon, R.H. Hydroxamate flotation of malachite. Can. Metall. Q. 1979, 18, 125–129. [Google Scholar] [CrossRef]
- Peterson, H.D.; Fuerstenau, M.C.; Rickard, R.S.; Miller, J.D. Chrysocolla flotation by the formation of insoluble surface chelates. Trans. Am. Inst. Min. Eng. 1965, 232, 388–392. [Google Scholar]
- Yoon, R.H.; Hilderbrand, T.M. Purification of Kaolin Clay by Froth Flotation Using Hydroxamate Collectors. U.S. Patent 4629556 A, 16 December 1986. [Google Scholar]
- Hope, G.A.; Woods, R.; Parker, G.K.; Buckley, A.N.; Mclean, J. A vibrational spectroscopy and XPS investigation of the interaction of hydroxamate reagents on copper oxide minerals. Miner. Eng. 2010, 23, 952–959. [Google Scholar] [CrossRef]
- Zhao, G.; Zhong, H.; Qiu, X.; Wang, S.; Gao, Y.; Dai, Z.; Huang, J.; Liu, G. The DFT study of cyclohexyl hydroxamic acid as a collector in scheelite flotation. Miner. Eng. 2013, 49, 54–60. [Google Scholar] [CrossRef]
- Zhang, X.; Du, H.; Wang, X.; Miller, J.D. Surface chemistry aspects of bastnaesite flotation with octyl hydroxamate. Int. J. Miner. Process. 2014, 133, 29–38. [Google Scholar] [CrossRef]
- Oprea, G.; Mihali, C.; Danciu, V.; Podariu, M. The study of 8-hydroxyquinoline and salicylaldoxime action at the malachite flotation. J. Min. Metall. A Min. 2004, 40, 49–63. [Google Scholar]
- Jain, V.; Rai, B. Density functional theory computations for design of salicylaldoxime derivatives as selective reagents in solvent extraction of copper. Trans. Indian Inst. Met. 2016, 69, 135–141. [Google Scholar] [CrossRef]
- Xu, H.; Zhong, H.; Tang, Q.; Wang, S.; Zhao, G.; Liu, G. A novel collector 2-ethyl-2-hexenoic hydroxamic acid: Flotationperformance and adsorption mechanism to ilmenite. Appl. Surf. Sci. 2015, 353, 882–889. [Google Scholar] [CrossRef]
- Liu, G.; Huang, Y.; Qu, X.; Xiao, J.; Yang, X.; Xu, Z. Understanding the hydrophobic mechanism of 3-hexyl-4-amino-1,2,4-triazole-5-thione to malachite by ToF-SIMS, XPS, FTIR, contactangle, zeta potential and micro-flotation. Colloids Surf. A Physicochem. Eng. Asp. 2016, 503, 34–42. [Google Scholar] [CrossRef]
- Hiemenz, P.; Rajagopalan, R. Principle of Colloid and Surface Chemistry, 3rd ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 499–533. [Google Scholar]
- Sillen, L.G.; Martell, A.E.; Bjerrum, J. Stability Constants of Metal-Ion Complexes; Chemical Society: London, UK, 1971; pp. 153–161. [Google Scholar]
- O’Brien, E.C.; Farkas, E.; Gil, M.J.; Fitzgerald, D.; Castineras, A.; Nolan, K.B. Metal complexes of salicylhydroxamic acid (H2Sha), anthranilic hydroxamic acid and benzohydroxamic acid. Crystal and molecular structure of [Cu(phen)2(Cl)]Cl·H2Sha, a model for a peroxidase-inhibitor complex. J. Inorg. Biochem. 2000, 79, 47–51. [Google Scholar] [CrossRef]
- Ramesh, V.; Umasundari, P.; Das, K.K. Study of bonding characteristics of some new metal complexes of salicylaldoxime (SALO) and its derivatives by far infrared and UV spectroscopy. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 1998, 54, 285–297. [Google Scholar] [CrossRef]
- Natarajan, R.; Fuerstenau, D.W. Adsorption and flotation behavior of manganese dioxide in the presence of octyl hydroxamate. Int. J. Miner. Process. 1983, 11, 139–153. [Google Scholar] [CrossRef]
- Sreenivas, T.; Manohar, C. Adsorption of Octyl Hydroxamic acid/salt on cassiterite. Min. Proc. Extr. Metall. Rev. 2000, 20, 503–519. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Rao, F.; Song, S. Comparison of Adsorption of Phenol O-O and N-O Chelating Collectors at the Malachite/Water Interface in Flotation. Minerals 2017, 7, 20. https://doi.org/10.3390/min7020020
Li Z, Rao F, Song S. Comparison of Adsorption of Phenol O-O and N-O Chelating Collectors at the Malachite/Water Interface in Flotation. Minerals. 2017; 7(2):20. https://doi.org/10.3390/min7020020
Chicago/Turabian StyleLi, Zhili, Feng Rao, and Shaoxian Song. 2017. "Comparison of Adsorption of Phenol O-O and N-O Chelating Collectors at the Malachite/Water Interface in Flotation" Minerals 7, no. 2: 20. https://doi.org/10.3390/min7020020
APA StyleLi, Z., Rao, F., & Song, S. (2017). Comparison of Adsorption of Phenol O-O and N-O Chelating Collectors at the Malachite/Water Interface in Flotation. Minerals, 7(2), 20. https://doi.org/10.3390/min7020020





